Considerations for management strategy evaluation for small pelagic fishes
Abstract
Management strategy evaluation (MSE) is the state-of-the-art approach for testing and comparing management strategies in a way that accounts for multiple sources of uncertainty (e.g. monitoring, estimation, and implementation). Management strategy evaluation can help identify management strategies that are robust to uncertainty about the life history of the target species and its relationship to other species in the food web. Small pelagic fish (e.g. anchovy, herring and sardine) fulfil an important ecological role in marine food webs and present challenges to the use of MSE and other simulation-based evaluation approaches. This is due to considerable stochastic variation in their ecology and life history, which leads to substantial observation and process uncertainty. Here, we summarize the current state of MSE for small pelagic fishes worldwide. We leverage expert input from ecologists and modellers to draw attention to sources of process and observation uncertainty for small pelagic species, providing examples from geographical regions where these species are ecologically, economically and culturally important. Temporal variation in recruitment and other life-history rates, spatial structure and movement, and species interactions are key considerations for small pelagic fishes. We discuss tools for building these into the MSE process, with examples from existing fisheries. We argue that model complexity should be informed by management priorities and whether ecosystem information will be used to generate dynamics or to inform reference points. We recommend that our list of considerations be used in the initial phases of the MSE process for small pelagic fishes or to build complexity on existing single-species models.
| 1. INTRODUCTION | 1168 |
| 2. APPROACH | 1170 |
| 3. CURRENT STATUS OF MSES FOR SPFS | 1170 |
| 4. WHAT ECOLOGICAL PROCESSES NEED TO BE CONSIDERED WHEN SIMULATING SPF DYNAMICS FOR MSE? | 1171 |
| 4.1 Variation in vital rates | 1172 |
| 4.1.1 Recruitment | 1172 |
| 4.1.2 Other vital rates | 1174 |
| 4.2 Spatial considerations | 1175 |
| 4.3 Multispecies interactions | 1176 |
| 4.4 Considerations for modelling assessment error and reference points in the estimation model | 1178 |
| 5. SUMMARY AND RECOMMENDATIONS | 1179 |
| ACKNOWLEDGEMENTS | 1180 |
| CONFLICT OF INTEREST | 1180 |
| DATA AVAILABILITY STATEMENT | 1180 |
| REFERENCES | 1180 |
1 INTRODUCTION
Ecosystem-based fisheries management (EBFM) has been widely proposed as a way to incorporate ecological knowledge in order to conserve ecosystems, improve the resilience of fisheries, and reduce error in assessments and management actions relative to goals. One area where EBFM has gained traction is in the management of commercial fisheries for lower-trophic-level prey species, which can reduce food availability for predators. For this reason, management advice about major prey species is starting to include EBFM considerations, such as predation, climate drivers and habitat needs (see Anstead et al., 2021; Marshall et al., 2019). One set of such prey species are small pelagic fishes (hereafter referred to as SPFs). By definition, SPFs are small- to intermediate-sized pelagic species such as sardines, anchovies and herrings that are a primary food source for several marine predators (Alder et al., 2008; Butler et al., 2010; Cury et al., 2000; Furness, 2003; Overholtz et al., 2000; Szoboszlai et al., 2015). Although our focus is on fish, many of the considerations developed here also apply to invertebrate species such as squid and krill with similar ecological roles as SPFs in marine ecosystems. In addition to supporting valuable fisheries for reduction, human consumption and feed for aquaculture (Froehlich et al., 2018; Ma et al., 2019; Metian, 2009; Tacon & Metian, 2008), SPFs serve as energy conduits in marine food webs, transferring energy from lower-trophic-level phytoplankton and zooplankton to higher-trophic-level predators (Pikitch et al., 2014), and exert top–down control on zooplankton in several ecosystems (Cury et al., 2000; Lynam et al., 2017). SPFs might therefore be one group of species for which ecosystem considerations in management are particularly important (e.g. Pikitch et al., 2014).
Simulation approaches provide one way to use ecosystem information to explore the robustness of management strategies (Figure 1; FAO, 2009; Möllmann et al., 2014). One such approach that has been identified as particularly useful is Management Strategy Evaluation (MSE; Perryman et al., 2021; Punt et al., 2016; Smith, 1994). Management strategy evaluation uses simulation to compare the performances of candidate management strategies in relation to a set of management objectives and priorities defined by stakeholders (Punt, Butterworth et al., 2016). Management strategy evaluation has been operationalized broadly for management, for example, regulating marine mammal harvest and bycatch (Punt & Donovan, 2007; Wade, 1998), the management of fish (De Oliveira & Butterworth, 2004; Punt & Hobday, 2009) and shark fisheries (Smith et al., 2007), management of multispecies fisheries (Fulton et al., 2014), and for evaluating harvest rules intended to mitigate seabird bycatch (Tuck, 2011). Management strategy evaluation has also been used fairly extensively in the design of management systems for SPFs (e.g. for sardine (Sardinops sagax, Clupeidae) and anchovy (Engraulis encrasicolus, Engraulidae) in South Africa, de Moor & Butterworth, 2016; and for Atlantic menhaden (Brevoortia tyrannus, Clupeidae) in the USA, SEDAR, 2020).
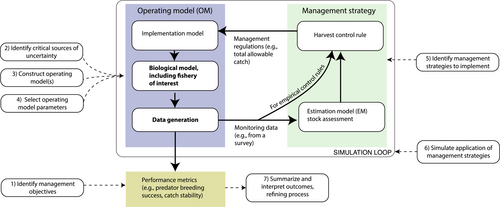
Management strategy evaluation is particularly powerful because it can inform management advice even when most of the underlying ecological processes impacting management effectiveness are not fully understood. Even when not operationalized for management to compare alternative harvest control rules as described above, MSE can be used to examine the robustness of existing management procedures to (possibly poorly understood) sources of uncertainty (e.g. survey frequency; Punt et al., 2020), or to the use of simplified models in decision-making (e.g. Lam et al., 2019; Surma et al., 2018). Management strategy evaluation is useful in these situations because it can explicitly account for ecological process uncertainty, as well as uncertainty related to monitoring, assessments and implementation (Plagányi, 2016). Some MSEs based on multispecies or ecosystem models have had direct impacts on management; for example, Kaplan et al. (2019) and Fulton et al. (2019), who showed the value of including EBFM considerations in management strategies. Nevertheless, despite the resources available with advice for conducting MSEs (Punt, Butterworth et al., 2016; Rademeyer et al., 2007; and others), MSE has not yet been widely adopted for management at the ecosystem level. This is due to several factors. The management goals of EBFM, including balancing spatial and species tradeoffs, are often not well-articulated (Essington et al., 2016; and examples in Koehn et al., 2020). Most ecosystem models are not designed at a scale or level of detail to provide the degree of precision required by management, that is, they typically are not designed for tactical decision-making (see Plagányi et al., 2014) or are not focussed on a specific management question (Essington and Plagányi 2014). Here, we provide technical advice on ecosystem considerations for MSEs. SPF fisheries provide an excellent case study on the use of MSE within an EBFM context because of SPF’s key role in marine ecosystems.
Simulation approaches for SPFs may require special consideration because of their unique role in marine food webs. They are known to have highly variable productivity, which, together with their short life spans, can lead to rapid and extreme fluctuations in abundance, as well as a high degree of uncertainty in their reference points related to carrying capacity (e.g. Chavez et al., 2003; Trochta et al., 2020). They also display large-scale aggregation behaviours that can lead to density-dependent catchability, sensitivity to exploitation, and localized depletion for dependent predators (Pitcher, 1995). All these characteristics must be considered in an ecosystem context, as SPFs are often key points of energy transfer in marine food webs and remain so throughout their life cycles (Cury et al., 2000). This means that predator–prey linkages and ecosystem structure are important considerations for building population models and in articulating management priorities and tradeoffs. Thus, crafting MSEs for SPFs is a particularly demanding process and will benefit from expert guidance.
An MSE approach requires users to develop a model of the system that covers the plausible range of scenarios, given the available information. Identifying situations where ecological processes could differ greatly from each other can show where more ecological information (diets, predators, etc.) may be required. A special consideration for all MSEs should be whether the level of complexity and the spatio-temporal scale of the models being used can most effectively answer the management question at hand and address the ecosystem objectives of EBFM (Plagányi et al., 2014). Finally, because of their ecological role, SPFs require a careful evaluation when it comes to management objectives and the interpretation of outputs, two other essential steps in the MSE process.
The MSE process involves the steps as outlined by Punt, Butterworth et al. (2016): (1) Identifying the management objectives and how these are represented in performance measures; (2) Identifying critical sources of uncertainty to which a management strategy must be robust; (3) Constructing one or more candidate (operating) models that represent the system to be managed, including the biology of the system, the way that data are collected from the system, and how the regulatory output from the management strategy is implemented; (4) Selecting operating model parameters and quantifying parameter uncertainty; (5) Identifying management strategies that could be implemented; (6) Simulating the application of the management strategies; and (7) Summarizing and interpreting the outcomes of these simulations, and refining the process if necessary (Figure 1).
In this study, we primarily focus on the second step of the MSE process, reviewing and summarizing critical sources of uncertainty that should be considered in the development of MSEs specifically for SPFs. We briefly review commercial SPF fisheries worldwide and use examples from several large marine ecosystems to demonstrate how important sources of uncertainty vary globally. We also discuss how, in some cases, these sources of uncertainty can be incorporated in population models that can be used in an MSE framework as an operating model (OM). This synthesis is intended to serve both directly as advice for those interested in developing MSEs for SPFs and indirectly as a case study for how EBFM approaches might be incorporated further into an MSE framework.
2 APPROACH
We invited scientific experts on SPFs from twelve countries (Canada, Chile, China, Denmark, France, Iceland, Norway, Poland, Portugal, Spain, the UK and the USA) to a workshop to discuss important sources of uncertainty about the ecology of SPFs, the data used to estimate their abundance, and how these data inform management strategies. This workshop was part of the International Council for the Exploration of the Sea North Pacific Marine Science Organization (ICES/PICES) Symposium on Drivers of Dynamics of Small Pelagic Fish Resources held in Victoria, British Columbia in March 2017. The experts comprised 28 scientists from universities and governmental research institutes with experiential knowledge of specific stocks of SPFs, their ecology and management, and quantitative tools for management. Participants were invited to answer two questions: (1) What ecological processes have been/can be/should be considered when simulating SPF dynamics for MSE? and (2) How does the importance of these processes and our ability to incorporate them in MSEs vary among ecosystems and among species? Participants were encouraged to consider data types and data limitations, scientific capacity (e.g. whether there is a full stock assessment), ecological role, ecosystem type, predation and environmental drivers. Group members were asked to discuss knowledge from their specific locations, as well as broader patterns common to SPFs globally, and to consider monitoring, estimation and implementation uncertainty. We synthesized the results of this discussion and identified the key areas of uncertainty, for which we incorporate examples from the Humboldt Current, California Current, Gulf of Alaska, the Mediterranean Sea and a collection of Northeast Atlantic stocks that include the Baltic Sea, Bay of Biscay, Barents Sea, North Sea and Norwegian Sea (Table 1).
| Theme | Must consider | Should consider | Key questions |
|---|---|---|---|
| Variation in vital rates |
Temporal changes in biological parameters such as somatic growth and natural mortality (All) Environmental shifts associated with productivity regimes (Humboldt Current, GoA) Depensatory dynamics when abundance is low (CA Current) |
Variation in functional responses with environmental conditions, such as bottom–up effects dominating under different environmental conditions (Humboldt Current) |
Does climate impact vital rates? If so, which ones? Do multispecies interactions impact SPF vital rates and vary temporally? Are SPF vital rates density-dependent? |
| Spatial considerations | Fishery stock identification (Northeast Atlantic stocks, Mediterranean Sea) | Spatial variation in abundance, productivity, growth and/or natural mortality (All) |
Are there spatial predator–prey interactions that are important to management objectives for the system? Does the SPF exhibit expansion–contraction effects as population size changes? Does the SPF stock span multiple jurisdictions spatially? Does productivity vary spatially? |
| Multispecies interactions | Predator needs and dynamics (Humboldt Current, CA Current) | Intraspecific competition, interspecific competition among forage species (Humboldt Current, CA Current, Mediterranean Sea) |
Does the SPF stock interact with other SPF stocks? Are there key predators of the SPF that are an important consideration based on management objectives? Are there other key interactions impacting the stock? |
| Assessment error and reference points | Ability of the survey or assessment to capture dynamics (CA Current, GoA, Mediterranean Sea) | Error due to spatial structure or dynamics (All) |
Are there spatial dynamics that will generate bias in reference points or biomass estimates? Is there spatial structure in the survey that may not capture spatial structure in the population? |
Note
- More detailed region-specific considerations are summarized in Siple and Koehn (2017). Northeast Atlantic stocks = Baltic Sea, Bay of Biscay, Barents Sea, North Sea, and Norwegian Sea; CA Current = California Current; GoA = Gulf of Alaska; All = CA Current, GoA, Humboldt Current, Mediterranean Sea; Northeast Atlantic stocks.
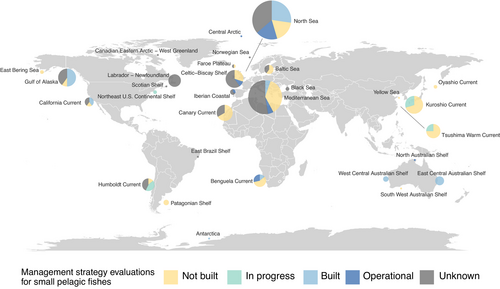
To assess the state of MSEs for SPFs in light of the discussion, we also reviewed existing SPF stocks worldwide and compiled an updated list of commercially fished small pelagic stocks with and without MSEs, thereby updating de Moor et al. (2011). Starting with the list of stocks included in the RAM legacy database (RAM Legacy Stock Assessment Database, 2018; Ricard et al., 2012), we added stocks from the Mediterranean and Baltic Seas, Australasia, Africa and China to extend geographical coverage and compile a full list (Table S1; Figure 2). Finally, we synthesized the outcomes to determine a set of guiding considerations for MSE of SPFs.
3 CURRENT STATUS OF MSES FOR SPFS
Our review of existing SPF stocks and MSEs illustrates that although there are existing MSEs for SPFs in some locations, not many are in use by management (not “operational”). For the majority of SPF stocks, the existence of a MSE is unknown or a MSE is currently not built (108/153 stocks globally; Figure 2). There are 10 stocks for which MSEs for SPFs are currently in progress. These results suggest that this review may be timeous to provide guidance for developing MSEs for SPFs with the hope of subsequent operationalization. Existing MSEs, especially those that are currently operational (35/153 stocks globally; 11 of which are operational in management), provide examples to guide future development of closed-loop simulations for other SPF stocks (Table 2). [Correction added on 25 June 2021, after first online publication: Section 3 has been updated in this version]
| Broader topic | Specific consideration | Example of use in an existing MSE | Example of use in an existing MSE—species or stock | Reference | Example of use (E) or technical guidance (G) |
|---|---|---|---|---|---|
| Variation in vital rates | Recruitment | Random assignment of 3 different stock–recruitment models | Northeast Atlantic mackerel | ICES (2020c) | E |
| Recruitment | Use AIC-weighted average of 3 stock–recruit models (Ricker, Beverton-Holt, segmented regression), with an AR−1 correlation for error | Norwegian spring-spawning herring | ICES (2018b) | E | |
| Recruitment | Estimate the stock–recruit relationship differently for "boom" and "bust" years | South African sardine | de Moor et al. (2011) | E | |
| Recruitment | Approximate recruitment variation based on environmental drivers that occur at the same timescale | Pacific sardine in California | Hurtado-Ferro and Punt (2014) | Ea | |
| Recruitment | Incorporate environmental drivers mechanistically | Several | Punt et al. (2014) and Haltuch, Brooks et al. (2019) | G | |
| Recruitment | Incorporate forecasts of environmental drivers explicitly in control rules, where appropriate | Pacific sardine in California | Tommasi et al. (2017) | Eb | |
| Recruitment | Incorporate low-recruitment scenarios for robustness testing | South African sardine; Bay of Biscay anchovy | de Moor et al. (2017); Sánchez et al. (2019) | E | |
| Growth | Several | This paper | – | ||
| Fecundity | Several | THIS paper | – | ||
| Age-at maturity | Several | This paper | – | ||
| Natural mortality | Include different mortality and mortality variation scenarios in robustness tests | Several | Punt, Butterworth et al. (2016), Rademeyer et al. (2007) and DFO (2019) | G | |
| Natural mortality | Use a multispecies model to estimate natural mortality | Several | ICES (2020a); ICES (2020b) | E | |
| Spatial patterns | Recruitment | Model spatial differences in carrying capacity implicitly rather than modelling recruitment differences in a spatially explicit model | Several | This paper | – |
| Migration | Where appropriate, link spatial models to oceanographic models (i.e. "end-to-end" ecosystem models) | Pacific sardine and northern anchovy | Fiechter et al. (2014) | Ec | |
| Predator needs | Explicitly model space or population structure of predators and prey using a MICE model | Pacific sardine and northern anchovy | Punt, MacCall et al. (2016) | E | |
| Predator needs | Model spatial dynamics using a proxy that can mimic spatial patterns; use MICE model | South African sardine | de Moor et al. (2017) and Robinson et al. (2015) | E | |
| Multiple processes simultaneously | Model structure explicitly using MICE models | Pacific sardine and northern anchovy | Punt, MacCall et al. (2016) | E | |
| Multiple processes simultaneously | Allocate stock to different subareas, with mixing, based on parallel spatial process | South African sardine | de Moor et al. (2017) | E | |
| Stock covering multiple jurisdictions | Include plausible ranges for historical and future catches or jurisdictions without data | Several | This paper | – | |
| Multispecies interactions | Competition within forage guild | Incorporate scenarios for competition, apparent competition and different drivers where appropriate | Several | This paper | – |
| Predator–prey relationship | Include different plausible values for functional response parameters if shape of relationship unknown | Several | This paper | – | |
| Predator–prey relationship | Use meta-analysis to parameterize functional relationships to account for uncertainty in strength or functional form of relationship | Several | This paper | – | |
| Accounting for predator needs | Explicitly include predators in operating model using a MICE model, if data are sufficient to fit one | Atlantic herring; Pacific sardine and northern anchovy | Deroba and Bence (2008) and Punt, MacCall et al. (2016) | E | |
| Accounting for predator needs | Use more complex ecosystem models to determine reference points that satisfy predator needs before testing them | Several; Atlantic menhaden | Pikitch et al. (2012), Smith et al. (2011) and SEDAR (2020) | E |
Note
- Not all of these examples were used explicitly in management decision-making. Cases where the specific consideration influenced the selection of a management strategy are indicated as footnotes.
- a Recruitment assumptions were key because the harvest control rule includes sea surface temperature, which is (with noise) a driver of the stock–recruitment function.
- b Temperature impacted the harvest guideline being tested in the simulation. This simulation was a research exercise, so no harvest guideline was formally adopted based on these results.
- c Movement and migration rates informed by the environment were shown to influence availability to the fleet.
4 WHAT ECOLOGICAL PROCESSES NEED TO BE CONSIDERED WHEN SIMULATING SPF DYNAMICS FOR MSE?
- Variation over time in vital rates, such as growth, mortality or age-at-maturity, especially as a function of predator abundance or density dependence;
- Spatial patterns for predators and prey, including migration, multiple stocks and stock mixing, spatial differences in predator needs (e.g. for central place foragers), and spatial differences in the implementation of control rules; and
- Species interactions among SPFs and between SPFs and their predators.
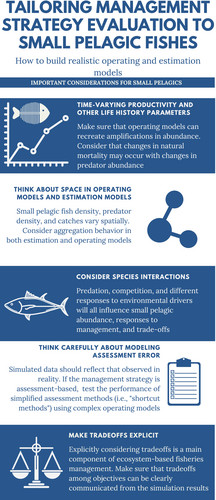
Considering important areas of uncertainty can improve MSEs in two ways: (i) by creating a more realistic set of scenarios across which to evaluate management performance by generating dynamics similar to (or plausible for) those observed in real stocks; and (ii) by adequately characterizing uncertainties for robustness tests.
- Considerations for estimating assessment errors and reference points in the estimation model (EM)
4.1 Variation in vital rates
Changes in vital rates were identified as a key consideration across all five geographical areas discussed during the workshop (Table 1), with changes in fecundity, natural mortality and age-at-maturity potentially leading to low-frequency variation in productivity, a pattern observed in many stocks, including some SPFs.
4.1.1 Recruitment
The appropriate functional form of the stock–recruit (SR) relationship is notoriously difficult to estimate, particularly for SPFs (van Deurs et al., 2020; Subbey et al., 2014), but it is important for OMs to describe past and future recruitment. The two key questions for stock–recruit relationships in SPFs are as follows: (1) Is there a density-dependent effect, and if so, at what stock sizes? and (2) What is the magnitude and time scale of recruitment variability?
SPF dynamics are characterized by large fluctuations in population size (“boom and bust” dynamics) that arise from a high degree of recruitment variation and a short life history. These dynamics are thought to be a result of responses to environmental variability (Bartolino et al., 2014; Borja et al., 2008; Checkley et al., 2017; Lindegren et al., 2013), natural mortality (Jacobsen & Essington, 2018) or a combination of environmental variability and fishing (Essington et al., 2015; Fu et al., 2018; Lindegren et al., 2013; Pinsky & Byler, 2015). Discussions about SPF recruitment typically emphasize the effect of environmental processes on survival of early life stages (Somarakis et al., 2019; Zimmermann et al., 2019). These processes operate at low frequency, leading to regimes of high and low population productivity (Szuwalski et al., 2014; Vert-pre et al., 2013). Thus, the parameters of the stock–recruit relationship are thought to be non-stationary, and distinguishing which parameter is time-varying is difficult. Finally, because it is very difficult to distinguish whether spawning biomass is being driven by recruitment variation or vice versa for SPFs, decisions about how recruitment variation is modelled will affect interpretations about the impact of fishing (Szuwalski et al., 2019). Thus, recruitment variation is an important consideration both in how reality is represented in the OM and in how the results of the MSE are interpreted.
Methods for modelling recruitment for SPFs include using multiple stock–recruitment functions (as in the MSE for Northeast Atlantic mackerel [Scomber scombrus, Scombridae]; ICES, 2019c), estimating stock–recruit parameters for boom and bust years separately and conservatively projecting using only the “bust” year parameters in projections (de Moor et al., 2011) or including periodic “boom” years too, and approximating recruitment variation based on environmental drivers that occur at the same time scale (e.g. Hurtado-Ferro & Punt, 2014). One could also estimate different stock–recruit functional forms for alternative OMs, particularly when, given the high recruitment variability, no single functional form fits the historical data best. For cases where environmental variation is thought to drive recruitment variation, Haltuch, Brooks et al. (2019) provide several suggestions for how environmental drivers can be incorporated into MSEs. We direct readers to De Oliveira and Butterworth (2005) and De Oliveira et al. (2005) for warnings about using environmental indices as predictors of recruitment success, and Punt et al. (2014) for suggestions about incorporating environmental uncertainty in simulation approaches. Explicit integration of environmental drivers can also allow an assessment of the robustness of management strategies to hypothesized directional changes in recruitment in response to climate change (e.g. Haltuch et al., 2019).
4.1.2 Other vital rates
Other vital rates can also vary over time in SPF stocks. Existing MSE advice includes robustness trials to variation in natural mortality (Punt, Butterworth et al., 2016; Rademeyer et al., 2007), which is particularly important for SPFs given their role as prey. We discuss natural mortality below under “Multispecies interactions” and discuss the other vital rates here.
Time-varying vital rates may be particularly important for SPFs because of the sensitivity of their dynamics to interacting effects of predation and fishing mortality. Growth, fecundity and age-at-maturity can vary temporally for SPFs (Hunter et al., 2019; van der Lingen et al., 2006; SEDAR, 2020; Silva et al., 2006; Smoliński, 2019). Disease outbreaks, parasites and environmental conditions such as hypoxia also vary over time (de Moor et al., 2017) and can affect life history. OMs should be able to reproduce historically observed variations in abundance in order to produce realistic future projections, and time-varying parameters may be required to achieve this. Changes in predator needs can lead to temporal changes in natural mortality (see “Multispecies interactions” section). Though estimates of these time-varying parameters are not always readily available, processes that are plausible a priori but lack data or conclusive evidence should not necessarily be excluded from investigations. In cases where environmental drivers might affect both recruitment and another vital rate such as growth, we recommend that modellers conduct analyses to identify correlations between biological parameters and so they can be included in the OM. Punt et al. (2014) offer further guidance for using MSE to evaluate the impact of environmental variation on management performance when environmental drivers influence changes in abundance or parameter values.
4.2 Spatial considerations
Spatio-temporal dynamics are an essential component of how SPFs behave and how predators and fisheries exploit them. Spatial issues include hyperstability, migration, stock mixing, spatial differences in predator needs, particularly for central place foragers susceptible to localized depletion, and spatial differences in the implementation of harvest control rules, such as when multiple countries share the same resource. How these are represented in the OM will depend on the research question and the performance objectives for the stock. Stock identification (the identification of biologically distinct stocks for the purposes of management) and the spatial mixing between these management units are an issue for several stocks, including: Pacific herring (Clupea pallasii, Clupeidae) in the Gulf of Alaska whose growth and fecundity vary spatially; Northeast Atlantic sprat (Sprattus sprattus, Clupeidae) (Hunter et al., 2019; McKeown et al., 2020; Quintela et al., 2020); anchovy and sardine in the Mediterranean Sea (Fiorentino et al., 2014); two stocks of Atlantic herring (Clupea harengus, Clupeidae), namely, western Baltic spring-spawning herring and central Baltic herring (ICES, 2018a); and South African sardine (de Moor et al., 2017; Weston et al., 2015).
Many SPFs undergo range contractions when abundance is lower (i.e. the “basin model” of MacCall, 1990), which can affect productivity if it results in depensatory dynamics (Holling, 1959; Liermann & Hilborn, 2001; Swain & Benoît, 2015; Walters & Kitchell, 2011). Conversely, range collapses might be explained using information about biomass, changes in recruitment or spatial vulnerability to disease (e.g. MacCall, 1990). These behaviours can also affect the precision and accuracy of abundance indices when range expansions in high-abundance years result in surveys missing a proportion of the stock (Barange et al., 2009; Hilborn & Walters, 1992; MacCall et al., 2016). For example, in the California Current, where sardine habitat appears to extend northward at high population sizes and under specific oceanographic conditions, the extent of the spring acoustic survey for sardine depends on an environmentally based estimate of spawning habitat extent to ensure that all potential spawning habitat is sampled (Zwolinski et al., 2011). While expansion–contraction effects can be modelled empirically, there is seldom a mechanistic way to model them.
Recruitment can also vary spatially, and the limitations of recruitment by habitat may drive dynamics. For example, optimal spawning habitat for Pacific sardine is associated with specific oceanographic conditions (Nieto et al., 2014; Reiss et al., 2008; Weber & McClatchie, 2010; Zwolinski et al., 2011). Recruitment can be spatially correlated because of sensitivity to local environmental conditions (e.g. in north Atlantic and northeast Pacific herring stocks; Zheng, 1996). Stocks can also be expected to be less productive at their margin of distribution if they follow the basin model (MacCall, 1990; Saraux et al., 2014). Alternatively, the underlying stock–recruit relationship might change with local depletion because density-dependent effects occur at small spatial scales (e.g. Casini et al., 2011). An OM may need to consider local dynamics if they lead to emergent patterns at the stock scale (MacCall et al., 2019; Punt, MacCall et al., 2016). This is possible with some ecosystem models that have small-scale dynamics and have been used for MSE (e.g. Fulton et al., 2011). As the stock–recruit relationship varies spatially, so will carrying capacity, which might be easier to model implicitly using depensatory stock–recruitment relationships instead of explicitly with a spatial model (Liermann & Hilborn, 2011). Spatial models linked to oceanographic models (i.e. “end-to-end” ecosystem models) can generate spatio-temporal dynamics in recruitment, but conditioning these such models is so resource-intensive that applying them at a resolution relevant to management is often not an option.
Interactions between predators and SPFs are also inherently spatial, and predator populations may not have access to the entire region of SPF distribution. Spatial models can capture some of the dynamics and behaviour of predators that forage from a fixed location like a breeding colony (e.g. Barrett et al., 2006). Some spatial structure can be captured using Models of Intermediate Complexity (MICE; Plagányi et al., 2014) with limited spatial structure (e.g. Punt, MacCall et al., 2016; Robinson et al., 2015). Alternatively, spatial structure could be approximated by modelling an (annually varying) proportion of the population to be within the foraging area and thus subject to the predation of a predator. Short time steps (e.g. monthly or quarterly) may be required to effectively model individual prey movement.
If fishing pressure differs throughout a SPF’s distribution range, the MSE may require spatial structuring in the OM to adequately account for these differences. The effect of fishing activities on the dynamics and movement of SPFs should be considered in spatial models (Sydeman, Thompson et al., 2017; Okamoto et al., 2020). Plausible spatial distributions of fishing activity can be generated using random utility models of fisher behaviour and port-level landings (e.g. Rose et al., 2015). While explicit modelling of areas is preferable where data permit, in some cases, selectivity can be used as a proxy for area (the “areas as fleets” approach; Cope & Punt, 2011; though some simulation studies now indicate that this approach is sub-optimal). For example, spatially disaggregated fishing effort may be adequately reflected by age-disaggregated selectivity if the SPFs move through different areas as they grow (e.g. South African anchovy de Moor & Butterworth, 2016). SPFs often occupy an area that spans the jurisdictions of multiple countries. For example, Pacific sardine are fished across Canada, the USA and Mexico (Punt, MacCall et al., 2016). The South Peru–North Chile anchovy stock is managed independently between Peru and Chile, with different assessments (Canales et al., 2020). In cases where the stock is not managed cooperatively across its range, management outcomes for the whole stock will depend on the combined effects of each country's management plan. While the benefits of cooperative management across the stock's range have been documented for some taxa (e.g. Pacific hake [Merluccius productus, Merlucciidae]; Hamel et al., 2015), SPFs are often large stocks, the majority of which are fished by more than one country (139 of 224 stocks, based on FAO wild capture production from 2019). Additionally, the need to work cooperatively in a short timeframe is unique to SPFs, given that acquiring data can sometimes take longer than the time for which the fish is available to the fishery (for example, recruitment fisheries for age-0 individuals). In cases where data are not available for all jurisdictions, simulations can include alternative plausible ranges for historical and future catches for countries for which dynamic management strategies are not available, such that fishery impacts on the entire stock can be modelled. Attempting to model dynamics across jurisdictions can identify data gaps where more information should be gathered (Fulton et al., 2011).
The choice of spatio-temporal dynamics in the OM depends on the research question, the scale of the dynamics, the resolution of the data, and the objectives of the strategic management decision being investigated. For example, the OM could explicitly include space or stock structure if users want to evaluate management strategy performance with respect to benefits to central place foragers such as seabirds (e.g. sardine and anchovy in the California Current, Punt, MacCall et al., 2016; South African sardine, de Moor et al., 2017). Alternatively, if users want to evaluate performance with respect to spatially-dependent harvest rates or implementation error (e.g. overshooting quotas), such as when multiple countries with different management structures harvest the same straddling stock, the OM could (minimally) be structured to represent the stock distributed by country. Spatial differences in recruitment, natural mortality, growth and fleet dynamics may necessitate a spatially explicit model. Choosing the appropriate spatial scale to represent is a central challenge, as misspecification can lead to incorrect representation of recruitment and of the management values that are used to estimate reference points (Kapur et al., n.d.). Other considerations include conceptual decisions about the hypotheses being tested and tactical decisions about how mortality and recruitment occur over space (Punt, 2019).
Not all spatial dynamics can or should be included in an MSE. One example of this is the case of sardine in South Africa, where the harvest control rule implemented after MSE testing provides total sardine quotas (spatially disaggregated by west/south coast in some years). In addition to this harvest control rule, an island closure experiment has restricted purse seine fishing around some key African penguin breeding islands (e.g. Ross-Gillespie & Butterworth, 2020; Sherley, 2020). In this case, the relatively small spatial scale of the 20-km radius around the penguin breeding islands was not explicitly modelled in the MSE but under EBFM, the impact of fishing on penguins is still being evaluated (de Moor, 2018). Spatial issues are likely to become even more urgent in the future, as climate change continues to influence the movement and distribution of SPFs. Management strategy evaluations that allow decision-makers to balance tradeoffs for multiple jurisdictions will be useful.
4.3 Multispecies interactions
SPFs are characterized by their role as prey for a diverse assemblage of predators, including predatory fish, marine mammals and seabirds (Engelhard et al., 2014; Pikitch et al., 2014; Smith et al., 2011). The availability of alternative prey for predators is a key consideration, but there is considerable uncertainty surrounding interactions between prey species and how these impact predators.
Properly characterizing the ecological relationships among multiple SPF stocks and how predators might respond to fluctuations in their prey is an important step in generating realistic dynamics for each species and determining the outcomes for fisheries that harvest them and predators that may depend on more than one species. Negative correlations between species abundance can be evidence of competition, apparent competition or opposite drivers. Thus, if there is little information or conflicting information about intraguild relationships, OMs with multiple forage species may need to include a range of possible options. For example, sardine–anchovy relationships can appear to be positive (McClatchie, 2012) or negative (Chavez et al., 2003), and different biological and non-biological processes can be invoked to explain the same observations (Hosack et al., 2013; Siple et al., 2020). Competitive relationships between SPFs can also change over the life cycle of a species, as in the Norwegian Sea where Atlantic mackerel feed on herring larvae (Allan et al., 2021; Skaret et al., 2015), while the adults of both species compete for planktonic prey on their feeding grounds (Nikolioudakis et al., 2019). There are several possible scenarios for intraguild relationships in fisheries where SPFs are part of a multispecies fishery. Considering different interactions between exploited SPF stocks could help to capture dynamics associated with competition or apparent competition due to food availability.
Predator–prey relationships—particularly functional responses and predation rates—may be one of the most important ecosystem processes driving the dynamics of SPFs. If possible, empirical data on functional response relationships (the shape of the relationships between predator demographic rate and prey abundance) should be used to parameterize predator responses (Koehn et al., 2021). These data may be available from meta-analyses, for example, seabird functional responses to changes in forage fish abundance (Cury et al., 2011) or from case studies of individual populations (e.g. Robinson et al., 2015; Suryan et al., 2006). Alternatively, statistical relationships representing the presence and strength of pairwise species interactions, including competition, can be estimated directly from time-series data using multivariate autoregressive models (Ives et al., 2003; Lindegren et al., 2009). However, even in data-rich cases, the shape of predator functional responses or estimated interaction terms are subject to uncertainty (Sherley et al., 2015; Sydeman, Piatt et al., 2017). Hence, alternative OMs might include different shape parameter values for the functional response.
Realized prey is a function of preferred prey, spatial overlap and the availability of other prey items (Blanchard et al., 2014). If predators eat non-preferred prey when it is abundant but a more preferred prey if available, a single-predator, single-prey model would be unable to sufficiently capture the plausible range of predation rates experienced by the prey (e.g. Pinnegar et al., 2003; Plagányi et al., 2014). Care should also be taken as to the importance of the focal SPF in the ecosystem. While one or two species of SPF are the key forage species in some ecosystems (i.e. “wasp-waisted” ecosystems; Cury et al., 2000) SPFs can be part of a diverse forage category, in which case models with predators must be flexible enough to characterize the dynamics of other forage species and the potential for key predators to prey-switch (e.g. with sardine and anchovy in the California Current; Koehn et al., 2016). Ecosystem models with the appropriate degree of structure can be helpful in distinguishing whether a given species is a “key” component or not (Plagányi & Essington, 2014). Incorrect assumptions about functional responses could lead to over- or under-estimation of the impacts of SPF fisheries on predators (Plagányi & Butterworth, 2005). Therefore, it is important to consider uncertainty in the strength and shape of predator–prey functional responses when assembling the reference set of OMs and robustness tests. The effects of a disturbance or a large increase in predator abundance may not propagate through the food web in a manner that is consistent across ecosystems, and the observed strength of trophic cascades depends on the response of the particular SPF to changes in food availability, as well as density dependence (Heath et al., 2014). These processes can be represented in OMs implicitly in the form of time-varying natural mortality and uncertainty in stock–recruit relationships, respectively.
The number of species, sites or ecosystem components included in the OM is important decisions to consider for SPFs because they are never at the top of the food chain; thus, there is no natural limit on model complexity. Accounting for additional non-predator–prey food web relationships may result in more realistic OMs than single-species models, although sometimes it suffices to model ecosystem components such as predation with a realistic range of natural mortalities (Deroba et al., 2018) or allow the OM to explicitly account for predator needs (Fulton et al., 2014; Pikitch et al., 2012). Theoretically, including more species increases the likelihood that the OM better represents the real world, provided the ecological processes are well understood, but the increased number of parameters required for a more complex OM involves evaluating an ever-larger set of robustness trials, which can quickly become prohibitive when parameters are unknown and cannot be estimated reliably. This may be the case when there are one or more forage species without stock assessments. In some cases, other analyses can be used to quantify the benefits of including additional ecological information to models (e.g. economic analyses; Essington et al., 2018) and to optimize the degree of model complexity (Collie et al., 2016), given the model error, that is, the uncertainty in the input variables propagating to the model output (Saltelli, 2019).
When OMs include predators as drivers of prey abundance, it is important for OMs to generate plausible levels of variability in predator and prey abundances if the objectives of management are based on desired levels of predator reproduction or abundance. The latter has been the primary way that multispecies interactions have been considered in MSEs for SPFs so far. For example, the MSE for Atlantic herring in the USA evaluated control rules based on the needs of seabirds and piscivorous fish (Deroba et al., 2018; Feeney et al., 2018) and represents one case where an MSE approach has been used explicitly to support EBFM (Townsend et al., 2019). The MSE for California Current sardine and anchovy included performance metrics related to the abundance of predators (sea lions and brown pelicans) and the probability that those predators fell below abundance thresholds (Punt, MacCall et al., 2016). Penguin feeding has also been included in the MSE for South African sardine, where performance metrics included the projected impact of alternative sardine management strategies on penguin dynamics based on the estimated relationship with sardine abundance (de Moor, 2018; Robinson et al., 2015).
Generating realistic amounts of variation in natural mortality in single-species models for SPFs may be difficult for some stocks. Proxies such as predator abundance may be used to bracket a realistic range of values and develop plausible scenarios for low-frequency variability in natural mortality. For example, in the Barents Sea, cod abundance is used to simulate capelin mortality, and in the North Sea, a multispecies assessment model is used to calculate natural mortality for several species (ICES, 2020a,b). Hazen et al. (2019) review the ways that predator diet, movement, life history, reproduction and demography can be used to make inferences about unobserved ecosystem components including the abundance of SPFs (fish and invertebrates) and provide guidance for how to employ them to detect changes at different timescales. It is important to ensure that predator abundance proxies for natural mortality are linked to the life-history parameter that is most likely to be actually involved in the relationship. For instance, changing predator abundance may be more appropriate to link to depensatory recruitment than natural mortality if predators select younger prey.
The complexity of the OM should be informed by the research question and management priorities and how ecosystem information is to be used, that is, to generate dynamics or to inform ecosystem reference points. Complex ecosystem models are useful for making strategic management decisions but are generally not recommended for use as operating models in an MSE (Punt, Butterworth et al., 2016; Sainsbury et al., 2000). They can, however, be used to determine reference points or harvest rates that might satisfy predator needs (Pikitch et al., 2012; Smith et al., 2011), before those reference points are evaluated in a single-species MSE. MICE models are more practical for making tactical, practical and quantitative management decisions. For example, Smith et al. (2015) used a small pelagics-specific variant of an Atlantis ecosystem model (Atlantis-SPF) to evaluate reference points for the Commonwealth Small Pelagic Fishery; for northern British Columbia herring. Surma et al. (2018) applied an ecosystem model to ascertain ecological outcomes for harvest control rules, while socio-economic impacts were assessed by industry and community members for their societal desirability (Lam et al., 2019). Pikitch et al. (2012) added variability to Ecosim food web models to test a broad range of harvest control rules. On the other hand, MICE models can address both tactical and strategic questions, because they can be conditioned on data, provide estimates of current abundance and exploitation while retaining important ecological interactions. Predator effects on prey can be modelled explicitly when data are available to condition a MICE model (e.g. the NWACS-MICE model built for Atlantic menhaden; SEDAR, 2020). These simpler models can be beneficial to use as OMs, as they illustrate how a management strategy performs in complex situations without the uncertainty and computational burden associated with more complex ecosystem models.
4.4 Considerations for modelling assessment error and reference points in the estimation model (EM)
The OM should generate pseudo-data about the biological system that match what would be available in reality. In practice, these data would either be used directly in a harvest control rule (empirical management strategies; for example advice for North sea horse mackerel is based on changes in survey indices; ICES, 2019a, b, c), as input to an assessment when the harvest control rule depends on assessment model outputs (model-based Management Strategies), or both, when the control rule depends on both data and results from an assessment. For model-based management strategies, the Estimation Model (EM) should either be the existing or proposed assessment method to be used to manage the stock in question; or generate uncertainty similar to the existing or proposed assessment method.
The inclusion of spatially explicit and/or time-varying components in the OM will assist the evaluation of the performance of a management strategy for spatially structured SPFs relative to its objectives. In particular, performance metrics using reference points may require spatially explicit or time-varying reference points (RPs). RPs are benchmarks to which stock level and fishing intensity are compared for management purposes (e.g. BMSY). RPs for determining stock status should be chosen appropriately given the “boom and bust” dynamics exhibited by many SPF populations. Some harvest control rules are linked to RPs. In some cases, externally estimated RPs (i.e. not estimated within an assessment) are used in management and can easily be input as time-invariant values in the MSE. In other instances, harvest control rules depend on annually estimated reference points output from the latest assessment (the EM) and time-varying changes to the RPs calculated by the EM should be considered. Where spatially explicit advice is to be provided, the EM would need to estimate spatially explicit reference points or spatially explicit relative stock status to inform the harvest control rule. This would ensure that performance of dynamic or spatially explicit management strategies is evaluated given realistic errors in the estimation of stock status and reference points.
EMs that include other ecosystem components are rare, but in some cases, they may help to meet new EBFM-related challenges. Tommasi et al. (2017) found through an MSE process that harvest guidelines explicitly incorporating sea surface temperature predictions as a driver of future SPF productivity increased biomass and yield and reduced the probability of biomass or yield falling below accepted thresholds when combined with harvest restrictions at low biomass. Environmental conditions are used in some harvest guidelines to ensure catch quotas are responsive to recruitment variation (e.g. in Pacific sardine; Kuriyama et al., 2020) and have been proposed in others as a way to avoid bias in biomass estimates (Peruvian anchoveta; IMARPE, 2019). Predator data collection could improve the characterization of uncertainty in EMs of SPFs and could allow predator abundance (Hollowed et al., 2000) or predator diet composition (Velarde et al., 2015; Zador & Yasumiishi, 2018) to be used as an indicator of prey abundance and distribution within management strategies. Predator diets may also be used as an indicator for SPF community composition (Elliott et al., 2015; Sydeman, Thompson et al., 2017). However, approaches that use ecosystem information in EMs for SPFs are rare and the OM would need to be constructed to generate data that reflects the uncertainty associated with using such information for management purposes (De Oliveira et al., 2005).
5 SUMMARY AND RECOMMENDATIONS
A key step in the development of any MSE is identifying uncertainties that are likely to be consequential. Sources of uncertainty are plentiful and input from experts is crucial for capturing the full range of scenarios that should be explored and identifying areas of focus (Punt, Butterworth et al., 2016). The process of identifying major sources of ecological uncertainty will require input from experts with knowledge of the ecosystem, the fishery and the quantitative models used for decision-making. Knowing these sources of uncertainty can help analysts interpret MSE results across ecosystems, even if data are insufficient to confirm whether certain plausible hypotheses can be supported. They can also identify “blind spots” where uncertainties are not known but could be impactful. Our study is intended to serve as a guide for those interested in applying MSE to a stock of SPF by providing concrete suggestions to incorporate more ecological processes into MSEs. Here, we share the results of a broader discussion among experts from several systems to demonstrate how uncertainties could be incorporated in models used for MSE.
Ecosystem processes arise at nearly every stage in the MSE process. In the construction of an OM, ecological information can be used in two primary ways: (1) to identify important sources of process and observation uncertainty, which management strategies should ideally be robust to and (2) to ensure the OM produces realistic population dynamics, such that the data generated from the OM are indistinguishable from the actual data, including some of the biases and known issues with the actual data. Our list of considerations (Table 1; Figure 3) should facilitate future modelling in support of management goals, particularly for those who are undertaking an MSE focussed on SPFs. It can be used to build model complexity, then identify robustness tests where, for example, parts of that model complexity are uncertain. For SPFs, identifying management objectives (step 1 of the MSE process); and interpreting results and communicating tradeoffs to stakeholders (step 7) often involve the consideration of EBFM priorities and ecological processes. The SPF-specific challenges of identifying EBFM priorities are documented in several case studies (Drew et al., 2021; Koehn et al., 2020).
A key decision in the design of any MSE is the choice of which tool(s) are appropriate in scope and scale for the research question; this manuscript will aid scientists in making such decisions. The models typically used to evaluate ecosystem outcomes for fisheries management have not been used extensively in simulation frameworks such as MSE because of the computational burden of running many simulations with complex ecosystem models such as Atlantis. However, some processes can be represented using modifications to a single-species model or MICE model. Accounting for some ecosystem processes will also be key to developing new ways to explicitly address tradeoffs (e.g. between the harvest of predators and prey; Koehn et al., 2017), a consideration inherent to EBFM (Link, 2010). We advise analysts constructing an OM to build in complexity in a way that is informed by the management priorities and whether the ecosystem information is being used to generate realistic dynamics, inform ecosystem reference points, or both.
Not all of the elements discussed here are necessary to include directly in an MSE for SPFs. Including too many sources of uncertainty is likely to lead to results that are difficult to interpret, and considering implausible sources of uncertainty could lead to the selection of an incorrect management strategy (Punt, Butterworth et al., 2016). Furthermore, there are costs to collecting ecosystem information (e.g. Essington et al., 2018) and incorporating it in an MSE. When information is not available about a specific interaction or ecosystem, we recommend meta-analysis as a tool for identifying a realistic range of values and robustness testing, especially if analysts are familiar with the studies therein. Finally, we encourage researchers carrying out MSEs for SPFs to clearly identify which hypotheses can be investigated given the available data. Making sure the OM can be conditioned on available data will build credibility during a management focussed MSE process and allow for the use of the MSE to support tactical decision-making and management. Although it is not straightforward to account for substantial missing data in an MSE, MSE can be tailored to address certain data limitation issues. However, MSE should not be limited to hypotheses that are supported by the data. For example, while depensation is often not supported by data (because of insufficient contrast), it should be considered for inclusion in the set of hypotheses on which the MSE is based unless it can be excluded given the likely substantial impact of depensation of the performance of candidate management strategies.
Other sources of uncertainty are not discussed at length here but merit further investigation and methodological development. Climate change will lead to ecological changes at several scales, the emergent patterns of which will likely drive SPF populations in ways that are difficult to predict. Future studies may need to consider dynamic interactions between the processes discussed here. We have not considered the societal uncertainties that can arise from the diverse sources of knowledge and values of fishery stakeholders and citizens (Lam et al., 2019). These societal uncertainties can influence the identification of management objectives, performance measures and management strategies considered in participatory MSE processes (Fulton et al., 2014). They also can affect the implementation of and compliance with management strategies recommended from MSEs, adding another layer of complexity and uncertainty to be considered in MSE best practices (Punt, Butterworth et al., 2016).
This review demonstrates the remarkable diversity of systems and management needs for SPFs globally. SPFs present unique challenges to management, but some of these can be addressed if management strategies are evaluated subject to an appropriate and defensible set of uncertainties. Future evaluations of management strategies for SPFs would benefit from a consideration of their unique ecological properties, as suggested throughout this study. Discussion among experts in the ecosystem of interest is also crucial for identifying sources of uncertainty for the simulation process. SPFs represent a case study for how information from these discussions can be leveraged in an MSE framework, which is key for producing robust management decisions that can be used for EBFM.
6 ACKNOWLEDGEMENTS
M. Siple was supported by a Pew Foundation fellowship and a James S. McDonnell Postdoctoral Fellowship (grant # 220020561). M. E. Lam was funded by the Research Council of Norway Marinforsk Programme (project # 303663). S. Sánchez-Maroño was funded by the Dirección de Pesca y Acuicultura del Gobierno Vasco (Spain). S. Surma was funded by the Natural Sciences and Engineering Research Council of Canada (grant #6564). TM. Canales was funded by the CONICYT PIA/BASAL FB 0002. The authors thank Hiroyuki Kurota for advice about Japanese stocks and Cristian Canales Ramirez for advice about Chilean stocks. Timothy E. Essington, Isaac Kaplan, Cody Szuwalski, Jennifer Boldt, Jaclyn Cleary and Mary Hunsicker are thanked for reviewing an earlier version of this manuscript. We thank two anonymous reviewers and the editor for comments on this manuscript. The authors also thank the following peers for participating in the workshop discussion: Ricardo Amoroso, University of Washington. Maria Angela Barbieri, Subsecretaría de Pesca y Acuicultura. Sophie Bertrand, Institut de Recherche pour le Développement. Jennifer Boldt, Fisheries and Oceans Canada. Jaclyn Cleary, Fisheries and Oceans Canada. Sherri Dressel, Alaska Department of Fish and Game. Isaac Kaplan, NOAA Northwest Fisheries Science Center. W. Scott Pegau, Prince William Sound Science Center. Alexandra Silva, Portuguese Institute for Sea and Atmosphere. Merete Tandstad, Food and Agriculture Organization of the United Nations. John Trochta, University of Washington. Carl Walters, University of British Columbia.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
Open Research
DATA AVAILABILITY STATEMENT
This manuscript contains data aggregated from other studies; the compiled information is provided in Table S1. The code used to produce Figure 2 is available at https://github.com/mcsiple/ff-pices.




