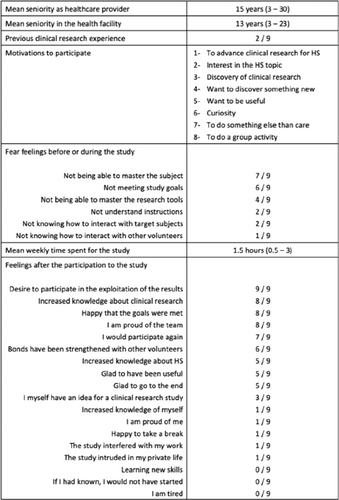
12th EHSF Conference 2023 Extended Abstracts
HS Nursing School
Let us talk about sex (nursing school)
Joanne L. Reeves
Erasmus Medical Center, Department of Dermatology, Rotterdam, Netherlands
Sexual health and a positive body image are important aspects of quality of life that can be affected by hidradenitis suppurativa (HS). HS is a chronic, or long-term, auto-inflammatory skin disease that affects intimate areas of the body, like the armpits, inframammary and groin areas.1 Sex is a basic human need that is has been to shown to be of vital importance for mental health and overall quality of life. The symptoms of HS can affect one's ability to interact socially and have intimate relationships. IA study examining sexuality in patients with HS found that 71% of participants stated that HS negatively affected their relationship.2 While we know from clinical experience that many patients have questions about sexual health they may be hesitant to start this conversation. Not only patients but also nurses find it difficult to discuss sexual health. A study from Saunamaki et al. in 2010 showed that although 90% of nurses understood how patients' disease might affect their sexuality, 80% did not take time to discuss sexual concerns, and 60% did not feel confident in their ability to address patients' sexual concerns.3 As a nurse it our role to help bridge this gap and make the patient feel comfortable and confident enough to discuss their sexual heath. This can be done by initiating the conversation and taking time to discuss their sexual health needs. Sexual health needs can range from practical information over ways to maintain intimacy during flares to tips on how to discuss HS with a new partner.
- Hidradenitis suppurativa: Signs and symptoms. American Academy of Dermatology. Available at https://www.aad.org/public/diseases/a-z/hidradenitis-suppurativa-symptoms. Accessed 10/8/2020.
- Cuenca-Barrales C, Molina-Leyva A. Sexuality in Patients with Hidradenitis Suppurativa: Beliefs, Behaviours and Needs. Int J Environ Res Public Health. 2020;17:8808.
- Saunamäki N et al. Discussing sexuality with patients: nurses' attitudes and beliefs. J Adv Nurs. 2010;66:1308–16.
Invited lectures
The place of surgery in HS
Philippe Guillem1,2
1Department of Surgery, Clinique du Val d'Ouest, Lyon, France; 2RésoVerneuil, Paris, France
Hidradenitis suppurativa (HS) is well recognized as a medico-surgical disease. But what is the meaning of such a sentence, even though it is frequently placed in the frontispiece of the publications-monuments? The meaning is obviously not the same depending on whether it is theorized in written recommendations o2r experienced on a daily basis. Knowing moreover that this experience of reality is necessarily different depending on whether one is the patient, the dermatologist or the surgeon. In the reality of a world where the first specialized contact is almost always a dermatologist, and where potentially powerful medical treatments have arrived, are arriving or will arrive, the question of the surgery in HS is relevant and particularly topical.
- The place of the (dermato)surgeon: her/his role in HS diagnostic and treatment of HS, in the meticulous organization of the postoperative wound care, in comorbidities screening and management, in research, in patients and HCPs education … Clearly, this is not the place of a specialized worker but of a quality partner in the duo with the dermatologist.
- The place of surgery as a full offer of care, cheap, curative, effective and long-lasting, well accepted by the patient. Clearly a credible place for surgery … provided that it meets quality requirements and therefore standardization and education.
- The place of the different surgical techniques: the place of incision-drainage is obviously not the same as the place of wide excision. Clearly, a multi-faceted place for surgery, nourished by a constant concern for technical adequacy to patients needs.
- The place of surgery in relation to other therapeutic alternatives: why, how and when combine surgery (curative but devoted to the management of specific lesions rather than of the whole disease) to other treatments such as antibiotics or biologics (suspensive but with systemic action). Clearly, a place of complementarity rather than opposition.
HS was apparently first described by two surgeons (Alfred Velpeau and Aristide Verneuil) in the 19th century. The most universally used classification was invented by a dermato-surgeon in 1989 (Harry Hurley). The legacy of surgery is consequently heavy in HS. The current place of surgery in HS is however not a place of honour. It is a place of choice. It is not a place left by the dermatologist; it is a place that the (dermato)surgeon must take her/himself, through specialized and renewed education, through high-quality research studies, through an evidence-based dialogue with dermatologists, and mostly through permanent and benevolent listening of the patient.
Oxidative stress and ROS: How they influence the inflammation in HS
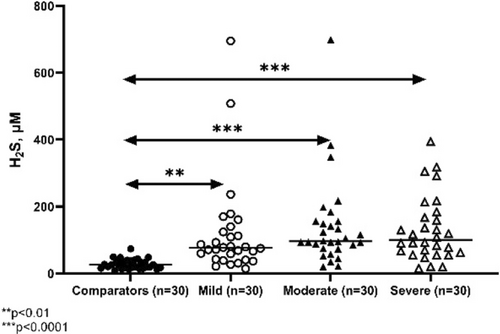
Francesca Prignano
Department of Health Sciences, Section of Dermatology, Florence University, Florence, Italy
Oxidative stress is an imbalance between the reactive oxygen species (ROS) and antioxidants. ROS is a collective term that may be divided into (i) two-electron (non-radical) ROS and (ii) free radical ROS. ROS generation depends on various endogenous and exogenous factors. Oxidative stress was involved in the pathogenesis of several skin disease, such as psoriasis, in which the ability of anti-tumour necrosis factor (TNF)-α to reduce the blood altered redox status was demonstrated. Considering this, the aim of this study was to investigate the blood redox status in patients affected by hidradenitis suppurativa (HS) and the effects of anti-TNF-α therapy on ROS levels. Blood samples of 30 HS patients (before and after 6 months of anti-TNF-α treatment) and 15 controls were collected. Oxidative stress markers included: lipid peroxidation estimation, total antioxidant capacity (TAC, using Oxygen Radical Absorbance Capacity [ORAC] method), and intracellular ROS levels (using flow cytometry analysis). Intracellular ROS production between HS patients treated with anti-TNF-alpha and controls did not show any difference, except for neutrophil intracellular ROS. The plasma lipid peroxidation was not different in the two groups (pre- and post-therapy) but higher than in controls, whilst TAC was significantly higher in treated patients. Considering that altered NADPH oxidase (NOX) may not be responsible for ROS production in HS, alternative ROS sources might sustain HS pathogenesis. Even though the relative contribution of these alternative ROS sources remains unclear, tobacco smoking, mechanical stress, high body mass index and skin microbiota might all account for the reported altered redox status in HS patients. Many trigger factors playing a role in HS pathogenesis might be inducers of ROS production (especially smoking). Furthermore, treatment with anti-TNF-α in HS patients might not be able to reduce ROS production and to activate the antioxidant capacity, thus eventually leading to its loss of efficacy.
Pathogenesis: new concerns from basic science and strength of acquired knowledge
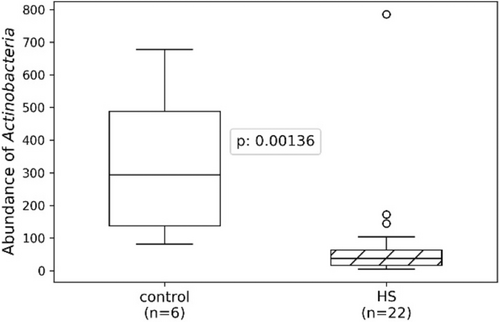
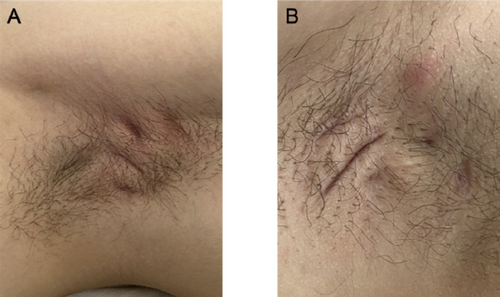
The microbiological difference between hidradenitis suppurativa tunnels and crohn's disease perianal fistulas
Hans Christian Ring1, Andreas Nordholm-Carstensen2, Kurt Fuursted3, Jonathan Thorsen4,5, Thomas Bjarnsholt6, Lene Bay6, Ditte M. L. Saunte7,8, Gregor B.E. Jemec7, Simon F. Thomsen1,9
1University of Copenhagen, Department of Dermatology, Bispebjerg Hospital, Copenhagen, Denmark; 2University of Copenhagen, Digestive Disease Center, Surgical division, Copenhagen, Denmark; 3Statens serum Institut, Department of microbiology and infection control, Copenhagen, Denmark; 4COPSAC, Copenhagen Prospective Studies on Asthma in Childhood, Copenhagen, Denmark; 5University of Copenhagen, Novo Nordisk Foundation Center for Basic Metabolic Research, Copenhagen, Denmark; 6University of Copenhagen, Costerton Biofilm Centre, Copenhagen, Denmark; 7University of Copenhagen, Department of Dermatology, Zealand University Hospital, Roskilde, Denmark; 8University of Copenhagen, Department of Clinical, Medicine, Faculty of Health and Medical Sciences, Copenhagen, Denmark; 9University of Copenhagen, Department of Biomedical Sciences, Copenhagen, Denmark
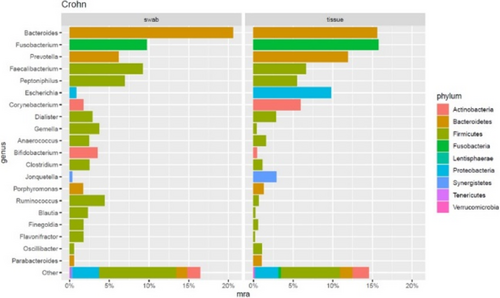
FIGURE 1 Distribution of the top 20 most abundant genera found in Crohn's Disease (CD) fistulas. Barplot showing the distribution of the 20 most abundant genera found in HS tunnels. The barplot shows that both fistula tissue and anal swabs are dominated by the same three species Bacteroides spp, Fucobacterium spp. and Prevotella spp.
Acknowledgement: The Department of Dermatology, Zealand University Hospital, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Ring HC et al. The microbiome of tunnels in hidradenitis suppurativa patients. J Eur Acad Dermatol Venereol 2019;33:1775–80.
Hippo pathway drives excessive fibrosis in hidradenitis suppurativa
Kelsey R. van Straalen1, Feiyang Ma2, Marta Calbet3, Mehrnaz Gharaee-Kermani1,2, Rachael Wasikowski1, Allison C. Billi1, P S Tsou1, Lam C. Tsoi1, Johann E. Gudjonsson1
1University of Michigan, Department of Dermatology, Ann Arbor, USA; 2University of Michigan, Division of Rheumatology, Deptartment of Internal Medicine, Ann Arbor, USA; 3Almirall SA, R&D Center, Sant Feliu de Llobregat, Barcelona, Spain
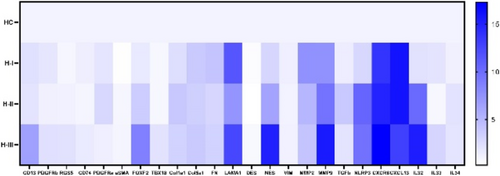
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disease characterized by a massive immune cell infiltrate, tissue, destruction and extensive fibrosis. We aimed to elucidate the role of fibroblasts in the pathophysiology of HS. Single-cell RNA-sequencing was performed on chronic lesional skin samples of 5 HS patients and skin samples of 7 controls. Ex vivo experiments were performed on primary dermal fibroblasts from chronic HS lesions (n = 5). Fibroblast subclustering revealed 6 subsets (SFRP2+, COL11A+, SFRP4+, LSP1+, RAMP1+, and CXCL13+). Two of these, SFRP4+ and CXCL13+, were specifically derived from HS samples. The SFRP4+ subset was identified as myofibroblasts and displayed prominent pro-fibrotic characteristics. Development and activation of this subset was found to be driven by key HS-associated inflammatory cytokines (IFNγ, IL-1β, and TNF). Analysis of the expression of transcription factors within the SFRP4+ subset identified several upregulated components of the Hippo pathway (YAP, WWTR1, TEAD1-4). To determine the functional relevance of the Hippo pathway in HS fibroblasts, primary dermal fibroblasts were stimulated with TRULI (promoting the Hippo pathway) and verteporfin (inhibiting the Hippo pathway). Verteporfin significantly reduced both protein and RNA expression of collagen I and to a lesser extent smooth muscle actin in HS FBs. Verteporfin stimulation also significantly inhibited HS FB contractility in the gel contraction assays and resulted in a significant dose-dependent reduction of both proliferation and migration of HS FBs. TRULI treatment resulted in a non-significant increased RNA expression of smooth muscle actin and collagen I. Treatment with TRULI also significantly increased proliferation but failed to further increase either migration or gel contraction. Overall, these results identify a central role for the Hippo pathway in promoting myofibroblast activation in HS and provides pre-clinical evidence that modulation of the Hippo pathway can reverse the pro-fibrotic phenotypes of myofibroblasts in HS.
Targeting the NLRP3 inflammasome reduces inflammation in hidradenitis suppurativa skin
Barry Moran1, Conor Smith1, Alexandra Zaborowski3, Mark Ryan4, Jozsef Karman10, Robert W. Dunstan4, Kathleen M. Smith10, Roisin Hambly5, Jana Musilova6, Aurelie Fabre7, Margaret O'Donnell8, Karsten Hokamp9, Kingston H. Mills1, William J. Housley4, Desmond Winter3, Brian Kirby5, Jean M. Fletcher1,2
1Trinity College Dublin, School of Biochemistry and Immunology, Dublin, Ireland; 2Trinity College Dublin, School of Medicine, Dublin, Ireland; 3St Vincent's University Hospital, Department of Surgery, Dublin, Ireland; 4AbbVie Bioresearch Center, Immunology Discovery Research, Worcester, USA; 5St Vincent's University Hospital, Department of Dermatology, Dublin, Ireland; 6University College Dublin, Education and Research Centre, Dublin, Ireland; 7St Vincent's University Hospital, Department of Histopathology, Dublin, Ireland; 8St Vincent's Private Hospital, Dublin, Ireland; 9Trinity College Dublin, Department of Genetics, Dublin, Ireland; 10AbbVie Bioresearch Center, Immunology Systems Computational Biology, Cambridge, USA
Treatment for the debilitating, chronic skin disease hidradenitis suppurativa (HS) is inadequate in many patients. Despite an incidence of approximately 1%, HS is underrecognized and underdiagnosed, with a high morbidity and poor quality of life. Characterizing HS skin immune cells, their transcriptome and secretome will lead to a better understanding of its pathogenesis, providing a rationale for new therapeutic strategies. In this study we employed single cell RNA sequencing (scRNA-Seq) to analyse gene expression in immune cells isolated from involved HS skin compared with healthy skin. Flow cytometry was used to quantify, in absolute numbers, and characterize the main immune cell populations. Secretion of inflammatory mediators from skin explant cultures was measured using multiplex assays and ELISA. (scRNA-Seq) identified a significant enrichment in the frequency of plasma cells, Th17 cells and dendritic cell subsets in HS skin, and the immune transcriptome was distinct and much more heterogenous when compared with healthy skin. Flow cytometry analysis revealed significantly increased numbers of T cells, B cells, neutrophils, dermal macrophages, and dendritic cells in involved HS skin. Genes and pathways associated with Th17 cells, IL-17, IL-1β, and the NLRP3 inflammasome were enhanced in HS skin, particularly in samples with a high inflammatory load. Inflammasome constituent genes principally mapped to Langerhans cells and a subpopulation of dendritic cells. The secretome of HS skin explants contained significantly increased concentrations of inflammatory mediators, including IL-1β and IL-17A, and culture with an NLRP3 inflammasome inhibitor significantly reduced the secretion of these and other key mediators of inflammation. These data provide a rationale for targeting the NLRP3 inflammasome in HS using small molecule inhibitors currently in trials for other indications.
Acknowledgement: This publication has emanated from research supported in part by a research grant from Science Foundation Ireland (SFI) under the SFI Strategic Partnership Programme 15/SPP/3212 and research support from AbbVie Inc. (JF, KHGM). Grant support was provided by the City of Dublin Skin and Cancer Hospital Charity (JF). RH is currently supported by an academic training grant under the Irish Clinical Academic Training Programme, supported by the Wellcome Trust and the Health Research Board (203 930/B/16/Z). We wish to thank all the people living with HS and healthy controls who contributed samples to this study.
New pharmaceutical technology from germany: The HS 3D-SEBOSKIN model
Christos C. Zouboulis1, Xiaoxiao Hou1, Henriette von Waldthausen1, Konstantin C. Zouboulis2, Georgios Nikolakis1, Amir M. Hossini1
1Brandenburg Medical School Theodor Fontane and Faculty of Health Sciences Brandenburg, Staedtisches Klinikum Dessau, Departments of Dermatology, Venereology, Allergology, and Immunology, Dessau, Germany; 2University of Oxford, Department of Chemistry and Kavli Institute for Nanoscience Discovery, Oxford, UK
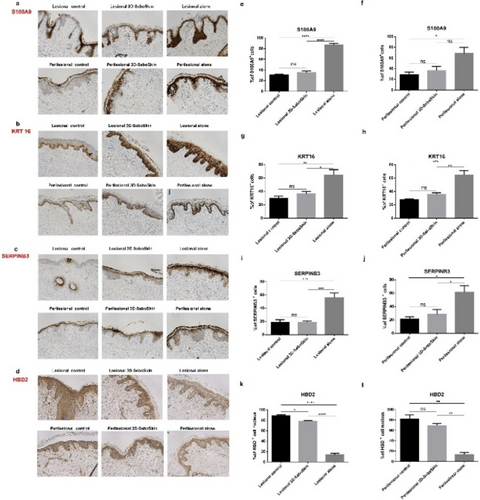
FIGURE 1 HS 3D-SeboSkin: Coculture of HS skin explants (lesional skin, perilesional skin) with SZ95 sebocytes. Expression of S100A9 (a), KRT16 (b), SERPINB3 (c) and HBD2 (d). before and after 3 days of coculture with/without SZ95 sebocytes in direct contact. Lesional and perilesional skin at time of surgery were regarded as control. Representative set of pictures (magnification, ×100) of S100A9 (e, f), KRT16 (g, h), SERPINB3 (i, k) and HBD2 (l, m) staining analysis. Results are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns, non-significant.
Acknowledgement: The Departments of Dermatology, Venereology, Allergology and Immunology, Dessau Medical Center, Dessau, Germany are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Nikolakis G et al. Ex vivo human skin and SZ95 sebocytes exhibit a homeostatic interaction in a novel co-culture contact model. Exp Dermatol 2015;24:497–502.
- Zouboulis CC et al. Alterations in innate immunity and epithelial cell differentiation are the molecular pillars of hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2020;34:846–61.
- Zouboulis VA et al. Hidradenitis suppurativa and comorbid disorder biomarkers, druggable genes, new drugs and drug repurposing – A molecular meta-analysis. Pharmaceutics 2022;14:44.
- Hou XX et al. 3D-SeboSkin model for human ex vivo studies of hidradenitis suppurativa/acne inversa. Dermatology 2022;238:236–43.
Epidemiology, Registries, Genetics (clinical phenotypes, syndromes)
Syndromic hidradenitis suppurativa
Angelo V. Marzano1,2,3
1Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Dermatology Unit, Milan, Italy; 2University of Milan, Department of Pathophysiology and Transplantation, Milan, Italy; 3European Hidradenitis Suppurativa Foundation, Dessau, Germany
Hidradenitis suppurativa (HS) is a chronic-relapsing, highly debilitating autoinflammatory skin disease of terminal hair follicles, clinically characterized by nodules, abscesses, and fistulas. HS may be classified into sporadic, familial, or syndromic forms. The latter include HS cases associated either with keratinization disorders or autoinflammatory diseases such as pyoderma gangrenosum, acne, and hidradenitis suppurative (PASH), pyogenic arthritis, pyoderma gangrenosum, acne, and hidradenitis suppurative (PAPASH), psoriatic arthritis, pyoderma gangrenosum, acne, hidradenitis (PsAPASH), and pyoderma gangrenosum, acne, suppurative hidradenitis, and ankylosing spondylitis suppurative (PASS). Syndromic presentations are extremely rare and represent the prototype of severe, treatment-refractory HS. Moreover, syndromic HS may be associated with several comorbidities, including obesity, inflammatory bowel diseases, and arthritis. Although significant advances have been made in unravelling their genetic background, a univocal genotype–phenotype correlation is currently lacking.1 Based on the findings of a study on syndromic HS involving 4 PASH and 1 PAPASH patients, genetically disrupted keratinization is likely to contribute to the pathogenesis of skin inflammation in syndromic HS.2 Moreover, in a recent, whole-exome sequencing (WES) study conducted on 10 unrelated syndromic HS patients (i.e., 4 PASH, 3 PAPASH, and 3 PASH/SAPHO overlap), three clinical settings have emerged based on presence/absence of gut and joint inflammation, contributing to a first step towards a much-needed preliminary genotype–phenotype correlation. Interestingly, the 4 individuals with PASH had gut inflammation and showed variants in NOD2, OTULIN, and GJB2 genes. Two PAPASH and the 3 PASH/SAPHO overlap patients with joint inflammation showed variants in NCSTN, WDR1, PSTPIP1 and NLRC4. A patient with PAPASH presenting with a mixed phenotype of gut and joint inflammation also had a variant in NLRC4.3 Collectively, these findings corroborate the polygenic autoinflammatory nature of syndromic HS which is closely linked to joint and gut inflammation. As WES is becoming a key tool for correctly diagnosing and classifying these complex conditions, dermatologists should be aware of clinical features and comorbidities hinting at the presence of these entities.
- Garcovich S et al. PASH, PAPASH, PsAPASH, and PASS: The autoinflammatory syndromes of hidradenitis suppurativa. Clin Dermatol 2021;39:240–7.
- Brandao L, Moura R, Tricarico PM, et al. Altered keratinization and vitamin D metabolism may be key pathogenetic pathways in syndromic hidradenitis suppurativa: a novel whole exome sequencing approach. J Dermatol Sci 2020;99:17–22.
- Marzano AV, Genovese G, Moltrasio C, et al. Whole-Exome Sequencing in 10 Unrelated Patients with Syndromic Hidradenitis Suppurativa: A Preliminary Step for a Genotype–Phenotype Correlation. Dermatology 2022;238:860–9.
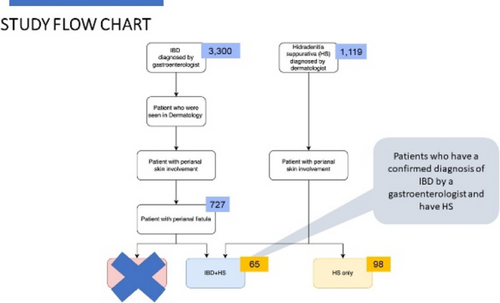
Frequency and predictors of inflammatory bowel disease in patients with hidradenitis suppurativa - Results from the german HS registry (HS-BEST)
Natalia Kirsten1, Kathrin Gehrdau1, Matthias Augustin1, Christos C. Zouboulis4, Andreas Pinter5, Falk G. Bechara6, Dagmar Presser7, Frenz Ohm8
1University Medical Center Hamburg-Eppendorf, Institute for Health Services Research in Dermatology and Nursing (IVDP), Hamburg, Germany; 2University Medical Center Hamburg-Eppendorf, Institute for Health Services Research in Dermatology and Nursing (IVDP), Hamburg, Germany; 3University Medical Center Hamburg-Eppendorf, Institute for Health Services Research in Dermatology and Nursing (IVDP), Hamburg, Germany; 4Dessau Medical Center, Brandenburg Medical School Theodor Fontane, Clinic of Cutaneous and Veneral Diseases, Dessau, Germany; 5University Hospital Frankfurt am Main, Clinic of Cutaneous and Veneral Diseases, Frankfurt, Germany; 6Ruhr-University Bochum, Clinic of Cutaneous and Veneral Diseases, Bochum, Germany; 7University Hospital Würzburg, Clinic of Cutaneous and Veneral Diseases, Würzburg, Germany; 8University Medical Center Hamburg-Eppendorf, Institute for Health Services Research in Dermatology and Nursing (IVDP), Hamburg, Germany
Associations between hidradenitis suppurativa (HS) and inflammatory bowel disease (IBD) have been described several times. The risk of IBD is significantly higher in patients with HS than in the general population. However, there are few studies that have investigated possible predictors for the presence of IBD in patients with HS. Data sets from 568 patients from the German multicentre HS registry (HS-Best) were included in the analysis. In addition to determining the prevalence of IBD, possible predictors for the existence of IBD were also investigated. The following variables were included in the analysis: Age, gender, BMI, Hurley stage, HS-PGA, presence of fistula, localisation of lesions and existence of other chronic inflammatory diseases. In addition, the prescribing behaviour of antibiotics in patients depending on the presence of concomitant IBD was investigated, as well as the 4 and 12-week drug survival of the antibiotics. Of 568 patients, 55.9% were female. The mean age was 38.3 (±11.7) years; the mean BMI was 29.6 (±6.8). At the inclusion visit, the mean HS-PGA was 2.02 (±1.5). The prevalence of IBD was 3.9%; of these, 2.6% (n = 15) had Crohn's disease, 1.2% (n = 7) had ulcerative colitis. In addition, 2.1% (n = 12) patients had irritable bowel syndrome. The following potential predictors of IBD occurrence were identified: female gender, higher number of chronic inflammatory comorbidities. Age, BMI, Hurley stage, HS-PGA, presence of fistula or particular locations of inflammatory lesions showed no significant association. Interestingly, there was an association between axillary and chest lesions and more frequent occurrence of IBDs (p < 0.05). Overall, antibiotics were prescribed less frequently in patients with HS; however, there was no significant difference in drug survival of antibiotics prescribed for week 4 and week 12. We found an overall prevalence for IBD in our cohort of 3.9% percent. Considering our results, we were able to identify women and people with other chronic inflammatory comorbidities as risk groups. Another interesting aspect was that there was no difference in drug survival of antibiotics between people with and without IBD.
Acknowledgement: The Departments of Dermatology, Venereology, Allergology and Immunology, Dessau Medical Center, Dessau, Germany are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
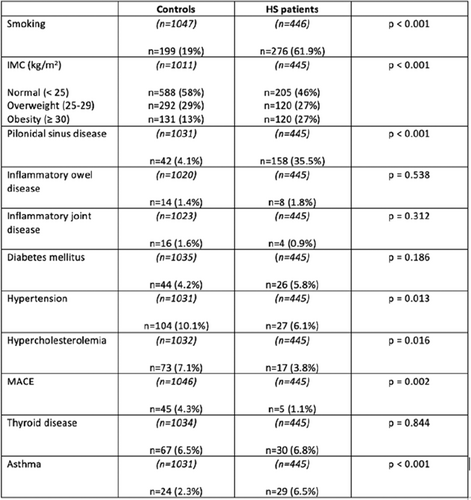
Belgian patients of the European Registry For Hidradenitis Suppurativa (ERHS): Data, scores and phenotypes since 2015
Anne-Sophie Sarkis, Stephanie Heudens, Mathieu Daoud, Mathilde Daxhelet, Farida Benhadou, Mariano Suppa, Laura Nobile, Jalila Karama, Hassane Njimi, Jonathan White, Veronique del Marmol
Université Libre de Bruxelles, Dermatology Department, HUB - Hôpital Erasme, Brussels, Belgium
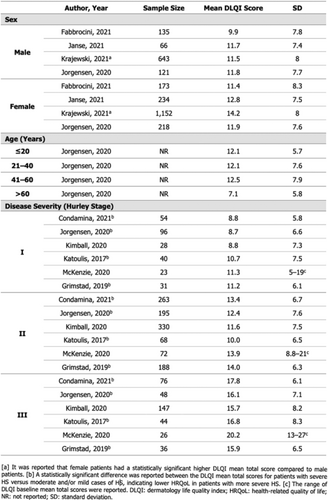
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease, characterized by painful and recurrent lesions in apocrine gland-bearing skin areas. With its wide range of clinical presentations, it is a heterogenous disease, which makes assessment and data collection difficult.1 Questionnaires with detailed items like the one developed for the European Hidradenitis Supurativa Foundation (EHSF) at the Hôpital Erasme (Université Libre de Bruxelles, Belgium),2,3 are useful to study this heterogenous disease and the associated comorbidities. The ERHS registry was created in 2015 and updated in 2019. It includes a “First Visit” questionnaire and a “Follow up” questionnaire. Data are recorded in an opensource software, Redcap. The questionnaires include sections for socio-demographic data, medical and HS history, clinical examination and the treatment plan. The physical examination is filled with detailed items, allowing the identification of several different phenotypes4 and an automatic calculation of different scores.5 Measures of the psychological impact of the disease and patient quality of life are recorded, notably with the DLQI. At present, 606 patients are included in the ERHS (62% women, 38% men). The mean age at first visit is 27 years, with an average diagnostic delay of 6.35 years. Tobbaco use is present in 71% of patients (former and current smokers). A family history of HS is noted in 42% of our population. Severity of the disease is quantified in 544 patients: Hurley I, II and III scores proportions are respectively 28.3%, 52.4% and 19.3% in this cohort. The mean International HS Severity Score System (IHS4) score is 7.34. The mean value of other scores is: 21.7 for the modified Sartorius Score 2007, 7.4 for the Severity Assessment of HS (SAHS), 10.5/30 for the Dermatology Life Quality Index (DLQI). This registry also enables determination of relative phenotype proportions in our cohort. Comorbidities documented in this HS cohort include 43.8% of patients with depression and 27.8% with arthritis, such as rheumatoid arthritis or ankylosing spondylitis. As for cardiovascular risk factors, obesity was found in 33.5% of patients, hypertension in 10.6%, diabetes mellitus in 6.4% and dyslipidemia in 12.4%. The prevalence of the main comorbidities observed in our cohort is higher than the prevalence reported in the HS literature and the general European population. Finally, data on substance use showed: 24.6% of patients reporting cannabis use, 8.8% report daily alcohol consumption, and 4% report use of other recreational drugs. The ERHS and the questionnaires allow systematic and larger data collection, including detailed comorbidities, phenotypes and severity of disease. Analysis of this large database will contribute to better understanding and managing HS, in a time where new therapeutic options are becoming available.
Acknowledgement: The Dermatology Department, HUB - Hôpital Erasme, Brussels, Belgium is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Sabat R et al. Hidradenitis suppurativa. Nat Rev Dis Primer 2020;6:18.
- Daxhelet M et al. Establishment of a European Registry for hidradenitis suppurativa/acne inversa by using an open source software. Vol 30, Journal of the European Academy of Dermatology and Venereology. Wiley; 2015, pp 1424–6.
- Daxhelet M et al. European Registry for Hidradenitis Suppurativa: State of play. J Eur Acad Dermatol Venereol 2021;35:e274-e276.
- Frew JW et al. Inter-rater reliability of phenotypes and exploratory genotype–phenotype analysis in inherited hidradenitis suppurativa. Br J Dermatol 2019;181:566–71.
- Ingram JR et al. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process. Br J Dermatol 2016;175:263–72.
Hidradenitis phenotypes based on elementary lesions: Description of a mixed phenotype with slowly progressive behaviour
Antonio Martorell, Francisco Navarro, Gemma Ochando-Ibernón, Gonzalo Garcia-Fadrique, Virginia Sanz-Motilva
Hospital de Manises, Department of Dermatology. Hidradenitis Suppurativa, Valencia, Spain
Acknowledgement: Funded by the II Call for research support for emerging research groups of the Hospital de Manises.
- Martorell A et al. Defining hidradenitis suppurativa phenotypes based on the elementary lesion pattern: results of a prospective study. J Eur Acad Dermatol Venereol 2020;34:1309–18.
- Frew JW et al. Inter-rater reliability of phenotypes and exploratory genotypephenotype analysis in inherited hidradenitis suppurativa. Br J Dermatol 2019;181:566–71.
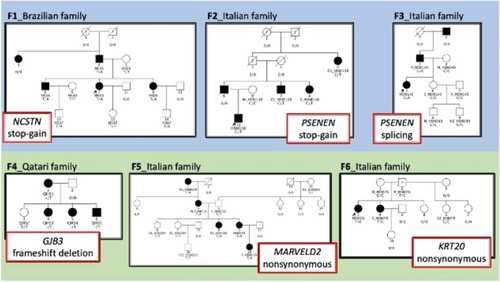
iPSCS derived skin organoids to understand the role of PSENEN genetic mutations in hidradenitis suppurativa
Cécile Nait Meddour1, Chiara Moltrasio2,5, Paola Maura Tricarico3, Juliette Berthier4, Marianne Gervais Taurel4, Stephane Jamain1, Angelo V. Marzano2,5, Michele Boniotto1
1Université Paris Est Créteil, Institut Mondor De Recherches Biomédicales - Translational Neuropsychiatry, Créteil, France; 2Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Dermatology Unit, Milan, Italy; 3University of Trieste, Department of Advanced Diagnostics, Trieste, Italy; 4Université Paris Est Créteil, Institut Mondor De Recherches Biomédicales - Biologie of Neuromuscular systems, Créteil, France; 5Università degli Studi di Milano, Pathophysiology and Transplantation, Milan, Italy
Genetic studies have convincingly associated Hidradenitis Suppurativa (HS) with rare loss-of-function (LOF) mutations in subunits of the gamma-secretase complex such as NCSTN, PSEN1, PSENEN and APH-1 (Duchatelet et al., 2020). However, the impact of LOF mutations in these genes is still unclear, and potential treatment discovery is hindered because of the lack of suitable in-vitro skin models. We identified a novel Italian family where HS was transmitted as an autosomal dominant tract. One member of this 3-generations family was recruited at the Dermatology Unit of the Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico of Milan (Italy), and whole exome sequencing analysis was performed on 2 affected members of this family and one unaffected one. We found a novel heterozygous nonsense mutation in PSENEN (p.Arg39X, 19:35746474 C>T ref: GRCh38.p13) that segregate with the disease, and it has already been reported in another HS family from India (Ralser et al., 2017). To study the activity of this mutation in hair follicle structure, we have developed hair-bearing skin organoids from induced pluripotent stem cells (iPSC) obtained from the proband. Hair follicle-derived keratinocytes were obtained from ten plucked hairs. Induced Pluripotent Stem cells (IPSCs) were generated from keratinocytes by Sendai virus infection using the CytoTune iPS 2.0 Sendai Reprogramming kit. Skin organoids were generated following the protocol described by Lee et al. Hair follicle structure will be studied by 3D imaging after organoids clearing. Keratinocytes derived from skin organoids will be extracted after overnight digestion with dispase, grown in a selective medium, and PSENEN expression assessed by Q-PCR and Western Blot analyses. We showed a decreased expression of PSENEN in keratinocytes derived from HS-patient than in keratinocytes isolated from skin-organoids derived from two IPSCs control lines, thus confirming that the p.Arg39X causes haploinsufficiency of the gene. We then reprogrammed hair follicles-derived keratinocytes and obtained IPSCs carrying the PSENEN mutation. The novel iPSC line (IMRB_08_01) expressed pluripotency markers as shown by q-PCR, immuno-fluorescence and FACS analyses. This iPSC line showed a normal karyotype and was able to differentiate intothe 3 germ layers, thus confirming the pluripotency of these cells. Skin-organoids derived from these IPSCs are growing, and hair follicle structure will be studied by immunostaining using an anti-KRT17 to visualize the outer root sheet and an anti- KRT71 to show the inner root sheath. We have generated a novel IPSCs line from a patient suffering from HS bearing a LOF mutation in PSENEN. Skin organoids derived from this IPSCs line will be studied to understand the role of gamma-secretase mutations in HS and to screen for potential treatments for this family.
Genetic analysis of 100 hidradenitis suppurativa patients by whole exome sequencing
Kevin Muret1, Francette Jean-Louis2, Claire Hotz3, Eric Bonnet1, Pierre Wolkenstein3, Jean-Laurent Casanova4, Yves Lévy2, Jean-François Deleuze1, Sophie Hue2
1Université Paris-Saclay, Centre National de Recherche en Génomique Humaine (CNRGH), Evry, France; 2UPEC, INSERM U955 team 16, Créteil, France; 3APHP, Dermatology Department, Créteil, France; 4The Rockefeller University, St. Giles Laboratory of Human Genetics of Infectious Diseases, New York, USA
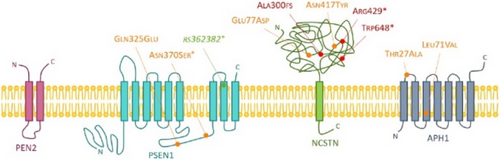
FIGURE 1 10 mutations of interest found in the ɣ-secretase complex. The names of the mutations follow the HGVS codification and the variant denominations, except for the rs362382 identifier which affects an exon-intron junction and not a coding region. The impacts of variants are predicted by the VEP tool, illustrated by the green, orange and red colours for a weak, moderate and strong effect respectively.
Comorbidities
Occlusive follicular diseases and HS
Ditte M.L. Saunte1,2
1Zealand University Hospital, Department of Dermatology, Roskilde, Denmark; 2University of Copenhagen, Department of Clinical Medicine, Faculty of Health Science, Copenhagen, Denmark
A defect in the follicular epithelial proliferation (hyperkeratosis) leading to follicular occlusion is believed to contribute to the pathogenesis of hidradenitis suppurativa (HS). Other disorders involving follicular occlusion seem to affect a greater proportion of HS patients. This study was planed to give an overview of occlusive follicular diseases associated with HS presenting a literature review. Common diseases such as acne and folliculitis are often seen in patients with HS.1 The clinical picture with perifolliculitis of the scalp, dermal abscesses, sinus tract development, and secondary scarring alopecia as seen in the disease dissecting cellulitis (DC) (syn. folliculitis et perifolliculitis capitis abscendes et suffoidens) may resemble HS and is associated with HS.2 The localized disease, pilonidal sinus/cysts, also have a high prevalence and may be associated with HS.1 When acne conglobata, pilonidal cyst and DC are seen concomitantly with HS, it is reported as the follicular occlusion tetrad.3,4 A pilot study of keratosis pilaris (KP) recently suggested that KP also may be more common in patients with HS. Another disease, the rare genodermatosis, Dowling-Degos, has been associated with HS alone, and also together with both squamous cell carcinoma and multiple keratoacanthomas.5 Although many disorders with follicular occlusions present a very different clinical picture, many are associated with HS. This session aims to provide an overview.
Acknowledgement: The Department of Dermatology, Zealand University Hospital, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Garg A et al. Comorbidity screening in hidradenitis suppurativa: Evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol 2022;86:1092–101.
- Federico A et al. Are dissecting cellulitis and hidradenitis suppurativa different diseases? Clin Dermatol 2021;39:496–9.
- Pesce A et al. Pilonidal disease, hidradenitis suppurativa and follicular occlusion syndrome: a diagnostic challenge. Eur Rev Med Pharmacol Sci 2018;22:4755–6.
- Vasanth V, Chandrashekar B. Follicular occlusion tetrad. Indian Dermatol Online J 2014;5:491.
- Supekar B et al. Dowling-Degos Disease with Follicular Involvement Associated with Hidradenitis Suppurativa: A Manifestation of Follicular Occlusion Phenomenon? Indian J Dermatol 2020;65:295–8.
Hidradenitis suppurativa and female infertility: A cross sectional pilot study
Cecilia E. Medianfar1, Rune K. Andersen1,3, Christoffer Kursawe Larsen1, Sara K. Saunte1, Ditte M.L. Saunte1,2, Gregor B.E. Jemec1,2
1Zealand University Hospital Roskilde, Department of Dermatology, Roskilde, Denmark; 2University of Copenhagen, Department of Clinical Medicine, Faculty of Health Science, Copenhagen, Denmark; 3University of Copenhagen, Department of Immunology and Microbiology, Copenhagen, Denmark
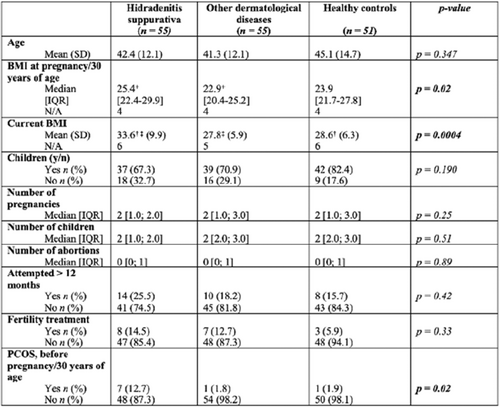
TABLE 1 Univariate analysis on basic demographics, fertility and fecundity. IQR: Interquartile range, SD: Standard deviation. +Indicates significant difference in BMI at pregnancy/30 years of age between HS group and other derm. diag. group. † Indicates significant difference in current BMI mean between HS group and healthy group ‡ Indicates significant difference in current BMI time mean between HS group and other derm. diag. group.
Acknowledgement: The Department of Dermatology, Zealand University Hospital, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
HS and IBD not only a comorbidity
Hessel H. van der Zee
Erasmus University Medical Center, Department of Dermatology, Rotterdam, Netherlands
In 1991, the co-occurrence of hidradenitis suppurativa (HS) with inflammatory bowel disease (IBD) was first reported.1 IBD consist of ulcerative colitis and Crohn's disease. However, co-occurrence of two diseases does not necessarily imply an association. But the association between HS and IBD especially Crohn's disease is well established. About 3.3% of HS patients have comorbid IBD which means that IBD is 4–8 times more frequent in HS than could be expected by chance.2 Moreover, in patients with IBD the co-occurrence of HS is estimated to be as high as 23%.3 Patients with HS and IBD does not seem to have a clinical distinct phenotype of HS.2
Acknowledgement: The Department of Dermatology, Erasmus University Medical Center, Rotterdam, Netherlands is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Ostlere LS et al. Hidradenitis suppurativa in Crohn's disease. Br J Dermatol 1991;125:384–6.
- Deckers IE et al. Inflammatory bowel disease is associated with hidradenitis suppurativa: Results from a multicenter cross-sectional study. J Am Acad Dermatol 2017;76:49–53.
- van der Zee HH et al. The prevalence of hidradenitis suppurativa in 1093 patients with inflammatory bowel disease. Br J Dermatol 2014;171:673–5.
HS and atopic dermatitis
Andrea Chiricozzi1,2
1Università Cattolica del sacro Cuore, Dermatology, Rome, Italy; 2Fondazione Policlinico Gemelli IRCCS, Dermatology, Rome, Italy
Limited evidence is currently available about the association between atopic dermatitis and hidradenitis suppurativa. It is emerging in the latest years as clinical reports and epidemiologic studies suggested a potential association between these two skin conditions, though the eventually shared pathogenic mechanisms are not elucidated.
Diagnostic tools, imaging: Their increasing impact on HS diagnosis, classification, and treatment
Role of ultra-high frequency and echoangio software in hidradenitis suppurativa
Ximena Wortsman1,2,3
1Universidad de Chile, Department of Dermatology, Santiago, Chile; 2Pontificia Universidad Catolica de Chile, Department of Dermatology, Santiago, Chile; 3Institute for Diagnostic Imaging and Research of the Skin and Soft Tissues, Santiago, Chile
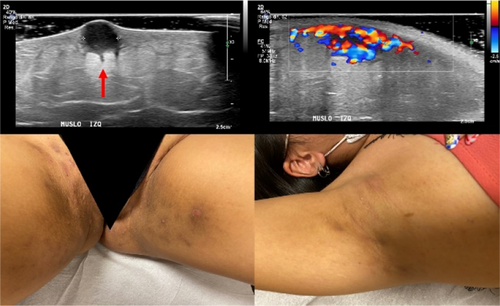
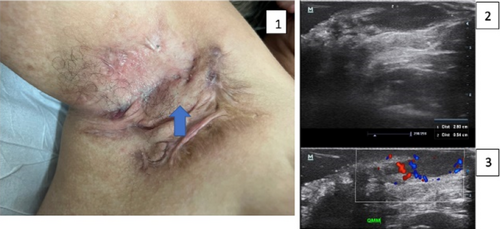
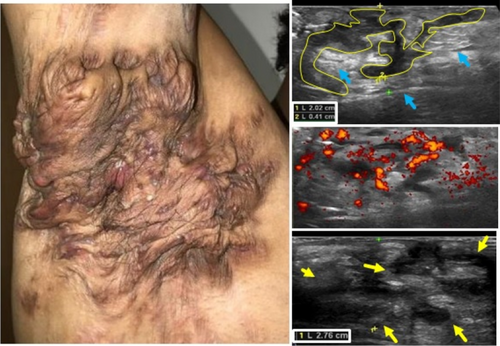
A prompt Hidradenitis Suppurativa (HS) diagnosis is needed to manage the disease and avoid progression. So far, the diagnosis of this condition is often late and could take even up to 10 years. Ultrasound has been reported to be a useful imaging modality for supporting the diagnosis, staging, and management of HS.1 This validated imaging technique improves clinical staging by detecting subclinical structural alterations of the tissues and their vascularity.1,2 Therefore, it is possible to perform better management with more precise anatomical information. Ultrahigh-frequency ultrasound uses special devices with probes that present frequencies up to 70 MHz, showing an axial spatial resolution closer to histology and going up to 30 μm. Early signs of HS have been reported in the literature with this new ultra-high-resolution technology, which is also an interesting and real-time window into the pathophysiology of the disease.3,4 New echoangio software, besides colour or power Doppler for tracking inflammation, allows us to improve the detection of the degree of lesional vascularity in HS. Moreover. Power Doppler has been reported as a biomarker of the activity of the disease.5 Ultrahigh-frequency ultrasound and echoangio software support the early diagnosis and tracking of the activity of HS.
- Wortsman X et al. Ultrasound in-depth characterization and staging of hidradenitis suppurativa. Dermatol Surg 2013;39:1835–42.
- Martorell A et al. Ultrasound as a diagnostic and management tool in hidradenitis suppurativa patients: a multicentre study. J Eur Acad Dermatol Venereol 2019;33:2137–42.
- Wortsman X et al. Seventy-MHz Ultrasound Detection of Early Signs Linked to the Severity, Patterns of Keratin Fragmentation, and Mechanisms of Generation of Collections and Tunnels in Hidradenitis Suppurativa. J Ultrasound Med 2020;39:845–57.
- Oranges T et al. Advanced evaluation of hidradenitis suppurativa with ultra-high frequency ultrasound: A promising tool for the diagnosis and monitoring of disease progression. Skin Res Technol 2020;26:513–9.
- Grand D et al. Doppler ultrasound-based noninvasive biomarkers in hidradenitis suppurativa: evaluation of analytical and clinical validity. Br J Dermatol 2021;184:688–96.
The role of imaging technologies in hidradenitis suppurativa
Skaidra Valiukeviciene1,2, Erikas Mazeika1,2, Vaiva Jariene1,2
1Lithuanian university of health sciences (LSMU), Department of Skin and Venereal Diseases, Kaunas, Lithuania; 2Hospital of LSMU Kauno Klinikos, Department of Skin and Venereal Diseases, Kaunas, Lithuania
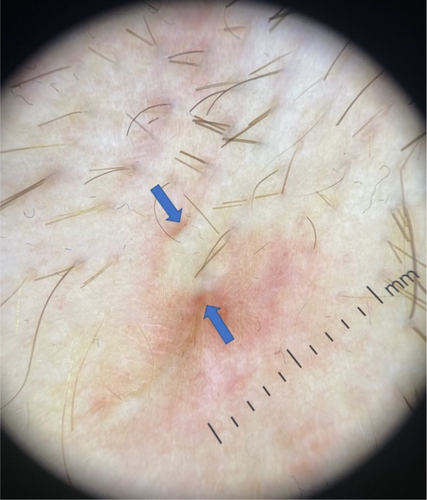
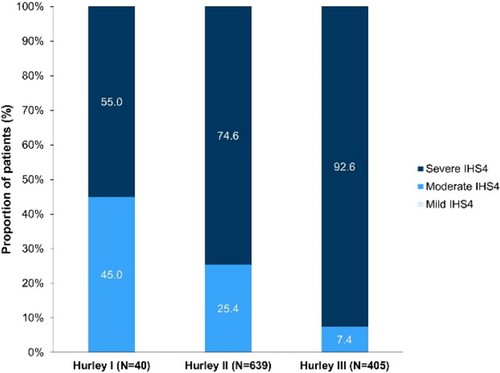
Until recently, clinical evaluation of the skin lesions is the gold standard in the diagnosis and staging of hidradenitis suppurativa (HS). The comprehensive literature review [Elkin K, 2020] of the past decade has shown that ultrasound (US) is well-characterized and has the greatest range of use, while magnetic resonance imaging (MRI) has a role in severe, anogenital HS lesions. An ultrasound staging (SOS-HS) using frequencies from 7 to 18 MHz has been proposed to evaluate the severity of the disease [Wortsman X, 2013]. We analysed PubMed database's abstracts of all retrieved articles during the period 2019–2022 containing the terms HS and non-invasive imaging tools including US, MRI, medical infrared thermography (MIT), positron emission tomography (PET/CT), computed tomography (CT), and whole-body photo-documentation. A recent cross-sectional multicentre study has shown that US is a powerful, not painful imaging tool for diagnostic characterization of HS lesions, except less than 0.1 mm. It modifies clinical staging and therapeutic management in HS by detecting subclinical disease [Martorell A, 2019; Wortsman X, 2022]. The inter-observer interclass correlation was found between US and the Sartorius, HiSCR nodule, abscess, and draining fistula count. The scores went from having ‘good’ rater agreement for Sartorius and HiSCR nodule and abscess count to ‘poor’ rater agreement for HiSCR draining fistula count to ‘excellent’ rater agreement among these scores [Lyons AB, 2022]. Based on the prospective study the power-Doppler US (PD-US) can assess the decrease of vascularization in HS lesions after the reduction of inflammation due to immunomodulatory therapies, and fistulas fibrosis to define better when a surgical approach - besides the medical treatment is required [Caposiena Caro RD, 2021]. A cohort study has indicated that pre-operative US improves surgical margin delimitation and can lower recurrence rates at 24 weeks in HS patients [Caposiena Caro RD, 2020]. A retrospective study compared the most significant lesions in patients with HS using high-frequency ultrasound (HFUS) (18–22 MHz) and ultra-high frequency ultrasound (UHFUS) (48 and 70 MHz) and found that UHFUS better than HFUS detects drop-shaped hair follicles, micro-tunnels, and microcysts [Oranges T, 2020]. Another study has shown that US-estimated epidermal thickness and dermal tunnel diameter have high analytical validity with corresponding histological measurements. PD-US intensity demonstrated a high correlation with dermal CD3+ and CD11c + cell counts, pain scores, abscess, and nodule count, International HS Severity Scoring System score, and the number of draining tunnels [Grand D, 2021]. Eventually, due to the variability of HS lesions and their localization in skin folds, standardized photographic documentation of HS candidate skin areas was proposed and its combination with MIT is useful to detect inflammation severity in HS lesions [Zouboulis CC, 2019]. A recent multicentre case–control study found that PET/CT can visualize cutaneous foci in clinically unapparent sites and estimate the burden of HS [Loo CH, 2020]. A higher than 18 MHz frequency ultrasound is strongly recommended for HS imaging in conjunction with a physical examination to evaluate disease severity in HS patients, particularly those classified as Hurley II and Hurley III. The MRI is recommended in severe, anogenital lesions. The MIT can be considered as an additional imaging tool, and PET/CT is not recommended because needs more evidence.
Acknowledgement: The Department of Skin and Venereal Diseases, Hospital of LSMU Kauno Klinikos, Kaunas, Lithuania is a health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
AI-aided automatic severity scoring system for hidradenitis suppurativa
Thi Thuy Nga Nguyen1, Alessandra Cartocci2, Hoc T. Huynh4, Loan T. Tong3, Milad Mozafari5, Tran Khoi Dang1, Tien Zung Nguyen1
1Torus Actions SAS, R&D Department, Toulouse, France; 2University of Siena, Department of medical biotechnologies, Siena, Italy; 3Taureau Artificial Intelligence JSC, R&D Department, Hanoi, Vietnam; 4Tomas Bata University in Zlin, Faculty of Applied Informatics, Zlin, Czech Republic; 5Research Center Cerveau Et Cognition, NeuralAI group, Toulouse, France
Hidradenitis Suppurativa (HS) is a chronic inflammatory skin disease that has a prevalence between 0.03% and 4% of the population.1 It is a debilitating disease that can cause pain, depression, and poor quality of life, even more than other diseases such as psoriasis and eczema.
- Segment all the HS lesions
- Classify individual HS lesions into different types (papule, nodule, abscess, drainage, fistula, scar, etc.)
- Separate colliding lesions of different types (e.g. separate a fistula from a colliding abscess)
- Calculate the local severity of each lesion
- Measure the extensivity (diameter and surface area) of each lesion as well as of the whole HS in an anatomical body part.
- Combine all the above scores together into a final score of our system via a mathematical formula
- Emulate other common scoring systems in use, again by combining the above scores together, but using other mathematical formulas.
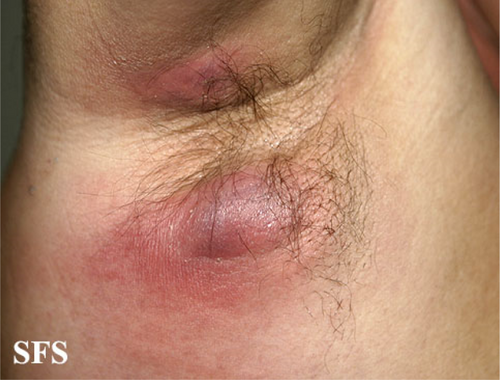
FIGURE 1 An example of HS (image is taken from https://www.atlasdermatologico.com.br/). Two abscesses with different sizes could be clearly seen in the image.
Acknowledgement: This work is financially supported by BelleTorus Corp. and Torus Actions SAS.
- Calao M et al. Hidradenitis Suppurativa (HS) prevalence, demographics, and management pathways in Australia: A population-based cross-sectional study. PLoS One 2018;13:e0200683.
- Zouboulis, C. et al. Development and validation of IHS4, a novel dynamic scoring system to assess hidradenitis suppurativa/acne inversa severity. Br J Dermatol 2017;177:1401–9.
- Kimball AB et al. Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol 2014;171:1434–42.
- Kimball AB et al. HiSCR (Hidradenitis Suppurativa Clinical Response): A novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol 2016;30:989–94.
In vivo-ex vivo correlation of hidradenitis suppurativa anatomical lesions using ultra-high frequency ultrasound and histopathological evaluation
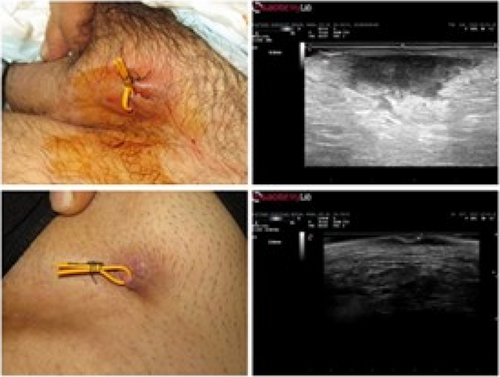
Giammarco Granieri1, Flavia Manzo Margiotta1, Alessandra Michelucci1, Agata Janowska1, Cristian Scatena2, Marco Romanelli1, Valentina Dini1
1University of Pisa, Department of Dermatology, Pisa, Italy; 2University of Pisa, Department of Histopathology, Pisa, Italy
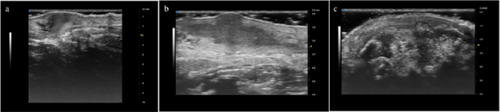
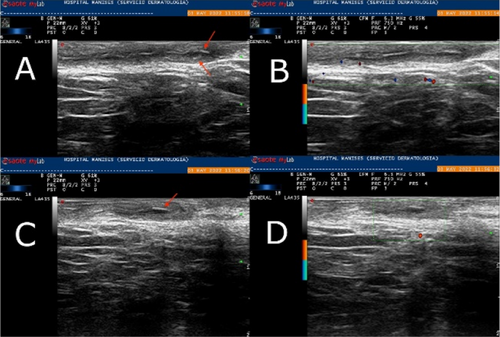
Hidradenitis suppurativa (HS), also known as acne inversa is a chronic inflammatory disease of the follicular pilosebaceous units (FPSUs). It is characterized by numerous lesions indicative of pathology that can be viewed both clinically and by histology and ultrasonography. In particular, ultrasonographic examination is very useful in characterizing patients who are candidates for surgery, who have permanent lesions such as tunnels, and patients who require medical therapy alone. the characterization of these lesions by Ultra-High Frequency Ultrasound (UHFUS) is very useful but needs numerous correlation studies with clinical and histological findings. In September 2022, a 42-year-old male patient with HS, Hurley III, at the axillary level came to our clinic. The patient had no additional comorbidities. He underwent wide-local excision in October 2022, with pre-surgical mapping performed with a 70 MHz probe with an axial resolution of 30 micrometres. After surgery, the anatomical piece was again evaluated US with tracing of tunnels and nodular lesions appreciable on clinical and instrumental examination. Images of histologic sections were subsequently acquired at the demarcated landmarks, and correlation between the ultrasound and histopathologic images was performed. A total of 5 lesions were examined that included 3 tunnels and 2 inflammatory nodules. On ultrasound examination with a 70 MHz probe, the tunnels appeared as hyperechogenic lesions surrounded by hypoechogenic halo immersed in the dermal context, which was hypoechogenic. The different echogenicities appreciated appeared concordant to the spatial arrangement of the inflammatory and pro-fibrotic infiltrate analysed at histopathological sections. The inflammatory nodules presented as hyperechogenic granularity structure and characterized by hypoechogenic inner lesions with hyperechogenic margin and superficial outlet. Histopathological and ultrasonographic images show excellent anatomical correlation of the analysed lesions. This evidence suggests the role of UHFUS with a 70 MHz probe as a valuable tool for in vivo analysis of HS anatomy. Particularly by means of real-time ultrasound and in a completely non-invasive manner, we were able to explore pre-surgery the presence of lesions that could only be detected by histological examination. Our study showed that through US study both in vivo and after surgical excision, useful correlations between clinical, histology and UHFUS can be obtained that can provide valuable help to clinicians in current practice.
Outcome measures, quality of life and unmet needs
IHS4-55
Thrasyvoulos Tzellos1,2
1European Hidradenitis Suppurativa Foundation e.V., Dessau, Germany; 2Nordland Hospital Trust, Department of Dermatology, Bodø, Norway
Validated, inclusive and easy-to-use outcome measures for hidradenitis suppurativa are essential both in the clinical trial setting and clinical practice. The continuous IHS4 is a validated tool that dynamically assesses nodules/abscesses/draining tunnels and classifies disease severity as mild/moderate/severe. However, dichotomous outcomes are often required for clinical trials reporting. This study was conducted to develop and validate a dichotomous outcome based on IHS4 that can be used in clinical trial settings and day-to-day clinical practice. De-identified data from the PIONEER-I and -II studies were accessed through Vivli. Potential IHS4 thresholds were analysed using baseline to Week 12 data from adalimumab-and placebo-treated hidradenitis suppurativa patients in the PIONEER-I trial. The final threshold was chosen based on its ability to discriminate between patients treated with adalimumab or placebo and its association withreduction in inflammatory lesions. The final threshold was validated using data from baseline to Week 12 from adalimumab- and placebo-treated hidradenitis suppurativa patients in both the PIONEER-II and the combined PIONEER-I and -II studies. The best performing cut-off for the IHS4 was a 55% reduction of the IHS4 score (IHS4-55). Patients who achieved the IHS4-55 had an odd's ratio of 2.00 [95% CI 1.26–3.18, p = 0.003], 2.79 (95% CI 1.76–4.43, p < 0.001) and 2.16 (95% CI 1.43–3.29, p < 0.001) for being treated with adalimumab rather than placebo in PIONEER-I, PIONEER-II and the combined dataset, respectively. Additionally, the achievement of the IHS4-55 was associated with a significant reduction in inflammatory nodules, abscesses and draining tunnels in all analysed datasets. IHS4-55, a novel dichotomous IHS4 version, based on a 55% reduction of the total score was developed. The IHS4-55 performs similarly to the HiSCR in discriminating between adalimumab-and placebo-treated hidradenitis suppurativa patients and shows significant associations with reductions in lesion counts. Moreover, the IHS4-55 addresses some of the HiSCR drawbacks by dynamically including draining tunnels in a validated manner. By allowing the analysis of hidradenitis suppurativa patients with an abscess and nodule count below 3 but many draining tunnels, this outcome measure will improve inclusivity in clinical trials.
Quality of life impairment in HS patients and reported outcomes: Pain index app
Łukasz Matusiak
Wroclaw Medical University, Department of Dermatology, Venereology and Allergology, Wrocław, Poland
Pain is the symptoms, which patients with HS consider play a crucial role in the reduction of their quality of life. HS-related pain includes nociceptive and neuropathic pain, and perception seems to be influenced by disease severity, depression and anxiety, regarded as different points of the same continuum rather than different entities. A nociceptive, acute skin pain is observed during flare-ups, linked to the local activation of innate and adaptive immune systems cells involving various proinflammatory cytokines (e.g. TNF-α, IL-1β, IL-6, IL-17, IL-23) and chemokines. The unrestricted and chronic immune response resulting in pyroptosis and irreversible tissue destruction is responsible for development of neuropathic pain as a consequence of the nervous system structures damage, or sometimes of prolonged, unremitting nerve stimulation.
Pain assessment can provide valuable information about the impact of the HS on the patient's well-being, as well as guide treatment decisions and evaluate the effectiveness of interventions.
To assess pain severity in HS sufferers, healthcare providers usually use VAS or NRS in weekly or monthly intervals with the intrinsic disadvantage of exact symptom severity recalling by the patients. In addition, the chronic central sensitization worsens the capacity of acute pain recall after a period of time. In order to avoid the disadvantages of retrospective evaluation and to optimize the acute skin pain evaluation in clinical trials, a ‘Pain index’ was recently proposed.1 The proposed pain assessment is based on a numeric rating scale (NRS: 0, no pain; 10, most severe pain), assessed daily and multiplied by the duration in days at the end of the month calculated as a 30-day period (0–30 days). The result, referred to as the ‘Pain Index’ (with a total scale of 0–300 points) was assessed as a patient-reported outcome measure instrument exhibiting statistically significant correlations with disease severity and QoL scales, including IHS4, HS-PGA and DLQI.
- Zouboulis CC. Pain Index: a new prospective hidradenitis suppurativa patient-reported outcome measure instrument. Br J Dermatol 2021;184:1203–4.
Medical treatment: Place in therapy of systemic “historical” drugs and new advancements from clinical trials
Anti-TNF-α treatment: From the success of pioneer I and II studies to the results with anti-IL-17 monoclonal antibodies
Christos C. Zouboulis1,2
1Brandenburg Medical School Theodor Fontane and Faculty of Health Sciences, Staedtisches Klinikum Dessau, Departments of Dermatology, Venereology, Allergology and Immunology, Dessau, Germany; 2European Hidradenitis Suppurativa Foundation e.V., Dessau, Germany
The central pathogenic events in hidradenitis suppurativa (HS) are considered to be the occlusion of the hair follicle due to an abnormal differentiation and proliferation of follicular keratinocytes and a perifollicular lymphohistiocytic infiltration. The hair follicle occlusion region involved is located between the ductus seboglandularis and the orifice of the apocrine gland duct, which is neighbouring the bulge area.1 The immune response in HS includes a differential expression of several innate immunity markers in serum and the skin,2 whereas tumour necrosis factor-α (TNF-α) seems to be one of the most important ones, being upregulated in blood, lesional and perilesional skin. TNF-α is a pro-inflammatory cytokine that has a central role in many inflammatory conditions. Despite the fact that TNF-α is not a HS specific biomarker, a fivefold increase in lesional skin relative to control HS skin has been assessed. The significant results of two similarly designed, multicenter, double blind, placebo-controlled trials (PIONEER I and II) have led to the approval of adalimumab, a fully human monoclonal anti-TNF-α antibody, as the first drug for the treatment of moderate-to-severe HS.3 HiSCR achievement rate at week 12 was significantly higher (p < 0.05) for patients randomized to weekly adalimumab versus placebo (41.8% vs. 26.0% in PIONEER I, p = 0.003; and 58.9% vs. 27.6% in PIONEER II, p < 0.001). A reduction of the number of inflammatory nodules and abscesses (AN count) to 0–2 at week 12 was achieved by 51.8% of HS patients receiving weekly adalimumab versus 32.2% under placebo (p < 0.05). Long-term adalimumab administration has shown a sustained improvement of skin lesions and pain in a part of the treated patients. Similar results have been obtained by the injectable anti-TNF-α antibody infliximab in smaller studies. Interestingly, adalimumab treatment was not associated with reduction of TNF-α lesional skin expression or serum concentration. In contrast, ex vivo studies of TNF-α concentration detected a marked TNF-α reduction in cultured HS lesional tissue and the culture supernatants under adalimumab treatment, like inhibition of soluble TNF receptor I was found in patients serum. Adalimumab did not affect keratinocyte differentiation-associated protein expression in lesional skin maintained ex vivo and tunnels after long-term patient treatment. The need of better visualization of drug effectiveness on HS has led to the development of new outcome measurement instruments evaluating the three primary HS lesions, namely inflammatory nodules, abscesses and draining tunnels, such as IHS4 and the dichotomous IHS4-55 as well as the consideration of anti-inflammatory molecules, which also influence keratinocyte differentiation. The rationale for selecting interleukin (IL)-17 as a HS therapy target is based on the central role of IL-17 in the pathophysiology of HS, although, like TNF-α, IL-17 is also not a HS specific biomarker. Strong expression of IL-17A and presence of IL-17 producing T cells was detected in lesional skin of HS patients. At the molecular level, an IL-17 signature and dysregulation of T-helper type 17 cytokines in HS lesional skin was demonstrated. HS patients showed imbalances in the T-helper 17 cell axis. The pro-inflammatory isoforms IL-17A, IL-17C and IL-17F have been identified in HS lesions. At last, overexpression of the IL-17 receptor was detected in obese smokers with HS. Phase II and III clinical studies with the IL-17A/F dual inhibitor bimekizumab4 or the IL-17A inhibitor secukinumab,5 respectively, have been successful in the treatment of moderate-to-severe HS [bimekizumab: HiSCR reduction at week 12 57.3% vs. placebo 26.1%; secukizumab: HiSCR reduction at week 16 45.0% vs. placebo 33.7% (SUNSHINE study), 42.3% vs. placebo 31.2% (SUNRISE study), including a reduction of IHS4]. Ex vivo studies did not detect effectiveness of secukinumab on IL-1β expression, however, it would be interesting to investigate whether anti-IL17 antibodies might reduce the number of draining tunnels and be also able to downregulate IL-1B-associated genes, such as IL1R1, IL1RN, IFNG, IL6, IL18, IL18R1, IL32, IL33, IL36A, IL36B, IL36G, IL36RN, IL37, TLR2, TLR3, TLR4, S100A7, S100A7A, S100A8, S100A9 and S100A122 and their protein expression and release ex vivo and in vivo, which might indicate an additional drug effectiveness on the probable HS initiator, the abnormal differentiation of the hair follicle keratinocytes.
Acknowledgement: The Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin – ALLOCATE Skin group).
- Zouboulis CC et al. Alterations in innate immunity and epithelial cell differentiation are the molecular pillars of hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2020;34:846–61.
- Zouboulis VA et al. Hidradenitis suppurativa and comorbid disorder biomarkers, druggable genes, new drugs and drug repurposing – A molecular meta-analysis. Pharmaceutics 2022;14:44.
- Kimball AB et al. Two phase 3 trials of adalimumab treatment of hidradenitis suppurativa. N Engl J Med 2016;375:422–34.
- Glatt S et al. Bimekizumab in moderate-to-severe hidradenitis suppurativa: A phase 2, double-blind, placebo-controlled randomized clinical trial. JAMA Dermatol 2021;157:1279–88.
- Kimball AB et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomized, placebo-controlled, double-blind phase 3 trials. Lancet 2023;401:747–61.
The lesson from guidelines in antibiotic treatment in HS
Kelsey R. van Straalen1,2
1Erasmus University Medical Center, Laboratory for Experimental Immunodermatology, Department of Dermatology, Rotterdam, Netherlands; 2University of Michigan Medical School, Department of Dermatology, Ann Arbor, USA
Traditionally hidradenitis suppurativa (HS) has been treated with systemic antibiotics, which remain the first-line medical therapy to date. Both tetracyclines and the combination of clindamycin + rifampicin are cornerstone systemic antibiotic therapies recommended in all current guidelines and consensus statement.1,2 However, evidence for their efficacy of these treatments is drawn from small studies, often without validated outcomes. In recent years several studies have emerged challenging both the indication and use of these therapies. In 2021 a Europe-wide prospective study by Van Straalen et al. demonstrated that clindamycin (300 mg twice daily) in combination with rifampicin (600 mg a day) showed similar efficacy as tetracyclines regardless of disease severity.3 With HiSCR rates of respectively 40.1% and 48.2% of patients after 12 weeks (p = 0.26) this study paved the way for the use of tetracyclines in moderate–severe patients, the removal of combination therapy as first line treatment, and earlier initiation of biologic treatment. In addition, combined treatment with rifampicin and clindamycin has been shown to significantly reduce the clindamycin plasma concentration in patients with HS, albeit with unknown clinical significance.4 Interestingly, a study by Caposiena Caro et al. including 60 patients demonstrated no significant difference in clinical response among patients treated with either clindamycin monotherapy (n = 30, 56.5% HiSCR achievement) or combined treatment with clindamycin and rifampicin (n = 30, 63.3% HiSCR achievement) after 8 weeks of treatment.5 Clindamycin monotherapy however showed a significantly better response rate in mild–moderate patients (either IHS4 category mild–moderate or Hurley I and II stage disease) than severe patients.5 Overall, this suggests that our current guidelines need to be overhauled to include these new insights. Tetracyclines could be considered as the first-line treatment in all patients, including those with moderate to severe disease. Clindamycin monotherapy might be best suited for mild–moderate patients, with rifampicin + clindamycin combination therapy reserved for severe, individual cases.
- Zouboulis CC et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015;29:619–44.
- Zouboulis CC et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol 2019;33:19–31.
- van Straalen KR et al. The efficacy and tolerability of tetracyclines and clindamycin plus rifampicin for the treatment of hidradenitis suppurativa: Results of a prospective European cohort study. J Am Acad Dermatol 2021;85:369–78.
- Join-Lambert O et al. Dramatic reduction of clindamycin plasma concentration in hidradenitis suppurativa patients treated with the rifampin-clindamycin combination. Eur J Dermatol 2014;24:94–5.
- Caposiena Caro RD et al. Clindamycin versus clindamycin plus rifampicin in hidradenitis suppurativa treatment: Clinical and ultrasound observations. J Am Acad Dermatol 2019;80:1314–21.
Photodynamic therapy with RLP068/CL as a new advancement in local treatment of HS
Elia Rosi, Maria T. Fastame, Ilaria Scandagli, Gianmarco Silvi, Prisca Guerra, Giulia Nunziati, Antonella Di Cesare, Francesca Prignano
University of Florence, Department of Health Sciences, Section of Dermatology, Florence, Italy
Hidradenitis suppurativa (HS), also known as acne inversa or Verneuil's disease, is a chronic (auto)inflammatory disease primarily affecting apocrine gland-rich areas of the body. HS treatment remains challenging, as well as defining the optimal treatment strategy for each patient.1 Photodynamic therapy (PDT) has been used for HS patients over the last few years with mixed results.2 In this context, our study sought to assess the effectiveness, tolerability, and safety of red light PDT with RLP068/Cl versus topical clindamycin in patients affected by HS. Patients attending our outpatient-clinic with symmetrical groin or axilla HS lesions (inflammatory nodules or abscesses) treated with adalimumab or about to start topical clindamycin were consecutively enrolled. Each patient was candidate to receive throughout 6 weeks topical clindamycin on one lesion and red light PDT with RLP068/Cl on the contralateral lesion. The therapeutic effectiveness was evaluated based on four response categories: complete response, good response, partial response, and no response. A total of 15 patients were enrolled. Approximately 30% of lesions treated with red light PDT with RLP068/Cl had a complete response at 6 weeks compared with approximately 13% of those treated with conventional topical clindamycin. No significant differences in terms of efficacy were found between red light PDT group and topical clindamycin group. Patients reported significant pain reduction for PDT-treated lesions at 3 weeks in comparison with those treated with topical clindamycin. Treatment was well tolerated, and no patients discontinued PDT. The effective mechanisms of PDT might depend on its ability of killing bacteria also in a biofilm. Indeed, bacteria seems to have a key role in HS pathogenesis.3 Considering this, in our study, the photoactivation of RLP068 (a new tetracationic Zn(II) phthalocyanine photosensitizer derivative) resulted in a rapid, broad range, bactericidal effect.4 Red-light PDT with RLP068/Cl is time-consuming for both patients and clinicians, but it may be a new adjuvant/alternative safe treatment in patients with poor response to therapy and with a high number of exacerbations/year. Furthermore, it could be faster in reducing pain compared with topical clindamycin. Finally, PDT seems not to induce antimicrobial resistance.
- Rosi E et al. Insights into the Pathogenesis of HS and Therapeutical Approaches. Biomedicines 2021;9:1168.
- Reshetylo S et al. Systematic review of photodynamic therapy for the treatment of hidradenitis suppurativa. Photodermatol Photoimmunol Photomed 2023;39:39–50.
- Rosi E et al. Consistency of Bacterial Triggers in the Pathogenesis of Hidradenitis Suppurativa. Vaccines (Basel) 2023;11(1):179.
- Mannucci E et al. Photodynamic topical antimicrobial therapy for infected foot ulcers in patients with diabetes: a randomized, double-blind, placebo-controlled study—the D.A.N.T.E (Diabetic ulcer Antimicrobial New Topical treatment Evaluation) study. Acta Diabetol 2014;51:435–40.
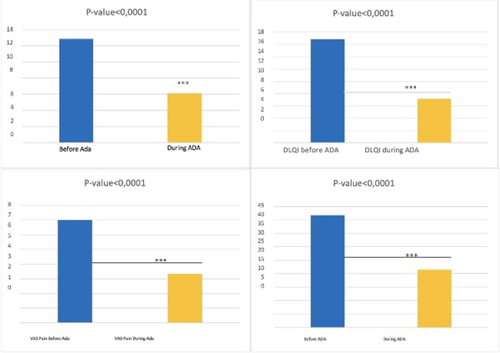
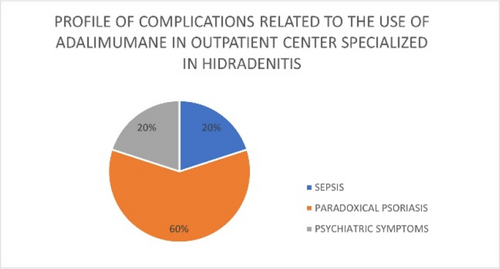
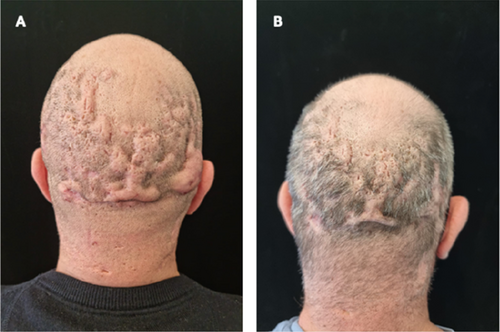
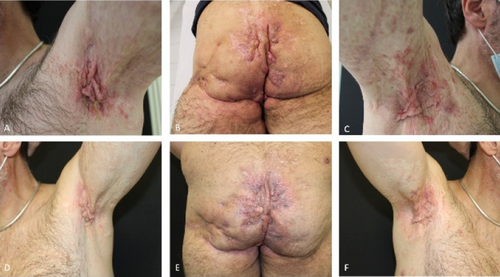
Switching from adalimumab originator to biosimilar in patients with hidradenitis suppurativa results in losses of response: Data from the German HS registry HSBEST
Frenz Ohm1, Natalia Kirsten1,6, Kathrin Gehrdau1, Brigitte Stephan1, Nesrine Ben-Anaya1, Andreas Pinter2,6, Falk G. Bechara3,6, Dagmar Presser4,6, Christos C. Zouboulis5,6, Matthias Augustin1,6
1University Medical Center Hamburg-Eppendorf, Institute for Health Services Research in Dermatology and Nursing (IVDP), Hamburg, Germany; 2University Hospital Frankfurt am Main, Department of Dermatology, Venereology and Allergology, Frankfurt, Germany; 3Ruhr-University Bochum, Department of Dermatology, Venereology and Allergology, Bochum, Germany; 4University Hospital Würzburg, Department of Dermatology, Venereology and Allergology, Würzburg, Germany; 5Brandenburg Medical School Theodor Fontane and Faculty of Health Sciences Brandenburg, Staedtisches Klinikum Dessau, Departments of Dermatology, Venereology, Allergology and Immunology, Dessau, Germany; 6European Hidradenitis Suppurativa Foundation (EHSF). V., Dessau, Germany
Since its approval, adalimumab biosimilar ABP 501 can be used alternatively to adalimumab originator (ADAO) in the treatment of hidradenitis suppurativa (HS). However, effectiveness and safety data, especially after switching from ADAO to ABP 501, remain scarce. We aimed to investigate the impact of switching from ADAO to ABP 501 on disease severity and the occurrence of adverse events (AEs) in patients with HS. We analysed data on patients enrolled in the German HSBest registry, who were on remission maintenance therapy with ADAO and then switched to ABP 501, following drug regulations. Evaluation outcomes were assessed at three time points (baseline of originator (t0), prior to switching to biosimilar (t1) and 12 to 14 weeks after switching (t2)) and included patient-reported AEs and disease severity using the International Hidradenitis Suppurativa Severity Score System (IHS4) score. A total of 94 patients were switched from ADAO to ABP 501. Mean age was 39.3 (± 13.2) years, 49% were male. The mean Hurley score was 2.26 (± 0.7). The patients had received ADAO for a mean of 17.6 (± 12.7) months before switching to biosimilar. Overall, 33.3% (n = 31) of the patients developed AEs and/or loss of response (LoR) within 12 to 14 weeks after switch. Of these, 61.3% (n = 19) experienced a LoR but no side effects, 22.6% (n = 7) a LoR combined with side effects and 16.1% (n = 5) side effects only. The median duration of therapy under originator was 19.0 (± 13.3) months without significant differences between patients with and without AEs. Our study showed that switching HS patients from adalimumab originator to biosimilar ABP 501 does significantly affect treatment effectiveness. As a result, switching patients, who are on remission maintenance therapy, should be viewed critically.
Acknowledgement: The Department of Dermatology, Venereology and Allergology, University Hospital Würzburg and the Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin – ALLOCATE Skin group).
Drug survival of metformin in patients with hidradenitis suppurativa
Jose Carlos Pascual1, Marina Senent-Valero1, Iris González-Villanueva1, Nolia Jara1, Ruben Hernández-Quiles1, Verónica Sánchez-García1, Alica Matijasevich2
1University General Hospital, Alicante Institute for Health and Biomedical Research (ISABIAL), Dermatology, Alicante, Spain; 2Universidade de Sâo Paulo, Medicina Preventiva FMUSP, Sâo Paulo, Brazil
There is little scientific evidence on the use of metformin in hidradenitis suppurativa (HS). To our knowledge there are no studies on the survival of metformin in HS. The objective of the study was to assess the drug survival of metformin for HS in a real world-A retrospective cohort, single-centre study was conducted in Spain in HS-patients treated with metformin. We included all patients treated with metformin for HS between January 2015 to December 2021, and who were follow-up for a minimum time of 12 months, irrespective of whether they continued on metformin or not. Patients with any Hurley stage were included. Demographic, disease-related and adverse events were obtained by review of medical records and our HS database. Drug survival analyses were performed through Kaplan–Meier survival. Additionally, Cox regression analyses models were used to determine predictors for a longer drug survival. We identified 96 HS patients treated with metformin. Mean age (SD) was 37.0 (11.7) years. 88 out 96 were women (91.7%). Most patients (92.7%) were classified as Hurley II. 63 (65.5%) patients were obese and 24 (25%) were overweight. 56 (58.3%) patients were smokers or ex-smokers. 22.9% had metabolic syndrome and 19.3% of the women associated polycystic ovary syndrome. The most frequent locations were the groin (74.0%) and the axilla (54.2%). Median drug survival was 12 months (IQR 5.3–22.8). Overall drug survival at 12 and 24 months was 51% and 21.9% respectively. The maximum survival time for metformin was 84 months. Adverse effects of metformin were described in 27 patients (28.1%), most of them gastrointestinal (92.1%). Male sex [HR (IC95%) 2.4 (1.1–5.2)], smoking [HR 1.9 (1.1–3.2)] and location of lesions in the abdomen [HR 2.2 (1.1–4.1)] were associated with an increased risk of metformin withdrawal in the univariate analysis. However, this association was not maintained after multivariate analysis. Drug survival of metformin is high in HS. Half of patients remain on medication after 12 months of treatment. None of the study variables were associated with a higher survival rate.
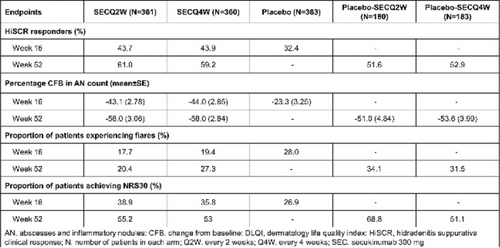
Secukinumab in moderate to severe hidradenitis suppurativa: Week 16 results of hurley stage II and III from the sunshine and sunrise phase 3 randomized trials
Alexa B. Kimball1, Afsaneh Alavi2,3, Gregor B.E. Jemec2,4, Christos C. Zouboulis2,5, Magdalena B. Wozniak6, Lorenz Uhlmann7, Angela Llobet-Martinez7, Shoba Ravichandran8, Angelo V. Marzano2,9, Ziad Reguiai2,10
1Harvard Medical School and Beth Israel Deaconess Medical Center, Dermatology, Boston, USA; 2European Hidradenitis Suppurativa Foundation e.V., Dessau, Germany; 3Mayo Clinic, Dermatology, Rochester, USA; 4Zealand University Hospital Roskilde, Dermatology, Roskilde, Denmark; 5Brandenburg Medical School Theodor Fontane and Faculty of health Sciences Brandenburg, Staedtisches Klinikum Dessau, Departments of Dermatology, Venereology, Allergology and Immunology, Dessau, Germany; 6Novartis Ireland Limited, Dublin, Ireland; 7Novartis Pharma AG, Basel, Switzerland; 8Novartis Pharmaceuticals Corporation, East Hanover, USA; 9Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico; Universita Degli Studi Di Milano, Dermatology, Pathophysiology and Transplantation, Milan, Italy; 10Polyclinic Courlancy Bezannes, Dermatology, Reims, France
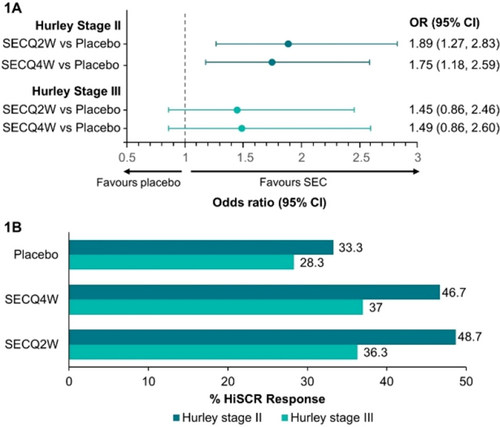
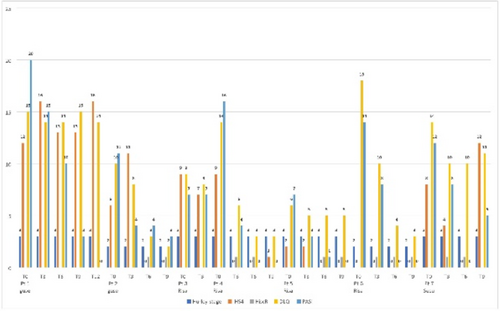
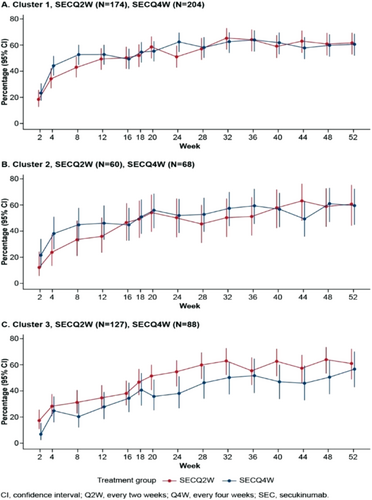
FIGURE 1 HiSCR odds ratio and response rate for patients with Hurley stage II and III. (A) Forest plot demonstrating odds ratio of HiSCR response for secukinumab versus placebo (B) Clustered bar chart showing HiSCR response rate (%) for secukinumab versus placebo. CI, confidence interval; HiSCR, Hidradenitis Suppurativa Clinical Response; OR, odds ratio; SEC, secukinumab; SECQ2W, secukinumab 300 mg every 2 weeks; SECQ4W, secukinumab 300 mg every 4 weeks.
Acknowledgement: The study was sponsored by Novartis Pharma AG. The Departments of Dermatology, Venereology, Allergology and Immunology, Dessau Medical Center, Dessau, Germany and the Department of Dermatology, Zealand University Hospital Roskilde, Roskilde, Denmark are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Samantha R et al. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 2020;82:1045–58.
- Saunte DM et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol 2015;173:1546–9.
- Kokolakis G et al. Delayed Diagnosis of Hidradenitis Suppurativa and Its Effect on Patients and Healthcare System. Dermatology 2020;236:421–30.
- Marzano AV et al. Evidence for a ‘window of opportunity’ in hidradenitis suppurativa treated with adalimumab: a retrospective, real-life multicentre cohort study. Br J Dermatol 2021;184:133–40.
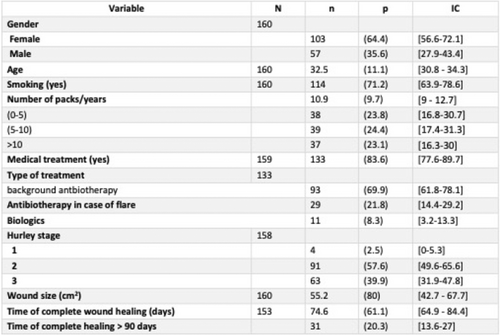
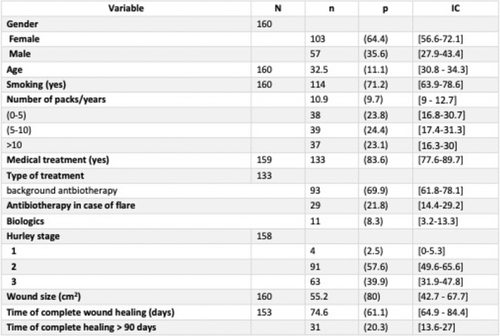
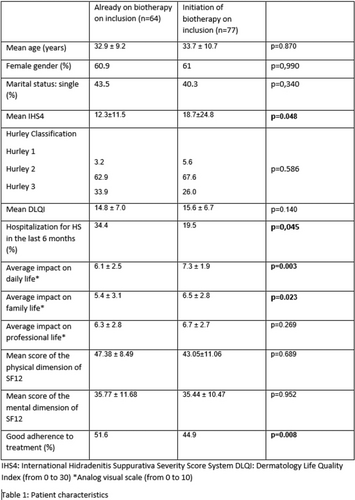
Efficacy of secukinumab in patients with hidradenitis suppurativa based on the international hidradenitis suppurativa severity scoring system (IHS4): A post hoc analysis of the SUNSHINE and SUNRISE trials
Christos C. Zouboulis1,2, Athanassios Kyrgidis1,3, Afsaneh Alavi1,4, Gregor B.E. Jemec1,5, Antonio Martorell1,6, Angelo V. Marzano7,8, Hessel H. van der Zee1,9, Elisa Muscianisi10, Torben Kasparek11, Teresa Bachhuber12, Magdalena B. Wozniak12, Christine-Elke Ortmann11, Iryna Lobach11, Shoba Ravichandran10, Thrasyvoulos Tzellos1,13
1European Hidradenitis Suppurativa Foundation (EHSF), Dessau, Germany; 2Brandenburg Medical School Theodor Fontane and Faculty of Health Sciences Brandenburg, Staedtisches Klinikum Dssau, Departments of Dermatology, Venereology, Allergology and Immunology, Dessau, Germany; 3Papanikolaou General Hospital of Thessaloniki, Aristotle University of Thessaloniki, Department of Maxillofacial Surgery, Thessaloniki, Greece; 4Mayo Clinic, Department of Dermatology, Rochester, USA; 5Zealand University Hospital, Roskilde and Health Sciences Faculty, University of Copenhagen, Department of Dermatology, Copenhagen, Denmark; 6Hospital de Manises, Department of Dermatology, Valencia, Spain; 7Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Dermatology Unit, Milan, Italy; 8Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy; 9Erasmus Medical Center, Department of Dermatology, Rotterdam, Netherlands; 10Novartis Pharmaceuticals Corporation, East Hanover, USA; 11Novartis Pharma AG, Basel, Switzerland; 12Novartis Ireland Limited, Dublin, Ireland; 13Nordland Hospital Trust, Department of Dermatology, Bodø, Norway
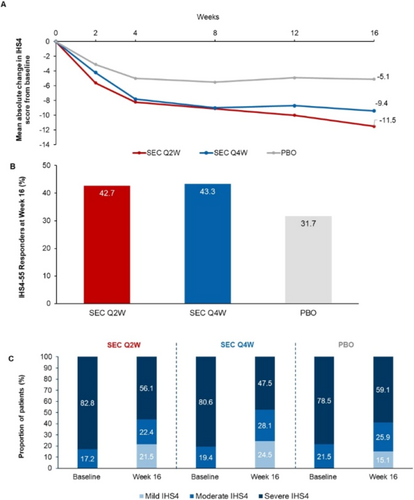
FIGURE 1 Mean absolute change from baseline in IHS4 score up to Week 16 (A); IHS4-55 response at Week 16 (B); shift in the IHS4 severity grade from baseline to Week 16 (C) [full analysis set – observed]. The IHS4-55 is a dichotomous outcome based on a 55% reduction in the total IHS4 score. IHS4, International Hidradenitis Suppurativa Severity Scoring System; PBO, placebo; Q2W, every 2 weeks, Q4W, every 4 weeks; SEC, secukinumab.
Acknowledgement: The study was sponsored by Novartis Pharma AG. The Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau, Dessau, Germany, the Department of Dermatology, Zealand University Hospital Roskilde, Roskilde, Denmark and the Department of Dermatology, Erasmus Medical Center, Rotterdam, Netherlands are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Kimball AB et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989–94.
- Zouboulis CC et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177:1401–9.
- A study to evaluate the safety and efficacy of PF-06650833, PF-06700841, and PF 06826647 in adults with hidradenitis suppurativa. ClinicalTrials.gov identifier: NCT04092452.
- Evaluation of Sonelokimab for the Treatment of Patients With Active Moderate to Severe Hidradenitis Suppurativa. ClinicalTrials.gov identifier: NCT05322473.
- Tzellos T et al. Development and validation of IHS4-55, an IHS4 dichotomous outcome to assess treatment effect for hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2023; 37:395–401.
Assessment of the performance of the IHS4-55 using data from a phase 2 study of bimekizumab in patients with hidradenitis suppurativa
Thrasyvoulos Tzellos1,2, Athanassios Kyrgidis1,3, Afsaneh Alavi1,4, Kelsey R. van Straalen1,5, Ingrid Pansar6, Leah Davis7, Christos C. Zouboulis1,8
1European Hidradenitis Suppurativa Foundation, e.V., Dessau, Germany; 2Nordland Hospital Trust, Department of Dermatology, Bodø, Norway; 3Aristotle University of Thessaloniki, Papanikolaou General Hospital of Thessaloniki, Department of Maxillofacial Surgery, Thessaloniki, Greece; 4Mayo Clinic, Department of Dermatology, Rochester, USA; 5University of Michigan, Department of Dermatology, Ann Arbor, USA; 6UCB Pharma, Brussels, Belgium; 7UCB Pharma, Morrisville, USA; 8Brandenburg Medical School Theodor Fontane and Faculty of Health Sciences Brandenburg, Staedtisches Klinikum Dessau, Departments of Dermatology, Venereology, Allergology and Immunology, Dessau, Germany
Acknowledgement: This study was funded by UCB Pharma. Medical writing services were provided by Costello Medical, funded by UCB Pharma. The Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau, Dessau, Germany are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Zouboulis CC et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol 2017;177:1401–9.
- Tzellos T et al. Development and validation of IHS4-55, an IHS4 dichotomous outcome to assess treatment effect for hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2023; 37:395–401.
- Vellaichamy G et al. Patient-reported outcomes in hidradenitis suppurativa. G Ital Dermatol Venereol 2019;154:137–47.
- Glatt S et al. Efficacy and Safety of Bimekizumab in Moderate to Severe Hidradenitis Suppurativa: A Phase 2, Double-blind, Placebo-Controlled Randomized Clinical Trial. JAMA Dermatol 2021;157:1279–88.
Adalimumab in conjunction with surgery compared with adalimumab monotherapy for hidradenitis suppurativa; a randomized controlled trial in a real-world setting (#90)
Pim Aarts, Johanna C. van Huijstee, Hessel H. van der Zee, Martijn B. van Doorn, Kelsey R. van Straalen, Errol P. Prens
Erasmus University Medical Center, Department of Dermatology, Rotterdam, Netherlands
Acknowledgement: The Department of Dermatology, Erasmus Medical Center, Rotterdam, Netherlands is a health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
Impact of secukinumab on the need for rescue surgical intervention in patients with moderate to severe hidradenitis suppurativa: Post-hoc analysis of week 16 data from sunshine and sunrise trials
Hessel H. Van der Zee1,2, Jacek C. Szepietowski1,3, Christopher Sayed1,4, Philippe Guillem1,5, Iltefat H. Hamzavi1,8, Stephanie Goldberg9, Clarice Field10, Christine-Elke Ortmann11, Iryna Lobach11, Magdalena B. Wozniak10, Angela Llobet-Martinez11, Shoba Ravichandran12, Teresa Bachhuber11, Falk G. Bechara1,13
1European Hidradenitis Suppurativa Foundation, Dessau, Germany; 2Erasmus Medical Center, Department of Dermatology, Rotterdam, Netherlands; 3Wroclaw Medical University, Department of Dermatology, Venereology and Allergology, Wroclaw, Poland; 4University of North Carolina, School of Medicine, Department of Dermatology, North Carolina, USA; 5Clinique du Val d'Ouest, Department of Surgery, Ecully, France; 6RésoVerneuil, Paris, France; 7Groupe de Recherche en Proctologie de la Société Nationale Française de Coloproctologie (SNFCP), Paris, France; 8Multicultural Dermatology Center, Henry Ford Hospital, Department of Dermatology, Detroit, USA; 9Mary Washington Healthcare, Fredericksburg, USA; 10Novartis Ireland Limited, Dublin, Ireland; 11Novartis Pharma AG, Basel, Switzerland; 12Novartis Pharmaceuticals Corporation, East Hanover, USA; 13Ruhr-University Bochum, Department of Dermatology, Venereology and Allergology, Bochum, Germany
Hidradenitis suppurativa (HS) is a follicular skin disease characterized by deep dermal inflammatory nodules, abscesses, and tunnels; chronic inflammation of these lesions eventually leads to irreversible tissue destruction and scarring.1 These manifestations make HS a difficult-to-treat disease that requires a multifaceted treatment approach inclusive of pharmacological therapies (e.g., antibiotics, steroids, biologics) as well as surgical intervention,2 in an orchestrated manner. HS treatment guidelines recommend surgery on a case-by-case basis based on patients' clinical features.3 Surgery can be indicated for acute lesions in a rescue capacity to relieve localized symptoms of pain and discomfort, however, incision and drainage (I&D), a common rescue surgery, carries a high risk of recurrence4 and thus, is a mostly symptomatic therapy. It is unknown whether the use of biologics can affect the need for rescue surgery to be performed on acute painful lesions. SUNSHINE (NCT03713619) and SUNRISE (NCT03713632) were identical Phase 3 randomized, placebo-controlled, multi-center clinical trials which assessed the clinical efficacy of secukinumab, a monoclonal antibody selectively neutralizing IL-17A, in patients with moderate to severe HS. Here, we report post-hoc analyses conducted to evaluate the requirement of rescue surgical intervention (I&D or excision) up to Week 16 in patients enrolled in the SUNSHINE and SUNRISE trials. Patients randomized at baseline received secukinumab 300 mg every week for an induction period of 5 weeks, followed by every 2 (SECQ2W) or 4 weeks (SECQ4W), or placebo (PBO) in a 1:1:1 ratio. During the trials, investigators were permitted to, at any time, perform a rescue surgical intervention (I&D or excision) or administer rescue therapy (intra-lesional corticosteroid administration) for acute painful single lesions requiring immediate intervention. Other pre-planned HS-related surgeries (e.g., wide excisions) were not permitted. Analysis of rescue surgical interventions up to Week 16 was performed on the observed pooled data of the SUNSHINE and SUNRISE trials. Overall, 3.5% (38/1084) of patients in SUNSHINE and SUNRISE received at least one rescue surgical intervention during the placebo-controlled period up to Week 16. Incidence of rescue surgical intervention (Figure 1) was numerically lower in those receiving secukinumab compared to placebo (SECQ2W, 2.2% [8/361]; SECQ4W, 3.1% [11/360]; Any SEC, 2.6% [19/721]; PBO, 5.2% [19/363]). Time-to-first rescue surgical intervention (mean days [standard deviation) was longer with SECQ2W dosing compared to placebo, however, average time was similar between the Any SEC group and placebo (SECQ2W, 55.3 days [38.5]; SECQ4W, 39.0 days [33.6]; Any SEC, 45.8 days [35.7]; PBO, 45.1 days [27.8]).
Acknowledgement: The study was sponsored by Novartis Pharma AG. The Department of Dermatology, Erasmus Medical Center, Rotterdam, Netherlands are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Sabat R et al. Hidradenitis suppurativa. Nat Rev Dis Primers 2020;6:18.
- Zouboulis CC et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015;29:619–44.
- Gulliver W et al. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord 2016;17:343–51.
- Kohorst JJ et al. Surgical Management of Hidradenitis Suppurativa: Outcomes of 590 Consecutive Patients. Derm Surg 2016;42.
Janus kinase 1 inhibition with povorcitinib (INCB054707) in hidradenitis suppurativa: Efficacy and safety during the open-label extension period of a phase 2 study
Joslyn S. Kirby1, Martin M. Okun2, Afsaneh Alavi3,4, Falk G. Bechara5, Christos C. Zouboulis3,6, Kurt Brown7, Leandro L. Santos7, Annie Wang7, Alexa B. Kimball8, Martina J. Porter8
1Penn State Health Milton S. Hershey Medical Center, Hershey, USA; 2Fort Memorial Hospital, Fort Atkinson, USA; 3European Hidradenitis Suppurativa Foundation (EHSF), Dessau, Germany; 4Mayo Clinic, Rochester, USA; 5Ruhr-University Bochum, Bochum, Germany; 6Brandenburg Medical School Theodor Fontane and Faculty of Health Sciences Brandenburg, Staedtisches Klinikum Dessau, Departments of Dermatology, Venereology, Allergology and Immunology, Dessau, Germany; 7Incyte Corporation, Wilmington, USA; 8Harvard Medical School and Beth Israel Deaconess Medical Center, Boston, USA
Acknowledgement: The Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau, Dessau, Germany are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Zouboulis CC et al. Development and validation of IHS4, a novel dynamic scoring system to assess hidradenitis suppurativa/acne inversa severity. Br J Dermatol 2017;177:1401–9.
- Tzellos T et al. Development and validation of IHS4-55, an IHS4 dichotomous outcome to assess treatment effect for hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2023; 37:395–401.
Surgical treatment: What is to consider a specific procedure for HS?
The use of seton drainage for hidradenitis suppurativa tunnels
Sara K. Saunte1,4, Peter T. Riis1,4, Jacob K. Poulsen2, Morten H. Thomsen2, Nina A. Frederiksen2, Ditte M.L. Saunte1,3, Gregor B.E. Jemec1,3
1Zealand University Hospital, Department of Dermatology, Roskilde, Denmark; 2Zealand University Hospital, Department of Surgery, Køge, Denmark; 3University of Copenhagen, Department of Clinical Medicine, Faculty of Health Science, Copenhagen, Denmark; 4Shared, first, authorship, Denmark
One characteristic of hidradenitis suppurativa (HS) is the appearance of tunnels. These hollow structures most often link the skin surface with the dermal-subcutaneous border, and are thought to be caused by inflammation and subsequent scarring. They may be inflamed, in which case they cause pain and malodorous discharge that may have prolonged course. Current management often involves surgery.1 Structural similarities exist between inflammatory bowel disease, in particular Crohn's Disease and HS. Seton drainage of anal fistulae is well-documented in Crohn's Disease where it is used to alleviate symptoms.2 For HS it has been suggested as an alternative treatment option for subcutaneous tunnels in HS, as it may diminish tissue damage as to surgery.3 The aim of this study is to review the effect and describe the satisfaction of patients with HS treated with setons. A retrospective study with a follow-up interview.
Data collected included patient characteristics (gender, age, BMI, Hurley stage, years with HS). The number of weeks having a seton was noted and the satisfaction with the procedure was assessed on a 0–10 numeric rating scale (NRS, 0 = unsatisfied, 10 = satisfied). The patient reported effect of seton insertion was studied using an impact scale: −3 to 3 (−3: very negative, −2: moderately negative, −1: slightly negative, 0: no impact, 1; slightly positive, 2; moderately positive, 3; very positive). A total of 13 patients (4 females, 9 males) were enrolled in the study. The mean age was 56.5 years (range 34–74 years). The mean BMI was 29.7 (range 18–61). Three patients reported a positive family history of HS and the mean time living with HS was 15.4 years (range 5–36). A total of 6/13 of the patients had Hurley stage II and 7/13 Hurley stage III. The mean time from the seton procedure to the interview was 95.8 weeks (range 12–374). The median overall satisfaction with the procedure was NRS 7.5 (IQR 5–9) and 12/13 of the patients would recommend the procedure to another patient in the same situation. The median of impact, of the setons, on pain was 1 (IQR 0–3) which refers to the setons having a slightly more positive impact on the experienced pain than before the insertion of seton. The setons did not have any effect on activity level of the patient. Our data suggest that setons reduce the experience of pain in HS patients. Self-reported satisfaction is high and most of the patients would recommend the procedure to other HS patients. The usage of seton should be considered for patients with symptomatic HS tunnels. It is tissue sparing, pain-relieving and has a high satisfaction among the patients.
Acknowledgement: The Department of Dermatology, Zealand University Hospital Roskilde, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Mikkelsen PR et al. Recurrence rate and patient satisfaction of CO2 laser evaporation of lesions in patients with hidradenitis suppurativa: A retrospective study. Dermatol Surg 2015;41:255–60.
- Motamedi MAK et al. Long-term outcomes after seton placement for perianal fistulas with and without Crohn's disease. Colorectal Dis 2021;00:1–9.
- Lajevardi SS, Abeysinghe J. Novel Technique for Management of Axillary Hidradenitis Suppurativa Using Setons. Case Rep Surg 2015;2015:369657.
Management of hidradenitis suppurativa tunnels using drainage setons: Retrospective, multicentric study
Javier Fernández-Vela1, Jorge Romaní1, Fernando Cabo2, María Pousa2, Gemma Camiña-Conforto3, Eva Vilarrasa3, Antonio Guilabert1
1Hospital General de Granollers, Department of Dermatology, Granollers, Spain; 2Complexo Hospitalario de Ourense, Department of Dermatology, Ourense, Spain; 3Hospital de la Santa Creu i Sant Pau, Department of Dermatology, Barcelona, Spain
- Martorell A et al. Defining Fistular Patterns in Hidradenitis Suppurativa: Impact on the Management. Dermatol Surg 2019;45:1237–44.
Wound care for patients with hidradenitis suppurativa: Pearls and pitfalls from a specialist clinic
Hannah E. Wainman1,2, Maria Barfoot2
1University of Bristol, Medical School, Bristol, UK; 2Gloucestershire Hospitals NHS Foundation Trust, Department of Dermatology, Gloucester, UK
- Moloney S. The challenges of wound management for hidradenitis suppurativa. Br J Nurs 2022;31:S34–S41.
Difficult cases, resistant to treatment
Doc, my adalimumab is not working anymore, what to do next?
Errol Prens
Erasmus MC, Department of Dermatology, Rotterdam, Netherlands
Acknowledgement: The Department of Dermatology, Erasmus MC, Rotterdam, Netherlands is a health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Kimball AB et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med 2016;375:422–34.
- Prens LM et al. Adalimumab and infliximab survival in patients with hidradenitis suppurativa: a daily practice cohort study. Br J Dermatol 2021;185:177–84.
- Ring HC et al. Drug survival of biologics in patients with hidradenitis suppurativa. JAMA Dermatol 2022;158:184–8.
New surgical aproach in hidradenitis suppurativa surgical treatment: Co-graft of acellular dermal matrix and split thickness skin graft—A new reconstructive surgical method
Marcin Gierek
Center for Burns Treatment, Severe Burns Department, Siemianowice Slaskie, Poland
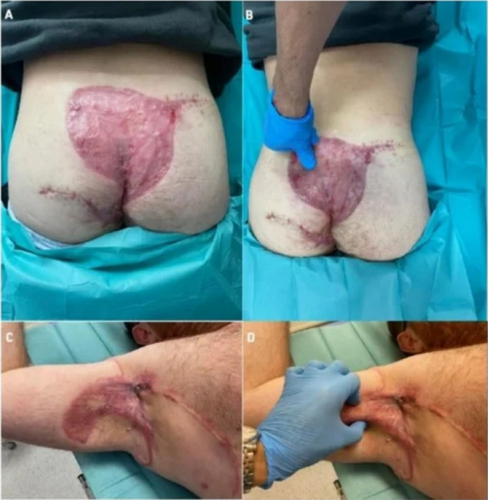
FIGURE 1 New Surgical Aproach - Co-graft of ADM and STSG. This image presents final results of surgical reconstruction with co-graft of ADM and STSG according to wide extensive resection (follow up 2.5 months). We reached good aesthetic results and very elastic scars. A)—Final result of reconstructed bottocks, (B)—Elastic scar in the “pinch” test, (C)—Final result of reconstructed right axilla, (D)—“Pinch” test in right axilla (elastic scar). Legend: ADM—Acellular Dermal Matrix, STSG—Split thickness skin graft.
- Zouboulis CC et al. Hidradenitis suppurativa/acne inversa: Criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology 2015;231:184–90.
- Lipsker D et al. The ABC of hidradenitis suppurativa: A validated glossary on how to name lesions. Dermatology 2016;232:137–42.
- Chen W, Plewig G. Should hidradenitis suppurativa/acne inversa best be renamed as “dissecting terminal hair folliculitis”? Exp Dermatol 2017;26:544–7.
- Gierek M et al. Impact of Hidradenitis Suppurativa Surgical Treatment on Health-Related Life Quality. J Clin Med 2022;11:4327.
- Nguyen TV et al. Hidradenitis suppurativa: An update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol 2021;35:50–61.
Hidradenitis suppurativa in transgender patients: A case series
Anne-Claire Fougerousse1,2, Pierre-Andre Bécherel2,3, Ziad Reguiai2,4, on behalf the GEM ResoVerneuil
1Military Teaching Hospital Begin, Department of Dermatology, Saint Mandé, France; 2GEM, ResoVerneuil, Paris, France; 3Hopital Privé d'Antony, Department of Dermatology, Antony, France; 4Polyclinic Courlancy Bezannes, Department of Dermatology, Reims, Germany
Gender incongruence or dysphoria is a relative common condition, with 1.8% of children in the United States identifying as transgender. Hormone therapy is the mainstay of treatment for masculinisation or feminisation. Adverse effects and risks are classically described with testosterone including acne and alopecia. Feminizing treatment with estrogens and antiandrogens are not usually associated to cutaneous side effects. Three cases de novo hidradenitis suppurativa (HS) in patients under masculinizing hormone therapy (MHT) are described in the literature.1,2 We report 6 new cases of HS, concerning both male and female hormones administration in transgender patients. A 23-year-old transgender man (non-cis gender, assigned female at birth) presented with 4 years history of severe acne of face and trunk, and painful nodules of the inguinal folds. He also underwent surgery for a pilonidal cyst. His Body Mass Index (BMI) was normal (<25 kg/m2). Acne and HS Hurley 1 lesions developed after the initiation of MHT with testosterone enantate, on stable dosage for 4.5 years. Severe acne was treated with isotretinoin 1 year after initiation of MHT. Testosterone levels were in normal range. Doyxcyclin 200 mg daily was initiated. After 4 months, he reported no HS flare. No modification of MHT was proposed. A 18-year-old transgender man (non-cis gender, assigned female at birth) had a personal history of HS Hurley 1C since he was 13, partially controlled with cyclins. His BMI was normal. He has not experienced any flare since the stabilization of MHT (5 years). A 48-year-old transgender woman (non-cis gender, assigned male at birth), with personal history of Crohn disease treated with sulfasalazine presented with history of mammary nodules and abscesses. Her BMI was normal. HS lesions developed after initiation of feminizing hormone therapy (FHT) (cyproterone acetate). HS management required clindamycin –rifampicin, cyclins, adalimumab and surgery for a draining fistula. Despite all these treatments HS remained active with frequent flares. A 17-year-old transgender woman (non-cis gender, assigned male at birth) had a personal history of HS Hurley 2 with abscess associated to a severe acne conglobata, treated with infliximab after failure of antibiotics (clindamycin/rifampicin, clindamycin/ofloxacin, doxycyclin) and oral isotretinoin. Her BMI was normal. FHT has been started after infliximab. HS evolution remained severe even after optimisation of infliximab dose (10 mg/kg every 4 weeks). A 36-year-old transgender woman (non-cis gender, assigned male at birth), with history of severe acne and normal BMI developed HS Hurley 2 lesions in axillary and inguino-perineal areas, 1 year after initiation of FHT (cyproterone acetate and oestrogens). She was treated with cyclins and hair removal laser without efficacy, then with adalimumab with a good control despite a few flares after 12 months. No modification of hormonal treatment was performed. A 32-year-old transgender woman (non-cis gender, assigned male at birth), developed HS Hurley 1C 8 months after FHT (cyproterone acetate and triptoreline). Treatment with hair removal laser, clindamycin and levofloxacin was insufficient and she was then treated with adalimumab with a good control maintained at 15 months. No modification of hormonal treatment was performed. Impact of hormones in the pathophysiology of HS is still debated, but androgens are thought to play a role, with evidence mostly based on epidemiologic association. HS comorbidities with elevated androgens as polycystic ovarian syndrome or obesity are also associated with elevated level of proinflammatory cytokines. Oestrogen metabolites (16α estrogens) are known to modulate local immune responses in other inflammatory diseases and could be mechanistically important in some HS patients. In this series, we reported 5 cases of transgender patients for whom modification of hormonal balance led to onset or exacerbation of HS. Our patients had no familial history of HS, were not overweight or obese. It is therefore possible that hormonal changes, whatever their direction (sudden excess of male or female hormones), might promote HS. Collecting other observations of HS in transgender patients will help us to better understand this particular association and to clarify the role of hormones in HS.
- Ramos-Rodríguez D t al. Hidradenitis suppurativa in a transgender man. Clin Exp Dermatol 2021;46:1305–6.
- Buonomo M et al. Development or exacerbation of hidradenitis suppurativa in two transgender men after initiation of testosterone therapy. Br J Dermatol 2021;184:1192–4.
Pyogenic granuloma and hidradenitis suppurativa
Maria L. Musumeci, Carlo Gerbino, Francesco Lacarrubba, Andrea Calogero Trecarichi, Giuseppe Micali
University of Catania, Department of Dermatology, Catania, Italy
Hidradenitis suppurativa (HS) is a common chronic-recurrent inflammatory cutaneous disorder affecting body areas rich in apocrine glands. Hallmark features of the disease are painful, inflammatory lesions (nodules, abscesses, fistulas, scars) located predominantly in the axillae, groins, and perineal/perianal areas. Follicular papules/pustules and simple/double pseudo-comedones have been infrequently reported. Also, the occurrence of multiple pyogenic granulomas (PG) in HS has been reported. PG, also known as lobular capillary haemangioma, is a common benign vascular tumour with unclear pathogenesis. Various factors have been associated to PG development, the most frequent being trauma, vascular malformations, drugs, pregnancy and infections. This lesion may affect all age groups, without gender prevalence. It generally appears as a solitary painless red and shiny skin overgrowth, with an eroded surface and easily bleeding. The occurrence of PG in HS patients has seldom been investigated, therefore we report our clinical experience in adult/paediatric HS patients. Clinical, dermatoscopic and histological findings of PG from HS paediatric and adult patients are presented and discussed.
A case of hidradenitis suppurativa, conglobate acne and dissecting cellulitis of the scalp successfully treated with secukinumab
Natale Schettini, Lucrezia Pacetti, Simone Cavaliere, Vincenzo Bettoli
University of Ferrara, Section of Dermatology and Infectious Diseases, Department of Medical Sciences, Ferrara, Italy
The authors report the case of a 24-year-old patient affected by Hidradenitis Suppurativa (HS), especially involving axillary, inguinal and pubic areas, Conglobate Acne of the face and Dissecting Cellulitis of the Scalp (DCS) (Figure 1). At the age of 16, being acne the manifestation with the highest clinical severity and impact on quality of life, a therapy with oral isotretinoin was started. This treatment brought to a clinical benefit on lesions of the face but did not have a considerable efficacy on HS and DCS. Also repeated courses of antibiotics, such as tetracycline, rifampicin, and clindamycin, showed no improvement. Due to the gradual worsening of HS, at the age of 19, adalimumab was started but even after 15 months of therapy no clinical response was reported. A treatment with dapsone was proposed, but the glucose-6-phosphate dehydrogenase deficiency contraindicated the intake of the drug. Meanwhile, a further deterioration of the clinical conditions was observed. The patient indeed presented a severe form of HS with numerous nodules and sinus tracts on axillae, groins and pubic region, Hurley III, and IHS4 score was 30. Nodules and abscesses were located also on the occipital region of the scalp. In December 2020, an off-label treatment with Secukinumab was initiated with a loading dose of 5 weekly injections of 300 mg followed by a dose of 300 mg every 4 weeks. A gradual improvement in the clinical presentation was reported already after a few months of therapy. Now the patient presents a reduction in the number of HS lesions with an IHS4 score of 9. Furthermore, also DCS showed a considerable improvement with an almost complete resolution of the inflammatory manifestations.
- De Bedout V et al. Treatment Dissecting Cellulitis of the Scalp With Secukinumab. J Drugs Dermatol 2021;20:776–7.
Paradoxical reactions during treatment with biological agents: Reports of 3 cases
Aikaterini I. Liakou1, Magdalini Kalamata1, Eythymia Agiasofitou1, Lydia S. Tsamtsouri3, Andreas Tsantes2, Eleni Chatzidimitriou1, Soultana Vladeni1, Alexandros I. Stratigos1
1Andreas Syggros Hospital, 1st University Clinic of Cutaneous and Veneral Diseases, Athens, Greece; 2Agios Savas Hospital, Department of Biopathology, Athens, Greece; 3National and Kapodistrian University of Athens, Department of Hygiene Epidemiology and Medical Statistics, Athens, Greece
We report 3 cases of paradoxical reactions to biologic agents. Patient 1: a 40-year-old male patient with chronic plaque psoriasis in the legs, who was treated with esters since 2016 (discontinuation due to gastrointestinal symptoms), he suffers from a mild HS localized only in the armpits, without specific treatment. In 2020 he switched to adalimumab and he reports a severe exacerbation of both HS (groin, gluteal) and psoriasis spread in the hole body after the 5th injenction. We was switched to secukinumab that improved both psoriasis and HS. Patient 2: a 32-year-old male patient with HS who started treatment with adalimumab in 2018. Due to lack of response after 2 years of treatment with adalimumab and occasional p.o. antibiotics (doxycycline, clindamycin, rifampicin) he switched to biosimilar and developed a psoriasiform rash in his legs and arms, while his HS remained the same. He was switched to secukinumab and his HS was improved. Patient 3: a 32-year-old male patient with Crohn's disease from 2014, under treatment with adalimumab, developed HS in 2016. In 2018, due to poor response to treatment, he was switched to Ustekinumab which improved his HS but triggered a severe flare of the crohn's disease and the patient underwent surgical removal of parts of his small intestine. He was switched to adalimumab (biosimilar) which keeps Crohn's disease under remission but leades to flares of HS. The flares are managed with antibiotis and occasional use of either per os or intralesional corticosteroids.
Advanced wound management approaches in hidradenitis suppurativa postsurgical lesions: The use of an oxygen-enriched oil-based medical device with prolonged release of reactive oxygen species
Alessandra Michelucci, Flavia Manzo Margiotta, Giammarco Granieri, Marco Romanelli, Valentina Dini
University of Pisa, Department of Dermatology, Pisa, Italy
Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the hair follicle, treated with a combination of medical and surgical approaches. Wide surgical excision may allow definitive resolution of the active inflammation as well as the removal of scarring tissue. The modality of wound healing following radical excision depends on the site and size of the lesions and influences disease recurrence rate. No guidelines are available in literature about optimal post-surgical wound healing.1 The aim of our study was to evaluate the effectiveness of an oxygen-enriched oil-based medical device with prolonged release of Reactive Oxygen Species (ROS) in the post-surgical management of HS lesions. We enrolled 20 patients with multi refractory, mild to severe HS. All the patients underwent a wide surgical excision of the lesions and then were randomized into two groups, differentiated on the basis of the dressing used in the second intention healing. The first group included 10 patients treated according to the principles of HS-Tissue, Inflammation, Moisture, Edge (TIME).2 In the second group, 10 patients were treated with an oxygen-enriched oil-based medical device with prolonged release of ROS. Patients were assessed once a week for 4 weeks and during each visit a comprehensive wound assessment was provided through the measurement of wound size, Wound Bed Score (WBS) and pain.3
Wound size decreased in all the evaluated lesions (p-value <0.001) as well as the pain measured through the Numerical Rating Scale (NRS) (p-value <0.001). Both groups showed an improvement in WBS assessment (p-value <0.001). No statistically significant differences were found in terms of reduction in mean area, mean NRS, and mean WBS among the two groups analysed. The gel's oleic composition provided a correct moist microenvironment, while the sustained-release of ROS improved the process of angiogenesis, cell proliferation and collagen synthesis. Moreover, the constant and prolonged release of ROS appeared to be able to inhibit bacterial and fungal proliferation.4 We can conclude that the constant ROS-releasing oxygen-enriched oil-based medical device could be included into the HS-TIME and can be considered as a valid alternative to silver dressings, if not well tolerated by the patient.
- Chetter IC et al. Patients with surgical wounds healing by secondary intention: A prospective, cohort study. Int J Nurs Stud 2019;89:62–71.
- Oranges T et al. HS-TIME: A Modified TIME Concept in Hidradenitis Suppurativa Topical Management. Wounds 2019;31:222–7.
- Falanga V et al. Wound bed score and its correlation with healing of chronic wounds. Dermatol Ther 2006;19:383–90.
- Dunnill C et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J 2017;14:89–96.
Ultrasonography-guided intralesional diode laser for the treatment of hurley II hidradenitis suppurativa: Results from a pilot study with 46 procedures
Philippe Guillem1,2, Christelle Enault1, Virginie Vlaeminck-Guillem3,4
1Clinique du Val d'Ouest, Department of Surgery, Ecully, France; 2RésoVerneuil, Paris, France; 3CHU, Department of Biology, Lyon, France; 4Lyon 1 University, INSERM 1052, CNRS 5286, CRCL, Lyon, France
Because of the burden of complete excision followed by secondary intention healing, minimally invasive treatments of pilonidal sinus disease (PSD) have been developed, including intralesional application of a radial fibre connected to a diode laser set. With a 1-year recurrence rate around 5% and therefore almost similar to that of lay-open primary excision, laser-assisted sinus closure trends to become the first choice treatment in simple PSD. Whether this mini-invasive technique could be useful in hidradenitis suppurativa (HS) remains to be determined. The aim of the study was to evaluate the early outcomes after intralesional diode laser for HS lesions. After receiving informed consent, we prospectively included patients with chronic non-complicated Hurley II HS lesions who were addressed for surgical treatment. Under general anaesthesia, the fistula tract was identified through inspection, palpation and, for some patients, ultrasonography (US). External orifices were widen or open if closed to allow sharp debridement using a curette and intralesional injection of povidone-iodine. A radial-emitting laser probe (Leonardo® dual 45 diode laser, wavelength 980–1470 nm, continuous energy of 10 W) was introduced in the fistula tract and pushed to the stop. The laser was then activated and pulled back at an approximate speed of 1 mm per second until evacuation. The whole procedure was repeated once. A simple dressing was applied and patients were instructed to change it daily until complete healing. Paracetamol and NSAID were prescribed to be used postoperatively on demand. We included 34 patients (24 women, 71%), median age 29.5 yrs (IQR: 24–39); 20 (59%) were current smokers and the median BMI was 25 (22.7–28.6). Treatment of 46 lesions were planned but one intermammary procedure was eventually cancelled because of the risk of skin burns and necrosis of a too superficial fistula (deroofing was performed). The locations were axillary (n = 17), inguinal (n = 16), perianal (n = 4), inner tight (n = 3), buttocks (n = 3) and others (n = 2). Treated lesions evolved for a median 2 yrs (1–4); previous incisions and/or excisions were reported in 5 and 7 patients, respectively. The median length of the fistula tract was 20.5 mm (12–30). Median applied energy was 274 J (182–420) in median time of 28 sec (19–43). The median procedure duration was 7 min (6–11). US was performed intraoperatively for 22/45 lesions (49%). Except two patients discharged at day 1 after surgery, all patients were outpatients. Pain at day 0 was scored at 0 or 39 lesions and 1 for 6. The median pain at day 2 was 2 (1–3). Early postoperative complications occurred for 12 lesions (27%) including bleeding (n = 1, stopped by simple compression), abscess (n = 4 treated by antibiotics and local care in 3 and deroofing in 1, burn-induced necrosis of the external orifice (n = 3, local care) and inflammatory flares (n = 4, antibiotics). With a limited follow-up of 99 days (ranges: 8–193), recurrence was observed in 5 patients (11%) and treated by deroofing (n = 1), excision (n = 1), or long course of oral antibiotics (n = 2); therapeutic decision pending for the last. This prospective pilot study suggests that intralesional diode laser could be an option for managing Hurley II HS lesions. The procedure is easy to performed (outpatient clinic) and well-tolerated. Because direct visual control of the fistula tract is lost as compared to deroofing and excision, intraoperative US appeared helpful. The postoperative wound care is clearly lightened. Both complication rate (27%) and the recurrence rate (11%), higher than those observed for PSD, suggest that a finer selection of patients is necessary in terms of inflammation stage, tract length and depth, and perhaps other patients and disease characteristics to be determined in larger and multicenter studies. Whether perioperative antibiotics are required should for example be ascertained. The observation that results were not as satisfying for HS than for PSD may be interpreted as different pathophysiological mechanisms including intrinsic (HS) or extrinsic (PSD) inflammation processes. Intralesional diode laser should be evaluated in the management of simple HS fistula tracts. It could be considered as an alternative to existing minor surgical procedures including deroofing and a convenient way to reduce postoperative wound care. Further studies are required to standardized perioperative management (potentially including perfect inflammation control) and indications.
Posters - Research
Biomarkers for hidradenitis suppurativa risk: Vitamin D receptor single nucleotide polymorphisms
Cristina Membrive-Jiménez2, Carlos Cuenca-Barrales1, Jose María Gálvez-Navas2, Alejandro Molina-Leyva1, Salvador Arias-Santiago1, Alberto Jiménez-Morales2
1Hospital Universitario Virgen de las Nieves, Granada, Spain, Department of Dermatology. Hidradenitis Suppurativa Unit., Granada, Spain; 2Hospital Universitario Virgen de las Nieves, IBS Granada, Granada, Spain, Department of Pharmacy. Pharmacogenetic Unit., Granada, Spain
Hidradenitis suppurativa (HS) is considered one of the dermatological pathologies that most affects the patient's quality of life. Alterations in the vitamin D receptor (VDR) gene play a key role in the modification of vitamin D uptake, modifying its function and increasing susceptibility to HS. The aim of this study was to evaluate the association of polymorphisms in the VDR gene and the risk of HS in a Caucasian population. A retrospective case–control study was conducted comprising 57 HS patients and 128 controls of Caucasian origin from Southern Spain. The genotypes were determined by Taqman® PCR Real Time. Controls were matched to cases on age and sex (2:1). Their clinical, socio-demographic, and pathological characteristics are detailed in Table 1. The median age of the patients was 50 (40–57), years. The group of cases consisted of 20 women (35.09%) and 37 men (64.91%). Statistical analysis revealed that the T allele of the VDR (BsmI) polymorphisms rs1544410 (p = 0.0001; OR = 2.47) and the A allele of the VDR-FoxI polymorphisms rs2228570 (p = 0.06; OR = 0.64) were associated with an increased risk of HS. Furthermore, these associations were confirmed in the analysis of other genetic models: VDR-BsmI rs1544410 of the genotypic model (p = 0.0006; TT vs. CT vs. CC), the additive model (p = 0.0001), the dominant model (p = 0.002; T vs. CC) and the recessive model (p = 0.002; C vs. TT); VDR-FoxI rs2228570 of the additive model (p = 0.075). No influence of TaqI (rs731236), ApaI (rs7975232), and Cdx2 (rs11568820) polymorphisms on the risk of developing HS was observed in the studied patients. The VDR (BsmI) polymorphisms (rs1544410) was significantly associated with the development of HS. According to it, this SNP could be used as a risk biomarker for the disease mentioned. Otherwise, no influence was found of the polymorphisms TaqI (rs731236), ApaI (rs7975232), and Cdx2 (rs11568820) on the risk of developing HS in our patients. Further studies should be done in order to understand and to confirm the role of VDR polymorphisms in HS.
Acknowledgement: In gratitude to the staff collaborating with this work, which is part of a research study conducted between the Dermatology Department and the Pharmacogenetics Unit of the Pharmacy Department of the Hospital Universitario Virgen de las Nieves.
Cytochrome P450 genes epigenetics should direct and instruct the therapeutical approach
Giovanni Damiani1, Uppala Radhakrishna2
1University of Milan, Department of Biomedical, Surgical and Dental Sciences, Milan, Italy; 2Oakland University William Beaumont School of Medicine, Department of Obstetrics and Gynaecology, Royal Oak, USA
Hidradenitis suppurativa (HS) is a chronic inflammatory disease frequently unresponsive to medical treatments. The exact mechanism remains elusive and no biomarkers have been identified to predict drug resistance at baseline. Since cytochrome P450 (CYPs) enzymes play a pivotal role in drug-metabolism, we decided to evaluate the epigenetic profile of CYP genes in HS patients. In this case–control study we compared 24 HS patients with 24 age-, sex-, ethnicity- matched controls. DNA samples were extracted from peripheral blood samples and analysed using the Infinium MethylationEPIC BeadChip array to detect methylation profiles in CYP genes.1,2 Differential methylation was assessed by comparing the ß-values for the cytosine at each CpG locus. False discovery rate (FDR) p-value, methylation status, and area under the ROC Curve (AUC) were calculated. Results: A total of 13 differently methylated CpG sites (p-value <0.05 and methylation differences ±0.05) of 13 unique CYPs (11 hypomethylated and 2 hypermethylated) were found only in HS patients. The most significant association was in the CYP19A1 gene (p = 8.0 × 10–6) encoding the aromatase enzyme involved in oestrogen biosynthesis. Furthermore, CYP26C1, CYP26B1, and CYP26C1 are involved in retinoid resistance, CYP2C19in clopidogrel resistance, while CYP2R1 is responsible for Vitamin D deficiency, and CYP2C19 is linked to depression and suicide ideation. CYPs methylation profiles may identify new endotypes resistant to specific therapies in HS, introducing a new era of precision medicine. CYPs may also become future targets for treating HS more efficiently as they elevate.
- Radhakrishna U et al. Cytochrome P450 genes mediated by DNA methylation are involved in the resistance to hidradenitis suppurativa. J Invest Dermatol 2022;22:S0022-202X(22)01939-X.
- Radhakrishna U et al. Methylated miRNAs may serve as potential biomarkers and therapeutic targets for hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2022;36:2199–2213.
Novel genetic variants and genes involved in the susceptibility to hidradenitis suppurativa, the lesson from familiar cases
Paola Maura Tricarico1, Chiara Moltrasio2,3, Lucas André Cavalcanti Brandao4, Ronald Rodrigues Moura1, Cecilia Del Vecchio1, Roberta Siqueira5, Hassan Riad6, Michele Boniotto7, Angelo V. Marzano8, Sergio Crovella9
1Institute for Maternal and Child Health - IRCCS Burlo Garofolo, Department of Advanced Diagnostics, Trieste, Italy; 2Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Dermatology Unit, Milan, Italy; 3University of Trieste, Department of Medical Surgical and Health Sciences, Trieste, Italy; 4Federal University of Pernambuco, Department of Pathology, Recife, Brazil; 5Clinical Hospital, Federal University fo Pernambuco, Dermatology Unit, Recife, Brazil; 6Hamad Medical Corporation, Department of Dermatology, Doha, Qatar; 7University Paris Est Créteil, 7INSERM, IMRB, Translational Neuropsychiatry, Créteil, France; 8Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy; 9University of Qatar, Department of Biological and Environmental Sciences, Biological Sciences Program, College of Arts and Sciences, Doha, Qatar
Acknowledgement: This work was supported by a Biomolecular Analyses for Tailored Medicine in AcneiNversa (BATMAN) project, funded by ERA PerMed (JTC_2018), by Starting Grant (SG-2019-12369421) founded by the Italian Ministry of Health, by grants (RC16/2018 and RC03/2020) from the Institute for Maternal and Child Health IRCCS ‘Burlo Garofolo funded by the Italian Ministry of Health and by a grant from Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico of Milan (protocol No.487_2020).
- Fitzsimmons JS et al. Evidence of genetic factors in hidradenitis suppurativa. Br J Dermatol. 1985;113:1–8.
- Moltrasio C et al. Hidradenitis Suppurativa: A Perspective on Genetic Factors Involved in the Disease. Biomedicines 2022;10:2039.
- Marzano AV et al. Whole-Exome Sequencing in 10 Unrelated Patients with Syndromic Hidradenitis Suppurativa: A Preliminary Step for a Genotype–Phenotype Correlation. Dermatology 2022;238:860–9.
- Tricarico PM et al. A rare loss-of-function genetic mutation suggest a role of dermcidin deficiency in hidradenitis suppurativa pathogenesis. Front Immunol 2022;13:1060547.
Epigenetic modulations are implicated in the disruption of the circadian rhythm inhidradenitis suppurativa
Uppala Radhakrishna1, Uppala Ratnamala2, Aaren Vedangi3, Rakesh Rawal5, Devendrasinh Jhala4, Giovanni Damiani6
1Green Cross Blood Bank, Molecular Diagnoscics, Ahmedabad, India; 2Gujarat University, Department of Life Sciences, Ahmedabad, India; 3ICON Krishi Institute Medical Sciences, Department of Clinical Research, Visakapatnam, India; 4Gujarat University, Department of Zoology, Ahmedabad, India; 5Gujarat University, Department of Biomedical Sciences, Ahmedabad, India; 6University of Milan, Clinical Dermatology, Milan, Italy
Circadian rhythms are 24-h cycles of physical, mental, and behavioural changes that affect sleep patterns and other biological mechanisms. Genetic and epigenetic factors affect the internal molecular feedback loops that determine circadian rhythms. Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease with multifactorial causes, including genetics, epigenetics, and immunology, associated with multiple comorbidities. To study the methylation profiles of circadian clock genes associated with HS susceptibility in blood DNA from HS patients, we analysed 24 HS cases and 24 healthy controls using the Infinium MethylationEPIC BeadChip array coupled with detailed bioinformatics and statistical methods. A total of 339 differentially methylated CpG sites were identified, encompassing 339 circadian clock genes including 272 hypomethylated, 67 hypermethylated CpGs. The disruption of circadian rhythms affects a number of rhythms, including sleep–wake rhythms and mental health disorders. SIGLEC5, RORA, CLUAP1, POLR1E and BIRC3 are the top five genes associated with HS. Gene Ontology analysis showed that 20 canonical signalling pathways related to HS with HS gene enrichment at probability values ≤0.01 were: Circadian rhythm, Wnt signalling pathway, Pathways in cancer, insulin secretion, Hedgehog signalling pathway. These multiple CpG associated with circadian rhythms may help improve the HS therapeutical management refraining its progression. Furthermore, interventions targeting circadian rhythm may act synergically with both surgery and clinical therapies to improve the overall patient outcome.
Unique B cell signature differentiates hidradenitis suppurativa from other inflammatory skin diseases psoriasis and atopic dermatitis
Conor Smith1, Roisin Hambly2,3, Solene Gatault3,4, Luis F. Iglesias-Martinez4, Sean Kearns6, Helen Rea3,5, Vivien Marasigan2,5, Kate Lynam-Loane6, Shivashini Kirthi2, Rosalind Hughes2, Jean M. Fletcher8,1, Walter Koch4,9, Brian Kirby2,3
1Trinity College Dublin, School of Biochemistry & Immunology, Trinity Biomedical Sciences Institute, Dublin, Ireland; 2St Vincent's University Hospital, Department of Dermatology, The Charles Centre, Dublin, Ireland; 3University College Dublin, Charles Institute of Dermatology, Dublin, Ireland; 4University College Dublin, Systems Biology Ireland, Dublin, Ireland; 5St Vincent's University Hospital, Clinical Research Centre, Dublin, Ireland; 6St Vincent's University Hospital, Clinical Research Centre, Dublin, Ireland; 7St Vincent's University Hospital, Clinical Research Centre, Dublin, Ireland; 8Trinity College Dublin, School of Medicine, Dublin, Ireland; 9University College Dublin, Conway Institute of Biomolecular and Biomedical Research, Dublin, Ireland
Acknowledgement: C. Smith likes to thank the British Society of Immunology for providing travel funding to attend the EHSF 2023 conference.
Characterization of an ex-vivo skin model of hidradenitis suppurativa: Pharmacological validation and evaluation of HS endotypes. HIDRASMEX-I study (hidra skin model ex-vivo-I)
Núria Riera-Martí1, Carla Carcasona2, Lluís Boix2, Ana Blanco2, Marta Calbet2, Amadeu Gavaldà2, Jorge Romaní3
1Consorci Sanitari Parc Taulí, Department of Dermatology, Sabadell, Spain; 2Almirall R&D Center, Barcelona, Spain; 3Hospital General de Granollers, Department of Dermatology, Granollers, Spain
Hidradenitis suppurativa (HS) is a chronic and inflammatory skin disease of the hair follicle which lacks reliable in vitro and in vivo models for research on pathogenesis and therapeutics. Potential ex-vivo human models using lesional HS and control healthy skin have been proposed,1 which intends to re-create the in vivo situation. HS treatment is challenging and should ideally take into account different therapeutic approaches for the phenotypes or endotypes that have been described.2 In a collaborative study with Almirall R&D Department, we established and validated a previously described ex-vivo skin model for HS, to characterize endotypes in terms of gene expression and potential pharmacological treatments. Twenty-three lesional skin samples were obtained from HS patients with chronic active disease (Hurley stage II or III) and representing the two endotypes described in the disease by González-Manso et al.2 after a clustering analysis. The day after surgery, 4 mm punch biopsies of the samples were placed in punched-out holes in a transwell membrane of a 12-well plate with the epidermis exposed to air and the dermis immersed in culture media. After that, the skin biopsies were cultured for 24 h at 37°C 5% CO2 atmosphere, and prednisolone and secukinumab (anti-IL17A) were added separately to the culture media. Gene expression in skin homogenates was analysed using an array card including 24 genes. The control group was healthy tissue samples obtained from breast or abdominal reduction surgeries. Additionally, cryosections were obtained for histological examination to prove evaluate skin integrity. HS skin samples showed an increase in gene expression of IFN gamma, IL-17A, IL-17F, IL-17C, IL-22, CXCR5, IGHG1, IGHG3, IGHM, IL1b, IL21, IL36A, IL6, CXCL8, IL-19, IL23A, IL36G, COL1A1 and CXCL13. High variability was observed between individuals. Additionally, our study also showed that prednisolone and secukinumab significantly inhibited gene expression of various cytokines in lesional HS skin. The anti-inflammatory effect of prednisolone was observed for IL17-A, IL-17F, IL-1β, CXCL8, IL-6, IL-19, IL-36A and IL-22, which are important cytokines in the pathogenesis of HS. Secukinumab also demonstrated significant downregulation of inflammatory markers related with IL-17 signalling pathway, specially IL19 and IL36A. When considering gene expression depending on the HS endotype, lesion site and HS severity, data showed no major differences in terms of gene expression. In this study, HS lesional skin showed an increase in gene expression of several markers which could be relevant in disease pathogenesis. High variability was observed between individuals increasing the number of samples required to obtain significant results in the model. No major differences between endotypes were observed with the analysed gene expression panel. Prednisolone at 10 μg/mL and secukinumab at 200 nM reduced expression of several markers. All these data are consistent with the clinical data reported in the literature, which allows the model to be used in the validation of therapeutic hypothesis.
- Vossen ARJV et al. The anti-inflammatory potency of biologics targeting tumour necrosis factor-α, interleukin (IL)-17A, IL-12/23 and CD20 in hidradenitis suppurativa: an ex vivo study. Br J Dermatol 2019;181:314–23.
- González-Manso A et al. Hidradenitis Suppurativa: Proposal of Classification in Two Endotypes with Two-Step Cluster Analysis. Dermatology 2021;237:365–71.
Extensive lymphocyte clonal expansion occurs in the tertiary lymphoid structures in hidradenitis suppurativa
Catherine P.-J. Lu
New York University, New York, USA
Hidradenitis suppurativa (HS) is a severe chronic inflammatory disease of human apocrine gland-bearing skin. Treatment of HS is extremely challenging due to limited understanding of the critical inflammatory mechanisms underpinning its pathogenesis. The mechanisms responsible for sustaining the chronic inflammation and rapid recurrence after surgical removal or biologics treatment remain elusive. Using single cell and spatial transcriptomics, we demonstrate the presence of tertiary lymphoid structures (TLSs) with clearly defined B and T cell zones and that the lymphocyte proliferation occurs exclusively in TLSs adjacent to the tunnels in HS lesions. Further, through V(D)J clonal analyses, we showed that polyclonal expansions of IL17A+ and IFNG+ cells occur predominantly in the TLSs, as well as that extensive clonal expansion plasma cells sharing common VHJH usage across patients systemically circulate in the body. We further identified the distinct populations of inflammatory fibroblasts that provide critical cytokines for the formation of TLSs and exhibit features similar to fibroblastic reticular cells (FRCs) and marginal reticular cells (MRCs) in the secondary lymphoid organs. Mechanistically, we show that HS lesional fibroblasts can be stimulated to upregulate cytokines to recruit immune cells and that complex immune-mesenchymal interactions contribute to their local aggregation. Our work provides significant advance in understanding the local immune milieu that sustained the chronic inflammation in HS.
Acknowledgement: The authors are grateful for the support from New York University Hansjörg Wyss Department of Plastic Surgery and Sanofi Innovation Award.
Pilot study of gene expression profiling of skin in hidradenitis suppurativa patients after adalimumab treatment
Elisabetta Botti1, Mariavittoria Cannizaro1, Maria L. Musumeci2, Elena Campione1, Giuseppe Micali2, Luca Bianchi1
1Tor Vergata University Hospital, Department of Dermatology, Rome, Italy; 2University of Catania, Department of Dermatology, Catania, Italy
Hidradenitis suppurativa (HS) is a chronic cutaneous inflammatory disease. The immunopathogenesis is a complex and not fully clarified. Anti-tumour necrosis factor, adalimumab is the only biological theraphy approved for therapeutic use. The mechanisms of anti-TNF-α in lesional HS skin are not fully understood. We performed a pilot study to investigate events in skin from HS patients after treatment with adalimumab by use of bioinformatics tools. We used the Affymetrix Human GeneChip Clariom S to analyse gene expression in biopsies taken from lesional (L) and no lesional (NL) HS skin before and 3 months after initiation of treatment with adalimumab and healthy normal skin (HN). We found that 4000 genes were differentially expressed between L and HN skin and 388 genes between pre treatment and post treatment lesional skin. Using the lists of differentially expressed transcripts with fold change 1.5, we performed an enrichment analysis for the Gene Onthology categories, in order to identify particular Molecular Functions, or Biological Processes that occur with a higher frequency than expected. Comparing post ADA lesional and Pre ADA lesional skin we found that principal differentially expressed genes belong to processes linked to the immune response, diapedesis and also to the cellular matrix organization.
Immunological profile in patients with severe hidradenitis suppurativa: Evolution after biological treatment
Cristina Ciudad Blanco1, Ofelia Baniandrés Rodríguez1, Rebeca Kennedy Batalla2, Rafael Correa Rocha2
1Hospital General Universitario Gregorio Marañón, Dermatology, Madrid, Spain; 2Hospital General Universitario Gregorio Marañón, Immuno-Regulation Laboratory, Madrid, Spain
Patients with hidradenitis suppurativa present alterations in innate and acquired immunity, which manifest with elevations of certain cell populations and interleukins.1-5 After treatment with biologic drugs, changes in the immunological profile occur.1 The objective of the study is to characterize the immunological profile of patients with severe hidradenitis suppurativa and to study the response of the immune system using flow cytometry after biological treatment. This is a prospective observational study comparing a cohort of patients with severe hidradenitis suppurativa and healthy controls. The immunological study includes the plasma concentration of cytokines (IL-1β, 2, 4, 6, 10, 12, 13, 17A, IFN-γ, TNF-α, GM-CSF), the characterization of the leukocyte populations (leukocytes, granulocytes, monocytes, NK cells, dendritic cells, NKT, CD4, CD8 lymphocytes, double positive, double negative, B cells) and in vitro study of functionality and cellular response.5 Patients between 18 and 40 years of age, with normal weight and naive to biological treatment will be included. Samples have been obtained from 5 patients and 5 controls. The highest responses are Th1 in all patients, Th9 was also greatly increased in 2 patients and Th17 in 5, although it was always less activated than Th1. In all of them, the greatest expression of cytokines is due to an increase in TNF-α and IFN-γ, in 2 patients the increase in IL-2 was also very marked. In 2 patients, the response of the innate immune system was increased with an increase in neutrophils and NK and NKT cells. In the other 3 patients, the innate immune system was much less activated. After 1 month of treatment with adalimumab (160 mg induction in week 0, 80 mg in week 2 and 40 mg weekly from week 4), Th1 levels were reduced, while Th2 and Th17 responses continued to increase. TNF-α production was reduced by 50% and there was an increase in IL-10 production by CD4 and CD8 cells. In patients with inflammation of the innate immune system, there was a reduction in the number of circulating granulocytes, neutrophils, NK cells, and NKTs. The 5 included patients have shown clinical improvement after treatment with adalimumab, reaching HiSCR at 12 weeks. The study continues to include more patients and perform measurements at 6 and 12 months of treatment, so it is too early to draw conclusions. All patients present significant elevations of TNF-α and IFN-γ, which are significantly reduced after a month of treatment. But they are very non-specific data, more studies are needed to know which pathways are altered and how they are modified after biological treatment.
- Lowe M et al. Immunopathogenesis of hidradenitis suppurativa and response to anti-TNF-alfa therapy. JCI Insight 2020;25:e139932.
- Raimondo et al. Immunopathogenesis of chronic inflammatory skin diseases: novel molecular targets and biomarkers. Life 2022;12:891.
- Nguyen T et al. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol 2021;35:50–61.
- Vellaichamy G et al. Insights from gamma-secretase: functional genetic of hidradenitis suppurativa. J Invest Dermatol 2021;141:1888–96.
- Thomi R et al. Association of hidradenitis suppurativa with T Helper 1/T Helper 17 Phenotypes: a semantic map analysis. JAMA Dermatol 2018;154:592–95.
Metformin restores immune and metabolic dysregulation in hidradenitis suppurativa
Andreea Petrasca1, Roisin Hambly2, Niamh Kearney2, Aoife O'Rourke1, Conor Smith1, Mohamed Ismaiel3, Czara Kennedy3, Alexandra Zaborowski3, Desmond Winter3, Brian Kirby2, Jean M. Fletcher1
1Trinity College Dublin, School of Biochemistry & Immunology, Trinity Biomedical Sciences Institute, Dublin, Ireland; 2St. Vincent's University Hospital, Department of Dermatology, Dublin, Ireland; 3St Michael's Hospital, Department of Surgery, Dublin, Ireland
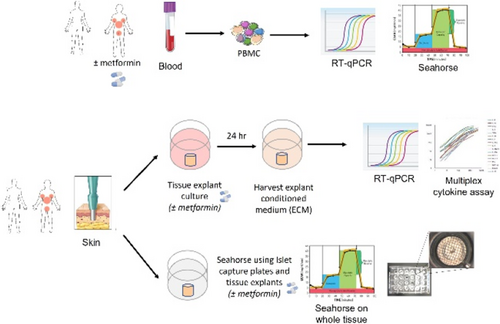
FIGURE 1 Methods. We isolated PBMC from the blood of healthy individuals, untreated HS patients and metformin-treated HS patients and carried out RT-qPCR and Seahorse metabolic assays. Furthermore, we used skin biopsies to set up 24 h explant cultures in the presence or absence of ex vivo metformin and carried out RT-qPCR, Seahorse analysis and multiplex cytokine assays before and after metformin treatment.
Skin barrier alterations are not a characteristic feature of hidradenitis suppurativa
Krisztian Gaspar1, Orsolya Somogyi1,6, Zsolt Dajnoki1,2, Lilla Szabó1,6, Zoltán Hendrik3, Christos C. Zouboulis4, Klaudia Dócs5, Péter Szűcs5, Katalin Dull1,2, Dániel Törőcsik1,2, Anikó Kapitány1,2, Andrea Szegedi1,2
1University of Debrecen, Department of Dermatology, Faculty of Medicine, Debrecen, Hungary; 2University of Debrecen, ELKH-DE Allergology Research Group, Debrecen, Hungary; 3University of Debrecen, Department of Pathology, Faculty of Medicine, Debrecen, Hungary; 4Brandenburg Medical School Theodor Fontane, and Faculty of Health Sciences Brandenbrg, Staedtisches Klinikum Dessau, Departments of Dermatology, Venereology, Allergology and Immunology, Dessau, Germany; 5University of Debrecen, Department of Anatomy, Histology and Embryology, Faculty of Medicine, Debrecen, Hungary; 6University of Debrecen, Petrányi Gyula Doctoral School, Debrecen, Hungary
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease of the apocrine gland-rich (AGR) skin regions with a well-characterized Th1/Th17 cytokine milieu. Keratinocytes (KCs) seem to be the crucial drivers of the initial pathogenic steps of HS. However, the possible role of permeability barrier alterations in activating KCs during the development of HS has not been clarified. We compared the permeability barrier of non-lesional HS (HS-NL) and lesional HS (HS-L) skin with healthy AGR regions and investigated the major permeability barrier elements at the mRNA (RT-qPCR) and protein (immunohistochemistry) levels. Stratum corneum (SC) components related to cornified envelope formation (FLG, LOR, KRT1, KRT10, TGM5), corneocyte desquamation (KLK5, KLK7, KLK14), and (corneo)desmosome organization (DSG1, DSC1, PKP1, CDSN) were analysed along with tight junction (TJ) molecules (CDH1, CLDN1, OCLN) and barrier alarmins (KRT6A and KRT16). Permeability barrier function was also investigated via transepidermal water loss (TEWL) measurements. The distribution of junction structures was visualized using confocal microscopy. At the gene level, none of the investigated molecules were significantly altered in HS-NL skin, while 11 molecules were significantly altered in HS-L skin, compared with healthy AGR skin. At the protein level, the investigated molecules in HS-NL skin were similar to healthy AGR skin, while in HS-L skin only slight, and inconsequential differences (KRT1 and KLK5 decreased, KLK7, KRT6, and DSG1 increased) were detected. No significant differences in TEWL or in the expression of junction structures could be observed. The absence of differences in HS-NL and the only slight barrier alterations in HS-L skin suggest that the permeability barrier is not significantly damaged in HS skin and permeability barrier alterations are not the driver factors of KCs' activation in this disease.
Acknowledgement: The ELKH-DE Allergology Research Group, Department of Dermatology, Faculty of Medicine, University of Debrecen is a Centre of Excellence for Hungarian Academy of Science. The Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin – ALLOCATE Skin group).
Neutrophils and the IL-17 axis play a key pathological role in hidradenitis suppurativa
Matthew Page, Hannah Edwards, Remi Okoye, Stevan Shaw
UCB Pharma, Slough, UK
Hidradenitis suppurativa (HS) is a painful and complex inflammatory skin disease, characterized by dense neutrophilic infiltration of the dermis and extensive pathogenic tissue remodelling, leading to formation of abscesses, fistulas and tunnels.1 The aetiology and pathobiology of the disease is yet to be fully elucidated with only tumour necrosis factor (TNF) and interleukin-17 (IL-17) pathways confirmed as pathological drivers of HS. Other pathways thought to drive HS pathobiology include the inflammasome-related cytokine IL-1 that is upregulated in HS lesional tissue.2 The objective of the study was to understand the role of IL-17A and IL-17F in promoting HS pathogenesis. A combination of immunohistochemistry staining and RNA-seq of HS lesional and non‑lesional tissue was used to explore the roles of IL-17A and IL-17F expression in HS pathobiology. Human complex in vitro functional assays were utilized to investigate the effects of IL‑17A and IL-17F inhibition. RNA-seq was also conducted on skin biopsies taken at baseline in a phase 2 study, with additional biopsies taken after 12 weeks of bimekizumab treatment (640 mg at Week 0, then 320 mg every 2 weeks). In HS lesional tissue, both IL-17A and particularly IL-17F were elevated compared with non-lesional skin and were predominantly restricted to the dermis. An associated influx of neutrophils was observed in the deep infiltrate of HS lesions with up‑regulation of genes that induce neutrophil migration (CXCL8), neutrophil activation (CSF3), and broad effects on neutrophil biology, including IL1B. Monogenic defects in the IL-1 pathway lead to neutrophilic dermatoses.3,4 Using functional human assays, inhibition of IL-17A and IL-17F reduced inflammatory genes associated with neutrophil biology and inhibited neutrophil migration to a greater extent than IL-17A alone. In patients with HS achieving HiSCR50 (≥50% reduction in total abscess and inflammatory nodule count, with no increase from baseline in abscess or draining tunnel count) at Week 12 of a phase 2 study, gene sets affecting neutrophil-related functions, including neutrophilic chemokines, were significantly suppressed after treatment with bimekizumab. Although IL-17A and IL-17F have overlapping biology, recent publications suggest that expression of these isoforms are differentially regulated by STAT5-inducing cytokines.5 We extended these findings to show that inflammasome-related cytokines, such as IL-1, also strongly enhanced IL-17F production by Th17 cells and innate lymphocytes. Together these data support the hypothesis that both IL-17A and IL-17F play a central role in this neutrophilic dermatosis and the presence of IL-1 may partly explain the high expression of IL-17F in lesional HS tissue.
- Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med 2012;366:158–64.
- Sabat R et al. Hidradenitis suppurativa. Nat Rev Dis Primers 2020;6:18.
- Aksentijevich I et al. An autoinflammatory disease with deficiency of the interleukin-1–receptor antagonist. N Engl J Med 2009;360:2426–37.
- Kolivras A et al. Histologic patterns and clues to autoinflammatory diseases in children: What a cutaneous biopsy can tell us. Dermatopathology (Basel) 2021;8:202–20.
- Cole S et al. Interleukin (IL)-12 and IL-18 synergize to promote MAIT cell IL-17A and IL-17F production independently of IL-23 signalling. Front Immunol 2020;11:585134.
Role of gamma-secretase pathway in the pathogenesis of hidradenitis suppurativa and its correlation with Alzheimer's disease
Elisa Molinelli1, Daniela Passarella2, Caterina Licini3, Oriana Simonetti1, Francesca Paparesta4, Donatella Brancorsini4, Gaia Goteri4, Monica Mattioli Belmonte3, Claudio Russo2, Carola Porcile2, Annamaria Offidani1
1Polytechnic University of Marche, Department of Clinical and Molecular Sciences, Dermatologic clinic, Ancona, Italy; 2University of Molise, Department of Medicine and Health Sciences, Campobasso, Italy; 3Polytechnic University of Marche, Department of Clinical and Molecular Sciences, Histology institute, Ancona, Italy; 4Polytechnic University of Marche, Department of Biomedical Sciences and Public Health, Institute of Pathological Anatomy and Histopathology, Ancona, Italy
Hidradenitis suppurativa (HS) is a chronic, debilitating, inflammatory cutaneous disease, which is clinically characterized by painful skin nodules, abscesses, draining sinuses, and disfiguring scars on the intertriginous areas. It is associated with several comorbidities (inflammatory bowel disease, arthritis, type I and II diabetes mellitus, lipids/atherosclerosis/adipogenesis and cancer risk) and it is characterized by significant morbidity, functional impairment, and poor quality of life.1 The aetiology of HS is still unclear. Thirty-four unique mutations in patients with HS have been described, mainly involving the gamma secretase–Notch pathway. It is known that mutations in gamma-secretase component particularly in two presenilin genes (PSEN1 and PSEN2) cause early-onset familial Alzheimer's disease (FAD).2–5 The aim of our research was to characterize HS gamma secretase mutants compared to FAD mutants and to test their activity over different substrates. We evaluated in vitro the presence and processing of PSEN1 in the gamma-secretase complex, and the activity of wild type versus mutated (FAD and HS) gamma-secretase over different substrates. We also analysed by immunohistochemistry samples and fresh tissue samples from patients affected by sporadic HS and familial HS, using healthy skin as control, looking at gamma-secretase complexes as well as signatures derived from substrate's fragments. We report our preliminary results investigating the role of the gamma-secretase pathway in the pathogenesis of HS.
- Zouboulis CC et al. What causes hidradenitis suppurativa? 15 years after. Exp Dermatol 2020;29:1154–70.
- Esme P et al. Increased prevalence of family history of Alzheimer's disease in hidradenitis suppurativa: Cross-sectional analysis of 192 HS patients. Dermatol Ther 2020;33:e14219.
- Barthelson K et al. PRESENILIN 1 Mutations Causing Early-Onset Familial Alzheimer's Disease or Familial Acne Inversa Differ in Their Effects on Genes Facilitating Energy Metabolism and Signal Transduction. J Alzheimers Dis 2021;82:327–47.
- Li A et al. Analysis of hidradenitis suppurativa-linked mutations in four genes and the effects of PSEN1-P242LfsX11 on cytokine and chemokine expression in macrophages. Hum Mol Genet 2019;28:1173–82.
- Pink AE et al. γ-Secretase mutations in hidradenitis suppurativa: new insights into disease pathogenesis. J Invest Dermatol 2013;133:601–7.
An insight into the microbic world of hidradenitis suppurativa
Gianmarco Silvi, Elia Rosi, Antonella Di Cesare, Francesca Prignano
University of Florence, Department of Health Sciences, Section of Dermatology, Florence, Italy
Hidradenitis suppurativa (HS) is a chronic inflammatory disease affecting the apocrine gland bearing areas of the skin. The role of microbic agents in HS is still very controversial. The main hypothesis is that skin microbic flora may trigger and maintain chronic inflammation in HS lesions. Indeed, in HS the follicular plugging and rupture of follicular epithelium may induce the spreading of bacteria into the dermis. Bacterial antigens may stimulate the activation of keratinocytes and macrophages, with consequent release of pro- and anti-inflammatory cytokines and chemokines. Moreover, the efficacy of antimicrobial drugs in HS, such as clindamycin or tetracyclines, supports the relevance of bacterial colonization.1,2 Studies concerning the bacteriology of HS are very heterogeneous, in terms of methodology and data interpretation; thus, results are often conflicting. Isolates from HS lesional samples are frequently polymicrobial, with concomitant presence of both skin commensal and possibly pathogenic species. Among aerobic species, S. Aureus seems to be associated with late-stage HS lesions, as well as with smoking.3 S. lugdunensis is an aerobic Coagulase Negative Staphylococcus (CNS) that is found frequently in HS advanced suppurating lesions and may play a role as a pathogen.4 Anaerobic species isolated from HS lesional samples consist in S. epidermidis (Gram positive CNS) and Gram negatives of the Bacteroidetes family, including Prevotella spp, which may often be detected in HS tunnels.5
In general, anaerobic species seem to be prevalent in early HS lesions, while inflammatory nodules and abscesses are often colonized by both anaerobic and aerobic bacteria. Furthermore, there may be a correlation between the type of bacteria isolated and the localization of HS lesions. For example, species of the Enterobacteriaceae family are frequently detected in the gluteal and inguinal regions.3 The microbic landscape of HS is very complex and still largely unknown. However, a systematic categorization of the different microbial profiles detected in HS lesions, may have significant implications for a more personalized therapeutical approach.
- Matusiak Ł. et al. Bacteriology of Hidradenitis Suppurativa – Which Antibiotics Are the Treatment of Choice? Acta Derm Venereol 2014;94:699–702.
- Hessam S. et al. Microbial Profile and Antimicrobial Susceptibility of Bacteria Found in Inflammatory Hidradenitis Suppurativa Lesions. Skin Pharmacol Physiol 2016;29:161–7.
- Nikolakis G. et al. Bacterial Colonization in Hidradenitis Suppurativa/Acne Inversa: A Cross-Sectional Study of 50 Patients and Review of the Literature. Acta Derm Venereol 2017;97:493–8.
- Guet-Revillet H. et al. Bacterial Pathogens Associated with Hidradenitis Suppurativa, France. Emerg Infect Dis 2014;20:1990–8.
- Ring HC. et al. The Microbiome of Tunnels in Hidradenitis Suppurativa Patients. J Eur Acad Dermatol Venereol 2019;33:1775–80.
Modulation of hydrogen sulfide in hidradenitis suppurativa
Christina Damoulari, Theodora Kanni, Panagiotis Koufargyris, Konstantina Katrini, Dimitra Stergianou, Konstantinos Leventogiannis, Evangelos J. Giamarellos-Bourboulis
National and Kapodistrian University of Athens, 4th Department of Internal Medicine, Athens, Greece
- Renieris G et al. Serum hydrogen sulfide and outcome association in pneumonia by the SARS-CoV-2 coronavirus. Shock 2020;54:633–7.
- Manandhar S et al. Hydrogen sulfide and its interaction with other players in inflammation. Adv Exp Med Biol 2021;1315:129–59.
Modulation of gut microbiome in hidradenitis suppurativa
Theodora Kanni, Emmanuil Stylianakis, Christina Damoulari, Sofia Ktena, Dimitra Stergianou, Georgia Damoraki, Evangelos J. Giamarellos-Bourboulis
National and Kapodistrian University of Athens, 4th Department of Internal Medicine, Athens, Greece
Neutrophil-derived B-cell activating factor supports B/plasma-cell persistence and function in skin lesions in hidradenitis suppurativa/acne inversa
Robert Sabat1, Deimante Simaite2, Johann E. Gudjonsson3, Theresa-Charlotte Brembach1, Torben Krause1, Gabriela Salinas4, Katrin Witte1, Georgios Kokolakis1, Christos Nikolaou1, Thomas Leeuw2, Béma Coulibaly7, Clément Levin7, Kamran Ghoreschi6, Natascha Rill1, Matthias Herrmann2, Eckart Bartnik2, Hans-Dieter Volk5, Kerstin Wolk1
1Charité – Universitätsmedizin Berlin, Psoriasis Research and Treatment Center, Dermatology/Medical Immunology, Berlin, Germany; 2Sanofi-Aventis Deutschland GmbH, Frankfurt, Germany; 3University of Michigan Medical School, Department of Dermatology, Ann Arbor, USA; 4University Medical Center Göttingen, NGS-Integrative Genomics Core Unit, Institute of Human Genetics, Göttingen, Germany; 5Charité - Universitätsmedizin, Berlin, Institute of Medical Immunology, Berlin, Germany; 6Charité - Universitätsmedizin, Berlin, Department of Dermatology, Venereology and Allergology, Berlin, Germany; 7Sanofi Aventis Recherche Developpement, Molecular Histopathology & Bio-Imaging, R&D, Vitry-sur-Seine, France
Hidradenitis suppurativa (HS), also referred to as acne inversa, is a chronic-inflammatory disease, characterized by painful inflamed nodules, abscesses, and pus-draining fistulas appearing in the skin of axillary, inguinal, and perianal areas.1 Medicamentous treatment options for HS remain very limited, with the anti-tumour necrosis factor-alpha antibody adalimumab as the only approved systemic treatment so far. HS lesions contain a heterogeneous infiltrate of immune cells. However, little was known so far about the mediators that support the activity and long-term persistence of the immune cells in the lesions. This study aimed to characterize mediators supporting the B/plasma-cell in the HS skin lesions. For this purpose, the following methods were applied: Skin samples from several cohorts of HS patients and different control cohorts were assessed by RNA-sequencing, RT-qPCR, flow-cytometry, and immunohistofluorescence. Blood and cultured skin biopsies, keratinocytes, dermal fibroblasts, neutrophilic granulocytes (neutrophils), monocytes, and B-cells were used for mechanistic studies. Complex systems biology approaches were applied to evaluate bulk and single-cell RNA-sequencing data. Our results showed that the proportions of B/plasma cells, neutrophils, CD8+T cells, M0 and M1 macrophages were elevated in HS lesions compared to skin of healthy intertriginous and perilesional areas. There was an association between B/plasma cells, neutrophils, and B-cell activating factor (BAFF). BAFF was abundant in HS lesions, particularly in nodules and abscesses. Among the cell types in HS lesions, myeloid cells were the main BAFF producers. Mechanistically, granulocyte colony-stimulating factor in the presence of bacterial products was the major stimulus for neutrophils' BAFF secretion. Lesional upregulation of BAFF receptors was attributed to B (TNFRSF13C) and TNFRSF13B) and plasma cells (TNFRSF17). Characterization of the lesional BAFF pathway revealed numerous molecules involved in migration/adhesion (e.g., CXCR4, CD37, CD53, SELL), proliferation/survival (e.g., BST2), activation (e.g., KLF2, PRKCB), and ROS production (e.g., NCF1, CYBC1) of B/plasma cells. Correspondingly, upregulation of a selection of these elements in HS lesional skin was demonstrated in HS lesional skin compared to control skin of healthy donors and patients with other inflammatory skin diseases and could be reversed by ‘therapeutic’ BAFF neutralization ex vivo. Furthermore, increased intercellular adhesion and SELL (L-Selectin) expression was confirmed in isolated, BAFF-exposed B-cells. In summary, neutrophil-derived BAFF seems to support B/plasma-cell persistence and function in HS lesions and might represent a new promising therapeutic target for HS. Since biological drugs that inhibit the BAFF activity are already approved (belimumab) or under clinical development for other medical indications (ianalumab, blisibimod), there is hope of a timely evaluation of these drugs in view of the reduction of the profound medical need that exists for HS.
Acknowledgement: The authors thank Véronique Pécheux, Annette Buss, and Malte Rozmarynowicz for excellent technical assistance and Rose K.C. Moritz for help in interpreting histological alterations of HS lesions. Different parts of this study were partly supported by grants from Sanofi-Aventis Deutschland GmbH in the scope of the strategic master project of Charité, BIH, and Sanofi-Aventi.
- Sabat R et al. Hidradenitis suppurativa. Nat Rev Dis Primers 2020;6:18.
Value of blood biomarkers of systemic inflammation in patients with hidradenitis suppurativa
Valdemar W. Nielsen1, Nikolaj Holgersen1, Hans Christian Ring1, Alexander Egeberg1,2, Jacob P. Thyssen1,2, Simon F. Thomsen1,3
1Bispebjerg Hospital, Department of Dermato-Venereology & Wound Healing Centre, Copenhagen, Denmark; 2University of Copenhagen, Department of Clinical Medicine, Copenhagen, Denmark; 3University of Copenhagen, Department of Biomedical Sciences, Copenhagen, Denmark
Hidradenitis suppurativa (HS) is a systemic inflammatory disease but little is known about biomarkers of disease severity. We investigated whether blood biomarkers of systemic inflammation were associated with disease severity and explored risk factors for elevated levels of inflammation biomarkers. A total of 662 adult outpatients with HS from a dermatological university department were included. Demographic factors, body mass index (BMI), comorbidities, and biomarkers in blood were examined. Disease severity was measured by Hurley stage and Hidradenitis Suppurativa Score (HSS). We calculated the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), monocyte/lymphocyte ratio (MLR), platelet/neutrophil ratio (PNR), pan-immune-inflammation value (PIV) and systemic inflammation index (SII). SII was calculated as neutrophil count x platelet count/lymphocyte count. The median age was 38.7 years (IQR 28.3–51.2) and 62.8% were female. A total of 36.6%, 49.8%, and 13.6% of patients had Hurley stage I, II and III, respectively. The median HSS was 16 (IQR 9–30.3). Several inflammatory markers correlated positively with HSS, including C-reactive protein (CRP) (r = 0.392, p < 0.001), erythrocyte sedimentation rate (ESR) (r = 0.437, p < 0.001), NLR (r = 0.16, p < 0.001), MLR (r = 0.11, p = 0.004), PIV (r = 0.28, p < 0.001), and SII (r = 0.213, p < 0.001). PNR correlated negatively with HSS (r = 0.224, p < 0.001). PLR did not correlate with HSS (r = 0.008, p = 0.847). SII was significantly higher in patients with comorbidities, including diabetes (955.7 vs. 750.7, p = 0.023), inflammatory bowel disease (IBD) (1081.4 vs. 733.8, p < 0.001), hypertension (1006.6 vs. 736.1, p < 0.001), and in smokers (796.9 vs. 664.6, p = 0.025). We found no difference in SII among patients with inflammatory arthritis (751.5 vs. 767.4, p = 0.94) or obesity (747.6 vs. 782.8, p = 0.49). Conclusions: Several blood biomarkers of systemic inflammation correlated with disease severity in patients with HS, especially CRP and ESR. These biomarkers can aid in the assessment of the systemic burden of severe HS disease and to detect comorbidities.
Hidradenitis suppurativa-like epidermis model
Isabel Haferland1, Andreas Pinter1, Tanja Roßmanith2, Sandra Diehl1, Claudia Bürger1, Tanja Ickelsheimer1, Roland Kaufmann1, Anke König1
1University Hospital Frankfurt am Main, Department of Dermatology, Venereology and Allergology, Frankfurt, Germany; 2Fraunhofer, Institute for Translational Medicine and Pharmacology ITMP, Frankfurt, Germany
Hidradenitis Suppurativa (HS) is a chronic inflammatory skin disease of the sebaceous gland unit. The initial manifestation usually occurs after puberty, with the highest disease activity between 30 and 40 years of age. Inflammatory nodules, abscesses, and even fistula tracts form at the predilection sites. Causal treatment options are lacking due to incompletely understood pathophysiology. However, based on research, some pathophysiological processes have already been elucidated, including the involvement of various immune cells and cytokines or fistula tract formation1. HS research comprises in vitro, ex vivo, and clinical studies. In general, in vitro experiments use primary cells from the skin or blood of HS patients2. To conduct ex vivo studies, punch biopsies, called explants, are cultured in a transwell system. Among the cell culture models, this system most accurately reflects the native tissue3. Besides, regarding animal models, there is a discussion about whether previously established models for other diseases are suitable for research in HS4. The epidermis model is another in vitro model used in research for other inflammatory diseases5. As far as we know, no epidermis model has yet been developed for HS. However, epidermis models will represent an excellent addition to the already established ex vivo explants for studying biomarkers or drugs. Therefore, we examined whether primary keratinocytes from lesional HS skin form multi-layered epidermis models. We cultured keratinocytes submersed in a transwell system and lifted them to the air-liquid interface to induce differentiation. After culturing, we evaluated the HS epidermis models according to differentiation markers and inflammatory patterns. Immunohistochemical staining suggested differentiation similar to native tissue. Further, we detected the level of the pro-inflammatory cytokines IL-1β and TNF-α in the medium of the epidermis models.
- Sabat R et al. Hidradenitis suppurativa. Nat Rev Dis Primer 2020;6:18.
- Jones D et al. Inherent differences in keratinocyte function in hidradenitis suppurativa: Evidence for the role of IL-22 in disease pathogenesis. Immunol Invest 2018;2;47(1):57–70.
- van der Zee HH et al. Adalimumab (antitumour necrosis factor-α) treatment of hidradenitis suppurativa ameliorates skin inflammation: an in situ and ex vivo study. Br J Dermatol 2012;166:298–305.
- van der Zee HH et al. Can Animal Skin Diseases or Current Transgenic Mice Serve as a Model for Hidradenitis Suppurativa? Dermatology 2012;225:9–13.
- Moon S et al. In Vitro Models Mimicking Immune Response in the Skin. Yonsei Med J 2021;62:969.
Optimized human skin organ-culture conditions for pre-clinically exploring the impact of candidate therapeutics on hidradenitis suppurativa samples ex vivo
Joana R. Viola1, Markus Fehrholz1, Karin I. Pappelbaum1, Tamas Biro1, Thomas Meyer2, Falk G. Bechara2, Sylke Schneider-Burrus3, Ralf Paus1, Janin Edelkamp1, Marta Bertolini1
1Monasterium Laboratory, Skin and Hair Research Solutions GmbH, Münster, Germany; 2Klinik für Dermatologie, Venerologie und Allergologie, St. Josef Hospital, Bochum, Germany; 3Center for Dermatosurgery, Havelklinik, Berlin, Germany
Given the severe impairment of affected patients' quality of life, the effective management of hidradenitis suppurativa (HS) remains a major unmet medical need. While the primary pathobiology of this eminently chronic inflammatory skin disease, which starts as an infundibulitis, remains unclear, clinically relevant preclinical research platforms are urgently needed to evaluate the efficacy of novel candidate HS therapeutics. Several lesional HS skin organ culture assays have been reported, but are poorly characterized. Therefore, we have sought to standardize and characterize HS skin organ culture conditions for the ultimate goal to use it as a preclinical research and drug testing tool. Full-thickness 4 mm punches from lesional (nodule- or fistula-containing) and peri-lesional skin were collected from HS patients undergoing surgery and organ-cultured under different conditions for 24, 48 or 72 h. Initially, vehicle or adalimumab (the anti-TNFa inhibitor) were administred to HS lesional samples containing fistula in a serum free medium ex vivo. Transcriptomic prolifing after 24 h was performed by RNAseq, while tissue integrity and T-cell numbers were assessed after 48 hrs in organ culture perilesional and nodule-containing lesional punches by Haematoxylin & Eosin (H&E) immunohistochemistry and CD3/CD4/CD8 immunofluorescence microscopy. RNAseq analysis under serum-free culture conditions showed that adalimumab treatment decreases the transcription of HS-relevant alarmins and antimicrobial peptides (e.g., S100A7A/A9, CAMP, DEFB4A), and other HS biomarkers, in particular LCN2 and IL17C. However, tissue integrity was rapidly lost within 48 h of organ culture, even though T-cells were still detectable in peri-lesional and lesional skin samples. To increase tissue viability, different culture conditions were explored, i.e. different media supplements, including human serum, and a period of serum starvation to avoid interaction of serum components with administered therapeutic antibodies. 24, 48, or 72 h organ-cultured samples were compared to non-cultured HS samples to depict the preservation of disease phenotype ex vivo, using histochemistry or immunofluorescence microscopy to evaluate skin integrity as well as protein expression of CD3 (T-cells) CD66b (neutrophils), CD19 (B-cells), Keratin 15, Keratin 17, Lipocalin2 (LCN2), and ELISA for determining CCL20 release into the medium. This showed that HS-associated immune phenotype, CCL20 release into the culture medium from fistula-containing lesional versus peri-lesional samples, and other HS disease activity markers were best preserved over 72 h in organ-cultured lesional HS samples using a defined, standardized, serum-supplemented proprietary medium. A short period of serum starvation at the initiation of organ culture, when a drug would need to be administered ex vivo, did not impair skin integrity or HS phenotype, thus providing a therapeutic window in which biologics can be administered in absence of serum. Therefore, we reveal here standardized conditions that permit organ-culture of HS peri- and -lesional punches for at least 72 h without impairing tissue integrity, and that CCL20 release into the medium can serve as a sensitive and quantifiable marker for evaluating disease activity. Our optimized and standardized assay system is ideally suited for transcriptomic, proteomic, and immunopathology analyses in situ for probing the efficacy of novel candidate HS therapeutics under clinically relevant conditions ex vivo as well as for dissecting mechanisms of drug action and for identifying biomarkers that hep to distinguish responder from non-responder HS patients.
Serum amyloid A: A potential new marker of severity in hidradenitis suppurativa
Michela Iannone1, Giorgia Salvia1, Cristian Fidanzi1, Matteo Bevilacqua1, Agata Janowska1, Riccardo Morganti2, Marco Romanelli1, Valentina Dini1
1University of Pisa, Department of Dermatology, Pisa, Italy; 2University of Pisa, Department of Clinical and Experimental Medicine, Section of Statistics, Pisa, Italy
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease with systemic inflammation and high impact on quality of life. Treatment strategies are still inadequate with a lack of inflammation biomarkers. We conducted a prospective study to assess the correlation between Serum Amyloid A (SAA) levels and active lesion count; disease severity; Dermatology Life Quality Index (DLQI); smoking; BMI and lesion sites. Forty-one patients (M/F: 22/19) were enrolled. Demographic, clinical, laboratory, and therapeutic data were assessed at baseline on patients not under treatment or in wash-out from systemic treatment for at least 2 weeks. Associations were investigated by univariate and multivariate analyses. SAA levels were significantly associated with number of nodules (p = 0.005), abscesses (p < 0.001), fistulas (p = 0.016) and severe IHS4 (p = 0.088 and r = 0.514). Gluteal localization was correlated with high values of mSartorius and severe IHS4. We recommend assessment of SAA levels to monitor therapeutic response in patient with HS in order to prevent disease's flare and potential complications.
Introduction of large language models for biomarker detection in hidradenitis suppurativa – Chances and risks
Viktor A. Zouboulis1,2
1Universitaetsklinikum Hamburg-Eppendorf (UKE), Faculty of Medicine, Hamburg, Germany; 2European Hidradenitis Suppurativa Foundation e.V., Dessau, Germany
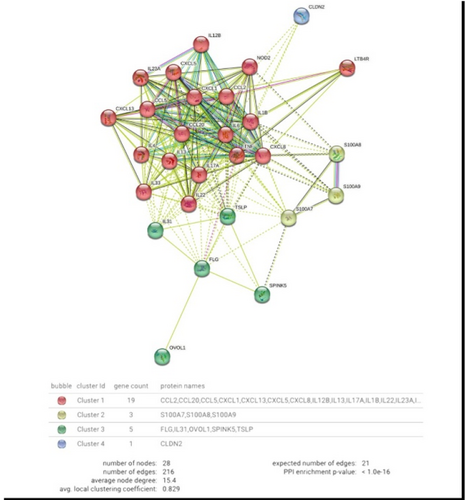
FIGURE 1 Artificial Intelligence in HS research. String-DB protein network of the 60 (28 unique) by ChatGPT suggested biomarkers for HS, AD, Crohn's disease, ulcerative colitis, RA, psoriasis; network nodes represent proteins; edges represent protein–protein associations; k-means clustering with 4 clusters.
- McDermott JE et al. Challenges in biomarker discovery: combining expert insights with statistical analysis of complex omics data. Expert Opin Med Diagnostics 2013;7:37–51.
- ChatGPT. Optimizing Language Models for Dialogue, 30.11.2022. https://openai.com/blog/chatgpt/ (Accessed: 11 January 2023).
- Zouboulis VA et al. Hidradenitis Suppurativa and Comorbid Disorder Biomarkers, Druggable Genes, New Drugs and Drug Repurposing—A Molecular Meta-Analysis. Pharmaceutics 2022;14:44.
- Szklarczyk D et al. The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 2021;49:D605–12.
- Singhal K et al. Large Language Models Encode Clinical Knowledge. Nature 2023 Jul 12. doi: 10.1038/s41586-023-06291-2.
The polygenic architecture of hidradenitis suppurativa revealed by a first meta-analysis GWAS
Atlas Khan1, Lam C. Tsoi2, Johann E. Gudjonsson3, Errol P. Prens3, Theodore G. Drivas4, Marylyn D. Ritchie4, Amir Hossein Saeidian5, Hákon Hákonarson5, Nick Dand6, Jonathan Barker6, Michael Simpson6, Jake Saklatvala6, Brian Kirby7, Kelsey R. van Straalen2, Lynn Petukhova1, The Hidradenitis Suppurativa Genetics Consortium
1Columbia University, New York, USA; 2University of Michigan, Ann Arbor, USA; 3Erasmus University Medical Center, Rotterdam, Netherlands; 4University of Pennsylvania, Philadelphia, USA; 5Children's Hospital of Philadelphia, Philadelphia, USA; 6King's College London, London, UK; 7University College Dublin, Charles Institute of Dermatology, Dublin, Ireland
The Hidradenitis Suppurativa Genetics Consortium (HSGC) was founded in 2021. Our mission is to use human genetic studies as a starting point to discover hidradenitis suppurativa (HS) disease mechanisms, to identify and prioritize drug targets, and to improve the accuracy and utility of an HS diagnosis. Here, we present the first results of this large collaborative model, in which we perform a meta-analysis of five HS GWAS containing data for 1667 cases and 215 033 controls. No locus exceeded our stringent threshold for genome-wide significance (p < 5 × 10−8). This confirms our initial findings, presented at the 9th Conference of the EHSF, in which an HS GWAS of 600 cases and 80 000 controls indicated that HS is not an autoimmune disorder and that its genetic architecture lacks large-effect disease alleles1. Our meta-analysis results similarly empirically demonstrate that resolving the polygenic architecture of HS will require samples sizes of at least 2000 to 4000 cases. Ultimately, tens of thousands of participants from diverse genetic ancestries will be needed to generate clinically meaningful genetic evidence and polygenic risks scores, which will require cooperation and coordination among many diverse stakeholders2. Therefore, the HSGC is committed to respectful engagement of all stakeholders, easy and streamlined collaboration requiring only summary statistics generated with standardized protocols, open sharing of data, and widespread dissemination of results. We have already on-boarded additional collaborators and our next meta-analysis is expected to contain at least an additional 2500 HS cases, exceeding our immediate goal of 4000 cases.
- 9th Conference of the European Hidradenitis Suppurativa Foundation, 5–7 February 2020, Athens, Greece. Experimental dermatology. 2020;29(S1):36.
- Jabbour A et al. Clinical translation of hidradenitis suppurativa genetic studies requires global collaboration. Br J Dermatol 2022;186:183–5.
Inflammatory and fibrosis related gene expression patterns in hidradenitis suppurativa lesions
Saba Khoshbakht1, Selin Kolsuz2, Ayşenur Botsalı3, Ercan Çalışkan3, Seçil Vural4
1KOC University, Research Center for Translational Medicine (KUTTAM), Istanbul, Turkey; 2KOC University, School of Medicine, Istanbul, Turkey; 3University of health sciences, Department of Dermatology, Gulhane Faculty of Medicine, Kecioren, Turkey; 4KOC University, School of Medicine, Department of Dermatology, Istanbul, Turkey
Candidate variants in genes involved in immune response to microbes and in inflammatory pathways in hidradenitis suppurativa with associated features
Snaigune Miskinite1, Maia Delage2, Olivier Join-Lambert3, András N. Spaan4, Xavier Mariette5, Nicolas Picard6, Emmanuelle Bourrat7, Jean-Laurent Casanova4, Aude Nassif2, Alain Hovnanian4
1University Paris Cité, INSERM UMR1163, Laboratory of Genetic Skin Diseases, Imagine Institute, Paris, France; 2Institut Pasteur, Paris, France; 3France 3- Centre Hospitalo-universitaire de Caen, Caen, France; 4Rockfeller Institute, New York, USA; 5Bicêtre Hospital, Department of Rheumatology, Kremlin-Bicêtre, France; 6University Paris Cité, Imagine Institute, Paris, Germany; 7Saint-Louis Hospital, Department of Dermatology, Paris, France
Hidradenitis Suppurativa (HS) is a frequent, chronic and inflammatory dermatosis that presents with nodules, abscesses, sinus tracts and fistulas in apocrine gland-bearing areas. The disease is responsible for a very heavy burden with a high unmet medical need. The lack of clear understanding of the pathogenesis is a major obstacle to improve treatment. The existence of familial cases (30%–42%) and the identification gamma-secretase complex gene mutations in a small subset of families support the genetic basis of HS. We previously established a prevalence of gamma-secretase gene mutations in a large European cohort of 188 HS patients at 6.4%, suggesting a large genetic heterogeneity. Here we report 8 non-related patients presenting with HS and associated features, who carry rare variants (MAF < 10−5) likely to be pathogenic in genes involved in antibacterial activity, innate immunity and inflammatory pathways. We analysed 201 familial and sporadic cases from an active cohort of 1600 HS patients. We used targeted next generation sequencing (NGS) with an in-house custom panel in a majority of patients, including all known GSC genes and 74 candidate genes involved in the Notch pathway, in autoinflammation and in autoimmunity. We identified 8 distinct rare variants, all of which except one are heterozygous, in 8 unrelated patients. A rare missense variant in the RNF213gene was identified in one patient with HS (Hurley II) and acne fulminans. A frameshift variant in IFIH1 was found in one familial case of HS (Hurley stage III) and Crohn disease and a missense variant in the same gene was identified in one sporadic case of SAPHO with HS and acne fulminans. A frameshift variant in IRF2BP2 was detected in one family with PASH syndrome (including acne fulminans). A missense variant in OTULIN was identified in a patient with HS and Crohn's disease. A homozygous frameshift variantin LACC1 was found in a patient affected with PAPASH syndrome. Finally, whole exome sequencing identified a heterozygous missense variant in NLRP1 in one sporadic case affected with HS (Hurley stage II), pachyonychia congenita and multiple sclerosis; and a large hemizygous deletion removing several exons of the XIAP gene in one male patient affected with HS, Crohn, pyoderma gangrenosum and familial Mediterranean fever.
The single nucleotide substitutions which we identified are predicted to be damaging by in silico tools (Polyphen2/Sift/CADD) and/or are located in critical domains of the corresponding proteins. Variants in IFIH1, IRF2BP2 and LACC1 are predicted to be damaging because they lead to a frameshift resulting in a premature stop codon. The IFIH1 and IRF2BP2 variants co-segregate with the disease phenotype in the corresponding families. The NLRP1 variant identified in a sporadic case is absent from all healthy members of the patient's family. Co-segregation study in the remaining 6 families is on-going. The rare variants we report occur in genes playing a pivotal role in antibacterial autophagy (RNF213 encoding an E3 ubiquitin ligase), PAMPS recognition (IFIH1 or MDA5), NF-kB regulation (OTULIN), NOD2 pathway (LACC1 and XIAP), type I interferon signalling (IRF2BP2, encoding a co-repressor of IRF2) and the inflammasome (NLRP1). Pathogenic variants in these genes have previously been identified in other auto-inflammatory diseases including severe Crohn disease (IFIH1), common variable immunodeficiency syndrome (IRF2BP2), rheumatoid and juvenile arthritis (LACC1), OTULIN-related auto-inflammatory syndrome (ORAS) (OTULIN), multiple sclerosis (NLRP1) and in inborn errors of immunity. We hypothesize that the variants that we report could either confer an increased susceptibility to HS or even underlie monogenic forms of syndromic HS. Functional studies are required to support their possible implication in HS, as well as testing additional HS patients with and without associated features for variants in these genes and related pathways. These findings expand the spectrum of genes potentially involved in HS pathogenesis and support the notion that HS is a highly genetically heterogeneous disease involving genes playing a role in immune response to microbes.
Posters – Epidemiology and Registries
Epidemiology and comorbidity of hidradenitis suppurativa in Korea for 17 years: A Korean nationwide population-based cohort study
Ji Hae Lee2, Jong Won Lee1, Yeon-Woo Heo1, Solam Lee1
1Yonsei University Wonju College of Medicine, Department of Dermatology, Wonju, South Korea; 2St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Department of Dermatology, Suwon, South Korea
Hidradenitis suppurativa (HS) is a chronic autoinflammatory skin condition and is associated with several comorbidities. Previous studies report variable prevalence rates of HS, depending on the methodology. However, the exact prevalence remains unknown and the majority of published studies included largely Caucasian cohorts. Epidemiological data on hidradenitis suppurativa (HS) for Asian populations are limited. This study aimed to analyse the change in the prevalence and incidence of HS over 17 years in South Korea using the Korean National Health Insurance Service (NHIS) database. This study also evaluated the comorbidities in patients with HS.
Population-based data from the NHIS database of Korea were obtained between January 2003 and December 2019. The incidence rate was calculated for patients with HS per 100 000 people-year. Odds ratios were estimated to determine the association between comorbidities and HS during the study period. Hazard ratios for the risk of incident comorbidities were obtained using the Cox regression model. A total of 45 511 patients with HS and 910 220 controls matched for age, sex, insurance type, and income level were included. The patients with HS were predominantly male (62.83%), and the mean age was 36.68 ± 18.63 years. The incidence rate of HS per 1 000 000 person-years in Korea increased from 11.69 in 2003 to 78.78 in 2019. The annual prevalence per 1 000 000 people also increased from 34.68 in 2003 to 140.10 in 2019, showing a similar trend. The incidence of HS tended to increase in both sexes. Many comorbidities, including atopic, metabolic and end-organ, autoimmune/inflammatory, and psychiatric diseases were associated with HS at baseline. Allergic diseases, hypertension, diabetes mellitus, dyslipidemia, myocardial infarction, chronic hepatitis and cirrhosis, chronic kidney disease, inflammatory bowel diseases, rheumatoid arthritis, vitamin D deficiency, and psychiatric diseases, were associated with HS. The incidence and prevalence of HS in Korea have increased over the past 17 years and patients with HS have an increased prevalence and risk of comorbidities. Physicians need to keep in mind and closely monitor these comorbidities in patients with HS.
Updated findings from the global hidradenitis suppurativa COVID-19 registry
Haley B. Naik1, Raed Alhusayen2, Sandra Guilbault3, John Ingram4, Michelle A. Lowes5, Angelo V. Marzano6,7, Bente Villumsen8, Christine Yannuzzi9
1University of California, San Francisco, Dermatology, San Francisco, USA; 2University of Toronto, Medicine, Toronto, Canada; 3Hope for HS, Troy, USA; 4Cardiff University, Dermatology & Academic Wound Healing, Cardiff, UK; 5The Rockefeller University, New York, USA; 6Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Dermatology, Milan, Italy; 7Università degli Studi di Milano, Pathophysiology and Transplantation, Milan, Italy; 8Patientforeningen HS Danmark, Copenhagen, Denmark; 9HS Warriors, Port Richey, USA
As the pandemic persists and novel COVID-19 variants emerge, dermatologists balance the benefit of achieving effective hidradenitis suppurativa (HS) control with biologics therapy with their potential morbidity and mortality risk in the context of comorbidities associated with HS and severe COVID-19. The objective of this study was to determine whether biologic use predicted COVID-19 outcomes in patients with HS over the course of the COVID-19 global pandemic. We examined data from self-reported entries to the Global HS COVID-19 Registry from 4/5/2020–9/14/2022. Eligible cases had health care provider (HCP)-confirmed diagnosis of HS, HCP-confirmed diagnosis of COVID-19 based on a positive test or symptom screening questions, and at least 20% survey completion. COVID-19 severity was assessed by hospitalization and oxygen support requirement. Threshold dates used for COVID-19 vaccine availability, delta variant strain dominance and omicron variant strain dominance were 12/14/20, 6/1/21 and 9/26/21, respectively. HS flares were self-reported. Data were compared with Fisher's exact and Pearson χ2. Multivariable regression adjusted for demographics and comorbidities. 397 self-reported entries were included in the study. 16.1% (64/397) respondents reported biologic use at the time of COVID-19 infection. 1 death was reported into the registry. There was no difference in adjusted odds of hospitalization (aOR: 1.01, 95% [CI 0.26, 3.87], P = 0.16) between respondents on biologics versus not, but patients on biologics more frequently required respiratory support (aOR:1.66, 95% [CI 0.60, 4.6], P = 0.03). Patients using biologics more frequently reported HS exacerbation with COVID-19 infection (biologic: 56.3%; nonbiologic: 32.1%, P < 0.001), and more frequently reported other COVID-19 complications (59.4% vs. 42.6%, P = 0.01), including shortness of breath. This association was not observed for patients treated with only disease modifying anti-rheumatic drugs, antibiotics, or if biologics were discontinued in the setting of COVID-19 infection. There were no differences in hospitalization (P = 0.22) or respiratory support (P = 0.66) based on continuation of biologics with COVID-19. COVID-19 vaccination status was reported in 176 entries. 86% (152/176) of respondents received at least one dose of a COVID-19 vaccine, 51% (78/152) of whom reported receiving at least one booster dose. The most reported vaccines among respondents included: Pfizer (48%, 73/152), Moderna (26%, 39/152), and AstraZeneca (14%, 22/152). While there was no difference in odds of hospitalization after COVID-19 vaccine availability (aOR = 0.49, 95% CI [0.16, 1.52], P = 0.1), respondents had lower odds of requiring oxygen assistance (aOR = 0.49, 95% CI [0.16, 1.52], P = 0.03) after COVID-19 vaccines became available. Similarly, there was no difference in odds of hospitalization after the delta (aOR = 0.15, 95% CI [0.25, 2.22], P = 0.1) and omicron (aOR = 0.33, 95% CI [0.88, 1.25], P = 0.06) variants became the dominant global COVID-19 strains. However, respondents reported higher odds of requiring oxygen assistance after delta (aOR = 1.15, 95% CI [0.46, 2.90], P = 0.03) and lower odds after omicron (aOR = 0.90, 95% CI [0.35, 2.31], P = 0.04) variant dominance. Respondents did not report any difference in HS flare frequency pre- and post-COVID-19 vaccine availability (aOR = 1.18, 95% CI [0.71, 1.96], P = 0.17), however, flares were more frequently reported after delta (aOR = 1.61, 95% CI [1.04, 2.51], P < 0.05) and omicron (aOR = 2.1, 95% CI [1.36, 3.26], P < 0.005) variant dominance. After COVID-19 vaccines became available, the majority of respondents reported COVID-19 vaccination and booster. While HS patients on biologics were more likely to require oxygen support in the setting of COVID-19 infection, that risk decreased after COVID-19 vaccine availability. Hospitalization and respiratory support requirement did not differ between those who continued versus discontinued biologics in the setting of COVID-19 infection. Delta and omicron variants were not associated with an increased risk of hospitalization, although delta variant was associated with increased risk of oxygen support requirement. HS flares were more frequently reported in patients on biologics and after both the delta and omicron variants became dominant strains worldwide. Our findings from the largest international cohort of HS patients with COVID-19 offer meaningful support to provide COVID-19 vaccination and continue biologic therapy for HS treatment.
Acknowledgement: The authors are grateful to the people with HS who contributed data to registry, and the Hidradenitis Suppurativa Foundation and Canadian Hidradenitis Suppurativa Foundation for continued funding support. We would also like to acknowledge Dr. Nancy K. Hills for her assistance with statistical analyses, and Maia Paul, Margaret Kudlinski and Hannah Balter for administrative assistance with registry management.
Prevalence of hidradenitis suppurativa in Poland
Klaudia P. Knecht-Gurwin1, Łukasz Matusiak1, Jacek C. Szepietowski1, Gregor B.E. Jemec2, Dorra Bouazzi2
1Wroclaw Medical University, Department of Dermatology, Venereology, and Allergology, Wroclaw, Poland; 2Zealand University Hospital, Department of Dermatology, Roskilde, Denmark
Hidradenitis suppurativa (HS) is a persistent, detrimental disorder afflicting apocrine glands regions of the skin. The skin alterations embrace solitary, painful, deep-seated inflamed nodules, abscesses and bridged scars. The pathophysiology of this entity encompasses magnitude of interactions between inflammatory, genetic and environmental factors1. HS epitomizes an instance of a very debilitating ailment that instigates a significant depletion of quality of life. Allegedly, 7 years passes for the diagnosis to be established2. Undoubtedly, the mentioned above diagnostic delay and, as a result, the delay of the implementation of the proper treatment contribute to widespread outbreaks of the illness. Untreated disease leads to the occurrence of intricate complications, such as metabolic syndrome, cardiovascular disorders and immune-mediated inflammatory disorders (IMIDs). Robust data of the prevalence of HS remains inconsistent. The meta-regression analysis revealed an overall prevalence of 0.4%. The reported point prevalence of HS worldwide is between 0.0003% and 4.1%.3 This glaring discrepancy is presumably elicited by heterogeneous measurement methods and populations being studied. Registry-based studies denote a low prevalence of HS, whereas survey studies demonstrate a substantially higher prevalence in the range of 1%–2%. The highest prevalence of 4.1% was based on an in-person examination by dermatologists among young adults in Denmark. The study conducted in Germany put forward the incidence of HS as 0.3%. According to a survey study carried out in the UK, HS exhibits a 0.77% point prevalence. Moreover, Australian clinicians have utilized a validated HS-screening questionnaires, thereby estimating prevalence of HS at 0.67%.4 Unravelling the incidence of HS is still yet to be elucidated. The aim of our study was to expound on and subsume the prevalence of HS in Polish population. Initial epidemiologic aspects revealed by the registration of patients in the public healthcare data shed some light on the incidence of HS in Poland. Scrutinizing the data of National Health Fund, in the period of 2014 to 2016, there were registered the number of 367, 373 and 440 HS patients in the consecutive years. The estimated prevalence indicated 0,001%. This data significantly differs from those mentioned above. Seemingly, the disparity stems from imprecisely translated the ICD-10 code of this disorder as “multiple abscesses of axillary apocrine sweat glands”. Furthermore, detectability and awareness of HS among dermatologist in Poland seems to be high, but they are not the only physicians that patients refer to. In the study we have conducted, participants were persons accompanying patients administered to hospital. A questionnaire created by the center coordinating international cooperation was utilized. Individuals who have been tested positive with the screening questionnaire (containing questions about common HS symptoms, sex, age, BMI) underwent a physical examination by a dermatologist. The representative group was estimated at 385 people. The size of the study group was 930 people. People who, according to the questionnaire, could potentially suffer from HS, underwent a physical examination to verify the initial assessment. In addition, 10% of participants who did not report symptoms were tested to determine the false-negative rate. 13/930 respondents (1.4%; 95% CI 0.64%–2.15%) were diagnosed with HS. Women constituted 38.46% of patients and men 61.54%. The mean age at diagnosis was 38 years for women and 36.88 years for men. The average BMI was 26.55 kg/m2 in women and 29.61 kg/m2 in men. According to the Hurley classification, lesions of Hurley I severity were diagnosed in 7 patients and Hurley II in 6 patients. In women, HS lesions occurred more frequently in many locations (most often on the buttocks - 80%) and in men in 1 or 2 locations (most often in the genital area - 50%). Data from the National Health Fund apprehend HS as a seldom entity, with the prevalence of 0.001%. The results of the current study show a significant underestimation of the prevalence of this disease. Presumably, it alludes to the fact that this disorder is not correctly diagnosed among various clinicians. The expected outcome of the study is a better understanding of the prevalence of HS. In consequence, the insufficiency in the diagnostic pathway of HS, especially in the early detection and treatment, may be reduced. More comprehensive approach would enhance detectability and awareness of HS, thereby leading to earlier treatment implementation and preventing the complications that may occur due to the diagnostic delay.
Acknowledgement: The Department of Dermatology, Zealand University Hospital, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Saunte DML, Jemec GBE. Hidradenitis Suppurativa: Advances in Diagnosis and Treatment. JAMA 2017;318:2019–32.
- Lee EY et al. What is hidradenitis suppurativa? Can Fam Physician 2017;63:114–20.
- Jemec GB et al. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996;35(2 Pt 1):191–4.
- Jfri A et al. Prevalence of Hidradenitis Suppurativa: A Systematic Review and Meta-regression Analysis. JAMA Dermatol 2021;157:924–31.
Characterizing hidradenitis suppurativa in arabs: Analysis of two cohorts in Israel
Jen A. Barak Levitt1, Shira Barmatz5, Shira Fisch5, Yoss Taieb5, Adam Dalal5,3, Dana Tzur6,8, Arnon D. Cohen2,4, Daniel Mimouni5,3, Emmilia Hodak5,3, Shany Sherman5,3
1Ha'Emek Medical Center, Department of Dermatology, Afula, Israel; 2Clalit Health Services, Department of Research and Information, Chief Physician Office, Clalit Health Services, Tel Aviv, Israel, Tel Aviv, Israel; 3Tel Aviv University, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel, Tel viv, Israel; 4Ben-Gurion University of the Negev, Siaal Research Center for Family Medicine and Primary Care, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel, Beer Sheva, Israel; 5Rabin Medical Center, Division of Dermatology, Rabin Medical Center – Beilinson Hospital, Petach Tikva, Israel, Petach Tikva, Israel; 6Tel Aviv University, Department of Behavioural Sciences, Ariel University, Ariel, Israel, Tel Aviv, Israel; 7Sheba Medical Center, Department of Dermatology, Sheba Medical Center, Tel HaShomer, Ramat Gan, Israel, Tel Hashomer, Israel; 8Shalvata Mental Health Center, Shalvata Mental Health Center, Hod Hasharon, Israel
FIGURE 1 Treatments by ethnic group (Institution-based cohort). The landscape of treatments for hidradenitis suppurativa (HS) based on the institutional cohort, according to ethnicity. For each treatment administered, the relative percentage of Arab patients (cyan) and Jewish patients (blue) is presented. The dashed line refers to the relative percentage of each ethnic group in the population of HS patients. The asterisks represent statistical significance of p < 0.05.
Prevalence and epidemiology of patients with hidradenitis suppurativa in the Faroe Islands
Amanda T. Brosbøl1, Dorra Bouazzi1, Gregor B.E. Jemec1,2, Ditte M.L. Saunte1,2
1Zealand University Hospital Roskilde, Department of Dermatology, Roskilde, Denmark; 2Landssjukrahusid, Dermatology Clinic, Torshavn, Faroe Islands; 3Faculty of Health Science, University of Copenhagen, Department of Clinical Medicine, Copenhagen, Denmark
Hidradenitis Suppurativa (HS) is a chronic, painful inflammatory skin condition1. Patients develop suppurating nodules and/or abscesses which can lead to the formation of tunnels and scarring2. There is a knowledge gap on the prevalence and the epidemiology of the disease in many countries, which leads to delayed diagnosis and treatment initiation. The study objective is to estimate the prevalence of HS in the Faroe Islands, and to describe the epidemiological characteristics of the included HS patients. A retrospective registry-based study determining the prevalence of HS by national registers covering the entire population of the Faroe Islands. The sample cohort consists of a registry including all patients referred to a dermatologist. Demographic/clinical data and history of treatment were extracted to a predefined Excel sheet by two investigators (AB & DB) separately for quality control. Frequencies were calculated and data were tested for normality using the Shapiro–Wilk test. In cases where no Hurley stage was recorded and photos of the lesions were available, the Hurley stage was identified by (DB&DMS) using the Hurley classification. A total of 45 patients diagnosed with HS were identified based on the main population of 48.865 people. The prevalence of HS was estimated to 0.1%, and 78% were women. The average age was 33 years, with a diagnostic delay of 10 years. Approximately, a third (29%) were overweight (mean BMI 34.1) and 16 (36%) were smokers. The majority of the patients (64%) had Hurley stage II, 24% Hurley stage I, and 4% Hurley III, respectively. Thirty-six (80%) patients were affected in the groin and 30 (66%) were affected in the axilla. Twenty-one (47%) of the patients had received treatment before referral to a dermatologist, with a systemic antibiotic as the most common treatment. All patients except one received a topical treatment after diagnosis. The most frequently used systemic treatment after diagnosis was a systemic antibiotic (tetracyclines represented 60%, and a combination of rifampicin/clindamycin represented 29%). One (2%) patient had received adalimumab. The number of shifts in systemic treatment was calculated with a median of 1 (range 0–5). Six (13%) patients had performed surgical procedures. The prevalence of HS in the national registries on the Faroe Islands is 0.1%. The diagnostic delay of 10 years indicates a low awareness of the diagnosis among healthcare providers. A characterization of HS patients on the Faroe Islands may contribute to earlier diagnosis, treatment initiation, and awareness of HS.
Acknowledgement: The Department of Dermatology, Zealand University Hospital, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Jfri AH et al. Hidradenitis Suppurativa: Comprehensive Review of Predisposing Genetic Mutations and Changes. J Cutan Med Surg 2019;23:519–27.
- Zouboulis CC et al. Hidradenitis Suppurativa/Acne Inversa: Criteria for Diagnosis, Severity Assessment, Classification and Disease Evaluation. Dermatology 2015;231:184–190.
Prevalence of hidradenitis suppurativa: Results from the french team of the GHiSA project
Samantha Gaspard1, Ines Evain1, Myriam Fioretta1, Christelle Enault1, Sylvia Math1, Anne-Sophie Gadat1, Marion Clerbout1, Christelle Chavrier1, Marianne Beuque1, Virginie Vlaeminck-Guillem3,4, Philippe Guillem1,2
1Clinique du Val d'Ouest, Department of Surgery, Ecully, France; 2RésoVerneuil, Paris, France; 3CHU, Department of Biology, Lyon, France; 4Lyon 1 University, INSERM 1052, CNRS 5286, CRCL, Lyon, France
The exact prevalence of hidradenitis suppurativa (HS) remains controversial. In France, a figure of 1% is usually used based on a 2008 study. The GHiSA (Global Hidradenitis Suppurativa Atlas) is a collaborative international study proposing a standardized approach to evaluate HS prevalence in more than 50 centers around the world. It proposes a validated screening questionnaire and objective validation of the findings in the screened population of adults accompanying patients. The aim of the study was to assess the prevalence of HS in France using the standardized methodology of the GHiSA project. A validated screening questionnaire was prospectively proposed to healthy adults accompanying patients coming to the Clinique du Val d'Ouest (Lyon, France) for hospitalization or for consultation at the Médicentre (a consultation centre attached to the Clinique du Val d'Ouest). Two screening questions were asked: “Do you have recurrent boils of the skin?” and “Have you for the past 6 months had 2 or more boils/abscesses in any of the locations” typically affected in HS. Data gathering stopped when the pre-specified number of 500–1000 accompanying persons has been reached. In addition to the GHiSA methodology, adult patients were also proposed the same questionnaire during the same period. Positive accompanying persons and patients were clinically examined. Correlations between eventual HS diagnosis and clinical characteristics were assessed using univariate and multivariate analysis. Between March and November 2022, we collected 525 accompanying adults and 560 patients. 25 accompanying adults (5.5%) answered at least yes or unsure to the screening questions. Out of them, HS diagnosis was eventually made for 18 subjects (3.4% of the cohort; 95% CI: 1.9–5.0%). A similar prevalence was observed in the patients cohort: 3.6% (95% CI: 2.0–5.1%). Out of the 28 subjects with eventual HS diagnosis, 15 (39%) said they had already been diagnosed. The 28 HS-diagnosed subjects had lesions in the inguinal (n = 26, 68%), axillary (n = 17, 45%), gluteal (n = 14, 37%), genital (n = 10, 26%) and submammary (n = 8, 21%) areas. They were classified as Hurley I (n = 22, 58%) and Hurley II (n = 16, 42%). Except for the age, the two cohorts were not different for the other baseline characteristics (gender, BMI, tobacco smoking) and therefore pooled to explore risk factors for HS diagnosis. The proportion of subjects with HS diagnosis was higher in women (4.3% vs. 2.1%, NS) and smokers (8.3% vs. 2.3%, p < 0.001). No link was observed with neither BMI (as a continuous variable), nor overweight or obesity (binary variables). A younger age was associated with a higher HS risk. Multivariate analysis confirmed the lack of influence of gender and BMI and showed smoking and age as independent predictors of HS diagnosis. This explorative, cross-sectional, descriptive study, based on a prospective recruitment discloses an unexpected high prevalence of HS (3.4%) in a cohort of patients-accompanying adults. The similar prevalence observed in the other cohort (patients, 3.6%) reinforces this result. No Hurley III disease was diagnosed suggesting that most of undiagnosed patients in the general population have mild to moderate disease. The reasons underlying the significant increase when comparing to the previously published French study remain to be determined; potentially in the light of comparisons with other GHiSA project centres. They potentially include greater freedom of speech and, in a related way, better dissemination of information on the disease (grouping of patients in associations, explosion of social networks, information campaigns, improved primary and secondary training of healthcare providers), which has led to an apparent increase. However, an increase in the risk factors for the disease cannot be ruled out. In this respect, although the role of tobacco is well confirmed by this study, its consumption has been decreasing in France since 2014 (date from which national data are available) and cannot explain the increased prevalence of HS. In the HS patient population, an F/M sex ratio of 3–4 is usual and men are known to have more severe forms. It was therefore not expected that female sex would not have a predictive role on the risk of HS. This implies that the population that comes to consult the referral centres may not be representative: men in general may be under-represented, whereas there may be an over-representation of severe forms among the men who consult. In the French centre in Lyon, the prevalence of HS is 3.4% in a cohort of accompanying adults. It is 3.6% in a parallel cohort of patients. Young age and smoking status (but not gender or BMI) were independent predictors of a diagnosis of HS. Further studies are needed to understand the (apparent?) increase in the prevalence of HS in France.
Epidemiology and clinical features of adult patients with hidradenitis suppurativa in Germany: Baseline characteristics from the german hidradenitis suppurativa registry HSBest
Frenz Ohm1, Matthias Augustin1,7, Kathrin Gehrdau1, Andreas Pinter2,7, Falk G. Bechara3,7, Dagmar Presser4,7, Maurizio Podda5, Christos C. Zouboulis6,7, Natalia Kirsten1,7
1University Medical Center Hamburg-Eppendorf, Institute for Health Services Research in Dermatology and Nursing (IVDP), Hamburg, Germany; 2University Hospital Frankfurt am Main, Department of Dermatology, Venereology and Allergology, Frankfurt, Germany; 3Ruhr-University Bochum, Department of Dermatology, Venereology and Allergology, Bochum, Germany; 4University Hospital Würzburg, Department of Dermatology, Venereology and Allergology, Würzburg, Germany; 5Hospital Darmstadt, Department of Dermatology, Venereology and Allergology, Darmstadt, Germany; 6Brandenburg Medical School Theodor Fontane and Faculty of Health Sciences Brandenburg, Staedtisches Klinikum Dessau, Departments of Dermatology, Venereology, Allergology and Immunology, Dessau, Germany; 7European Hidradenitis Suppurativa Foundation (EHSF), Dessau, Germany
Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic recurrent, painful, inflammatory skin disease that leads to a significant reduction in quality of life. The German Hidradenitis suppurativa registry HSBest collects real-world data on patient characteristics, disease severity and therapy in the long-term course. The aim was to describe the baseline characteristics collected in the German, prospective, non-interventional HSBest registry. Patients with a minimum age of 18 years and clinically diagnosed HS are enrolled in HSBest. Standardized physician and patient electronic case report forms (eCRF) are obtained at enrolment and every 12 weeks collecting data about clinical and demographic aspects, quality of life and treatment. The analysed baseline data were collected at inclusion visit between March 2020 and July 2022 at the Institute for Health Services Research in Dermatology and Nursing (IVDP) of the University Medical Center Hamburg-Eppendorf. A total of n = 561 patients were included. The majority of participants were female (58.4%) and of Caucasian origin (83.7%), had moderate to severe disease (Hurley stage II or III; 76%), and had a history of surgery for their HS (75.1%). The average registry patient was obese with a BMI of 30.3, and 54.3% of the patients reported regular tobacco smoking. The following prevalences of comorbidities were evident: depression (25.6%), pilonidal sinus (24.4%), inflammatory bowel disease (13.1%), arthritis (13%), psoriasis (10.1%), polycystic ovary syndrome (9.5%) and diabetes (6.1%). In this registry cohort of HSBest, the majority of patients suffered from a moderate to severe disease manifestation combined with severe distress. In addition to disease-specific skin lesions, high prevalences of comorbidities were striking. A large proportion of patients are characterized by a disease-promoting lifestyle, reflected in obesity and nicotine abuse.
Acknowledgement: The Departments of Dermatology, Venereology and Allergology, University Hospital Wuerzburg and the Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin – ALLOCATE Skin group).
Hidradenitis suppurativa reference center in Brazil: 10-year analysis of hospital de clínicas da unicamp, characterizing the profile of patients and treatments
Renata F. Magalhães, Thaís D.S. Martins, Juliana Y. Massuda Serrano
State University of Campinas (UNICAMP), Department of Medical Clinic, Dermatology Division, Campinas, Brazil
Hidradenitis suppurativa affects around 0.41% of the Brazilian population1, but it is believed that the prevalence is higher due to underdiagnosis and lack of recognition of the broad clinical spectrum2. There is a delay in diagnosis and difficulty in accessing treatments, especially in public health services. Unicamp's HS outpatient clinic began in 2003 and today has 168 registered patients. reference in the interior region of the state of São Paulo, Brazil. The objectives of this study were to analyse the profile of patients treated at the dermatology clinic of a tertiary hospital, the most used treatments and the results obtained with the interventions. We analysed 70 medical records of patients with HS who had follow-up data for more than 12 months, treated in the last 10 years at the dermatology outpatient clinic of HC - UNICAMP. The data surveyed were: gender, age, age at disease onset, affected areas, smoking, obesity, associated comorbidities, treatment used and therapeutic responses. To assess the effectiveness of the therapy used, the method used was the IGA (Investigator Global Assessment) on a scale of 0–3, with IGA = 0 total improvement of lesions and IGA3 presence of activity (0 = no complaints, no lesions – 3 = symptomatic disease with a high activity). A reduction in IGA was considered a response to treatment, reaching IGA = 0 or 13. The age of the patients ranged between 16 and 61 years, with a median of 32 years; age at onset of HS between 8 and 49 years, with a median of 18.5 years. The majority (60%) were female. As for severity, according to Hurley's classification, Hurley 1, 12.9%; Hurley 2, 31.43% and Hurley 3, 55.71%. Regarding the areas affected by HS, the most common were: armpits 87.1%; groin/inguinal 74.3%; intergluteal region 25.7%; breast region 17.1%; abdominal region 10%; dorsal region 8.57 and facial region 5.71%. Regarding BMI, 43 cases were evaluated, and it ranged from 17.4 kg/m2 to 58 kg/m2, with a median of 30.96 kg/m2. Almost half had obesity, 51.16% and 26.5% of whom were smokers. Among the other comorbidities evaluated, in order of prevalence: acne (22.86%); diabetes mellitus (18.57%); hypothyroidism (17.14%); pilonidal cyst (15.71%); systemic arterial hypertension (12.86%); joint involvement, mainly spondylitis and sacroiliitis (11.43%); pyoderma gangrenosum (11.43%); trisomy 21 (10%); follicular occlusion triad (5.71%); intestinal involvement (5.71%). Of the 70 patients participating in the study, 51.4% had a good response in at least 6 months of treatment (IGA 0/1). Comparing the antibiotics, sulfamethoxazole-trimethoprim and doxycycline were the most used, especially in moderate–severe cases (Hurley 2 and 3). Sulfamethoxazole-trimethoprim was used in 84.3% of cases and obtained a higher percentage of good response, in 35%; followed by doxycycline in 50% of patients with improvement in 23% of cases. Systemic corticosteroids were used in 35.7%, with improvement in 24% of patients, for a period of one to 12 weeks. Retinoids were used in 37% of patients, 65.4% of which were Hurley 3 with improvement in 34.6% of cases. Isotretinoin was used more often because it treated many cases of young women. Partial or extensive surgery was performed in 45.7% of patients, with significant improvement in 59.4%. Immunobiological treatment was used in 34.3% of the patients, 94% Hurley 3, with adalimumab being the most used. Of those who used this immunobiological, 87.5% had a significant improvement. Other immunobiologicals were used as a second line, such as infliximab, secukinumab and tocilizumab, due to the diagnosis of rheumatoid arthritis, ankylosing spondylitis, Crohn's disease, or due to failure of adalimumab. Data show that patients arrive late at the reference center, as more than half of the cases were Hurley 3. According to the 2019 Brazilian consensus, sulfa was widely used because it is available in the public system and has good results and safety, rifampicin was not used in this cohort of patients, due to its restricted access due to the high prevalence of tuberculosis and risk of mycobacterial resistance. Isotretinoin was indicated in cases of acne and folliculitis dissecans of the scalp, in smaller doses and for a long time; acitretin in cases of multiple cysts and signs of follicular occlusion in men or women without risk of pregnancy; metformin if cases if signs of metabolic syndrome, overweight, diabetes, insulin resistance or hyperandrogenism4. Antibiotics and systemic corticosteroids were widely used in the first approach to moderate Hurley 1 and 2 cases. Immunobiologicals were indicated for the most severe cases of HS, most of which were Hurley 3, showing that patients are arriving late at the reference center. There is a need for new treatment options accessible to patients with HS in Brazil and access to more complex surgery.
- Ianhez M et al. Prevalence of hidradenitis suppurativa in Brazil: a population survey. Int J Dermatol 2018;57:618–20.
- Tricarico PM et al. An integrated approach to unravel hidradenitis suppurativa etiopathogenesis. Front Immunol 2019;10:1–13.
- Garg A et al. Development and initial validation of the HS-IGA: a novel hidradenitis suppurativa-specific investigator global assessment for use in interventional trials. Br J Dermatol 2022;187:203–10.
- Magalhães RF et al. Consensus on the treatment of hidradenitis suppurativa - Brazilian Society of Dermatology. An Bras Dermatol 2019;94(2 Suppl 1):7–19.
Prevalence of crohn's disease among paediatric patients with hidradenitis suppurativa
Nicole Mastacouris, Andrew Strunk, Amit Garg
Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Department of Dermatology, New Hyde Park, USA
Hidradenitis suppurativa (HS) and Crohn's disease (CD) are chronic, recurrent, inflammatory disorders with shared genetic susceptibility and immunologic features. Population-based studies have established the association between CD and HS in adults1,2. Whether a similar association is present among children and adolescents is unclear. Literature on this topic is limited to single-center studies and registries which often lack a general population control group for comparison.3-5 To address this gap, we aimed to compare prevalence of CD among children and adolescents with HS to prevalence in a general population sample in the United States. This was a cross-sectional study of the IBM Explorys database, a multi-health system data analytics and research platform comprised of electronic health records from over 40 healthcare networks across the United States. The study population included patients between ages 5 and 17 years having at least two encounters in the database between January 1, 2015 and December 31, 2019 (period of interest), and at least 6 months of observable person time in the database within this period. Patients with HS were identified by at least one ICD-9 (705.83) or ICD-10 (L73.2) diagnosis code on or prior to December 31st, 2019. The control cohort comprised a 15% random sample of patients in the database without a diagnosis code for HS. Primary outcome was diagnosis of CD during or before the study period, based on at least two diagnosis codes. Prevalence of CD was compared between HS patients and controls using logistic regression. Unadjusted and adjusted models were fit to describe the crude association as well as the influence of potential confounders. Adjusted models included age, sex, race, Medicaid insurance status (proxy for socioeconomic status), body mass index, smoking status, and number of healthcare encounters (to account for surveillance bias). A total of 3003 paediatric patients with HS and 402 947 paediatric control patients met inclusion criteria during the study period. Median (IQR) age in HS patients and controls were 17 (15, 17) and 12 (8, 16) years, respectively. Approximately 82% of paediatric HS patients were female compared to 50% of patients without HS. The majority of patients in each cohort were White (56% in HS, 72% in controls), followed by Black (33% in HS, 14% in controls), Hispanic/Latino (2.0% in HS, 2.6% in controls), and Asian (0.7% in HS, 1.6% in controls). Prevalence of obesity was 58% and 23% among patients with HS and non-HS controls, respectively. Crohn's disease was prevalent in 0.66% of HS patients and 0.11% of control patients (unadjusted OR 6.29; 95% CI 4.01–9.86). In adjusted analysis, odds of CD were 4.54 (95% CI 2.82–7.31) times higher in paediatric HS patients compared to controls. In subgroup analysis among patients aged 15–17 years, prevalence was 0.73% in HS patients and 0.20% in control patients (unadjusted OR 3.63, 95% CI 2.25, 5.86). After adjusting for covariates, odds of CD were 4.78 (95% CI 2.88–7.93) times higher among HS patients aged 15–17 years compared to controls of the same age group. This large population-based analysis describes high strength of association between CD and HS among children and adolescents. The low absolute prevalence of CD in this group should also be taken into context with this association when counselling patients.
- Garg A et al. Overall and Subgroup Prevalence of Crohn Disease Among Patients With Hidradenitis Suppurativa: A Population-Based Analysis in the United States. JAMA Dermatol 2018;154:814–8.
- Schneeweiss MC et al. Occurrence of inflammatory bowel disease in patients with chronic inflammatory skin diseases: a cohort study: Classification: Epidemiology. Br J Dermatol. 2022;187:692–703.
- Liy-Wong C et al. Hidradenitis Suppurativa in the Paediatric Population: An International, Multicenter, Retrospective, Cross-sectional Study of 481 Paediatric Patients. JAMA Dermatol 2021;157:385–91.
- Lloyd-McLennan AM et al. Prevalence of inflammatory bowel disease among paediatric patients with hidradenitis suppurativa and the potential role of screening with faecal calprotectin. Pediatr Dermatol 2021;38:98–102.
- Tiri H et al. Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol 2018;79:514–9.
Prevalence of atherosclerosis in hidradenitis suppurativa on coronary computed tomography angiography
Niamh Kearney1,2, Brian O'Riordan3, Rosalind Hughes1,5, Collette McCourt2, Donal O'Kane2, Jonathan Dodd3,4, Brian Kirby1,5
1St. Vincent's University Hospital, Department of Dermatology, Dublin, Ireland; 2Belfast Health and Social Care Trust, Department of Dermatology, Belfast, UK; 3St. Vincent's University Hospital, Department of Radiology, Dublin, Ireland; 4University College Dublin, School of Medicine, Dublin, Ireland; 5University College Dublin, Charles Institute of Dermatology, Dublin, Ireland
Hidradenitis suppurativa (HS) is associated with increased risk of major adverse cardiac events (MACE). To date, coronary atherosclerosis in HS has only been assessed using carotid intima media thickness measurements on ultrasound as a surrogate marker. Coronary computed tomography angiography (CCTA) is the primary non-invasive imaging tool for evaluating coronary artery disease. Our aim was to evaluate the prevalence of coronary atherosclerosis using CCTA in HS and compare this to population norms. We recruited 20 consecutive patients from a HS specialty clinic. Information was collected on demographics and HS disease severity. All patients were screened for cardiovascular disease risk factors. CCTA was completed in line with radiology departmental protocol in our centre. The patient cohort included 16 females (80%) and 4 males (20%) with a mean age of 38.7 years. Patients had moderate to severe disease Hurley stage 2 and 3 (n = 19, 95%) with mild disease activity using IHS4 (median 3). The majority of patients were current (n = 10, 50%) or former (n = 4, 20%) smokers. A 1st degree family history of cardiovascular disease was reported by 5 patients (25%). The mean BMI and waist circumference were elevated at 35.4 kg/m2 and 102 cm with 11 patients fulfilling diagnostic criteria for metabolic syndrome (55%). There was a high prevalence of cardiovascular disease risk factors with hypertension, dyslipidaemia and insulin resistance noted in 17 patients (85%), impaired fasting glucose in 3 patients (15%) and type 2 diabetes in 2 patients (10%). The median coronary artery calcium (CAC) score was 0 (IQR 0, range 0–198) with calcified coronary plaque present in 2 patients (10%). In this cohort, using standardized age, race and gender-matched population-based centiles, the probability of a CAC score >0 is 7%. Thus, patients with HS were 3% more likely to have CAC on CCTA but this was not statistically significant (relative risk 1.03, 95% CI 0.884–1.21, p = 0.642). Large healthcare database studies have demonstrated that patients with HS are more likely to develop MACE. In this pilot study of CCTA imaging in HS, patients were more likely to have coronary atherosclerotic plaque than the general population but this was not significant. The non-significant numerical increase in atherosclerotic plaque seen in this study may be explained by the small cohort of predominantly young female patients and lag period from the development of risk factors to the development of plaque. These patients have not developed atherosclerotic plaque yet despite a significant burden of traditional cardiovascular disease risk factors. Our results demonstrate a clear opportunity for clinicians to intervene early in patients with HS to reduce the risk of MACE. All clinicians who treat HS should screen for and treat modifiable cardiovascular disease risk factors in all patients early.
Comparative prevalence of pilonidal sinus disease in the general population and in patients with hidradenitis suppurativa: Results from a french prospective case–control study
Ines Evain1, Myriam Fioretta1, Christelle Enault1, Sylvia Math1, Anne-Sophie Gadat1, Marion Clerbout1, Christelle Chavrier1, Marianne Beuque1, Samantha Gaspard1, Virginie Vlaeminck-Guillem3,4, Philippe Guillem1,2
1Clinique du Val d'Ouest, Department of Surgery, Ecully, France; 2RésoVerneuil, Paris, France; 3CHU, Department of Biology, Lyon, France; 4Lyon 1 University, INSERM 1052, CNRS 5286, CRCL, Lyon, France
Association between pilonidal sinus disease (PSD) and hidradenitis suppurativa (HS) is well recognized. It is however often based on clinical findings and retrospective analysis of cohorts of HS patients and data from prospective case–control studies are scarce. PSD prevalence in the French general population is even unknown. The aim of the study was to compare the prevalence of PSD in the French general population and a cohort of HS patients. As part of the GHiSA project (estimation of HS prevalence in a control population through a validated screening questionnaire), we prospectively constructed a cohort representative of the general French population and including control patients referred to the Clinique du Val d'Ouest (Lyon, France) for consultation or hospitalization for a pathology other than HS, and those accompanying patients coming for such situations. To avoid a family aggregation bias, we excluded accompanying persons from the same families as the control patients. At the same time, we included all consecutive HS patients coming to the HS-specialized centre of the healthcare institution for consultation or hospitalization. The HS patients identified after clinical validation among the control patients and accompanying persons were included in the HS cohort. The clinical data collected included basic demographic data (age, sex, body mass index, current smoking status), as well as the presence of a PSD and the notion of a family history of PSD (regardless of the degree of relationship). Patients with missing PSD value (yes/no) were excluded from the study. Between March and November 2022, we collected 1031 controls (503 accompanying adults and 528 patients). A diagnosis of PSD (current or past) was reported by 42 of the 1031 controls, giving a prevalence of 4.1% (95% CI: 2.9%–5.3%). PSD was significantly more frequent in men (25/375) than in females (17/656): 6.7 vs. 2.6% (p = 0.001; relative risk = 1.74, 95% CI: 1.29–2.35). Its prevalence was significantly increased in smokers (8.3% vs. 3.1%; p = 0.001; RR = 3.38, 95% CI: 2.50–4.59) and in controls with a family history of PSD (20.6% vs. 2.8%; p < 0.001; RR = 7.59, 95 CI: 5.28–10.90). BMI was also found as a positive predictor of PSD (RR = 1.07; 95% CI: 1.05–1.10, p < 0.001). Multivariate analysis confirmed male gender, smoking and family history of PAS as independent predictors of PSD in control subjects. A diagnosis of PSD was made in 158 of the 445 HS patients included during the same period of time, representing a prevalence of 35.5% (95% CI: 31.0–40.0%). In this HS cohort, PSD was significantly more frequent in males (44.6% vs. 30.6%; p = 0.003) and in patients with PSD family history (60.2% vs. 28.1%). BMI proved to be a positive predictor of PSD in the HS cohort (RR = 1.03; 95% CI: 1.05–1.10, p < 0.001), while PSD prevalence was not different in HS smokers and HS non-smokers: 35.3% versus 35.9% (p = 0.396). When combining both control and HS cohorts, independent predictors of PSD were male gender (RR = 2.22, 95% CI: 1.54–3.20), BMI (RR = 1.05, 95% CI: 1.01–1.080), PSD family history (RR = 5.21, 95% CI: 1.01–1.08). Having a diagnosis of HS was also a strong independent predictor of PSD (RR = 8.88, 95% CI: 5.79–13.61), confirming the close link between HS and PSD. Conversely (along with female gender, BMI, smoking status), having a personal and/or family history of PSD was an independent predictor of HS: RR = 8.73 (95% CI: 5.71–13.4) and RR = 2.27 (95% CI: 1.48–3.49), respectively. This explorative, cross-sectional, descriptive study, based on a prospective recruitment suggests a high 4% prevalence of PSD in the general population. The risk of PSD increases with male gender, smoking status, BMI and PSD family history. The widely-accepted acquired origin theory, known from the Karydakis's HxFxV formula (free Hair; intergluteal vacuum Force; skin Vulnerability) can be assessed in the light of these risk factors: male gender may be correlated with increased free hair, BMI with vacuum force), and smoking with skin vulnerability. The higher PSD prevalence in HS patients (35%) as well as identification of HS as an independent predictor of PSD suggest that HS is specifically involved in PSD pathophysiology. It is likely that HS predisposes to PSD through an action to all three items of the Karydakis's formula. Prevalence of pilonidal sinus disease is higher in HS patients (35%) than in the general population (4%). The very close relationship between these two entities implies that it is imperative to search for one (questioning and clinical examination) in the presence of the other. The search for a family history of PSD should also be a useful concern in the diagnostic process.
Comparative prevalence of comorbidities in the general population and in patients with hidradenitis suppurativa: Results from a french prospective case–control study
Sylvia Math1, Anne-Sophie Gadat1, Marion Clerbout1, Christelle Chavrier1, Marianne Beuque1, Samantha Gaspard1, Ines Evain1, Myriam Fioretta1, Christelle Enault1, Virginie Vlaeminck-Guillem2,3, Philippe Guillem1,4
1Clinique du Val d'Ouest, Department of Surgery, Ecully, France; 2CHU, Department of Biology, Lyon, France; 3Lyon 1 University, INSERM 1052, CNRS 5286, CRCL, Lyon, France; 4RésoVerneuil, Paris, France
Risk of hidradenitis suppurativa occurrence increases with familial history of smoking
Marianne Beuque1, Samantha Gaspard1, Ines Evain1, Myriam Fioretta1, Christelle Enault1, Sylvia Math1, Anne-Sophie Gadat1, Marion Clerbout1, Christelle Chavrier1, Virginie Vlaeminck-Guillem3,4, Philippe Guillem1,2
1Clinique du Val d'Ouest, Department of Surgery, Ecully, France; 2RésoVerneuil, Paris, France; 3CHU, Department of Biology, Lyon, France; 4Lyon 1 University, INSERM 1052, CNRS 5286, CRCL, Lyon, France
The statistical association between smoking and hidradenitis suppurativa (HS) is well documented, but essentially through the (objective and demonstrative) high frequency of the proportion of (former and current) smokers in retrospective cohorts of patients. Prospective studies are scarce and several grey areas remain, such as the molecular and cellular mechanisms involved or the links between the quantity of tobacco consumed and the onset, severity or evolution of the disease. The role of passive smoking is moreover still very little studied. The aim of the study was to compare the prevalence of individual and familial smoking in the French general population and a cohort of HS patients using a prospective, cross-sectional study. As part of the GHiSA project (estimation of HS prevalence in a control population through a validated screening questionnaire), we prospectively constructed a cohort representative of the general French population and including control patients referred to the Clinique du Val d'Ouest (Lyon, France) for consultation or hospitalization for a pathology other than HS, and those accompanying patients coming for such situations. To avoid a family aggregation bias, we excluded accompanying persons from the same families as the control patients. At the same time, we included all consecutive HS patients coming to the HS-specialized centre of the healthcare institution for consultation or hospitalization. The HS patients identified after clinical validation among the control patients and accompanying persons were included in the HS cohort. The clinical data collected included basic demographic data (age, gender, body mass index, family history of HS), as well as the individual current smoking status and a history of familial smoking (especially in mothers and fathers). Between March and November 2022, we collected 1047 controls (507 accompanying adults and 540 patients) and 446 HS patients with complete data regarding age, gender and individual current smoking status). As expected, HS patients were significantly more frequently smokers than controls: 61.8 (95% CI: 57.3%–66.3%) versus 19.1% (16.7%–21.5%), p < 0.001. Of note, the proportion of smokers was also found to be significantly higher in subjects (either controls or patients) with a family history of HS: 54.7% versus 29.5%, p < 0.001. HS patients also significantly had a higher proportion of family history of smoking: 57.7% versus 23.4% when evaluated regardless of the degree of relationship of the smoker(s) in the family, 50.8% versus 17.9% for first-degree relatives, and 38.2% versus 11.2% for mother and/or father (all: p < 0.001). In HS patients with a parental (mother and/or father) history of smoking, age at disease onset was significantly lower than in HS patients without this parental history: 19.9 ± 6.4 versus 21.8 ± 9.7 years (p = 0.006). Mean IHS4 was also higher: 7.6 ± 9.9 versus 6.3 ± 8.8, although not significantly. Since smoking and HS were significantly associated with both male gender, BMI, family history of HS, and family history of smoking, we evaluated the role played by all these confounding factors in HS occurrence through multivariate analysis. While gender was eventually removed from the regression, smoking in mother/father was found to be an independent predictor of HS (OR: 2.91, 95% CI: 2.08–4.08), along with individual smoking (OR: 5.59; 4.17–7.50), family history of HS (OR: 9.33; 5.70–15.26) and BMI (OR: 1.09; 1.06–1.12), all p < 0.001. Through a prospective cross-sectional study, we here found that a family history of smoking was associated with an increased risk of HS. Having smoker mother and/or father was a significant predictor factor, independent of other characteristics such as gender, family history of HS and overweight. Despite obvious limitations including recall bias or the lack of precisions about the exact familial tobacco consumption in terms of quantity and duration of cigarette use or in terms of exact consumption at the moment of HS occurrence in index cases, the two main results of our study (increased HS risk and earlier disease onset with family history of smoking) suggests a role of passive smoking as an independent triggering factor of HS. The mechanisms by with parental smoking therefore determines HS occurrence in child remain to be elucidated. More precisely, what is the part of a role of social model exercised by parents on their children, the part of genetic mechanisms of susceptibility to HS and the part of epigenetic modifications induced by tobacco? In this cross-sectional study, HS was not only associated with an individual history of smoking but also with a familial history of smoking (especially in mother and/or father), suggesting a role of passive smoking in disease occurrence. Facing the difficulty of smoking quitting in the general population and HS patients, our results offers additional arguments for individual and familial counselling.
Prevalence of polycystic ovarian syndrome among children and adolescents with hidradenitis suppurativa
Nicole Mastacouris, Andrew Strunk, Amit Garg
Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Department of Dermatology, New Hyde Park, USA
Hidradenitis suppurativa (HS) and polycystic ovarian syndrome (PCOS) are chronic disorders affecting similar patient populations with overlapping metabolic comorbidities. Previous population-based studies have established a significant association between HS and PCOS in adults1,2. However, the strength of this association among the paediatric population is not well characterized. Existing literature is limited to small retrospective studies and HS registries, often lacking a control group.3-5 Herein, we aimed to compare prevalence of PCOS among children and adolescents with HS to prevalence in a general population sample in the United States. This was a cross-sectional study of the IBM Explorys database, a multi-health system data analytics and research platform comprised of electronic health records from over 40 healthcare networks across the United States. The study population included patients between the ages of 9 and 17 having at least 1 encounter in the database between January 1, 2018 and December 31, 2019 (period of interest) and at least 12 months of observable person time in the database. Patients with HS were identified by at least 1 ICD-9 (705.83) or ICD-10 (L73.2) diagnosis code for HS on or prior to December 31st, 2019. The control cohort comprised a 15% random sample of patients in the database without a diagnosis code for HS. The primary outcome was diagnosis of PCOS during or before the study period, based on at least one ICD-9 (256.4) or ICD-10 (E28.2) code. Prevalence of PCOS was compared between patients with HS and controls using logistic regression. Unadjusted and adjusted models were fit to describe the crude association as well as the influence of potential confounders. Adjusted models included age, sex, race, Medicaid insurance status (proxy for socioeconomic status), body mass index, type II diabetes mellitus, obstructive sleep apnea, and number of healthcare encounters (to account for surveillance bias due to increased contact with the healthcare system among patients with HS). A total of 1136 paediatric patients with HS and 79 884 paediatric control patients met inclusion criteria during the study period. Median (IQR) age in HS patients and controls were 16 (15, 17) and 13 (11, 16), respectively. The majority of patients in each cohort were White (52% in HS, 71% in controls), followed by Black (37% in HS, 16% in controls), Other race (9.5% in HS, 11% in controls), and Asian (0.5% in HS, 2% in controls). Prevalence of obesity was 57% and 24% among patients with HS and non-HS controls, respectively. Prevalence of type II diabetes mellitus was 1.6% and 0.3% among patients with HS and non-HS controls, respectively. Prevalence of obstructive sleep apnea was 3.4% and 0.8% among patient with HS and non-HS controls, respectively. PCOS diagnosis was prevalent in 4.0% of HS patients and 0.3% of control patients (unadjusted OR 12.63; 95% CI 9.15–17.43). In adjusted analysis, odds of PCOS were 3.16 (95% CI 2.23–4.47) times as high in paediatric HS patients compared to controls. In subgroup analysis among patients aged 15–17, prevalence was 4.7% in HS patients and 0.8% in control patients (unadjusted OR 6.51; 95% CI 4.64–9.14). After adjusting for covariates, odds of PCOS were 2.97 (95% CI 2.07–4.28) times as high among HS patients aged 15–17 compared to controls of the same age. Among HS patients and controls, obese patients were more likely to have PCOS compared to non-obese patients (prevalence ratio of 5.31 (95% CI 2.06–13.70) in HS patients, 9.10 (95% CI 6.73–12.29) in controls). This large population-based analysis found a strong association between PCOS and HS among children and adolescents while controlling for BMI and other potential confounders. Our results indicate that paediatric HS patients, particularly those with signs of hyperandrogenism, may benefit from screening for PCOS.
- Garg A et al. Hidradenitis Suppurativa Is Associated with Polycystic Ovary Syndrome: A Population-Based Analysis in the United States. J Invest Dermatol 2018;138:1288–92.
- Kimball AB et al. The Comorbidity Burden of Hidradenitis Suppurativa in the United States: A Claims Data Analysis. Dermatol Ther (Heidelb) 2018;8:557–69.
- Seivright JR et al. Physical and psychosocial comorbidities of paediatric hidradenitis suppurativa: A retrospective analysis. Pediatr Dermatol 2021;38:1132–6.
- Liy-Wong C et al. Hidradenitis Suppurativa in the Paediatric Population: An International, Multicenter, Retrospective, Cross-sectional Study of 481 Paediatric Patients. JAMA Dermatol 2021;157:385–91.
- Braunberger TL et al. Hidradenitis suppurativa in children: The Henry Ford experience. Pediatr Dermatol 2018;35:370–3.
Posters – Clinics and Diagnostics
When sebo-cystomatosis mimicks HS
Giulia Nunziati, Elia Rosi, Ilaria Scandagli, Gianmarco Silvi, Prisca Guerra, Antonella Di Cesare, Francesca Prignano
University of Florence, Department of Health Sciences, Section of Dermatology, Firenze, Italy
Steatocystoma multiplex (SM) is a benign pilosebaceous unit disease which shares overlapping clinical features with hidradenitis suppurativa (HS). We report five cases (3 males; 2 females) showing clinical aspects consistent with both SM and HS. Regarding anamnestic data, median age at first visit was 31 years (IQR: 23.5–44.5); four patients were current smokers, three patients had a personal history of acne and one of the female patients had a previous diagnosis of precocious puberty. A positive family history of HS was documented only in one patient, while none of them was aware of familiar cases of SM. Disease severity was assessed clinically and with ultrasound and scored according to IHS4 and Hurley staging system. All these patients presented multiple asymptomatic yellow-coloured to skin coloured papules and/or nodules of varying size localized mainly on the upper trunk, on the groin and on the buttocks. Some of them also showed inflammatory nodules, abscesses and sinus tracts involving cutaneous regions rich in apocrine glands, such as axillary-mammary region and groin. By ultrasound we distinguished steatocystomas, which appear as well-defined hypoecoic nodules with mild posterior acoustic enhancement, from nodules, abscesses and sinus tracts. Steatocystomas were located in the dermis and subcutis in 20% of cases and they were only dermal in 80% of cases. Interestingly, one patient showed a steatocystoma lesion with a positive power doppler signal, suggesting a diagnosis of Steatocystoma Multiplex Suppurativa. All patients were initially treated with oral clindamycin 450/600 mg/day for 6–8 weeks, with good response in 3 cases. Two patients underwent CO2 laser excision of steatocystoma located on gluteal region. In two patients biologic therapy with adalimumab was initiated. HS and SM can often share similar clinical features and sometimes these diseases can coexist in the same patient, as previously described in the literature.1-4 In order to distinguish between these two diseases and to make a precise diagnosis, we suggest ultrasound as an additional instrumental useful tool for differential diagnosis, not excluding histopathological examination if necessary.
- Hollmig T, Menter A. Familial coincidence of hidradenitis suppurativa and steatocystoma multiplex. Clin Exp Dermatol 2010;35:e151-2.
- Atzori L et al. Steatocystoma multiplex suppurativa associated with hidradenitis suppurativa successfully treated with adalimumab. J Eur Acad Dermatology Venereol 2019;33(suppl 6):42–4.
- Zussino M et al. Coexistence of steatocystoma multiplex and hidradenitis suppurativa: Assessment of this unique association by means of ultrasonography and Colour Doppler. Ski Res Technol 2019;25:877–80.
- Fletcher J et al. Coexistence of Hidradenitis Suppurativa and Steatocystoma Multiplex: Is It a New Variant of Hidradenitis Suppurativa? J Cutan Med Surg 2021;25:586–90.
Characteristics of patients with paediatric onset of hidradenitis suppurativa
Anne-Claire Fougerousse1, Ziad Reguiai2, François Maccari3, Philippe Guillem4, Aude Nassif5, Nathalie Beneton6, Elisa Cinotti7, Céline Girard8, Raphaele Binois9, Jean-Luc Perrot10, On behalf of the GEM ResoVerneuil
1Military Teaching Hospital Begin, Department of Dermatology, Saint Mandé, France; 2Polyclinic Courlancy Bezannes, Department of Dermatology, Reims, France; 3Private practice, Dermatology, La Varenne Saint Hilaire, France; 4Clinique du Val d'Ouest, Visceral Surgery, Ecully, France; 5Institut Pasteur, Department of Dermatology, Paris, France; 6CH, Department of Dermatology, Le Mans, France; 7University of Siena, Department of Medical, Dermatology Unit, Surgical and Neuro.Sciences, Siena, Italy; 8CHU, Department of Dermatology, Montpellier, France; 9CH, Department of Dermatology, Orléans, France; 10CHU, Department of Dermatology, Saint Etienne, France
Paediatric onset of hidradenitis suppurativa HS occurs in 2.2 to 38.3% of cases. Differentiation with adult-onset HS is controversial with recent data suggesting a clinical spectrum comparable in the two populations in studies with small number of paediatric onset HS, and a high frequency of comorbidities in a retrospective study of 481 patients with paediatric onset HS1,2. Epiver was a prospective multicentric cohort study including 1428 HS patients, objective of which was to describe the epidemiology of HS3. In this ancillary study we compared the demographic and clinical characteristics of HS patients according to the age of onset (paediatric: <8 years, or adult). Among the 1428 patients included in the Epiver study, age of onset of HS was available for 1384, with 528 patients with paediatric onset (mean age at inclusion 28.7 ± 10 years) and 856 patients with adult onset of HS (mean age at inclusion 36.5 ± 10.5 years, p < 0.001). Mean age of onset of HS symptoms was 14.5 ± 2.1 years in paediatric onset group, and 25.7 ± 7.7 years in adult onset group. Mean age of diagnosis was 24.1 ± 8.9 years in paediatric onset group, and 31.9 ± 9.3 years in adult onset group. Paediatric onset group comprised more women (70.3%) compared to adult onset group (56.1%, p < 0.001). More patients in the paediatric onset group had familial history of HS (28.2% vs. 22.8%, p = 0.024) or familial history of pilonidal cyst (14.6% vs. 10.1%, p = 0.011). More patients in the adult onset group were smokers (82.1% vs. 67.8%, p < 0.001). No significant difference in cannabis use was identified between the two groups, with 20.5% of cannabis users in the paediatric onset group and 19% in the adult onset group. Numerically more patients in the adult onset group had familial history of chronic inflammatory rheumatism (6.2% vs. 3.8%, p = 0.051). There was no difference according to BMI between the two groups (mean BMI 27.45 ± 8.26 in paediatric onset group, 27.82 ± 8.27 in adult onset group, p = 0.122). Repartition according to Hurley stage was similar between the two groups (with respectively 46.7 and 42.8% Hurley I stages, 39.4 and 40.5% Hurley II and 14 and 16.7% Hurley III in paediatric onset and adult onset HS groups). Patients with paediatric onset HS had more frequently HS lesions in inguinal folds (76.7% vs. 71.4%, p = 0.029), mammary region (6.1% vs. 3.3%, p = 0.013), face (29.5% vs. 22.9%, p = 0.006), trunk (18.6% vs. 13.7%, p = 0.015) and legs (5.9 vs. 3.3%, p = 0.020). Patients with adult onset HS had more frequently HS lesions in genital area (36.1 vs. 28.6%, p = 0.004) and scalp (4.4 vs. 2.3%, p = 0.036). Pain (evaluated by visual analogic scale from 0–10) was more important in the paediatric onset group (5.7 ± 3.3) than in the adult onset group (5.3 ± 3.3, p = 0.047). Impact on quality of life (evaluated with Dermatology Life Quality Index) was important but comparable between the two groups (12.7 ± 7.3 in paediatric onset group versus 12.4 ± 7.3 in the adult onset group, p = 0.450). More patients in the adult onset group had associated inflammatory bowel disease (4.7% vs. 1.7%, p = 0.004), dyslipidaemia (8.4 vs. 3.2%, p < 0.001) and diabetes mellitus (5 vs. 1.1%, p < 0.001). Frequency of associated diseases as pilonidal cyst (33.3% in paediatric onset group, 30.5% in adult onset group), acne (respectively 36.9 and 33.6%), chronic inflammatory rheumatism (respectively 5.5 and 4.9%), hypertension (respectively 5.1 and 7.6% were comparable between the two groups. Our study confirms the feminine predominance and the frequency of familial HS history in paediatric onset HS. Contrary to the large retrospective study1 (with a predominance of North America centers) we did not show a high prevalence of comorbidities in patients with paediatric onset HS. In our study the mean diagnostic delay was 9.6 years in the paediatric onset group, much longer than in the study of Liy-Wong et al. [1] (2 years) and closer to the average diagnostic delay in France (8.4 years4). No difference of severity (evaluated by Hurley staging or DLQI) was identified according to the age of onset of HS. In our study, localization of HS lesions differs according to the age of onset with more patients with follicular lesions (face, trunk, legs) and lesions of the inguinal folds or mammary area in the paediatric onset group. Limitations of our survey include recollection bias given the nature of the study, and the lack of a control group. However, the strength of this study is its large sample size and the examination by physicians implicated in HS management.
Acknowledgement: To all members of ResoVerneuil who participate to this study.
- Liy-Wong C et al. Hidradenitis Suppurativa in the Paediatric Population: An International, Multicenter, Retrospective, Cross-sectional Study of 481 Paediatric Patients. JAMA Dermatol 2021;157:385–91.
- Garcovich S et al. Paediatric Hidradenitis Suppurativa: A Cross-Sectional Study on Clinical Features and Treatment Approaches. J Cutan Med Surg 2022;26:127–34.
- Perrot JL et al. How to Define Mild to Severe Hidradenitis Suppurativa? A Simple New Tool Based on Latent Class Analysis of EPIVER Data Study. Clin Cosmet Investig Dermatol 2022;15:1091–103.
- Loget J et al. Errance médicale des patients atteints d'hidradénite suppurée: un problème majeur et persistant. Étude « R-ENS Verneuil » [Misdiagnosis of hidradenitis suppurativa continues to be a major issue. The R-ENS Verneuil study]. Ann Dermatol Venereol 2018;145:331–8.
The natural history study of hidradenitis suppurativa: A large-scale multicenter study in turkey
Erkan Alpsoy1, Bilge Fettahlıoglu Karaman2, Deniz Demirseren3, Levent Cınar4, Nida Kacar5, Aylin Turel Ermertcan6, Emel Bulbul Baskan7, Derya Ucmak8, Kifayet Mammadli1, Fadime Kılınç9, Serkan Yazici7, Selami Aykut Temiz9, Tugba Özkök Akbulut10, Arzu Ataseven9, Aysun Şikar Aktürk11, Hayriye Sarıcaoğlu7, Meltem Türkmen12, Fatmagul Gülbaşaran13, Burhan Engin14, Hatice Kaya Ozden15, Koray Durmaz9, Muge Guler Ozden16, Hilal Ozdemir16, Bengu Cevirgen Cemil17, Sezgi Sarıkaya Solak18, İbrahim Halil Yavuz19, Göknur Ozaydın Yavuz19, Münevver Guven20, Algun Polat Ekinci21, Tugba Atıcı21, Didem Didar Balci22, Aylin Oztürk22, İlknur Kıvanç Altunay23, Ezgi Ozkur23, Ece Ugurer23, Ayse Serap Karadag24, Goknur Kalkan17, Bilgen Erdogan17, Savas Yayli25, Leyla Baykal Selcuk26, Levent Donmez27, Aslı Bilgic1
1Akdeniz University, Department of Dermatology, Antalya, Turkey; 2Çukurova University, Department of Dermatology, Adana, Turkey; 3Ankara Bilkent State Hospital, Department of Dermatology, Ankara, Turkey; 4Erciyes University, Department of Dermatology, Kayseri, Turkey; 5Pamukkale University, Department of Dermatology, Denizli, Turkey; 6Celal Bayar University, Department of Dermatology, Manisa, Turkey; 7Uludag University, Department of Dermatology, Bursa, Turkey; 8Dicle University, Department of Dermatology, Diyarbakır, Turkey; 9Necmettin Erbakan University, Department of Dermatology, Konya, Turkey; 10Health Sciences University, Haseki Training and Research Hospital, Department of Dermatology, Istanbul, Turkey; 11Kocaeli University, Department of Dermatology, Kocaeli, Turkey; 12Health Sciences University, İzmir Bozyaka Training and Research Hospital, Department of Dermatology, İzmir, Turkey; 13Dokuz Eylül University, Department of Dermatology, İzmir, Turkey; 14İstanbul University-Cerrahpaşa, Cerrahpaşa Faculty of Medicine, Department of Dermatology, İstanbul, Turkey; 15Kocaeli Derince Tarining and Research Hospital, Department of Dermatology, Kocaeli, Turkey; 16Ondokuzmayıs University, Department of Dermatology, Samsun, Turkey; 17Dış Kapı Yıldırım Beyazıt Training and Research Hospital, Department of Dermatology, Ankara, Turkey; 18Trakya University, Department of Dermatology, Edirne, Turkey; 19Van Yüzüncü Yıl University, Department of Dermatology, Van, Turkey; 20Aydın Adnan Menderes University, Department of Dermatology, Aydın, Turkey; 21İstanbul Capa University, Department of Dermatology, İstanbul, Turkey; 22Tepecik Training and Research Hospital, Department of Dermatology, İzmir, Turkey; 23Sisli Etfal Training and Research Hospital, Department of Dermatology, Istanbul, Turkey; 24Arel University, Faculty of Medicine, Memorial Health Group, Atasehir Hospital, Department of Public Health, Istanbul, Turkey; 25Koc University, Department of Dermatology, Istanbul, Turkey; 26Karadeniz Teknik University, Department of Dermatology, Trabzon, Turkey; 27Akdeniz University, Department of Public Health, Antalya, Turkey
Information on the natural history of hidradenitis suppurativa (HS) is still limited; the chronology of disease symptoms and their gender differences have not yet been fully elucidated. In this study, we aimed to investigate the natural course of HS in a large number of patients. This large-scale multi-center study included 827 consecutive patients (528 males, 299 females) from 27 centers. The disease symptoms for each patient were retrospectively recorded in the time order of the manifestations. Sociodemographic and clinical characteristics of the patients were collected. Disease severity was documented as Hurley staging (HSt) and Physician's Global Assessment score (HS-PGA). The average age was 33.80 ± 11.72 (range 12–74) years (women, 30.20 ± 10.59; men, 35.84 ± 11.84; p < 0.001). The mean age of disease onset was earlier in women than in men (22.42 ± 9.28 vs. 27.06 ± 20.56, p < 0.001). The mean disease duration was 91.17 ± 83.64 months. Most of the patients were in HSt II (n = 405, 49%) and HS-PGA moderate group (n = 268, 32.4%), and disease severity was higher in men compared to women (HSt, p < 0.0001; HS-PGA, p < 0.0001). Active smoking, regular alcohol consumption, and other disorders comprising follicular occlusion tetrad [acne conglobata (25.6%), pilonidal sinus (25.0%), dissecting cellulitis (5.2%)] were also more common in males. Of the 3 most common symptoms [purulent discharge (81%), abscess (78.5%) and pain (75.7%), purulent discharge (p = 0.038)] was significantly more frequent in males. The mean duration of abscess (3.53 ± 3.41 weeks) was significantly shorter in women than in men (women, 3.11 ± 2.65; men, 3.75 ± 3.75, p = 0.01). The first 5 years of the disease were the most active disease-period in 67.5% of the patients. Most patients (46.4%) described the high activity of the disease (abscesses, inflammatory nodules in at least 3 months of the last 6 months). Stress (57%) was the most common aggravating factors, followed by hyperhidrosis (50.3%), tight clothing/friction (46.2%), hot weather-summer time (44.4%) and shaving/hair removal (29.1%). Menstruation was also described as an aggravating factor in 43.5% of women. Stress (p = 0.032), putting on weight (p = 0.022) was a more common aggravating factor in women compared to men. The most important factor improving the disease symptoms was antibiotics (55.5%), followed by spontaneous opening/resolution (37.1%), loose cotton clothing/ keeping the area clean/ dry and cool environment (34.6%), relaxed/stress-free environment/sun and fresh air (29.4%) and bath/shower and swimming (29.3%), respectively. While biological therapy was shown as significantly higher in men as an alleviating factor (p < 0.001), laser hair removal (p = 0.003) and pregnancy (0.016) were more common alleviating factors described in women. Axilla (80.8%) and inguinal region (64.2%) were the most frequently involved sites in both genders. The gluteal region (38%) followed these involvements in men and the mammalian regions (48.8%) in women. The involvement of the axilla was earlier in men than in women (p < 0.001). Multivariate analysis showed that male sex, increased age, early age of disease onset, involvement of inguinal area and noncompliance with the treatment protocol are independent factors for the disease severity. The disease starts earlier in women and is more severe in men. Environmental factors such as smoking and alcohol consumption, which were found to be high in male patients in our study, may have played a role in the frequency and severity of the disease. Although the activity of the disease is continuous, it is much more evident in the early years. Chronologically, the gluteal region in men and the mammalian region in women is the most common onset sites after the axilla and inguinal region. This study confirms the idea that environmental and hormonal factors may play an important role in the course of the disease, probably with other endogenous or exogenous factors. Also, our results emphasize that early and effective treatment in the early stages of the disease can prevent or reduce the disease progression, especially in risk groups such as the male gender.
Screening of hidradenitis suppurativa in patients with axial spondyloarthritis
Rosa Fornons-Servent1, Laura Farrán2, Xavier Juanola2, Jaime Notario1
1Hospital Universitari de Bellvitge, Department of Dermatology, Hospitalet del Llobregat, Barcelona, Spain; 2Hospital Universitari de Bellvitge, Department of Rheumatology, Hospitalet del Llobregat, Barcelona, Spain
Spondyloatritis (SpA) and hidradenitis suppurativa (HS) are both chronic inflammatory conditions that share several patho-physiological features, risk factors and response to some treatments. HS was reported to be more prevalent in patients with axial SpA than in the general population but there are few reports on how to screen HS in patients with SpA1. This study was conducted to determine the prevalence of HS in patients with axial SpA with a self-screening questionnaire confirming the diagnosis with a posterior dermatologic evaluation. We performed a cross-sectional study with a self-screening questionnaire that was sent to all patients of the rheumatology cohort of axial SpA of tertiary referral Hospital Universitari de Bellvitge fulfilling the ASAS axial SpA criteria that consent participate. The questionnaire was based on validated diagnostic HS questions2,3 translated to Spanish and accompanied by a brief informative description of HS and prototypical pictures of HS lesions of the Hurley stages of the axilla3. First question is about the presence of recurrent HS typical lesions in typical locations. Second and third questions were about the presence of outbreaks of boils during the last 6 months and its frequency. Being under anti-TNF treatment was also contemplated in the results interpretation. The diagnosis was confirmed with a dermatologic evaluation. A total of 233 patients were asked to participate, 205 patients accepted and 72.2% (148/205) of the questionnaires were eligible for analyses. Included patients had a mean age of 52.2 ± 12.4 years, 32.5% were females, 19.6% were smokers, 67.5% had overweight or obesity, the mean symptom duration was 23 ± 13 years and 79% were HLA-B27 positive. Based on questionnaire answers we select 9 patients who were visit in dermatology department and HS diagnosis was confirmed in 4 cases. Patients who did not answer positive in the second and third question but were under anti-TNF treatment were also selected for dermatology evaluation, that represent 2 of the 9 selected patients and 1 of them was finally diagnosed of HS. The prevalence of HS in patients with SpA in our series was 2.7%. Patients with HS and SpA had a mean age of 43.2 ± 12.5 years, 2/4 were females, 3/4 were smokers, 3/4 had overweight or obesity, the mean SpA symptom duration was 14.7 ± 2.6 years, 3/4 was HLA-B27 positive, all of them were Hurley I in the moment of the evaluation and the mean delay in diagnosis was 14.3 years. Statistically significant differences were observed in the percentage of smokers and the mean SpA symptom duration. In our cohort of axial SpA the prevalence of HS is superior than in the general population4. Using a self-screening questionnaire could be useful to select the patients to refer for a dermatological evaluation to confirm the diagnosis of HS. Anti-TNF treatment for SpA could control the HS clinical manifestations. For these reason, patients already under treatment with anti-TNF with history of typical HS lesions in typical locations should be evaluated despite a negative answer in the questionnaire about the recurrence of outbreaks of boils in the last 6 months.
- Rondags A et al. High prevalence of hidradenitis suppurativa symptoms in axial spondyloarthritis patients: A possible new extra-articular manifestation. Semin Arthritis Rheum 2019;48:611–7.
- Vinding GR et al. The prevalence of inverse recurrent suppuration: a population-based study of possible hidradenitis suppurativa. Br J Dermatol 2014;170:884–9.
- Esmann S et al. Questionnaire-based diagnosis of hidradenitis suppurativa: specificity, sensitivity and positive predictive value of specific diagnostic questions. Br J Dermatol 2010;163:102–6.
- Jfri A et al. Prevalence of Hidradenitis Suppurativa: A Systematic Review and Meta-regression Analysis. JAMA Dermatol 2021;157:924–31.
Autosomal dominant hyperimmunoglobulin E syndrome: Differential diagnosis or part of a new syndromic hidradenitis suppurativa?
Axel Villani1,2, Aurelien Fonseca3, Virginie Vlaeminck-Guillem4,5, Philippe Guillem6,7
1CHU, Department of Dermatology, Lyon, France; 2Lyon 1 University, CIRI, Lyon, France; 3Technipath, Department of Pathology, Lyon, France; 4CHU, Department of Biology, Lyon, France; 5Lyon 1 University, INSERM 1052, CNRS 5286, CRCL, Lyon, France; 6Clinique du Val d'Ouest, Department of Surgery, Ecully, France; 7RésoVerneuil, Paris, Germany
Hidradenitis suppurativa and endocrinological comorbidities: A multidisciplinary, joint approach
Juan Garcias-Ladaria1, Guillermo Serra Soler2, Ines Gracia-Darder1, Juan J. González Malmierca1, Ana Martin-Santiago1
1Hospital Universitari Son Espases, Department of Dermatology, Palma, Spain; 2Hospital Universitari Son Espases, Department of Endocrinology, Palma, Spain
Hidradenitis suppurativa (HS) is associated with metabolic syndrome (MS), obesity, diabetes, dyslipidemia, hypertension, and polycystic ovary syndrome (PCOS).1 All these comorbidities, added to the high prevalence of cigarette smoking among HS patients, increase the risk of cardiovascular events and death2. In addition, some comorbidities like obesity, insulin resistance and hyperandrogenism derived from PCOS are involved in the pathophysiology of HS3,4. Therefore, the correct diagnosis and management of these comorbidities is of upmost interest. In our Dermatology Department, HS patients with endocrinologic comorbidities, especially obesity and PCOS, are selected for a multidisciplinary consultation with an endocrinologist. Herein, we aim to describe our experience. All patients attended for HS in the multidisciplinary consultation were included in this prospective observational study. At the first visit the following variables were collected: sex, age, height, smoking habit, personal history of diabetes, dyslipidemia, hypertension and PCOS, familial history of HS and treatments used. At the first and each follow-up visit, lesions were counted, weight, body mass index, BMI, and blood pressure are registered, and patients were asked to answer the following patient reported outcome measures (PROM): numeral rating (NRS) for pain and itch, and DLQI. Obese patients (i. e. BMI > 30) and women with signs of hyperandrogenism were investigated for insulin resistance (IR) and sexual hormones alterations in blood. Other laboratory investigations like hemogram, glucose, lipids and acute phase reactants were investigated if required by the clinical context. For patients with at least 3 visits the following outcomes were investigated: percentage of patients achieving HiSCR, change in IHS4 and PROMS, rate of RI remission, and change in BMI (only for obese). From June 2019 to October 2022, 71 patients (71.4% women, mean age 36.6) were attended. Eleven (15.7%) presented hypertension, 14 (20%) dyslipidemia, 11 (15.7%) diabetes, 15 (21.7%) PCOS. At the first visit, 63 (90%) patients were at least overweighted (BMI > 25), 53 (75.7%) were obese (BMI > 30). According to the National Cholesterol Education Program ATP III criteria5, 35 patients (49.3%) had MS. Of 54 patients investigated, 22 (40.7%) had IR and 16 of 37 investigated (43.2%) presented alterations in sexual hormones. Two new diagnoses of diabetes were made. Aside from skin directed therapies, hypocaloric diet was prescribed in 25 patients, metformin in 31, antiandrogen contraceptives in 15 and Glucagon 1-like peptide (GLP1) analogues in 14. Other treatments prescribed were simvastatin (3), fluoxetine (2), empaglifozine (1), pioglitazone (1), ezethrol (1), and spironolactone (1). Hormonal therapy was adjusted in a transgender patient.
Sixteen patients (75% women) had 3 or more visits (median follow-up 574 days, range 252–1134). Twelve of them (75%) reached HiSCR. All HS scores improved: IHS4 (median 4.5 to 0.5), DLQI (8 to 3), NRS for pain (1.5 to 0) and NRS for itch (2.5 a 2). Six out of 8 (75%) with IR normalized their parameters, and, in obese patients, median BMI was reduced from 39 to 35.7. The multidisciplinary approach with an endocrinologist was useful and efficient for the management of metabolic disease in HS patients. Not only two new diagnoses of diabetes were made, but also oral antidiabetic drugs, lipid-lowering medications and antidepressants, which are not part of the usual dermatologic therapeutic armamentarium, were successfully introduced or adjusted. Taking care of metabolic disease in HS patients is important for the management of HS, but, above all, it is crucial to prevent major causes of morbidity and mortality in our patients.
- Stefanadi EC et al. Metabolic syndrome and the skin: a more than superficial association. Reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr 2018;10:9.
- Tzellos T et al. Cardiovascular disease risk factors in patients with hidradenitis suppurativa: a systematic review and meta-analysis of observational studies. Br J Dermatol 2015;173:1142–55.
- Vilanova I et al. Insulin resistance in hidradenitis suppurativa: a case–control study. J Eur Acad Dermatol Venereol 2018;32:820–4.
- Kaleta KP et al. Metabolic Disorders/Obesity is a Primary Risk Factor in Hidradenitis Suppurativa: An Immunohistochemical Real-World Approach. Dermatology 2022;238:251–9.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97.
Colonoscopy findings in hidradenitis suppurativa
Nasro A. Isaq1, Mika Takaichi1, Laura E. Raffals2, Edward E. Loftus2, Caroline L. Matchett2, Nisha B. Patel2, Afsaneh Alavi1
1Mayo Clinic, Dermatology, Rochester, USA; 2Mayo Clinic, Department of Gastroenterology, Rochester, USA
- Sabat R et al. Hidradenitis suppurativa. Nat Rev Dis Primers 2020;6:18.
- Zouboulis CC et al. What causes hidradenitis suppurativa? 15 years after. Exp Dermatol 2020;29:1154–70.
- Garg A et al. Overall and subgroup prevalence of Crohn disease among patients with hidradenitis suppurativa: a population-based analysis in the United States. JAMA Dermatol 2018;154:814–8.
- Goldburg SR et al. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 2020;82:1045–58.
- Phan K et al. Prevalence of inflammatory bowel disease (IBD) in hidradenitis suppurativa (HS): systematic review and adjusted meta-analysis. Int J Dermatol 2020;59:221–8.
Periodontal status in patients with hidradenitis suppurativa
Beata Jastrząb1, Barbara Paśnik-Chwalik2, Tomasz Konopka2, Piotr K. Krajewski1, Jacek C. Szepietowski1, Łukasz Matusiak1
1Wroclaw Medical University, Department of Dermatology, Venereology and Allergology, Wroclaw, Poland; 2Wroclaw Medical University, Department of Periodontology, Wroclaw, Poland
Hidradenitis suppurativa (HS) is a chronic, inflammatory disease of apocrine gland-bearing skin, characterized by recurrent nodules, abscesses and sinus tracts. The aetiology underlying HS is still not fully understood, but genetics, hormonal, lifestyle and microbial factors seem to play the role in the development of the disorder. Periodontitis is a chronic inflammatory disease of the of the tooth-supporting apparatus, caused by bacterial infection of dental plaque and excessive host response. The relationship between periodontitis and autoimmune skin diseases such as psoriasis, pemphigus, pemphigoid, and lichen planus has been recognized. Nevertheless, current literature on oral health in HS individuals is limited. The primary purpose of the study was to assess the prevalence of periodontitis and to characterize the oral microbiome in HS patients. Fifty-five HS patients and fifty-five healthy controls were enrolled in the study. The presence of periodontitis was assessed in all patients during the periodontal evaluation. The quantitative analysis of the nine crucial periodontal pathogens (A. actinomycetemcomitans, P. gingivalis, T. denticola, T. forsythia, P. intermedia, P. micros, F. nucleatum, E. nodatum, and C. gingivalis) and total bacteria count was conducted by DNA copy number measurement. HS patients were significantly more frequently diagnosed with periodontitis than healthy controls (45.5% vs. 14.5%). Moreover, the mean total bacteria count was significantly higher in the HS samples than that in healthy ones (p < 0.05). The majority of periodontal pathogens were more commonly isolated in individuals with HS compared to controls. The most frequent bacteria detected in HS group were T. denticola (70.9%), C. gingivalis (67.3%), P. micros (41.8%) and T. forsythia (40.0%), while among healthy controls C. gingivalis (34.5%), T. denticola (20.0%) and P. micros (18.2%) were most commonly isolated pathogens. There was no correlation between number of DNA copies of periodontal pathogens and HS severity assessed with both Hurley and HS4 scales. This is the first study assessing the periodontal status in HS individuals. The present research revealed higher prevalence of periodontitis as well as periodontal pathogens in HS patients compared to healthy controls. Nevertheless, further investigations are needed to shed the light on possible role of periodontal pathogenic bacteria in HS development.
If PASH syndrome is not difficult enough!
Prisca Guerra, Elia Rosi, Ilaria Scandagli, Gianmarco Silvi, Giulia Nunziati, Antonella Di Cesare, Francesca Prignano
University of Florence, Department of Health Sciences, Section of Dermatology, Florence, Italy
PASH is a rare autoinflammatory condition, which consists of pyoderma gangrenosum (PG), acne conglobata and hidradenitis suppurativa (HS).1 The latter is often associated with other comorbidities, such as Crohn's disease (CD), and psoriasis. The occurrence of all these components in a single patient is challenging for both the patients as well as their physicians. Herein, we present the case of a 54-year-old female, affected by PASH syndrome, CD, and psoriasis resistant to several medical therapies. She presented to our outpatient dermatologic clinic complaining of an exacerbation of her HS lesions after 1 year on guselkumab therapy. She had a diagnosis of PASH syndrome since 2021, after 10 years of HS course. At our observation, she presented a recurrence of PG, an exacerbation of HS lesions on the pubic, inguinal, perianal, and axillary regions and erythematous, scaly plaques on both lower limbs (PASI = 6.3). The patient also referred a recurring fever. Previous treatments included both antibiotics and biologicals (adalimumab, etanercept, ustekinumab, infliximab originator and guselkumab). In an attempt to treat all four conditions, a second course of infliximab biosimilar (5 mg/kg given as an intravenous infusion followed by additional 5 mg/kg infusion doses at 2 and 6 weeks after the first infusion, then every 8 weeks thereafter) in association with linezolid (600 mg twice daily) was initiated. After 12 weeks of treatment, the patient's HS lesions improved, and she obtained an almost complete remission of her PG. Infliximab is a chimeric human-murine monoclonal antibody that binds with high affinity to both soluble and transmembrane forms of TNFα and it is approved for the treatment of moderate to severe psoriasis in adults and, according to the HS ALLIANCE working group, may be considered a second line for HS. Linezolid is a synthetic bacteriostatic agent of the oxazolidinone class, and it is approved by the FDA and EMA for ABSSSI. To the best of our knowledge, this is the first case describing the use of the association of infliximab and linezolid in a patient affected by PASH syndrome, CD and psoriasis.
- Huang J et al. Pyoderma Gangrenosum, Acne, and Hidradenitis Suppurativa Syndrome: A Case Report and Literature Review. Front Med (Lausanne) 2022;9:856786.
Psychiatric co-morbidity in patients with hidradenitis suppurativa: A cross sectional study examining demographic differences and burden of disease
Nikolaj Holgersen1, Valdemar W. Nielsen1, Yiqiu Yao1, Hans Christian Ring1, Alexander Egeberg1,2, Jacob P. Thyssen1, Simon F. Thomsen1,3
1Bispebjerg and Frederiksberg hospital, Department of Dermatology-Venereology, Copenhagen, Denmark; 2University of Copenhagen, Department of Clinical Medicine, Copenhagen, Denmark; 3University of Copenhagen, Department of Biomedical Sciences, Copenhagen, Denmark
Little is known about the demographic, clinical characteristics, and disease burden in hidradenitis suppurativa (HS) patients with psychiatric co-morbidity (PCM). The objective of this study was to examine the differences in demographic and clinical characteristics, co-occurring diseases, and disease burden in HS patients with and without PCM. 657 consecutive, adult HS patients from a dermatological university outpatient clinic were included. Data was obtained through interview and clinical examination. Disease burden was based on Hurley Stage, and the dermatology life quality index (DLQI) questionnaire. PCM was based on self-reported data by patients. 177 patients (26.9%) reported having current or previous PCM. Of the different PCM's 16.4% (n = 108) had depression/bipolar disorder, 8.7% (n = 57) anxiety, 2.7% (n = 18) ADHD/ADD, 2.6% (n = 17) schizophrenia, 2.1% (n = 14) PTSD, 2.0% (n = 13) OCD/Tourette's, 2.0% (n = 14) any personality disorder, and 0.9% (n = 6) an eating disorder. 7.9% (n = 52) had more than one PCM. Patients with PCM were associated with being obese (BMI > = 30 kg/m2) (44.9 vs. 35.0%) OR = 1.51 (1.06–2.15) p = 0.022, unemployed (52.5 vs. 20.8%) OR 4.23 (2.92–6.11) p < 0.001 and active or previous smoker (89.8 vs. 73.5%) OR 3.19 (1.88–5.40) p < 0.001. Patients with depression/bipolar disorder were more likely to be female (73.1 vs. 61.7%) OR 1.70 (1.07–2.70) p = 0.026. Overall, there was no significant difference between Hurley stages p = 0.777 or higher DLQI-score p = 0.079 in patients with and without PCM. Selectively, patients with depression/bipolar disorder above the age of 30 were associated with having obstructive lung disease (11.7 vs. 5.3%) OR 2.38 (1.02–5.52) p = 0.043 whereas patients with schizophrenia were associated with having acne (35.3 vs. 15.4%) OR 2.99 (1.07–8.34) p = 0.036 and diabetes (23.5 vs. 7.5%) OR 3.80 (1.18–12.24) p = 0.026. Patients with HS have a high prevalence of PCM, particularly depression/bipolar disorder and anxiety. The PCM group is characterized by more obesity, smoking and unemployment. PCM subgroups showed a higher prevalence of specific non-psychiatric comorbidities.
Analysis of HS patient demographics and clinical data in Lithuania
Tadas Raudonis1, Vitalij Cernel2, Ruta Ganceviciene1, Christos C. Zouboulis3
1Vilnius University, Centre of Dermatovenereology, Vilnius, Lithuania; 2Vilnius University, Faculty of Medicine, Vilnius, Lithuania; 3Brandenburg Medical School Theodor Fontane and Faculty of Health Sciences Brandenburg, Staedtisches Klinikum Dessau, Departments of Dermatology, Venereology, Allergology and Immunology, Dessau, Germany
Hidradenitis suppurativa is a lifelong disease affecting the hair follicle and apocrine glands with severe impact on quality of life and recurrent course. As HS significantly compromises quality of patient's life, it is crucial that diagnosis should be identified without delays and decrease the burden of such patients on the healthcare system. Objectives of the study were to evaluate the main demographic data of HS patients along with differences in clinical presentation. HS patients presenting to a reference Centre of Dermatovenereology in Lithuania were assessed according to EHSF guidelines at various stages of disease and treatment. Data was processed using MS Excel and IBM SPSS 26.0. Normal distribution of the data was assessed using the Shapiro–Wilk test. The homogeneity of the groups was evaluated using the chi-square test. Differences are considered statistically significant when the value is below the significance interval of 0.05 (p < 0.05). The study included 47 patients, 44.7% (n = 21) of them – female. The average age of the subjects was 39.72 ± 13.297 years, average BMI – 28.41 ± 6726. 59.57% (n = 28) had comorbidities, such as psoriasis, inflammatory bowel disease, joint pain, metabolic disease, dislipidemia, hypertension. Only 17% (n = 8) had a family history of HS, but a strong positive correlation was found between family history of inflammatory diseases (70.2% (n = 33)) and the severity of HS (r = 0.69 p < 0.05). 59.57% (n = 28) are active or previous smokers, with an average of 27.11 pack years. Among Hurley III patients (n = 11), 90% (n = 10) were smokers. Average disease onset was 26 ± 7456 years, patients sought medical care at 29 years, and the mean time to diagnosis was 5.4 D ± 7.75 years. 70.2% (n = 33) were previously misdiagnosed. On average, subjects had 6.17 ± 6.98 painful days over the last 4 weeks. The average intensity of pain according to the VAS scale was 5.43 ± 3.33 points. Mean DLQI at baseline was 8.9 ± 7.436. 27% (n = 13) were on biological therapy 12.76% (n = 6) were on systemic antibiotics, 14.89% (n = 7) – both biologics and systemic antibiotics, 44.68% (n = 21) were treated only with topicals. Patients on biologics had a mean DLQI of 7.3, systemic antibiotics - 10.5, others – 7. Patients on biologic treatment had a mean IHS4 of 7.38 at the beginning of treatment, 3.22 at follow up (p < 0.05). Meanwhile, for patients not on biologics, the initial IHS4 score was 6.21, and 5.42 at follow-up (p > 0.05). 29.78% (n = 14) had recurrent inflammatory lesions in the last 4 weeks with no differences between patients on biologics or systemic antibiotics. The majority of HS patients were obese and more than half had comorbidities. 70% had a family history of inflammatory diseaseses which correlate with the severity of HS. 60% of patients were active or previous smokers, majority of Hurley III patients were smokers. Majority of the patients were misdiagnosed and had to wait for approximately 5 years for correct HS diagnosis. Patients on biologic treatment had higher improvement of IHS4 compared to other treatment modalities.
Acknowledgement: The Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin – ALLOCATE Skin group).
Association of dowling-degos disease in hidradenitis supurativa patients
Patricia Garbayo-Salmons1, Jose Carlos Pascual2, Antonio Martorell3, Eugenia Agut-Busquet4, Jorge Romaní de Gabriel5
1Consorci Sanitari Parc Taulí, Department of Dermatology, Sabadell, Spain; 2Hospital General Universitario de Alicante, Department of Dermatology, Alicante, Spain; 3Hospital de Manises, Department of Dermatology, Manises, Spain; 4Consorci Sanitari Parc Taulí, Department of Dermatology, Sabadell, Spain; 5Hospital General de Granollers, Department of Dermatology, Granollers, Spain
Dowling-Degos disease (DDD) is a rare benign autosomal dominant genodermatosis presenting with reticulated flexural hyperpigmentation. Although hidradenitis suppurativa (HS) is the most well-known comorbidity associated with DDD, little is known about the clinical presentation and prognosis of HS in DDD. We retrospectively reviewed a multicentric cohort of 32 patients with concomitant HS and DDD (group 1), and we assessed possible differential factors compared to a control group of 636 patients with isolated HS (group 2). Overall patients in group 1, 43.8% were men with a mean BMI of 27.9 kg/m2. Regarding family history, 75% reported history of HS and 50% of DDD. Moreover, 84.4% were active smokers and they all shared a Fitzpatrick skin type over III. Clinical exploration revealed main involved areas were axillae (75%), groins (71.9%) and trunk (68.8%). In some cases, freckle-like macules were predominant over reticulate macules. Biopsy was performed in all cases, confirming diagnosis suspicion of DDD. Genetic study was accomplished in 46.9% patients, with identification of the following pathogenic mutations: POFUT-1 (21.9%), NCSTN (15.6%), PSENEN (3.1%) and APH1B (3.1%). Significant differences were observed between group 1 versus 2 in terms of basal Hurley: I (15.6 vs. 41.6%), II (53.1 vs. 32.2%) and III (31.3 vs. 26.2%); and HS phenotype at diagnosis: LC1 (31.3% vs. 41.12%), LC2 (50% vs. 23.05%) and LC3 (18.8% vs. 35.8%). Control group comprised patients from a monographic HS clinic in a tertiary hospital, thus representing a more severe subset of patients. We present the largest series of patients with DDD and concomitant HS to date. Our results suggest a higher basal Hurley and a higher prevalence of follicular phenotype in patients with concomitant DDD and HS when compared to patients with isolated HS.
Serum uric acid levels in hidradenitis suppurativa: A case–control study
María José Sánchez Pujol1, Alexandre Docampo2, Ines Poveda3, Iris Gonzalez-Villanueva2, Francisca Sivera4, Jose Carlos Pascual2
1Hospital Marina Baixa, Department of Dermatology, Villajoyosa, Spain; 2Hospital General Universitario de Alicante, Department of Dermatology, Alicante, Spain; 3Hospital de Elda, Department of Dermatology, Elda, Spain; 4Hospital de Elda, Department of Rheumatology, Elda, Spain
According to recent publications, elevated levels of uric acid (UA) may be associated with metabolic syndrome and increased cardiovascular risk, and UA levels appear to decrease in the phases of acute inflammation. To date, no studies have been published on the association between hidradenitis suppurativa (HS) and serum UA levels. We performed a single-centre case–control study, recruiting patients with moderate to severe HS, and healthy controls with the objective of comparing the prevalence of hyperuricaemia in HS patients and in healthy controls. We included 200 people with HS and 100 healthy controls, matched to the cases. The participants with HS were more likely than the controls to have obesity (P < 0.001) and metabolic syndrome (P < 0.001). The prevalence of hyperuricaemia was 18% in the HS patients and 13% in the controls, with no statistically significant differences between the two groups. In the multivariate analysis, the variables associated with hyperuricaemia were male sex (OR 2.3; 95% CI 1.13–4.68; P = 0.022), BMI (OR 1.09; 95% CI 1.01–1.15; P = 0.025) and metabolic syndrome (OR 3.8; 95% CI 1.3–11.2; P = 0.014). We found no association between hyperuricaemia and HS. No difference was observed between the prevalence of hyperuricaemia in people with hidradenitis suppurativa and in the general population.
Hidradenitis suppurativa and food insecurity: A stratified analysis
Shivani Jain1, Aditya Sood2, Sokol Tushe2, Ryan Dieudonne2, Lauren Orenstein3
1Louisiana State University Health Sciences Center-New Orleans, School of Medicine, New Orleans, USA; 2Emory University, School of Medicine, Atlanta, USA; 3Emory University School of Medicine, Department of Dermatology, Atlanta, USA
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that affects 1%–4% of the population1. While previous research has raised the importance of exploring social determinants of health in HS, little exploration has been done to assess which factors are important to consider2,3. Prior research has shown an association between obesity and HS, and emerging studies highlight an association between food insecurity and obesity2,4. As such, this study aimed to investigate the association between HS and food insecurity, while adjusting for the potential confounders of age, sex, and race. Individuals ≥18 years old with self-reported diagnosis of HS as well as those without HS were identified through the All of Us database. The final sample included 127 individuals with HS for whom demographic information was available on age, sex, and race and who completed the Children's HealthWatch Hunger Vital SignTM screening survey (validated for use among adults in 2017), as well as 381 random non-HS controls for whom the same metrics were available. After stratifying data by food security status, univariate and multivariate logistic regression models were run to analyse the effects of age, sex, and race between those with and without HS. Additionally, separate models were run to account for effect modification by age, sex, and race on food security status in just those individuals with HS. Demographic analysis showed that among those with HS the mean age was 51.9 years, and 84% were female, 66% were Caucasian, and 17% were African-American. Logistic regression analyses showed that food insecurity was significantly associated with HS (OR unadjusted = 3.47 [2.08, 5.78]; OR adjusted 2.55 [1.46, 4.45]), even after adjusting for confounders of age, sex, and race. Among those with HS, sex (OR: 0.53 [0.20, 1.48]) was not found to have a significant association with food security status. However, for those with HS who racially identified neither as white nor as black, there was a significantly increased association with also being food insecure (OR: 3.04 [1.02, 9.06]). This study supports an association between HS and food insecurity. There are several limitations to this study, including the use of a self-selected population that may not be fully representative of the larger HS population and may limit the generalizability of findings. Recent literature suggests that HS goes underdiagnosed and understudied among African-Americans3,5. Although the All of Us database seeks to increase sampling population diversity, its current sample may also be affected by this bias. Further research is needed to elucidate the relationship between HS and food insecurity and how factors such as race may modify it. This can help inform potential interventions to decrease food insecurity among those with HS.
Acknowledgement: The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085 U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276. In addition, the All of Us Research Program would not be possible without the partnership of its participants.
- Sabat R et al. Hidradenitis suppurativa. Nat Rev Dis Primers 2020;6:18.
- Kromann CB et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol 2014;94:553–7.
- Vaidya T et al. Examining the race-specific prevalence of hidradenitis suppurativa at a large academic center; results from a retrospective chart review. Dermatol Online J 2017;23:13030.
- Keenan GS et al. Household Food Insecurity, Diet Quality, and Obesity: An Explanatory Model. Obesity (Silver Spring) 2021;29:143–9.
- Price KN et al. Race and Ethnicity Gaps in Global Hidradenitis Suppurativa Clinical Trials. Dermatology 2021;237:97–102.
Early impairment of endothelial and myocardial function in patients with hidradenitis suppurativa
Ignatios Ikonomidis1, Georgios Pavlidis1, Ourania Efthymiou2, Konstantinos Katogiannis1, Konstantina Diamanti2, Georgia Pappa2, Ioanna Tseneklidou2, Gerasimos Filippatos1, Alexander Katoulis2
1National and Kapodistrian University of Athens, Medical School, 2nd Department of Cardiology, “Attikon” General University Hospital, Athens, Greece; 2National and Kapodistrian University of Athens, Medical School, 2nd Department of Dermatology and Venereology, “Attikon” General University Hospital, Athens, Greece
Hidradenitis suppurativa (HS) is a chronic, recurrent, debilitating, inflammatory skin disease, manifested by painful, deep-seated, inflamed lesions of the apocrine gland-bearing areas of the body (axillae, submammary folds, inguinal and anogenital region). HS has been associated, among others, with an increased cardiovascular risk. Our aim was to investigate the endothelial, arterial and left ventricular (LV) myocardial function in patients with severe HS. Twenty-eight, previously untreated patients (mean age: 36 ± 13 years) with Hurley stage III disease and a mean score of 19 ± 8 on the International Hidradenitis Suppurativa Severity Score System (IHS4) scale, were studied. Twenty healthy sex-, age- and body mass index -matched individuals, who were also weighted for common cardiovascular risk factors, served as the control group. Both patients and controls were evaluated for: a) the perfused boundary region (PBR) of the sublingual microvessels with a diameter of 5–25 μm, using a dedicated camera (Microscan, GlucoCkeck). An increased PBR value is considered as a marker of decreased thickness of the endothelial glycocalyx; b) the carotid-femoral pulse wave velocity (PWV-Complior); c) the flow-mediated dilation (FMD) of the brachial artery; and d) the LV global longitudinal strain (GLS), using the speckle-tracking echocardiography. Compared to healthy controls, HS patients had increased PBR value (2.32 ± 0.19 vs. 1.99 ± 0.14 μm, p < 0.001) and decreased FMD (5.7 ± 2.9 vs. 10.3 ± 1.9%, p < 0.001) and GLS values (−18.6 ± 2.8 vs. −22.5 ± 2.3%, p < 0.001). In contrast, similar PWV values (9.2 ± 2 vs. 9.1 ± 1.9 m/s, p = 0.937) were recorded between patients and controls. Increased IHS4 score (more severe disease) was associated with increased PBR values (r = 0.42, p = 0.026) and decreased FMD values (r = −0.37, p = 0.045). Patients with HS show early impairment of endothelial and LV myocardial function, which correlate with the severity of the underlying systemic inflammation.
Paradoxical psoriasis in patients with hidradenitis suppurativa
Gemma Martin-Ezquerra, David Pesque, Helena Escola, Marta Ferran, Ramon Pujol Vallverdu
Hospital del Mar, Department of Dermatology, Barcelona, Spain
The induction or exacerbation of psoriasis (PSO) in patients treated with tumour necrosis factor (TNF) antagonists is a well-established phenomenon. It is calculated that 2 to 5% of treated patients develop psoriasis-like skin lesions called paradoxical PSO. The pathogenesis of this reaction seems to involve a disruption in cytokine balance by TNF blockade, allowing unopposed interferon alpha production in genetically predisposed individuals1. As it frequently requires cessation of the drug, paradoxical psoriasis is a challenge in the management of patients with chronic inflammatory diseases, such as inflammatory bowel disease, rheumatoid arthritis and hidradenitis suppurativa (HS). Patients often respond to conventional PSO treatments and may have resolution of the lesions over time without interruption of biologic therapy. Anti-TNF discontinuation usually results in lesion resolution. Patients with severe lesions, like erythrodermic or pustular presentations, should cease TNF antagonist use. PSO and HS although different diseases share pathogenic features. Studies of prevalence have shown varying results: A study from Korea found that among their 28 516 inhabitants with HS, the prevalence of PSO was 3.86%.2 In Denmark, the 6.4% of HS patients had PSO3. We present five cases of paradoxical PSO in HS-patients treated with TNF antagonists (Table 1). We included three women and 2 men, middle-aged. None of them had previous PSO or family history of the disease. Psoriasis developed varying from 14 days after the initiation of the TNF antagonist to 14 months. Two cases corresponded to pustular psoriasis, and three to plaque type psoriasis. Management varied from adding topical treatment to modifying the TNF antagonist for another biological drug (secukinumab or ustekinumab) and in one case, methotrexate was added. Paradoxical psoriasis while on TNF antagonist treatment is not an uncommon side effect. Management can vary among severity and preferences of each patient.
TABLE 1 Characteristics of the series of patients with paradoxical PSO.
| Gender, Age | Hurley | Previous PSO | Familiar history of PSO | TNF antagonist | Time of appearance | Type of PSO | Outcome |
|---|---|---|---|---|---|---|---|
| M, 33 | III | No | No | Adalimumab | 12 months |
Plaque-type PASI 3 |
Resolution with topical treatment |
| F, 29 | II | NO | No | Adalimumab | 21 days | Pustular psoriasis | Stop adalimumab. Secukinumab |
| F, 35 | III | No | No | Adalimumab | 14 months |
Plaque type PASI 4 |
Improvement with topical treatment |
| F,46 | II | No | No | Adalimumab | 6 months |
Plaque type PASI 4 |
Stop adalimumab. Ustekinumab |
| M, 50 | III | No | No | Infliximab | 6 months | Pustular | Addition of methotrexate |
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumour necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum 2010;40:233–40.
- Lee JH et al. Prevalence and comorbidities associated with hidradenitis suppurativa in Korea: a nationwide population-based study. J Eur Acad Dermatol Venereol 2018;32:1784–90.
- Kjaersgaard Andersen R et al. Psoriasis as a comorbidity of hidradenitis suppurativa. Int J Dermatol 2020;59:216–20.
Overlapping inflammatory diseases in hidradenitis suppurativa patients and their families
Elif E. Gülbezer1, Adil Özcanlı2, Defne Baskurt3, Seçil Vural2
1Koç University School of Medicine, Division of Rheumatology, Department of Internal Medicine, Istanbul, Turkey; 2Koç University School of Medicine, Department of Dermatology, Istanbul, Turkey; 3Koç University School of Medicine, Istanbul, Turkey; 4Koç University School of Medicine, Department of Dermatology, Istanbul, Turkey
Hidradenitis suppurativa (HS) is an inflammatory disorder characterized by chronic deep-seated nodules, sinus tracts, and scars in the intertriginous areas. A significant decrease in quality of life accompanies chronic pain of active lesions. Association with inflammatory bowel disease (IBD) and spondyloarthropathies suggest an inflammatory signature overlap with rheumatological disorders. In this study, we aim to investigate inflammatory conditions in a cohort of HS patients. Consecutive HS patients presented to the dermatology outpatient clinic between 2021–2022 are included in the study. Among the HS patients who applied to dermatology between 2021 and 2022, patients with arthralgia and low back pain were referred to the rheumatology department for evaluation. Family history was taken in detail for inflammatory conditions. Acute phase reactants were analysed. The group comprised 55 HS patients, including sixteen Hurley stage I (27.2%), 24 Hurley stage II (41.8%), and 14 (23.6%) Hurley stage III patients. The mean age of onset for HS was 28.8 (min:14, max:56). 51% (N = 28) were women. Family history for HS was present in 14 (25.4%) patients. Twenty-four (43.6%) of 55 HS patients who applied to the dermatology outpatient clinic had an autoinflammatory/autoimmune disease diagnosis or inflammatory symptom/finding other than HS (Table 1). Sixteen (29%) patients described arthralgia and were referred to the rheumatology outpatient clinic. Among the HS patients, 32.7% had a significant autoimmune or autoinflammatory condition other than HS in 1st or 2nd-degree relatives. Positive family history for inflammatory processes other than HS was present in 10 (41.6%) patients with an additional inflammatory condition and 8 (25.8%) patients without a different inflammatory disease. Family history of inflammatory disorders include (Familial Mediterranean fever, acne conglabata, inflammatory bowel disease, psoriasis, Takayasu arteritis, amyloidosis, ankylosing spondylitis, uveitis, erythema nodosum, Behçet's disease and vitiligo. Serum amyloid A levels (SAA) were calculated in 16 patients, and 62.5% had elevated SAA levels. Autoinflammatory rheumatological diseases or rheumatological symptoms were present in a significant proportion of HS patients. Prominent family history of inflammatory diseases suggests common genetic pathways in this spectrum of diseases. In addition, detailed history taking, including rheumatological symptoms in HS patients, is valuable and crucial in determining the extent of inflammation.
TABLE 1 Inflammatory features of patients with autoimmune or autoinflammatory findings other than HS.
| Patients with autoimmune or autoinflammatory findings other than HS | n = 24 | % |
|---|---|---|
| Inflammatory joint pain or peripheral arthritis | 10 | 41.6 |
| Sacroiliitis/ankylosing spondylitis | 7 | 29.1 |
| Familial Mediterranean fever (FMF) | 5 | 20.8 |
| Recurrent oral aphthosis | 5 | 20.8 |
| Inflammatory low back pain | 4 | 16.6 |
| Uveitis | 3 | 12.5 |
| Papulopustular lesion | 3 | 12.5 |
| Inflammatory bowel disease | 2 | 8.3 |
| Genital aphthae | 2 | 8.3 |
| Psoriasis | 2 | 8.3 |
| Erythema nodosum | 1 | 4.1 |
| Heel pain | 1 | 4.1 |
| Inflammatory neck pain | 1 | 4.1 |
| Gout | 1 | 4.1 |
| Sarcoidosis | 1 | 4.1 |
| Autoimmune hepatitis | 1 | 4.1 |
| Type 1 diabetes mellitus | 1 | 4.1 |
Hidradenitis suppurativa occurs in a specific familial context: Results from a french prospective case–control study
Anne-Sophie Gadat1, Marion Clerbout1, Christelle Chavrier1, Marianne Beuque1, Samantha Gaspard1, Ines Evain1, Myriam Fioretta1, Christelle Enault1, Virginie Vlaeminck-Guillem3,4, Philippe Guillem1,2
1Clinique du Val d'Ouest, Department of Surgery, Ecully, France; 2RésoVerneuil, Paris, France; 3CHU, Department of Biology, Lyon, France; 4Lyon 1 University, INSERM 1052, CNRS 5286, CRCL, Lyon, France
Hidradenitis suppurativa (HS) is known to be associated with several inflammatory (bowel, joints, asthma…), follicular (pilonidal sinus disease…), metabolic (obesity, diabetes, dyslipidemia, thyroid diseases…) and cardiovascular (high blood pressure, stroke, myocardial infarction…) comorbidities. Clinical practice suggests that these comorbidities are not only carried by the patients but also by their relatives. The aim of the study was to assess prevalence of several HS comorbidities in the relatives of control subjects and HS patients using a prospective, cross-sectional study. As part of the GHiSA project (estimation of HS prevalence in a control population through a validated screening questionnaire), we prospectively constructed a cohort representative of the general French population and including control patients referred to the Clinique du Val d'Ouest (Lyon, France) for consultation or hospitalization for a pathology other than HS, and those accompanying patients coming for such situations. To avoid a family aggregation bias, we excluded accompanying persons from the same families as the control patients. At the same time, we included all consecutive HS patients coming to the HS-specialized centre of the healthcare institution for consultation or hospitalization. The HS patients identified after clinical validation among the control patients and accompanying persons were included in the HS cohort. All subjects were proposed to fulfil a questionnaire including questions about several comorbidities including bowel and joint inflammatory diseases, asthma, pilonidal sinus disease, obesity, diabetes, dyslipidemia, thyroid diseases, high blood pressure, stroke, myocardial infarction and the last two grouped in a MACE item (major adverse cardiovascular events). Between March and November 2022, we collected 1047 controls (507 accompanying adults and 540 patients) and 446 HS patients. Univariate analyses showed that all HS comorbidities present as a familial history were associated with HS, except dyslipidemia and stroke. HS diagnosis was significantly more frequent in patients with a family history of pilonidal sinus disease (relative risk = 5.37), inflammatory joint disease (RR = 3.79), inflammatory bowel disease (RR = 3.04), obesity (RR = 2.52), diabetes mellitus (RR = 2.20), thyroid disease (RR = 1.74), high blood pressure (RR = 1.58), myocardial infarction (RR = 1.55) and MACE (RR = 1.41). Multivariate analysis showed that a family history of pilonidal sinus disease (RR = 4.73), inflammatory joint disease (RR = 2.72), inflammatory bowel disease (RR = 2.22), diabetes mellitus (RR = 1.80), and thyroid disease (RR = 1.59) were all independent predictors of HS. As high as 30% of HS patients report a family history of HS. A mutation in one of the gamma-secretase genes and transmitted in an autosomal dominant mode has been found in only a very small proportion of familial cases (about 5%, frequently in association with a particular phenotype). The current pathophysiological hypothesis is rather that of a polygenic disease with a HS phenotype (extremely variable) resulting from a combination of genetic and probably epigenetic events. Despite obvious methodological biases (self-questionnaire with recall bias), our study shows that HS really does occur in a particular family context since family histories as varied as PSD, inflammatory digestive or rheumatological disorders, metabolic abnormalities (obesity, diabetes) or thyroid diseases are significant independent risk factors. Interestingly, the diversity of these intra-familial co-morbidities covers different aspects of the pathophysiology of HS: skin, hair follicles and their interaction with skin bacteria for PSD, auto-inflammation for obesity and digestive, rheumatological and thyroid diseases, and metabolic disorders for obesity and diabetes. All these clinical situations may present as a familial aggregation due to either genetic phenomena or a family-specific lifestyle. Family history plays a particular role in the occurrence of HS. What parents potentially pass on to their children is not unequivocal and includes diverse susceptibilities involving both hair follicle physiology, auto-inflammation mechanisms and metabolic disorders. Further studies are required to determine whether these susceptibilities are part of a genetic or a lifestyle background.
Inflammatory comorbidities in hidradenitis suppurativa: A retrospective monocentric study
Flaminia Antonelli, Dalma Malvaso, Andrea Chiricozzi, Ketty Peris
Università Cattolica del sacro Cuore, Dermatology, Roma, Italy
Prevalence of abdominal aortic aneurysm in patients with hidradenitis suppurativa in the VA health care system
Zachary Wendland1,2, Katelyn J. Rypka1,2, Claire Herzog3, Lindsey Greenlund3, Travis Fulk4, Noah I. Goldfarb5,6
1University of Minnesota, Department of Dermatology, Minneapolis, USA; 2Minneapolis Veterans Affairs Health Care System, Department of Dermatology, Minneapolis, USA; 3University of Minnesota, Medical School, Minneapolis, USA; 4Southern Illinois University, School of Medicine, Springfield, USA; 5University of Minnesota, Department of Medicine and Dermatology, Minneapolis, USA; 6Minneapolis Veterans Affairs Health Care System, Department of Medicine and Dermatology, Minneapolis, USA
Hidradenitis suppurativa (HS) is an inflammatory condition characterized by painful nodules, abscesses and draining tunnels involving body folds. Elevated interleukin (IL)-17 levels have been found in both the skin and blood of patients with HS1. Abdominal aortic aneurysm (AAA), a focal dilation of the aorta, is a potentially life-threatening condition, thought to be partially driven by IL-17 inflammation2 and has been found to have increased prevalence in patients with psoriasis, an IL-17-mediated skin disease3. To date, no studies have explored the relationship between AAA and HS. The objective of our study was to ascertain if a diagnosis of HS is associated with an increased prevalence of AAA compared to patients without HS within the Veterans Affair Health Care System (VAHCS). A retrospective, cross-sectional study of VAHCS patients using the VA Informatics and Computing Infrastructure (VINCI) database was conducted between January 1, 2017 – December 31, 2021. 7 465 613 veterans were included in our analysis of AAA, with 43 647 veterans having at least one diagnosis of HS by ICD9 (705.83) or 10 (L73.2). Our cohort of patients were largely white (78.0%), men (89.6%) greater than 65 years of age (52.9%). Overall, patients with HS were 1.33 times more likely than non-HS patients to have associated AAA diagnosis (adjusted odds ratio [aOR] = 1.26–1.40; p < 0.001) (3.82% vs. 3.66%), after adjusting for sex, age, race, obesity, tobacco use, diabetes mellitus type II and cardiovascular comorbidities. Male patients, greater than age 65, with a history of tobacco use and without a diagnosis of HS (population currently screened for AAA), had a prevalence of AAA of 9.1. This was compared to male patients, greater than age 65, with a history of HS, and without a diagnosis of tobacco use, which had a prevalence of AAA of 6.5%. The cross-sectional study design limited our understanding of the cause-and-effect relationship between HS and AAA. In addition, this study only evaluated prevalence of AAA, and did not investigate the association between HS and AAA rupture and mortality. Overall, our study demonstrates a statistically significant association between HS and AAA (aOR = 1.333 [1.26–1.40]; p < 0.001). Currently, the European Society of Cardiology (ESC) and the European Society for Vascular Surgery (ESVS) guidelines recommend a one-time screening for AAA using ultrasound in all men greater than 65 years old with a history of tobacco use4. While the prevalence of AAA in this demographic of patients does seem to be influenced by the presence/absence of HS, it does not appear to be clinically significant enough to warrant or suggest any changes to current guidelines. Future studies are needed on the cause-and-effect relationship of HS and AAA, as well as on the associated risk of rupture and mortality.
- Frew JW et al. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Res 2018;7:1930.
- Thanigaimani S et al. Potential of Disease-Modifying Anti-Rheumatic Drugs to Limit Abdominal Aortic Aneurysm Growth. Biomedicines 2022;10:2409.
- Khalid U et al. Nationwide Study on the Risk of Abdominal Aortic Aneurysms in Patients With Psoriasis. Arterioscler Thromb Vasc Biol 2016;36:1043–8.
- Cornell M, Perea G. Which patients should we screen to detect an aneurysm of the abdominal aorta? Eur Soc Cardiol; published 29.07.2020, accessed 12.12.2022. https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-18/which-patients-should-we-screen-to-detect-an-aneurysm-of-the-abdominal-aorta#:~:text=Considering%20all%20these%20data%2C%20both,)%20%5B1%2C17%5D.
Extrafacial demodicosis in a patient with hidradenitis suppurativa
Gavrail R. Poterov1,2, Rositsa V. Lavcheva1,2, Radostina S. Deliyska1,2, Tanya T. Gancheva1,2, Karen L. Manuelyan1,2, Evgeniya H. Hristakieva1,2
1UMHAT Prof. Dr. Stoyan Kirkovich, Department of Dermatology and Venereology, Stara Zagora, Bulgaria; 2Trakya University, Medical Faculty/Dermatovenereology, Stara Zagora, Bulgaria
Hidradenitis Suppurativa (HS) is а chronic inflammatory skin disease, the aetiology of which is still not fully understood. Many risk factors and comorbidities play a role in HS. Treatments such as antibiotics and biologics (Adalimumab, Infliximab) are used to reduce the severity of the disease and the frequency of the flare-ups. Demodex mites are ectoparasites often found in the hair follicles on the face. They can cause a rash with follicular scales, erythema and pruritus. A connection has been established between Demodex mites and rosacea. In rare cases, extrafacial involvement may also be observed. The antimicrobial peptide (LL-37) participates in inflammation and in the regulation of hair growth, and also has an effect on cell proliferation in angiogenesis. In rosacea, the Demodex mites stimulate Toll-like receptor 2, resulting in an increased production of LL-37. The levels of this peptide are also increased in HS. We present a female patient in her 50s with severe HS, who was treated with long courses of antibiotics and 2 months of adalimumab. The comorbidities and risk factors included diabetes, arterial hypertension and smoking. On a regular follow-up appointment, the patient complained of a new itchy rash on her chest and abdomen. Erythematous miliary papules with mild desquamation were observed. The patch test was negative. No bacteria or Candida spp. were cultured. A standard test for demodicosis was positive. The patient was treated with intravenous metronidazole (500 mg b.i.d.) plus ivermectin (200 mcg/kg once weekly for 2 weeks) with a favourable clinical response. This case is an unusual presentation of demodicosis in a female polymorbid patient with severe HS. Long-term use of local and systemic antibiotics in patients with HS might lead to dysregulation of the skin microbiota. Immunosuppression, as well as the presence of common risk factors such as smoking, diabetes, and hypertension also observed in our patient, could also contribute to the development of extrafacial demodicosis. All these factors, along with the increased LL-37 in both HS and demodicosis, could suggest a possible link between the two conditions.
Positivity rates of contact dermatitis patch testing in hidradenitis suppurativa patients
Ning McKenzie1, Lauren Schaefer2, Jill Killian2, Molly Youssef2, Afsaneh Alavi2
1Mayo Clinic Alix School of Medicine, Department of Dermatology, Phoenix, USA; 2Mayo Clinic, Department of Dermatology, Rochester, USA
Hidradenitis Suppurativa (HS) is an inflammatory skin condition that causes painful and debilitating draining tunnels and nodules in folded skin. The pathophysiology is complex and not fully understood, but it has increased prevalence in women, African Americans, smokers, and patients with increased BMI1. Patients with HS are exposed to numerous dressings, topical and oral therapies to control the continuous drainage and inflammation. A case–control study was performed comparing patients with HS who have received patch testing within the Mayo Clinic hospital system (17 total) matched with psoriasis patients using sex, age, and performed patch testing. Using the positivity definition of day 5 reaction grades 2, 3 and 4, we reported the allergens that have a nonzero positivity rate in HS patients and compared them to the overall positivity rates to the psoriasis patients. The allergens that had the highest positivity rates in HS patients are listed in Table 1. Out of the 27 allergens analysed, 22 of the allergens had higher positivity rates in HS than psoriasis patients. This is the first data on the rates of positive contact dermatitis patch testing in HS patients, and it shows specific allergens that are higher than the control group of psoriasis patients. Even though irritant contact dermatitis is very common in HS patients, insights into allergic contact dermatitis in HS patients can improve patient education and quality of life through avoidance of common allergens.
TABLE 1 Allergens with high positive rates in HS patients.
| Allergens | % positive in HS patients | % positive in psoriasis patients |
|---|---|---|
| Nickel (ll) sulfate hexahydrate 2.5% | 41.2 | 29.4 |
| Neomycin sulfate 20% | 25.0 | 18.8 |
| Quaternium 15 1% | 20.0 | 0.0 |
| 2-Bromo-2-nitropropane-1,3-diol 0.5% | 18.2 | 7.7 |
| Gold (I) sodium thiosulfate dihydrate 0.5% | 18.2 | 23.1 |
- Goldburg S et al. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 2020;82:1045–58.
Hidradenitis suppurativa and pemphigus: A difficult to treat combination
Florentina S. Delli, Aikaterini Tsentemeidou, Katerina Bakirtzi, Efstratios Vakirlis, Maria Smaragdi, Elena Sotiriou
Aristotle University of Thessaloniki, First Department of Dermatology-Venereology, Thessaloniki, Greece
A potential association between pemphigus and hidradenitis suppurativa (HS) has been recently discussed in literature, as a result of reports documenting the coexistence of the two diseases. We report the case of a woman with severe pemphigus vulgaris (BSA = 42%, PDAI = 58) diagnosed in 2010, successfully treated with standard corticosteroid schema for 4 years. The initial clinical presentation was dominated by multiple mucosal and skin erosions, while vegetating erosions were observed in the axillae. When pemphigus control was achieved, the clinical presentation changed. Both axillae and inguinal areas presented with multiple painful deep-seated nodules, abscesses and draining fistulas, lesions compatible with HS. Adalimumab 40 mg weekly was started in 2016 with subsequent excellent control of both diseases until September 2022. Pemphigus then relapsed and 1.5 mg/kg/day of methylprednisolone per os was started, which however had no effect on oral erosions. Only after adalimumab had been discontinued, did 1.5 mg/kg/day of methyl prednisolone per os succeed in bringing about the epithelization of skin and oral mucosal lesions. The patient refused rituximab therapy. Pemphigus is a recently described potential comorbidity of HS. It is possible that a common pathogenetic background is responsible for coexistence of the two diseases.
Association of hidradenitis suppurativa (HS) with myeloid cells dysplasias [myelodysplastic (MDS) or myeloproliferative syndromes (MPS)]: 4 cases
Pierre-Andre Bécherel1, Julie Blanc1, Melanie Dehais1, Jean-Christophe Moreno1, Marina Thomas1,5
1Antony Hospital, Dermatology and Clinical Immunology, Antony, France; 2Antony Hospital, Dermatology and Clinical Immunology, Antony, France; 3Antony Hospital, Dermatology and Clinical Immunology, Antony, France; 4Antony Hospital, Dermatology and Clinical Immunology, Antony, France; 5Antony Hospital and L'Yvette Clinique, Dermatology and Clinical Immunology, Antony, France
In auto-inflammatory diseases (AID), there is now a known association with myeloid lineage diseases: in Vexas syndrome, polychondritis, or Sweet syndrome for example. The pathophysiology of such associations might relie on mutations in the ubiquitin pathway1. HS belongs also to the spectrum of auto-inflammatory diseases: upregulation of interleukin 1β, interleukin-36, caspase-1, and NLRP3 and dysregulation of the Th17/Treg cell axis have been demonstrated, suggesting that autoinflammation is a key event in the pathophysiology of the disease2. Notably, HS may be associated with other AID such as inflammatory bowel diseases or pyoderma gangrenosum, highlighting again the importance of autoinflammation in HS. We present 4 HS cases, associated with myeloid lineage diseases, as described now in other AID. This association is probably not fortuitous. Four patients were included in this short series, 2 men, 2 women. Mean age: 39 (36–46). All were classified as Hurley II. For all of them, the hematologic disease had begun before HS (3,6 years before in average). 2 myelodysplastic syndromes (MPS) (myeloid chronic leukaemia and myelofibrosis); 2 MDS (multilineage dysplasias). Mean IHS4 at inclusion: 12 (8–14). Only 1 smoker. Treatments used for the underlying hematologic disease: ruxolitinib, azacytidine or imatinib. The antibiotics failed in all patients (doxycycline and association of clindamycin/levofloxacin), and they were all switched to adalimumab. Success in 3 patients with an 18-month treatment duration on average currently and mean IHS4 reached and maintained: 4. Failure in the other case, switched to secukinumab, with success (12 months treatment currently), IHS4 reached: 5. Good control of the underlying hematologic disease, with no interaction with the biologics. HS is very complex and can be considered as an auto-inflammatory disease and might share some pathophysiological issues with other AID (Vexas, relapsing polychondritis, …), especially when associated with hematologic disorders. In these other AID, mutations in the ubiquitin genes have been discovered both in myeloid cells and in infiltrating cells in the dermis1. The subsequent overexpression of the IFN pathway is supposed to play an important part in the pathogenesis. IFN should probably be avoided in these hematologic patients and JAK inhibitors preferred to treat both HS and the underlying hematologic disease. This is at our knowledge a rarely reported association in HS. The next step should be to search for the ubiquitin mutations in these particular patients, which could be added to the already known pro-inflammatory gene mutations (NOD2, LPIN2, NLRP3, NLRP12, PSMB8, MVK, IL1RN, …).
- Beck DB et al. Disorders of ubiquitylation: unchained inflammation. Nat Rev Rheumatol 2022;18:435–47.
- Nomura T. Hidradenitis Suppurativa as a Potential Subtype of Autoinflammatory Keratinization Disease. Front Immunol 2020;11:847.
Hidradenitis suppurativa restricted to the pubic region in a woman with early menopause
Ivana Tusheva, Katerina Damevska
Ss Cyril and Methodius University in Skopje, University Clinic of Dermatology, Skopje, North Macedonia
- Cucu CI et al. Hidradenitis suppurativa in postmenopause. Acta Endocrinol (Buchar) 2021;17:274–7.
- Abu Rached N et al. The Role of Hormones in Hidradenitis Suppurativa: A Systematic Review. Int J Mol Sci 2022;23:15250.
- Zouboulis CC et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol 2019;33:19–31.
Antiphospholipid syndrome associated to severe hidradenitis suppurativa
Virginia R. Lopez Gamboa1, Mariana Kuyumllian2
1Private practice, Dermatology, Buenos Aires, Argentina; 2Rossi, Department of Radiodiagnosis and Imaging, Buenos Aires, Argentina
- David CB et al. Hidradenitis Suppurativa associated with systemic lupus erythematosus: a case report. Medicine (Baltimore) 2018;97:e0186.
- Saunte DM et al. Diagnostic delay in hidradenitis suppurativa is a global problem. BJD 2015;173:1546–49.
- Wortsman X et al. Ultrasound in-depth characterization and staging of hidradenitis suppurativa. Dermatol Surg 2013;39:1835–42.
Paediatric hidradenitis suppurativa: Follow up after 6 years. A descriptive study
Verónica Mora-Fernández, Julio Bassas-Vila
Hospital Universitari Germans Trias i Pujol, Dermatology Department, Badalona, Spain
Hidradenitis suppurativa (HS) is uncommon in patients of paediatric age1 and studies about its presentation and prognosis are limited2. There are no standardized guidelines about its treatment. Prognostic factors and evolution remain unknown3. A retrospective data collection was performed with patients first visited in 2016 and after 6 years follow-up. We reviewed medical record available. Inclusion criteria was diagnosis of HS before 18 years of age. Follow up of 12 paediatric HS patients was performed: 3 male and 9 female patients aged from 12 to 17 years old (mean age 14.3) with a mean BMI of 24.6 (range 17–31). Only one patient was a smoker. The 42% (5/12) of patients had family story of HS and the mean evolution time since onset was 2.6 years (range 1 to 7 years). The modified Hurley scale was distributed in 25% (3/12) for H1a, 16% (2/12) for H1b, 25% (3/12) for H1c, 16% (2/12) for H2a and 16% (2/12) for H2c. After 6 years, the modified Hurley scale was distributed in 50% (6/12) for H1a, 8% (1/12) for H1b, 25% (3/12) for H1c and 17% (2/12) for H3. The 17% (2/12) of patients had an HS progression, 58% (7/12) maintained his modified Hurley scale and the 25% (3/12) had an improvement of his HS. The most common phenotype was axilomammary (83%) and no changes on phenotype had been identified over the follow up. All patients had received topical antibiotics while the 75% (9/12) received one or more oral antibiotics (88% doxycycline, 11% amoxicillin, 11% azithromycin, 11% clarithromycin, and 11% trimethoprim sulfamethoxazole). A 17% (2/12) received zinc gluconate and only one patient received oral retinoids. After 6 years follow up, the 66% (8/12) received oral antibiotic in which doxycycline was prescribed in half of cases (4/8), combination of rifampicin and clarithromycin or doxycycline was prescribed in 37.5% (3/8) of cases and trimethoprim sulfamethoxazole was prescribed in 25% (2/8) of cases. Only two patients received biologic treatment: one was treated with adalimumab and the other received adalimumab and secukinumab after failure of the first. Only 2 patients received surgical treatment during the follow up. In our case series follow up we found a female predominance similar to other paediatric studies4,5. The 58% of patients (7/12) had no progression or improvement, suggesting that early HS is not a predictive factor. We also notice that not all patients were successfully treated following the guidelines due to parents' decisions or mild side effects. Although in both cases of progression, all treatments available were prescribed. To note, BMI was over the mean and the higher in both cases (29 and 31 respectively). It is important to highlight that only two patients received surgical treatment during this period, taking in mind that the presence of fistulae was in 33% (4/12) of cases since the first visit. In the paediatric population, it is very common to be concerned about overtreatment and since information in this age group is limited, no active treatment is usually prescribed. On the other hand, pre-teen symptom onset was observed in half of our patients, making paediatric HS an uncommon childhood disease that should be detected among paediatricians due to the importance of an early diagnosis2.
- Garcovich S et al. Paediatric Hidradenitis Suppurativa: A Cross-Sectional Study on Clinical Features and Treatment Approaches. J Cutan Med Surg 2022;26:127–34.
- Seivright J et al. Paediatric hidradenitis suppurativa: epidemiology, disease presentation, and treatments. J Dermatolog Treat 2022;33:2391–93.
- Choi E et al. Hidradenitis suppurativa in paediatric patients. J Am Acad Dermatol 2022;86:140–47.
- Liy-Wong C et al. Hidradenitis Suppurativa in the Paediatric Population: An International, Multicenter, Retrospective, Cross-sectional Study of 481 Paediatric Patients. JAMA Dermatol 2021;157:385–91.
- Riis PT et al. Clinical characteristics of paediatric hidradenitis suppurativa: a cross-sectional multicenter study of 140 patients. Arch Dermatol Res 2020;312:715–24.
Clinical burden of hidradenitis suppurativa (HS) with draining tunnels
John Ingram1, Gregor B.E. Jemec2, Angelo V. Marzano3,4, Errol P. Prens5, Sylke Schneider-Burrus6, Isabel Truman7, Ana C. Hernandez Daly8, Robin Jha8, Hyemin Song8, Alexa B. Kimball9
1Cardiff University, University Hospital of Wales, Division of Infection and Immunity, Cardiff, UK; 2Zealand University Hospital, Department of Dermatology, Roskilde, Denmark; 3Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Dermatology Unit, Milan, Italy; 4Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy; 5Erasmus University Medical Center, Department of Dermatology, Rotterdam, Netherlands; 6Center for Dermatosurgery, Havelklinik, Berlin, Germany; 7Adelphi Real World, Bollington, UK; 8Boehringer Ingelheim International GmbH, Ingelheim Am Rhein, Germany; 9Harvard Medical School and Clinical Laboratory for Epidemiology and Applied Research in Skin (CLEARS), Department of Dermatology, Beth Israel Deaconess Medical Center, Boston, USA
Affecting an estimated 0.3%–1.0% of people worldwide, hidradenitis suppurativa (HS) is a debilitating chronic, inflammatory skin disease associated with a considerable clinical burden. Patients present with painful skin lesions typically affecting skin folds in the axillary, groin, gluteal and perianal body regions. These lesions include inflammatory nodules (IN), abscesses, and draining tunnels (dT). While the overall burden of HS has previously been described, there are no data on the impact of dT on clinical burden. The objective of the study was to explore the clinical burden of moderate-to-severe HS in patients presenting with and without dT. This retrospective study was conducted using data from the Adelphi HS Disease Specific Programme (DSP™) across the United States, France, Germany, Italy, Spain and the United Kingdom. Real-world clinical data were collected from November 2020 to April 2021 through a combination of physician surveys, medical record data extraction (also completed by the physician), and patient surveys. All physicians in the study were dermatologists who were actively involved in the management of HS. Patients were classified as having moderate-to-severe HS, based on physician assessment. Descriptive statistics were assessed using StataCorp. (Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC. 2021). Of the 580 patients with moderate-to-severe HS included in this study, 46% (n = 264) had dT. Demographics were similar between the two groups. For patients with and without dT, mean age was 38.9 and 33.3 years, 55.3% and 57.6% were female, and mean BMI was 28.6 and 28.4 kg/m2, respectively. The most common comorbidities reported by in patients with HS were obesity, depression, acne, anxiety, and dyslipidaemia. Compared with patients without dT, a higher proportion of patients with dT had deteriorating or unstable disease in the 12 months prior to study initiation (51.6% vs. 35.1%). Patients with dT were also likely to have more skin lesions (nodule/abscesses); half (50.4%) of patients with dT had 2–5 abscesses, compared with 33.5% of patients without dT. Moreover, a higher proportion of patients with dT had ≥6 IN (18.6% vs. 6.3%) and scarring (92.1% vs. 71.2%). Patients with dT also experienced more inflammation (73.5% vs. 63.5%), drainage of lesions (62.1% vs. 40.0%), malodorous drainage (40.5% vs. 21.3%), and depression (29.6% vs. 18.1%). A greater proportion of patients with dT had lesions affecting the anus/perianal skin (24.6% vs. 13.3%) and genitalia (39.8% vs. 26.6%). The most common treatments for HS that patients with dT received at the time of data collection were systemic antibiotics (49.6% of patients with dT; 48.8% of patients without dT) and biologics (40.6% of patients with dT; 27.5% of patients without dT). Patients with dT were also more likely to receive treatment with systemic/intralesional corticosteroids (17.7% vs. 8.5%). A considerable proportion of patients were eligible to receive biologics but were not receiving them at the time of data collection (58.4% of patients with dT; 30.3% of patients without dT), often because the physician wanted to exhaust other treatment options first. Moreover, a higher proportion of physicians felt unsatisfied with the current available treatments for patients with dT (61.4% vs. 51.2%). While some patients with HS had been treated with surgical incision and drainage (48.5% of patients with dT; 31.3% of patients without dT), many had never received any surgical intervention (40.5% of patients with dT; 60.8% of patients without dT). To date, this study is the first to explore the effect of dTs on the clinical burden of HS. Overall, our findings show that HS patients with dT experience a more substantial clinical burden than patients without dT, highlighting an unmet need for more effective disease management approaches, such as biologics and surgery, in this population.
Acknowledgement: The study was supported and funded by Boehringer Ingelheim. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE) and did not receive payment related to the development of this abstract. Boehringer Ingelheim was given the opportunity to review the abstract for medical and scientific accuracy, as well as intellectual property considerations. Isabella Goldsbrough, PhD, of OPEN Health Communications (London, UK) provided writing, editorial, and formatting support, which was contracted and funded by Boehringer Ingelheim. The Department of Dermatology, Zealand University Hospital, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
Hidradenitis suppurativa and keloids with macro comedones: An aberrant presentation of hidradenitis suppurativa
Daniel S. Andrade1, Kaique Arriel1, Artur A. Duarte1, Renata F. Magalhães2, Dimitri L.F. SIlva1
1Santo Amaro Medical School (UNISA), Department of Dermatology, São Paulo, Brazil; 2State University of Campinas (UNICAMP), Department of Dermatology, Campinas, Brazil
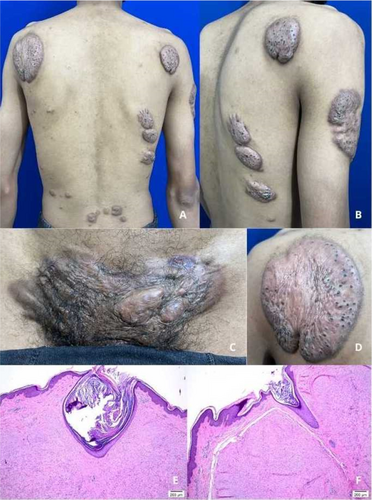
FIGURE 1 Hidradenitis Suppurativa and keloids with macrocomedones. A, B, D: Keloids on the trunk with macro comedones over its entire surface; C: Keloids, comedones and fistulas in the pubic area; E, F: Anatomopathological examination showing fibrous proliferation in the superficial dermis, in a nodular arrangement and intersected by bands of hyaline collagen, in the midst of which there are dilated follicular isthmuses with macrocomedones.
- Martorell A et al. Actualización en hidradenitis supurativa (I): epidemiología, aspectos clínicos y definición de severidad de la enfermedad, Actas dermo-sifiliográficas 2015;106:703–15.
- Sabat R et al. Hidradenitis suppurativa. Nature Rev Dis Primers 2020;6:18.
- Jfri A et al. Association of hidradenitis suppurativa and keloid formation: A therapeutic challenge JAAD Case Reports 2019;5:675–8.
- Lee S-Y et al. IL-17 Induced Stromal Cell–Derived Factor-1 and Profibrotic Factor in Keloid-Derived Skin Fibroblasts via the STAT3 Pathway. Inflammation 2020;43:664–72.
- Magalhães RF et al. Consensus on the treatment of hidradenitis suppurativa - Brazilian Society of Dermatology. An Bras Dermatol 2019;94(2 Suppl 1):7–19.
Investigation of hidradenitis suppurativa flares following COVID-19 vaccination: A cross sectional study
Aikaterini I. Liakou1, Andreas Tsantes2, Eythymia Agiasofitou1, Lydia S. Tsamtsouri3, Eleni Chatzidimitriou1, Soultana Vladeni1, Magdalini Kalamata1, Alexandros I. Stratigos1
1Andreas Syggros Hospital, 1st University Clinic of Cutaneous and Veneral Diseases, Athens, Greece; 2Agios Savas Hospital, Department of Biopathology, Athens, Greece; 3National and Kapodistrian University of Athens, Department of Hygiene Epidemiology and Medical Statistics, Athens, Greece
Vaccination against infections had been associated in the past with flares of autoimmune diseases. In the complex pathogenesis of hidradenitis suppurativa many immune pathways are involved, thus vaccination against Covid-19 virus could be associated with flares or even onset of the disease. The aim of the study is to detect and describe the flares and the new cases of HS after vaccination against COVID-19 infection. A cross sectional study was conducted, where 89 patients of the hidradenitis suppurativa department of Andreas Syggros hospital, were included. The collection of demographic data as well as the associated with HS data were obtained through the use of a questionnaire. Of the 89 patients that were included in the study, 74 (83.1%) had received at least 1 dose of mRNA vaccine against the Covid-19 infection, whereas 15 (16.9%) patients had not received any doses. From the 74 patients that were vaccinated, 19 (25.7%) reported flare after the vaccination, while 2 (2.7%) reported onset of hidradenitis after vaccination. Of the patients that experienced exaggeration of their condition, 11 (14.9%) were men and 8 (10.8%) were women. The results of this study demonstrate that a significant number of patients developed a flare of the disease after the administration of the Covid-19 vaccine. Those results underline the need for conduction of more studies with larger sample size, in order to determine whether there is a causal connection between vaccination and exaggeration or initiation of hidradenitis suppurativa.
Clinical practice gaps in the diagnosis and management of hidradenitis suppurativa
Elaine D. Bell1, Alessia - Piazza2, Alexa B. Kimball3
1Medscape Education Global, London, UK; 2Medscape Education Global, London, UK; 3Harvard Medical School, Department of Dermatology, Faculty of Medicine, Boston, USA
- 447 clinicians completed the activity: 20% dermatologists, 30% PCPs, 11% emergency medicine physicians, 10% obstetricians/gynaecologists and 23% surgeons
- 76% of dermatologists, 54% of PCPs and 56% of other clinicians saw 1 to 10 patients a month with HS; 8% of dermatologists, 3% of PCPs and 5% of other clinicians and saw 11 to 20 patients per month
- Approximately 50% of clinicians did not recognize that HS occurs more often in females compared with males or can occur in patients in their first or second decade of life
- Most clinicians (89% of dermatologists, 82% of PCPs and 78% of other clinicians) underestimated the number of patients with HS who had been misdiagnosed for 15 years or more
- 90–95% of clinicians underestimated the mean time between symptom onset and diagnosis as described in the UNITE study
- Approximately 30% of dermatologists, 60% of PCPs and 63% of other clinicians underestimated the number of inflamed lesions experienced by patients in a 6 month period
- 45% of dermatologists could not identify what Hurley stage 2 means in terms of disease severity with regards to abscesses, sinus tracts and scarring
- 35% of dermatologists, 39% of PCPs and 47% of other clinicians underestimated the risk of suicide in patients with HS compared with the general population
- Only 56% of dermatologists correctly identified topical clindamycin, oral tetracyclines, adalimumab and wide local excision as treatments that are strongly recommended for HS management across multiple international guidelines
- 76% of dermatologists correctly identified topical antibiotics as the preferred treatment for a patient with mild localized HS (Hurley stage 1 with a PGA of clear, minimal or mild) with few lesions and no sinus tracts
- 79% of dermatologists correctly recommended adding or changing to a disease modifying therapy for a patient with sinus tracts in the inguinal region and inflamed lesions on the buttocks who had visited the ER on 4 occasions that year for draining of lesions & pain medication, and who is currently treated with antibiotics
- Only 60% of dermatologists, 26% of PCPs and 35% of other clinicians were mostly or very confident in diagnosing HS
- Only 43% of dermatologists, 19% of PCPs and 24% of other clinicians were mostly or very confident in managing patients with HS.
This activity uncovered significant clinical gaps in knowledge, competence and confidence related to the burden of disease in HS, the patient experience of HS, severity assessment of HS and recommended treatment strategies in the management of HS. These results support the need for further education on evolving best practice for the diagnosis, assessment and management of HS, targeted at dermatologists and other clinicians who encounter patients with HS. This would be beneficial in supporting clinicians to translate knowledge into clinical practice in order to optimize outcomes for patients.
Acknowledgement: Supported by an independent educational grant from UCB Pharma https://www.medscape.org/viewarticle/962792.
- Saunte DM et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol 2015;173:1546–9.
- Chernyshov PV et al Quality of life in hidradenitis suppurativa: an update. Int J Environ Res Public Health 2021;18:6131.
Ectopic hidradenitis suppurativa: How difficult is it to diagnose and manage?
Patrícia Amoedo1, Gilberto Rosa1, Pedro Matos1, Filomena Azevedo1, Carmen Lisboa1,2
1Centro Hospitalar Universitário de São João, Department of Dermatology and Venereology, Porto, Portugal; 2Faculty of Medicine, University of Porto, Pathology Department, Microbiology Division, CINTESIS, Porto, Portugal
Hidradenitis suppurativa (HS) is a chronic, relapsing, inflammatory disease of the follicle, characterized by deep-seated abscesses with sinus tracts, in areas with a high density of apocrine glands, like axillae and anogenital, inguinal, and inframammary regions1,2. However, the reason for this distribution is unknown and rare cases of HS in non-apocrine areas, such as the face, abdomen, chest, tight, knee, foot and scalp have been reported.2-4 Here we report a case series of 4 patients with ectopic HS. All patients were male, aged between 25–49 years old (mean 36.5 ± 10.97), with severe HS (Hurley III, IHSA score >8 and HS-PGA: 4–5), involving atypical areas. Specifically, the dorsum of the foot (Patient 1); lateral aspects of buttocks, pre-sternal area, and arms (patient 2); face, trunk, calcaneus, and dorsal surface of 3rd toe of the right foot (patient 3); and penis, trunk, posterior aspect of the thigh and peristomal (patient 4). In patient 4, penile fistulae led to urethral stenosis requiring ileovesicostomy. Also, some lesions resemble pyoderma gangrenosum (PG), but histology ruled out this diagnosis. All patients except one (patient 2) had a typical distribution at the HS onset, with subsequent appearance of ectopic lesions over the course of the disease. Patient 2 has never developed lesions in classic locations. One patient was overweight and a smoker (patient 4) and another was an ex-smoker with family history of HS (patient 2). Only patient 4 had comorbidities, namely autoimmune thyroiditis, and sleep apnea. In general, oral antibiotics (doxycycline, clindamycin, rifampicin, and amoxicillin/clavulanic acid) and acitretin alone were ineffective, with some value as adjuvants. In all cases, therapy with adalimumab was initiated due to the failure of conventional therapies. All patients had partial remission, the longest of 3.5 years. However, the two patients under treatment for longer need a switch to ustekinumab after secondary failure. A dose increment of adalimumab to 80 mg weekly was tried in one patient, without success. The pathogenesis of HS is poorly understood and is a continuous source of debate. Previous theories considered infundibulum hyperkeratosis as the primary event, leading to follicle obstruction, dilatation, rupture, and inflammation of the surrounding dermis2. However, histopathology studies revealed that a subclinical inflammatory state precedes these changes5. It has been suggested that metalloproteinases (MMPs) overexpression in patient's skin may alter the extracellular matrix (ECM) and produce inflammation5. In ectopic cases, it has been suggested that friction may be the cause of hyperkeratosis1,2. Also, friction itself can induce an increase in MMPs, with subsequent ECM remodelling and release of pro-inflammatory mediators.5 Thus, perhaps there is no real predilection for apocrine-rich areas, but for greater friction-areas, such as intertriginous areas. However, in the cases we present here, the location of the lesions, although atypical, is not clearly related to friction. Genetic and environmental factors, such as tobacco and obesity, also contribute to the development of the disease1,2. Curiously, in this case series, only one patient was obese and a smoker and another had family history of HS, but two of them have no risk factors, which may suggest that there are other unknown predisposing factors involved. On the other hand, they all suffer from severe HS, and, in some extent, severity is genetically determined, as it tends to be similar between relatives. Then, we question whether, in severe forms, the genetic predisposition alone can trigger the disease regardless of the presence of friction or other risk factors, being ectopic location a marker of severity. Additionally, cases of HS located in an amputation stump, caesarean section scar, and striae distensae have been reported, posing the question of a Koebner effect2. We question if the peristomal lesion of patient 4 fits into this description. Ectopic HS can be difficult to diagnose, especially at presentation. Their lesions may resemble acne vulgaris, carbuncle, Crohn's disease, folliculitis, furuncle, granuloma inguinale, pilonidal cyst, and PG, and should be considered in the presence of recalcitrant nodules/abscess and fistulae1. Regarding the treatment, since the ectopic forms are typically severe, conventional treatments are usually insufficient, requiring biological treatment. However, in our experience, biological treatment tends to decrease inflammatory burden but does not inhibit occasional disease flares. In addition, most of these ectopic cases involved regions that are difficult to manage by surgery.
- Boer J, Mihajlovic D. Boils at Frictional Locations in a Patient with Hidradenitis Suppurativa. Acta Dermatovenerol Croat 2016;24:303–4.
- Gutierrez N, Cohen PR. Ectopic Hidradenitis Suppurativa: Case Report and Review of Literature. Cureus 2021;13:e12966.
- Rondags A et al. Ectopic hidradenitis suppurativa on the dorsal foot of a road maker. JAAD Case Rep 2017;3:429–31.
- Nisar S et al. Extensive hidradenitis suppurativa with dorsal foot involvement: a case report. J Surg Case Rep 2019;(11):rjz349.
- Boer J, Jemec GBE. Mechanical forces and Hidradenitis Suppurativa. Exp Dermatol 2021;30:212–15.
Reasons for consultation in a tele mentoring project for hidradenitis suppurativa in latin america
Virginia R. Lopez Gamboa1, Claudio Greco2, Mario Bittar3
1Private practice, Dermatology, Buenos Aires, Argentina; 2Hospital Dr Norberto Piacentini, Dermatology Department, Buenos Aires, Argentina; 3Hospital JN Lencinas, Dermatology Department, Mendoza, Argentina
Project ECHO® (Extension for Community Healthcare Outcomes) is a tele mentoring platform for varied specialties and pathologies over the world1. In Argentina there have been successful ECHO experiences for dermatology since 2015, regarding psoriasis and atopic dermatitis2. Considering Argentina is a vast country with difficult access to specialty care and HS is a rare and hard to treat dermatosis3, in 2020 we launched the project focused on Hidradenitis Suppurativa (HS). It has two options for participation: monthly free Zoom® meetings to discuss clinical HS cases and review up to date knowledge of the disease; and a WhatsApp® group for physicians that require real time clinical support from HS specialists. Although initially the project was aimed only for Argentina, over the past months, we have opened ECHO HS for any physician interested in HS in Latin America. The main objective of this study was to assess the clinicians´ reasons for consultation in ECHO HS over 2022. We reviewed the cases and topics discussed over the monthly meetings and conducted a short survey through the Whatsapp® group using Google forms. It included the type of preferred interaction (Zoom® or Whatsapp®), the reasons for consultation (diagnostic confirmation, deciding on treatment plans, biologic patient preparation and use, or referrals) and most useful Zoom interaction (clinical cases, updates, or both). Only complete surveys were included in the study. Over the last year, 12 meeting were conducted to discuss 16 clinical cases, and update 7 relevant topics. At the end of the study, the instant messaging group included 226 participants, of which 211 physicians were based in Argentina, 10 in Chile, 4 in Colombia and 1 in Spain. Most of the participants were dermatologists (n = 221) and 4 were radiologists specialized in dermatologic sonography. We received 62 complete surveys, of which the majority were female physicians (n = 49, 79%) living in Argentina (n = 61, 98%). Most preferred form of participation was both Zoom® and Whatsapp® (n = 41, 61%), while 18 (29%) chose only Whatsapp® and 3 (5%) Zoom® meetings. Regarding the reasons for consultation multiple answers were allowed, and the results were distributed as follows: 44 physicians used the group for referrals, 32 for decisions regarding treatment plans, 27 for biologic patient preparation and use and 22 for diagnostic confirmation. With relation to the topics discussed in the meeting, 97% (n = 60) answered that they preferred both clinical cases and updates. As seen by the results of this study, referring to colleagues is the most needed form of consultation as well as receiving guidance on treatment plans, while meetings are meaningful for clinical case discussion and relevant topic updates. This study demonstrates that tele mentoring in HS is useful for clinicians, allowing further research on barriers to accessing specialty care and treatment in our region.
- ECHO project. https://hsc.unm.edu/echo/. Download date: 12/19/2022.
- Mazzuoccolo DL et al. High impact of a national psoriasis telementoring clinic on medical practices for patients in underserved areas. Dermatol, Ther. 2021;1:e14575.
- Lavieri A et al. Consenso Nacional de Hidradenitis Supurativa. SAD 2019. https://sad.org.ar/wp-content/uploads/2019/12/Consenso-Hidradenitis-Supurativa2019-14112019.pdf Download date: 12/19/2022.
Could dermoscopy be a helpful instrument to improve hidradenitis suppurativa diagnosis accuracy?
Anna Pirogova, Natalya P. Teplyuk
Sechenov University, Department of Dermatology, Moscow, Russia
- Lacarrubba F et al. Double-ended pseudocomedones in hidradenitis suppurativa: clinical, dermoscopic, and histopathological correlation. First Ed, Wiley Blackwell 2018;22:491–3.
- Micali G (ed) Hidradenitis suppurativa: a diagnostic atlas. First Ed, Hoboken, NJ: Wiley, 2017: 87–9.
Correlation of clinical and ultrasonography with colour doppler disease severity staging in skin of colour patients of hidradenitis suppurativa
Sophia Rao1, Sunil Dogra1, Sendhil M. Kumaran1, Tarun Narang1, Anindita Sinha5
1Post Graduate Institute of Medical Education and Research, Department of Dermatology, Venereology and Leprology, Chandigarh, India; 2Post Graduate Institute of Medical Education and Research, Department of Dermatology, Venereology and Leprology, Chandigarh, India; 3Post Graduate Institute of Medical Education and Research, Department of Dermatology, Venereology and Leprology, Chandigarh, India; 4Post Graduate Institute of Medical Education and Research, Department of Dermatology, Venereology and Leprology, Chandigarh, India; 5Post Graduate Institute of Medical Education and Research, Department of Radiodiagnosis and Imaging, Chandigarh, India
Hidradenitis suppurativa (HS) is a recurrent chronic inflammatory skin disease of the apocrinegland bearing areas of the body. Physical examination underestimates the severity as palpation of lesion is limiting because of the inflammation. This often causes misinterpretation of the extent of the lesions, leading to error in the assignment of the stage. Therefore, advances in ultrasonography (USG) in HS is being recently tried to overcome the limitations of just clinical assessment in HS. Implementation of USG using Sonographic Scoring of HS (SOS-HS) staging and Colour Doppler to visualize fistulous tracts, the type of vascularisation in HS will give a great aid in significant improvement in management of HS patients. Forty six patients above 18 years of age with the clinical diagnosis of HS were enrolled in this study. All patients had undergone clinical examinations, USG with Colour Doppler study. The clinical examination was a single session physical examination for the detection of nodules, fistulas and abscesses in the sites involved like axillae, mammary, periumbilical, inguinal, buttocks, thighs and perianal regions. The USG with colour Doppler was performed by an experienced radiologist on ESOATE MYLAB 9 machine with high frequency linear probe (L+ 8–24 MHz). The ultrasonographic data was analysed on basis of detection of widening of hair follicles, abnormal thickening, echogenicity of the skin layers, types of lesions, vascularity, lesion location and extent in all axes.
The disease severity was determined according to the Hurley staging system1 and SOS-HS staging2 using a 3 point scale (I-III). Vascularization was assessed according to a four category system3. Fistulous tracts were classified using colour Doppler into three types4. Fibrotic scarring of fistulous tracts and edema was further graded into grades 0,1 and 24. Patterns in fistulous tracts were classified as: peripheral, internal and mixed4. All the USG and colour Doppler parameters (SOS-HS Staging, degree of vascularization, type of fistulous tracts, pattern of fistulous tracts, grading of fibrotic scarring and edema of fistulous tracts) were correlated with clinical Hurley staging. Comparisons of values of skewed data was done via Kruskall Wallis test in case >2 groups. For categorical data comparisons was made using Fisher's exact test. Spearman's correlation coefficient was applied to study the correlation of all the above mentioned parameters and p < 0.05 was considered significant. A group of 46 patients with HS were included from July 2021 to November 2022. The majority were male (56.5%; N = 26), aged 28.07 ± 7.28 years, with disease duration of 4.64 ± 3.44 years, and BMI of 26.82 ± 4.90. The main affected locations were axilla (60.9%) and groin (21.7%) regions. History of acne was present in 76.1% and 13.1% subjects had HS in one or more family members. Comparing clinical Hurley staging with USG SOS-HS; patients in stage I hurley corresponded to 46.7% in stage I, 40% in stage II and 13.3% in stage III on SOS-HS. Similarly stage II hurley corresponded to 38.5% in stage II and 61.5% in stage III on SOS-HS. While hurley stage III corroborated to 100% in stage III SOS-HS. A high association was observed between Hurley and SOS-HS staging (p < 0.001). Overall, 18 patients (74.8%) had more severe disease by SOS-HS versus Hurley Staging system. Comparing clinical Hurley staging with degree of vascularity on colour Doppler; patients in stage I hurley had absent vasularization in 20%, minimal vascularization in 46.7% and moderate vascularization in 33.3%. Similarly, patients in stage II hurley had minimal vasularization in 26.9%, moderate vascularization in 50% and 23.1% had severe vascularization. Patients in hurley stage III had moderate and severe vascularization in 20% and 80% respectively. A significant association was observed between Hurley staging and degree of vascularization (p < 0.002). Overall, 23.1% patients had more severe vascularization by Colour Doppler versus Hurley Staging system. Similarly, a significant association was seen between clinical Hurley staging and Fibrotic scarring of fistulous tracts on colour Doppler (p < 0.002). Overall, 47.9% had more severe fibrotic scarring by Colour Doppler. A significant association was also seen between clinical Hurley staging and edema of fistulous tracts on colour Doppler (p < 0.006). Overall, 95.9% patients had more severe fibrotic scarring by Colour Doppler. No significant association was observed between hurley staging and type and pattern of fistulous tracts on Colour Doppler. The present study is the first one demonstrating correlation between clinical and USG with colour Doppler disease severity staging in skin of colour patients of HS. Implementation of USG with colour Doppler can modify the clinical staging and therapeutic management in HS by detecting subclinical disease. It will also positively impact the quality of life of these patients.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach In: Roenigk RK, Roenigk HH (eds). Dermatologic Surgery: Principles and Practice. Marcel Dekker, New York, 1989:729–39.
- Wortsman X et al. Ultrasound In-Depth Characterization and Staging of Hidradenitis Suppurativa. Dermatol Surg 2013;39:1835–42.
- Caposiena Caro RD et al. Power Doppler ultrasound assessment of vascularization in hidradenitis suppurativa lesions. J Eur Acad Dermatol Venereol 2018;32:1360–7.
- Wortsman X et al. Colour Doppler ultrasound assessment of morphology and types of fistulous tracts in hidradenitis suppurativa (HS). J Am Acad Dermatol 2016;75:760–7.
UHFUS with 70 MHZ probe for the early anatomic detection of follicle obstruction in patients affected by hidradenitis suppurativa
Valentina Dini1, Alessandra Michelucci1, Flavia Manzo Margiotta1, Giammarco Granieri1, Teresa Oranges1,2, Marco Romanelli1
1University of Pisa, Department of Dermatology, Pisa, Italy; 2Meyer Children's Hospital, Department of Paediatrics, Dermatology Unit, Florence, Italy
- Nguyen TV et al. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol 2021;35:50–61.
Evaluation of hidradenitis suppurativa through high-field magnetic resonance and 3D reconstruction: Experience of a case
Clarissa Canella1,2, B. Flávio Luz2, Gerson Ribeiro3, Carolina Ávila de Almeida5, Jéssica Povill5, Luiza D'Almeida4, Heron Werner1,3
1Clinica Alta Excelência Diagnóstica, Department of Radiodiagnosis and Imaging, Rio de Janeiro, Brazil; 2Universidade Federal Fluminense, Department of Dermatology, Niteroi, Brazil; 3Pontifícia Universidade Católica, Department of Biodesing Lab DASA/PUC, Rio de Janeiro, Brazil; 4Santa Casa de Misericórdia do Rio de Janeiro, Department of Dermatology, Rio de Janeiro, Brazil; 5Diagnóstico das Américas S/A – DASA, Department of Radiodiagnosis and Imaging, Rio de Janeiro, Brazil
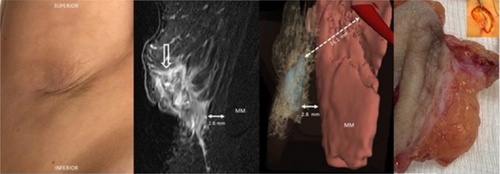
FIGURE 1 Clinical imaging, magnetic resonance imaging and 3D reconstruction of hidradenitis suppurativa. 40-year-old female presenting hidradenitis suppurativa. MRI using surface-coil on a sagittal T2-fat saturation sequence of the fistula (open arrow), allowing the evaluation of its distance from the muscle (MM). Note that on MRI it is difficult to differentiate the fistula from the surrounding inflammatory tissue and it is not possible to visualize the axillary artery. Segmentation of MRI images using 3D Slicer software allows a better differentiation between the fistula (in blue) and the surrounding inflammatory tissue and the distance between the fistula and the axillary artery (in dark red).
- Jabbary Lak F et al. Non-contrast-enhanced 3-tesla magnetic resonance imaging using surface-coil and sonography for assessment of hidradenitis suppurativa lesions. Acta Derm Venereol 2020;100:1–7.
- Srisajjakul S et al. Magnetic Resonance Imaging of Hidradenitis Suppurativa: A Focus on the Anoperineal Location. Korean J Radiol 2022;23:785–93.
- Zabala-Travers S. Biomodeling and 3D printing: A novel radiology subspecialty, Annals of 3D Printed Medicine. 2021;4:100038.
A non invasive assessment with a fluorescence device for the detection of colonized hidradenitis suppurativa lesions
Alessandra Michelucci, Flavia Manzo Margiotta, Giammarco Granieri, Marco Romanelli, Valentina Dini
University of Pisa, Department of Dermatology, Pisa, Italy
- Goldburg SR et al. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 2020;82:1045–58.
- Vossen ARJV et al. Hidradenitis Suppurativa: A Systematic Review Integrating Inflammatory Pathways Into a Cohesive Pathogenic Model. Front Immunol 2018;9:2965.
- Ring HC et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol 2017;176:993–1000.
- Rennie MY et al. Understanding Real-Time Fluorescence Signals from Bacteria and Wound Tissues Observed with the MolecuLight i:X™. Diagnostics (Basel) 2019;9:22.
Protocol for the use of ultrasound in hidradenitis suppurativa
Ariany T.A.S. Denofre1, Mirena Ide1, Cintia A. Souza1, Rachel P. Dertkigil2, Renata F. Magalhães1
1University of Campinas - Unicamp, Department of Clinical Medicine – Dermatology Division, Campinas, Brazil; 2University of Campinas - Unicamp, Department of Radiology, Campinas, Brazil
Ultrasound emerged as an important tool for evaluation of Hidradenitis Suppurativa (HS). It shows subclinical lesions, revealing the real gravity stage of the patient, which is very important for a disease that can be devastating.1–3 The aim of this study was to review literature about ultrasound and HS to create a protocol for the use of ultrasound. Besides that, images of clinical and ultrasonographic lesions were collected (Figure 1) to create an atlas for medical education. A revision of the literature was conducted in the BVS, EMBASE, SCOPUS, Web of Science and PUBMED with keywords: hidradenitis suppurativa, ultrasound, colour doppler. To guarantee high quality of data, articles were chosen based on the Qualis Periodic rank from Sucupira Platform (Brazil), opting for the highest ranked journals, remaining 19 articles and 2 published books. The data was extracted and unified in one protocol to guide the use of ultrasound in the HS and its findings. The objective of ultrasound exam is to have an early and precise diagnosis. The clinical evaluation underestimates the severity of the disease, especially in most critically ill patients, probably because of inflammation. It allows diagnosis of subclinical lesions, graduates the inflammatory activity, classification of the type of fistula and determination of surgical margins of post-surgery recurrence. It is also possible to select lesions to infiltrate with triamcinolone, providing better responses and accuracy. It has a high correlation with histological findings, with Doppler intensity related to neutrophilic infiltrate4. The reduction of the Doppler is the first sign of clinical response to treatment. Association of ultrasound use on surgical determination of the margins and biologics are the best to avoid recurrence.
Disease severity evaluation: Use of clinical scores (Hurley, IHS4, DLQI, VAS) and ultrasound severity scores (SOS-HS and/or HS-US-PGA).
Interval between exams: At the first medical appointment, 8 weeks after antibiotics begin, week 4, 12 and 24 after biological begin, 12 weeks after triamcinolone infiltration and 6 months after surgery.
Ultrasound exam: Explain procedure to the patient. Use ultrasound after clinical exam with inspection and palpation of the lesions. When possible, same examiner, trained in ultrasound and dermatology. Always use linear probe, between 5–22 MHz, operating with copious amount of gel on lesion or probe. Softly position probe to perpendicularly capture lesioned and perilesional area in two axes. Ultrasound evaluation on at least two sites, preferably in axillary and inguinal region, in all symptomatic areas. When multiple fistulae, select the biggest. If necessary, compare with contralateral area. Analyse vascular pattern with Doppler ultrasound of lesioned and perilesional area.
Ultrasound findings: widening of the hair follicles, thickening and/or abnormal echogenicity of the dermis, pseudocystic nodules, fluid collections, fistulous tracts. The presence of 3 or more findings makes the diagnosis of HS. Pseudocystic nodules and fluid collections are equivalent to nodules and abscesses clinically. Hair tracts are other additional finding defined. Fibrosis is characterized as hypoechoic areas. Reactive lymph nodes are more common during infections, with an oval well-defined shape, normally 1 cm, with cortical thickening and central vascularization.
Edema graduation: Grade 1 with hyperechoic hypodermis and Grade 2 with hyperechoic hypodermis with anechoic fluid between lobules. Edema is related to inflammation.
Types of fistulae classified by ultrasound: Type 1 with low fibrosis and low or high edema; Type 2 with high fibrosis and low edema and Type 3 with high fibrosis and high edema. Type 2 and 3 are related to a more severity stage of the disease.
Doppler Ultrasound use: grades the inflammation and disease activity, with high correlation with symptoms. It can be peripheral, internal, and mixed. Intensity is graded as minimal, moderated, and high. Nodules and abscesses commonly show peripheral pattern. Simple fistulae show peripheral and mixed pattern and complex and chronical fistulae, mixed pattern. The reduction of the Doppler sign is related with improvement of the lesions clinically.
Clinical and Surgery resolution: Location of fistulae is related with the clinical response – dermal and dermal-epidermal fistulae have more clinical resolution. Complex and hypodermal fistulae (Type 2 and 3) only with surgery. To determine the surgical margins, it is suggested to use 0,5 cm of margin and then explore it with ultrasound. Increase the area until there is no subclinical lesion detected with the ultrasound5.
- Wortsman X et al. Ultrasound in-depth characterization and staging of hidradenitis suppurativa. Dermatol Surg Off Publ Am Soc Dermatol Surg 2013;39:1835–42.
- Martorell A et al. Ultrasound as a diagnostic and management tool in hidradenitis suppurativa patients: a multicentre study. J Eur Acad Dermatol Venereol J Eur Acad Dermatol Venereol 2019;33:2137–42.
- Lacarrubba F et al. Ultrasonography in the pathway to an optimal standard of care of hidradenitis suppurativa: the Italian Ultrasound Working Group experience. J Eur Acad Dermatol Venereol 2019;33(suppl 6):10–4.
- Zarchi K et al. Pain and inflammation in hidradenitis suppurativa correspond to morphological changes identified by high-frequency ultrasound. J Eur Acad Dermatol Venereol 2015;29:527–32.
- Cuenca-Barrales C et al. Pre-operative ultrasound planning in the surgical management of patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2020;34:2362–7.
“Lesion Journey” in hidradenitis suppurativa: Clinical and ultrasonographic correlations
Andrea Sechi1, Guido Mioso2, Luigi Naldi1, Bianca Maria Piraccini3
1San Bortolo Hospital, dermatology unit, Vicenza, Italy; 2University of Padova, dermatology unit, Padova, Italy; 3University of Bologna, dermatology unit, Bologna, Italy
- Kurzen H et al. What causes hidradenitis suppurativa? Exp Dermatol 2008;17:455–6.
Posters – Outcome measures
Understanding disease burden, unmet needs, treatment pathways and outcomes of patients with hidradenitis suppurativa in England: Rationale and design of a retrospective, non-interventional study
John Ingram1, Kave Shams2, Ellie Rashidghamat3, Annette Brown4, Paula Pamies4
1Cardiff University, Division of Infection and Immunity, Cardiff, UK; 2University of Leeds, Leeds Centre for Dermatology and Leeds Institute of Rheumatic and Musculoskeletal Medicine, Leeds, UK; 3St John's Institute of Dermatology, Department of Dermatology, London, UK; 4Novartis Pharmaceuticals UK Ltd, London, UK
Acknowledgement: The authors thank Rajib Kishore Hazam, PhD, and Nihal Maremanda, PhD (Novartis Healthcare Pvt. Ltd., India), for editorial and medical writing support, which was funded by Novartis Pharmaceuticals UK Ltd, London, in accordance with the Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
- Sabat R et al. Hidradenitis suppurativa. Nat Rev Dis Primers 2020;6:18.
- Zouboulis CC. The socioeconomic burden of hidradenitis suppurativa/acne inversa. Br J Dermatol 2019;181:7–8.
- Ingram JR et al. Unmet clinical needs and burden of disease in hidradenitis suppurativa: real-world experience from EU5 and US. J Eur Acad Dermatol Venereol 2022;36:1597–605.
- Kokolakis G et al. Delayed diagnosis of hidradenitis suppurativa and its effect on patients and healthcare system. Dermatology 2020;236:421–30.
- Ingram JR et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol 2018;178:917–24.
Hidradenitis supurative: Study on factors influencing the course and severity of the disease
Lais P. De Oliveira, Luiza F. Vieira D'almeida, Katherine Carranza, Rubem D. Azulay, Vitoria Azulay, Mariana M. Rechuan
Institute of Dermatology Professor Rubem David Azulay, Department of Dermatology, Rio de Janeiro, Brazil
- Boer J et al. Boils at frictional locations in a patient with hidradenitis suppurativa. Acta Dermatovenerol Croat 2016;24:303
- Bui T-L et al. Hidradenitis suppurativa and diabetes mellitus: A systematic review and metaanalysis. J Am Acad Dermatol 2018;78:395–402.
- Canoui-Poitrine F et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol 2009;61:51–7.
- Cartro A, Driscoll MS. Comorbidities of hidradenitis suppurativa: a review of the literature. Int J Womens Dermatol 2019;5:330–4.
- Chapman S et al. Cutaneous squamous cell carcinoma complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Alp Pannonica Adriat 2018;27:25–8.
Influence of hormonal factors on women with hidradenitis suppurativa
Anne-Claire Fougerousse1, Ziad Reguiai2, François Maccari3, Philippe Guillem4, Aude Nassif5, Nathalie Beneton6, Elisa Cinotti7, Céline Girard8, Raphaele Binois9, Jean-Luc Perrot10, on behalf of the GEM Resoverneuil
1Military Teaching Hospital Begin, Department of Dermatology, Saint Mandé, France; 2Polyclinic Courlancy Bezannes, Department of Dermatology, Reims, France; 3Private practice, Dermatology, La Varenne Saint Hilaire, Germany; 4Clinique du Val d'Ouest, Visceral Surgery, Ecully, France; 5Institut Pasteur, Department of Dermatology, Paris, France; 6CH, Department of Dermatology, Le Mans, France; 7University of Siena, Department of Medical, Dermatology Unit, Surgical and Neuro.Sciences, Siena, Italy; 8CHU, Department of Dermatology, Montpellier, France; 9CH, Department of Dermatology, Orléans, France; 10CHU, Department of Dermatology, Saint Etienne, France
Hidradenitis suppurativa (HS) affects 3 women for 1 men, but contrary to other chronic inflammatory skin diseases such as psoriasis, impact of homonal factor on HS has poorly been evaluated. Epiver was a prospective multicenter cohort study including 1428 HS patients, objective of which was to describe the epidemiology of HS1. We performed a subanalysis, including all women (n = 884) to evaluate the impact of hormonal factors (pregnancy, post partum, menopause, menstrual cycle) on HS and to describe the use of contraception and number of pregnancies in women with HS. Among the 884 women in the Epiver study, the mean age was 33.1 ± 11.1 years, with 690 women of childbearing age (18–45 years), 47 women under 18 years. Mean age of onset of HS was 20.9 ± 8.5 and mean age of diagnosis was 28.6 ± 10 years. 452 (51.1%) women have been pregnant at least once with a mean of 2.0 ± 1.0 pregnancies per women. For the entire population (n = 884) the mean number of pregnancy per women was 1 ± 1.2. 446 women had a contraception: oral n = 286 (64.4%) of which cyproterone acetate n = 31, physical contraception (condom, diaphragm) n = 18 (4.1%), intrauterine device n = 104 (23.4%) (of which 33 hormonal IUD), implant n = 35 (7.9%). Eighty women were menopausal. Among the 452 women with history of pregnancy, 61.4% reported no impact of pregnancy on HS activity, 23.3% reported an improvement and 15.3% a worsening; 57.6% reported no impact of post partum on HS activity, 40.3% a worsening and 2.1% an improvement. Among the 80 menopausal women, 72.1% reported no impact of menopause on HS activity, 19.7% a worsening and 8.2% an improvement. 63.1% of women reported no impact of menstrual cycle on HS activity, 0.4% an improvement and 36.5% a worsening, mostly (68.5%) in second part of the menstrual cycle. In our study, 6 out of 10 women reported no impact of hormonal factors on HS activity. For women with modification of HS activity correlated to hormonal factors, 60% presented an amelioration during pregnancy, 95% a worsening during post partum and 70% a worsening after menopause (but the number of post menopausal women was limited). Contraceptives methods used by women in our study were not comparable of theses of French general population with an overrepresentation of oral contraception (64.4% vs. 36.5% in general population). This could be explained by the favourable impact of antiandrogens and oestrogens on HS. Number of pregnancy per women in our study was much lower than in French general population (mean number of child per women in France: 1.87). Several reasons can ben hypothesized: worries about carrying a baby because of HS or its treatments, more HS women being single compared to general population, impact of HS on fertility (directly or due to comorbidties as polycystic ovary syndrome). Limitations of our survey include recollection bias given the nature of the study, and the lack of a control group. However, the strength of this study is its large sample size. Our study brings more informations about the impact of hormonal factors on women with HS. It underlines a much lower fertility rate compared to general population. Deeper investigations are needed to understand the reasons.
Acknowledgement: To all memebers of ResoVerneuil who participate to this study.
- Perrot JL, et al. How to Define Mild to Severe Hidradenitis Suppurativa? A Simple New Tool Based on Latent Class Analysis of EPIVER Data Study. Clin Cosmet Investig Dermatol 2022; 15:1091–103.
The study on monitoring the course of hidradenitis suppurativa patients
Vaiva Jariene1,2, Paulina Cekanauskaite1,2, Vesta Kucinskiene1,2, Skaidra Valiukeviciene1,2
1Lithuanian university of health sciences (LSMU), Department of Skin and Venereal Diseases, Kaunas, Lithuania; 2Hospital of LSMU Kauno Klinikos, Department of Skin and Venereal Diseases, European Reference Network for Rare and Complex Diseases of the Skin (ERN- Skin) member, Kaunas, Lithuania
The Hospital of Lithuanian University of Health Sciences Kauno Klinikos is an approved member of the European Reference Network on Rare and Undiagnosed Skin Disorders (ERN-Skin) since 2017. Our center participates in “Acquired Immunological Low Prevalence and Complex Adult Diseases of the skin (ALLOCATE SKIN)” thematic group which focuses on inflammatory skin conditions, including hidradenitis suppurativa (HS). Therefore, we assign treatment and monitoring to patients according to the valid European HS guidelines1. We aimed to evaluate the epidemiological data and the clinical results of long-term monitoring of HS patients. This study is a continuation of a previous research with a new patients data that were collected2. Our research includes a total of 37 patients treated in 2020 September – 2021 December. Depersonalized data of the patients were collected by using European Hidradenitis Suppurativa Foundation first and follow up questionnaires in Redcap® database online platform. The follow up visits there every 3 months. Non-parametric tests such as Kruskal Wallis and Mann–Whitney U test were used for assessing interdependence between qualitative data. Correlations were ranked using Spearman's correlation coefficient (r). According to our study female-to-male ratio were 0.75:1. The mean duration of diagnosis delay was 5.6 years (SD ±7). The most common comorbidity was acne 51.4% (n = 19) and the prevalence was higher among men (p = 0.049). Other common comorbidities were pilonidal cyst 32.4% (n = 12), arthritis 16.2% (n = 6), psoriasis 8.1% (n = 3). Clinical examination at the baseline visit revealed that 41% of all patients were of Hurley I stage, 46% of Hurley II and 5.4% of Hurley III stage. According to our research data, the severity of the disease did not depends on patients sex, smoking status or BMI. Patients on the first visit received treatment based on European guidelines1. On the first visit 64.9% (n = 24) of the patients received local treatment, 3.2% (n = 16) were treated with long-term systemic antibiotics and surgical treatment (local excisions) were used for 2.7% (n = 2). None of the patients received biological treatment after the first visit. The average of DLQI score on the first visit were 9.1 points, IHS4 score were 9.4 points. After receiving first line treatment average DLQI score decreased to 8.1 points, IHS4 score decreased to 8.7 points. By using adequate treatment IHS4 scores were statistically significant lower (p = 0.002). It was determined that higher BMI statistically significant correlated with lower DLQI change after the first treatment (r = −0.58, p = 0.004). Based on the results of this research and comparison with other authors data a few recommendations can be established. A specialized center ensures effective treatment of HS, therefore, it is recommended to monitor the patients and to ensure long-term multi-specialty care1. Continuous special training of doctors about the clinical manifestations of HS and the severity of the disease is required in order to avoid late onset diagnosis. Collected research data may be useful in the future for further scientific analyzes or clinical trials.
Acknowledgement: The Department of Skin and Venereal Diseases, Hospital of LSMU Kauno Klinikos, Kaunas, Lithuania is a health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Zouboulis CC et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015;29:619–44.
- Kucinskiene V et al. Clinical Characteristics of Hidradenitis Suppurativa Patients from a Reference Centre in Kaunas, Lithuania. Dermatol Basel Switz 2020;236:66–70.
Can hidradenitis suppurativa patients classify their own lesions by means of a digital lesion identification scheme?
Michael Schultheis2, Petra Staubach-Renz2, Stephan Grabbe2, Fareed Khoury3, Uwe KIrschner3, Georgios Nikolakis1
1Brandenburg Medical School Theodor Fontane and Faculty of Health Sciences Brandenburg, Staedtisches Klinikum Dessau, Departments of Dermatology, Venereology, Allergology and Immunology, Dessau, Germany; 2University Medical Centre, Johannes Gutenberg University, Department of Dermatology, Mainz, Germany; 3Dermatology Outpatient Office Dr. Uwe Kirschne, Mainz, Germany
Hidradenitis suppurativa (HS) differs widely with respect to its clinical presentation. Literature imposes different phenotypes potentially implying different treatment modalities1,2. However, evaluating, documenting and digitalizing lesions as basis for phenotyping can often be time-consuming for professionals in the daily medical setting. In contrast, the VOICE project, a survey including 1.299 participants in 14 countries, shows that HS-patients are willing to share their experiences and data on past treatments and other characteristics3. In order to link this information to disease phenotypes in the future, the goal of this study was to develop a set of descriptive definitions and associated images of HS lesions, which serve as a digital tool to enable patients to identify their own lesion types. The “Lesion Identification Scheme for Acne Inversa” (LISAI) developed in this study includes 11 main skin lesions of HS. The schemes for physicians and patients were then implemented in a specific software. All participating physicians were trained on the physician LISAI in order to ensure consistent professional assessment. Upon patient consent, the physician used the software to document the lesions identified. Patients subsequently logged into the patient-version of the software from the convenience of their home and selected the lesions they identified on themselves. Afterwards the correlation between professionals and patients was tested. For seven lesion types, correlation coefficients were statistically significant. A large/strong correlation between patients and physicians was found for the draining fistulas (0.593) and double-ended comedones comedo (0.501). For five other lesion types, correlation was medium/moderate, namely the inflammatory nodule (0.365), abscess (0.303), accordion like−/ bridged scar (0.454), epidermal cyst (0.325) and pilonidal sinus (0.394). HS-patients demonstrate high willingness to share their experiences and data. Therefore, a self-assessment scheme, as the developed LISAI, can be a valuable tool to enrich patient surveys by the identification of lesion types e.g. as basis for phenotyping. In the present study, the focus was on examining reliability and agreement only, and the LISAI is not yet tested for its potential applicability for the exact number of lesions which could be a valuable additional field of usage to get a better understanding of structures in flares as well as to assess treatment effects.
Acknowledgement: The authors wish to thank all the patients who participated in LISAI despite the difficulties caused by the COVID-19 pandemic. The Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau, Dessau, Germany are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Dudink K et al. Prevalence and Clinical Characteristics of Hidradenitis Suppurativa Phenotypes in a Large Dutch Cohort. Dermatology 2022;238:600–2.
- Kirby JS. Unravelling the Heterogeneity of Hidradenitis Suppurativa with Phenotype Schema. J Invest Dermatol 2021;141:1136–8.
- Garg A et al. Evaluating patients' unmet needs in hidradenitis suppurativa: Results from the Global Survey Of Impact and Healthcare Needs (VOICE) Project. J Am Acad Dermatol 2020;82:366–76.
Power doppler signal morphology as predictive factor of failure to adalimumab in hidradenitis suppurativa patients
Raffaele Dante Caposiena Caro1, Elisabetta Botti2, Giulia Bazzacco1, Sara Lambiase2, Michele Pauluzzi1, Chiara Pensa2, Luca Bianchi2, Iris Zalaudek1
1Azienda sanitaria universitaria Giuliano Isontina (ASU GI)., Dermatologia e Venereologia, Trieste, Italy; 2Università di Tor Vergata, Dermatologia e Venereologia, Roma, Italy
Ultrasound (US) is a real-time non-invasive that in the last few years has been applied in the study of Hidradenitis Suppurativa (HS), being useful for a better and clearer identification of clinical and subclinical lesions. Power Doppler describes the blood flow patterns in real time, using colour to display the strength of the Doppler signal. It shows greater sensitivity compared to conventional colour Doppler and offers a useful tool to achieve a more complete knowledge and a proper management of the disease.1-5 The objective of the study was to evaluate the correlation between vascular distribution at power Doppler ultrasound and the response to adalimumab. A retrospective multi-centric analysis of the power-Doppler ultrasound examinations, achieved between January 2021 and December 2022, of patients with HS at our departments was performed. All patients started biologic therapy with adalimumab 160 mg initial dose (Day 1), a second dose 2 weeks later (Day 15) of 80 mg, then a third dose of 40 mg (Day 29) and subsequent doses 40 mg every week. US assessment was realized at baseline, after 12 and 24 weeks of therapy. The study included 213 sinus tracts assessed with US (Esaote MyLabOne, 18 MHz) in 61 adult patients with Hurley II-III HS. Patients with mixed vascularization at baseline achieved a poorer treatment response to adalimumab with a complete lesion healing (no signs of clinical-ultrasonographic inflammation/drainage) in the 2.6% and 5.3% of cases, respectively after 12 and 24 weeks of therapy. Whereas, fistulas with peripheral vascularization achieved a complete lesion healing in the 47.5% and 64.6% of cases, respectively at 12th and 24th week of treatment. Power Doppler offers a useful real-time and non-invasive tool to achieve a more complete knowledge and a proper management of the disease. It may be helpful to identify less likely responsive lesions to medical treatment, anticipating the need for surgical excision. Further, extensive cohort studies regarding the implementation of US in patients with HS on treatment are necessary to confirm our observations.
- Wortsman X. Can Ultrasound Examinations Generate Pain in Hidradenitis Suppurativa Patients? Results from a Multicentric Cross-Sectional Study. Dermatology 2023;239:277–82.
- Lacarrubba F et al. Ultrasonography in the pathway to an optimal standard of care of hidradenitis suppurativa: the Italian Ultrasound Working Group experience. J Eur Acad Dermatol Venereol 2019;33(suppl 6):10–14.
- Caposiena Caro RD et al. Power Doppler ultrasound assessment of vascularization in hidradenitis suppurativa lesions. J Eur Acad Dermatol Venereol 2018;32:1360–7.
- Martorell A et al. Defining Fistular Patterns in Hidradenitis Suppurativa: Impact on the Management. Dermatol Surg 2019;45:1237–44.
- Caposiena Caro RD et al. Clinical and Power-Doppler ultrasound features related with persistence of fistulous tracts under treatment with adalimumab in hidradenitis suppurativa: 4 years of follow-up. Dermatol Ther 2021;34:e14804.
Comparison between clinical grade scales for hidradenitis suppurativa and the clinical-sonographic scoring system scale (SOS-HS)
Carolina Ávila de Almeida1, Jéssica Povill1, Mario Chaves2, Luiza D'Almeida3, B. Flávio Luz4, Clarissa Canella4,5
1Diagnóstico das Américas S/A – DASA, Department of Radiodiagnosis and Imaging, Rio de Janeiro, Brazil; 2Universidade do Estado do Rio de janeiro, Department of Dermatology, Rio de Janeiro, Brazil; 3Santa Casa de Misericórdia do Rio de janeiro, Department of Dermatology, Rio de Janeiro, Brazil; 4Universidade Federal Fluminense, Department of Radiodiagnosis and Imaging, Niteroi, Brazil; 5Clinica Alta Excelência Diagnóstica, Department of Radiodiagnosis and Imaging, Rio de Janeiro, Brazil
- Preda-Naumescu A et al. Hidradenitis suppurativa: pathogenesis, clinical presentation, epidemiology, and comorbid associations. Int J Dermatol 2021;60:e449-58.
- Napolitano M et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol 2017;10:105–15.
- Wortsman X. Strong validation of ultrasound as an imaging biomarker in hidradenitis suppurativa. Br J Dermatol 2021;184:591–2.
Psychometric assessment of the HiSQOL in adolescents with moderate-to-severe hidradenitis suppurativa
Joslyn S. Kirby1, Samar Hasan2, John Ingram2
1Penn State Health, Department of Dermatology, Hershey, USA; 2Cardiff University, University Hospital of Wales, Division of Infection & Immunity, Cardiff, UK
Hidradenitis suppurativa (HS) can have a large negative impact on quality of life (QOL). The Hidradenitis Suppurtiva Quality Of Life (HiSQOL) scale was developed to validly and reliably measure HS-specific HRQOL for adults with HS. Additionally, there is a need to identify scales for use with adolescents with HS, both in trials and in clinical care. The objective of this study was to investigate the validity and reliability of the HiSQOL, a patient-reported instrument, for measurement of HS-specific QOL for adolescents with moderate-to-severe HS. This was a qualititive study and following relevant ethics board approval, interviews were conducted with HS patients aged 11 to <18 years-old at a single center in the United States (n = 10) and in Cardiff, United Kingdom (n = 2). The interviews included both concept elicitation followed by cognitive debriefing of instruments. A subset of participants also completed (scored) both the HiSQOL and the Children's Dermatology Life Quality Index (CDLQI) (n = 6). Qualitative analysis was conducted to identify themes. Psychometric properties including Cronbach alpha and Pearson Correlation were assessed on the subset with scores from both instruments. Our findings are derived from 12 participants, who were 75% (n = 9) female, age range was 12–17 years. Hurley stage was II or III in all participants (100%). The face validity of the HiSQOL was confirmed in the cognitive debriefings as participants indicated the instrument was exciting and assessed relevant symptoms and impacts of the condition. For the Symptom subscale, patients did not identify any missing items. For the Psychosocial subscale, current items were endorsed by participants, while frustration or anger were suggested as additions by four participants. For two items on sexual activity and desire, two of the older US teen participants endorsed the items. Participants suggested rephrasing of the items to capture social relationships (playing, hanging-out with friends) or early romantic social activities (hanging out with someone special). In the UK, the research ethics committee recommended the items on sexual activity for participants below 16 be removed and for those 16 years of age in the UK, the items were optional. For the Activities subscale, the item for exercise was suggested for rephrasing to indicate sports or gym. Quantitative analyses on the subset showed positive results. Content validity was supported by a strong Pearson correlation between the HiSQOL and CDLQI (r = 0.92; p = 0.01). The reliability for HiSQOL, using the Cronbach alpha, was also strong (a = 0.98). The HiSQOL demonstrates initial validity and reliability for adolescents with HS, in addition to adults. Participants highlighted a few items that could be adjusted. Further work will develop and confirm a robust measure of HS-specific HRQOL for adolescents in clinical trials.
HiSQOL mini: A shortened version of the hidradenitis suppurativa quality of life (HiSQOL) instrument
Samar Hasan1, Tim Pickles2, Bente Villumsen3, Angela Gibbons4, Barry McGrath5, Joslyn S. Kirby6, John Ingram1
1Cardiff University, University Hospital of Wales, Department of Dermatology, Cardiff, UK; 2Cardiff University, University Hospital of Wales, Centre for Trials Research, Cardiff, UK; 3Patientforeningen HS Danmark, Copenhagen, Denmark; 4Patient Partner, Cardiff, UK; 5HS Ireland, Hidradenitis Suppurativa Association, County Clare, Ireland; 6Penn State Health, Department of Dermatology, Pennsylvania, USA
Hidradenitis Suppurativa Quality of Life (HiSQOL) instrument is a validated Hidradenitis Suppurativa (HS) -specific quality-of-life-measure1. It consists of 17 items in three sub-domains: activity-adaptations, symptoms, and psychosocial impact. HiSQOL-mini was developed for situations where time constraints exist, like busy clinics. We obtained data from Treatment of Hidradenitis Suppurativa Evaluation Study (THESEUS), a UK-wide observational study that followed 150 participants over 1 year. HiSQOL score was measured at baseline and at 3-monthly follow-up visits for 1 year. Statistical analysis using Spearman's ρ was used to assess correlation of items with corresponding sub-domain and total HiSQOL scores at each of the visits. In the symptoms and psychosocial domains, pain and feeling down or depressed respectively most often displayed the highest correlation with the sub-domain and total scores across the 5 timepoints. In the activity-adaptations sub-domain, walking was closely followed by getting dressed. We created a focus group of HS patients and clinicians with expertise in HS. There was consensus among patients that pain and feeling down or depressed well represented their sub-domains. The group agreed that since activity-adaptations domain has more items, both walking and getting dressed items should be included in HiSQOL-mini. A final analysis correlating HiSQOL-mini with total HiSQOL-17 scores and Patient Global Assessment (PtGA) scores showed high correlation between HiSQOL-mini and both HiSQOL-17 at all timepoints (ρ > 0.93) and PtGA (ρ > 0.65). HiSQOL-mini is highly correlated with HiSQOL and PtGA and might serve as a shortened version where HiSQOL is less feasible. We acknowledge that these data were from one patient cohort and more validation is necessary.
- Kirby JS et al. The Hidradenitis Suppurativa Quality of Life (HiSQOL) score: development and validation of a measure for clinical trials. Br J Dermatol 2020;183:340–8.
A systematic literature review of health-related quality of life and other patient reported outcomes across key subgroups in hidradenitis suppurativa
Alexa B. Kimball1, Joslyn S. Kirby2, John Ingram3, Tanja Tran4, Ingrid Pansar4, Valerie Ciaravino5, Damon Willems4, Anne Mary Lewis-Mikhael6, Amit Garg7
1Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, USA; 2Penn State University, Department of Dermatology, Hershey, USA; 3Cardiff University, Department of Dermatology, Division of Infection & Immunity, Cardiff, UK; 4UCB Pharma, Brussels, Belgium; 5UCB Pharma, Colombes, France; 6ICON plc, Dublin, Ireland; 7Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, USA
Acknowledgement: This study was funded by UCB Pharma. Medical writing support was provided by Costello Medical.
- Ingram JR et al. Unmet clinical needs and burden of disease in hidradenitis suppurativa: real-world experience from EU5 and US. J Eur Acad Dermatol Venereol 2022;36:1597–605.
- Hongbo Y et al. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol 2005;125:659–64.
- Hawker GA et al. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain Scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63(suppl 11):S240-52.
Validation of the HS-IGA: A novel hidradenitis suppurativa-specific investigator global assessment for use in interventional trials
Amit Garg1, Carla Zema2, Valerie Ciaravino3, Robert Rolleri4, Luke Peterson4, Llenalia Garcia5, Tyler Massaro4, Gregor B.E. Jemec6, Joslyn S. Kirby7, Linnea Thorlacius6, John Ingram8
1Donald and Barbara Zucker School of Medicine at Hofstra Northwell, New Hyde Park, USA; 2Zema Consulting, Madison, USA; 3UCB Pharma, Colombes, France; 4UCB Pharma, Morrisville, USA; 5UCB Pharma, Slough, UK; 6Zealand University Hospital, Department of Dermatology, Roskilde, Denmark; 7Penn State Milton S Hershey Medical Center, Department of Dermatology, Hershey, USA; 8Cardiff University, University Hospital of Wales, Division of Infection and Immunity, Cardiff, UK
Few well-developed simple measures exist to capture hidradenitis suppurativa (HS) severity and response to interventions for use in trials, which may hinder drug development. Recently, HiSTORIC described the development and initial validation of an investigator global assessment in HS (HS-IGA) to measure disease severity and responsiveness to an intervention. Objective of the study was to assess the psychometric properties of the HS-IGA using a third trial dataset. Blinded data from UCB Pharma's HS0001 phase 2 randomized double-blind placebo-controlled active-reference arm trial (NCT03248531) were used to assess psychometric properties of the HS-IGA, including, convergent/divergent validity, test–retest reliability, responsiveness and predictive validity. A total of 88 adult participants with moderate to severe HS randomized and dosed in HS0001 were included in the analysis. The main outcomes were HS-IGA score at pre-specified timepoints up to 12 weeks post-randomisation. The HS-IGA score showed strong convergent validity with IHS4 and HS-PhGA scores at baseline (Spearman correlation 0.86 [p < 0.001] and 0.74 [p < 0.001], respectively) and at Week 12 (0.73 [p < 0.001] and 0.64 [p < 0.001], respectively). The HS-IGA scores showed very good test–retest reliability (ICC = 0.92) between scores assessed during pre-dosing visits at screening and baseline. At Week 12, HS-IGA responders were significantly associated with HiSCR50/75/90 responders (χ2 of 18.45 [p < 0.001], 18.11 [p < 0.001], and 20.83 [p < 0.001], respectively). The HS-IGA score showed good predictive validity compared with HiSCR50/75/90 (AUC 0.69/0.73/0.85) and with HS-PhGA clear or minimal (AUC 0.71) response at Week 12. The HS-IGA demonstrates very good psychometric properties, and thus it may be considered for use as an endpoint in clinical trials for HS. The HS-IGA represents a valuable clinician-reported outcome to support drug development efforts on behalf of patients with HS.
Acknowledgement: This study was funded by UCB Pharma. Editorial services were provided by Costello Medical. The Department of Dermatology, Zealand University Hospital, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
Comparison of the international hidradenitis suppurativa severity scoring system (IHS4) and hurley staging in hidradenitis suppurativa disease severity assessment: A post hoc analysis of the SUNSHINE and SUNRISE phase 3 trials
Christos C. Zouboulis1,2, Errol P. Prens1,3, Christopher Sayed1,4, Alejandro Molina-Leyva1,5, Vincenzo Bettoli1,6, Marco Romanelli1,7, Jacek C. Szepietowski1,8, Torben Kasparek9, Iryna Lobach9, Magdalena B. Wozniak10, Christine-Elke Ortmann9, Teresa Bachhuber9, Elisa Muscianisi11, Shoba Ravichandran11, Thrasyvoulos Tzellos1,12
1European Hidradenitis Suppurativa Foundation (EHSF), Dessau, Germany; 2Brandenburg Medical School Theodor Fontane and Faculty of Health Sciences Brandenburg, Staedtisches Klinikum Dessau, Departments of Dermatology, Venereology, Allergology and Immunology, Dessau, Germany; 3Erasmus University Medical Center Rotterdam, Department of Dermatology, Rotterdam, Germany; 4University of North Carolina at Chapel Hill, Department of Dermatology, Chapel Hill, USA; 5Hidradenitis Supurativa Unit. Ibs Granada Hospital Universitario Virgen de Las Nieves, Granada, Spain; 6University of Ferrara, Section of Dermatology and Infectious Diseases, Department of Medical Sciences, Ferrara, Italy; 7University of Pisa, Department of Dermatology, Pisa, Italy; 8Wroclaw Medical University, Department of Dermatology, Venereology and Allergology, Wrocław, Poland; 9Novartis Pharma AG, Basel, Switzerland; 10Novartis Ireland Limited, Dublin, Ireland; 11Novartis Pharmaceuticals Corporation, East Hanover, USA; 12Nordland Hospital Trust, Department of Dermatology, Bodø, Norway
Acknowledgement: This investigation was sponsored by Novartis Pharma AG. The Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau, Dessau, Germany and the Department of Dermatology, Erasmus Medical Center, Rotterdam, Netherlands are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Zouboulis CC et al. Hidradenitis suppurativa/acne inversa: Criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology 2015;231:184–90.
- Hurley H. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HHJr (eds) Dermatologic Surgery: Principles and Practice. Marcel Dekker, New York, 1989; 729–39.
- van der Zee HH, Jemec GB. New insights into the diagnosis of hidradenitis suppurativa: clinical presentations and phenotypes. J Am Acad Dermatol 2015;73(suppl. 1):S23–6.
- Zouboulis CC et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol 2017;177:1401–9.
A core outcome set for hidradenitis suppurativa trial outcomes: An update from the historic initiative
Linnea Thorlacius1, John Ingram2, Bente Villumsen3, Joslyn S. Kirby4, Amit Garg5, Gregor B.E. Jemec1
1Zealand University Hospital, Roskilde, Denmark, Department of Dermatology, Roskilde, Denmark; 2Institute of Infection & Immunity, Cardiff University, University Hospital of Wales, Heath Park, Cardiff, UK; 3The Patients' Association HS Denmark, Copenhagen, Denmark; 4Penn State Hershey Medical Center, Department of Dermatology, Hershey, USA; 5New York Medical College, Department of Dermatology, New York, USA; 6Zealand University Hospital, Roskilde, Denmark, Department of Dermatology, Roskilde, Denmark
Until 2017, there was no consensus on core outcome domains for hidradenitis suppurativa (HS). Numerous outcome measure instruments exist, with a total of 30 instruments used in the 12 randomized controlled trials included in a Cochrane review from 20161. Heterogeneity in the use of outcomes in HS limits evidence synthesis, including meta-analysis, and likely leads to outcome reporting bias because of selective reporting of more favourable outcomes2. Furthermore, the most frequently used instruments emphasize clinical features with limited incorporation of patient-reported outcomes, despite recommendations emphasizing the importance of the patient perspective in outcomes research. The HISTORIC initiative was formed in 2016 to tackle these issues by developing a Core Outcome set (COS) of domains (“what” to measure) and instruments (“how” to measure) relevant to all major stakeholders, including patients, to be recommended for use in all subsequent HS clinical trials3. To reach consensus on what to measure, six stakeholder groups participated in a Delphi process which included five anonymous e-Delphi rounds and four face-to-face consensus meetings to reach consensus on the final core domains1. To reach consensus on how to measure, a workgroup was created for each core domain to identify the most suited instrument to measure each domain. This process involves reviewing exciting validation data, performing new validation studies or developing new instruments as outlined by COMET/COSMIN4. A total of 41 patients and 52 HCPs from 19 countries in four continents participated in the consensus process to identify six core domains: pain, physical signs, HS specific quality of life, global assessment, progression of course and symptoms1. The pain workgroup has included a daily NRS pain scores in a clinical study to get more data on validity and feasibility aspects. The physical signs workgroup has reviewed exciting validation data and has developed a novel candidate instrument. Hidradenitis suppurativa quality of life (HiSQOL) was developed to measure HS specific quality of life and different validation studies are completed and ongoing5. A single gloabal item covering overall effect on quality of life has also been developed. A novel investigator global assessment instrument (HS-IGA) has been developed and validated in different datasets. For progression of course, qualitative, literature and Delphi studies have been performed to define a flare in HS. More quantitative flare studies are undergoing. Delphi exercise has tried to define a recurrence after HS surgery. In the symptoms workgroup, different fatigue instruments have been reviewed and a content validation study of PROMIS-SF 8a for is undergoing. The group is also working on developing an instrument to measure drainage. A final core outcome measurement set of instruments should be within reach in the next few years. Routine adoption of the COS in future HS trials will ensure that outcomes of importance to both patients and HCPs are collected and at the same time facilitate evidence synthesis and lower the risk of outcome reporting bias.
Acknowledgement: The Department of Dermatology, Zealand University Hospital, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Thorlacius L et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol 2018;179:642–50.
- Clarke M. Standardizing outcomes for clinical trials and systematic reviews. Trials 2007;8:39.
- Williamson PR et al. The COMET Handbook: version 1.0. Trials 2017;18(suppl 3):280.
- Prinsen CA et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” - a practical guideline. Trials 2016;17:449.
- Kirby JS et al. The Hidradenitis Suppurativa Quality of Life (HiSQOL) score: development and validation of a measure for clinical trials. Br J Dermatol 2020;183:340–8.
Severity of hidradenitis suppurativa is assessed differently by the patient and the practitioner
Myriam Fioretta1, Christelle Enault1, Sylvia Math1, Anne-Sophie Gadat1, Marion Clerbout1, Christelle Chavrier1, Marianne Beuque1, Samantha Gaspard1, Ines Evain1, Virginie Vlaeminck-Guillem3,4, Philippe Guillem1,2
1Clinique du Val d'Ouest, Department of Surgery, Ecully, France; 2RésoVerneuil, Paris, France; 3CHU, Department of Biology, Lyon, France; 4Lyon 1 University, INSERM 1052, CNRS 5286, CRCL, Lyon, France
While Hurley classification and IHS4 score are the most two scores used in published studies, there is still a need to identify the best score to assess severity of hidradenitis suppurativa. It has been well recognized that patient-reported outcome has to be taken into account but whether this could be translated in a validated score usable in routine practice remains to be demonstrated. For example, how and why patients estimate the severity of their disease is not known. The aim of the study was to evaluate (1) how HS patients estimate the severity of their disease, (2) what they consider as the source of the severity and (3) how consistent the patient and the physician estimations of HS severity are. We prospectively included all consecutive HS patients coming to a HS-specialized centre. Patients were asked to fill in a questionnaire including their estimation of the severity of the disease (classified as “mild”, “moderate” or “severe”) and the determinants of this estimation (importance of pain or discharge; aspect, size or number of lesions; impact on marital life, family life or work). In parallel, the severity of the disease was assessed by the same practitioner (PG) on the basis of Hurley's classification, the IHS4 score (separated into 3 classes of increasing severity), the HS-PGA score (with stages 0, 1 and 2 grouped into a single “mild” class and stages 4 and 5 into a single “severe” class) and the DLQI (mild if DLQI≤5, moderate if 6 ≤ DLQI≤10; severe if DLQI>10). Between March and November 2022, we collected questionnaires of 292 HS patients. There were 194 women (66%) and 98 men (34%), with a mean age of 35 and a mean BMI of 28. Out of them 184 (63%) were smokers and 63 (23%) had a family history of HS. Patients self-evaluated their disease as mild, moderate and severe in 18% (n = 48), 48% (n = 128) and 34% (n = 90), respectively. Were considered as determinants of HS severity: pain for 88% of the patients, size of lesions for 70%, number of lesions for 67%, aspect of lesions for 65%, impact on work for 61%, discharge for 51%, impact on marital in 38%, and impact of family life for 19%. Other determinants spontaneously reported by patients (response to an open question) were psychological impact (n = 15), reduced mobility (n = 14), fatigue (n = 7), feeling of stigma (n = 7), daily life (n = 4), the need to specifically cover the lesions with appropriate dressings or clothing (n = 4), and (1 occurrence each) chronicity, frequency of flare-ups, difficulty managing hair, irritability, odour, sleep difficulties and lack of awareness of the disease. Compared to clinician-derived severity scores, patient-estimated severity was consistent with the Hurley classification, the IHS4 classification, the HS-PGA and the DLQI only in 35%, 40%, 35% and 47%, respectively. Most often, the differences are underestimates by the practitioner (90% for Hurley classification, 76% for IHS4 and 87% HS-PGA), except for DLQI (overestimation in 78% of the discrepancies). No relation was observed between the patient-physician discrepancies and patient’ or disease's characteristics. Although this study has several limitations including biases in patient self-reporting of severity, it strongly suggests that the tools usually recorded by the clinician are consistent with patient's evaluation in only 35 to 47%, with DLQI being the least bad tool. The reasons underlying these discrepancies are unclear and further studies are required to assess them more precisely.
Speed of progression: A new clinical parameter for therapeutic decision making in hidradenitis suppurativa
Eva Vilarrasa1,2, Silvia Vidal2, Flavia Bittencourt2, Luis Puig1,2, Ignasi Gich3
1Hospital de la Santa Creu i Sant Pau, Dermatology, Barcelona, Spain; 2Hospital de la Santa Creu i Sant Pau, Research Institute, Barcelona, Spain; 3Hospital de la Santa Creu i Sant Pau, Clinical Epidemiology and Public Health Department, Barcelona, Spain
Hidradenitis suppurativa (HS) is an inflammatory, recurrent and debilitating skin condition. The progression from mild to moderate and severe forms of HS is crucial for the proper management of patients with HS, and is critical in therapeutic decision making. But the risk factors associated with this progression remain to be elucidated. Disease severity in HS is a determining factor in the therapeutic decision and it is reflected in the consensus guidelines. However, in many cases there is still confusion about whether or not to initiate systemic biologic therapy and the appropriate time to do so. In addition, there is a lack of clinical parameters to help us know which patients are at high risk of progression to severe forms. In this sense, we have developed a clinical variable that could help to recognize those patients who have a higher rate of progression and would be candidates for an early or more hard-hitting approach: speed of progression. This parameter results from the ratio between the severity of HS (measured through the International Hidradenitis Suppurativa Severity Score System -IHS4-)1 and the time of disease progression (in years). This score gives us a numerical result and reflects the speed of progression of the disease in each patient. Moreover, as recent studies showed that some inflammatory biomarkers, such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), total neutrophil, lymphocyte and platelet count, neutrophil-lymphocyte ratio (NLR), and platelet-lymphocyte ratio (PLR) may be associated with disease severity and disease activity,2-3 these parameters were also evaluated. The objective of the study was to define speed of progression as a new clinical prognostic parameter to assess severity progression in HS, and correlate it with clinical evolution, response to systemic treatment and with routinely obtained inflammatory serum biomarkers, already validated1,2. Medical files of 130 patients who were referred to the outpatient HS center in the Department of Dermatology, Hospital Sant Pau, from 2019 and 2022 were included. Clinical, demographical and biochemical data of patients were prospectively collected and evaluated. The speed of progression rate was calculated by the ratio of IHS4 to the time of disease progression (date of first reported HS lesion to first diagnosis of HS). Speed of progression was significantly correlated with ESR levels (r = 0.286, P = 0.008), CPR levels (r = 0.428, P = 0.003), total neutrophil count (r = 0.467, P = 0.001) and NLR (r = 0.496, P = 0.0001). In addition, it was significantly associated with a shorter time to initiation of the first systemic treatment and the use of combined therapy. No positive correlation was found between speed of progression and body mass index or smoking status- Besides, there were significant positive correlations among ESR levels, CPR levels, total neutrophil count, total platelet count, total lymphocyte count, and NLR with IHS4, as previously reported. Time of disease progression inversely correlated with total neutrophil count (r = −0.1999, p = 0.0298), but no significant correlation was observed between time of disease progression and the other inflammatory parameters evaluated in the study. Speed of progression is a novel and easy-to-calculate clinical parameter that emerges as a promising prognostic factor for disease progression.
- Zouboulis CC et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol 2017;177:1401–9.
- Gambichler T et al. Complete blood collection-based systemic inflammation biomarkers for patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2022;36:1593–6.
- Miller IM et al. Leukocyte Profile in Peripheral Blood and Neutrophil-Lymphocyte Ratio in Hidradenitis Suppurativa: A Comparative Cross-Sectional Study of 462 Cases. Dermatology 2016;232:511–9.
Baseline knowledge of dermatology specialists/residents on hidradenitis suppurativa and immediate learning outcome after their participation at a european academy of dermatology and venereology school
Viktor A. Zouboulis1,2, António F. Massa2,3, Georgios Nikolakis2,4, Errol P. Prens2,5, Jacek C. Szepietowski2,6, Thrasyvoulos Tzellos2,7, Christos C. Zouboulis2,4
1Universitaetsklinikum Hamburg-Eppendorf (UKE), Faculty of Medicine, Hamburg, Germany; 2European Hidradenitis Suppurativa Foundation e.V., Dessau, Germany; 3Centro Hospitalar de Vila Nova de Gaia/Espinho, Department of Dermatology, Vila Nova de Gaia, Portugal; 4Staedtisches Klinikum Dessau, Brandenburg Medical School Theodor Fontane and Faculty of Health Sciences, Departments of Dermatology, Venereology, Allergology and Immunology, Dessau, Germany; 5Erasmus University Medical Center, Department of Dermatology, Rotterdam, Netherlands; 6Wroclaw Medical University, Department of Dermatology, Venereology and Allergology, Wroclaw, Poland; 7Nordland Hospital Trust, Department of Dermatology, Bodø, Norway
During the last three European Academy of Dermatology and Venereology (EADV) Schools on hidradenitis suppurativa (HS; Wroclaw, 2019; Athens 2020; and Porto, 2022) an educational programme-associated questionnaire with 50 course-relevant questions has been distributed to the participants at the beginning and the end of the EADV School1,2. Purpose of the activity was to assess the baseline knowledge on HS and the learning outcome of the 2-day theoretical and practical course of the respective EADV Schools as well as to compare the educational vanue of the three Schools. The participation data of the EADV HS Schools was 5 specialists and 23 residents (20 female, 8 male) In 2019 and 4 specialists and 20 residents (17 female, 7 male) in 2020, as previously reported [1,2]. In 2022, 32 residents (24 female, 8 male) participated at the HS School. The individual baseline HS knowledge and the immediate learning outcome after the end of the School were evaluated in a voluntary, anonymous, prospective interventional study. The Kruskall-Wallis test was used for the comparison of the baseline knowledge of the participants of the three Schools and the learning outcome after the Schools. The Wilcoxon paired samples test has been used to assess the global outcome of each School. Twenty-four participants out of 28 (85.7%) have filled both questionnaires and provided their results for evaluation in 2019; 19/24 (79.2%) in 2020 and 32/33 (97.0%) in 2022. The baseline HS knowledge among the School participants was 35.5/50 correct answers (median value; range 25–46) in 2019, 35/50 (range 26–42) in 2020 and 34.5/50 (range 24–41) in 2022. At the end of the School, 42/50 correct answers (range 37–48; Hodges-Lehmann median difference 6/50; p < 0.0001) were provided by the participants in 2019, 40/50 (range 32–46; Hodges–Lehmann median difference 3/50; p < 0.0007) in 2020 and 39/50 (range 22–44; Hodges–Lehmann median difference 5/50; p < 0.000005) in 2022. All 24 study participants improved their immediate knowledge outcome in 2019. The improvement ranged from 4.3% (from 46 to 48 correct answers) to 84.0% (from 25 to 46 correct answers). Fifteen of 19 study participants improved their immediate learning outcome in 2020. The improvement ranged from 2.4% (from 42 to 43 correct answers) to 57.7% (from 26 to 41 correct answers), whereas 4 participants did not show any improvement (38, 38, 40 and 42 correct answers before and after the course, respectively). In 2022, 29 participants improved their immediate knowledge outcome; 2 participants did not show any improvement (31 and 34 correct answers before and after the course) and one worsened (from 26 to 22 corrects answers, −13.4%). The improvement in 2022 ranged from 2.4% (from 41 to 42 correct answers) to 54.2% (from 24 to 37 correct answers). There was neither a baseline global HS knowledge difference among the three assessed years nor a difference of the global learning outcome after the courses. The study shows a constant improvement of the immediate knowledge on HS after an EADV HS School over the years. It underlines the educational value of this endeavour in increasing the thematic knowledge level of both dermatology residents and specialists. Such studies can objectively assess and compare the efficacy of educational activities in human diseases.3-5
Acknowledgement: We thank Mr. Marc Somja, EADV employee for his help for the organization of the EADV Schools on HS in 2019 and 2020 and Ms. Marina Binarelli, EADV employee for her help for the organization of the EADV HS School in 2022. The Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau and the Department of Dermatology, Erasmus University Medical Center, Rotterdam, Netherlands are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin – ALLOCATE Skin group).
- Zouboulis CC et al. Learning efficacy of the EADV School on hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2019;33:e359–60.
- Zouboulis CC et al. Further evidence for the immediate knowledge improvement through EADV Schools on hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2020;34:e852-3.
- Fielding DW, Regehr G. A call for an integrated program of assessment. Am J Pharm Educ 2017;81:77.
- Gotfryd AO et al. Assessment of a learning strategy among spine surgeons. Global Spine J 2017;7:33–8.
- Flores EK, Hess R Jr. Comparing teaching methods on skin disorders using standardized patients dressed in moulage versus paper cases. Am J Pharm Educ 2018;82:6636.
The excellent inter-rater reliability of IHS4 corroborates its aptitude as primary outcome measurement instrument for large clinical studies in hidradenitis suppurativa
Viktor A. Zouboulis1,2, Bor Hrvatin Stancic3, Anastasia Abaitancei4, Maria J. Guimarães5, Inês Lobo Lobo6, António F. Massa7,2, Georgios Nikolakis8,2, Miguel Nogueira6, Ayhan O. Özdemir9, Anna Pirogova10,2, Errol P. Prens11,2, Jacek C. Szepietowski12,2, Ivana Tusheva13, Thrasyvoulos Tzellos14,2, Christos C. Zouboulis8,2
1Universitaetsklinikum Hamburg-Eppendorf (UKE), Faculty of Medicine, Hamburg, Germany; 2European Hidradenitis Suppurativa Foundation e.V., Dessau, Germany; 3University Medical Centre Ljubljana, Department of Dermatology, Ljubljana, Slovenia; 4Universitaet Transilvania, Department of Dermatology, Brașov, Romania; 5Hospital de Braga, Department of Dermatology and Venereology, Braga, Portugal; 6Centro Hospitalar Universitário do Porto, Serviço de Dermatovenereologia, Porto, Portugal; 7Centro Hospitalar de Vila Nova de Gaia/Espinho, Department of Dermatology, Vila Nova de Gaia, Portugal; 8Staedtisches Klinikum Dessau, Brandenburg Medical School Theodor Fontane and Faculty of Health Sciences, Departments of Dermatology, Venereology, Allergology and Immunology, Dessau, Germany; 9Sağlık Bakanlığı-Turkish Ministry of Health, Istanbul, Turkey; 10I.M. Sechenov First Moscow State Medical University, Department of Dermatology, Moscow, Russia; 11Erasmus University Medical Center, Department of Dermatology, Rotterda, Netherlands; 12Wroclaw Medical University, Department of Dermatology, Venereology and Allergology, Wroclaw, Poland; 13Ss. Cyril and Methodius University, Clinic of Dermatovenerology, Skopje, Germany; 14Nordland Hospital Trust, Department of Dermatology, Bodø, Norway
Core outcome domains represent a major need in hidradenitis suppurativa/acne inversa (HS) as reliable disease staging systems and outcome measurement instruments (OMIs). Despite this need, a review of diverse staging systems and 30 OMIs, which have been applied in 12 randomized controlled trials reports that only three studies investigated inter-rater reliability1. A previous own prospective study on inter-rater and intrarater reliability and agreement2,3 of four widely used HS staging systems [Hurley score, refined Hurley staging, International HS Severity Scoring System (IHS4)4 and HS Physician's Global Assessment] has evidenced the importance of HS staging by a simple rater evaluation of physical signs, which is obscure in large clinical studies. Based on the obtained results, IHS4 in particular, but also HS-PGA, can be recommended for staging patients with HS. In-the-between, IHS4 has been validated as a reliable OMI for clinical studies and real world data analysis. Therefore, the calculation of the inter-rater reliability (IER) of multiple raters, especially such with minor experience in HS, is of paramount importance for the final introduction of IHS4 as primary OMI in large clinical studies. Ten patients (4 female, 6 male; mean ± standard deviation age 43.9 ± 17.2 years; body mass index 20.4–44.9) with a clinically confirmed diagnosis of HS5 and widely differing disease severity (mean IHS4 3.6–50.7) were included. Raters were 31 dermatology residents from 16 countries, who participated in the European Academy of Dermatology and Venereology (EADV) HS School (held 28–30 November 2022 in Porto, Portugal). The study protocol and the instruments to be evaluated [IHS4, Hurley score, Dermatology Life Quality Index (DLQI)] were provided to the raters before rating. All raters assessed the same patients. Inter-rater reliability (IER) was assessed by Cronbach's α and Kendall's W tests. Results were interpreted using George and Mallery scale (>0.9 excellent; > 0.8 good; > 0.7 acceptable; > 0.6 questionable; > 0.5 poor; < 0.5 unacceptable).3 Calculations were carried out using the DataTab statistics calculator 2023 (Seiersberg, Austria; https://datatab.net) and the GIGA Calculator (Georgiev GZ. Correlation Coefficient Calculator. https://www.gigacalculator.com/calculators/correlation-coefficient-calculator.php URL). Correlations among the instruments were calculated using the Wilcoxon test for paired samples.
Cronbach's α was calculated 0.99 among the 31 raters after clinical examination of the 10 HS patients and Kendall's W was 0.931 (p = 0.00007). Therefore, we concluded that the IHS4 IER was excellent. Interestingly, the unweighted evaluation of inflammatory nodules, abscesses and draining tunnels markedly reduced the Kendall's W to 0.857. Moreover, the evaluation of inflammatory nodules and abscesses only, led to an unacceptable Kendall's W of 0.180. There was a significant correlation of IHS4 with Hurley staging, both regarding the grouped mean (p = 0.002) and median values (p = 0.006). There was also a significant correlation of DLQI with IHS4 (p = 0.047) and Hurley stage (p = 0.046). Our results demonstrate the excellent inter-rater reliability (IER) of IHS4 among multiple raters with minor experience in HS and corroborate its paramount role as primary OMI for large clinical studies in HS.
Acknowledgement: We thank Ms. Marina Binarelli, EADV employee for her help for the organization of the EADV HS School in 2022 and the Serviço de Dermatovenereologia, Centro Hospitalar Universitário do Porto, Porto for the organization of the hands-on course. The Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau and the Department of Dermatology, Erasmus University Medical Center, Rotterdam, Netherlands are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin – ALLOCATE Skin group).
- Ingram JR et al. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process. Br J Dermatol 2016;175:263–72.
- Zouboulis CC et al. Inter-rater and intrarater agreement and reliability in clinical staging of hidradenitis suppurativa/acne inversa. Br J Dermatol 2019;181:852–4.
- Gisev N et al. Interrater agreement and interrater reliability: key concepts, approaches, and applications. Res Social Adm Pharm 2013;9:330–8.
- Zouboulis CC et al. Development and validation of IHS4, a novel dynamic scoring system to assess hidradenitis suppurativa/acne inversa severity. Br J Dermatol 2017;177:1401–9.
- Zouboulis CC et al. Hidradenitis suppurativa/acne inversa: Criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology 2015;231:184–90.
Potential predictors of cardiovascular risk improvement in patients with hidradenitis suppurativa treated with adalimumab: A pivotal study of factors associated with carotid intima-media thickness reduction
Manuel Sanchez-Diaz1, Luis Salvador-Rodriguez1, Carlos Cuenca-Barrales3,1, Salvador Arias-Santiago1,2, Alejandro Molina-Leyva3,4
1Hospital Universitario Virgen de las Nieves, IBS Granada, Granada, Spain, Dermatology Unit, Granada, Spain; 2School of Medicine, University of Granada, Granada, Spain, Dermatology Department, Granada, Spain; 3Hospital Universitario Virgen de las Nieves, IBS Granada, Granada, Spain, Hidradenitis Suppurativa Clinic, Granada, Spain; 4European Hidradenitis Suppurativa Foundation, Dessau-Roßlau, Germany
Hidradenitis Suppurativa (HS) has been linked to higher cardiovascular risk (CVR) due to its inflammatory burden. There is little evidence on how biologic treatment could modify the cardiovascular risk of HS patients. The aims of the present study were to explore the modification of CVR in patients under adalimumab treatment and to explore the potential factors associated with CVR improvement. A prospective longitudinal study was performed. A cohort of patients with HS treated with adalimumab was followed-up. Carotid intima-media thickness (IMT), and other clinical and biochemical CVR factors were collected at baseline and 32 weeks after starting the treatment. Twenty-seven patients with severe HS were included. Overall, there were no differences in IMT between baseline (633 μm) and 32 weeks follow-up (634 μm). However, 40.7% (11/27) of the patients presented an improvement in IMT. This group (IMT responders) had a higher prevalence of dyslipidemia, diabetes mellitus, higher HbA1c levels, consumed more tobacco, and had higher BMI at baseline. Moreover, these patients had lower IHS4 scores at baseline and tended to have a greater IMT basal value, indicating a higher burden of subclinical atherosclerosis. Adalimumab treatment might benefit a subset of patients with HS in terms of cardiovascular risk reduction. In light of the results of the present study patients with classical cardiovascular risk factors, and those with higher burden of subclinical atherosclerosis and with less inflammatory load, may be more likely improve their IMT during adalimumab treatment.
Effectiveness, safety and potential predictors of response at week 16 in patients with severe hidradenitis suppurativa treated with secukinumab: A multicenter observational retrospective study
Pablo Fernandez-Crehuet Serrano3, Sofía Haselgruber-deFrancisco1, Alicia Padial-Gomez4, Fiorella Vasquez-Chinchay5, Maria D. Fernandez-Ballesteros6, Irene López-Riquelme6, David Jimenez-Gallo7, Juan M. Segura-Palacios8, Marisol Contreras-Steyls9, Guiovana F. Osorio-Gómez10, Juan Carlos Hernández-Rodríguez10, Carlos Cuenca-Barrales1, Alejandro Molina-Leyva1,2
1Hospital Universitario Virgen de las Nieves, IBS Granada, Granada, Spain, Hidradenitis Suppurativa Clinic, Granada, Spain; 2European Hidradenitis Suppurativa Foundation, Dessau-Roßlau, Germany; 3Hospital Reina Sofia de Córdoba and IMIBIC, Dermatology Department, Córdoba, Spain; 4Hospital Universitario de Jerez de la Frontera, Dermatology Department, Jerez de la Frontera, Spain; 5Hospital Universitario Virgen del Valme, Dermatology Department, Sevilla, Spain; 6Hospital Universitario Regional de Málaga, Dermatology Department, Málaga, Spain; 7Hospital Universitario Puerta del Mar, Dermatology Department, Cádiz, Spain; 8Hospital Costa del Sol, Dermatology Department, Marbella, Spain; 9Hospital Universitario Virgen de la Victoria, Dermatology Department, Málaga, Spain; 10Hospital Universitario Virgen del Rocio, Dermatology Department, Sevilla, Spain
Hidradenitis suppurativa is a chronic inflammatory disease affecting hair follicles with apocrine glands. Treatment includes medical and surgical interventions. Biologics drugs have a key role in the long-term anti-inflammatory treatment of moderate to severe patients due to their immunomodulatory properties. The objective of the study was to evaluate the effectiveness, safety and explore potential predictors of clinical response in patients with severe hidradenitis suppurativa treated with secukinumab after 16 weeks of treatment. Multicenter observational retrospective study. Patients treated with secukinumab 300 mg every 2 or 4 weeks who had completed at least 16 weeks of follow-up from 9 hospitals based in southern Spain (Andalusia) were included in this study. Treatment effectiveness was assessed using the Hidradenitis Suppurativa Clinical Response (HiSCR). Information about adverse events was collected and the therapeutic burden of the patients was calculated as the number of systemic medical treatments and surgical interventions (excluding incision and drainage) experienced until the start of secukinumab treatment. Forty-seven patients were included for analysis with a mean age of 40.9 (10.8) years, and a male: female ratio of 23:24. The mean duration of disease was 18.8 (8.5) years. According to Hurley stage, 46.8% (22/47) of the patients presented a Hurley stage II 53.19% (25/47) presented a Hurley stage III, the mean value of the International Hidradenitis Suppurativa Severity Scoring System (IHS4) at baseline was 21.12 (11.91). All patients had previously received biological treatment with at least one drug. Mean therapeutic burden was 6.89 (3.55) interventions prior to the use of secukinumab. At week 16, 48.93% (26/47) of patients achieved HiSCR response. Adverse events were present in 6.38% (3/47) of the patients. The multivariate analysis showed that female sex, lower BMI and a lower therapeutic burden were independently associated with a higher probability of HiSCR achievement. We observed favourable short-term effectiveness and safety of secukinumab in the treatment of severe hidradenitis suppurativa patients. Female sex, which in our geographical area is associated with less severe disease features, lower BMI and a lower therapeutic burden were associated with a higher probability of achieving HiSCR response.
Inflammatory tunnel “Railway Sign”: A new, independent, ultrasonographic predictor of adalimumab failure in patients with hidradenitis suppurativa
Piotr K. Krajewski1, Abdulhad Jfri2, Gemma Ochando-Ibernón3, Antonio Martorell3
1Wroclaw Medical University, Department of Dermatology, Venereology and Allergology, Wroclaw, Poland; 2Harvard Medical School, Boston, USA; 3Hospital de Manises, Department of Dermatology, Valencia, Spain
Skin ultrasound is a useful tool for diagnosis, staging, and monitoring of treatment response in patients with hidradenitis suppurativa. There have been reports of linear, hyperechoic structures within the cutaneous-epidermal inflammatory tunnels that would correspond to hair-shaft fragments. Nevertheless, in our experience, no hair was found during surgery and histological examination. Therefore, it has been hypothesized that the hyperechoic parallel structures observed in ultrasound may correspond to the granulation tissue arising inside the tunnel and/or the onset of pseudoepithelialization. This hypothesis was supported by the fact that the hyperechogenic lines were frequently parallel, indicating the epithelialized lumen within the tunnel The study aimed to check whether there is a correlation between the newly described ultrasonographic picture called by our group “railway sign” with the response to treatment with adalimumab. 102 lesions of the type B cutaneous-epidermal tunnels from 63 adult patients were analysed. Every lesion was examined clinically and ultrasonographically by an expert in the field. In 68 (66.67%) of the lesions, the ultrasound examination showed the presence of parallel hyperechogenic lines within the tunnel (“railway sign,” while in the remaining ones, the sign was absent. Subsequently, each patient started biological treatment according to international guidelines. Clinical and ultrasound assessments were made at 12 and 24 weeks.
Does BMI influence healing after hidradenitis suppurativa surgery?
Anne Cecile Ezanno1, Gaetan Texier2, Joffrey Marchi2, Anne-Claire Fougerousse3, GEM ResoVerneuil
1Military Teaching Hospital Begin, Department of Surgery, Saint Mandé, France; 2Epidemiology center and health public service, Marseille, France; 3Military Teaching Hospital Begin, Department of Dermatology, Saint Mandé, France
Influence of smoking on healing after HS excisions
Anne Cecile Ezanno1, Gaetan Texier2, Joffrey Marchi2, Anne-Claire Fougerousse3, GEM resoVerneuil
1Military Teaching Hospital Begin, Department of Surgery, Saint Mandé, France; 2Epidemiology center and health public service, Marseille, France; 3Military Teaching Hospital Begin, Department of Dermatology, Saint Mandé, France
Intermittent circadian fasting may improve surgical outcomes in hidradenitis suppurativa patients: A ethnomedical, multicenter, retrospective observational study
Giovanni Damiani1, Vittoria G. Bianchi2, Santo R. Mercuri2, Dennis McGonagle3, Uppala Radhakrishna4
1University of Milan, Milan, Italy; 2San Rafael University, Milan, Italy; 3Leeds University, Leeds, Germany; 4Oakland University William Beaumont School of Medicine, Royal Oak, USA
The assumption that diet may modulate systemic inflammation with a circadian pattern starts to find some evidence also in hidradenitis suppurativa (HS).1 Thus, we performed a multicenter, retrospective observational study to verify the potential modulation of surgical outcomes in patient undergoing intermittent circadian fasting (ICF) before, during and after surgery. We enrolled 61 HS patients (34 males and 37 females with an average age of 41.3 ± 5.96 yoa. All patients had moderate to severe HS (IHS4 19.13 ± 5.33) and were candidate to receive surgery since patients display unresponsiveness or partial repsonse to medical treatments (26 in Adalimumab and 35 antibiotics and retinoids). Surgery was performed before ICF in 26 (42.62%) patients, during ICF in 13 (21.31%) and after ICF in 22 (36.07%). The difference between the 3 ICF groups resulted statistically significant for the wound healing percentages, for both the Patient and Observer Scar Assessment Scale (POSAS), for the patient overall opinion as well as for the Dermatology Life Quality Index (DLQI) score (p-values ≤0.001). The statistically significance remained also comparing before ICF versus during and after ICF, suggesting that ICF exerts a beneficial and synergical effect on wound healing after surgery. ICF improves wound healing in HS patients that undergo surgery and circadian rhythm should be more studied also in HS clinical practice (chronomedicine) to maximize ongoing therapeutical strategies.
Acknowledgement: We thank the Italian Center for Precision Medicine and Chronic Inflammation for its logistical and practical support to the project.
- Damiani G et al. The Safety and Impact of a Model of Intermittent, Time-Restricted Circadian Fasting (“Ramadan Fasting”) on Hidradenitis Suppurativa: Insights from a Multicenter, Observational, Cross-Over, Pilot, Exploratory Study. Nutrients 2019;11:1781.
Posters – Therapeutics
Profile of patients treated with biotherapy for moderate to severe hidradenitis suppurativa in the OMCCI cohort
Anne-Claire Fougerousse1, Ziad Reguiai2, François Maccari3, Jean-Luc Perrot4, Edouard Begon5, Laure Mery-Bossard6, Domitille Thomas-Beaulieu6, Diane Pourchot6, Charlotte Fite7, Ines Zaraa7, Jessica Beaziz7, Dominique Lons-Danic7, Alexandra Patchinsky8, Anne-Laure Liegeon8, Céline Girard9, Guillaume Chaby12, Charline Garcia14, Elise Lang14, Antoine Badaoui15,1, Nathalie Quiles17, Charlotte Lepelley19, Daphné Denis19, Caroline Jacobzone-Leveque20, Josiane Parier3, Rima Mothy22, Anne-Sophie Dillies24, Pierre-Andre Bécherel25, on behalf the OMCCI group
1Military Teaching Hospital Begin, Department of Dermatology, Saint Mandé, France; 2Polyclinic Courlancy Bezannes, Department of Dermatology, Reims, France; 3Private practice, Dermatology, La Varenne Saint Hilaire, France; 4CHU, Department of Dermatology, Saint Etienne, France; 5Centre Hospitalier, Department of Dermatology, Pontoise, France; 6Centre Hospitalier, Department of Dermatology, Saint Germain en Laye, France; 7Hopital Saint Joseph, Department of Dermatology, Paris, France; 8CHR, Department of Dermatology, Metz-Thionville, France; 9CHU, Department of Dermatology, Montpellier, France; 10CHU, Department of Dermatology, Montpellier, France; 11CHU, Department of Dermatology, Amiens, France; 12CHU, Department of Dermatology, Amiens, France; 13CH, Department of Dermatology, Le Puy En Velay, France; 14CH, Department of Dermatology, Le Puy En Velay, France; 15Private practice, Dermatology, Paris, France; 16Hopital Saint Joseph, Department of Dermatology, Marseille, France; 17Hopital Saint Joseph, Department of Dermatology, Marseille, France; 18Private practice, Dermatology, Vannes, France; 19Private practice, Dermatology, Vannes, France; 20CH, Department of Dermatology, Lorient, France; 21Private practice, Dermatology, Beauvais, France; 22Private practice, Dermatology, Beauvais, France; 23Private practice, Dermatology, Estriées-Deniécourt, France; 24Private practice, Dermatology, Estriées-Deniécourt, France; 25Hopital Privé d'Antony, Department of Dermatology, Antony, France
Acknowledgement: To all members of the OMCCI group.
- Bertolotti A et al. Guidelines for the management of hidradenitis suppurativa: recommendations supported by the Centre of Evidence of the French Society of Dermatology. Br J Dermatol 2021;184:963–5.
Paradoxical skin reaction after anti-TNF treatment for inflammatory bowel disease: Should we include hidradenitis suppurativa?
Eleftheria Tampouratzi1, Spyridon Vrakas2, Aristotelis Mellos2, Ioannis Katsantonis1
1Tzaneio General Hospital, Department of Dermatology-Venereology, Pireus, Greece; 2Tzaneio General Hospital, Department of Gastroenterology, Pireus, Germany
Hydradenitis suppurativa (HS) is a chronic inflammatory disease that can be associated with inflammatory bowel disease (IBD) based on clinical, histopathological, pathogenetical and therapeutical similarities1,2. However cases of paradoxical HS, secondary to the use of biologic agents have been described in the literature3. We identified a total of 4 patients with HS among 52 with IBD presented in dermatological department from gastroenterologists for skin disease examination. A total of 52 patients with IBD during a 3.5 year old period (2016–2019) were examined for dermatological manifestations. Patient's age was ranging from 18 to 72 years old, 90% suffered from Crohn's disease and the rest 10% from urcerative colitis. Most patients 69% were under biological treatment. Among IBD patients, 4 (8%) were identified with HS (the other skin diseases are paradoxical psoriasis, viral and fungal infections, erythema nodosume and others). All of them were women. It should be emphasized that 3 of them were taking anti-TNF agents before the clinical presentation of HS. There are several case reports and retrospective studies highlighting the association between HS and CD. Therefore is would be strongly suggested that the HS development after CD is a natural disease progression by peculiar cutaneous manifestations4. Although anti-TNF agents could treat both IBD and HS, the timeline of appearance of HS in our patients after anti TNF treatment may suggest a paradoxical side effect as mentioned in recent literature. As in psoriasis, HS can develop while patient is under anti-TNF treatment for IBD due to a cytokine dysregulation as has been demonstrated with TNF antagonists and psoriasiform skin lesions. In these cases, it is indicated for treatment to switch or stop the anti-TNF treatment. Ustekinumab as anti IL-12/IL-23 antibody which has been used for psoriasis and anti -TNF induced psoriasiform skin lesions has been shown to be a reliable substance in CD treatment5. This may possibly provide an additional therapeutic implementation for anti IL-12/23 or anti IL-23 agents as a reliable alternative for patients with CD with or without HS, as already observed herein.
- Phan K et al. Prevalence of inflammatory bowel disease in hydradenitis suppurativa: systematic review and adjusted meta-analysis. Int J Dermatol 2020;59:221–8.
- Zhang M et al. Association of hydradenitis suppurativa with Crohn's disease. World J Clin Cases 2021;9:3506–16.
- Beraldo RF et al. Hydradenitis suppurativa as a paradoxical side effect of the use of adalimumab in patients with Crohn's disease? Clin Exp Gastroenterol 2020;13:293–8.
- Buchanan F et al. A controversional relationship between Crohn's disease and hydradenitis suppurativa: a case series and literature review. Cureus 2022;14:e23422.
- Takeda K et al. Ustekinumab treatment for hidradenitis suppurativa. J Dermatol 2019;46:1215–8.
Secukinumab as a therapeutic alternative for a kidney-transplant woman with coexisting systemic lupus and recalcitrant hidradenitis suppurativa
F. Javier Melgosa Ramos1, Ramón García-Ruiz1, Alvaro Aguado Vázquez1, Cecilia Alonso Díez1, Marta Galarreta Pascual1, Sergio Martín Jiménez1,2, Almudena Mateu Puchades1
1University Hospital Doctor Peset, Department of Dermatology, Valencia, Spain; 2University Hospital of Salamanca, Department of Pharmacy, Salamanca, Spain
Hidradenitis suppurativa (HS) is a chronic inflammatory immune mediated systemic disorder with a several impact in patient's life. HS is associated with multiple comorbidities, including auto-immune disorders, that sometimes limit the therapeutic approach1. Nowadays, adalimumab is the only approved biological treatment for moderate-to-severe HS, but sometimes patients are not suitable for the treatment with this drug. Adalimumab and other TNF alpha inhibitors have been involved in the development and worsening of systemic lupus in some patients2. In moderate-to-severe HS patients, the IL-17 pathway is overexpressed and its blockade has been proposed as a therapeutic alternative3. Moreover, IL-17 has been involved in the pathogenesis systemic lupus (including nephritis) and also in the progression and development of chronic kidney disease, so IL-17 inhibitors as secukinumab or bimekizumab might also play a role in the treatment of these diseases4. On this topic we present a 38-year-old kidney transplant woman with a well-controlled systemic lupus, and a severe miss-controlled HS not suitable for the treatment with adalimumab, in which secukinumab treatment allowed her to improve HS control without worsening the systemic lupus or kidney function5.
- Dauden E et al. Recommendations for the management of comorbidity in hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2018;32:129–44.
- Kazzi B et al. Adalimumab-Induced Lupus Nephritis: Case Report and Review of the Literature. Eur J Rheumatol 2022;9:108–10.
- Goldburg SR et al. Hidradenitis suppurativa: Current and emerging treatments. J Am Acad Dermatol 2020;82:1061–82.
- Rafael-Vidal C et al. Blocking IL-17: A Promising Strategy in the Treatment of Systemic Rheumatic Diseases. Int J Mol Sci 2020;21:7100.
- Al-Rawi KF et al. Relationship Between IL-2, IL-17 Concentrations, and Serum Creatinine Levels in Men with Chronic Kidney Diseases. Rep Biochem Mol Biol 2022;10:664–74
New-onset inflammatory spondyloarthritis after a wide excision procedure in a patient with long-term HS and on adalimumab therapy
Antoanela Carija1,3, Tonci Stipic3, Ana Bubic3, Mara Drnas3
1University of Split School of Medicine, Department of Dermatology, Split, Croatia; 2University hospital centre Split, Department of Dermatology, Split, Croatia; 3University hospital centre Split, Department of Dermatology, Split, Croatia; 4University of Split School of Medicine, Split, Germany
Several studies report a high prevalence of inflammatory arthritis among hidradenitis suppurativa (HS) patients1, the most common type linked to HS being spondyloarthritis2. However, the association remains inconsistent and with a wide range of prevalence of 3.7–52.5%3. Richette et al. found that inflammatory arthritis was more prevalent in patients with severe HS2. The exact mechanisms underlying the interaction between HS and inflammatory arthritis remain unknown. However, possible explanations include genetic susceptibility, immune dysregulation, cytokine abnormalities, and environmental factors4. We present a patient with extremely severe and active hidradenitis suppurativa, who developed severe abrupt inflammatory arthritis after a wide excision of a gluteal abscess. Our patient has been suffering from HS for more than 20 years; he has been under dermatological treatment for more than 15 years and on adalimumab therapy for more than 5 years. Before this surgery he did not report pain in his joints. Although the dermatologist advised the continuation of adalimumab therapy during the operation and in the postoperative recovery, the patient temporarily stopped the antiTNF-alpha inhibitor therapy. Five weeks after the operation and 5 weeks after the last application of adalimumab, the patient has developed severe inflammatory arthritis in large and small joints, which migrated and was most pronounced at rest. Dactylitis of the fourth finger of the left hand was also clinically observed. The patient was treated with NSAID and prednisone; adalimumab was reintroduced and a rheumatologist was consulted. In the case report, we present the findings and analyse the possible causes of the appearance of his inflammatory arthritis.
- Dauden E et al. Recommendations for the management of comorbidity in hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2018;32:129–44.
- Richette P et al. Hidradenitis suppurativa associated with spondyloarthritis— results from a multicenter national prospective study. J Rheumatol 2014;41:490–4.
- Almuhanna N et al. Association between Hidradenitis Suppurativa and Inflammatory Arthritis: A Systematic Review and Meta-Analysis. Dermatology 2021;237:740–7.
- Shah A et al. The critical role of macrophages in the pathogenesis of hidradenitis suppurativa. Inflamm Res 2017;66:931–45.
Recurrence rates of hidradenitis suppurativa lesions explored with pre-surgical mapping with UHFUS
Flavia Manzo Margiotta, Alessandra Michelucci, Giammarco Granieri, Agata Janowska, Marco Romanelli, Valentina Dini
University of Pisa, Department of Dermatology, Pisa, Italy
Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the hair follicle, whose treatment often requires surgical approach such as wide local excision. Ultra-high frequency ultrasound (UHFUS) is becoming progressively used in HS management, with new data highlighting its role in the precise definition of the margin of HS lesions. A presurgical mapping with UHFUS, in particular, offers the possibility to define in detail the extension of the tunnels and fistulas, allowing excellent precision in the identification of patients and the correct areas to undergo surgery. The aim of our work was then to analyse the recurrence rate of HS lesions, previously explored with presurgical mapping, who underwent wide local excision and were treated with second intention healing. We conducted a single center retrospective study, enrolling 19 patients affected by HS and treated with a wide excision surgery. All the patients were analysed through a pre-surgical US mapping with a 22 MHz and a 70 MHz probe and underwent a wide local excision of the selected lesions. We performed a weekly visit until Week 4 and then a week every 3 months until Week 52. At every visit we evaluated clinically and geographically any sign of relapse on the site of lesion. We then calculated the pain through the VAS analogue scale. Our population consisted of 6/19 (31.6%) males and 13/19 (68.4%) females with an average age of 47.5 years and medium HS-Hurley score of 2.5. Inguinal region was affected in 9/19 (47.4%) patients, armpits in 4/19 (21.1%) patients, pubic region in 3/19 (15.8%) patients, gluteal and perianal region in 2/19 (10.5%) patients and sternal region in 1/19 5.2%) patient. After wide local excision, 11/19 (57.9%) patients were treated with standard therapy according to the principles of HS-TIME, while 3/19 (15.8%) patients received a prolonged-release oxygen free radical oleic matrix and 5/19 (26.3%) patients were treated with ultra-portable Negative Pressure Wound Therapy (NPTW). The mean follow-up time was 13 months and we registered only 2 cases (10.5%) of clinical relapse in a recurrence mean time of 10 months. Total remission of the disease was then achieved in 17/19 (89.5%) patients. At week 0, mean VAS value was 7, which decreased at Week 36 (VAS = 2.5) and during the last follow-up visit (VAS = 0.5). HS management still represents a clinical challenge in particular for its surgical approach. The definition of the correct anatomical margins of the lesions to be treated cannot be accurately explored with the physical examination alone. In particular, clinical examination often struggles to recognize the presence of tunnels, which represent the pathophysiological substrate for the recurrence of pathology and can be useful to discriminate between the need of a medical or surgical approach. In particular, the 22 MHz probe allowed us to identify lesions in depth, while the 70 MHz probe permitted us to identify lesion margins. The advantages of double presurgical mapping are different. It allows us to precisely identify anatomical changes, even detecting subclinical lesions, which if not properly removed, lead to disease recurrence. Our remission rate was higher than data discussed in literature (89.5% vs. 49%), even if our observation time was shorter (13 months vs. 36.2 months) and will have to be extended in the next studies. Our recurrence rate also appears lower even in cases of first intention closure after surgery (10.5% vs. 13.0%). To conclude, UHFUS is a valid tool in determining the anatomical extension of HS lesions, allowing patients who undergo surgery to reduce the relapse rate on the treated areas.
Successful management of hidradenitis suppurativa complicated by squamous cell carcinoma with adalimumab: A case report
Amir Samadian, Miroslav Nečas, Hana Jedličková
Masaryk University, First Department of Dermatology-Venereology, Brno, Czech Republic
- Dufour DN et al. Hidradenitis suppurativa: a common and burdensome, yet under-recognized, inflammatory skin disease. Postgrad Med J 2014;90:216–21.
- AbbVie's HUMIRA® (Adalimumab) Receives First and Only U.S. Food and Drug Administration Approval for Moderate to Severe Hidradenitis Suppurativa | AbbVie News Center – available in the Internet (cited 12.12.2022). https://news.abbvie.com/news/abbvies-humira-adalimumab-receives-first-and-only-us-food-and-drug-administration-approval-for-moderate-to-severe-hidradenitis-suppurativa.htm
- Krathen MS et al. Pharmacologic Immunomodulation and Cutaneous Malignancy in Rheumatoid Arthritis, Psoriasis, and Psoriatic Arthritis. J Rheumatol 2010;37:2205–15.
- Lapins J et al. Incidence of Cancer Among Patients With Hidradenitis Suppurativa. Arch Dermatol 2001;137:730–4.
- Constantinou C et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg 2008;74:1177–81.
One year disease outcomes in patients with hidradenitis suppurativa treated with TNF-alpha inhibitors
Rositsa V. Lavcheva1,2, Gavrail R. Poterov1,2, Radostina S. Deliyska1,2, Tanya T. Gancheva1,2, Karen L. Manuelyan1,2, Evgeniya H. Hristakieva1,2
1UMHAT Prof. Dr. Stoyan Kirkovich, Department of Dermatology and Venereology, Stara Zagora, Bulgaria; 2Trakya University, Medical Faculty/Dermatovenereology, Stara Zagora, Bulgaria
The treatment of Hidradenitis Suppurativa (HS) is challenging and even with appropriate treatments, flare-ups are common. Most of the current guidelines include the use of TNF-alpha inhibitors as inflammatory modulators for patients with severe HS. The objective of the study was to evaluate the treatment outcome in patients with HS on TNF-alpha inhibitor according to HS severity, phenotype and comorbidities. A retrospective analysis of 396 patients with HS, registered at the Dermatology Clinic and Expert Center for HS at the University Hospital in Stara Zagora, Bulgaria was performed. A total of 36 patients with moderate to severe HS were started on TNF-alpha inhibitor (аdalimumab). Patients were assessed by HS localization, phenotype, severity (Hurley stage, International Hidradenitis Score-IHS4), treatment outcome (HiSCR) and relevant comorbidities at baseline, week 12, week 24 and week 52. Out of 396 patients with HS, 36 were started on adalimumab – 32 males (89%) and 4 females (11%). At baseline, according to clinical presentation, the patients were distributed in the following phenotype groups: regular – 19 (53%), follicular – 3 (8%), syndromic – 3 (8%), conglobate – 2 (6%), PG-like – 5 (14%), anogenital – 4 (11%). Hurley stage assessment was as follows: Hurley II – 12 (33%), Hurley III – 24 (67). The most common comorbidities were obesity – 11 (31%), diabetes – 6 (17%), pyoderma gangrenosum – 5 (14%), and Crohn's disease – 2 (6%). Clinical improvement and IHS4 score reduction were observed in 23 patients (64%) at week 12 and 32 patients (89%) at week 24. After 1 year, seven patients (19%) had discontinued treatment – three had undergone surgical treatment, one had dropped on his own wish, one had developed vasculitis, and two had had no improvement of the HS. A fatal outcome had occurred in one patient, who developed acute meningitis and purulent ventriculitis 4 months after starting the treatment. Adalimumab was continued for at least 52 weeks in 28 patients (78%). In this group, 20 patients required hospitalization, and/or additional antibiotic treatment or surgical treatment after 12 weeks of treatment. In 8/36 patients (22%) complete HS remission was observed for the 6 months before week 52 and they required no additional treatment with systemic antibiotics. At baseline, six of these patients (75%) were in Hurley stage II and two (25%) were in Hurley stage III. Adalimumab could lead to improvement of HS but most patients would require additional treatment. Six of the eight patients who achieved full remission were in an earlier Hurley II stage of the disease. Early assessment and treatment might increase the chance of better treatment results.
Successful treatment of hidradenitis suppurativa with roflumilast: A case report with 10 months of follow-up
Maria Blomberg1, Hans Christian Ring2, Alexander Egeberg2,3, Claus Zachariae1, Simon F. Thomsen2,4, Mette Gyldenloeve1
1Herlev and Gentofte hospital, Department of Dermatology and Allergy, Copenhagen, Denmark; 2Bispebjerg and Frederiksberg hospital, Department of Dermato-Venereology and Wound Healing Centre, Copenhagen, Denmark; 3University of Copenhagen, Department of Clinical Medicine, Copenhagen, Denmark; 4University of Copenhagen, Department of Biomedical Sciences, Copenhagen, Denmark
Few effective treatments exist for hidradenitis suppurativa (HS), and drug survival for adalimumab and other off-label biologics seems to be limited1. Roflumilast is a selective phosphodiesterase 4 (PDE4) inhibitor, that acts on cytokines involved in HS pathogenesis. It is up to 90 times more potent in inhibiting PDE4 isoforms than apremilast, another PDE4 inhibitor, which has shown efficacy against HS in earlier studies2,3. A side effect is weight loss, which is often favourable in the HS population. Generic versions of roflumilast are available in some countries for the cost of less than 2.50 USD daily. We here present the 10 months follow-up results of a 56-year-old male patient with severe HS (Hurley 3) treated successfully with roflumilast. Initially, the patient was treated with several different antibiotics, adalimumab (6 months) and infliximab (5 months) but failed to respond. Off-label therapy with oral roflumilast 500 μg once-daily was initiated. At 3 months check-up, marked improvement was found in several parameters, continuing until 10 months follow-up, including reductions in International Hidradenitis Suppurativa Severity Score System (IHS4) from 16 to 3, Dermatology Life Quality Index (DLQI) from 21 to 9, numerical rating scale (NRS)-pain from 8 to 3, and body mass index (BMI) from 31.7 to 26.6. Roflumilast may represent a novel treatment for HS. The drug seems to have several potential advantages including effect on both subjective and objective parameters, on comorbidity (weight) and it has relatively low costs. Larger studies are needed.
- Ring HC et al. Drug survival of biologics in patients with hidradenitis suppurativa. JAMA Dermatol 2022; 158:184–8.
- Vossen ARJV et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol 2019; 80:80–8.
- Walsh NM. Comparison of the modes of action of apremilast and roflumilast. PhD thesis, University of Glasgow, 2015.
Ertapenem for the treatment of hidradenitis suppurativa: A multicenter retrospective study. Description of candidate patients and administration route
Juan Manuel Segura Palacios1, Alberto Soto Moreno2, Ismael V-Millán1, Francisco Rivas Ruiz3, Carlos Cuenca Barrales2, Alejandro Molina Leyva2
1Hospital Costa del Sol, Department of Dermatology, Marbella, Spain; 2Hospital Virgen de las Nieves, Department of Dermatology. Hidradenitis Suppurativa Unit., Granada, Spain; 3Hospital Costa del Sol, Research Unit, Marbella, Spain
The use of intravenous ertapenem is included in the HS Alliance review as a treatment option in selected severe patients, but its use is at the discretion of the treating physician (evidence level 4, grade of recommendation C).1 It is essential to determine which severely affected HS patients would benefit and when would be the ideal time for its administration given the risk of randomly using a broad-spectrum antibiotic like ertapenem2. A multicenter retrospective cohort study wasc conducted in 3 hospitals in the south of Spain to evaluate the effectiveness and safety of treatment with ertapenem in patients with moderate to severe HS in the period between 2017 and 2022. Disease severity was assessed at baseline and follow-up at 6 weeks (end of treatment) and 12/16 weeks, using the International Hidradenitis Suppurativa Severity Score System (IHS4), Investigator Global Assessment (IGA) and pain visual analogue scale (VAS) score. The Wilcoxon rank test was used to evaluate paired differences with respect to the baseline assessment, establishing the level of statistical significance at p < 0.05. Fifteen patients with moderate to severe HS were included (20% Hurley II and 80% Hurley III). All included patients were male with a median age of 51 years. 40% of the patients had a family history of HS and 86.7% were smokers. Median body mass index was 30.1 (obesity), median time of disease evolution was 19 years and median number of affected body areas was 5. The administered dose of ertapenem was 1 g for 6 weeks and the route was 60% intramuscular, 33.3% intravenous and 6.7% mixed. All patients except one had previously received biological treatment, especially adalimumab (14/15). More than half of the patients (8/15) had undergone two or three biologics prior to treatment with ertapenem. The median IHS4 dropped from 28 to 18 (p = 0.008), median IGA changed from 5 to 4 (p = 0.011) and VAS from 9 to 2 (p = 0.004) after 6 weeks of ertapenem (end of treatment). No patient discontinued treatment and no adverse effects were recorded. No patient underwent combined surgery. After treatment with ertapenem most patients (11/15) started a new biological treatment and the other 4 patients underwent antibiotic consolidation treatment with rifampicin/moxifloxacin/metronidazole combination. Between 12 and 16 weeks from baseline, these parameters were collected again: median IHS4 16 (p = 0.001), median IGA 4 (p = 0.001), and median VAS 6.5 (p = 0.003). There was a statistically significant difference in favour of the use of the intramuscular route (IHS4 and VAS) although it is not very valuable due to the sample size. Parenteral administration of ertapenem to patients with HS to be a valid treatment option for seeking rapid improvement in cutaneous inflammation. Join-Lambert et al. reported a significant improvement in pain and drainage in patients treated with the daily administration of 1 g intravenous ertapenem3,4. The efficacy of ertapenem is probably related to its broad spectrum of activity against aerobic and anaerobic bacteria associated with the microbiome of the HS patients although it also has immunomodulatory effects that have not yet been well determined5. The experience with our patients has been a significant decrease in the IHS4, IGA and VAS score. As a novelty, the main route of drug administration was intramuscular, which has not been described to our knowledge to date in this type of patient. In our case series, the positioning of the use of ertapenem was in patients with an inflammatory phenotype with failure of between 1 and 3 previous biological treatments and fundamentally as a bridging cooling therapy to start another biological. Ertapenem could be reserved for patients with an inflammatory phenotype, with multiple affected areas that are not candidates for surgical treatment, with failure of a previous biologic, and as bridging therapy to start another biologic. Given the high bioavailability of intramuscular ertapenem, its use should be encouraged for greater convenience and to avoid complications associated with intravenous use.
- Zouboulis CC et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization – systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol 2019;33:19–31.
- Mendes-Bastos P et al. Ertapenem for the treatment of Hidradenitis suppurativa: how much do we need it? Actas Dermosifiliogr (Engl Ed) 2018;109:582–3.
- Join-Lambert O et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother 2016;71:513–20.
- Alikhan A et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol 2019;81:91–101.
- Braunberger TL et al. Ertapenem - a potent treatment for clinical and quality of life improvement in patients with hidradenitis suppurativa. Int J Dermatol 2018;57:1088–93.
Paradoxical psoriasiform reactions during treatment with adalimumab for hidradenitis suppurativa: Real-life experience and therapeutic response to other biological drugs
Lorenza Burzi, Federica Repetto, Simone Ribero, Luca Mastorino, Pietro Quaglino, Paolo Dapavo
University of Turin, Department Of Medical Sciences, Turin, Italy
Hidradenitis suppurativa (HS) is a painful chronic inflammatory skin disease of the hair follicle. Adalimumab, a tumour necrosis factor-α blocker (TNF-α), is the only approved biologic agent for the treatment of moderate to severe HS1. Paradoxical psoriasiform skin reactions are described in HS patients during treatment with anti-TNF-α agents2 such as to require discontinuation of the same. No guidelines are currently available for the management of this clinical condition. The aim of this paper is to describe the incidence and clinical features of paradoxical psoriasiform eruptions occurring during treatment with adalimumab in patients with hidradenitis suppurativa and to report real-life experience in management and the possible role of other biologic agents for the treatment of both conditions. We conducted a retrospective study at the Dermatology Department of the Turin University Hospital considering all patients affected by moderate to severe HS (Hurley stage II or III) treated with adalimumab at approved dosage who developed psoriasiform cutaneous eruption during treatment. Data were collected from January 2017 to November 2021. We evaluated the incidence rate of paradoxical psoriasis during treatment as well as clinical characteristics of patients who developed this reaction. Psoriasis has been evaluated according to the Psoriasis Area Severity Index (PASI) and HS according to the International Hidradenitis Suppurativa Severity Score System (IHS4) and the Hidradenitis Suppurativa Clinical response (HISCR) score. Severe skin eruptions discontinued adalimumab and 7 patients required switch to newly biological agents: four patients were treated with risankizumab, two with guselkumab and one received secukinumab, all at the approved dosage for psoriasis. We assessed the clinical response for both conditions at 3, 6 and 9 months.
- Zouboulis CC et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015;29:619–44.
- Dequidt L et al. Paradoxical psoriasiform reactions to antitumour necrosis factor-α drugs in hidradenitis suppurativa. Br J Dermatol 2018;178:281–3.
Guselkumab in the treatment of severe hidradenitis suppurativa, a promising role?
Lorenza Burzi, Federica Repetto, Alice Ramondetta, Giulia Rozzo, Matteo Licciardello, Simone Ribero, Pietro Quaglino, Paolo Dapavo
University of Turin, Department Of Medical Sciences, Dermatology Clinic, Turin, Italy
- Dequidt L et al. Paradoxical psoriasiform reactions to antitumour necrosis factor-α drugs in hidradenitis suppurativa. Br J Dermatol 2018;178:281–3.
- Schlapbach C et al. Expression of the IL- 23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol 2011;65:790–8.
- Casseres RG et al. Guselkumab in the treatment of hidradenitis suppurativa: a retrospective chart review. J Am Acad Dermatol 2019;81:265–7.
- Kearney N et al. Successful use of guselkumab in the treatment of severe hidradenitis suppurativa. Clin Exp Dermatol 2020;45:618–9.
Switching from adalimumab originator to biosimilars in hidradenitis suppurativa: What's beyond cost-effectiveness?
Gabriele Roccuzzo, Giulia Rozzo, Lorenza Burzi, Federica Repetto, Paolo Dapavo, Simone Ribero, Pietro Quaglino
University of Turin, Department Of Medical Sciences, Dermatology Clinic, Turin, Italy
In the last years, adalimumab biosimilars have represented an accessible alternative to the originator agent in the treatment of moderate to severe hidradenitis suppurativa thanks to their positive impact on health care budgets1. These anti-TNF-alpha drugs represent the only biologic agents currently approved for moderate to severe hidradenitis suppurativa (HS). Though biosimilars are validated according to the same standards of pharmaceutical quality, safety, and efficacy that apply to the reference medicine, their performance may differ from the originator, due to different manufacturing processes, excipients, and packaging2. As of today, studies investigating the switch from adalimumab originator to biosimilar, following pharmacoeconomic policies, are lacking as only few reports have addressed this issue3,4. Herein we present a real-life setting retrospective study aimed at assessing the safety and efficacy of this switch in 37 patients, evaluated for 12 months in terms of IHS4 (International Hidradenitis Suppurativa Severity Score System) and HiSCR (Hidradenitis Suppurativa Clinical Response). At adalimumab originator first prescription (T0), 78.4% of the patients were classified as Hurley 2 and 21.6% as Hurley 3, with a mean basal IHS4 of 16.51 ± 9.6. No patient discontinued adalimumab originator since first prescription, remaining an average of 52.7 ± 30.4 weeks on therapy. All 37 patients were subsequently switched to the biosimilar following local pharmacoeconomic policies. At the time of drug switch (Ts), mean ISH4 had improved to 6.6 ± 7.2 (p < 0.00001). All patients reached the 3-month analysis after the switch (T3) and no significant difference was observed compared to Ts in terms of ISH4 (p = 0.81). During the year of follow up, 16 patients (43.2%) discontinued treatment (8 due to treatment inefficacy, 5 due to severe injection site pain and 3 for unspecified reasons). The overall mean time on biosimilar was 44.4 ± 17.6 weeks. No severe side effects were observed. The analysis between Ts and last biosimilar administration showed no substantial differences as for ISH4 improving (p = 0.64552) nor HiSCR achievement rates (p = 0.50233). In the subset of patients who discontinued the biosimilar due to inefficacy, cutaneous worsening was represented by higher mean ISH4 (i.e., 8.75 ± 8.22 at T3 and 14.86 ± 7.97 at time of discontinuation), yet without achieving statistical significance (p = 0.12852). Out of the 16 patients who discontinued the biosimilar, 12 were subsequently switched back to adalimumab originator. Currently, 8 patients are still receiving beneficial treatment with the originator, while 5 have been lost to follow up and 3 have been switched to off-label anti IL17 due to poor disease control, in accordance with literature evidence5. Overall, no significant differences emerge between adalimumab originator and biosimilar in terms of clinical response following non-medical switch. High discontinuation rates (43.2%) raise questions on patients' compliance to the new drug regimen, as severe pain at the injection site represents a substantial cause of biosimilar discontinuation (i.e., 31.5% of the cases). In selected cases, rechallenge with adalimumab originator may represent a valid option, as 66.6% (n = 8) of the patients who switched back to the former agent have benefited in terms of tolerability and efficacy. Carefully integrating pharmacoeconomic policies with a thorough assessment regarding the benefit–risk ratio of a nonmedical switch from originator to biosimilars remains essential to provide each HS patient with the best therapeutic option.
- Gallo G et al. Efficacy of switching from adalimumab originator to adalimumab biosimilar in moderate to severe psoriasis patients: a real-life experience in a tertiary referral Centre. Australas J Dermatol 2021;62:e431-2.
- Montero-Vilchez T et al. Switching from Adalimumab originator to biosimilar: clinical experience in patients with hidradenitis suppurativa. J Clin Med 2022;11:1007.
- Patil S. Low-dose adalimumab biosimilar (ZRC-3197) in the treatment of hidradenitis suppurativa. Indian J Dermatol Venereol Leprol 2018;84:745–7.
- Ricceri F et al. Clinical experience with adalimumab biosimilar imraldi in hidradenitis suppurativa. Dermatol Ther 2020;33:e14387.
- Ribero S et al. Effectiveness of Secukinumab in the treatment of moderate–severe hidradenitis suppurativa: results from an Italian multicentric retrospective study in a real-life setting. J Eur Acad Dermatol Venereol 2021;35:e441-2.
Drug survival of anti IL17 and IL23 agents after adalimumab failure in hidradenitis suppurativa: A single center experience
Federica Repetto, Gabriele Roccuzzo, Lorenza Burzi, Luca Mastorino, Paolo Dapavo, Simone Ribero, Pietro Quaglino
University of Turin, Department Of Medical Sciences, Dermatology Clinic, Turin, Italy
Anti-TNF-alpha adalimumab has represented the main biologic therapy for the treatment of moderate to severe Hidradenitis Suppurativa (HS) since 2015, with reported response rates up to 82%.1 Unfortunately, patients with unsatisfactory responses to this therapy still represent a therapeutic challenge in clinical practice. As the development of inflammatory nodules, abscesses, and fistulas has been proved to be triggered not only by TNF-alpha, but also by other cytokines, such as IFN-γ, IL-1, IL-17, and IL-12/23, selectively targeting these other mediators reasonably represents a promising therapeutic option2. Recent studies have proved that anti-IL17 and IL-23 biologics, originally approved for the treatment of psoriasis, can play a role in tackling the inflammatory cascade responsible for HS, leading to clinical improvement3-5. As of today, however, studies investigating the off-label swap to anti-IL17 or anti-IL23 agents, following first line therapy with adalimumab, are lacking. Herein, we describe the results of a retrospective study carried out in a real-life setting at the Dermatology clinic of the University of Turin aimed at evaluating the safety and efficacy of this swap in HS patients. Patients were evaluated every 12 weeks according to IHS4 (International Hidradenitis Suppurativa Severity Score System) and HiSCR (Hidradenitis Suppurativa Clinical Response). At adalimumab first prescription (T0), 42.3% of the patients were classified as Hurley-2 and 57.7% as Hurley-3. At the time of swap (Ts), 16 patients were started on anti-IL17 secukinumab, whilst 10 patients on anti-IL23 agents (risankizumab n = 7, guselkumab n = 3), with mean IHS4 in the two groups of 21.1 (range 10–40) and 17.1 (range 8–40) (p = 0.357), respectively. The groups were comparable for all the evaluated parameters, with no difference in terms of basal mean IHS4 values (i.e., 22.4 and 22, p = 0.952) nor Hurley score (i.e., 11 patients Hurley-2 and 15 Hurley-3). Drug choice was based according to literature evidence and was not influenced by any specific clinical feature. During the follow up time, 8 patients (50%) in the anti-IL17 and 1 patient (10%) in the anti-IL23 groups discontinued treatment due to inefficacy (p = 0.037), respectively. As for clinical responses, a statistically significant difference between the two groups was found at 6 months in terms of HiSCR (p = 0.0272). At the 12-month follow up analysis, mean IHS4 scores of 5.1 (range 0–20) and 14.1 (range 0–30) were recorded in the IL-23 and IL-17 groups, respectively, with statistically significant improvement compared to respective baseline only achieved in the former (p = 0.0027 vs. p = 0.0926). No severe side effects were observed in neither group. According to these data, some interesting observations can be made. Firstly, both interleukin-inhibitor families play a role in improving clinical scores in patients with HS refractory to anti-TNF-alpha, yet with a statistically significance only found in the anti-IL23 group. Secondly, higher discontinuation rates in the anti-IL17 group throughout the year of follow-up, along with a statistically significant different clinical response achievement rate in the anti-IL23 group at 6 months, should not be overlooked. Though the small sample size of this study, along with its retrospective nature, prevents us from drawing definitive conclusions regarding which biologic agent should be prescribed as first choice after adalimumab failure, carefully integrating pre-clinical evidence with real-life data remains essential in pursuing the best therapeutic option for each HS patient.
- Gulliver W et al. Real-world effectiveness of adalimumab in patients with moderate-to-severe hidradenitis suppurativa: the 1-year SOLACE study. J Eur Acad Dermatol Venereol 2021;35:2431–9.
- Markota Čagalj A et al. New and Emerging Targeted Therapies for Hidradenitis Suppurativa. Int J Mol Sci 2022;23:3753.
- Repetto F et al. Efficacy and Safety of Risankizumab in Hidradenitis Suppurativa: A Case Series. Acta Derm Venereol 2022;102:adv00780.
- Burzi L et al. Guselkumab in the treatment of severe hidradenitis suppurativa, a promising role? Dermatol Ther 2021;34:e14930.
- Ribero S et al. Effectiveness of Secukinumab in the treatment of moderate–severe hidradenitis suppurativa: results from an Italian multicentric retrospective study in a real-life setting. J Eur Acad Dermatol Venereol 2021;35:e441-2.
A multi-failure case of hidradenitis suppurativa with recurrent fever and flogistic state
Ilaria Scandagli, Elia Rosi, Gianmarco Silvi, Giulia Nunziati, Prisca Guerra, Antonella Di Cesare, Francesca Prignano
University of Florence, Department of Health Sciences, Section of Dermatology, Firenze, Italy
Hidradenitis suppurativa (HS) is a chronic relapsing inflammatory skin disease clinically characterized by recurrent, deep-seated, painful, subcutaneous nodules, sinus tracts and hypertrophic scarring. HS lesions are localized in the apocrine gland-bearing areas such as the axillae, buttocks, as well as the genital and perineal areas. The major goals of treatment in HS are reduce inflammation, and reduce the formation of new lesions and associated symptoms.
Strategies are applied to treat HS both medical and surgical, according to the different stages of the disease. We report of a case of HS Hurley Stage III in an adult man who failed all available treatments (combination therapy with topic clindamycin and oral doxycycline, numerous courses of oral clindamycin and adalimumab). Our patient after an initially success with adalimumab lasting approximately 10 months developed worsening of his skin lesions and systemic symptoms like anorexia, evening fever and abnormal laboratory tests showing evidence of inflammation: increase of C reactive protein, erythrocyte sedimentation rate, fibrinogen and white blood cells.
We decided to switch to another biologic, an anti-IL17 monoclonal antibody, secukinumab, approved for psoriasis, psoriatic arthritis, ankylosing spondylitis but with favourable results in HS, as proved by the trials Sunrise and Sunshine. In our patient, secukinumab has demonstrated efficacy with progressive improvement in the signs of cutaneous and systemic inflammation and a favourable safety profile.
Topical and intralesional photodynamic therapy for hidradenitis suppurativa: A retrospective case series study of the university hospital son espases
Ines Gracia-Darder, Luis Javier Del Pozo Hernando, Ana Llull Ramos, Susana Romero Delgado, Almudena De la Fuente Risueño, Juan J. González Malmierca, Juan Garcias-Ladaria
Hospital Universitari Son Espases, Department of Dermatology, Palma De Mallorca, Spain
Photodynamic therapy (PDT) is an interesting therapeutic alternative for hidradenitis suppurativa (HS) with a good safety profile, but its effectiveness has yet to be demonstrated due to the lack of standardization of the treatment, the use of different photodynamic agents and light sources, and the absence of randomized controlled trials. We present a series of patients with HS treated with PDT using topical and intralesional photosensitizers. Patients with HS treated with PDT using topical and intralesional photosensitizers in our institution from July 1, 2021 6 to December 1, 2022 were included. Before the procedure, all lesions were evaluated with dermatologic ultrasonography. Lesions were characterized as nodules or fistulas. Fistulas were classified according to Martorell et al.1. In superficial lesions, 5-aminolevulinic acid (ALA, Ameluz®) or methyl aminolevulinate (MAL, Metvix®) were used as topical sensitizers. In deeper lesions (always less than 8 mm maximum depth) intralesional ALA 1% or 2% gel was instilled under local anaestesia with mepicavaine 2%. After 3 h of incubation, illumination was administered using a LED-based red-light source of 635 nm (BF-RhodoLED®), with a fluence of 37 J/cm2 per session. Patients experiencing improvement but no remission were offered other sessions at 4 weeks intervals. Pain during the procedure and other possible side effects were registered. Therapy outcome was evaluated at follow-up visits, 3 and 6 months after therapy. According to the persistence of symptoms and/or lesions, the result was categorized: resolution, improvement, or no change/worsening. Twenty-eight locations in 23 patients (43% men) were treated. The mean age of the patients was 39 years. The treated locations were groin (11, 39%), armpits (6, 21%), buttocks (5, 18%), pubis (2, 7%), submammary region (2, 7%), scrotum (1, 4%), sacral region (1, 4%). The average number of sessions carried out was 1,6. The lesions treated were inflammatory nodules in 3 cases (11%) and fistulas in 25 (89%). Sexteen fistulas were dermoepidermal (64%), 6 dermal (24%) and 3 complex (12%). In 21 sessions (75%) the photosensitizer was applied intralesionally and a cream was used in 7. Examination with Wood's light prior to irradiation showed moderate fluorescence in 55% of cases. The median pain VAS (visual analogy scale) during the irradiation was 4.8 points. No other side effects were recorded. Twenty-five treatments were evaluated at 3 months of follow up: 2 (8%) resolved the lesions, 21 (84%) improved, and 2 (8%) did not improve. At 6 months 19 patients were evaluated: 6 (32%) resolved, 4 (21%) improved partially and 9 (47%) did not improve. There were no notable differences between the patients in terms of pain, fluorescence, number of sessions, and efficacy between patients treated with topical and intralesional PDT. PDT has been proposed as an alternative option for HS, recommended by North American HS Guidelines PDT with a C strength of recommendation and level II/ II evidence. The therapeutic effect of PDT in HS may be due to its direct cytotoxic effect on bacteria, biofilm and sebaceous glands, as well as on the induction of an immunomodulatory response. PDT has some advantages that make it an attractive therapeutic option due to its ability to treat multiple lesions simultaneously, being minimally invasive, having few adverse effects, and good tolerance. In our study PDT was an effective therapeutic option for the treatment of inflammatory nodules and fistulas in HS, although several sessions may be necessary to avoid recurrence, as we observed that some of the patients that initially improved, had recurred before 6 months. In accordance with our results, other authors have reported successful treatment of HS lesions with PDT using topical ALA and MAL2. Regarding the use of intralesional photosensitizers, there are only two studies3,4, using methylene blue, reporting good results, measured by DLQI and Sartorius scale. The penetration capacity of the light emitted by the 635 nm PDT lamp is 5–10 mm. We propose to treat HS lesions with PDT according to their depth, as the topical MAL after 3 h of incubation reaches 2 mm depth and ALA 0,3–0,6 mm. Topical PDT could be used in lesions <2 mm depth, while lesions up to 8–10 mm could be treated with intralesional ALA with lamp irradiation. A third option for lesions deeper than 8 mm could be the use of intralesional ALA and intralesional diode laser of 630 nm.
PDT seems to be cost-effective, safe, and well-tolerated local therapy for Hurley I-II patients with both acute inflammatory lesions and fistulas, although more prospective studies, with higher sample sizes and standardized outcomes are needed to provide more scientific evidence.
- Martorell A et al. Defining Fistular Patterns in Hidradenitis Suppurativa: Impact on the Management. Dermatol Surg 2019;45:1237–44.
- Gracia Cazaña T et al. Systematic Review of Light-Based Treatments for Hidradenitis Suppurativa. Actas Dermosifiliogr (Engl Ed) 2020;111:89–106.
- Agut-Busquet E et al. Photodynamic therapy with intralesional methylene blue and a 635 nm light-emitting diode lamp in hidradenitis suppurativa: A retrospective follow-up study in 7 patients and a review of the literature. Photochem Photobiol Sci 2016;15:1020–8.
- Gamissans M et al. Ultrasound-guided photodynamic therapy with intralesional methylene blue and a 635 nm light-emitting diode lamp in hidradenitis suppurativa: A retrospective study of 41 patients. Photodermatol Photoimmunol Photomed 2022;38:12–18.
Short term safety and effectiveness of intralesional galvanic current in the treatment of draining tunnels of patients with hidradenitis suppurativa: Preliminary results
Alberto Soto-Moreno1, Carlos Cuenca-Barrales1, José Antonio García-Vidal2,3, Francesc Medina-Mirapeix2,3, Salvador Arias-Santiago1, Alejandro Molina-Leyva1,4
1Hospital Universitario Virgen de las Nieves, IBS Granada, Granada, Spain, Dermatology Department, Hidradenitis Suppurativa Unit., Granada, Spain; 2University of Murcia, Department of Physiotherapy, Murcia, Spain; 3Virgen de la Arrixaca University Clinical Hospital, Research Group Fisioterapia y Discapacidad, Institute of Biomedical Research (IMIB), Murcia, Spain; 4European Hidradenitis Suppurativa Foundation, Dessau-Roßlau, Germany
Hidradenitis Suppurativa (HS) is a chronic disease with uncovered therapeutic needs at present, most notoriously in lesion-directed therapies (intralesional corticosteroids, photodynamic therapy, laser), where the percentage of lesion resolution ranges between 44% and 81%. The recurrent nature of the disease, with spontaneous outbreaks of lesions producing unpleasant symptoms such as pain, suppuration or malodor, even in patients under maintained systemic treatment, makes it necessary to have effective local treatments for the control of these lesions.
The application of galvanic current (GC) by percutaneous needle has demonstrated its safety and efficacy in humans in the treatment of lesions structurally similar to those of HS such as mammary fistulas. Its mechanism of action is based on the production of a stimulus capable of provoking cell necrosis and inducing a new activation of the inflammasome to reinitiate the inflammation control process and also with a potential positive effect on the bacterial biofilms present in some lesions. Open label study to evaluate the efficacy and safety of the application of intralesional galvanic current in the treatment of draining tunnels in patients with hidradenitis suppurativa. Patients were evaluated 4 weeks after the treatment administration. Effectiveness was assessed as combined remission: simultaneous presence of ultrasound remission (reduction of at least 75% of the lesion area in the two axes) and clinical remission (absence of suppuration). Adverse effects were assessed by telephone call 48 h after the intervention and at week 4. Seven patients were included. The mean age of the participants was 41 years (35–47), the male:female ratio was 2:4. Baseline ultrasonographic dimensions were mean longitudinal diameter was 32 mm (13.77), mean transverse diameter was 14.42 mm (5.93), mean depth was 4 mm (2.14). Mean baseline suppuration was assessed with a visual analogue scale (VAS) of 3.85 (4). Four weeks after GC administration, the mean longitudinal diameter was 12.5 mm (14.5), reduction of 62.5% with respect to baseline; the mean transverse diameter was 8.3 mm (7.9), reduction of 42.86%; and the depth was 2.54 mm (2.8), reduction of 36.5%. A combined remission was observed in 42.8% (3/7) of the patients. The mean suppuration at 1 month after treatment was 1 in VAS (66.6% reduction from baseline). The pain of the treatment was assessed with a mean VAS of 0. Four participants reported mild dysesthesia and itching at 48 h. Galvanic current may constitute a safe and effective treatment for draining tunnels in patients with hidradenitis suppurativa.
Retinoids use for hidradenitis suppurativa: A practice survey
Anne-Claire Fougerousse1,2, Germaine Gabison3,2, GEM ResoVerneuil2
1Military Teaching Hospital Begin, Department of Dermatology, Saint Mandé, France; 2GEM, ResoVerneuil, Paris, France; 3Private practice, Dermatology, Saint Maurice, France
Acknowledgement: To the members of ResoVerneuil who participate to this study.
Paradoxical hidradenitis suppurativa induced by adalimumab biosimilar successfully treated with guselkumab in a psoriasis patient
Fabrizio Martora, Matteo Megna, Gabriella Fabbrocini
Università di Napoli, Dermatologia e Venereologia, Naples, Italy
- Ruggiero A et al. Paradoxical Hidradenitis Suppurativa during Biologic Therapy, an Emerging Challenge: A Systematic Review. Biomedicines 2022;10:455.
- Berman HS et al. Guselkumab in the treatment of concomitant hidradenitis suppurativa, psoriasis, and Crohn's disease. J Dermatol Treat 2021;32:261–3.
- Kovacs M, Podda M. Guselkumab in the treatment of severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2019;33:e140-1.
- Jørgensen AR et al. Guselkumab for hidradenitis suppurativa in a patient with concomitant Crohn's disease: Report and systematic literature review of effectiveness and safety. Clin Case Rep 2020;8:2874–7.
- Burzi L et al. Guselkumab in the treatment of severe hidradenitis suppurativa, a promising role? Dermatol Ther 2021;34:e14930.
How adalimumab impacts on antibiotic prescriptions in patients affected by hidradenitis suppurativa: A 1-year prospective study and retrospective analysis
Fabrizio Martora, Matteo Megna, Gabriella Fabbrocini
Università di Napoli, Dermatology Unit, Naples, Italy
- Chiricozzi A et al. The Hidradenitis Suppurativa (HS) “Multidisciplinary Unit”: a rationale and practical proposal for an organized clinical approach. Eur J Dermatol 2018;28:274–5.
- Kimball AB et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med 2016;375:422–34.
Tumour necrosis factor-α inhibitor prescription patterns for hidradenitis suppurativa in the united states veterans affairs healthcare system
Katelyn J. Rypka1,2, Zachary Wendland1,2, Claire Herzog3, Lindsey Greenlund3, Fatai Y. Agiri4, Cristina Perez4, Scott L. DuVall4, Lauren Orenstein5, Julie A. Lynch4, Noah I. Goldfarb1,2
1Minneapolis Veterans Affairs Health Care System, Department of Dermatology, Minneapolis, USA; 2University of Minnesota, Department of Dermatology, Minneapolis, USA; 3University of Minnesota Medical School, Minneapolis, USA; 4VA Salt Lake City Healthcare System, VA Informatics and Computing Infrastructure, Salt Lake City, USA; 5Emory University, Department of Dermatology, Atlanta, USA
An increase in the age- and group-specific incidence of hidradenitis suppurativa (HS) has been observed among women, 18–29-year-olds, and African Americans1. Recently, a publication using the Explorys database found low rates of biologic use and statistically significant differences in tumour necrosis factor alpha inhibitors (TNFα-i) prescription patterns between sexes, races, and age groups2. There is prominent concern regarding the provision of services and exacerbation of disparities in access to care for HS patients. To date, there have been no studies on the prevalence of HS in the United States (US) Veteran Affairs health care system (VAHCS). We aimed to determine whether there are similar disparities in TNFα-i, specifically Adalimumab and Infliximab, prescribing rates in the VAHCS compared to civilian healthcare systems in the US. A retrospective cross-sectional and cohort study among adult VAHCS patients with HS was performed to evaluate the prevalence of HS in the US VAHCS and estimate potential discrepancies in the prescription of Adalimumab or Infliximab for patients with HS stratified by age, race, ethnicity, biologic sex, obesity, and tobacco use. The cohort was stratified by age, gender, race, ethnicity, obesity, and tobacco use. The inclusion criteria required patients to be 18-years of age or older, have a diagnosis of HS (ICD-9: 705.83 and/or ICD-10: L73.2), qualify for VAHCS care for 1 year before and after HS diagnosis (index date) and have at least one encounter 6 months pre- and post-index date during the study period (January 1, 2010 through December 31, 2021). Patients were excluded if they were started on a biologic prior to their diagnosis of HS. 35 589 individuals, with a diagnosis of HS, were found in the VAHCS. Of HS patients in the VAHCS, 7.0% had been prescribed either Adalimumab or Infliximab. The majority of patients with HS were White (52%), male (76%), with a BMI >30 (51%), and a history of tobacco use (51%). The largest age group was 45–64 years of age (47%). Female patients were 1.21 times more likely to receive a prescription for Adalimumab or Infliximab than male patients (95% confidence interval [CI]: 1.10–1.33). HS patients had a higher likelihood of being prescribed Adalimumab or Infliximab if they were Black (odds ratio [OR] = 0.41; 95% CI: 0.37–0.45) or obese (OR = 1.21; 95% CI: 1.11–1.33) compared to their counterparts. The overall demographic profile of patients with HS in the VAHCS differs significantly from what is observed in the general US civilian population, with higher prevalence in males and older adults 45–64 years of age. Overall, a lower percentage of patients with HS within the VAHCS had been prescribed biologics, however, the rates were higher for veterans with HS compared to the US civilian population (~2%).2 In the veteran population, patients that identified as female and black were more likely to be prescribed biologics. It is unknown whether these differences in TNFα-i prescribing is due to confounding variations in severity.
- Garg A et al. Incidence of hidradenitis suppurativa in the United States: A sex- and age-adjusted population analysis. J Am Acad Dermatol 2017;77:118–22.
- Orenstein LAV et al. Low prescription of tumour necrosis alpha inhibitors in hidradenitis suppurativa: A cross-sectional analysis. J Am Acad Dermatol 2021;84:1399–1401.
Certilizumab on treating hidradenitis suppurativa: A promising treatment option
Asem Shadid1, Saud Alobaida2, Yousef Binamer2
1King Fahad Medical City, Department of Dermatology, Riyadh, Saudi Arabia; 2King Faisal Specialist Hospital and Research Centre, Department of Dermatology, Riyadh, Saudi Arabia
Hidradenitis suppurativa (HS), is a chronic inflammatory skin condition that affects apocrine gland-bearing skin. The management of HS with biologics has expanded significantly over the past few years. Certolizumab pegol (CZP) is a pegylated (polyethylene glycol) antigen-binding fragment (Fab) of a recombinant humanized anti-TNF-α monoclonal antibody, which is approved for psoriasis, rheumatoid arthritis, ankylosing spondylitis and Crohn's disease. In recent years many reports have been merging on the use of Certolizumab in treating Hidradenitis suppurativa. The electronic database MEDLINE was searched through PubMed in February 2022 using the following search terms: Certolizumab “[All Fields] OR “certolizumab pegol”[All Fields] AND “Hidradenitis suppurativa”[All Fields]. Our search revealed that Certolizumab was used in 6 case reports to treat Hidradenitis suppurativa with a total of 7 patients. In conclusion, to this date Certolizumab pegol is FDA-approved for the treatment of psoriasis, rheumatoid arthritis, ankylosing spondylitis, and Crohn's disease, but not for HS. There are few cases in the literature discussing the use of certolizumab in HS, all of which, shows a good and promising response with no reported side effects. Certolizumab was used as an alternative to the approved medications in cases of failure to therapy or contraindication (e.g., pregnancy). However, the efficacy and safety of certolizumab in treating HS patients is still not well-established and larger trials are needed.
Adalimumab combined with apremilast, an effective treatment to rescue HS patients after secondary failure to adalimumab
Blanca D. Ley, Maria C. Gonzalez, Carmen G. Acebes, Agustina S. Rodriguez
Hospital Universitario del Sureste, Dermatology, Madrid, Spain
Adalimumab combined with apremilast, An effective treatment to rescue HS patients after secondary failure to adalimumab. Hidradenitis suppurativa (HS) is relatively common, with the prevalence of 0.05% to 4.10%, yet many patients receive inadequate treatment. As far as today, adalimumab is the only biological treatment approved for Hidradenitis suppurativa (HS), being affective in near 60% of the patients1. Apremilast is a small-molecule inhibitor of phosphodiesterase 4 with an intracellular mechanism of action that increases levels of cyclic adenosine monophosphate (cAMP) indicated for the oral treatment of moderate to severe plaque psoriasis and for the treatment of psoriatic arthritis. Recently, treatment with apremilast has demonstrated clinically meaningful improvement in moderate HS in two different Randomized controlled trial2,3 and in two case series of nine4 and seven5 patients. We present a case of a forty-two-year-old male with HS since he was twenty. His condition was very severe, developing numerous active, inflamatory large interconnected lesions in both axilla. After several surgeries and antibiotic treatments, we decided to start adalimumab at the doses approved for HS. The patient showed a fast response, achieving the HiSCR50, two mounts after adalimumab was introduced. This response was also maintained in time, as no new lesions appeared during the first 2 years of treatment. After that, several inflammatory nodules and two new fistulas appeared in both axilla, showing a loss of response to adalimumab. At that point we decided not to stop adalimumab treatment, introducing apremilast 60 mg per day in association instead. Of note, after 3 months of adalimumab-apremilast treatment, all the inflammatory lesions disappeared, no new lesions appeared and the patient was very satisfied with the treatment. This case points to the benefit of the combination of apremilast with adalimumab, which seems to be a good option to HS patients after secondary failure to adalimumab.
- Kimball AB et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med 2016;375:422–34.
- Vossen ARJV et al. Apremilast for moderate Hidredenitis suppurativa. Results of a Randomized controlled trial. J Am Acad Dermatol 2019;80:80–8.
- Kerdel FR et al. Apremilast for the treatment of mild to moderate hidradenitis suppurativa in a retrospective open-label phase 2 study. J Drugs Dermatol 2019;18:170–6.
- Weber P et al. Apremilast in the treatment of moderate to severe hidradenitis suppurativa: A case series of 9 patients. J Am Acad Dermatol 2017;76:1189–91.
- Garbayo-Salmons P et al. Hidradenitis Suppurativa Treated With Apremilast: A Case Series. Actas Dermosifiliogr (Engl Ed) 2021;10:936–9.
Ozenoxacin effectiveness in hidradenitis suppurativa
Pablo Fernandez-Crehuet, Delia Diaz-Ceca, Damian Moreno-Mesa, Irene Rivera-Ruiz, Miguel J. Cencerrado, Cesar Guijarro-Sanchez, Jose L. Fernandez-Crehuet
Hospital Reina Sofia de Córdoba and IMIBIC, Dermatology, Cordoba, Spain
Hidradenitis suppurativa (HS) is a morbid, recurrent, chronic inflammatory, skin condition that presents a challenge to clinical therapy. It is characterized by disbalance in the imunoregulatory T-subsets within cell mediated immune response in patients with predisposing genetic factors. Although bacterial infection is not the starter fact in hidradenitis suppurativa, it is well known that plays an essential role in maintaining inflammation1,2. The presence of Enterobacteriaceae, Staphylococcus and gastrointestinal flora have been evidenced in hidradenitis suppurative lesions in previous works. Beta-lactams and fluorquinolones are well-documented effective agents against these microorganisms. As systemic tetracyclines and quinolones have also demonstrated potent anti-inflammatory effect, they are widely accepted in the treatment of the hidradenitis suppurativa3,4. However, non-studies have been published about using topical quinolones in this entity to the date. Ozenoxacin is a highly potent topical antimicrobial approved for superficial skin infection treatment caused by S. aureus. This non-fluorinated quinolone acts by inhibiting DNA gyrase A and topoisomerase IV and affects supercoiling, supercoil relaxation, chromosomal condensation, chromosomal decatenation and many others. The objective of the study was to evaluate the effectiveness and usefulness of 5-days course of topical ozenoxacin 1% cream, in patients with hidradenitis suppurativa attended in Dermatology Department of Reina Sofia Hospital in Cordoba (Spain). Observational retrospective study. Patients were treated with ozenoxacin 1% cream twice a day for 5 days. According to other simultaneous treatment, they were included in three groups: no other treatment (naive), biological therapy (adalimumab, secukinumab and guselkumab) and oral antibiotic. No changes in coexisting treatment were accepted. We used surveys and pictures to evaluate the response to ozenoxazin. Pain was measured by visual analogy scale, grade of suppuration from 0 to 4 was referred by the patient, decreasing size of abscess and fistula and outbreak duration before and after the use of topical ozenoxacin were registered. Hurley stage, localization of the lesions and previous use of topical clindamycin were also included. SPPS program with Wilcoxon and Kolmogórov-Smirnov tests were used for statistical analyses. Forty-four patients were included with a mean age of 36.21 years. Axillary and inguinal areas were predominant locations and 40% of the patients presented Hurley stage III. Thirty-eight patients (86%) were previously treated with topical clindamycin with partial or lack of response. Mean pain after the use of ozenoxacin significatively decreased from 7.24 to 4.03. Mean suppuration also decreased from 3.58 to 1.97. Statistically significant differences with clindamycin use results were found5. Decreased size of abscesses/fistulas was evidenced in 57.9% of the patients but not enough significance was observed in outbreaks duration. Concomitant treatment presented influence in pain decreased after using topical ozenoxacin. Topical ozenoxacin is approved for impetigo treatment in adults and children older than 6 months. Our study suggests that this non-fluorinated quinolone could decreased pain and suppuration of the lesions in patients with hidradenitis suppurativa especially those with mild Hurley stages and with lack of previous use of antibiotics. Although further studies are required, we propose the use of topical ozenoxacin in patients with hidradenitis suppurativa as useful alternative therapy in these patients.
- Matusiak Ł et al. Bacteriology of hidradenitis suppurativa - which antibiotics are the treatment of choice? Acta Derm Venereol 2014;94:699–702.
- Bettoli V et al. Antibiotic Treatment of Hidradenitis Suppurativa. Dermatol Clin 2016;34:81–9.
- Gulliver W et al. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord 2016;17:343–51.
- Zouboulis CC et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015;29:619–44.
- Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol 1983; 22:325–8.
Long term efficacy of secukinumab in HS patients: A case series
Dalma Malvaso, Flaminia Antonelli, Andrea Chiricozzi, Ketty Peris
Università Cattolica del sacro Cuore, Dermatologia e Venereologia, Rome, Italy
Introduction. Hidradenitis suppurativa (HS) is a chronic disabling inflammatory skin disease that, in moderate to severe cases, usually requires the use of systemic antibiotics, retinoids, and immunosuppressants/immunomodulants including adalimumab, the first-approved anti-tumour necrosis factor-alpha (TNF)-α agent. Nevertheless, the presence of several conditions poses a limit to the prescription of adalimumab and require the off-label use of immune-targeted therapies, including secukinumab, a human monoclonal immunoglobulin G1 kappa antibody that selectively binds to interleukin (IL)-17A. Indeed, patients with HS present imbalances in the T-helper cell axis 17 (Th17) cells, with high serum levels of proinflammatory cytokine IL-17A. We report the case of 3 HS patients referred to our clinic successfully treated with long-term secukinumab. A 28-year-old woman patient with HS and SLE is treated with off-label secukinumab associated with hydroxychloroquine. The presence of autoimmune conditions, particularly systemic lupus erythematosus (SLE), may represent a contraindication to the use of TNF-α inhibitors due to the risk of drug-induced lupus. At our observation (Figure 1a), the patient reported a worsening of disease associated with a relevant disease burden (Hurley II; IHS4: 12; pain-Numeric Rating Scale [NRS]: 7/10; itch-NRS: 3/10; Dermatology Life Quality Index [DLQI]: 15). Because adalimumab was considered contraindicated, off-label subcutaneous secukinumab injections at the dosage of 300 mg at weeks 0, 1, 2, 3, and 4, followed by monthly maintenance dosing, were prescribed. As soon as week 16 of secukinumab therapy (Figure 1b), improvement of disease severity was observed (IHS4: 8, pain-NRS: 0/10, and itch-NRS: 3/10; DLQI:5; HiSCR: positive). Efficacy and safety of the treatment were maintained throughout the follow up period (week 104 IHS4: 1, pain-NRS: 0/10, and itch-NRS: 3/10; DLQI:5; HiSCR: positive).
Complications related to the use of adalimumab in specialized outpatient center in hidradenitis suppurativa
Mariana M. Rechuan, Laise Lorena C. Santos, Vitoria Azulay, Marcos Paulo G.G. Bouchuid, Luiza F. Vieira D'Almeida
Santa Casa da Misericoirdia do Rio de Janeiro, Instituto de Dermatologia Professor Rubem David Azulay, Rio de janeiro, Brazil
- Lu J-W et al. Efficacy and safety of adalimumab in hidradenitis suppurativa. Medicine 2021;100:e26190.
- Goldburg SR et al. Hidradenitis suppurativa. Journal of the American Academy of Dermatology 2020;82:1045–58.
- Aubin F. Adalimumab. Ann Dermatol Venereol 2019;146:478–82.
- Shivaji UN et al. Review article: managing the adverse events caused by anti-TNF therapy in inflammatory bowel disease. Alim Pharmacol Therapeutics 2019;49:664–80.
- Boletim Epidemiológico de Tuberculose – 2022 — Português (Brasil) [Internet]. www.gov.br. Available from: https://www.gov.br/aids/pt-br/centrais-de-conteudo/publicacoes/2022/boletim-epidemiologico-de-tuberculose-2013-2022/view
Efficacy of two different ixekizumab protocols in the management of recalcitrant hidradenitis suppurativa
Flavia Manzo Margiotta, Alessandra Michelucci, Giammarco Granieri, Cristian Fidanzi, Marco Romanelli, Valentina Dini
University of Pisa, Department of Dermatology, Pisa, Italy
A new ultraportable negative pressure wound therapy in the management of hidradenitis suppurativa post surgical lesions: A case report
Giammarco Granieri, Alessandra Michelucci, Flavia Manzo Margiotta, Matteo Bevilacqua, Marco Romanelli, Valentina Dini
University of Pisa, Department of Dermatology, Pisa, Italy
Hidradenitis suppurativa (HS) is a chronic inflammatory disease that often requires a surgical approach with secondary intention closure. The aim of our work was to explore the clinical improvement obtainable through the use of a new ultra-portable Negative Pressure Wound Therapy (NPWT) device in the post-surgical management of HS lesions. A 23-year-old male patient affected by axillary and intergluteal HS in Hurley IIC was admitted at our clinic in August 2022. The presence of a scar area with a tunnel in the right axilla was assessed, for which surgery was scheduled in October 2022. After the wide local excision, closure by secondary intention was opted and applications of silver hydrofiber and thin polyurethane foam with silicone interface were performed twice a week. Due to the slow progression of wound healing, an ultra-portable NPWT was applied at T28 and it was changed at T30, T34 and T38. At each visit, the wound was evaluated in terms of area, perimeter, depth, Wound Bed Score (WBS) and Numerical Rating Scale (NRS) for the pain quantification. All numerical evaluations were performed considering advanced dressing (T0–T28) and NPWT (T28–T38) timelines. NPWT resulted in higher WBS improvement than advanced dressings (ΔWBS 6 vs. ΔWBS 2). Furthermore, NPWT determined a greater reduction of area (ΔArea 5 cm2 vs. ΔArea 4 cm2), perimeter (Δperimeter 18 mm vs. Δperimeter 14 mm) and maximum depth (Δdepth 2 mm vs. Δdepth 0 mm) as well as a better improvement of NRS pain (ΔNRS 4 vs. ΔNRS 3). NPWT represents a highly effective clinical tool in the management of post-surgical HS wounds, allowing a fast improvement in terms of clinical measurements and pain reduction.
A patient with hidradenitis suppurativa and psoriasis treated with brodalumab who developed pyoderma gangrenosum: A paradoxical reaction?
Giorgia Salvia, Alessandra Michelucci, Flavia Manzo Margiotta, Matteo Bevilacqua, Marco Romanelli, Valentina Dini
University of Pisa, Department of Dermatology, Pisa, Italy
Pyoderma gangrenosum (PG) is a rare neutrophilic dermatosis characterized by rapidly developing painful ulcers typically at sites of trauma, especially on the lower extremities. Even if the pathogenesis of the disease is not yet fully understood, several autoimmune comorbidities such as inflammatory bowel disease, hematologic cancer and rheumatoid arthritis have been associated with PG. Moreover, it has been reported that several drugs can be associated with the development of PG-like lesions eventually occurring years after the start of the drug therapy. We reported a clinical case of a 43-year-old female with a diagnosis of hidradenitis suppurativa (HS) psoriasis and psoriatic arthritis who was admitted to our department in December 2021 with a painful ulcerative lesion on the lower extremity. The patient was under treatment with Brodalumab from June 2021, since the previous psoriasis and HS therapies with Infliximab and Adalimumab led to a loss of efficacy on the cutaneous lesions. During the visit, a first diagnosis of an insect bite was done, but due to the absence of clinical improvement after antibiotic therapy, a biopsy was performed and we made a diagnosis of PG. We decided to start intravenous steroids and cyclosporine. After 2 days, the patient presented a new erythematous lesion on the right breast that rapidly developed in a necrotic ulcer and required a radical mastectomy of the right breast. At discharge, the patient re-started Adalimumab therapy (40 mg subcutaneous injections every 2 weeks). At the following checkup, we noticed an improvement of plaque psoriasis, psoriatic arthritis and HS as well as the resolution of the lower leg PG lesion. Several drugs have been associated with the development of PG. The exact mechanism by which each drug causes this inflammatory skin condition is not known but it is thought to be multifactorial. The key steps involved in the pathogenesis of PG-like lesions seems to be correlated with neutrophil migration, dysregulation of the inflammatory response and keratinocyte apoptosis. Brodalumab is a monoclonal antibody that inhibits IL-17 by blocking IL-17 receptor A. It is feasible that inhibition of IL-17 receptor by Brodalumab may induce a disruption in the cytokine balance in predisposed individuals. Furthermore, cases of drug-induced PG by other biologic agents targeting IL-17 have been reported in the literature. It is worth mentioning that several cases of biologic-induced PG happened after the switching of one biologic agent to another possibly due to the dysregulation of the cytokine milieu. To conclude, we reported the case of a possible Brodalumab-induced PG in a patient affected by psoriasis and HS.
Nonsteroidal anti-inflammatory drugs and the risk of hidradenitis suppurativa
Marianne Clerbout1, Christelle Chavrier1, Marianne Beuque1, Samantha Gaspard1, Ines Evain1, Myriam Fioretta1, Christelle Enault1, Sylvia Math1, Anne-Sophie Gadat1, Virginie Vlaeminck-Guillem3,4, Philippe Guillem1,2
1Clinique du Val d'Ouest, Department of Surgery, Ecully, France; 2RésoVerneuil, Paris, France; 3CHU, Department of Biology, Lyon, France; 4Lyon 1 University, INSERM 1052, CNRS 5286, CRCL, Lyon, France
Although the subject is not specifically covered, the medical literature gives varied instructions on the use of systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients with hidradenitis suppurativa (HS). Some authors accept the use of NSAIDs for the treatment of HS flares while others contraindicate their prescription for HS as well as for intercurrent or associated pathologies. The aim of the study was to explore the potential link between NSAIs and HS through a prospective, cross-sectional study comparing control subjects and HS patients. As part of the GHiSA project (estimation of HS prevalence in a control population through a validated screening questionnaire), we prospectively constructed a cohort representative of the general French population and including control patients referred to the Clinique du Val d'Ouest (Lyon, France) for consultation or hospitalization for a pathology other than HS, and those accompanying patients coming for such situations. To avoid a family aggregation bias, we excluded accompanying persons from the same families as the control patients. At the same time, we included all consecutive HS patients coming to the HS-specialized centre of the healthcare institution for consultation or hospitalization. The HS patients identified after clinical validation among the control patients and accompanying persons were included in the HS cohort. All subjects were proposed to fulfil a questionnaire including questions about the use of NSAIDs (yes/no) during their life and during the last 3 months, the type of the molecule(s) consumed and the reason for taking them. Between March and November 2022, we collected 1025 controls (501 accompanying adults and 524 patients) and 286 HS patients. NSAIDs has been already consumed during life of 668 controls (65.2%) and 233 HS patients (72.4%), p = 0.017. Taking NSAIDs in the 3 months preceding the questionnaire was more frequent in HS patients than in controls: 41.5% versus 34.5%, respectively (p = 0.026). However, the way NSAIDs were taken differed between the two groups with more daily uses in controls than in HS patients: 38.5% versus 11.1%, respectively (p = 0.028). Among HS patients consuming NSAIDs, 71 (30.5%), 77 (33%) and 85 (36.5%) consumed 1, 2 or ≥3 different molecules (vs. 41%, 35.5% and 23.5% in the controls, respectively; p < 0.001). The five most consumed NSAIDs were in both groups and in order ibuprofen, aspirin, ketoprofen, diclofenac and flurbiprofen. Only ibuprofen, ketoprofen and flurbiprofen were individually more consumed in HS patients than in controls. The most frequent reasons for NSAI consumption were headache (29.5% of all consumers), arthromylagia (24.1%), period pain (19.3% of female consumers), backpain (15.3%), and migraine (11.6%). Period pain has more frequently been put forward in female HS patients than in female controls: 30.8% versus 14.6% (p < 0.001). Similarly more HS patients consumed NSAIDs for fever than controls: 7.2% versus 2.6% (p = 0.002). By contrast, more controls consumed NSAIDs for arthromyalgia and back pain than HS patients: 26.3 s 17.9% (p = 0.012) and 17.2 versus 10.3% (p = 0.015). As high as 17.5% of HS patients consumed NSAIDs for HS flares. We found a correlation between use of NSAIDs use (ether during patient's life or during the last 3 months) and HS severity, with a significant progressive consumption while severity increased as assessed through the Hurley score (I: 79%, II: 67%, III: 43%). Similar results were observed with IHS4 score or HS-PGA. No significant correlation was observed between NSAID use and either DLQI, pain, discharge or pruritus VAS. Whether HS was patient-reported as stable, improved or worsened was not influenced by NSAIDs consumption. Although possible biases related to its design (self-questionnaire) and the fact that taking NSAIDs is possibly already influenced by medical recommendations not to take them; our study is the first one to explore NSAIDs consumption in HS patients. It shows that HS patients consume more NSAIDs than controls. The reasons for NSAID consumption were different between the two cohorts. Increased flurbiprofene consumption (a NSAID usually prescribed for period pain) and the increased use of NSAIDs for period pain in HS patients suggest that HS female patients experience increased period pain. Some of them also have endometriosis, a gynecologic inflammatory condition that has not been reported as a HS comorbidity but could be addressed. Conversely, we failed to find an increased NSAIDs consumption for joint or back pain in HS patients, a result that could be expected due to the well-known association of HS and arthropathy. At least, no correlation was found between NSAID consumption and HS severity, arguing against a role of NSAIDs as triggering factor for HS or HS flare.
Factors associated with patients self-reported response to treatments in hidradenitis suppurativa
Christelle Chavrier1, Marianne Beuque1, Samantha Gaspard1, Ines Evain1, Myriam Fioretta1, Christelle Enault1, Sylvia Math1, Anne-Sophie Gadat1, Marion Clerbout1, Virginie Vlaeminck-Guillem3,4, Philippe Guillem1,2
1Clinique du Val d'Ouest, Department of Surgery, Ecully, France; 2RésoVerneuil, Paris, France; 3Lyon 1 University, INSERM 1052, CNRS 5286, CRCL, Lyon, France; 4CHU, Department of Biology, Lyon, France
The appearance of phase II-III trials evaluating the benefit of some biologics in HS has made it clear that the therapeutic evaluation initiated with this type of treatment should be extended, in order to be able to make comparisons and issue recommendations for substantiated way, to other therapeutic measures (antibiotics, surgery). From this comparative perspective, an outcome that is just as important as the response rate is the identification of the factors that influence it positively or negatively. The aim of the study was to evaluate whether response to HS treatments could be predicted by simple clinical characteristics. All consecutive HS patients coming to a French HS-specialized centre for consultation or hospitalization were included from March to November 2022. They were proposed to answer a questionnaire including questions about their previous and present treatments and whether they considered this/these treatments as efficient in controlling HS. We also collected basic clinical patients characteristics (age at questionnaire, gender, age at disease onset, smoking status, BMI) as well as disease severity score: Hurley classification, IHS4 as a continuous variable, IHS4 as a discrete variable (mild if ISH4 < 4, moderate between 4 and 9 or severe if IHS4 ≥ 10), HS-PGA as a 0–5 discrete variable, HS-PGA as a binary variable (≤3 or ≥4). At the end of the study, 403 HS patients were included: 258 women (64%) and 145 men (36%), mean age 34 ± 11 years, mean BMI 27.4 ± 6.4 kg/m2, HS family history 24%, current smokers 64%, mean age at disease onset 21 ± 8 years. The treatments received by these patients were: short course(s) of oral antibiotics (n = 291, 72%), emergency surgery (n = 245, 61%), topical antibiotics (n = 243, 60%), surgical excision (n = 221, 55%), long course(s) of oral antibiotics (n = 191, 47%), zinc (n = 73, 18%), short and long course(s) of intravenous antibiotics (n = 33, 8% and n = 28, 7%, respectively) and biologics (n = 30, 7%). The patient-report response rate was 90% for surgical excision, 72% for emergency surgery, 52% and 49% for short and long course(s) of oral antibiotics, 47% for biologics, 42% and 29% for short and long course(s) of intravenous antibiotics, and 16% for zinc. Factors associated with response to short course(s) of oral antibiotics were continuous IHS4, discrete IHS4, and male gender, these last two being found independent predictors during multivariate analysis. Factors associated with response to topical antibiotics were discrete IHS4 and binary HS-PGA, which were found dependent on each other. A higher age at disease onset, male gender and response to previous short course(s) of oral antibiotics were all predictors of response to long course(s) of oral antibiotic, but only response to previous short course(s) of oral antibiotics proved to be independent. Factors associated with response to emergency surgery were response to previous short course(s) of oral antibiotics and severity assessed by either continuous ISH4, discrete IHS4 or binary HS-PGA. Co-analysed with either of the three severity score, response to previous short course(s) of oral antibiotics was the sole independent predictor. No specific factor was associated with response to surgical excision. No correlation was searched for biologics due to the too small number of patients treated. This evaluation, limited in time, of the therapeutic alternatives experienced by the HS patients, offers an idea of the way in which they were treated. We note the low proportion of patients treated with biologics, in relation to a 5-year delay for adalimumab reimbursement by social insurance in France. Topical antibiotics are widely used despite the lack of strong medical literature. The highest response rates were obtained with surgery, either emergent or planned, possibly overestimated due to the specialization of the HS centre. They however represent how adequate surgery can be considered from the patients' point of view. The lack of factors predictors of response to surgery is likely to be related to the high response rate, also suggesting that surgery can be offered in a large spectrum of situations. Severity was found to be the most frequent factor involved in response rate. Along with response to one treatment seems often related to the response to another one, this suggests that a significant proportion of HS patients could have recalcitrant disease. Development of new alternatives, including biologics (poorly represented in this study) remains therefore a major future challenge.
Long-term efficacy and safety of brodalumab in a patient with hidradenitis suppurativa and concomitant palmo-plantar psoriasis
Manfredo Bruni, Emanuele Vagnozzi, Maria Esposito, Maria Concetta Fargnoli
University of L'Aquila, Dermatology, Department of Biotechnological and Applied Clinical Sciences, L'Aquila, Italy
- Frew JW et al. A Systematic Review of Promising Therapeutic Targets in Hidradenitis Suppurativa: A Critical Evaluation of Mechanistic and Clinical Relevance. J Invest Dermatol 2021;141:316-24.e2.
- Witte-Handel E et al. The IL-1 pathway is hyperactive in hidradenitis suppurativa and contributes to skin infiltration and destruction. J Invest Dermatol. 2019;139:1294–305.
- Navrazhina K et al. Interleukin 17C is elevated in lesional tissue of hidradenitis suppurativa. Br J Dermatol 2020;182:1045–7.
- Frew JW et al. The effect of subcutaneous brodalumab on clinical disease activity in hidradenitis suppurativa: An open-label cohort study. J Am Acad Dermatol 2020;83:1341–8.
- Yoshida Y et al. Long-standing refractory hidradenitis suppurativa responded to a brodalumab monotherapy in a patient with psoriasis: A possible involvement of Th17 across the spectrum of both diseases. J Dermatol 2021;48:916–20.
Efficacy and safety of modified-release doxycycline 40 mg for the treatment of hidradenitis suppurativa
Gemma Camiña-Conforto1, Ruben Fuentes2, Luis Puig1, Eva Vilarrasa1
1Hospital de la Santa Creu i Sant Pau, Dermatology, Barcelona, Spain; 2Hospital de la Santa Creu i Sant Pau, Research Institute, Barcelona, Spain
Hidradenitis is an inflammatory disease with multiple possible therapeutic approaches, both medical and surgical. Treatment guidelines include antibiotics, doxycycline or combination of rifampicin and clindamycin being the most frequently used1,2. Modified-release doxycycline (MRD) has been used to treat moderate to severe acne inflammatory lesions, showing comparable efficacy and superior safety to doxycycline 100 mg3,4. MRD is currently approved for the treatment of rosacea, with good results, without affecting the commensal microflora or generating antibiotic resistances5. Antibiotic resistance is currently a worldwide concern, since it is one of the greatest threats to global health, and efforts are being made to try to prevent its development. WHO has been leading multiple initiatives to fight antimicrobial resistance. In this context, limiting the use of antibiotics as much as possible or using those that are associated with a lower rate of resistance is crucial. There is limited evidence on MRD 40 mg at sub-antimicrobial dose for treatment of patients with HS. This treatment regimen could provide clinical improvement with a lower rate of adverse events than higher doses, avoiding development of antibiotic resistance. The objective of the study was to prospectively evaluate the efficacy and safety of MRD 40 mg in patients with HS, in clinical practice. Prospective clinical study of adult patients with HS. Patients suitable for doxycycline treatment according to the European guidelines who were treated with MRD 40 mg daily for 28 days from April 2022 to December 2022 were included. Local Ethics Committee approval and patient informed consent were obtained. Age, gender, comorbidities, previous and concomitant treatment, severity of HS, adherence to treatment, clinical response, reported adverse events, recurrences and total duration of follow-up were considered for analysis. A total of 37 patients (19 males and 18 females, with a mean age of 33 ± 12,6 years) were evaluated. Regarding the severity of the disease, 9 patients were Hurley I, 23 Hurley II and 5 Hurley III. 17 (45.9%) were smokers and 9 (24.3%) presented an IMC >30. The most affected area was the groin, in 23 patients (62%), followed by the gluteal area (49%). 13.5% of patients were receiving chronic systemic biological treatment, while 24% received MDR in combination with topical or intralesional steroids. 85% patients completed 28 weeks of treatment, whereas 15% had a partial adherence to treatment. HiSCR50 was achieved in 80.6% of the treated patients, and AN count was reduced by 76.3%. EVA of pain also improved significantly from basal to week 28. MDR was well tolerated and no patient referred gastrointestinal adverse events or candidiasis. Clinical response was maintained in 70% of patients in a median of 6.8 ± 3.4 months of follow-up. The use of MRD has demonstrated significant clinical improvement of inflammatory lesions in patients with HS, along with a good safety profile.
- Zouboulis CC et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015;29:619–44.
- van Straalen KR et al. The efficacy and tolerability of tetracyclines and clindamycin plus rifampicin for the treatment of hidradenitis suppurativa: Results of a prospective European cohort study. J Am Acad Dermatol 2021;85:369–78.
- Leyden JJ et al. A randomized, phase 2, dose- ranging study in the treatment of moderate to severe inflam- matory facial acne vulgaris with doxycycline calcium. J Drugs Dermatol 2013;12:658–63.
- Moore A et al. Efficacy and Safety of Subantimicrobial Dose, Modified-Release Doxycycline 40 mg Versus Doxycycline 100 mg Versus Placebo for the treatment of Inflammatory Lesions in Moderate and Severe Acne: A Randomized, Double-Blinded, Controlled Study. J Drugs Dermatol 2015;14:581–6.
- Steinhoff M et al. Topical Ivermectin 10 mg/g and Oral Doxycycline 40 mg Modified-Release: Current Evidence on the Complementary Use of Anti-Inflammatory Rosacea Treatments. Adv Ther 2016;331 481–501.
Systemic corticoids as a salvage therapy in severe multi-resistant hidradenitis suppurativa
Pierre-Andre Bécherel1, Julie Blanc1, Melanie Dehais1, Jean-Christophe Moreno1, Marina Thomas1,2
1Antony Hospital, Dermatology and Clinical Immunology, Antony, France; 2L'Yvette Clinique, Dermatology, Longjumeau, France
In case of severe flares of HS, in patients already multi-treated especially under biological treatments, therapeutic means are lacking to relieve them quickly. Especially when the lesions are extensive and not easily accessible to surgery. We present here a series of 21 patients with severe flare-ups under infliximab and after many other failures, in which a short systemic corticosteroid therapy allowed a fast recovery. 21 consecutive patients were included in this open study. 15 men, 6 women. Mean age: 35 (28–42). 8 patients Hurley II, 13 Hurley III. All had an inflammatory phenotype, none with a follicular one. They had extensive lesions, more than 4 sites involved for each patient, and surgery was not a reasonable option. All of them were under infliximab 10 mg/kg (mean 7 months, 4–9) at time of inclusion, once a month, after both antibiotics (doxycycline, rifampicin, clindamycin, quinolones,…) and then adalimumab or secukinumab failures; 10 had already received ertapenem. Mean IHS4 at inclusion: 18 (16–24). Mean BMI: 28,2. 17 smokers (12,6 packets/year in average). 5 had anti-drug antibodies (anti-adalimumab or anti-infliximab). Induction was initiated at 60 mg prednisone po for 1 week, and then the dose was tapered: 10 mg every 3 days. The duration of prednisone therapy was 25 days in all cases (5 mg for the last 3 days). No side effects: no infection, no diabetes, no acute psychiatric complications. The relief was observed in 24 to 72 h in all cases: pain improvement, recovery of sleep, less draining, disappearance of the inflammatory signs and swelling. Infliximab was continued with the same intervals. In 14 patients, the efficacy of IFX was at least partially restored. HS is not an infectious disease, and whereas the use of NSAIDs is controversial, the use of corticoids is classical in resistant cases in local injections, the efficacy of which could be due partly to a systemic diffusion. In severe recalcitrant cases, when all known treatments have been used (antibiotics including ertapenem, biologics: anti-TNF or anti-IL-17), systemic corticoids appear to be efficient and safe as an emergency therapy, and able to restore-partly sometimes and for how long? - the effectiveness of IFX. The risk of worsening an infectious process with corticoids does not seem to be a concern in HS, inflammation being the main pathophysiological phenomenon involved. The long experience with local corticoid injections confirms the safety.
Photodynamic therapy with ZN(II) phthalocyanine (RLP068/CL) for hidradenitis suppurativa
Marina Venturini, Piergiacomo Calzavara-Pinton
University of Brescia, Dermatology, Brescia, Italy
Introduction: Several studies have been published since 2004 on the use of photodynamic therapy (PDT) to treat hidradenitis suppurativa. The use of superficial or interstitial illumination with 5-amino-levulinic acid (5-ALA) or methylene blue (MB) have been proposed. Injecting 5-ALA or MB followed by illumination with a fibre optic sensor placed inside the lesion appears to be a better method of treating these thick lesions. A novel photosensitizer, called RLP068/Cl, is a tetracationic Zn(II)phthalocyanine derivative that have demonstrated a good efficacy against surgical wound infections induced by a methicillin-resistant strain of Staphylococcus aureus and also against prosthetic joint infections-associated biofilms induced by Pseudomonas aeruginosa. We evaluated the efficacy and safety of topical PDT with RLP068/Cl 0.3% in gel formulation on 17 patients with hidradenitis suppurativa (HS), irradiated with red LED light source at 630 nm for 8 min (120 mW/cm corresponding to 60 J/cm2 of delivered light dose). The treatment was delivered twice a week for 6 weeks. A complete response was obtained in 47% of treated patients with resolution of inflammed lesions (nodules, abscesses), reduction of draining tunnels and improvement of pain and itch symptoms. The treatment was well tolerated without side effects. PDT with RLP068/Cl represent an interesting therapeutic option in the landscape of the future treatment of HS as monotherapy for mild HS or in association with biologics for moderate–severe HS.
- Vecchio D et al. Antimicrobial photodynamic therapy with RLP068 kills methicillin-resistant Staphylococcus aureus and improves wound healing in a mouse model of infected skin abrasion PDT with RLP068/Cl in infected mouse skin abrasion. J Biophotonics 2013;6:733–42.
- Pantò F et al. Efficacy and safety of photodynamic therapy with RLP068 for diabetic foot ulcers: a review of theliterature and clinical experience. Drugs Context 2020;9:2019-10-3.
- Mordon S. Treating hidradenitis suppurativa with photodynamic therapy. JnCosmet Laser Ther 2018;20:223–8.
Orismilast for mild to severe hidradenitis suppurativa: Preliminary data from osiris, a phase 2a, open-label, single-center, single-arm clinical trial
Camilla Goetzsche Frederiksen1, Farnam Barati Sedeh1, Elisabeth Taudorf1, Ditte M.L. Saunte1,2, Gregor B.E. Jemec1,2
1Zealand University Hospital, Roskilde and Health Sciences Faculty, University of Copenhagen, Department of Dermatology, Roskilde, Denmark; 2University of Copenhagen, Faculty of Health Science, Department of Clinical Medicine, Copenhagen, Germany
Acknowledgement: The authors would like to thank UNION Therapeutics for providing the investigational product (Orismilast). The Department of Dermatology, Zealand University Hospital, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Vinkel C, Thomsen SF. Hidradenitis Suppurativa: Causes, Features, and Current Treatments. J Clin Aesthet Dermatol 2018;11:17–23.
- Gulliver W et al. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord 2016;17:343–51.
- Zouboulis CC et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization – systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol 2019;33:19–31.
- Silverberg JI et al. Pharmacology of orismilast, a potent and selective PDE4 inhibitor. J Eur Acad Dermatol Venereol. 2023;37:721–9.
- Warren RB et al. Oral orismilast: Efficacy and safety in moderate-to-severe psoriasis and development of modified release tablets. J Eur Acad Dermatology Venereol 2023;37:711–20.
Efficacy and safety of the combined medical and surgical approaches in patients affected by hidradenitis suppurativa
Dalma Malvaso, Andrea Chiricozzi, Flaminia Antonelli, Barbara Fossati, Ketty Peris
Università Cattolica del sacro Cuore, Dermatologia e Venereologia, Rome, Italy
Hidradenitis suppurativa (HS) is a debilitating chronic skin disease; its therapeutic approach often requires combined medical and surgical treatment. The objective of the study was to assess the efficacy and safety of the surgical approach combined with different pharmacological treatments, evaluating the proportion of patients achieving the Hidradenitis Suppurativa Clinical Response (HiSCR), along with the incidence of postoperative complications, and local recurrence.
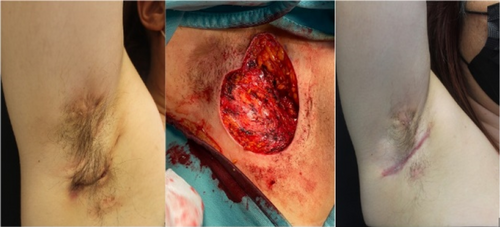
FIGURE 1 Surgery of HS lesion localized at right axilla in adalimumab-treated patient. Patient suffering from hidradenitis suppurativa Hurley III, preoperatively (A); during wide surgical excision of multiple fistulous tracts located in the right axilla (B); postoperative outcome 16 weeks after surgery (C).
How does the patient's life change after surgical treatment? Impact of hidradenitis suppurativa surgical treatment on health-related life quality
Marcin Gierek
Center for Burns Treatment, Severe Burns Department, Siemianowice Slaskie, Poland
- Zouboulis CC et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015;29:619–44.
- Marasca C et al. The pharmacology of antibiotic therapy in hidradenitis suppurativa. Expert Rev Clin Pharmacol 2020;13:521–30.
- Goldburg SR et al. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 2020;82:1045–58.
- Nguyen TV et al. Hidradenitis suppurativa: An update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol 2021;35:50–61.
- Dalgard FJ et al. The psychological burden of skin diseases: A cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol 2015;135:984–91.
A case of a therapy resistant hidradenitis suppurativa and underlying Crohn's disease
Merve Akdeniz, Sylke Schneider-Burrus
Havelklinik Berlin, Department of Dermatosurgery and Phlebology, Berlin, Germany
A 27-year-old male was referred to our department with recurrent inguinal, gluteal and scrotal abscesses and fistulas over the past 6 years. Previous treatments including multiple courses of systemic antibiotics (Clindamycin plus Rifampicin 300 mg bd, Doxycyclin 100 mg/d) had been unsuccessful. Under systemic treatment with Adalimumab 40 mg s.c. per week for 11 months the patient showed local progress of the disease; hence the medication was stopped. In preparation of a perianal wide excision operation of fistulas, the patient had received a protective colostoma. For further evaluation of other therapeutical options, the patient was then referred to our department. On first presentation, the patient was in reduced weight and poor general condition. Pain was reported as 10/10 (VAS), the DLQI was 28. Physical examination revealed communicating fistulas extending from the gluteal right via the sacral and perianal area to the gluteal left with secretion of little pus. Also, there were sinus tracts in the scrotum and inguinal on both sides. Moreover, multiple brownish papules presented over the entire body. There was a colostoma on the left lower abdomen. The patient reported no diarrhoea or other signs of an inflammatory bowel disease. Following extensive surgery of gluteal sinus tracts, the patient showed progress of the disease surrounding the operation wounds within 4 weeks after surgery. Histological examinations of the gluteal region revealed a significant chronic and active ulcerating, partly fistulating inflammation with epitheloid granulomas, Additionally, there was a newly developed phimosis with severe inflammation and leakage of pus. Histologically, the foreskin biopsy showed epitheloid granulomas, as well with similarity to a cutaneous sarcoidosis. In order to rule out a chronic intestinal bowel disease, a colonoscopy and gastroscopy were performed. The diagnosis of Crohn's disease was confirmed histologically and a systemic treatment with infliximab (5 mg/kg) was initiated. After 6 weeks of treatment with infliximab, the inflammation of the skin was regredient, the wounds healed well, no other surgery was necessary. This patient report shows that in therapy resistant cases of HS, especially if located perianal, a chronic inflammatory bowel disease can be underlying even if there are no evident clinical signs IBD.
Secukinumab in moderate to severe hidradenitis suppurativa: Analysis of pooled data from the SUNSHINE and SUNRISE phase 3 randomized trials
Afsaneh Alavi1, Ziad Reguiai2, Gregor B.E. Jemec3, Alice B. Gottlieb4, Magdalena B. Wozniak5, Lorenz Uhlmann6, Heng Fan7, Angela Llobet-Martinez6, Shoba Ravichandran8, Alexa B. Kimball9
1Mayo Clinic, Department of Dermatology, Rochester, USA; 2Polyclinique Courlancy-Bezannes, Dermatology department, Reims, France; 3Zealand University Hospital, Dermatology Department, Roskilde, Denmark; 4Icahn School of Medicine at Mount Sinai, Department of Dermatology, New York City, USA; 5Novartis Ireland Limited, Dublin, Ireland; 6Novartis Pharma AG, Basel, Switzerland; 7Shanghai Novartis Trading Limited, Shanghai, China; 8Novartis Pharmaceuticals Corporation, East Hanover, USA; 9Harvard Medical School and Clinical Laboratory for Epidemiology and Applied Research in Skin (CLEARS), Department of Dermatology, Boston, USA
Acknowledgement: This investigation was sponsored by Novartis Pharma AG. The Department of Dermatology, Zealand University Hospital, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Gottlieb AB et al. Long-term Safety of Secukinumab Over Five Years in Patients with Moderate-to-severe Plaque Psoriasis, Psoriatic Arthritis and Ankylosing Spondylitis: Update on Integrated Pooled Clinical Trial and Post-marketing Surveillance Data. Acta Derm Venereol 2022;102:adv00698.
The MIRA trial: A global, randomized, double-blind, placebo-controlled phase 2 trial to evaluate the efficacy and safety of the IL-17A- and IL-17F-inhibiting nanobody® sonelokimab in patients with active, moderate-to-severe hidradenitis suppurativa
Alexa B. Kimball1, Martina J. Porter1, Brian Kirby2,3, Errol P. Prens4, Falk G. Bechara5, Gregor B.E. Jemec6,7, James G. Krueger8, Jacek C. Szepietowski9, Joslyn S. Kirby10, Melinda J. Gooderham11, Nuala Brennan12, Eva Cullen12, Kristian Reich12,13
1Harvard Medical School and Beth Israel Deaconess Medical Center, Department of Dermatology, Boston, USA; 2St Vincent's University Hospital, Charles Department of Dermatology, Dublin, Ireland; 3University College Dublin, Charles Institute of Dermatology, Dublin, Ireland; 4Erasmus University Medical Center, Department of Dermatology, Rotterdam, Netherlands; 5Ruhr-University Bochum, Department of Dermatology, Venereology and Allergology, Bochum, Germany; 6Zealand University Hospital, Department of Dermatology, Roskilde, Denmark; 7University of Copenhagen, Health Sciences Faculty, Copenhagen, Denmark; 8The Rockefeller University, Laboratory of Investigative Dermatology, New York, USA; 9Wroclaw Medical University, Department of Dermatology, Venereology and Allergology, Wroclaw, Poland; 10Penn State Health Dermatology, Department of Dermatology, Hershey, USA; 11Probity Medical Research and Queen's University, SKiN Centre for Dermatology, Peterborough, Canada; 12MoonLake Immunotherapeutics AG, Zug, Switzerland; 13University Medical Center Hamburg-Eppendorf, Translational Research in Inflammatory Skin Diseases, Institute for Health Care Research in Dermatology in Nursing, Hamburg, Germany
Management of hidradenitis suppurativa (HS) is complex and challenging, and long-term prognosis for treated patients remains poor1. There is increasing scientific evidence to support IL-17A- and IL-17F-mediated inflammation as central to the pathogenesis of HS, with enrichment of Th17 cells in HS-involved skin and an observed upregulation of IL-17A and IL-17F at the mRNA and protein levels, especially in dermal tunnels2,3. Nanobodies® represent a new generation of antibody-derived targeted therapies, consisting of one or more antigen-binding variable regions of heavy-chain-only antibodies (VHH). Sonelokimab is a novel humanized Nanobody® consisting of three covalently linked VHH domains. With two domains, sonelokimab selectively binds with high affinity to IL-17A and IL-17F, thereby inhibiting the IL-17A/A, IL-17A/F, and IL-17F/F dimers that are central drivers of inflammatory disease4. Being small in size (~40 kDa) and containing a third domain binding to human albumin, sonelokimab is specifically designed to penetrate difficult-to-reach inflamed tissues and directly target sites of inflammation4. The MIRA trial will evaluate the clinical efficacy and safety of sonelokimab in patients with active, moderate-to-severe HS5. This is a 24-week global, randomized, prospective, parallel-group, double-blind, placebo-controlled Phase 2 trial expected to recruit approximately 210 participants with active, moderate-to-severe HS (NCT05322473).5 Participants will be randomized to receive either one of two sonelokimab doses, placebo, or adalimumab (active reference arm).5 The primary outcome will be the percentage of participants achieving a ≥75% reduction from baseline in total abscess and inflammatory nodule (AN) count, with no increase in abscess or draining fistula count (HiSCR 75) at Week 125. Secondary outcomes include HiSCR 50, IHS4 (which includes quantification of draining tunnels), as well as established scores on health-related quality of life and pain5. The MIRA trial is ongoing and is currently enrolling across seven countries, with the first patient randomized in May 20225. The MIRA trial is the first registered randomized controlled trial to use the greater threshold of clinical response of HiSCR 75 as its primary endpoint5. The results may provide further understanding on the role of IL-17A- and IL-17F-driven inflammation in the pathophysiology of HS.
Acknowledgement: The study was performed on behalf of MoonLake Immunotherapeutics AG, Zug, Switzerland. The Department of Dermatology, Zealand University Hospital Roskilde, Roskilde, Denmark and the Department of Dermatology, Erasmus Medical Center, Rotterdam, Netherlands are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Sabat R et al. Hidradenitis suppurativa. Nat Rev Dis Primers 2020;6:18.
- Navrazhina K et al. Epithelialized tunnels are a source of inflammation in hidradenitis suppurativa. J Allergy Clin Immunol 2021;147:2213–24.
- Lima AL et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol 2016;174:514–21.
- Papp KA et al. IL17A/F nanobody sonelokimab in patients with plaque psoriasis: a multicentre, randomized, placebo-controlled, phase 2b study. Lancet. 2021;397:1564–75.
- ClinicalTrials.gov. Identifier: NCT05322473.
Effect of secukinumab on draining tunnels in patients with moderate to severe hidradenitis suppurativa: Post hoc analysis of the SUNSHINE and SUNRISE phase 3 randomized trials
Falk G. Bechara1, Pierre-Andre Bécherel2, Ziad Reguiai3, Pedro Mendes-Bastos4, Joslyn S. Kirby5, Jennifer Hsiao6, Teresa Bachhuber7, Magdalena B. Wozniak8, Torben Kasparek9, Christine-Elke Ortmann10, Iryna Lobach11, Clarice Field12, Shoba Ravichandran13, Alexa B. Kimball14
1Ruhr-University Bochum, Department of Dermatology, Venereology and Allergology, Bochum, Germany; 2Antony Private Hospital, Antony, France; 3Polyclinique Courlancy-Bezannes, Dermatology Department, Reims, France; 4Hospital CUF Descobertas, Dermatology Centre, Lisboa, Portugal; 5Penn State Milton S Hershey Medical Center, Hershey, USA; 6University of Southern California, Department of Dermatology, Los Angeles, USA; 7Novartis Pharma AG, Basel, Switzerland; 8Novartis Ireland Limited, Dublin, Ireland; 9Novartis Pharma AG, Basel, Switzerland; 10Novartis Pharma AG, Basel, Switzerland; 11Novartis Pharma AG, Basel, Switzerland; 12Novartis Ireland Limited, Dublin, Ireland; 13Novartis Pharmaceuticals Corporation, East Hanover, USA; 14Harvard Medical School and Clinical Laboratory for Epidemiology and Applied Research in Skin (CLEARS), Beth Israel Deaconess Medical Center, Department of Dermatology, Boston, USA
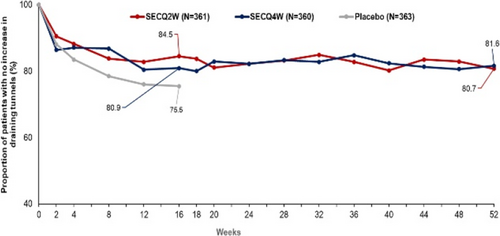
FIGURE 1 Proportion of patients reporting no increase in draining tunnels over time to Week 52. Line graphs showing the effects of SECQ2W, SECQ4W, and placebo from baseline up to Week 52 on draining tunnels in the SUNSHINE and SUNRISE trials (pooled data). Data are presented as observed. At Week 16, patients randomized to placebo were switched to receive SECQ2W or SECQ4W up to Week 52. Only patients on continuous secukinumab treatment for 52 weeks are represented in the graph beyond Week 16. Q2W, every 2 weeks; Q4W, every 4 weeks; SEC, secukinumab 300 mg.
Acknowledgement: The study was sponsored by Novartis Pharma AG.
- Ofenloch RF. Health-related quality of life in hidradenitis suppurativa. Br J Dermatol 2017;176:861–62.
- Kimball AB et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med 2016;375:422–34.
- Vanlaerhoven A et al. Hurley III Hidradenitis Suppurativa Has an Aggressive Disease Course. Dermatology 2018;234:232–3.
- Navrazhina K et al. Epithelialized tunnels are a source of inflammation in hidradenitis suppurativa. J Allergy Clin Immunol 2021;147:2213–24.
- Scholl L et al. Surgical Treatment of Sinus Tracts and Fistulas in Perianal Hidradenitis Suppurativa. J Cutan Med Surg 2018;22:239–24.
Burden of pain and use of pain medications in patients with HS: Real-world data from UNITE
Lauren Orenstein1, Kassim Rahawi2, Akash Danavar2, Michael Lane2, Raj Chovatiya3, Hadar Lev-Tov4, So Yeon Paek5, Hessel H. Van der Zee6, Christopher Sayed7
1Department of Dermatology, Emory University, Atlanta, USA; 2AbbVie Inc., North Chicago, USA; 3Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, USA; 4Dr. Phillip Frost Department of Dermatology & Cutaneous Surgery, University of Miami Miller School of Medicine, Miami, USA; 5College of Medicine, Texas A&M University and Division of Dermatology, Baylor Scott & White, Dallas, USA; 6Erasmus University Medical Center Rotterdam, Department of Dermatology, Rotterdam, Netherlands; 7Department of Dermatology, University of North Carolina School of Medicine, Chapel Hill, USA
Acknowledgement: AbbVie Inc. funded this study and participated in the study design; study research; collection, analysis and interpretation of data; and writing, reviewing and approving of this publication. All authors had access to the data, and participated in the development, review, and approval, and in the decision to submit this publication. Medical writing support was provided by Lisa Denny, PhD, and Janet Matsuura, PhD, of ICON (Blue Bell, PA) and was funded by AbbVie. The Department of Dermatology, Erasmus Medical Center, Rotterdam, Netherlands are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
A centre-based, ambulatory care concept for hidradenitis suppurativa improves disease activity, burden and patient satisfaction: Results from the randomized controlled trial EsmAiL
Michael Schultheis1, Petra Staubach-Renz1, Georgios Nikolakis2, Lukas Schollenberger3, Melanie Mauch4, Marion Burckhardt4,5, Marcus Heise6,7, Marina Zamsheva6,7, Alexandra Strobel7,9, Gero Langer6,7, Falk G. Bechara10, Uwe Kirschner11, Katharina Hennig1, Christian Kunte12, Matthias Goebeler13, Stephan Grabbe1
1University Medical Centre, Johannes Gutenberg University, Department of Dermatology, Mainz, Germany; 2Dessau Medical Center, Brandenburg Medical School Theodor Fontane, Department of Dermatology, Dessau, Germany; 3University Medical Centre, Johannes Gutenberg University, Interdisciplinary Centre for Clinical Trials, Mainz, Germany; 4German Society for Wound Healing and Wound Treatment, Gießen, Germany; 5Baden-Wuerttemberg Cooperative State University, School of Business and Health, Stuttgart, Germany; 6Martin Luther University Halle-Wittenberg, Institute for Health- and Nursing Science, Halle, Germany; 7Medical Faculty, Martin Luther University Halle-Wittenberg, Profile Centre of Health Sciences Halle, Halle, Germany; 8Medical Faculty, Martin Luther University Halle-Wittenberg, Institute of General Practice and Family Medicine, Halle, Germany; 9Medical Faculty, Martin Luther University Halle-Wittenberg, Institute of Medical Epidemiology, Biostatistics, and Informatics, Profile Area Clinical Studies & Biostatistics, Halle, Germany; 10Ruhr-University Bochum, Department of Dermatology, Venereology, and Allergology, Bochum, Germany; 11Dermatology Outpatient Office Dr. Uwe Kirschne, Dermatology, Mainz, Germany; 12Artemed Fachklinik München, Department of Dermatologic Surgery and Dermatology, Munich, Germany; 13University Hospital Würzburg, Department of Dermatology, Wuerzburg, Germany
Hidradenitis suppurativa (HS) is an inflammatory disease of the inverse skin regions that occurs particularly in young women and affects approximately 1% of the population. The disease is characterized by inflammatory nodules and abscesses that coalesce into fistulous ducts and diffuse scarring, leading to severe physical but also psychological impairment. Outpatient care is often inadequate and usually cannot prevent progression. EsmAiL was a two-arm, multicentre, prospective randomized controlled trial. It included 553 adults with HS. Inclusion criteria were at least three inflammatory lesions and at least moderate impact of the disease on quality of life. The control group (CG) remained in regular care, whereas the intervention group (IG) was referred to specialized so-called “acne-inversa-centres (AiZ)” which treated patients according to a trial-specific multimodal concept. Primary endpoint was HS disease activity measured by International Hidradenitis Suppurativa Severity Score System (IHS4). 279 patients were randomized to IG and 274 to CG. At the end of the 12-month intervention phase, participants in the IG (n = 203) achieved a mean improvement in the primary outcome measure IHS4 of 9.3 points while the decrease in the CG patients (n = 174) amounted to 5.7 points (p = 0.003). Patients receiving the new care concept also reported a significantly (p < 0.001) higher decrease in pain, DLQI and HADS compared to changes in the CG. Therapy satisfaction was also significantly higher in the IG than in the CG (p < 0.001). HS is a debilitating, complex disease that severely limits the personal and professional lives of those affected. There is a need to enable the outpatient sector to provide interdisciplinary and multi-professional care for HS. The establishment of so-called AiZ and standardized treatment algorithms in the ambulatory setting has a substantial, positive impact on the course of the disease and significantly improves patient satisfaction.
Acknowledgement: The authors wish to thank all the patients who participated in EsmAiL despite the extraordinary Covid-19 situation, as well as the medical staff of the AiZ caring for the patients during these especially challenging circumstances. The Departments of Dermatology, Venereology and Allergology, University Hospital Wuerzburg and the Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau are health care providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin – ALLOCATE Skin group).
Antibiotic resistances in hidradenitis suppurative patients treated with adalimumab
Terenzio Cosio1, Elisabetta Botti2, Cristiana Borselli2, Sara Lambiase2, Virginia Maffei2, Elena Campione2, Luca Bianchi2
1University of Rome Tor Vergata, PhD course in Microbiology, Immunology, Infectious Diseases, and Transplants (MIMIT) Microbiology Section, Department of Experimental Medicine, Rome, Italy; 2Tor Vergata University Hospital, Dermatology Unit, Rome, Italy
Hidradenitis Suppurativa (HS) is a chronic, inflammatory, recurrent, debilitating skin disease of the terminal follicles in apocrine gland-rich areas, characterized by painful, deep-seated, inflamed abscesses, nodules and fistulae. Genetics, innate and adaptive immune systems dysfunction against terminal follicle homeostasis and apocrine glands integrity, hormonal status and lifestyle are variously involved in HS pathogenesis1. Skin dysbiosis, biofilm-associated bacteria, bacterial infections or superinfections are debated in HS progression and correlated with localization, clinical manifestations and disease extension2. The effectiveness of antibiotics in HS, because of their antimicrobial, anti-inflammatory and immunomodulatory properties, suggests the role of triggering microbial factors. Duration and frequency of antibiotic use should balance the benefit with the risk of antibiotic resistance. Considering this view, we performed a monocentric, pivotal, open-label observational study in a tertiary centre in Italy to evaluate the presence of aerobic and anaerobic bacteria and related drug resistance from lesional swabs in patients with HS treated with adalimumab. A total of 37 patients treated with anti-TNF-α were enrolled. The 67.6% were male, while 32.4% were female. All the patients are smokers with a mean age of 38.56 (±11.92) years; a medium BMI of 28.11. The 70% of the population presented an axillary lesion, followed by 18% with a genital lesion, and then two lesions were recovered on the back trunk, front trunk and abdomen. Following the lesional skin swabs, 38 bacteria have been isolated. It was noted that the most isolated bacteria were Escherichia coli, Staphylococcus aureus and Proteus mirabilis, followed by Finegoldia magna, Enterobacteriaceae, Staphylococcus lungdunesis and Corynebacterium striatum. In the population study, the 38 bacteria presented different resistances, from 1 (39.5%) to 8 (2.6%). Evaluating which isolated bacteria presented resistance, it was noted that P. mirabilis had the highest drug resistance, followed by Staphylococcus aureus. It was pointed out that the most widespread resistance is levofloxacin (23.7%), followed by imipenem (15.8%), clindamycin and amoxicillin/clavulanate acid (13.2%). Focused on Actinomyces spp., they presented resistance to clindamycin and erythromycin. Interestingly, in the female population, Actinomyces oris was the only resistant bacteria, presenting vancomycin, metronidazole and clindamycin resistance. Of importance, the presence of resistance to ceftazidime/ avibactam also emerged from the population study, focusing attention on patients with HS as a possible reservoir for multi-resistant bacteria on the World Health Organization's List of Essential Medicines3. Considering the significant risk of further increases in resistance rates, AB should be administered only when clinically indispensable and whenever HS purulent material is available, acquiring bacterial antibiotic sensitivity profiles is recommended. Otherwise, an empirical choice should be made. Future prospective studies on AB resistance patterns, microbiome characteristics and their relationships with different HS therapies, including AB, immunomodulatory drugs and anti-TNF-a targeted therapies, are required.
- Goldburg SR et al. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 2020;82:1045–58.
- Mintoff D et al. The Clinical Relevance of the Microbiome in Hidradenitis Suppurativa: A Systematic Review. Vaccines 2021;9:1076.
- Hwang TJ et al. Reforming the World Health Organization's Essential Medicines List: Essential but Unaffordable. JAMA 2022;328:1807–8.
New insights on HS phenotypes and treatment response: A post hoc analysis of SUNSHINE and SUNRISE phase 3 trials
Anna Passera1, David Demanse1, Gregor B.E. Jemec2, Ginette A. Okoye3, Tiffany Mayo4, Jennifer Hsiao5, Vivian Y. Shi6, Martina J. Porter7, Angel S. Byrd3, Lorenz Uhlmann1, Xiaoling Wei8, Marc Vandemeulebroecke1, Shoba Ravichandran9, Elisa Muscianisi9
1Novartis Pharma AG, Basel, Switzerland; 2University of Copenhagen, Copenhagen, Denmark; 3Howard University College of Medicine, Washington, USA; 4University of Alabama at Birmingham, Birmingham, USA; 5University of Southern California, Department of Dermatology, Los Angeles, USA; 6University of Arkansas for Medical Sciences, Little Rock, USA; 7Beth Israel Deaconess Medical Center and Harvard Medical School, Department of Dermatology, Boston, USA; 8Novartis Pharma, Shanghai, China; 9Novartis Pharmaceuticals Corporation, East Hanover, USA
Acknowldgement: The authors thank Anne-Christine Potocki and Grace Cleary, employees of Novartis, for their support in data collection and revision. Medical writing assistance was funded by Novartis Pharma AG, Basel, Switzerland, in accordance with GPP4 guidelines. The study was sponsored by Novartis Pharma AG. The Department of Dermatology, Zealand University Hospital, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- van Straalen KR et al. Poor interrater reliability of hidradenitis suppurativa phenotypes. J Am Acad Dermatol 2018;79:577–8.
- Saunte DML, Jemec GBE. Hidradenitis Suppurativa: Advances in Diagnosis and Treatment. JAMA 2017;318:2019–32.
- Martorell A et al. Defining hidradenitis suppurativa phenotypes based on the elementary lesion pattern: results of a prospective study. J Eur Acad Dermatol Venereol 2020;34:1309–18.
- Kimball AB et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomized, placebo-controlled, double-blind phase 3 trials. Lancet 2023;401:747–61.
- Passera A et al. Baseline characteristics and subtype clusters in hidradenitis suppurativa: Exploratory analysis of SUNSHINE and SUNRISE studies. P0040. Presented at the 31st European Academy of Dermatology and Venereology (EADV) Congress; September 7–10, 2022; Milan, Italy.
Piperacillin/tazobactam treatment as a crisis therapy in hidradenitis suppurativa patients: A series of 10 patients
Aviv Barzilai1,2, Shir Tubiana1, Adam Dalal1, Sharon Baum1,2
1Sheba Medical Center, Dermatology, Ramat Gan, Israel; 2Tel Aviv University, Faculty of Medicine, Tel Aviv, Israel
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease that is usually present in skin folds. Most patients with moderate to severe disease require long-term antibiotic treatment or Adalimumab/other off-label treatments to control the disease. The clinical course in many patients is characterized by relapses, even when patients are on acceptable therapeutic modalities. When it erupts with a moderate to a high degree of severity, it greatly affects the patients' quality of life. Antibiotic treatment is common when the disease flares up and includes amoxicillin/clavulanic acid in mild cases, while Ciprofloxacin and Clindamycin are used in more severe cases. Nevertheless, some do not improve under this treatment. Piperacillin/tazobactam has a broad spectrum of antibacterial activity encompassing most Gram-positive and Gram-negative aerobic bacteria as well as anaerobic bacteria. The study aimed to evaluate the effectiveness of the piperacillin/tazobactam treatment in HS patients with Hurley stage III lesions who flared and do not improve under the conventional antibiotic treatment. We performed a retrospective analysis of the HS patients' files hospitalized at the department of Dermatology, Sheba Medical Center between 2020 and 2022. Ten patients who had failed conventional treatment for HS (antibiotic treatment or Adalimumab/other off-label treatments) were treated with piperacillin/tazobactam for 6–10 days and were followed up after this treatment for at least 3 months period were identified. Following piperacillin/tazobactam treatment, 4 patients demonstrated complete regression in erythematous nodules, without fluctuation or purulent discharge. Mild secretion was observed in 4 additional patients, while 2 patients demonstrated moderate secretion. During the follow-up period, 6 patients received conventional therapy for HS. One patient exacerbated after 2 months, 3 exacerbated after 3 months, and 2 remained stable without exacerbations over 8 months of follow-up. Among the 4 patients who did not receive conventional therapy, 3 remained stable. One patient did not demonstrate significant improvement with prolonged treatment with piperacillin/tazobactam and exacerbated less than a month later. Piperacillin/tazobactam is an effective treatment at the time of disease flare-up in HS patients with Hurley stage III lesions who do not respond to conventional treatment. Thus it should be considered as crisis therapy in these patients.
Switching from intravenous to subcutaneous infliximab in patients with hidradenitis suppurativa
Mar Luque, Lorenza Barboza, Alejandra Sandoval, Irene Fuertes
Hospital Clinic, Barcelona, Department of Dermatology, Barcelona, Spain
The tumour necrosis factor (TNF) antagonist Adalimumab a is currently the only biologic approved by the Food and Drug Administration for Hidradenitis Suppurativa (HS). The rate of therapeutic failure or loss of response to this drug in patients with moderate–severe HS is high. Multiple studies have assessed the efficacy of infliximab ev for the treatment of these patients especially at doses of 7.5 to 10 mg/kg every 4 weeks.1–4 The bioequivalence of intravenous (IV) CT-P13 with reference infiximab in terms of safety and efficacy has been established. Data from clinical trials in patients with rheumatoid arthritis, Crohn's disease or ulcerative colitis have shown that the safety and tolerability profile of subcutaneous (SC) CT-P13 is generally consistent with that established for IV CT-P13. Infections are the most frequently observed adverse drug reactions. With the exception of localized injection site reactions, which are more frequent with subcutaneous, there were no clinically meaningful differences in the tolerability profiles of SC CT-P13 and IV CT-P13. SC lCT-P13 is approved for use in adults in all indications where it is approved for IV CT-P13, based on clinical trials that showed that the subcutaneous formulation has non-inferior efficacy to IV CT-P13 in the treatment of rheumatoid arthritis and has non-inferior pharmacokinetics to the intravenous formulation in patients with inflammatory bowel disease.
HS usually debuts in adolescence and progresses in later years so most patients with moderate severe disease have work and family responsibilities that are affected by maintaining IV treatment every 4 weeks. In addition, situations such as the recent pandemic have put the focus on the interest of reducing hospital attendance for patients as much as possible and minimizing the burden on day hospitals. This dosing has the potential to improve patient comfort and reduce the burden on the healthcare system. As yet, we have no data on the efficacy and safety of Infliximab CT-P13 sc in patients with hidradenitis suppurativa. This change in dosage moves towards more individualized therapy, through a more convenient route of administration and a potential benefit on the serum concentration of the drug, leading to therapeutic flexibility and less dependence on infusion centers. We present our experience with 6 patients diagnosed with severe HS who have undergone a switch from intravenous to subcutaneous infliximab therapy.
- Ghias MH et al. High-dose, high-frequency infliximab: A novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol 2020;82:1094–101.
- Huguet JM et al. Subcutaneous Infliximab [CT-P13], a True Biologic 2.0. Real Clinical Practice Multicentre Study. Biomedicines 2022;10:2130.
- Smith PJ et al. Efficacy and Safety of Elective Switching from Intravenous to Subcutaneous Infliximab [CT-P13]: A Multicentre Cohort Study. J Crohns Colitis 2022;16:1436–46.
- Argüelles-Arias F et al. Switch to subcutaneous infliximab during the SARS-CoV-2 pandemic: preliminary results. Rev Esp Enferm Dig 2022;114:118–9.
Posters – Quality of life/nurse and patient reports
Hidradenitis suppurativa and psychopathology: The chicken or the egg?
Jorge Romaní1, Laura Ros2, Patricia Garbayo-Salmons3, Núria Riera-Martí3
1Hospital General de Granollers, Department of Dermatology, Barcelona, Spain; 2Hospital Mutua de Terrassa, Department of Psychiatry, Barcelona, Spain; 3Consorci Sanitari Parc Taulí, Department of Dermatology, Barcelona, Spain
- Phan K et al. Hidradenitis suppurativa and psychiatric comorbidities, suicides and substance abuse: systematic review and meta-analysis. Ann Transl Med 2020;8:821.
- Romani J et al. Hidradenitis suppurativa: results of the register of morbidity and use of health resources in the autonomous community of Catalonia, Spain. Exp Dermatol 2020;29:5.
A cross-sectional study of fatigue, depression and anxiety in patients with hidradenitis suppurativa compared to the general population of Denmark
Camilla G. Frederiksen1, Viktoria Sigsgaard1, Christoffer Kursawe Larsen1, Peter T. Riis1, Gregor B.E. Jemec1,2
1Zealand University Hospital Roskilde, Department of Dermatology, Roskilde, Denmark; 2University of Copenhagen, Department of Clinical Medicine, Copenhagen, Denmark
Acknowledgement: We thank all the included patients for their willingness to participate, time, and patience. The Department of Dermatology, Zealand University Hospital, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
- Frew JW et al. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Res 2018;7: 1930.
- Louati K, Berenbaum F. Fatigue in chronic inflammation - a link to pain pathways. Arthritis Res Ther 2015;17:254.
- Katz P. Causes and consequences of fatigue in rheumatoid arthritis. Curr Opin Rheumatol 2017:29:269–76.
- Riis PT et al. A pilot study of fatigue in patients with hidradenitis suppurativa. Br J Dermatol 2018;178:e42-e3.
- Matusiak L et al. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol 2010;90:264–8.
Satisfaction with life among patients with hidradenitis suppurativa: A cross-sectional study
Julia E. Rymaszewska, Piotr K. Krajewski, Joanna Maj, Jacek C. Szepietowski
Wroclaw Medical University, Department of Dermatology, Venereology and Allergology, Wroclaw, Poland
Hidradenitis suppurativa (HS) can negatively impact patients' satisfaction with life (SWL). The objective was to thoroughly analyse the SWL of the patients with HS in relation to the disease severity, sociodemographic factors, depressive and anxiety symptoms, the presence of psychopathological symptoms as well as the quality of life. 114 patients with HS (53.1% females; mean age 36.6 ± 13.1 years) were enrolled. Severity of the disease was measured using Hurley and IHS4 scales. The assessment of: SWL was based on the Satisfaction with Life Scale (SWLS); depressive symptoms measured with Patient Health Questionnaire-9 (PHQ9); anxiety symptoms with Generalized Anxiety Disorder-7 (GAD7); psychopathological symptoms with General Health Questionnaire (GHQ-28) and quality of life with Hidradenitis Suppurativa Quality of Life Scale (HiSQoL). Low SWL was found among 36 (31.6%) subjects. Moreover, 48 patients (42.1%) presented average SWL and the remaining ones showed high SWL – 30 patients (26.3%). No statistical significance was found in SWL between HS severity groups (Hurley, IHS4). Additionally, there was no correlation between SWL and IHS4. SWL of both male and female participants with HS were much alike (Male: 19.7 SD = 5.7; Female: 19.8 SD = 5.7). Therefore, no statistical significance was found in comparison of SWL in both groups. SWL did not correlate with the age, number of hospitalizations, and the duration of the disease. A strong, negative correlation between SWL and depressive symptoms (PHQ9) was found among our HS patients (r = −0.603, p < 0.001). Moreover, a strong, negative correlation was established between SWL scores and anxiety symptoms (GAD7) of HS participants (r = −0.579, p < 0.001). Additionally, there was a strong, negative correlation between SWL scores and psychopathological symptoms, measured by the (GHQ-28) (r = −0.651, p < 0.001). SWL correlated weakly, negatively with symptoms measured by HiSQoL experienced by the patients (r = −0.331, p = 0.011). SWL compared with the psychosocial part of HiSQoL questionnaire presented moderate, negative correlation among all patients (r = −0.478, p < 0.001). Lastly, SWL of our participants correlated negatively, weakly with problems with everyday activities measured by HiSQoL, such as: walking, sport, sleep, hygiene and dressing up (r = −0.366, p < 0.001). SWL is low in reasonable number of patients with HS. No relation was found between SWL and Hurley, as well as IHS4 scores. Similar results were obtained between male and female groups in relation to the SWL. Factors related to the underlying disease and sociodemographic factors did not affect the level of SWL reported by patients. Patients with low SWL presented depressive and anxiety symptoms. We noted that participants with low SWL were at risk of developing psychiatric disorders. Finally, the HS patients with low SWL had low quality of life.
Nurse-led annual consultations for patients with hidradenitis suppurativa: Impressions and characteristics
Stine H. Pedersen, Alberte A. Brogaard, Gregor B.E. Jemec
Zealand University Hospital Roskilde, Department of Dermatology-Venereology, Roskilde, Denmark
Hidradenitis Suppurativa is a painful and debilitating inflammatory skin disease. The psychosocial burden of HS is immense. In 2021, the Department initiated nurse-led annual consultations for patients with Hidradenitis Suppurativa (HS). The purpose of the annual consultations is to discuss themes and issues that the patients find bothersome to get a broader and holistic view of the patient. The patients are offered nurse-led annual consultations once annually. The consultations typically last 30 min. The purpose of this study is to characterize and describe the content of the nurse-led consultations. This is retrospective study. Electronic patient journals were screened for the content and evaluated for common topics of conversation. This study included 79 electronic patient journals. Forty-six percentage of the patients (26/57) reported that they had nothing to discuss. For the remaining various themes and topics were discussed during the consultations (Table 1). Blood pressure, pulse, height, weight and Body Mass Index (BMI) were measured for almost all patients. The patients primary purpose with the consultations were the discussion of disease-oriented topics including boils, pain, and treatment. The topics discussed during the consultations are mainly about: general condition (blood pressure, pulse, height, weight and BMI), treatment (topical, systemic, surgery etc.), and adjunctive measurements such as diet, smoking, alcohol, and exercise). From a holistic view, the consultations should include both content physical, psychological, social, and spiritual topics. The results shows that the focus mainly is physical topics. Topics like sexuality, psychological well-being and social problems was not discussed. Future nurse-led annual consultations will also focus on those issues.
TABLE 1 Main topics form nurse-led consultations.
| Main topic | |
|---|---|
| Patient perspective |
Nothing to discuss (42%) (26/57) Boils (16%) (9/57) Medicine (9%) (5/57) Pain (5%) (3/57) |
| General condition | Indication of good general condition (95%) (75/79) |
| Treatment |
Resorcinol (88%) (60/68) Systemic treatment (65%) (44/68) Medicine delivery (49%) (39/79) Surgery (49%) (36/73) Bandages (38%)(30/79) Other topical treatment options (19%) (13/68) Intense pulse light, for hair removal (17%) (13/76) |
| Prevention of complications |
Help with dietary changes (25%) (15/61) Help to stop smoking (24%) (10/42) Help with changes in exercise habits (8%) (6/77) Help to stop drinking alcohol (3%) (1/39) |
Acknowledgement: The Department of Dermatology, Zealand University Hospital, Roskilde, Denmark is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
Assessment of depression in hidradenitis suppurativa: How to improve the management of the patients
Sara Anton Fernandez, Hassane Njimi, Claire Van Damme, Véronique del Marmol, Farida Benhadou
University Libre of Brussels, Department of Dermatology Hôpital Erasme, Brussels, Belgium
Hidradenitis suppurativa (HS) is a chronic inflammatory disease associated to an important impact on the quality of life of the patients. Importantly, the burden caused by the disease is associated to a huge prevalence of depression and to a 3 times higher risk of suicidal behaviour in comparison to the general population. The main objective was to assess the prevalence of depression among a belgian cohort of HS patients. The second objective was to better characterize HS patients with depression versus patients no depression in this cohort. Adult patients with HS were recruited prospectively from the outclinic of dermatology at Erasme Hospital in Brussels, Belgium. In addition to demographic and medical data, two questionnaires were used; the Hidradenitis Suppurativa Quality of Life- 17 items (HiSQOL-17) to assess the impact of the disease on the quality of life and the Goldberg Depression Scale (GDS) to assess the depression item. 43 patients were recruited (39.5% men/60.5% women). 51% have reported suffering from depression related to their disease but only 22.7% were receiving a psychological support. In the cohort of patients with depression, the majority were female (63.6%), smoker (68.2%) and obese (45.5%). Among these patients, the onset of lesions was earlier (25 years ±10 vs. 27.3 years ±11), with a delayed diagnosis time (7.4 months ±4 vs. 4.3 months ±4), with a higher Hurley staging (50% had a Hurley stage II and 36.4% had stage III) and with a severe IHS4 score (36.4% vs. 23.8%). 45.5% reported an important alteration of HiSQOL-17 score and 18.2% reported an extremely impacted score. Surprisingly, 19.1% of patients who declared not to suffer from depression had a positive GDS (GDS > 21) compatible with depression with 9.5% reported severe depression. We observed in our Belgian cohort a high rate of depression and a low rate of psychological follow-up. Thanks to this preliminary results, we aim to increase awareness among dermatologists to actively look for signs of depression in patients suffering from HS in order to provide appropriated psychological support. The HiSQOL-17 and GDS scores represent relevant and easy-to-use scores to integrate in our daily practice to improve the management of HS patients.
Acknowledgement: The Department of Dermatology, Hôpital Erasme, Brussels, Belgium is a health care provider of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
Quality-of-life endpoints in women of childbearing age with hidradenitis suppurativa: A tertiary-care-centre-based study
Aikaterini Tsentemeidou1, Elena Sotiriou1, Katerina Bakirtzi1, Ilias Papadimitriou1, Themis Chatzi-Sotiriou3, Angeliki Panagopoulou1, Nikolaos Kougkas2, Aimilios Lallas1, Efstratios Vakirlis1
1Aristotle University of Thessaloniki, First Department of Dermatology-Venereology, Thessaloniki, Greece; 2Aristotle University of Thessaloniki, Fourth Department of Internal Medicine, Thessaloniki, Greece; 3University of Macedonia, Department of International and European Studies, School of Social Sciences, Humanities and Arts, Thessaloniki, Greece
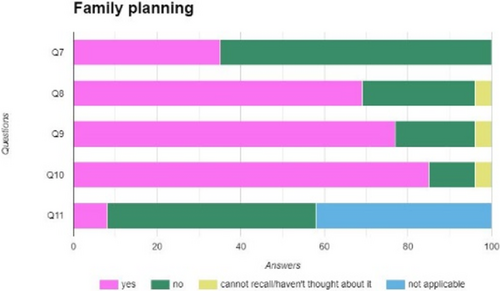
FIGURE 1 Effect of hidradenitis suppurativa on family planning among women of childbearing age. Q7 I have decided/am seriously thinking of not having children or having less children because of my disease, Q8 I am afraid that my disease will have an impact on my children's health and/or my ability to take care of them, Q9 I am afraid that pregnancy/the postpartum will have a negative impact on my disease, Q10 I am afraid that the medication I am taking will have an impact on my fertility or will harm my baby during pregnancy or lactation, Q11 I have faced problems with pregnancy, birth, postpartum or breastfeeding because of my disease (what kind of problems?), x axis: percentages.
- Revankar R et al. Shedding light on the impact of hidradenitis suppurativa on women and their families: A focus of the International Journal of Women's Dermatology. Int J Women's Dermatology 2021;7:661–3.
- Cuenca-Barrales C et al. Sexual Distress in Patients with Hidradenitis Suppurativa: A Cross-Sectional Study. J Clin Med 2019;8:532.
- Montero-Vilchez T et al. Reproductive potential and outcomes in patients with hidradenitis suppurativa: Clinical profile and therapeutic implications. Life (Basel) 2021;11:277.
Impact of hidradenitis suppurativa on major life changing decisions
Clara Ureña-Paniego1, Alberto Soto-Moreno1, Manuel Sanchez-Diaz1, Carlos Cuenca-Barrales1, Salvador Arias-Santiago1, Alejandro Molina-Leyva1,2
1Hospital Universitario Virgen de las Nieves, IBS Granada, Granada, Spain, Hidradenitis Suppurativa Clinic, Granada, Spain; 2European Hidradenitis Suppurativa Foundation, Dessau-Roßlau, Germany
Hidradenitis suppurativa (HS) is a chronic and recurring inflammatory disease affecting intertriginous skin. HS remains to be the skin condition with the greatest impairment in quality of life (QoL). Treatment with biologic therapies or surgery has shown to significantly improve QoL among these patients. Nevertheless, the long-term impact of HS on patients' major life changing decisions (MLCD) nor the role treatment might have on them have been studied until date. The aims of this study were to assess the impact of HS on MLCD and to explore if missing the medical or surgical window of opportunity of the disease was associated with a higher burden on MLCD. Transversal study. Patients with hidradenitis suppurativa were recruited at a specialized unit at Hospital Universitario Virgen de las Nieves (Granada). During the follow-up visit, the dermatologist evaluated disease severity, progression and loss of the window of opportunity in every participant. Patients were assessed via self-administered questionnaires exploring the impact of HS on major life decisions related to career choice, education, interpersonal relationships, desire to have children and sexuality, housing, travelling and life habits. 50 patients were included. 64% (32/50) were female and mean age was of 34.5 years. 38% (19/50) and 18% (9/50) of patients had missed the clinical and surgical window of opportunity for HS respectively. Patients who had missed the medical window of opportunity and those with higher Hurley stage considered that HS had impacted negatively on their job performance and modified their toxics habits. Higher Hurley scores also related to a greater impact on the desire to have children and sexuality. An earlier disease debut was associated with a greater effect on patients' education. HS substantially influences MLCD of patients. Early detection and proactive treatment could reduce the impact of the disease on MLCD.
Psychoeducational intervention in hidradenitis suppurativa through a patient engagement program
Francisco José Rodríguez-Cuadrado*, Marta Loro-Pérez*, Rita Cabeza-Martínez, Gaston Roustan-Gullón, Fernando Alfageme-Roldán
Hospital Universitario Puerta de Hierro Majadahonda, Department of Dermatology, Majadahonda, Spain
*The first and second authors have contributed equally to the manuscript.
- Matusiak L et al. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol 2010;90:264–8.
- Montero-Vilchez T et al. The Burden of Hidradenitis Suppurativa Signs and Symptoms in Quality of Life: Systematic Review and Meta-Analysis. Int J Environ Res Public Health 2021;18:6709.
- Machado MO et al. Depression and Anxiety in Adults With Hidradenitis Suppurativa: A Systematic Review and Meta-analysis. JAMA Dermatol 2019;155:939–45.
- Cartron A et al. Comorbidities of hidradenitis suppurativa: A review of the literature. Int J Womens Dermatol 2019;5:330–4.
- Arantón-Areosa L. Escuelas de pacientes. Enferm Dermatol 2010;4:54–5.
Sleeping disorders of patients suffering from hidradenitis suppurativa in relevance with sleeping quality
Tryfon Papadopoulos1,2, Anastasia Trigoni1,2, Elissavet Mingiani1,2, Parthena Meltzanidou1,2, Fragiski Tsatsou1,2, Elizabeth Lazaridou1,2
1Papageorgiou General Hospital, Second Department of Dermatology, Thessaloniki, Greece; 2Aristotle University of Thessaloniki, Second Department of Dermatology, Thessaloniki, Greece
- Physical Health
- Depression
- Cognitive - Functional Failure
- Rising Mortality
- Age
- Sex
- Smoking
- Medical Treatment
- Morbidity
- INDEX DLQI
- INDEX IHS4
- DLQI
- IHS4
- Medical Treatment we retrived the following results:
- Men 49.3%
- Women 40.3%
- Smokers 62.3%
- Non - smokers 37.7%97,1% on questions received
- Adalimumab 40 mg → 46.7%
- Adalimumab 80 mg → 7.46%
- Vibramycin 100 mg → 10.45%After the examination of lesions,
- 93% showed axillary lesions
- 62.8% showed gluteal lesions
- 44.2% showed femoral lesions According to DLQI
- 20 patients (29%) suffered 2–5 (minor impact)
- 12 patients (17.4%) suffered 6–10 (medium impact)
- 28 patients (40.6%) suffered 11–20 (major impact)
- 9 patients (13%) suffered 21–30 (acute impact) According to IHS4 (International Hidradenitis Suppurativa Scoring System) results as follows:
- ≤3, 23 patients, 33% moderate HS
- 4–10, 28 patients, 40.6%, medium severity HS
- ≥11, 18 patients, 26.1%, severe HS
- 7–9 h of sleep/day for adults (aged between 18 and 64 years)
- 7–8 h of sleep/day for adults (≥ 65 years)
- 50.7% women
- 49.3% men
Average age 36.15 years with standard deviation 11.93 and 68.1% of them were smokers. Moreover, 53.6% of the patients were BAD SLEEPERS while 46.4% were GOOD SLEEPERS. Results showed that sleeping quality is not assosiated with sex (x2 (1) = 0.35, p = 0.552) and age (r = 0.10, p = 0.406) of the patients. However, the sleeping quality of the patients is assosiated with smoking (x2 (1) = 3.87, p = 0.049) with the non smokers having better sleeping quality. Additionally, neither has been confirmed to be associated with DLQI (x2 (4) = 2.40, p = 0.662) and IHS4 (x2 (1) = 0.92, p = 0.632) nor with medical intake of Vibramysin (x2 (1) = 0.36, p = 0.547) and Adalimumab 40 mg and 80 mg (x2 (1) = 0.11, p = 0.737).
Quality of life, anxious and depressive symptoms and mental disorders in a sample of spanish hidradenitis suppurativa patients starting a biologic treatment
Marta Loro-Pérez, Francisco José Rodríguez-Cuadrado, Gaston Roustan-Gullón, Fernando Alfageme-Roldán
Hospital Universitario Puerta de Hierro Majadahonda, Servicio de Dermatología y Venereología, Madrid, Spain
Hidradenitis suppurativa (HS) is a chronic inflammatory disease that causes pain, itching, malodour and suppuration1. Quality of life of people diagnosed with HS is frequently diminished, this decrease being directly proportional to the severity of the symptoms2. When it comes to the psychological state of HS patients, they tend to experiment emotional distress. Depression, anxiety and substance abuse are frequent comorbidities of HS1. Patients show a tendency to feel less worthy of love as their symptoms worsen, however, sadness is not the only emotion triggered by the symptoms: it can be natural that they experiment irritation, anger and hopelessness leading them to feel more anxious2. The main objective of the present study is to evaluate the impact in the quality of life in a sample of patients with HS in Madrid, Spain, as well as the presence of symptoms of anxiety and depression. From September of 2021 to September of 2022, an evaluation on quality of life, anxiety and depression was conducted on every patien that started a treatment with a biologic drug to treat HS. Dermatology Life Quality Index (DLQI) was used to evaluate quality of life, and Hospital Anxiety and Depression Scale (HADS) was used to evaluate symptoms of anxiety and depression. Data for anxiety and depressive symptoms subanalysis was obtained with HADS subscales (HADS-A for anxious symptoms; HADS-D for depressive symptoms). Patients were asked if they currently had any psychological or psychiatric diagnosis for a mental disorder. In order to analyse the results, statistic software Stata/IC 16.0 was used. Out of 22 patients that completed the evaluation, 31.81% were males. Mean age of the sample was 41.4 (SD 12.54) and mean age at diagnosis was 19.8 (SD 9.31). Median value provided by DLQI evaluation resulted on 12 (IQR 14), which indicates that patients from this sample show a very large effect on their quality of life due to HS. As to the score provided by HADS evaluation, median value resulted on 16 (IQR 8). Median value on subscale HADS-A was 10 (IQR 8) and for HADS-D 5.5 (IQR 6). 10 patients (45.45%) were currently diagnosed with a mental disorder: 9.09% had been diagnosed for an eating disorder; 9.09% had a substance abuse disorder; 9.09% received a depressive disorder diagnosis; 9.09% were diagnosed with anxiety; 4.55% had a mixed anxiety-depressive disorder and 4.55% had an attention-deficit disorder. Patients with HS show a very large effect on their quality of life due to HS, similar to other skin conditions such as psoriasis or atopic dermatitis3,4. Results on anxiety and depressive symptoms indicate that this sample of patients is likely to show an anxiety or depressive disorder. Median results on HADS-A and HADS-D also seem to be higher in this sample of HS than in other samples of psoriasis patients5. As for the presence of mental disorders, anxiety, depression, substance abuse and bulimia were the most frequently diagnosed. These results lead to reflect on the importance of an evaluation of anxiety and depressive symptoms in HS patients, especially in cases where quality of life is very affected by the symptoms. Presence of eating disorders, characterized by body dysmorphic symptoms and an altered body image, should also be addressed when conducting an evaluation of psychological distress in HS patients, as well as substance abuse.
- Cartron A et al. Comorbidities of hidradenitis suppurativa: A review of the literature. Int J Womens Dermatol 2019;5:330–4.
- Esmann S et al. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Dermato-Venereologica 2011;91:328.
- Senthilnathan A et al. Even mild hidradenitis suppurativa impairs quality of life. Br J Dermatol 2011:181:838–9.
- Katugampola U et al. The Dermatology Life Quality Index: assessing the efficacy of biological therapies for psoriasis. Br J Dermatol 2007;156:945–50.
- Tribó MJ et al. Patients with moderate to severe psoriasis associate with higher risk of depression and anxiety symptoms: results of a multivariate study of 300 Spanish individuals with psoriasis. Acta Dermato-Venereologica 2019;99:417–22.
Signs, symptoms, clinical severity of hidradenitis supurativa and its impact on quality of life
Marcos Paulo G.G. Bouchuid, Katherine Alexandra C. Delgado, Vitoria Azulay, Rubem D. Azulay, João C.R. Avelleira, Luisa F. Vieria D'Almeida
Institute of Dermatology Professor Rubem David Azulay, Rio de Janeiro, Brazil
Hidradenitis suppurativa (HS) is an autoinflammatory disease whose chronicity and intensity of signs and symptoms generate a great impact on the quality of life of its sufferers, interfering with social, economic and personal life, which leads to high average scores on the Dermatology Life Quality Index (DLQI). Being a chronic disease, related to intense pain, bad odour and exudation, HS represents a great impact on the quality of life of its carriers1 It can interfere from social and economic to personal and sexual life, leading patients to a high risk of depression and suicide, probably related to this impact2. The objective of this study is to evaluate the association between Hurley's stage, anatomical regions and symptoms related to HS, and DLQI scores that indicate a greater impairment of the patients' quality of life. This was a descriptive, observational and cross-sectional study with retrospective analisis, whose data collection took place in the archived physical records and electronic records of the Meddit operating system treated at the Hidradenitis Suppurativa outpatient clinic at Instituto de Dermatologia Prof. Rubem David Azulay, from Santa Casa da Misericórdia do Rio de Janeiro (RJ), from January 2018 to January 2021. The inclusion criterion was the clinical diagnosis of HS reported in the medical records. Our exclusion criterion was the absence of clinical criteria for the diagnosis of HS. Clinical characteristics including location of the lesions (axillary, pre-sternal, inframammary, inguinal, genital, gluteal and perianal)2-4 presence of arthralgia and fatigue, Hurley stage and score on the DLQI questionnaire (attached) on the first query where tabulated referring to 224 physical and electronic medical records from patints with a confirmed diagnosis of Hidradenitis Suppurativa, according to clinical analysis. This data was posteriorly analysed in search for the correlation between signs and symptoms, clinical severity and life quality. Of the total number of HS cases in our study, 174 (78%) were female and 50 (22%) were male. The sample consisted of patients aged 8 to 70 years, with a mean age of 31.1 years (standard deviation 13.5). The intensity of fatigue was described as having a strong correlation with the Hurley stage, with approximately 40% of the studied patients classified as having clinically significant fatigue5. The factors related to HS studied were grouped according to their nature into three groups, namely: lesion sites, associated symptoms and Hurley stage. With regard to the injury sites group, we were able to observe the statistic relevance of the inframammary (Chi-Square Statistics = 11.25, degrees of freedom = 4, p-value = 0.02) and pre-sternal lesions (Chi-Square Statistics = 14.8, degrees of freedom = 4, p-value = 0.005) in correlation with higher DLQI scores, while other typical lesion sites of HS did not obtain the same result, such as the armpit, inguinal region, perianal, gluteus and genital. Our results differ from those found in the literature, where anogenital and inguinal lesions are seen as the major quality of life compromising ones1. After statistical analyses, it was shown that the presence of greater clinical severity, measured by the Hurley classification, correlates with reduced quality of life1. Considering that the classification at a higher Hurley stage is determined by the presence of intense inflammatory activity of the disease, with an important variety of subsequent local changes, a detailed analysis of these different components in search of their correlations with a better quality of life is recomended. The results obtained in our sample were not sufficient to significantly correlate the presence of arthralgia with a worse performance of patients in the DLQI questionnaire. We emphasize, however, that this symptom was present in our sample, and it is relevant to deepen its approach, with a view to a better understanding of its relationship with HS. On the other hand, correlation was found between fatigue and worse life quality, wich acknowledges evidence found in prior studies. Among our limitations, we find the fact that the study only addressed patients from a reference center for HS, which favours the oversampling of severe cases. HS is a life quality thretening desease by its multifactorial compromisisng of fisical and mental health. The search for uncommon lesion sites as well as fatigue aproach appears to be an effective way of achieving higher imporvement on patients quality of life and identifying those who can reach worse clinical features. Nonetheless, arthralgia was not demonstrate as directly determining life quality commitment, but was a prevalent simptom, that should be carefully aproached. We suggest new studies be done to deepen the searched criteria involvemente on HS Life quality commitment, and embrace new criteria.
- Ravn Jørgense A-H et al. Factors affecting quality of life in patients with hidradenitis suppurativa. Arch Dermatol Res 2020;312:427–36.
- Ingram JR et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa). Br J Dermatol 2019;180:1009–17.
- Alikhan A et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: Diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol 2019;81:76–90.
- Naasan H, Affleck A. Atypical hidradenitis suppurativa. Clin Exp Dermatol 2015;40:891–3,
- Deckers IE et al. The Handicap of Hidradenitis Suppurativa. Dermatol Clin 2016;34:17–22.
A global survey evaluating patients' unmet needs in hidradenitis suppurativa: Rationale, design and methodology
Georgios Kokolakis1, Philippe Guillem2, Eva Vilarrasa3, Ivette Alarcon4, Mei Go5, Craig Richardson4, Barry McGrath6, Joslyn S. Kirby7
1Universitätsmedizin Berlin, Department of Dermatology, Venereology and Allergology, Charité, Germany; 2Val d'Ouest, Surgery Department, Lyon, France; 3Hospital de la Santa Creu i Sant Pau, Dermatology Department, Barcelona, Spain; 4Novartis Pharma AG, Medical Affairs Department, Basel, Switzerland; 5Novartis Pharma AG, Patient Engagement Department, Basel, Switzerland; 6HS Ireland, Hidradenitis Suppurativa Association, County Clare, Ireland; 7Penn State Health Hershey Medical Center, Department of Dermatology, Hershey, USA
Hidradenitis suppurativa (HS), a chronic, painful, and debilitating inflammatory skin disorder1, affects physical, social, and emotional aspects of a patient's life. For example, HS patients experience greater pain and associated psychological comorbidities, including depression, anxiety, and risk of suicide, compared to those with other dermatological conditions. These challenges are further augmented by significant delays in diagnosis, with patients waiting on average 7 years from the time symptoms first appear, and limited access to adequate treatment. Taken together, these factors mean that HS has a profound impact on patients' quality of life (QoL).2,3 Evaluating how patients experience their disease from a physical, psychological, and social perspective and how they are managed within healthcare systems is key to identifying opportunities for targeted and effective improvements. Gaps currently exist in robust quantitative & qualitative evidence generation at patient population level, in particular regarding patients' experience of pain, fatigue, psychological comorbidities, and the impact of the disease on work and interpersonal relationships. The survey will evaluate the perspective on various aspects of HS patients to facilitate greater patient integration into healthcare policies and clinical decision-making to improve the QoL and wellbeing of people with HS around the world. This is a real-world survey including patients diagnosed with HS from six countries (USA, UK, Germany, France, Italy, and Spain). The survey will cover a population that is representative of the entire HS patient population and, for the first time, will also include undiagnosed patients who screen positive for diagnostic criteria for HS. It will be conducted between December 2022 and January 2023. To participate in the survey, patients (aged ≥18 years) must have a self-reported HS diagnosis at the time of data collection and must have not participated in surveys regarding HS in the last 4 weeks. A total of 625 adult patients with HS are planned to be interviewed and included in the survey. A quantitative online questionnaire composed of 70 questions will be completed by participants who qualify for the survey. The survey will use a combination of validated tools such as Dermatology Life Quality Index (DLQI) and Patient Activation Measure® (PAM-13), a 13-item survey that assesses an individual's knowledge, skills, and confidence in managing their health care, as well as newly defined questions that are tailored to the objectives of the survey and to the current gaps within the HS literature including questions regarding interpersonal relationships and family planning. A dedicated steering committee, composed of HS clinicians and patient research partners will also evaluate the data to understand, identify, and jointly implement the appropriate next steps for equity to access to health care. The primary endpoint is to assess patients' level of activation in terms of recognizing and managing their own health. The secondary endpoints include collection of data on the patient journey, which will be assessed through questions focusing on key elements such as (i) patients' perceptions of initial symptoms and their help-seeking behaviour; (ii) patients' perspectives on the actions that the consulted healthcare professional(s) (HCP[s]) undertook until referral to a specialist; (iii) impact of the disease on everyday experiences, social and personal life, self-esteem, and emotional state. The data from the survey will provide large-scale, worldwide, robust, quantitative as well as qualitative evidence, examining the full range of HS patient experience which hitherto is lacking for the HS population4. The results will provide the first available quantitative assessment to (i) determine the level of the patient's knowledge, skills, and confidence around HS, before and after diagnosis; (ii) inform and support clinical practice through end-to-end partnership with key patient organizations, patient advocates, and medical experts, uniting HCPs and patient communities; (iii) understand patients' perception and expectations towards available treatment options. The results of this survey will provide HS patient and medical communities with the resources and information required for better decision-making, that is based on strong shared evidence right from the start of a patient's journey when they first approach their health care specialists with HS symptoms. This will facilitate quicker diagnosis and more appropriate treatment plans to improve the lives of patients living with this debilitating disease.
Acknowledgement: The study was sponsored by Novartis Pharma AG.
- Reddy S et al. All-cause mortality among patients with hidradenitis suppurativa: A population-based cohort study in the United States. J Am Acad Dermatol 2019;81:937–42.
- Patel ZS et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep 2017;21:49.
- Garg A et al. Evaluating patients' unmet needs in hidradenitis suppurativa: Results from the Global Survey Of Impact and Healthcare Needs (VOICE) Project. J Am Acad Dermatol 2020;82:366–76.
- Naik HB et al. A call to accelerate hidradenitis suppurativa research and improve care-moving beyond burden. JAMA Dermatol 2019;155:1005–6.
Determinants of appointment absenteeism at a tertiary hidradenitis suppurativa outpatient clinic
Britt Nilausen1, Anne-liv R. Gerber1, Nikolaj Holgersen1, Valdemar Nielsen1, Hans Christian Ring1, Simon F. Thomsen1,2
1Bispebjerg and Frederiksberg hospital, Department of Dermatology, Copenhagen, Denmark; 2Department of Biomedical science, University of Copenhagen, Copenhagen, Denmark, Copenhagen, Denmark
Factors associated with absenteeism among patients with hidradenitis suppurativa (HS) have not previously been investigated. A better and broader understanding of which factors that may be associated with absenteeism in the HS clinic may optimize the treatment course. We therefore examined demographic and clinical determinants of appointment absenteeism at a tertiary HS outpatient clinic. All unique patient follow-up appointments during 12 months at a dermatological university outpatient clinic were included. Demographic and clinical data obtained at the first consultation prior to the missed appointment were compared between absent and non-absent patients. Of a total of 161 scheduled, consecutive HS patient follow-up appointments, 37 (23.0%) patients did not show up. Patients with absenteeism were more likely to be males (56.8% vs. 36.3%), OR = 2.30 (1.09–4.86) p = 0.028, of non-Caucasian descent (37.8% vs. 16.9%), OR = 2.99 (1.32–6.73) p = 0.008. Patients with a positive family history of HS were less likely to be absent (8.9% vs. 30.5%) OR = 0.22 (0.08–0.61) p = 0.004. Measurements for disease severity showed no statistically significant differences between absent and non-absent patients, including Hurley stage (p = 0.717), IHS4-score (p = 0.112), number of boils in the month prior to consultation (p = 0.855), DLQI (p = 0.688) and patient reported VAS on pain (p = 0.230) and influence on daily life (p = 0.992). HS patients with outpatient clinic absenteeism are more likely to be males and of non-Caucasian decent, whilst patients with a positive family history of HS are less likely to be absent. Both patient reported and clinical measures of disease severity did not seem to have an impact on absenteeism. Knowledge of these factors can be used to target sub-populations at risk of absenteeism and establish possible preventive interventions.
Paramedics and nurses against suppurative hidradenitis (PANASH): An example of nurse involvement in clinical research
Christelle Enault1, Sylvia Math1, Anne-Sophie Gadat1, Marion Clerbout1, Christelle Chavrier1, Marianne Beuque1, Samantha Gaspard1, Ines Evain1, Myriam Fioretta1, Virginie Vlaeminck-Guillem3,4, Philippe Guillem1,2
1Clinique du Val d'Ouest, Department of Surgery, Ecully, France; 2RésoVerneuil, Paris, France; 3CHU, Department of Biology, Lyon, France; 4Lyon 1 University, INSERM 1052, CNRS 5286, CRCL, Lyon, France
wHoiS
Giuseppina Pintori
HS Italian patient community Globalskin.org Italy, Cagliari, Italy
- assessing the mental and physical health of HS patients;
- medical care and support from the scientific community;
- political support, resolutions and recognition;
- increase awareness and knowledge of the public and society at large.
In particular, this survey aims to highlight the importance of recognizing disability status for patients with moderate or severe HS, as is the case for other conditions, according to European and national laws.