Buoyancy of post-fertilised Dissostichus mawsoni eggs and implications for early life history
Abstract
Gametes from gravid Antarctic toothfish (Dissostichus mawsoni) were combined in vitro and buoyancy measurements were made on fertilised eggs during early development. Eggs were strongly positively buoyant, indicating that they would ascend quickly in the water column and reside near or in association with the underside of sea ice, which covers most of the spawning habitat during winter. An association with sea ice may provide: protection from the turbulence of ice-free surface waters, a mechanism that modifies the velocity of advection by surface currents, and a habitat with a concentrated planktonic food source during spring months. An association with sea ice would also create spatial differences in egg advection patterns for eggs produced spanning a large geographic area. The effects of climate change on sea ice formation and melting patterns, or fishery-induced changes in the spatial density of adults could then influence juvenile advection patterns and influence survival.
1 INTRODUCTION
Antarctic toothfish, Dissostichus mawsoni Norman, 1937, spawn during winter months on seamounts, and potentially on the continental slope around Antarctica, likely at depths less than 1500 m (Hanchet et al., 2008; Parker & Grimes, 2010; Parker et al., 2019). Most of the area inhabited by D. mawsoni during winter is under sea ice that typically extends northward from the continent to at least 63°S latitude in the region (Fetterer et al., 2017). Because of this, reproduction and early life history of D. mawsoni is difficult to study throughout much of their range.
Based on information from the congener Patagonian toothfish (D. eleginoides), D. mawsoni eggs are likely pelagic, residing in the water column upon release (Mujica et al., 2016; North, 2002). The buoyancy of D. mawsoni eggs is unknown. Therefore, the precise advection of the eggs and their potential vertical distribution in the water column through time cannot be estimated. This is important information to be acquired for D. mawsoni because combined with the location of spawning, the depth distribution of eggs through time will determine the spatial distribution of larvae and juveniles. Moreover, the distribution of eggs and larvae is critical information for describing stock structure and potential connectivity with other populations, which is necessary for stock assessment and fishery management analyses (Mormede et al., 2014a,b). For example, particle advection simulations using oceanographic models indicate that eggs may remain at depths near 200 m and circulate within the Ross Gyre prior to recruiting to a demersal habitat (Ashford et al., 2012; Behrens et al., 2021; Hanchet et al., 2008).
A survey during the austral winter (June–July) of 2016 to the northern Ross Sea provided an opportunity to collect D. mawsoni eggs in the plankton and also to generate fertilised eggs using male and female D. mawsoni in spawning condition caught using bottom longlines. We used these developing eggs to measure egg density as basis to calculate in situ egg buoyancy, ascent rates and ultimately their vertical distribution.
2 METHODS
2.1 Ichthyoplankton samples
The survey was conducted from 1 June through 9 July 2016 in the vicinity of seamount features in the Pacific–Antarctic fracture zone of the Ross Sea between 170°E and 170°W longitudes and 62°–66°S latitudes. A ring net was deployed periodically to sample for D. mawsoni eggs. The net had a diameter of 60 cm and a length of 3 m, with 2-mm mesh size and was configured using a 10-kg depressor weight. Vertical tows were made from a depth of 500 m to the surface, and oblique tows from a depth of 200 m to the surface. An RBR duet™ logger was deployed with each plankton tow to obtain temperature and measure the maximum depth sampled. A Seabird™ CTD, sampling every 10 s, was deployed to a depth of 500 m at each station to obtain depth profiles for salinity and temperature.
2.2 Egg culture
Adult D. mawsoni were collected using bottom longlines at depths of 872–1915 m as detailed in Parker et al. (2019). Eggs from ripe female (loose, hyaline eggs) and male (freely expressing milt) D. mawsoni (CCAMLR gonad stage F4 and M4) were fertilised following spawning protocols based on standard finfish culture techniques (Cabrita et al., 2009). Fertilised eggs were cultured in flow-through 2-L conical containers, and a subsample was preserved daily for up to 4 days post-fertilisation. Surface water from vessel intake at 3 m depth was provided to the culture containers continuously, and water temperatures fluctuated from −0.5 to 5.0°C as the vessel moved north after the survey.
2.3 Molecular characterisation of eggs
Both wild-caught and fertilised eggs were characterised through standard barcoding by sequencing the 5′ region of the cytochrome c oxidase 1 (COI) gene. Analyses were performed at BOLD (http://www.boldsystems.org). The primers ‘C_FishF1t1’ and ‘C_FishR1t1’ (Ivanova et al., 2007) were used for the amplification.
2.4 Egg buoyancy
The density of D. mawsoni eggs from fertilisation experiments was measured using columns of salinity gradients produced by Martin Instruments Co. Ltd, and described by Coombs (1981). The salinity gradients in the columns were obtained from mixing in saline water from two salinity stock solutions of high and low salinity respectively made from freshwater with added aquarium sea salt. Nine calibrated glass floats of known density in the range from 1.0200 to 1.0270 g cm−3 (accurate to ±0.0002 g cm−3) were used to determine the precise density gradients in each of the columns, which spanned salinities from about 36‰ at the bottom to 24‰ at the top of the column. The water jacket around the columns was cooled from the seawater intake of the vessel. Hence, temperature of the system varied from 0.4 to 3.0°C during the experiments. The salinity gradients of the columns were determined from calculations of specific gravity of the glass floats and the temperature of the columns measured as the vertical location within the gradient.
Live eggs were introduced one at a time into the chamber and allowed to sink and stabilise at its location of neutral buoyancy for 15 min. The vertical position of the egg was then recorded together with the positions of the calibration floats and the temperature of the system. The vertical position of each egg in the salinity gradient column system was recorded daily along with positions of each calibrated float. Eggs of poor quality will become heavier and sink to the bottom through limited osmoregulation resulting in loss of water through the vitelline membrane (Jung et al., 2014; Stenevik et al., 2008).
The in situ egg density was derived from the neutral buoyancy values (expressed in salinity) from the salinity gradient column and the in situ temperature of the water column (Table 1). The in situ buoyancy at different depths, Δρ = ρwater−ρegg (in g cm−3), was derived from the in situ water density, ρwater (from Table 2), and the in situ egg density, ρegg.
| Depth | Temp | Egg-specific gravity (σt) | Egg buoyancy, (Δρ) (g cm−3) | ||||
|---|---|---|---|---|---|---|---|
| (m) | °C | Light fraction | Average | Heavy fraction | Light fraction | Average | Heavy fraction |
| 0 | −1.5 | 25.37 | 26.06 | 26.79 | 0.0020 | 0.0013 | 0.0006 |
| 20 | −1.5 | 25.37 | 26.06 | 26.79 | 0.0020 | 0.0013 | 0.0006 |
| 50 | −1.5 | 25.37 | 26.06 | 26.79 | 0.0021 | 0.0014 | 0.0007 |
| 75 | −1.0 | 25.36 | 26.05 | 26.78 | 0.0021 | 0.0015 | 0.0007 |
| 100 | −0.4 | 25.34 | 26.02 | 26.75 | 0.0022 | 0.0015 | 0.0008 |
| 150 | 1.0 | 25.27 | 25.96 | 26.68 | 0.0024 | 0.0017 | 0.0010 |
| 200 | 2.0 | 25.21 | 25.89 | 26.61 | 0.0024 | 0.0018 | 0.0010 |
| 300 | 2.0 | 25.21 | 25.89 | 26.61 | 0.0024 | 0.0018 | 0.0010 |
| 500 | 2.0 | 25.21 | 25.89 | 26.61 | 0.0025 | 0.0018 | 0.0011 |
| Depth (m) | Temperature (°C) | Salinity (‰) | σt |
|---|---|---|---|
| 0 | −1.5 | 34.0 | 27.36 |
| 20 | −1.5 | 34.0 | 27.36 |
| 50 | −1.5 | 34.1 | 27.44 |
| 75 | −1.0 | 34.2 | 27.50 |
| 100 | −0.4 | 34.3 | 27.54 |
| 150 | 1.0 | 34.5 | 27.64 |
| 200 | 2.0 | 34.6 | 27.65 |
| 300 | 2.0 | 34.6 | 27.65 |
| 500 | 2.0 | 34.7 | 27.73 |
2.5 Vertical distribution of the eggs
Antarctic toothfish likely spawn at depths of at least 500 m and likely more than 1000 m based on the bathymetric distribution of their seamount habitats (Hanchet et al., 2015). Based on egg size and the calculated in situ egg buoyancy distributions, ascent velocities from several spawning depths were calculated based on Equation (1). The vertical equilibrium distributions of the eggs in different conditions of turbulence of the mixed layer were calculated based on Equation (2). The modelling conditions for the region were based on hydrographic climatology for the survey area for July and taken from the NOAA National Centers for Environmental Information (https://www.nodc.noaa.gov/OC5/WOA09F/pr_woa09f.html).
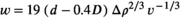 (1)
(1)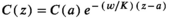 (2)
(2)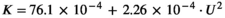
3 RESULTS
3.1 Ichthyoplankton samples
A total of 29 ichthyoplankton samples from June through July showed very few organisms larger than the 2-mm mesh size (mostly euphausiids, salps, copepods, amphipods and chaetognaths). However, on 2, 4 and 8 July 2016, a total of 19 eggs were captured during oblique plankton tows where the maximum net depth was 177 m but eggs were likely collected much closer to the surface (~63°S, ~172°E, bottom depths ~1200 m). Eggs of D. mawsoni were captured within 200 m of the surface. These eggs were not viable post-capture but were preserved and subsequently identified as D. mawsoni using genetic analysis (see below). The combination of running ripe and spent females, and eggs caught within 200 m of the surface suggested that, in 2016, D. mawsoni in the northern Ross Sea region began spawning in early July. Patagonian toothfish sampled during the survey were not in spawning condition at that time.
3.2 Species identification of eggs
Twelve eggs were available for the molecular characterisation: three collected with the plankton net (‘wild eggs’, field code: JAN 1601 P20 BIO 14) and nine obtained from in vitro fertilisation (‘fertilized eggs’, field code: JAN 1601 P20 R2). Egg vouchers were stored in the collections of the Italian National Antarctic Museum (MNA, Section of Genoa) under the numbers MNA-09620 to MNA-09622 (‘wild eggs’); MNA-09623 to MNA-09629 and MNA-09699 to MNA-09700 (‘fertilized eggs’).
Seven sequences were obtained from the twelve eggs that were available for sequencing. Two of these sequences corresponded to ‘wild eggs’ (MNA-09620 and MNA-09622) and five to ‘fertilized eggs’ (MNA-09623 to MNA-09625, MNA-09629 and MNA-09699) (Table 3). All the obtained COI sequences fell into the single Barcode Index Number (Ratnasingham & Hebert, 2013) Registry ‘BOLD:AAD3402’, which included 6 public BOLD sequences (Table 3) all corresponding to D. mawsoni. Two of these BOLD sequences corresponded to adult specimens that were determined on a morphological base by Andrew Stewart (Museum of New Zealand, Te Papa, Tongarewa). Each one of the sequences obtained in the present study was also blasted in GenBank (https://blast.ncbi.nlm.nih.gov/Blast.cgi). In all cases, the match was of 99%–100% with D. mawsoni and 96%–97% with the congener D. eleginoides (Table 3). In addition, D. eleginoides shows a different spawning season (Parker et al., 2019). Therefore, the wild eggs were from D. mawsoni.
| Project Code | BOLD Process ID | Museum voucher/Storing Institution | Barcoded species | BOLD BIN | Region | Latitude | Longitude | Depth (m) |
|---|---|---|---|---|---|---|---|---|
| FNZ | FNZ727-06 | NMNZ | D. mawsoni | BOLD:AAD3402 | RS | |||
| ANTFI | ANTFI127-06(**) | Bangor University | D. mawsoni | BOLD:AAD3402 | SO | −61.22 | −55.67 | |
| ANTFI | ANTFI160-06(**) | Bangor University | D. mawsoni | BOLD:AAD3402 | SO | −61.13 | −55.92 | |
| FNZB | FNZ969-07 (*) | P42671 (NMNZ) | D. mawsoni | BOLD:AAD3402 | RS | 1,119 | ||
| FKCI | EATFR006-12 | MNHN-IC−2001–1143 (MNHN) | D. mawsoni | BOLD:AAD3402 | ||||
| FNZC284-09 (*) | P43859 (NMNZ) | D. mawsoni | BOLD:AAD3402 | 402 |
| Project Code | BOLD Process ID | Museum voucher/Storing Institution | Egg BLAST results | BOLD BIN | Region | Latitude | Longitude | Depth (m) |
|---|---|---|---|---|---|---|---|---|
| tnb-CODE | BAMBI1219-18 | MNA−09620 (MNA) | 99%–100% D. mawsoni | BOLD:AAD3402 | RS | −63.13833 | 171.79450 | 200 |
| TNB-CODE | BAMBI1221-18 | MNA−09622 (MNA) | 99%–100% D. mawsoni | BOLD:AAD3402 | RS | −63.13833 | 171.79450 | 200 |
| TNB-CODE | BAMBI1222-18 | MNA−09623 (MNA) | 99%–100% D. mawsoni | BOLD:AAD3402 | RS | −63.13833 | 171.79450 | 200 |
| TNB-CODE | BAMBI1223-18 | MNA−09624 (MNA) | 99%–100% D. mawsoni | BOLD:AAD3402 | RS | −63.13833 | 171.79450 | 200 |
| TNB-CODE | BAMBI1224-18 | MNA−09625 (MNA) | 99%–100% D. mawsoni | BOLD:AAD3402 | RS | −63.13833 | 171.79450 | 200 |
| TNB-CODE | BAMBI1238-18 | MNA−09629 (MNA) | 99%–100% D. mawsoni | BOLD:AAD3402 | RS | −63.13833 | 171.79450 | 200 |
| TNB-CODE | BAMBI1239-18 | MNA−09699 (MNA) | 99%–100% D. mawsoni | BOLD:AAD3402 | RS | −63.13833 | 171.79450 | 200 |
- All these sequences fall within the BOLD BIN AAD3402, which refers to D. mawsoni. Acronyms for Storing Institution: MNA, Italian National Antarctic Museum (Section of Genoa), Genoa, Italy; NMNZ, Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand; MNHN, Muséum National d Histoire Naturelle, Paris, France. Acronyms for Region: SO: Southern Ocean; RS, Ross Sea. (*) Specimens that were determined by a taxonomist (Andrew Stewart); (**) sequences published in: Rock et al., (2007).
3.3 Egg culture
On the 2nd and the 9th of July, eggs from two running ripe female D. mawsoni (145 and 162 cm TL) were fertilised with milt from several running ripe males. The fertilised eggs from one female were cultured for 8 days and eggs from the other female cultured for 5 days. Embryo development appeared to cease after about 3 days and was most likely a result of increasing water temperature as the vessel moved north. Eggs were large and consistent in size within and between the two females (mean diameter = 3.76 mm, SD = 0.098 mm). The early development and microstructure of the eggs was described by Ghigliotti et al. (2018).
3.4 Egg buoyancy
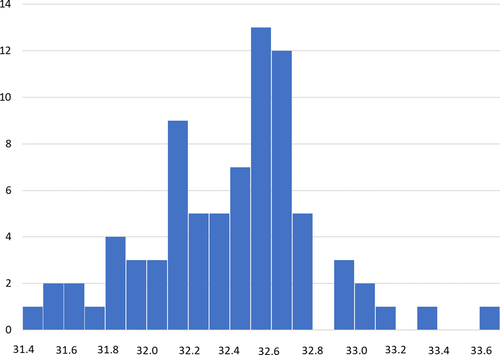
A total of 90 in vitro fertilised eggs were placed in the density gradient column spanning 10 days of post-fertilisation incubation (Figure 1). Mean neutral buoyancy was at a salinity of 32.40 ppt and values were normally distributed (Figure 2). Eggs showed no significant change in neutral buoyancy density during the period examined. The 5th percentile (i.e. eggs with the lowest density) had a mean neutral buoyancy at salinity of 31.55, while the 95th percentile (i.e. the eggs with the highest densities) were on the average neutrally buoyant at a salinity of 33.30 ppt.

In situ specific gravity of eggs was determined for several depths for (a) the lightest fraction of the eggs (neutrally buoyant at salinity 31.55), (b) the mean values (neutrally buoyant at a salinity of 32.40) and (c) the heaviest fraction of the eggs (neutrally buoyant at salinity of 33.30; Tables 1 and 2). As salinity of the water column decreases from about 34.7‰ at 500 m depth to about 34.0‰ in the surface layer, the resulting egg buoyancy also decreases as eggs ascend towards the surface. The average egg buoyancy (expressed as a difference in density) decreases from 0.0018 g cm−3 at 500 m depth to 0.0013 g cm−3 at the surface. The heaviest fraction of the eggs was substantially positively buoyant at 500 m depth (0.0011 g cm−3) but was also significantly positively buoyant at the surface (0.0006 g cm−3). The lightest fraction of the eggs was strongly positively buoyant at 500 m depth (0.0025 g cm−3).
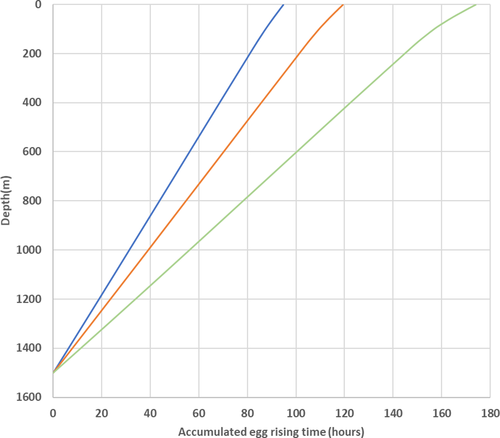
Average density eggs (and those lighter than average) reach the surface from an assumed spawning depth of 1500 m in 4–5 days (95 and 119 h, respectively), while the heaviest fraction of the eggs would take over a week to reach the surface layers from 1500 m depth (Figure 3).
3.5 Vertical distributions of eggs
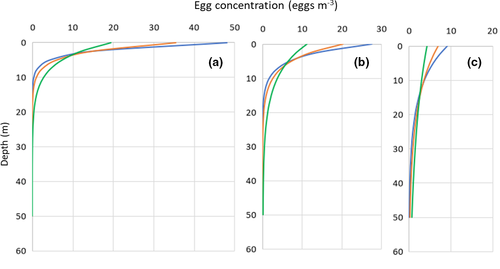
The modelled steady-state vertical egg distributions (i.e., the distribution after the eggs have ascended from the spawning depth and the vertical distribution is balanced between the buoyancy forces and the vertical turbulent mixing) under calm wind conditions is comparable to conditions under the sea ice (Figure 4a). Under such conditions, more than 95% of the modelled eggs is predicted to be found shallower than 10 m depth and only the heaviest eggs would mix down to 20–25 m depth. However, even for the heaviest eggs, more than 80% are predicted to be found shallower than 10 m depth.
Egg distributions in ice-free water exposed to winds from 5 to 12 m s−1 would be distributed deeper (Figure 4b,c). However, even in the open ocean, more than 95% of D. mawsoni eggs would be expected to be found shallower than 20 m depth for most wind situations which is quite shallow compared to most pelagic eggs (Sundby & Kristiansen, 2015).
4 DISCUSSION
Both the capture of wild eggs at depths shallower than 200 m and the densities calculated for fertilised eggs show that eggs of D. mawsoni are pelagic and would be found at increasing concentrations towards the surface forming a gradient modulated by turbulence of the mixed layer (Sundby, 1983). Although buoyancy measurements have not been conducted for D. eleginoides eggs, they have been described with similar vertical pelagic distribution (Evseenko et al., 1995) implying that they are also positively buoyant.
The calculated buoyancy of D. mawsoni eggs is typical of most other pelagic fish eggs. Most pelagic fish eggs (which are eggs positively buoyant in the upper mixed layer of the water column) range in buoyancy, Δρ, from 0.001 to 0.002 g cm−3 (Sundby, 1991; Sundby & Kristiansen, 2015). However, D. mawsoni eggs are large compared to other studied pelagic fish eggs (Baras et al., 2018; Barneche et al., 2018) and this gives the eggs higher ascent speeds than other pelagic eggs of similar buoyancy. Average ascent speeds of 2.7–3.6 mm s−1 clearly defines them at the upper range for pelagic eggs (Sundby & Kristiansen, 2015).
4.1 Implications of buoyant eggs
In comparison with large marine fish eggs from northern high latitudes, D. mawsoni eggs, with their pelagic distribution and exposure to the mixing layer, differ in vertical distribution. In high latitudes of the North Atlantic, such large marine eggs are most often vertically distributed mesopelagically implying that they are found at equilibrium distributions at greater depths below the turbulent mixed upper layer. Examples of species with mesopelagic egg distributions are Atlantic halibut Hippoglossus hippoglossus (Linnaeus, 1758) (Haug et al., 1984), Greenland halibut Reinhardtius hippoglossoides (Walbaum, 1792) (Ådlandsvik et al., 2001; Stene et al., 1999) and Baltic cod Gadus morhua Linnaeus, 1758 (Nissling et al., 1994; Nissling & Westin, 1991). The vertical distribution of D. mawsoni eggs estimated from the buoyancy measurements and egg size suggests that in the low-turbulence environment that covers most of the likely spawning areas, more than 95% of eggs would be within 10 m of the water–ice interface.
In the Northern hemisphere, mesopelagic eggs have thinner chorions than pelagic eggs. This might be an adaption to deep-sea calm conditions protected from the strain of breaking waves of the mixed layer. In contrast, the D. mawsoni eggs have extraordinarily thick chorions (Ghigliotti et al., 2018). Dissostichus mawsoni eggs, confined to the mixed layer by high buoyancy, have a high likelihood of coming into contact with the underside of sea ice. Hence, thick chorions might be an adaption to possible abrasion from the ice, similar to demersal species that have thick chorions such as eggs from lumpsucker (Cyclopterus lumpus; Davenport, 1985; Lønning et al., 1984), Atlantic salmon (Salmo salar; Aulstad & Gjedrem, 1973; Songe et al., 2016), sturgeon (Acipenser transmontanus; Cherr & Clark, 1982) and other Antarctic species (Notothenia coriiceps, Notothenia rossi, Lepidonotothen nudifrons, and Lepidonotothen larseni; Riel & Kock, 1989).
Because much of the potential spawning habitat (seamounts and the continental slope) is under sea ice during the winter period, and because their eggs are strongly buoyant, we hypothesise that D. mawsoni display an early life history strategy for egg development similar to that of Antarctic silverfish (Pleuragramma antarctica), where eggs incubate within the platelet ice matrix on the underside of the fast sea ice before hatching (Evans et al., 2012; Vacchi et al., 2004, 2012). However, in contrast to the coastal spawning habitat of P. antarctica, much of the potential spawning area for D. mawsoni would not be subject to the formation of platelet ice, lacking the supercooled water emanating from under an ice shelf (Langhorne et al., 2015).
The association of D. mawsoni eggs with the underside of sea ice could serve three functions: protection in a low-turbulence environment, a mechanism to avoid advection out of the region, and a habitat where sea ice-based productivity will generate a predictable food source after hatching.
4.2 Protection
The presence of sea ice creates a low-turbulence environment at the ice–water interface, especially towards the interior of a large expanse of sea ice (Eicken, 1992). In addition, the presence of numerous interstices on the underside surface may create refugia for numerous organisms in close association with sea ice, as already observed for larval and juvenile Antarctic krill (Euphausia superba, Thorpe et al., 2007).
The vertical distribution of D. mawsoni eggs, estimated from the buoyancy measurements and egg size, suggests that in the low-turbulence environment that covers most of the likely spawning areas, the vast majority of eggs would accumulate at the water–ice interface. The chorion of D. mawsoni eggs is thick for a pelagic egg, especially one so strongly buoyant, suggesting the large egg size and large yolk mass generate more buoyancy (Ghigliotti et al., 2018; Kjesbu et al., 1992). The thick chorion may also afford protection to counter abrasion of sea ice exposure and it likely creates further protection from freezing (Cziko et al., 2006).
Measurements of buoyancy for 6 wild D. mawsoni eggs at the eyed-embryo stage caught near the surface in September suggests similar buoyancy at this stage (Parker & Di Blasi 2020). Changes in density during embryonic development may affect their exposure to surface ice as described by Evans et al. (2012) for eggs of P. antarctica found in the platelet ice in the spawning area of Terra Nova Bay.
4.3 Retention
The presence of sea ice and its movement affects the dispersal pattern of buoyant eggs. The effect of wind friction on sea ice results in faster transport of pack ice around the Ross Gyre and changes the route of egg advection compared to that in ice free waters (Kimura, 2004). Eggs and larvae associated with pack ice would not be exposed to ice-free waters until November or early December when the sea ice breaks up and melts (Comiso et al., 2011, Smith et al., 2014; Thorpe et al., 2007). This effect may place Ross Sea larvae in a location after their first winter that aids in their subsequent transport or movement to the continental shelf of the Amundsen Sea (Ashford et al., 2012; Hanchet et al., 2008).
Hatching in D. eleginoides was estimated to occur approximately 3 months after fertilisation (Evseenko et al., 1995; North, 2002). If development time is similar in D. mawsoni, hatching would occur after the breakup and dispersal period in the Ross Sea begins, which occurs over much of the spawning habitat in October–November (Dotto et al., 2018; Smith et al., 2014). An association with sea ice may result in more juveniles retained in the Ross Gyre (Dunn et al., 2012; Rickard et al., 2010). A strategy to avoid significant eastward or northward advection could be important for an estimated 18-month juvenile pelagic phase (Hanchet et al., 2008) and could be a determinant of stock structure for this species. The effects would be most pronounced during the first few months after spawning as the second winter would be experienced by juveniles (when 11–14 months old and likely near 10 cm in length (La Mesa, 2007)) with swimming abilities enabling diel vertical migration or directed swimming which could reduce any association with sea ice.
4.4 Food source in under sea ice environment
As day length increases during spring months, primary production increases and a diverse assemblage develops within and on the underside of pack ice (Arrigo, 2014). The primary production provides the basis of a diverse sea ice-associated ecosystem that could provide a key food source for D. mawsoni larvae hatching in October–November (Eicken, 1992). An association of D. mawsoni eggs with sea ice could provide a mechanism to ensure that hatching larvae have a reliable food source compared to foraging in ice-free waters.
5 CONCLUSIONS
If D. mawsoni eggs are protected, retained and nurtured by association with sea ice, then changes to the timing and spatial patterns in the advection and dispersal of sea ice due to climate change could also influence the development and retention of D. mawsoni larvae in the region and have a large effect on recruitment dynamics for the population.
These results generate three areas of research needed to further understand the spawning dynamics of D. mawsoni and evaluate potential effects of fishing and climate change on those dynamics. First, the association of larvae with the underside of sea ice should be confirmed as it would significantly impact the distribution of D. mawsoni eggs and larvae. Second, although spawning may begin in the northern Ross Sea in early July, the temporal and spatial extent of spawning should be determined to inform the timing and starting locations for eggs and larvae. Observations from a survey in September 2019 suggested spawning had finished during August on the seamounts near 150°W on the Pacific–Antarctic ridge (Parker & Di Blasi 2020). These are especially important parameters in developing dispersal and recruitment models. Third, studies are needed to determine if the annual recruitment from the Ross Sea population is derived from particular geographic regions of spawning activity. These results could inform spatial population models to evaluate the effects of climate change and fisheries management approaches on the Ross Sea D. mawsoni population.
ACKNOWLEDGEMENTS
We thank the Italian National Programme of Antarctic Research (PNRA) for providing Davide Di Blasi with the opportunity to participate in the survey under the project DISMAS (PNRA Project 2015/B1.02). Barcoding was funded by the PNRA project TNB-CODE (‘Terra Nova Bay barCODing and mEtabarcoding of Antarctic organisms from marine and limno-terrestrial environments’, PI: S. Schiaparelli). We thank the on-board scientific observers Mike Prasad (New Zealand National observer) and Dawie Potgieter (CCAMLR international observer) for carrying out sample collection at the high sampling rates required for the survey. We also thank Talley’s Ltd and the crew of the FV Janas for their safety focus, co-operation and assistance throughout the survey. Funding for the survey was provided by New Zealand Ministry for Primary Industries project ANT201501.
CONFLICT OF INTERESTS
The authors confirm that they have no conflicts of interest to declare in the preparation or publication of this research.
AUTHOR CONTRIBUTIONS
SP and S Sundby conceived and designed the research and conducted analyses. DS, DD, and S Schiaparelli conducted the experiments. SP, S Sundby, LG, and S Schiaparelli wrote the manuscript. All authors read and approved the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




