
Synergistic impacts of sediment contamination and dam presence on river functioning
Summary
1. Dam presence is commonly associated with strong accumulation of polluted sediments. In spite of this context of multiple stressors, physical effects are often solely considered in the ecological assessment of the dam impacts.
2. We studied four ‘reservoir/downstream reach’ systems differing in levels of sediment contamination in reservoirs. Using assemblages and biotrait (i.e. ecological or biological attribute) responses of macroinvertebrate communities and leaf litter breakdown, we examined the individual effects and potential interactions between sediment contamination and dam presence along the gradient of ecotoxic pressure.
3. Leaf breakdown rates ranged from 0.0044° per day in the most contaminated reservoir to 0.0120° per day in the reference reservoir. Comparisons of community trait profiles among reservoirs highlighted a gradient of trait responses to sediment contamination.
4. In the absence of toxic contamination, the dam-induced modifications in biotraits of invertebrate assemblages were not related to a reduction of leaf litter breakdown. Conversely, contaminated sediment in reservoir induced strong functional disturbances (i.e. bioecological shifts and reduction of leaf litter breakdown) downstream of dams.
5. Key biotrait categories positively related to leaf litter breakdown rate have been identified. They corresponded mainly to shredders and/or small-sized (<0.5 cm) insects, using aquatic (e.g. crawlers) or aerial (e.g. fliers) active dispersal strategies. In addition, trait categories positively correlated to contamination level have been considered as ‘response’ traits. They corresponded to large-sized (>4 cm) species, having several generations per year (polyvoltin), using asexual reproduction and/or disseminating by drift (aquatic, passive).
6. In the current context of ecological continuity restoration, this study has identified the risks associated with the presence of historical contamination in the run-of-river reservoirs for downstream ecosystem health.
Introduction
The proliferation of industries in the 20th century has induced a strong increase in metal inputs in rivers which have led to large-scale degradation in freshwater communities. Despite increasing severity in environmental legislation and recent decrease of metal discharges, high concentrations of metals are still present in sediment, especially those that are fine-grained (Cairns et al., 1984; Chapman, 1990; Brumbaugh et al., 2007). In addition to this permanent toxic pressure, the increasing need of water has led to river hydromorphology management including an excessive construction of dams and weirs (Malmqvist & Rundle, 2002). These infrastructures drastically disturb sediment transport and resident biota, impair the physical and biological features of river channels (Petts, 1984; Stanford et al., 1996; Poff et al., 1997) and favour pollutant accumulation. Contaminated sediments are a significant, diffuse source of toxic contamination and represent a serious threat to freshwater ecosystem–ecological integrity and human health (Neal et al., 2005; Byrne, Reid & Wood, 2010). Even if the biological impacts of physical alterations on freshwater communities have been increasingly taken into account in the last decade, the ecological risk related to sediment contamination remains far less considered. However, in functional terms, sediments are an important part of bottom mosaics where invertebrates are involved in organic matter decomposition, nutrient cycling, carbon sequestration and pollutant degradation (Groffman & Bohlen, 1999; Adams & Wall, 2000; Austen et al., 2002). Contaminated sediment could impair not only resident invertebrate communities, but also communities far from dams, as toxicants can be transferred downstream during reservoir flush-outs or dam removal, leading to physical (e.g. interstitial space clogging) and chemical (e.g. metal toxicity) disturbances (Larsson, 1985; Asare et al., 2000; Coulthard & Macklin, 2003).
Sediment toxicity has been assessed by biotests which do not account for responses at the community level (Lafont et al., 2007). Traditionally, stream bioassessment methods have focused on benthic macroinvertebrate communities (Rosenberg & Resh, 1993) mainly involving taxonomy-based metrics that describe assemblage structure (Hering et al., 2006; Friberg et al., 2009). However, both ‘structure’ and ‘function’ should be simultaneously considered to efficiently assess the ecological integrity of rivers (Minshall, 1996; Gessner & Chauvet, 2002). Functional metrics can significantly contribute to a better assessment of stream ecosystem health because they (i) respond to many abiotic and biotic parameters, (ii) integrate environmental conditions over time and space, (iii) are more directly and easily translated in terms of ecosystem functioning than taxonomic metrics, (iv) simultaneously cover several habitats within streams and (v) are comparable at large spatial scales, even across ecoregions that differ in their taxonomic composition (Feio et al., 2010).
One of the most common methods for assessing pollutant impacts on ecosystem functioning is the measurement of leaf litter breakdown which can give evidence of functional changes in bacterial, fungal and invertebrate community composition and activity (Gessner & Chauvet, 2002; Woodward et al., 2012). It has been widely reported that leaf litter breakdown is reduced in waters impaired by metal contamination (Burton, Stanford & Allan, 1985; Maltby & Booth, 1991; Carlisle & Clements, 2005). Biotrait-based approaches can also fundamentally improve diagnosis of anthropogenic impacts by (i) providing mechanistic understanding and inference of potential cause–effect relationships between stressors and biological impairments (Dolédec & Statzner, 2008; Verberk, Siepel & Esselink, 2008; Archaimbault et al., 2010; Culp et al., 2010) and (ii) partially resolving effects of multiple stressors (Statzner & Bêche, 2010; Schäfer et al., 2011). Moreover, the influence of macroinvertebrate trait combinations on leaf litter breakdown has been insufficiently explored, even though they are likely to be more powerful than taxonomic metrics in detecting and understanding the mechanistic basis of community diversity effects on decomposition processes (Lecerf et al., 2006; Castela, Ferreira & Graça, 2008). The joint study of functional processes and biotrait changes in benthic communities would allow identification of the best response (to pressures) and effect (on ecosystem processes) traits to include in ecological and ecotoxicological risk assessment procedures for contaminated sediments of rivers.
Despite the ecological and ecotoxicological risks on resident and downstream communities, the run-of-river reservoirs have been very little studied. Yet, studying these systems provides an opportunity (i) to elucidate the mechanisms of community and ecosystem responses to multiple (i.e. physical and chemical) stressors and (ii) to improve predictions about downstream impact of dams, especially in the case of contaminated sediment. The main objectives of this study were (i) to distinguish functional changes specifically related to sediment contamination from those related to dam presence, (ii) to identify effects of multiple stressors (i.e. physical constraints and toxic contamination) on downstream communities and (iii) to compare taxonomic (taxa identity-based approach), functional (trait-based approach) and ecosystems (functional process approach) responses to these stressors. We hypothesised that sediment contamination in reservoirs enhances the impact of physical constraints on ecosystem functioning not only in the reservoirs but also in the downstream reaches due to downstream propagation.
Methods
Study sites
Four reservoir/downstream reach systems on the Ain and Bienne rivers belonging to the same ecoregion (Fig. 1, Northern Jura-Prealps, France, Wasson et al., 2002) were selected to examine leaf litter breakdown rates and variation in assemblages and mean biotrait profiles of macroinvertebrate communities in response to both dam presence and contaminated sediment. According to the French National Agency for Water and Aquatic Environments (ONEMA), the studied reservoirs are small (2–7 ha) and come from weirs along the watercourse. In spring, the average flows of the Ain and Bienne rivers at the study sites were 7.80 m3s−1(±3.44) and 5.27 (±3.80) m3s−1, respectively. For the reference system (S1: Up1/Re1/Do1), we added an upstream site (Up1) to isolate the physical impact from the effects of sediment contamination on both the reservoir and downstream communities. The mean wet width of the study sites ranged from 19 to 35 m in rivers and from 39 to 112 m in reservoirs. The average depth of study sites ranged from 29 to 49 cm in rivers and from 69 to 141 cm in reservoirs. Although the catchment of both rivers is mainly forested (Ain: 57.3% and Bienne: 72.6%) and agricultural (Ain: 36.0% and Bienne: 22.0%; dairy exploitations), historical industrial activities, mainly in the upstream sector of the Bienne river, and paper mills along the Ain river have led to the construction of dams and to the discharge of many industrial effluents. The physicochemical characteristics of sediments (Ekman grab, sampling depth of 20–30 cm) and water were measured by a specialised laboratory (LDA26, Valence, France) on samples collected during the spring field campaign according to international or national standards: for metals [ISO 11885 (Fe, Al, Mn, Zn); NF EN ISO 17294-2 (Cd, Cr, Co, Cu, Ni, Pb, As) and NF EN 1483 (Hg)] and for PAHs and PCBs (XP X33012 method). Concentrations of eleven metals, eight PCBs and 10 PAHs were investigated in sediments. PCBs were always below their quantification limit (5 μg kg DM−1). The level of sediment contamination in a given reservoir was defined according to its mean rank for each pollutant concentration (mol g−1). A higher rank represents a higher contamination level. The selected systems represented a significant gradient of sediment contamination in metals and PAHs (Friedman’ test –P < 0.001), from the reference reservoir (Re1) to the most contaminated reservoir (Re4). Table 1 summarises the main environmental characteristics of the study sites.
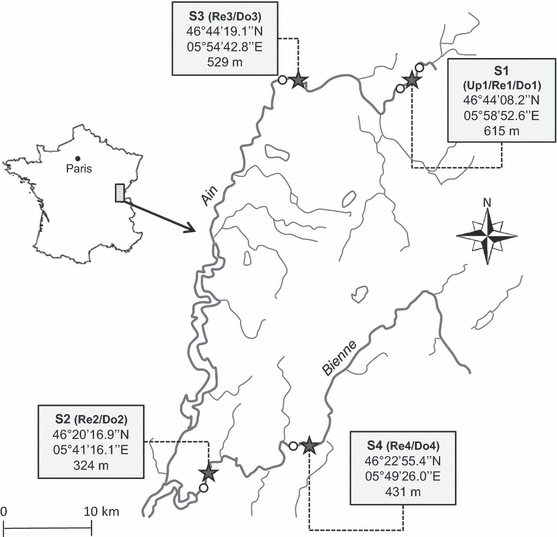
Location of the study systems in the Jura Mountains, eastern France. Stars correspond to the reservoir sites, and circles represent river sites upstream or downstream from the dams.
| Reservoir sites (n = 4) | River sites (n = 5) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Re1 | Re2 | Re3 | Re4 | Up1 | Do1 | Do2 | Do3 | Do4 | |
| Water chemistry | |||||||||
| pH | 8.10 | 8.50 | 8.10 | 8.20 | 8.10 | 8.20 | 8.15 | 8.35 | 8.20 |
| Dissolved oxygen | 12.40 | 12.50 | 11.90 | 11.40 | 11.30 | 11.30 | 11.00 | 9.80 | 10.50 |
| Conductivity (μS cm−1) | 416.00 | 329.00 | 305.00 | 364.00 | 412.00 | 411.00 | 347.00 | 361.00 | 325.00 |
| Calcium (mg L−1) | 82.00 | 68.00 | 64.00 | 74.00 | 82.00 | 82.00 | 70.00 | 75.00 | 65.00 |
| Magnesium (mg L−1) | 7.20 | 3.90 | 2.90 | 4.20 | 7.30 | 7.52 | 4.00 | 4.20 | 3.30 |
| Dissolved organic carbon (mg L−1) | 1.40 | 1.70 | 1.80 | 1.80 | 1.50 | 1.40 | 1.70 | 1.70 | 1.80 |
| Biological oxygen demand (mg L−1) | <0.50 | <0.50 | 0.60 | <0.50 | 0.50 | <0.50 | 0.50 | 0.70 | 0.60 |
| Phosphorus (mg L−1) | 0.006 | 0.01 | 0.01 | <0.005 | <0.005 | 0.01 | 0.02 | 0.01 | 0.02 |
| Metal contamination in sediments (mg kg DW−1) | |||||||||
| Aluminum (Al) | 3.7 × 103 | 2.5 × 103 | 2.1 × 103 | 4.4 × 103 | 0.8 × 103 | 1.2 × 103 | 1.0 × 103 | 0.7 × 103 | 0.7 × 103 |
| Cadmium (Cd) | <0.20 | <0.20 | 0.20 | 0.30 | <0.20 | <0.20 | <0.20 | <0.20 | 0.30 |
| Cobalt (Co) | 3.40 | 2.50 | 2.30 | 3.40 | 2.10 | 2.50 | 1.90 | 1.40 | 1.40 |
| Iron (Fe) | 9.9 × 103 | 4.9 × 103 | 3.9 × 103 | 7.0 × 103 | 4.5 × 103 | 7.3 × 103 | 4.4 × 103 | 1.7 × 103 | 2.2 × 103 |
| Lead (Pb) | 9.10 | 11.50 | 32.00 | 23.90 | 4.90 | 4.70 | 16.10 | 82.90 | 18.80 |
| Arsenic (As) | 10.60 | 4.60 | 4.90 | 5.90 | 8.70 | 11.40 | 3.50 | 4.10 | 3.30 |
| Chromium (Cr) | 21.30 | 14.90 | 32.80 | 12.80 | 9.20 | 12.90 | 6.30 | 4.90 | 5.00 |
| Copper (Cu) | 4.10 | 24.00 | 32.20 | 45.30 | 2.10 | 1.70 | 5.50 | 21.60 | 2.90 |
| Nickel (Ni) | 9.40 | 10.80 | 16.90 | 6.50 | 5.10 | 5.90 | 4.90 | 4.30 | 3.10 |
| Zinc (Zn) | 26.90 | 52.40 | 78.20 | 85.70 | 17.50 | 13.90 | 22.30 | 55.50 | 30.60 |
| PAH contamination in sediments (μg kg DW−1) | |||||||||
| Benzo(a)pyrene (BaP) | 23.00 | 62.00 | 535.00 | 286.00 | <10 | 197.00 | 46.00 | 67.00 | 44.00 |
| Benzo(a)anthracene (BaA) | 20.00 | 46.00 | 649.00 | 233.00 | <10 | <10 | 44.00 | 68.00 | 55.00 |
| Benzo(k)fluoranthene (BkF) | 12.00 | 28.00 | 206.00 | 121.00 | <10 | 90.00 | 22.00 | 36.00 | 31.00 |
| Benzo(ghi)perylene (BgP) | 5.00 | 47.00 | 43.00 | 133.00 | <10 | 153.00 | 28.00 | 46.00 | 36.00 |
| Fluoranthene (Fluo) | 46.00 | 127.00 | 1919.00 | 545.00 | <40 | 443.00 | 105.00 | 161.00 | 130.00 |
| Indeno(1,2,3-cd)pyrene (Ind) | 21.00 | 29.00 | 395.00 | 118.00 | <10 | 108.00 | 18.00 | 36.00 | 33.00 |
| Phenanthrene (Phen) | <50 | <50 | <50 | 180.00 | <50 | 119.00 | <50 | <50 | 63.00 |
| General information | |||||||||
| Surface (ha) | 2.0 | 3.7 | 3.0 | 7.0 | |||||
| Dam types | Broad-crested | Broad-crested | Broad-crested | V-notch | |||||
| Exploitation | Out of use | In use | In use | In use | |||||
Leaf litter breakdown
Senescent alder (Alnus glutinosa) leaves were collected from trees just before abscission in autumn 2009. Litter bags were made by placing 4 g (±0.026) of air-dried leaves into mesh bags (15 × 20 cm, 5 mm mesh size) to allow stream biota access. In each site, one iron bar was anchored to a sedimentary habitat, and four bags were fastened to each bar during spring field campaign (2010). Data loggers were placed with the litter bags on each site to record water temperature every thirty minutes during the experiment. Bags were retrieved after 40 days and returned to the laboratory. The leaves were individually rinsed with water from the corresponding stream to remove fine particulate matter and invertebrates. The remaining leaf material was oven-dried to constant mass (105 °C, 48 h) and weighed to the nearest 0.1 mg. Subsamples (500 mg) were ignited in a muffle furnace (550 °C, 4 h) to relate dry mass to ash-free dry mass (AFDM). Four additional leaf bags were kept in the laboratory before starting the experiment to estimate the initial oven-dried mass and AFDM of all leaf bags.
Macroinvertebrate communities
Macroinvertebrates were sampled during the spring campaign (2010) following two protocols. Macroinvertebrates in reservoirs were sampled using an original protocol (Colas, Archaimbault & Devin, 2011), whereby reservoirs were subdivided into three zones, termed ‘tail’, ‘middle’ and ‘dam’, reflecting their various hydraulic and hydro-morphological conditions. Communities of the banks and of the channel were sampled separately. Three to four samples were taken on the left and right banks of each zone using a Surber net. Macroinvertebrates in the channel were sampled by dredging from a boat perpendicular to a transect in each of the three areas defined earlier. Macroinvertebrates in river sites were sampled according to a normalised protocol (Multi-Habitat Sampling, norm XP T 90-333 in AFNOR, 2009) designed to fulfil the European Water Framework Directive (WFD) requirements. All samples were preserved in 4% formalin in the field and transferred to the laboratory where they were sieved and sorted. Organisms were mainly identified to genus level (using Tachet et al., 2000), except for some groups, which were only identified to higher taxonomic level [i.e. Diptera (family or tribe level) and Oligochaeta or Nematoda]. Individuals within each taxon were then counted. Taxa with fewer than three individuals caught during the sampling programme were not included in the analysis of faunal data. Due to the heterogeneity of site-sampling protocols, the abundances of taxa were transformed to densities, taking into account the sampled area for each sample unit, in order to allow between-sample and between-site comparisons. From taxonomic data (Table 2), we calculated site community mean biotrait profiles, thanks to 22 fuzzy-coded biological and ecological traits (Chevenet, Dolédec & Chessel, 1994; named biotraits hereafter) described for each taxon from the literature. The biological traits reflect the life history of taxa (e.g. ‘number of cycles per year’), their resistance and resilience abilities (e.g. ‘resistance forms’) and general morphological (‘body form’) or physiological (e.g. ‘respiration’, ‘feeding habits’) features of organisms (Usseglio-Polatera et al., 2000). Ecological traits mainly describe habitat preferences of taxa at different spatial scales (e.g. substratum, current velocity, temperature, pH, saprobity, longitudinal distribution). Each trait is described by a set of categories (named modalities). The mean weighted (by log-transformed abundances) trait profiles of site assemblages were calculated and expressed as relative abundance distributions of trait categories within the assemblages (Thioulouse et al., 1997).
| Taxon | Up1 | Re1 | Do1 | Re2 | Do2 | Re3 | Do3 | Re4 | Do4 |
|---|---|---|---|---|---|---|---|---|---|
| Allogamus* | + | + | + | + | + | + | + | ||
| Amphinemura* | + | + | + | + | + | + | + | + | + |
| Ancylus | + | + | + | + | + | + | + | + | |
| Antomyidae* | + | + | + | + | + | + | + | + | + |
| Asellus aquaticus* | + | + | + | + | + | ||||
| Atherix | + | + | + | + | + | + | + | + | + |
| Baetis | + | + | + | + | + | + | + | + | |
| Brychius* | + | + | + | ||||||
| Caenis* | + | + | + | + | + | ||||
| Ceratopogonidae | + | + | + | + | + | + | + | ||
| Chironomini* | + | + | + | + | + | + | + | + | + |
| Clinocerinae | + | + | + | + | + | + | + | + | |
| Dendrocoelum | + | + | |||||||
| Drusus* | + | + | |||||||
| Dugesia | + | + | + | + | + | + | |||
| Dytiscidae* | + | + | + | + | + | + | + | ||
| Ecdyonurus* | + | + | + | + | + | + | + | + | + |
| Elmis* | + | + | + | + | + | + | + | + | |
| Epeorus | + | + | |||||||
| Ephemera* | + | + | + | + | + | + | + | + | |
| Ephemerella | + | + | + | + | + | + | + | + | + |
| Erpobdella | + | + | + | + | |||||
| Esolus* | + | + | + | + | + | + | + | + | + |
| Gammarus* | + | + | + | + | + | + | + | + | |
| Glossiphonia | + | + | + | + | + | + | + | ||
| Glossosoma | + | + | + | + | |||||
| Gordius | + | + | + | ||||||
| Halesus* | + | + | + | + | + | ||||
| Helobdella | + | + | |||||||
| Hemereodromiinae | + | + | + | + | + | + | + | + | + |
| Heptagenia | |||||||||
| Hydra | + | + | + | + | |||||
| Hydracarina* | + | + | + | + | + | + | + | + | + |
| Hydraena* | + | + | + | + | + | + | |||
| Hydropsyche | + | + | + | + | + | + | |||
| Hydroptila | + | + | + | + | + | + | + | ||
| Lasiocephala* | + | + | + | + | + | ||||
| Lepidostoma* | + | + | + | ||||||
| Leptophlebia* | + | + | + | + | + | + | |||
| Leuctra* | + | + | + | + | + | + | + | + | + |
| Limnephilus* | + | + | + | + | |||||
| Limnius* | + | + | + | + | + | + | + | + | + |
| Limoniidae* | + | + | + | + | + | + | + | + | + |
| Liponeura | + | + | + | ||||||
| Lymnaeidae* | + | + | + | + | + | + | + | ||
| Micronecta | + | + | + | + | + | ||||
| Micropterna* | + | + | + | + | + | + | + | ||
| Nematoda | + | + | + | + | + | + | + | ||
| Oligochaeta | + | + | + | + | + | + | + | + | |
| Odontocerum* | + | + | + | + | |||||
| Orthocladiinae | + | + | + | + | + | + | + | + | |
| Oulimnius* | + | + | + | + | + | + | + | + | + |
| Paraleptophlebia* | + | + | + | + | |||||
| Perlidae* | + | + | + | + | |||||
| Perlodidae* | + | + | + | + | + | ||||
| Polycelis | + | + | + | + | + | + | + | ||
| Polycentropus | + | + | + | + | + | + | + | + | |
| Potamopyrgus* | + | + | |||||||
| Protonemura* | + | + | + | + | + | + | |||
| Psychoda* | + | + | + | + | + | + | + | + | |
| Psychomyia | + | + | + | + | + | + | + | + | + |
| Rhithrogena | + | + | + | + | + | + | + | ||
| Rhyacophila | + | + | + | + | + | + | + | ||
| Riolus* | + | + | + | + | + | + | + | + | + |
| Sericostoma* | + | + | + | + | + | + | + | + | + |
| Sialis | + | + | |||||||
| Simuliidae | + | + | + | + | + | + | + | ||
| Siphlonurus | + | + | + | + | + | + | |||
| Sphaerium | + | + | + | + | + | ||||
| Stenophylax* | + | + | + | + | |||||
| Tanypodinae | + | + | + | + | + | + | + | + | |
| Tanytarsini* | + | + | + | + | + | + | + | + | + |
| Tipulidae* | + | + | + | + | |||||
| Torleya | + | + | + | + | + | + | + | + | |
| Total abundance of shredders | |||||||||
| 0.2< frequency <0.5 | 165 | 787 | 1194 | 595 | 718 | 870 | 485 | 94 | 484 |
| 0.5 <frequency <0.7 | 425 | 611 | 46 | 132 | 74 | 216 | 164 | 44 | 68 |
| Frequency >0.7 | 552 | 882 | 33 | 112 | 238 | 435 | 569 | 32 | 223 |
Statistical analyses
Leaf breakdown rates (k) were estimated by fitting the AFDM data with the exponential model 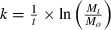 where (i) Mt is the leaf litter AFDM (g) remaining at time t, (ii) Mo is the initial AFDM at the beginning of the experiment and (iii) t is the sum of the mean daily temperatures (in degrees) during the whole experiment. The degree days model was used to account for potential differences in temperature between the reservoir and river sites. During the experiment, temperature ranged from 10.56 °C (±0.03) for S1 to 13.27 °C (±0.43) for S3 on the Ain river and from 12.46 °C (±0.13) for S2 to 12.61 °C (±0.66) for S4 on the Bienne river. Partial least-squares (PLS) regression (Abdi, 2003) analysis was carried out to identify abiotic factors significantly controlling leaf litter breakdown rates in reservoirs and downstream river reaches. The generated variable importance in the projection (VIP) values reflects the importance of each variable in the model, with VIP > 0.7 indicating important predictors (Eriksson et al., 1999). Synthetic variables concerning sediment contamination in metals and PAHs in reservoirs were added in the PLS analysis for leaf breakdown rates at downstream sites to examine the importance of contamination of associated reservoir sites for leaf breakdown rates downstream. These synthetic variables correspond to mean concentrations of PAHs and of metals (mol g−1 sediment) for each reservoir. The AFDM remaining were compared between stations by a one-way anova and a Tukey’s HSD post hoc test (after testing normality and homoscedasticity). In addition, simple linear models were built to examine the relationship between the metallic and the organic contaminations and leaf litter breakdown rates across all the stations. Contamination levels were determined using the mean concentrations (mol g−1 sediment) in ‘metals’ and ‘organic pollutants’ that emerged from the PLS analysis. To explore taxonomic responses, a complete-link clustering analysis was performed based on the Bray–Curtis similarity calculated between site assemblages. ‘Between-sites’ comparisons of trait category utilisation was performed with one-way anovas after arcsin (√p with p: proportion of each trait category in the community) transformation (normality and homoscedasticity being respected). Significant differences were evaluated after Bonferroni correction of P-values. Percentages of similarity (Jaccard distance) used for complete-link clustering analysis were calculated on the basis of trait categories exhibiting a significant ‘between-sites’ difference. Finally, Spearman correlation coefficients were used to explore relationships between trait categories, sediment contamination and leaf litter breakdown rates. We used Simca-P 9.0 (Umetrics AB, Umea, Sweden) for the PLS analyses and R software (R development Core Team, 2008) for the other analyses.
where (i) Mt is the leaf litter AFDM (g) remaining at time t, (ii) Mo is the initial AFDM at the beginning of the experiment and (iii) t is the sum of the mean daily temperatures (in degrees) during the whole experiment. The degree days model was used to account for potential differences in temperature between the reservoir and river sites. During the experiment, temperature ranged from 10.56 °C (±0.03) for S1 to 13.27 °C (±0.43) for S3 on the Ain river and from 12.46 °C (±0.13) for S2 to 12.61 °C (±0.66) for S4 on the Bienne river. Partial least-squares (PLS) regression (Abdi, 2003) analysis was carried out to identify abiotic factors significantly controlling leaf litter breakdown rates in reservoirs and downstream river reaches. The generated variable importance in the projection (VIP) values reflects the importance of each variable in the model, with VIP > 0.7 indicating important predictors (Eriksson et al., 1999). Synthetic variables concerning sediment contamination in metals and PAHs in reservoirs were added in the PLS analysis for leaf breakdown rates at downstream sites to examine the importance of contamination of associated reservoir sites for leaf breakdown rates downstream. These synthetic variables correspond to mean concentrations of PAHs and of metals (mol g−1 sediment) for each reservoir. The AFDM remaining were compared between stations by a one-way anova and a Tukey’s HSD post hoc test (after testing normality and homoscedasticity). In addition, simple linear models were built to examine the relationship between the metallic and the organic contaminations and leaf litter breakdown rates across all the stations. Contamination levels were determined using the mean concentrations (mol g−1 sediment) in ‘metals’ and ‘organic pollutants’ that emerged from the PLS analysis. To explore taxonomic responses, a complete-link clustering analysis was performed based on the Bray–Curtis similarity calculated between site assemblages. ‘Between-sites’ comparisons of trait category utilisation was performed with one-way anovas after arcsin (√p with p: proportion of each trait category in the community) transformation (normality and homoscedasticity being respected). Significant differences were evaluated after Bonferroni correction of P-values. Percentages of similarity (Jaccard distance) used for complete-link clustering analysis were calculated on the basis of trait categories exhibiting a significant ‘between-sites’ difference. Finally, Spearman correlation coefficients were used to explore relationships between trait categories, sediment contamination and leaf litter breakdown rates. We used Simca-P 9.0 (Umetrics AB, Umea, Sweden) for the PLS analyses and R software (R development Core Team, 2008) for the other analyses.
Results
Leaf breakdown rates
Site comparisons. Leaf breakdown rates ranged from 0.0044° per day in the most contaminated reservoir (Re4) to 0.0120° per day in the reference reservoir (Re1). Significant ‘between-sites’ differences (P < 0.001) in breakdown rates were evidenced, independently of the habitat type (i.e. reservoirs versus streams) but probably related to the sediment contamination level (Fig. 2). Three groups of sites were distinguished. The first group corresponded to the sites with the highest breakdown rate including the reference sites (S1) and the reservoir with an intermediate contamination level (Re2). The second group gathered the sites with lower breakdown rates (28% ± 10 of remaining AFDM) that are the three reaches downstream from contaminated reservoirs (Do2, Do3 and Do4) and one contaminated reservoir (Re3). The reservoir subjected to strong sediment contamination (Re4) exhibited a very low breakdown rate (55% of remaining AFDM).
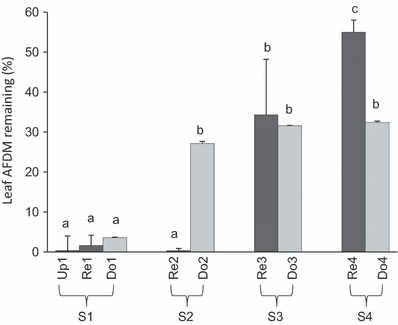
Mean percentages of leaf litter mass remaining after the 40-day litter bag experiment. Grey bars correspond to reservoir stations, clear grey bars to downstream stations, and for S1, the black bar to the upstream station. Letters correspond to significantly different groups identified by one-way anova and Tukey’s HSD post hoc test.
Physicochemical predictors of leaf litter breakdown rates. Although no clear pattern was evident between leaf litter breakdown rate and habitat type, the best physicochemical predictors of leaf litter breakdown differed in reservoirs versus downstream reaches (Table 3). In reservoirs, the best predictive descriptors were dissolved oxygen, zinc, benzo(ghi)perylene, magnesium, copper, water conductivity, calcium and nitrate concentrations. In downstream reaches, the parameters that best explained leaf litter breakdown rates were benzo(ghi)perylene, the biochemical oxygen demand (BOD), magnesium concentration, nickel, dissolved oxygen, water conductivity, benz(a)anthracene and metal concentration in associated reservoirs. Only four contaminants for the reservoirs and their downstream reaches were selected by the PLS for having an influence on leaf litter breakdown rates (VIP ≥ 0.7). However, dam contamination (both metals and organic compounds) exhibited high influence on breakdown rates observed downstream reaches. Considering the nine sites, leaf litter breakdown rates exhibited a strong negative relationship with metal contamination levels in sediments (n = 9, R2 = 0.74, P < 0.001, Fig. 3). No significant relationship was found with the PAH contamination levels.
| All sites (n = 9) | Reservoir sites (n = 4) | Downstream sites (n = 4) | ||||
|---|---|---|---|---|---|---|
| Loadings VIP | Component 1 (R2 = 63%) | Loadings VIP | Component 1 (R2 = 75%) | Loadings VIP | Component 1 (R2 = 84%) | |
| Y-Weights | +0.285 | +0.391 | +0.329 | |||
| Variables | ||||||
| Magnesium | 1.89 | 0.33 | 1.50 | 0.38 | 1.17 | 0.37 |
| Conductivity | 1.68 | 0.33 | 1.31 | 0.36 | 1.06 | 0.36 |
| Calcium | 1.6 | 0.32 | 1.29 | 0.36 | 0.95 | 0.34 |
| Nitrate | 1.54 | 0.31 | 1.06 | 0.26 | <0.7 | |
| Zinc | 1.49 | −0.24 | 1.71 | −0.36 | 0.83 | −0.30 |
| Copper | 1.29 | −0.21 | 1.49 | −0.32 | <0.7 | |
| Dissolved oxygen | 0.97 | 0.14 | 1.79 | 0.37 | 1.07 | 0.23 |
| Phosphorous | 0.91 | −0.25 | <0.7 | 0.80 | −0.32 | |
| Biological oxygen demand | 0.85 | −0.06 | <0.7 | 1.19 | −0.33 | |
| Fluoranthene | <0.7 | <0.7 | <0.7 | |||
| Benz(a)anthracene | <0.7 | <0.7 | 1.06 | −0.33 | ||
| Benzo(ghi)perylene | <0.7 | 1.67 | −0.39 | 1.24 | 0.22 | |
| Benzo(k)fluoranthene | <0.7 | <0.7 | <0.7 | |||
| Nickel | <0.7 | 0.88 | −0.25 | 1.07 | 0.18 | |
| Metals in dams | 1.04 | −0.33 | ||||
| PAHs in dams | 0.80 | −0.26 | ||||
- PLS, partial least-squares; VIP, variable importance in the projection.
- Loadings ≥0·3 are in boldface. Y-weights correspond to loadings of litter breakdown rate. Predictors with a negative sign contribute negatively to litter breakdown. Only predictors with VIP > 0·7 are included in the table.
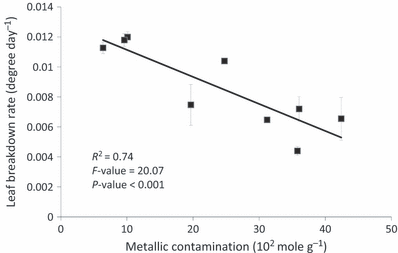
Linear relationship between leaf litter breakdown rate and mean concentration of metals in sediments (mol g−1).
Taxonomic and functional responses of macroinvertebrate communities
The cluster analysis (Fig. 4a) performed on the Bray–Curtis dissimilarity matrix between the assemblages of sites highlighted five groups of sites. The first group reflected the similarity between assemblages found upstream from the reference reservoir (Up1), downstream from the most contaminated reservoir (Do4) and downstream from the reservoir with an intermediate contamination (Do2). The second group associated assemblages of two contaminated reservoirs (Re2 and Re3) and of a site downstream from a contaminated reservoir (Do3). The last three groups isolated assemblages of the reference reservoir (Re1), of the most contaminated reservoir (Re4) and of the site downstream from the reference reservoir (Do1), respectively. The cluster analysis (Fig. 4b) performed on the Jaccard distance matrix between the mean biotrait profiles of stations highlighted three distinct groups of sites. The first group corresponded to the reference system, with a higher similarity between Up1 and Re1 than between Up1 and Do1. A second group highlighted the high similarity between the three reaches located downstream from contaminated reservoirs gathered with sites located in two intermediately contaminated reservoirs (Re2 and Re3). Finally, the third group isolated the most contaminated station, Re4. For precisely elucidating the bioecological shifts identified by the cluster analysis, we compared the trait profiles of:
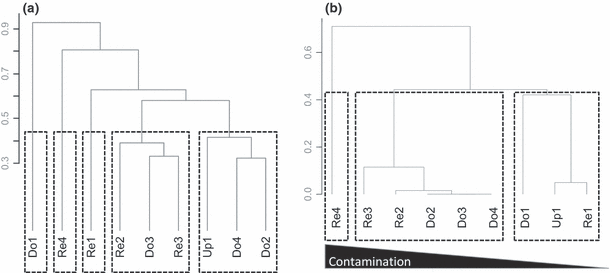
Dendrograms illustrating the results of the classification analyses performed on nine study-site macroinvertebrate assemblages according to their (a) taxonomic abundances using the complete-link method on a Bray–Curtis dissimilarity index matrix and (b) mean trait profiles using the complete-link method on a Jaccard distance matrix.
-
Re1 versus Re2/Re3 and Re1 versus Re4 to evaluate shifts related to the contamination gradient in reservoir sediment and similarly Do1 versus Do2/Do3/Do4 to compare downstream stations (see section Bioecological responses to contamination levels).
-
Up1 versus Re1 and Up1 versus Do1 to evaluate shifts only related to physical disturbance (see section Bioecological responses to dam presence).
Bioecological responses to contamination levels. Comparisons of community trait profiles among reservoirs (Table 4) highlighted a gradient of trait responses to sediment contamination. If few significant differences were observed between the reference and the two moderately contaminated reservoirs (similarity = 0.74), clear contrasts were evident between the reference and the highly contaminated reservoir (similarity = 0.44). Nevertheless, in contrast with leaf litter breakdown investigations, Re2 was associated with the biotrait profiles of stations exhibiting an intermediate contamination level (Fig. 4b). The reference reservoir community was characterised by a higher proportion of shredders and/or organisms without resistant forms, exhibiting affinity for gravel and living in the river channel of oligotrophic habitats. In contrast, faunal assemblages from the two moderately contaminated reservoirs consisted of a higher proportion of organisms that were thermophilic, deposit feeders, reproducing by free eggs or clutches, producing statoblasts or using larval diapause as resistance stages in harsh environmental conditions and/or disseminating by drift. The benthic assemblage of the most heavily impaired reservoir was composed of more organisms that were large-sized (>4 cm) with tegumental respiration. The relative abundances of parasites, limnophilic, polysaprobic organisms or species using cocoons as resistance stages were higher than in other reservoirs. Taxa found in these reservoirs are often described as colonising lentic (e.g. ponds) and eutrophic habitats. Finally, in both moderately and heavily contaminated reservoirs, shredder abundance significantly decreased, as well as that of organisms without a resistant form or living in the river. Sediment contamination also reduces the proportion of organisms with active dispersal. The ‘between-sites’ comparison of the downstream stations revealed significant differences between the reference station versus stations downstream from contaminated reservoirs. The functional similarity (1.0) of impaired downstream reaches was already observed in leaf decomposition rates. The benthic assemblages of impaired downstream reaches included many deposit feeders and acidification-sensitive organisms (Table 4). In contrast, the invertebrate assemblage of the reach downstream from the reference reservoir contained more shredders and predators, and more eurythermic organisms.
| Traits | Categories | Re1 | Re2 | Re3 | Re4 | Re1 versus Re2/Re3 | Re1 versus Re4 |
|---|---|---|---|---|---|---|---|
| Reservoir biotrait profiles | |||||||
| Aquatic stages | Larva | 0.38 (0.03) | 0.43 (0.04) | 0.40 (0.04) | 0.31 (0.07) | NS | ↘ |
| Current velocity | Fast | 0.21 (0.04) | 0.16 (0.01) | 0.16 (0.03) | 0.11 (0.05) | NS | ↘ |
| Current velocity | Medium | 0.32 (0.04) | 0.33 (0.03) | 0.32 (0.06) | 0.26 (0.04) | NS | ↘ |
| Current velocity | Null | 0.14 (0.07) | 0.15 (0.05) | 0.19 (0.09) | 0.28 (0.05) | NS | ↗ |
| Dispersal | Aerial active | 0.22 (0.11) | 0.21 (0.06) | 0.13 (0.07) | 0.12 (0.06) | NS | ↘ |
| Dispersal | Aerial passive | 0.04 (0.03) | 0.13 (0.06) | 0.13 (0.07) | 0.12 (0.06) | ↗ | ↗ |
| Dispersal | Aquatic active | 0.27 (0.08) | 0.23 (0.05) | 0.23 (0.07) | 0.17 (0.05) | NS | ↘ |
| Feeding habits | Deposit feeder | 0.28 (0.24) | 0.51 (0.12) | 0.43 (0.19) | 0.44 (0.24) | ↗ | NS |
| Feeding habits | Parasite | 0.01 (0.02) | 0.03 (0.02) | 0.03 (0.02) | 0.28 (0.31) | NS | ↗ |
| Feeding habits | Scraper | 0.29 (0.13) | 0.25 (0.06) | 0.23 (0.10) | 0.14 (0.11) | NS | ↘ |
| Feeding habits | Shredder | 0.35 (0.16) | 0.10 (0.07) | 0.17 (0.11) | 0.06 (0.06) | ↘ | ↘ |
| Food | Dead animal | 0.04 (0.03) | 0.01 (0.008) | 0.02 (0.02) | 0.009 (0.01) | ↘ | ↘ |
| Food | Vegetal detritus | 0.14 (0.08) | 0.14 (0.06) | 0.11 (0.06) | 0.03 (0.04) | NS | ↘ |
| Food | Macroinvertebres | 0.08 (0.05) | 0.05 (0.03) | 0.07 (0.04) | 0.28 (0.30) | NS | ↗ |
| Food | Microphytes | 0.31 (0.10) | 0.27 (0.04) | 0.27 (0.07) | 0.20 (0.07) | NS | ↘ |
| Locomotion | Crawler | 0.52 (0.21) | 0.50 (0.15) | 0.44 (0.20) | 0.27 (0.12) | NS | ↘ |
| Locomotion | Fixed | 0.04 (0.05) | 0.05 (0.03) | 0.08 (0.05) | 0.17 (0.12) | NS | ↗ |
| Locomotion | Flier | 0.03 (0.02) | 0.01 (0.01) | 0.008 (0.007) | 0.003 (0.007) | ↘ | ↘ |
| Longitudinal distribution | Hyporithron | 0.17 (0.03) | 0.18 (0.01) | 0.18 (0.03) | 0.13 (0.03) | NS | ↘ |
| Longitudinal distribution | Metarithron | 0.21 (0.04) | 0.19 (0.02) | 0.17 (0.02) | 0.13 (0.03) | ↘ | ↘ |
| Longitudinal distribution | Outside system | 0.07 (0.02) | 0.13 (0.03) | 0.11 (0.03) | 0.14 (0.04) | ↗ | ↗ |
| Maximal size | ≥4 cm | 0.15 (0.21) | 0.14 (0.14) | 0.18 (0.19) | 0.43 (0.19) | NS | ↗ |
| Reproduction | Free cluctches | 0.03 (0.04) | 0.12 (0.08) | 0.10 (0.09) | 0.09 (0.11) | ↗ | NS |
| Reproduction | Ovoviviparity | 0.19 (0.21) | 0.04 (0.02) | 0.15 (0.16) | 0.02 (0.03) | ↘ | ↘ |
| Resistance forms | Cocoons | 0.09 (0.13) | 0.09 (0.08) | 0.12 (0.12) | 0.26 (0.11) | NS | ↗ |
| Resistance forms | Diapause | 0.04 (0.03) | 0.15 (0.05) | 0.12 (0.05) | 0.10 (0.06) | ↗ | NS |
| Resistance forms | Egg statoblasts | 0.07 (0.07) | 0.21 (0.06) | 0.16 (0.08) | 0.03 (0.04) | ↗ | NS |
| Resistance forms | None | 0.79 (0.11) | 0.57 (0.09) | 0.64 (0.09) | 0.60 (0.11) | ↘ | ↘ |
| Respiration | Gill | 0.39 (0.22) | 0.39 (0.13) | 0.38 (0.18) | 0.12 (0.09) | NS | ↘ |
| Respiration | Plastron | 0.08 (0.06) | 0.03 (0.02) | 0.02 (0.02) | 0.008 (0.02) | ↘ | ↘ |
| Respiration | Tegument | 0.51 (0.23) | 0.54 (0.13) | 0.56 (0.20) | 0.86 (0.09) | NS | ↗ |
| Saprobity | Mesotrophic | 0.41 (0.05) | 0.46 (0.05) | 0.45 (0.05) | 0.34 (0.09) | NS | ↘ |
| Saprobity | Oligosaprobic | 0.32 (0.06) | 0.30 (0.03) | 0.31 (0.06) | 0.24 (0.06) | NS | ↘ |
| Saprobity | Xenosaprobic | 0.13 (0.05) | 0.12 (0.03) | 0.08 (0.04) | 0.06 (0.04) | NS | ↘ |
| Saprobity | α-mesosaprobic | 0.18 (0.09) | 0.19 (0.05) | 0.24 (0.07) | 0.29 (0.06) | NS | ↗ |
| Substrate preferences | Gravel | 0.18 (0.02) | 0.13 (0.02) | 0.14 (0.02) | 0.16 (0.02) | ↘ | NS |
| Substrate preferences | Litter | 0.11 (0.04) | 0.09 (0.009) | 0.09 (0.02) | 0.05 (0.02) | NS | ↘ |
| Substrate preferences | Mud | 0.06 (0.04) | 0.09 (0.03) | 0.09 (0.05) | 0.12 (0.02) | NS | ↗ |
| Substrate preferences | Roots | 0.09 (0.03) | 0.11 (0.03) | 0.11 (0.04) | 0.04 (0.03) | NS | ↘ |
| Substrate preferences | Sand | 0.10 (0.02) | 0.10 (0.02) | 0.10 (0.03) | 0.13 (0.02) | NS | ↗ |
| Temperature | Eurythermic | 0.68 (0.05) | 0.65 (0.03) | 0.68 (0.07) | 0.48 (0.15) | NS | ↘ |
| Temperature | Warm | 0.07 (0.05) | 0.19 (0.04) | 0.17 (0.04) | 0.14 (0.03) | ↗ | ↗ |
| Transversal distribution | Channel | 0.34 (0.11) | 0.21 (0.04) | 0.23 (0.09) | 0.12 (0.03) | ↘ | ↘ |
| Transversal distribution | Ponds | 0.07 (0.02) | 0.08 (0.03) | 0.09 (0.04) | 0.20 (0.08) | NS | ↗ |
| Trophic status | Eutrophic | 0.09 (0.10) | 0.15 (0.06) | 0.17 (0.09) | 0.24 (0.07) | ↗ | ↗ |
| Trophic status | Oligotrophic | 0.49 (0.09) | 0.38 (0.03) | 0.37 (0.06) | 0.42 (0.013) | ↘ | NS |
| Traits | Categories | Do1 | Do2 | Do3 | Do4 | Do1 versus Do2/3/4 |
|---|---|---|---|---|---|---|
| Downstream biotrait profiles | ||||||
| Food | Dead animal | 0.06 (0.03) | 0.02 (0.02) | 0.02 (0.01) | 0.009 (0.01) | ↘ |
| Feeding habits | Deposit feeder | 0.11 (0.04) | 0.31 (0.16) | 0.33 (0.14) | 0.39 (0.17) | ↗ |
| Locomotion | Flier | 0.04 (0.02) | 0.01 (0.01) | 0.006 (0.007) | 0.01 (0.01) | ↘ |
| Number of reproductive | < 1 | 0.11 (0.05) | 0.03 (0.02) | 0.03 (0.03) | 0.01 (0.02) | ↘ |
| Feeding habits | Predator | 0.18 (0.11) | 0.04 (0.03) | 0.08 (0.05) | 0.03 (0.01) | ↘ |
| Feeding habits | Shredder | 0.36 (0.09) | 0.16 (0.17) | 0.13 (0.09) | 0.10 (0.10) | ↘ |
| Low pH sensitivity | >4–4.5 | 0.04 (0.01) | 0.09 (0.02) | 0.09 (0.02) | 0.09 (0.02) | ↗ |
| Low pH sensitivity | >5–5.5 | 0.17 (0.02) | 0.21 (0.02) | 0.21 (0.02) | 0.21 (0.02) | ↗ |
| Low pH sensitivity | >6 | 0.46 (0.06) | 0.30 (0.05) | 0.30 (0.03) | 0.29 (0.03) | ↘ |
| Temperature | Eurythermic | 0.74 (0.05) | 0.64 (0.09) | 0.65 (0.05) | 0.59 (0.05) | ↘ |
- The difference was illustrated by an arrow, when the P-value (after Bonferroni correction) was significant: upward = ‘increasing in comparison with Re1 or Do1’ or downward = ‘decreasing in comparison with Re1 or Do1’.
Bioecological responses to dam presence. The macroinvertebrate bioecological trait profile of the reference upstream (Up1) and the reference reservoir (Re1) were highly similar (similarity = 0.98), even if flow reduction in the reservoir induced a significant decrease in macroinvertebrate taxa sensitive to low pH (Table 5). The dam induced a decline of taxa from springs (i.e. crenon) and a drastic decrease in taxa sensitive to organic contamination (oligosaprobic) and organisms sensitive to low pH without resistant forms and/or living in cold water. In contrast, an increase in species tolerant to organic contamination (α-mesosaprobic) and/or living in lowlands reaches, potentially using diapause or dormancy and feeding as predators, was observed for the downstream site. The global similarity proportion with the upstream station was 0.82.
| Traits | Categories | Up1 | Re1 | Do1 | Up1 versus Re1 | Up1 versus Do1 |
|---|---|---|---|---|---|---|
| Altitude | Lowlands | 0.55 (0.08) | 0.60 (0.09) | 0.72 (0.04) | NS | ↗ |
| Feeding habits | Predator | 0.04 (0.02) | 0.04 (0.03) | 0.18 (0.11) | NS | ↗ |
| Longitudinal distribution | Crenon | 0.12 (0.02) | 0.11 (0.02) | 0.75 (0.01) | NS | ↘ |
| Low pH sensitivity | >4–4.5 | 0.09 (0.03) | 0.05 (0.02) | 0.03 (0.01) | ↘ | ↘ |
| Low pH sensitivity | >6 | 0.29 (0.05) | 0.34 (0.04) | 0.46 (0.06) | NS | ↗ |
| Resistance forms | None | 0.78 (0.09) | 0.79 (0.01) | 0.59 (0.12) | NS | ↘ |
| Resistance forms | Diapause | 0.06 (0.04) | 0.04 (0.02) | 0.19 (0.08) | NS | ↗ |
| Saprobity | α-mesosaprobic | 0.11 (0.01) | 0.18 (0.04) | 0.23 (0.03) | NS | ↗ |
| Saprobity | Oligosaprobic | 0.37 (0.05) | 0.32 (0.06) | 0.28 (0.05) | NS | ↘ |
| Temperature | Cold | 0.33 (0.06) | 0.25 (0.04) | 0.15 (0.04) | NS | ↘ |
- The difference was illustrated by an arrow, when the P-value (after Bonferroni correction) was significant: upward = ‘increasing in Re1 or Do1’ or downward = ‘decreasing in Re1 or Do1’.
Towards response and effect biotrait categories
The study of the relationship between trait categories, sediment contamination and leaf breakdown rates has allowed identification of response and effect trait categories (Table 6). First, key biotrait categories related to the functional processes (i.e. negatively correlated to contamination and positively correlated to breakdown rate) have been identified. These trait modalities were ‘shredder’, ‘channel’ and ‘metarithron’ as habitat features; ‘<0.5 cm’ as maximal potential size and ‘aquatic active’ or ‘aerial active’ as dispersal strategies. All these trait categories are some bio/ecological attributes of key invertebrate taxa participating in leaf litter breakdown. In addition, a combination of trait categories was more indicative of disturbance, that is, was positively related to contamination and negatively related to leaf breakdown rate including ‘large’ (i.e. maximum size >4 cm), ‘polyvoltin’, ‘asexual reproduction’, ‘aquatic passive’ dispersal, ‘tegumental’ respiration mode and ‘microorganism’ as food. These trait categories mainly corresponded to taxa more tolerant to toxic and organic contamination, such as Chironomidae, Oligochaeta and Nematoda.
| Response categories | Effect categories | |||
|---|---|---|---|---|
| Metals | Organic | Rates | ||
| Maximal size (cm) | <0.5 | −0.76* | −0.67 | 0.76* |
| Maximal size (cm) | >4 | NS | 0.81* | −0.70 |
| Number of reproductive cycles | >1 per year | 0.72 | NS | −0.88** |
| Aquatic stages | Egg | −0.71 | NS | 0.73 |
| Reproduction | Asexual | 0.73 | 0.74 | −0.72 |
| Dispersal | Aquatic passive | NS | 0.76 | −0.69 |
| Dispersal | Aquatic active | −0.73 | −0.84* | 0.83* |
| Dispersal | Aerial active | −0.69 | −0.81* | 0.84* |
| Respiration | Tegument | NS | 0.85* | −0.75 |
| Respiration | Plastron | −0.93** | NS | 0.92** |
| Locomotion | Flier | −0.89* | NS | 0.90** |
| Locomotion | Crawler | −0.71 | −0.77 | 0.83** |
| Locomotion | Attached | NS | 0.78 | −0.86* |
| Food | Microorganism | 0.76 | 0.69 | −0.68 |
| Food | Dead plant | NS | −0.69 | 0.67 |
| Food | Dead animal | −0.72 | NS | 0.73 |
| Feeding habits | Shredder | −0.79* | NS | 0.82* |
| Transversal distribution | River channel | −0.79* | −0.76 | 0.88** |
| Longitudinal distribution | Metarithron | −0.73 | −0.75 | 0.86* |
- NS, no significant.
- Trait categories with a positive sign increase with the contamination for response trait categories and contributed positively to litter breakdown for effect trait categories.
- *P-value < 0.01; **P-value < 0.001.
Sensitivity of the different approaches in detecting disturbances
The different approaches used in this study allowed us to highlight the impacts of the different stressors independently (Table 7). The study of macroinvertebrate community structure did not reveal any structuring effect of the levels of sediment contamination and the presence of a dam. In contrast, the similarity of sites according to biotrait profiles has permitted us to distinguish dam impacts from those of sediment contamination as well as cumulative impacts of dam presence and contaminated sediment. Although dam presence did not significantly influence leaf litter breakdown, sediment contamination and the synergy of both perturbations led to disturbance of this ecosystem process.
| Approaches | |||
|---|---|---|---|
| Communities | Ecosystem | ||
| Taxonomic | Functional | Leaf breakdown | |
| Physical perturbation | No | Yes | No |
| Ecotoxic perturbation | No | Yes | Yes |
| Multiple stressor | No | Yes | Yes |
Discussion
Examining the ecological impact of selected dams on rivers allowed us to discriminate between physical effects related to flow alteration and effects due to toxicant accumulation in sediment. In particular, investigating biotrait profiles of benthic invertebrate assemblages has elucidated (i) the response of invertebrate communities to dam presence, (ii) the significant modifications in benthic assemblage biotraits due to sediment contamination and (iii) the cumulative effects of dam and sediment contamination on both biotraits and an ecosystem process, leaf litter breakdown.
Functional disturbances related to dam presence
The dam only slightly impaired reference reservoir communities (Re1), mainly leading to a decrease in acidification-sensitive taxa. Despite the changes in flow regime, the upstream reach assemblage trait was not significantly modified. In these small run-of-river reservoirs, the hydrological and hydromorphological constraints may be balanced by a greater availability of littoral habitats. In contrast, the dam impact was more pronounced in the downstream reference reach (Do1). First, the dam presence induced an increase in predators in the downstream community that may be related to an increase in Chironomidae below the dam (Bredenhand & Samways, 2009). In addition, the biotrait profiles in the downstream reach underlined a decrease in cold-water organisms that could indicate alteration in the seasonal temperature regime. The observed increase in the ability of organisms to develop resistant forms and to tolerate pollution revealed also the impact of dam on downstream reach communities. Dam presence was supposed to impair leaf litter breakdown in the reservoir and in the downstream reach by the modifications of (i) the availability and quality of detrital resources and (ii) the assemblages (i.e. invertebrates and microorganisms) involved in leaf litter breakdown. Indeed, flow changes in reservoirs have been assumed (i) to reduce the colonisation (Bärlocher, 1992; Chauvet, 1992) and activity (Ferreira & Graça, 2006) of microbial communities, potentially reducing leaf litter palatability to shredders and (ii) to increase litter clogging and burial in sediments (Naamane, Chergui & Pattee, 1999; Benfield et al., 2001). Despite these constraints, leaf litter breakdown rates measured in the reference reservoir (Re1) were not significantly different from those of its upstream reach (Up1). Similarly, and consistent with other previous studies (e.g. Casas et al., 2000; Muehlbauer et al., 2009), no physical impact on leaf litter breakdown rates was evidenced downstream the dams, in the absence of sediment contamination.
Functional disturbances associated with contaminated sediment
Intense siltation due to flow reduction and high historical contamination of sediment should lead to clearer trait-based responses of benthic communities to sediment contamination in reservoirs than in downstream reaches. This community response was characterised by a selection of adaptations in terms of feeding habits, resistant forms, dispersal, respiration and reproduction modes. In particular, deposit feeders were more abundant in contaminated reservoirs, while in the more heavily contaminated reaches parasites took the place of some shredders and scrapers. Feeding habits strongly determine organism exposure risk and sensitivity to toxicants (Vaal et al., 2000; Ducrot et al., 2005; Rabení, Doisy & Zweig, 2005). The vulnerability of shredders has been related to the direct impact of metatoxicity (Niyogi, Lewis & Mcknight, 2001; Carlisle & Clements, 2005) because this trophic group was composed of sensitive species (e.g. Ephemeroptera, Plecoptera and Trichoptera) or indirectly, because the contamination may alter the resource quality through microbial community alterations (Sridhar et al., 2001; Baudoin et al., 2008; Fernandes et al., 2009) and accumulation of contaminants in microorganisms on leaf litter (Farag et al., 1998; Schaller et al., 2011). This alteration of food quality used by macroinvertebrate communities under toxic conditions may have bottom-up effects on leaf breakdown process although many compensatory phenomena may exist, such as (i) shredders tolerant to toxic contamination or to poor quality resources or (ii) diet plasticity of other feeding groups making it possible to use (in the absence of major competitors) leaf litter as complementary resource (Callisto, Gonçalves & Graça, 2007). Our study showed a reduction of leaf litter breakdown rates with the increase in sediment metal contamination. Numerous studies have already reported impacts of river metal contamination on leaf litter decomposition (e.g. Carpenter, Odum & Mills, 1983; Maltby & Booth, 1991; Maltby & Crane, 1994; Carlisle & Clements, 2005). In addition to sediment contamination, dissolved oxygen availability could significantly influence leaf litter breakdown rate. Dissolved oxygen concentration may be a limiting factor in reservoirs (because of low flow and low current velocity), especially in interstitial habitats, influencing sediment pollutant bioavailability, bacterio-fungal colonisation and biological activity (Medeiros, Pascoal & Graça, 2009). Oxygen depletion also leads to qualitative and quantitative changes in macroinvertebrate and microbial assemblages (Ward et al., 1998) and then may impair leaf litter decomposition. Finally, because contamination by heavy metals occurs also through external contact (Paul & Meyer, 2001), the maximum size of organisms has been expected to be related to contamination level. Thus, the relative abundance of small organisms should decrease at high metal concentrations due to a higher surface/volume ratio (Dolédec & Statzner, 2008). The higher frequency of large organisms (>4 cm) in the most contaminated sites was consistent with this hypothesis. The biotrait shifts associated with flow modification and sediment contamination can potentially reduce the resilience of resident communities to disturbances. For example, the strong reduction of the dispersal abilities of organisms towards downstream may reduce resilience of downstream site communities and can affect the life cycles of many organisms (Brasher, 2003).
Cumulative impacts on downstream reach communities
Our results suggested that sediment contamination might induce a loss in resilience and resistance potential of reservoir communities (e.g. a decline in reproductive performances, reduced dispersal abilities and a loss in resource-harvesting efficiency) and, to some extent, a weaker resilience of downstream reach communities already subjected to physical constraints related to dam presence. Regardless of the level of sediment contamination in the reach, the biotrait profiles of reach communities sampled downstream from a contaminated reservoir were similar and clearly different from reach communities sampled downstream from the reference reservoir. In addition to changes in traits related to local physical constraints, communities located downstream from contaminated reservoirs were characterised by changes in a combination of traits (i.e. fewer K-strategists, more deposit feeders, fewer shredders and predators), assumed to be related, like litter breakdown alteration, to the cumulative impact of both dam presence and reservoir contamination.
Complementarity of approaches used
The structural changes (i.e. bioecological and taxonomic) related to physical and chemical impairment can affect ecosystem functioning by indirect effects (McGill et al., 2006; Zavaleta et al., 2009; Menezes, Baird & Soares, 2010), especially if the impaired traits are the main contributors to ecosystem functional properties (i.e. functional traits). The taxonomic study of faunal assemblages did not reveal the presence of any physical and ecotoxic disturbances and potential cumulative impacts of these two perturbations. In contrast, studying community biotrait profiles allowed us to identify trait categories responding both to dam presence and to sediment contamination. These ‘response traits’ might be correlated with the stressor effects on functional processes. Trait categories within communities that respond to metal contamination of sediments were identified and correlated to leaf breakdown rate. Examining biotrait profiles also allowed us to identify constraints (i.e. physical impact and low toxic contamination) that had no significant effects on leaf breakdown rate. Nelson (2000) demonstrated that community structure provided more information on stressor impacts (e.g. dam and metals) than did measurements of leaf litter breakdown rates because of (i) feedback mechanisms such as the replacement of intolerant shredders by tolerant ones (Schindler, 1987) and/or (ii) resistance of microorganisms to the stressors. Due to the high sensitivity of trait-based approaches for detecting the effects of physical and chemical stressors, we can assume that observed biotrait shifts are potential predictors of a disruption of functional processes (including leaf litter breakdown) in rivers. Because of increasing functional redundancy, a higher functional diversity of communities is considered as contributing more to ecosystem resistance and resilience. However, we hypothesised the existence of a threshold in community biotrait changes. When such a threshold is exceeded, the functional changes in benthic communities could become irreversible. The identification and definition of such ecological and functional thresholds, thanks to biotrait-based approaches and leaf litter breakdown monitoring, seems necessary in risk assessment of contaminated sediments and dam presence. It could certainly help to predict and to ensure ecosystem recovery, especially in the case of restoration practices (e.g. dam removal). Finally, our results lead us to wonder about the definition of a ‘functionally disturbed’ ecosystem. Must we consider that ecosystem functioning is disturbed if changes in biotraits within invertebrate assemblages can potentially induce a significant loss in community resilience even if such changes do not still affect measured functional processes (e.g. leaf litter breakdown rate), or only when measuring a significant effect on functional processes despite the level of changes in community trait combinations?
In conclusions, even if the ecological impact of dams has been increasingly studied in the last decades, those inherent to the cumulative effects of contaminated sediments have been scarcely and poorly examined. We have demonstrated that, in the absence of contaminated sediments, changes in biotrait profiles do not generate negative effects on ecosystem functioning processes. Sediment contamination alters the reservoir functioning and by propagation of the effects might reduce the resilience of downstream communities. Sediment contamination may also induce significant modifications in upper trophic-level composition (Fleeger, Carman & Nisbet, 2003). This study has highlighted the relevance of simultaneously using benthic invertebrate trait-based approaches and leaf breakdown process. These approaches combining the monitoring of both biotrait shifts and functional processes should be integrated into ecological and ecotoxicological risk assessment related to contaminated sediments and should be helpful for predicting the effects of multiple stressors. Run-of-river reservoirs constitute a relevant study framework for combined effects of physical and chemical stressors. In addition, the ‘good ecological potential’ of such systems, potentially defined by their sediment quality, must maintain both high biotrait diversity and ecosystem processes expected in good ecological conditions, the guarantee for downstream reach integrity already under physical stress. This definition of their ‘good ecological potential’ is particularly important in the context of ecological continuity restoration policy, including contaminated sediment resuspension risk evaluation. Omitting to assess the ecological and ecotoxicological risk related to sediment contamination in run-of-river reservoirs could lead to unexpected ecological consequences of ecological continuity restoration (e.g. dam removal). Indeed, the negative effects of contaminated sediment on habitat quality and functional processes (e.g. leaf litter breakdown) could deeply compromise ecosystem recovery (e.g. faunal recolonisation) and could lead to ‘physically restored’ but ‘functionally disturbed’ rivers.
Acknowledgments
This study was supported by the DIESE Programme from the National Research Agency – ANR PRECODD. The authors thank Eric Chauvet, Roger Jones and two anonymous reviewers for their suggestions that have significantly improved the quality of the manuscript. The authors thank Colas M., Bataillard C., Bouquerel J. and the Natural Park of Haut Jura for their help during field work.




