
Salamander diversity alters stream macroinvertebrate community structure
Summary
- Salamanders are abundant consumers in many fishless stream ecosystems, but few studies have explicitly examined their ecological role. Stream-dwelling salamander larvae are generalist predators of aquatic macroinvertebrates and may play an important role in structuring macroinvertebrate communities. The potential for emergent effects of multiple predator species suggests that changes in salamander diversity could alter their effects on macroinvertebrate communities, but this has not been tested.
- We used in-stream enclosures to manipulate the presence and diversity of the two most abundant salamanders, Eurycea wilderae (EWIL) and Desmognathus quadramaculatus (DQUA), in a southern Appalachian Mountain headwater stream to examine the impacts of salamander predation on macroinvertebrate communities. We were particularly interested in testing for potential diversity effects by comparing the effects of each species in monoculture to polycultures of both species (BOTH).
- Salamanders reduced macroinvertebrate abundance compared to the control treatment (CONTROL), but only when both species were present together. The general pattern of macroinvertebrate abundance among treatments was: CONTROL = EWIL = DQUA > BOTH, although no treatment significantly reduced the abundance of epibenthic taxa. The BOTH treatment also significantly altered macroinvertebrate community structure and reduced taxon richness by c. 57%. The effects of salamanders were particularly pronounced for chironomids (Diptera: Chironomidae), which comprised the majority of macroinvertebrate total abundance.
- Increased salamander diversity substantially altered macroinvertebrate communities, suggesting that niche complementarity or facilitation occurred despite the apparent functional similarity of these two species. Therefore, changes in salamander diversity may alter the effects of salamander communities on macroinvertebrates, which may cascade through food webs to affect stream ecosystem function.
Introduction
Amphibians are abundant consumers in many stream ecosystems, particularly small, fishless headwater streams, but their ecological role is poorly understood. Amphibians are declining worldwide (Stuart et al., 2004; Wake & Vredenburg, 2008), and the consequences of diminished or lost amphibian populations are likely to be significant because of their abundance and functional diversity in both aquatic and terrestrial ecosystems (Whiles et al., 2006). There is substantial evidence that amphibians serve key ecological roles in tropical headwater streams based on ecosystem responses to mass extinctions of anurans in Central America (Ranvestel et al., 2004; Whiles et al., 2006; Connelly et al., 2008; Colón-Gaud et al., 2009, 2010; Rugenski, Múrria & Whiles, 2012). This suggests that amphibians are important members of stream communities and may be overlooked consumers in non-tropical streams as well.
Salamanders, rather than anurans, are the most common amphibians in many North American headwater streams. Larvae of stream-breeding species are predators of aquatic invertebrates and can be extremely abundant in these small streams (Peterman & Truslow, 2008; Nowakowski & Maerz, 2009). The biomass and trophic position of these larvae suggest that they may influence stream ecosystem function through top-down effects (Davic & Welsh, 2004). However, the evidence is somewhat limited and unclear, as both negative (Davic, 1983; Huang & Sih, 1991a) and neutral (Reice & Edwards, 1986; Wooster, 1998) effects of salamander predation on macroinvertebrates have been reported. Additionally, although studies have examined interactions between salamanders, fish and macroinvertebrate prey (Huang & Sih, 1991b; Barr & Babbitt, 2007), potential interactions between multiple salamander species and macroinvertebrates have not been examined. Salamanders reach their highest abundance and diversity in fishless streams (Petranka, 1983, 1998; Barr & Babbitt, 2002; Lowe & Bolger, 2002), and interactions among salamander species may be important in determining their effects on macroinvertebrates in these habitats.
Multiple predator (i.e. predator diversity) effects on prey communities may differ from those observed for single species because of interspecific interactions between predators and prey (Sih, Englund & Wooster, 1998). For example, facilitation among predators can lead to risk-enhancing prey behaviour, so that the effects of multiple predator assemblages are greater than expected based on the performance of individual predator species (Soluk & Collins, 1988; Sih et al., 1998). Additionally, niche complementarity, in either diet or habitat, can also lead to increased resource capture in multiple predator assemblages (Ives, Cardinale & Snyder, 2005; Gable et al., 2012). Alternatively, predator effects will be weaker than expected if the presence of multiple predator species results in risk-reducing prey behaviour (Sih et al., 1998; Vance-Chalcraft & Soluk, 2005). Negative interactions among predator species, such as intraguild predation, may also dampen the effects of diverse predator assemblages (Huang & Sih, 1991b; Finke & Denno, 2005; Vance-Chalcraft et al., 2007). Therefore, predictions about the effects of multiple salamander species on macroinvertebrates based on studies that examined a single species may be misleading.
The Appalachian Mountains of western North Carolina are a hotspot of salamander diversity and abundance, and salamanders are likely to play key ecological roles in this area (Davic & Welsh, 2004). Larval black-bellied salamanders (Desmognathus quadramaculatus) and Blue Ridge two-lined salamanders (Eurycea wilderae) dominate larval salamander assemblages in many southern Appalachian Mountain streams. Both species are benthic, have similar habitat domains and are active gape-limited generalist predators of macroinvertebrates (Davic, 1991; Johnson & Wallace, 2005). Although their diets overlap, E. wilderae consume smaller prey species such as chironomids (Diptera: Chironomidae) and copepods (Davic, 1991; Johnson & Wallace, 2005) to a greater degree, presumably because of their comparably smaller size. These characteristics suggest that these two predators will have a larger impact on macroinvertebrate communities when both species are present than when either species is alone because of the potential for resource partitioning or facilitation (Ives et al., 2005; Schmitz, 2007). However, intraguild predation is common in salamander communities (Beachy, 1993, 1994, 1997), and the threat of intraguild predation may reverse these potential multiple predator effects (Huang & Sih, 1991b; Finke & Denno, 2005; Vance-Chalcraft et al., 2007).
Stream-breeding salamander populations are threatened by numerous stressors that may alter their diversity and abundance in stream ecosystems (Crawford & Semlitsch, 2008; Chatfield et al., 2009; Peterman & Semlitsch, 2009; Vazquez, Rothermel & Pessier, 2009; Milanovich et al., 2010; Peterman, Crawford & Semlitsch, 2011; Price et al., 2011). Because their ecological role is poorly understood within these ecosystems, the ecological consequences of altered salamander diversity are unclear. We therefore manipulated the presence of D. quadramaculatus and E. wilderae larvae in an Appalachian headwater stream to better understand the ecological consequences of differences in salamander diversity. We expected that predation by salamanders would generally reduce the abundance of macroinvertebrates and affect macroinvertebrate community structure, and that increased salamander diversity would alter these effects because of the potential for multiple predator effects among salamanders.
Methods
Study site
This experiment was conducted in Wolf Rock Branch, a fishless first-order tributary of Shope Fork within the Coweeta Hydrologic Laboratory, Macon Co., North Carolina, U.S.A. (35°2′N, 83°27′W). Wolf Rock Branch is at an altitude of c. 930 m and is a heavily shaded stream draining a mixed deciduous forest with a thick understory of rhododendron (Rhododendron spp.). The average wetted width of the experimental reach was 2.2 m with a discharge of c. 2.5 L s−1, although at least twice during the experiment heavy rains increased discharge substantially. Substratum consisted of a mixture of sand, gravel and cobbles with occasional boulders. Ambient nutrient levels were generally low (NH4-N < 10 μg L−1, PO4-P < 2 μg L−1), which is typical of streams in this area.
Experimental design
Enclosures to manipulate larval salamander composition (Fig. 1) were constructed of Acrylite® (Evonik Cyro LLC, Parsippany, NJ, U.S.A.) clear acrylic and 2-mm Nitex® mesh (Miami, FL, U.S.A.). The mesh was sufficiently small to keep salamander larvae from migrating into or out from the enclosures and large enough to allow movements of macroinvertebrates into and out of the enclosures. The enclosures were 0.5 m square to enclose a 0.25-m2 area and were 19.7 cm tall to allow water to flow through the enclosures without overtopping them during most flow conditions. The sides were constructed of 1.27-cm-thick acrylic with two large windows on each side. The windows were covered by the 2-mm Nitex® mesh held in place with silicone and 0.32-cm-thick acrylic fastened to the panels with stainless steel screws. The tops and bottoms of the enclosures were constructed of 0.95-cm-thick acrylic fastened to the sides using stainless steel screws. A 1 : 1 mixture of unbleached playground sand and gravel was added as substratum in enclosures to a depth of c. 2 cm. Four unglazed slate tiles were placed in each enclosure to provide cover for salamanders. Three g of air-dried red maple (Acer rubrum) leaves was placed under a tile in the centre of each enclosure to provide a detrital resource. We allowed macroinvertebrates to colonise enclosures for 7 days before adding salamanders. Enclosures were checked daily to remove leaf litter that had accumulated on the sides of enclosures and may have blocked water flow.

The experiment began on 10 November 2010 and ran for 25 days. The initial density of salamanders was 24 individuals m−2, which is within the observed ranges for these species (E. wilderae = 1–58 individuals m−2, D. quadramaculatus = 1–44 individuals m−2) in nearby streams (Keitzer & Gorforth, 2012). Treatments consisted of monocultures containing 6 individuals of the same species (E. wilderae = EWIL, D. quadramaculatus = DQUA), polycultures containing 3 individuals each of both species (BOTH), a control of no salamanders (CONTROL) and a cage control (CAGE) that did not contain mesh sides to examine the potential effects of mesh size on macroinvertebrate communities. Treatments were randomly assigned to an enclosure and replicated five times. Salamanders were captured with a dip net from within the experimental stream or from nearby streams at the Coweeta Hydrologic Laboratory. We attempted to limit intraguild predation by using smaller D. quadramaculatus, but because of the size differences between species (wet mass of E. wilderae: range = 0.06–0.30 g, mean = 0.12 g, wet mass of D. quadramaculatus: range = 0.12–2.18 g, mean = 0.64 g), it is possible that the threat of intraguild predation altered salamander behaviour in the BOTH treatment. There were no statistically significant differences in the sizes of either species between polyculture and monoculture treatments based on t-tests of the log-transformed wet masses of individual larvae. At the end of the experiment, all organic and inorganic material was removed from the enclosure and preserved in 70% ethanol. Surviving salamanders were counted and returned to their stream of capture.
Preserved material was gently rinsed through a 500-μm mesh sieve to remove macroinvertebrates, which were identified to the lowest practical taxonomic level, typically genus, under a dissecting microscope. Individuals were assigned to functional feeding groups (shredders, collector-gatherers, collector-filterers, predators and scrapers) according to Merritt, Cummins & Berg (2008). Chironomids (Diptera: Chironomidae) were identified as either Tanypodinae or non-Tanypodinae to separate predator and collector-gatherer functional feeding guilds, respectively. We also categorised macroinvertebrates more broadly as either chironomids or epibenthic taxa according to Englund, Sarnelle & Cooper (1999) to allow for comparisons of our results with their meta-analysis of fish and invertebrate predators.
Statistical analysis
An analysis of variance (anova) with a quasi-Poisson error distribution was used to examine treatment effects on the total abundance of macroinvertebrates, chironomids and epibenthic taxa. Significant differences were followed by planned contrasts to make pairwise comparisons of each treatment, a comparison of polycultures to both monocultures combined and a comparison of all salamander treatments to controls. A significant effect of the contrasts comparing polycultures to the monocultures was interpreted as evidence for predator diversity effects because it indicates that the effect of the diverse salamander treatment differed from when species were alone (sensu Bruno & O' Connor, 2005). The P-values of all post hoc comparisons were adjusted to control for the false discovery rate (Benjamini & Hochberg, 1995).
To examine the potential for niche differentiation among salamanders related to prey size selection, we examined the average dry mass of individual macroinvertebrates. The total lengths of individuals were measured under a dissecting microscope and used to calculate their dry mass based on published length–mass relationships (Benke et al., 1999). The mean dry mass of the macroinvertebrates (i.e. size) in an enclosure was then calculated and compared using anova. Data were natural-log-transformed to meet the normality assumptions of anova.
We used both taxon-level and functional trait–level approaches to examine the effects of salamander predation on macroinvertebrate community structure. We calculated the indicator values for each macroinvertebrate taxon to determine which taxa were associated with each treatment or combination of treatments (De Cáceres, Legendre & Moretti, 2010). Indicator values were calculated using the group-equalised point biserial correlation index (De Cáceres & Legendre, 2009). Permutation tests (n = 999 permutations) were used to test the significance of these relationships. The same procedure was repeated to calculate the indicator values of macroinvertebrate functional feeding groups.
A permutational multivariate analysis of variance (manova) on community dissimilarities was used to test the effects of treatments on macroinvertebrate community structure (permutations = 9999). Macroinvertebrate abundance was log + 1-transformed, and the Bray–Curtis distance metric of community dissimilarity was used for this analysis. Non-metric multidimensional scaling (NMDS) was used to visualise the separation of the macroinvertebrate communities subjected to each treatment.
We also calculated the reciprocal form of the Simpson diversity index and taxon richness of enclosures. An anova with a Poisson error distribution was used to examine the effects of salamander treatments on taxon richness. The effects of salamander treatments on the Simpson reciprocal diversity index were tested using anova.
A manova was used to test the effects of treatments on the abundance of the different aquatic macroinvertebrate functional feeding groups. Abundance data were log + 1-transformed to meet the normality assumptions of manova.
We calculated the predator impact of salamanders to compare our results to a meta-analysis of predator effects for invertebrate and fish predators (Englund et al., 1999). The predator impact (PI) of each salamander treatment was calculated as PI = ln (Ntrt/No), where Ntrt is the mean abundance of macroinvertebrates for a salamander treatment and No is the mean abundance of macroinvertebrates in controls (Cooper, Walde & Peckarsky, 1990; Englund et al., 1999). Thus, a negative PI value indicates that predation reduced the abundance of macroinvertebrates. We qualitatively compared the PI of salamanders to the PIs of fish and invertebrates using the COMPLETE data set of Englund et al. (1999) to place salamander predation into context of other aquatic predators. We did not conduct a formal meta-analysis due to a lack of data that met the selection criteria of Englund et al. (1999) for salamander predation effects. All analyses were performed using the R statistical environment version 2.15.1 (R Core Team, 2012) with the vegan (Oksanen et al., 2012), multcomp (Hothorn, Bretz & Westfall, 2008), indicspecies (De Cáceres & Legendre, 2009) and car (Fox & Weisberg, 2011) packages.
Results
One replicate each of the EWIL and BOTH treatments was lost as the result of multiple high flow events that occurred during the experiment. These same high flows washed the substratum out of CAGE enclosures, so we could not include that treatment in the analysis. Additionally, these high flows deposited large amounts of fine sediment in the remaining enclosures. We believe this, along with the high flow itself, may have resulted in relatively high mortality (c. 50%) of salamander larvae. Treatment effects on the number of larvae remaining at the end of the experiment were not detected by an anova with a Poisson error distribution (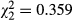 , P = 0.836), indicating that mortality was not related to differences in treatments and was most likely caused by other factors. Including the final density of salamanders as a covariate in analyses did not qualitatively affect the results.
, P = 0.836), indicating that mortality was not related to differences in treatments and was most likely caused by other factors. Including the final density of salamanders as a covariate in analyses did not qualitatively affect the results.
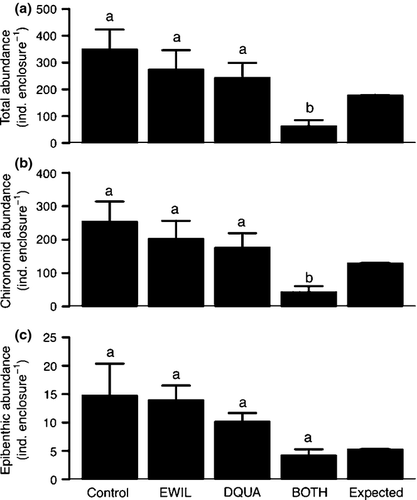
Despite potential issues associated with sediment deposition and high flows, a significant treatment effect was observed for the total abundance of macroinvertebrates (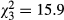 , P < 0.001, R2 = 0.47), chironomids (
, P < 0.001, R2 = 0.47), chironomids (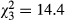 , P = 0.002, R2 = 0.5) and epibenthic taxa (
, P = 0.002, R2 = 0.5) and epibenthic taxa (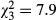 , P = 0.048, R2 = 0.37). The average (±1 SE) total macroinvertebrate abundance (individuals enclosure−1) was 350 ± 164 for CONTROL, 275 ± 143 for EWIL, 245 ± 121 for DQUA and 64 ± 44 for BOTH (Fig. 2). The average abundance for chironomids was 255 ± 59 for CONTROL, 203 ± 53 for EWIL, 176 ± 43 for DQUA and 44 ± 17 for BOTH. The average abundance for epibenthic taxa was 15 ± 6 for CONTROL, 14 ± 2.5 for EWIL, 10 ± 1.5 for DQUA and 4 ± 1 for BOTH. Pairwise comparisons indicated that among treatments, the pattern for the total abundance of macroinvertebrates and chironomids was CONTROL = EWIL = DQUA > BOTH. In contrast, no significant treatment effects were found for epibenthic taxa abundance. Planned contrasts showed that there was a diversity effect (i.e. EWIL + DQUA > BOTH) on the abundance of total macroinvertebrates and chironomids. There was also a significant effect of salamander predation in general (i.e. CONTROL > EWIL + DQUA + BOTH) for total macroinvertebrates and chironomids.
, P = 0.048, R2 = 0.37). The average (±1 SE) total macroinvertebrate abundance (individuals enclosure−1) was 350 ± 164 for CONTROL, 275 ± 143 for EWIL, 245 ± 121 for DQUA and 64 ± 44 for BOTH (Fig. 2). The average abundance for chironomids was 255 ± 59 for CONTROL, 203 ± 53 for EWIL, 176 ± 43 for DQUA and 44 ± 17 for BOTH. The average abundance for epibenthic taxa was 15 ± 6 for CONTROL, 14 ± 2.5 for EWIL, 10 ± 1.5 for DQUA and 4 ± 1 for BOTH. Pairwise comparisons indicated that among treatments, the pattern for the total abundance of macroinvertebrates and chironomids was CONTROL = EWIL = DQUA > BOTH. In contrast, no significant treatment effects were found for epibenthic taxa abundance. Planned contrasts showed that there was a diversity effect (i.e. EWIL + DQUA > BOTH) on the abundance of total macroinvertebrates and chironomids. There was also a significant effect of salamander predation in general (i.e. CONTROL > EWIL + DQUA + BOTH) for total macroinvertebrates and chironomids.
There was no evidence of niche partitioning among salamander species based on the average size of macroinvertebrates in enclosures. The average dry mass of individual prey in treatments was 0.18 ± 0.03 mg for CONTROL, 0.20 ± 0.03 mg for DQUA, 0.18 ± 0.02 for EWIL and 0.21 ± 0.03 for BOTH. These differences were not significantly different (F3,14 = 0.30, P = 0.83).
The indicator taxon analyses showed that non-Tanypodinae were significantly associated with the combination of CONTROL, DQUA and EWIL treatments (rg = 0.61, P = 0.05). No other taxa were significantly associated with any single treatment or combination of treatments. The Simpson reciprocal diversity index across treatments was generally low (mean = 2.17), indicating that communities were dominated by a few taxa. anova did not reveal a significant treatment effect for Simpson's reciprocal index (F3,14 = 0.56, P = 0.65, R2 = 0.1). In contrast, a significant treatment effect was observed for taxon richness (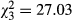 , P < 0.001, R2 = 0.46), which averaged 16 (±1.5) taxa across treatments. Pairwise comparisons demonstrated that the BOTH treatment was significantly lower than other treatments (Fig. 3). There was also a significant effect of salamander diversity on macroinvertebrate richness. These taxon-level differences were reflected in the permutational manova, which demonstrated a significant treatment effect for the structure of aquatic macroinvertebrate communities (F3,14 = 1.60, P = 0.028). A visual examination of the NMDS demonstrates that the macroinvertebrate community structure of the BOTH treatment was separated from all other treatments, which overlapped considerably among each other (Fig. 4).
, P < 0.001, R2 = 0.46), which averaged 16 (±1.5) taxa across treatments. Pairwise comparisons demonstrated that the BOTH treatment was significantly lower than other treatments (Fig. 3). There was also a significant effect of salamander diversity on macroinvertebrate richness. These taxon-level differences were reflected in the permutational manova, which demonstrated a significant treatment effect for the structure of aquatic macroinvertebrate communities (F3,14 = 1.60, P = 0.028). A visual examination of the NMDS demonstrates that the macroinvertebrate community structure of the BOTH treatment was separated from all other treatments, which overlapped considerably among each other (Fig. 4).

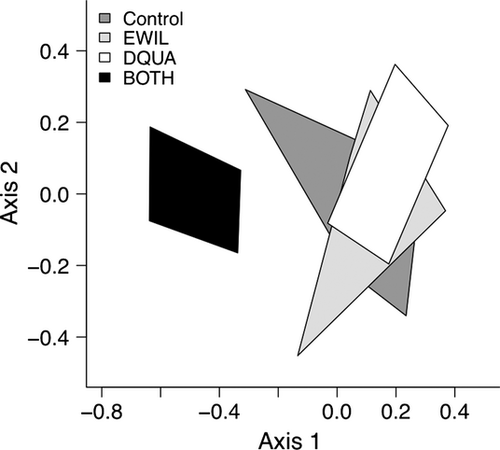
The collector-gatherer group was the most abundant functional feeding group across treatments, followed by predators, collector-filterers, shredders and scrapers. The proportions of collector-gatherer and predator taxa in the enclosure communities differed somewhat from studies in similar streams at Coweeta (Lugthart & Wallace, 1992; Whiles & Wallace, 1995; Wallace et al., 1999; Cross et al., 2006), although the overall order of the rankings is generally similar (Table 1). The BOTH treatment consistently reduced the density of aquatic macroinvertebrate functional groups (Fig. 5), but a manova failed to demonstrate a significant treatment effect (Wilks' λ = 0.201, F3,14 = 2.042, P = 0.057). However, the abundance of predators (rg = 0.62, P = 0.05) and collector-gatherers (rg = 0.61, P = 0.05) was significantly associated with the treatment grouping of CONTROL, DQUA and EWIL.
| Study | Functional Feeding Groups | ||||
|---|---|---|---|---|---|
| Collector-gatherer | Collector-filterer | Scraper | Shredder | Predator | |
| This study, CONTROL | 0.69 | 0.02 | 0.01 | 0.01 | 0.26 |
| This study, DQUA | 0.70 | 0.02 | 0.00 | 0.03 | 0.23 |
| This study, EWIL | 0.68 | 0.02 | 0.01 | 0.02 | 0.27 |
| This study, BOTH | 0.65 | 0.02 | 0.00 | 0.01 | 0.31 |
| Coweeta Streams | 0.85 | 0.01 | 0.01 | 0.03 | 0.12 |
- Data used for comparison are the average values for studies conducted in first- and second-order streams from Catchments 53, 54 and 55 at Coweeta Hydrologic Laboratory (Lugthart & Wallace, 1992; Whiles & Wallace, 1995; Wallace et al., 1999; Cross et al., 2006). We only used mean macroinvertebrate abundance data for pre-treatment years from mixed substratum habitats to calculate relative proportions. Treatments for this study were CONTROL = no salamanders, DQUA = monoculture of Desmognathus quadramaculatus, EWIL = monoculture of Eurycea wilderae, BOTH = polyculture of both salamander species.
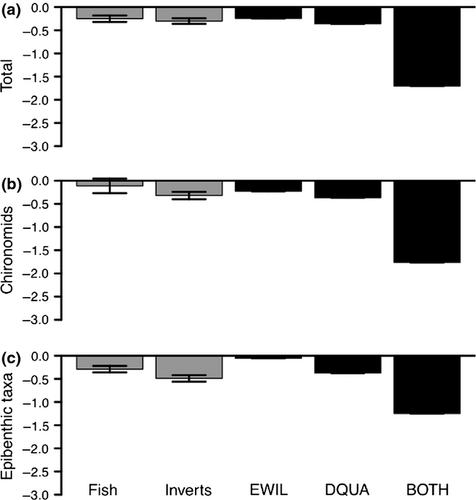
The mean predator impact on the total abundance of macroinvertebrates was −0.36 for the DQUA treatment, −0.24 for the EWIL treatment and −1.7 for the BOTH treatment. The predator impact on chironomids was −0.37 for the DQUA treatment, −0.22 for the EWIL treatment and −1.76 for the BOTH treatment. The predator impact on epibenthic taxa was −0.37 for the DQUA treatment, −0.06 for EWIL treatment and −1.25 for the BOTH treatment. Larval salamander effects on aquatic macroinvertebrates appeared similar to those of fish and invertebrate predators when in monoculture, but were much larger when both salamander species were present together (Fig. 6).
Discussion
Effects of salamander predation on macroinvertebrate communities
Predation by a single species of salamander had a minimal impact on macroinvertebrate communities, but effects became much more pronounced when both species were present. Their combined predation pressure reduced macroinvertebrate abundance by 82% compared to controls and 74% and 77% compared to monocultures of D. quadramaculatus and E. wilderae, respectively. While not always statistically significant, this pattern was remarkably consistent across chironomid and epibenthic taxa, as well as for the different functional feeding groups. Moreover, the combined predation pressure of both species reduced macroinvertebrate taxon richness by more than 50%, while neither species significantly affected taxon richness when alone. Predation by both species together substantially altered macroinvertebrate community structure. These effects were particularly pronounced for non-Tanypodinae (79% reduction), predators (77% reduction) and collector-gatherers (78% reduction). Because non-Tanypodinae comprised the majority of collector-gatherer abundance (98%), the effect on collector-gatherers was predominantly the result of predation on non-Tanypodinae. Non-Tanypodinae was also the most abundant taxon across treatments, on average comprising 66% of the macroinvertebrate community. Chironomids, including non-Tanypodinae, are a key dietary component of both salamander species (Davic, 1991; Johnson & Wallace, 2005), and it appears that the combined predation pressure of D. quadramaculatus and E. wilderae resulted in risk enhancement for non-Tanypodinae. While non-Tanypodinae still represented 60% of the total macroinvertebrate abundance when both salamander species were present, their reduction appeared to account for much of the difference in macroinvertebrate communities. Macroinvertebrate predators were 26% of macroinvertebrate abundance across treatments, and their reduction also contributed to differences in macroinvertebrate communities, although probably not to the same degree as the reduction in non-Tanypodinae.
The magnitude of the diversity effects compared to monocultures suggests that effects were non-additive (Sih et al., 1998) and were not the result of the sampling effect (sensu Huston, 1997). Instead, it appears that either niche complementarity or facilitation (i.e. prey risk enhancement) occurred when both species were present. It is possible that niche partitioning in diet or microhabitat preferences occurred when both species were present. These species consume similar prey, but differences in prey size selection may exist because D. quadramaculatus larvae are much larger than E. wilderae (Davic, 1991; Johnson & Wallace, 2005). We have also found that, while the microhabitat preferences overlap considerably, E. wilderae is more abundant in sandier substratum than D. quadramaculatus, which prefers gravel and cobble substratum (Keitzer & Goforth, unpublished data). Niche complementarity in either of these aspects can lead to greater overall resource capture (Ives et al., 2005; Gable et al., 2012) and may explain the substantial changes in macroinvertebrate community structure associated with the combined predation pressure of both species. It is not known whether facilitation occurs among salamander species, but it has been observed among other stream predators (e.g. Soluk & Collins, 1988; Nilsson et al., 2006) and may also explain the effect of salamander diversity on macroinvertebrate community structure. These mechanisms are not necessarily exclusive and more detailed experiments are needed to tease apart the causal mechanisms of the salamander diversity effect.
Of the proposed mechanisms, we believe that complementarity in diet based on prey size selection was the least likely, solely because of our experimental design. The small mesh (2 mm) used for the enclosures limited the immigration of larger macroinvertebrates into enclosures. Therefore, both species should have been forced to consume similarly sized prey, limiting the possibility for diet complementarity based on prey size. Two lines of evidence support this claim. First, the similarity of the macroinvertebrate communities exposed to predation by either salamander species when in monoculture indicates that the salamander species consumed similar prey species when separate. Secondly, the average size of macroinvertebrates was similar across treatments, suggesting that salamanders were not partitioning macroinvertebrates through prey size selection. Therefore, either facilitation or niche partitioning in another aspect of diet or microhabitat selection is the most likely causal mechanism of the salamander diversity effect in this experiment.
Comparison to previous studies of salamander predation
Previous studies of salamander predation on aquatic invertebrates have shown that a single salamander species can significantly impact macroinvertebrates (Davic, 1983; Huang & Sih, 1991a), but this is not always the case (Reice & Edwards, 1986; Wooster, 1998). It is difficult to compare our results to these studies because each study has employed a different experimental design, which undoubtedly accounts for at least some of the differences in results. For example, the studies by Davic (1983) and Huang & Sih (1991a) removed salamanders from areas within streams that were open to colonisation by multiple size classes of macroinvertebrates, while the mesh in our study limited colonisation of enclosures by larger size classes of macroinvertebrates. In contrast, Reice & Edwards (1986) used in-stream mesh enclosures but manipulated the presence of adults rather than larvae. Adult salamanders have a reduced ability to feed in aquatic environments and may not be expected to have a large impact on aquatic macroinvertebrates (Deban & Marks, 2002; Davic & Welsh, 2004). Huang & Sih (1991a) and Wooster (1998) examined the impacts of predation by different salamander and prey species, using single species of prey, in laboratory experiments with contrasting results. These inconsistencies in experimental design severely limit the comparability of salamander predation studies. There are over 50 salamander species with an aquatic larval stage (Petranka, 1998), and more studies employing consistent methodology on a larger number of species are needed to determine the generality of salamander predation effects.
Comparisons to other stream predator taxa
We found that the effects of either salamander species on macroinvertebrates were comparable to those of fish and macroinvertebrate predators (Englund et al., 1999). The foraging mode of stream predators influences their impacts on prey, and benthic-feeding predators, such as salamanders, typically have a larger impact than drift-feeding species (Dahl & Greenberg, 1996; Englund et al., 1999). This is further supported by the larger impact of salamanders on chironomids, which are generally more vulnerable to benthic predation than epibenthic taxa (Englund et al., 1999). Thus, salamanders had a similar impact on macroinvertebrates as other stream predators and may play an important role in structuring headwater stream macroinvertebrate communities.
While the predator impacts of both salamander species were comparable to other stream predators, the impacts of combined predation by both species were much larger. The meta-analysis of Englund et al. (1999) considered the effects of predator species in monoculture, and our results demonstrate the importance of considering multiple predator species because these effects can differ substantially from predation by a single species (Sih et al., 1998). Most prey species are subjected to multiple predators (Schoener, 1989), and it is therefore important to consider multiple predator effects to better understand the role of stream predator communities in structuring macroinvertebrate prey communities (Sih et al., 1998).
Potential limitations
The size of mesh and salamanders used in this experiment may have limited the generality of our results because it reduced the opportunity for size-based prey selection. Size-based predation is an important factor structuring stream food webs and may result in even greater effects of salamander predation on macroinvertebrate community structure if size differences among salamanders increase the potential for facilitation or niche partitioning (Woodward & Hildrew, 2002; Woodward et al., 2005). Alternatively, introducing larger size classes of salamanders may increase the potential for intraguild predation, which is common among many salamander species (Beachy, 1993, 1994, 1997). Intraguild predation can dramatically alter predation effects (Huang & Sih, 1991b; Finke & Denno, 2005; Vance-Chalcraft et al., 2007) and may play a key role in determining the effects of salamander predation on macroinvertebrate communities. Understanding the relative strengths of these different processes in natural salamander communities is vital to predicting the consequences of changing salamander diversity for headwater streams.
Immigration by prey species in an open system can swamp predation effects (Cooper et al., 1990), but this will depend on prey behavioural responses to predators (Sih & Wooster, 1994). While high prey exchange rates did not appear to swamp predation effects in this experiment, perhaps because the small mesh size (2 mm) of enclosures altered prey exchange rates (Cooper et al., 1990; Englund et al., 1999; but see Wooster, 1994; Dahl & Greenberg, 1996), it is possible that the salamander diversity effect was related to changes in prey immigration and emigration rates rather than consumption (Sih & Wooster, 1994). This would occur if exposure to both salamander species caused prey to increase emigration rates or decrease immigration rates relative to either salamander species alone, thus increasing the magnitude of the apparent impact of salamander predation (Sih & Wooster, 1994). If this were the case, then the diversity effect may only be relevant at the local patch scale (Englund, 1997). In other words, salamander predation may influence the distribution of macroinvertebrates within streams but not affect overall population dynamics at larger spatial scales. This is not to say that these effects are unimportant; stream predators have been shown to influence the patch-scale distribution and biomass of macroinvertebrate species (Peckarsky & Dodson, 1980; Lancaster, 1990; Gibson, Ratajczak & Grossman, 2004), which can impact ecosystem processes (Malmqvist, 1993; McIntosh, Peckarsky & Tayler, 2004; Woodward et al., 2008). Studies manipulating salamander abundance and diversity at multiple scales, as well as testing the behavioural response of macroinvertebrates to the presence of multiple salamander species, would help reveal potential scale dependency in predation effects.
In addition to potentially altering prey exchange rates, the mesh size in the experiment caused a substantial amount of fine sediment to be deposited in enclosures. Although sediment deposition appeared to be equally distributed across enclosures and treatments (S. Keitzer, personal observation), sedimentation can significantly affect macroinvertebrate communities (e.g. Angradi, 1999; Zweig & Rabeni, 2001; Matthaei et al., 2006; Larsen & Ormerod, 2010), and it is possible that it influenced the magnitude of predation impacts. For example, sedimentation may have reduced habitat complexity and increased predation rates (Brusven & Rose, 1981; Dahl & Greenberg, 1997), or it may have altered the macroinvertebrate community to taxa that are more vulnerable to salamander predation. We found that the macroinvertebrate communities in enclosures contained a much lower proportion of collector-gatherers (c. 20%) and a much higher proportion of predators (c. twofold) than expected when compared to natural stream communities, but a similar proportion of the remaining functional feeding groups was present (Lugthart & Wallace, 1992; Whiles & Wallace, 1995; Wallace et al., 1999; Cross et al., 2006). This suggests that either sedimentation or some aspect of the enclosure design influenced macroinvertebrate communities. However, it is not clear how these differences may have affected our results, and we believe that the predation effects in this study are still relevant because all of the experimental treatments appeared to be similarly affected.
In conclusion, while bottom-up effects on macroinvertebrates and salamanders have been well documented in Appalachian headwater streams (Wallace et al., 1999; Johnson & Wallace, 2005; Cross et al., 2006; Greenwood et al., 2007), our results indicate that top-down effects of salamander predation may also be important in structuring macroinvertebrate communities. Although these effects may be limited in their scale, we have provided evidence that salamanders are underappreciated consumers in headwater streams and that further research investigating their ecological role is warranted. Perhaps more importantly, we found that salamander diversity significantly alters the effects of salamanders on aquatic macroinvertebrate communities. Salamander populations and diversity are threatened by a multitude of factors, including habitat disturbance, disease and climate change (Crawford & Semlitsch, 2008; Chatfield et al., 2009; Peterman & Semlitsch, 2009; Vazquez et al., 2009; Milanovich et al., 2010; Peterman et al., 2011; Price et al., 2011). Because salamanders are common in fishless headwater streams throughout North America (Davic & Welsh, 2004), their importance and the significance of changes in their diversity may be extensive.
Acknowledgments
We thank Jay Beugly, Kevin Leet, Sam Nutile, Alejandra Principe and Tess Thoren for assistance with the experiment and processing samples and the staffs at Highlands Biological Station and Coweeta for logistical support. The Goforth laboratory, Roger Jones and two anonymous reviewers provided valuable comments that greatly improved earlier versions of this manuscript. A Highlands Biological Station Grant-in-Aid provided funding for this project. This research was conducted under a North Carolina Wildlife Resource Commission Wildlife Collection License (permit 10-SC00395), and animals were handled according to Purdue University Animal Care and Use Committee.




