Retyping and molecular pathology diagnosis of dyschromatosis universalis hereditaria
Abstract
Dyschromatosis universalis hereditaria (DUH) is characterized by diffuse symmetrically distributed hypopigmented macules mixed with hyperpigmentation. DUH is divided into three types by Online Mendelian inheritance in man (OMIM) that is, DUH1 (OMIM 127500), DUH2 (OMIM 612715) and DUH3 (OMIM 615402) according to the different linkage regions. Although each condition possesses corresponding phenotypic characteristics and the prognosis for each is somewhat different, these disorders are highly overlapped and difficult to differentiate in the clinical setting. Our latest study reveals a novel DUH subtype that presents a mild phenotype of pigmentation anomalies and is named PER3rs772027021 SNP related DUH or DUH4 by us, which make the DUH subtype can be further retyped. Heterozygous distribution or mosaic-like distribution of melanin is a newly discovered pathological features that is uniquely demonstrated in the affected layers of DUH1 and DUH4 patients. In this review, DUH is further divided into four subtypes according the causative genes and their mutational sites, and the mutation regions described in the previous reports. To make an accurate diagnosis, we suggest that Sanger sequencing or the target region sequencing (TRS) to the candidate causative genes related melanogenesis may be the most effective and convenient method of clinical diagnosis or/and prenatal diagnosis for DUH and DUH-like patients. More importantly, heterozygous distribution or mosaic-like distribution of melanin can be utilized for differential diagnosis of DUH. We also investigate the underlying molecular mechanism to form mosaic-like melanin in the affected layers of hyper- and/or hypo-pigmented macules from DUH1 and DUH4 patients. This review provides a molecular and pathological delineation of four types of DUH and aims to establish a concise diagnostic strategy to allow clinical dermatologists to make an accurate diagnosis.
1 BACKGROUND
Dyschromatosis universalis hereditaria (DUH; Online Mendelian inheritance in man [OMIM] 127 500) is a rare autosomal dominant genodermatosis, which was initially described by Ichikawa and Hiraga in two generations of two families in 1933.1 Asymptomatic hyperpigmented and hypopigmented macules extensively distributed over the trunk, limbs and sometimes face are the major phenotypes of this genodermatosis.2, 3 Lesions of irregular size and shape begin to appear in infancy or early childhood and almost the whole body including the face, trunk, extremities nails, hair and teeth in affected individuals are affected, but the palms and soles are not involved.2, 4, 5 Pigmentary anomalies on the trunk and extremities in the skin are the most dominant features of this disorder, and facial lesions are the second dominant features that can be seen in 50% of affected individuals, but the involvement of palms and soles is unusual.2 Mottled pigmentation can be seen on the oral mucosa and tongue and a diffuse hyper-pigmentation interspersed with spotty de-pigmented macules was showed on the palms and soles.5 Two Chinese DSH pedigrees in 20036 were identified by us show autosomal-dominant inheritance and have been diagnosed with DUH by a subsequent study.3 In 2008, the pathogenic gene in a second DUH with autosomal-recessive inheritance was mapped on region 12q21-q23 in the Arab population3 and the mutations in the ADAR gene were excluded. In 2013, a third DUH-related pathogenic region was mapped on 2q33.3-q36.13, and ACBC6 located at 2q35 confirmed the pathogenic gene of DUH.7 Therefore, DUH is divided into three types, DUH1 (OMIM 127500), DUH2 (OMIM 612715) and DUH3 (OMIM 615402) according to the different linkage regions located in the 6q24.2-q25.2, 12q21q23 and 2q35 regions, respectively, in which DUH1 and DUH3 are autosomal-dominant, while DUH2 is an autosomal recessive.8
ABCB6 and SASH1 are reported to be the pathogenic genes related to DUH.7-16 Although Wu et al.10 maintained that the DUH phenotypes caused by ABCB6 mutations showed more generalized mottled hyperpigmented macules mixed with hypopigmented macules arranged in a reticular pattern; however, the DUH phenotypes caused by SASH1 mutations are more likely to be confused with generalized lentiginosis. However, Cao et al. pointed out that although in the same family, the same mutation of SASH1 were shown in all of the affected individuals including the proband, nevertheless, the clinical manifestations among the affected individuals were different. Clinical manifestations are affected by skin colour, age and other environmental factors, such as ultraviolet (UV) exposure8 and our previous study also revealed that skin lesions were reported to become more pronounced after sun exposure,6 and SASH1 is involved in pigmentation upon UV exposure.13 Therefore, it is difficult to discriminate the subtypes of DUH based on clinical manifestations.
2 CLINICAL, HISTOLOGICAL AND GENETIC OVERVIEW OF THE RETYPING DUH
2.1 DUH1 (SASH1-associated DUH)
2.1.1 Clinical phenotypic characteristics of DUH1
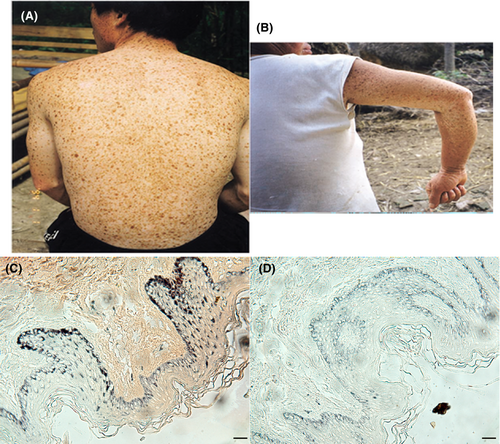
Generalized lentigines accompanied by mottled hyper- and hypopigmented macules are diffuse symmetrically distributed face, trunk, extremities nails, hair and teeth, which are the major phenotypic characteristics of DUH1.8 Systemic damage, including deafness, visual impairment and neurological symptoms are presented in some DUH cases.8 In 2003, two large families (a five-generation pedigree [Family A] and a three-generation pedigree [Family B]) from the Henan and Yunnan provinces of China segregating autosomal dominant cutaneous dyschromatosis were reported by our team. A mixture of hypopigmented and hyperpigmented macules of various sizes was shown on the trunks, arms, necks and faces of the affected individuals (Figure 1A,B). Biopsies of hypo- and hyperpigmented macules from the dorsa of the feet revealed basal melanosis and hypomelanosis.6 Nuber et al. reported that randomly distributed hyper- and hypo-pigmented skin mottled macules of variable shape and size were presented in the male proband from Bangladesh.17 Clinical presentations showed that hypopigmented spots were intermixed with the lentigines on the trunk and extremities, and there was mild dyschromatosis at the elbows and on the dorsa of the hands and feet in the SASH1-related DUH phenotypes.17 The proband who is a 27-year-old man from Henan Province, China, had multiple ‘freckles’ on his forehead beginning at 8 months of age and the lesions gradually increased and spread to his entire body at 10 years and his mother has a similar lentiginous phenotype.17
2.1.2 Mutations of the pathogenic gene of DUH1
We discovered two Chinese DUH pedigrees in 20036 showing autosomal-dominant inheritance and diagnosed patients with DUH.3 Three SASH1 variants (c.2000G>A, p.E509K; c.2019 T>C, p. L515P and c. 2126 T > G, p.Y551D) associated with DUH in two Chinese families and one American family were identified by our team. Additionally, increasing evidence suggests that SASH1 variants including a c.1761C>G (p.Ser587Arg) variant,9 a c.1553A>C (p.Q518P) one,10 and a c.1556G->A(p. S519N) one18 are associated with genodermatosis or DUH.9, 10, 18, 19
2.1.3 Mosaic-like melanin distribution different affected epithelial layers in the hyperpigmented macules is an important pathological feature of SASH1-associated DUH
Histologically, increased or decreased melanin pigmentation was found in the basal cells of hyperpigmented lesions or hypopigmented macules, respectively.2 In a large Chinese SASH1-associated DUH1 family, we investigated the melanin synthesis and distribution in the epithelial layers of the hyperpigmented and hypopigmented lesions from a Y551D-SASH1 mutation individual. Interestingly, melanin staining indicated that excessive synthesized melanin and less synthesized melanin were both demonstrated in both basal and superbasal layers, which constitutes the heterogeneous or mosaic-like distribution of melanin in different regions of the same hyperpigmented macule (Figure 1C). Less synthesized melanin was demonstrated in the basal layers of hypopigmented macules (Figure 1D).13, 14
2.2 DUH2 (an autosomal recessive DUH)
2.2.1 Clinical phenotypic characteristics of DUH2
DUH is also a clinically and genetically heterogeneous disorder. Most DUH affected individuals showed an autosomal dominant mode of inheritance, whereas some DUH families presented with an autosomal recessive inheritance pattern.3, 20 Stuhrmann et al. revealed that in a consanguineous Bedouin DUH family from Saudi Arabia with four affected and three unaffected sibs, this DUH was characterized by autosomal recessive inheritance.3 Two boys and two girls in this family showed symptoms of pigmentary anomalies. The sibs had multiple asymptomatic 2- to 5-mm macules of hypopigmented, depigmented and hyperpigmented, which were bilaterally symmetrical, and scattered all over the body including the back, hands, feet and face during infancy or early childhood. The palms and soles, mucous membranes, teeth and nails are normal.20
2.2.2 Mutations of the pathogenic gene of DUH2
Under the assumption of autosomal recessive inheritance and by genome-wide screening using an SNP array, Stuhrmann et al. had identified a novel locus for dyschromatosis on chromosome 12q21-q23, with a maximum LOD score of 3.4. A homozygous interval identified between the SNP markers rs1921045 and rs2373584, which suggested a candidate region of 18.9 cM on chromosome 12 was the only region with a significant linkage result.3
2.2.3 Melanin synthesis and distribution in the affected layers of the hyperpigmented macules from the DUH2 patients
No investigation including Stuhrmann's report3 reported that the melanin synthesis and distribution in the affected layers of the hyper- and hypo-pigmented macules in DUH2 patients.
2.3 DUH3(ABCB6-related DUH)
2.3.1 Clinical phenotypic characteristics of DUH3
Zhang et al. characterized a five-generation Chinese family with DUH, in which the disorder was inherited in an autosomal dominant manner. The proband was a 9-year-old boy who had normal skin at birth. Hyperpigmented and hypopigmented speckles appeared initially on his trunk at the age of 2 year, and then his face, neck and limbs were gradually involved by the extended speckles, and pruritus or pain was not found. Examination of the skin showed motley hyperpigmented and hypopigmented macules that nearly involved his whole body in a symmetrical pattern, and pigmentary anomalies on the face, neck, trunk and the dorsa of his hands and feet were most obvious; however his palms and soles, oral mucosa, hair, nails and teeth were affected.7 Generalized and random distribution of small hypo- and hyper-pigmented lesions were found on the hands and abdomen of patients in two ABCB6-related DUH families.16 Cui et al. investigated a Chinese family with typical features of autosomal dominant DUH and three unrelated patients with sporadic DUH and reported that the proband in this DUH family who was a 33-year-old female had cutaneous hypo- and hyper-pigmented macules(3–7 mm diameter) scattered over the entire body including the hands, feet, back, face and scalp. Her skin at palms and soles, mucus membranes, teeth, nails and hairs appeared normal. Her medical history indicated that the skin macules appeared within 2 months after birth and became darker when exposed to the sun.12
2.3.2 Mutations of the pathogenic gene of DUH3
A c.1067T4C (p.Leu356Pro) mutation in exon 3 of ABCB6 (ATP-binding cassette subfamily B, member 6) was identified in the DUH3 family, and two additional missense mutations a c.508A4G(p.Ser170Gly) mutation in exon 1 and a c.1736G4A(p.Gly579Glu) mutation in exon 12 of ABCB6 were found in two out of six patients in sporadic DUH3 patients.7 Two mutations in ABCB6 including a c.1358CT(p.Ala453Val) mutation and a c.964A.C(p.Ser322Lys) mutation were found in two Chinese DUH3 families.16 Cui et al. identified a missense mutation a c.1663 C>A(p.Gln555Lys) variant in ABCB6 in a Chinese family with typical features of the autosomal dominant DUH family. An additional mutation (g.776 delC, c.459 delC) in ABCB6 was found in an unrelated sporadic patient.12
2.3.3 Melanin synthesis and distribution in the affected layers of the hyperpigmented macules from the ABCB6-related DUH
Histopathologic analysis indicated that more melanin or melanized, mature melanosomes was detected in basal layers of the hyperpigmented areas than those within the hypopigmented areas from the proband with a p.Gln555Lys mutation on ABCB6 and empty immature melanosomes were found in the hypopigmented melanocytes.12
2.4 DUH4 (PER3rs772027021 SNP related DUH)
2.4.1 Clinical phenotypic characteristics of DUH4
Our group recently reported a novel DUH subtype with mild phenotypes, in which the hyperpigmented and/or hypopigmented phenotypes appeared in the affected individuals carrying the SNP PER3rs772027021. The phenotypes of pigmentary anomalies in the extended Chinese DUH family of four-generation in Hubei, China were found to be transmitted in an autosomal dominant manner.21 The proband in this DUH family had normal skin at birth. The lesions which were most obvious on her face, neck, trunk and dorsa of her hands and buttocks are presented in a symmetrical pattern. Hyperpigmented macules appeared initially on her face when she was a toddler, the hyperpigmented macules became larger as evenly distributed freckles, and the colour of these macules deepened. Hypopigmented macules began to appear on her neck, elbows, knees and phalangeal joints of the proband in early adolescence. Her palms and soles, oral mucosa, hair, nails and teeth were normal. In adulthood, irregularly shaped, asymptomatic hyper and hypopigmented macules were present over her face, neck, abdomen and back, dorsal aspects of the hands and arms, thighs, calves and hips (Figure 2A–C).21
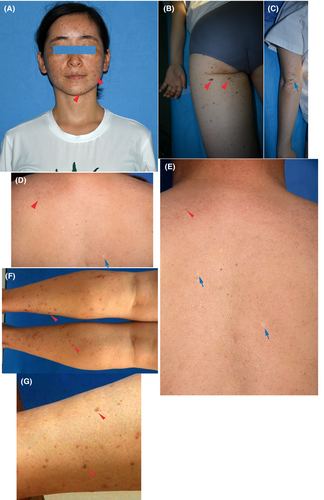
Other affected individuals all showed sporadic or disseminated hyperpigmented and/or hypopigmented macules on the limbs, trunk, back and auricle. The hypopigmented and hyperpigmented lesions of the proband's father were concentrated on his trunk, shoulder and back and his hyperpigmented lesions were diffusely distributed (Figure 2D,E). The hyperpigmented lesions of her aunt were concentrated on both lower limbs (Figure 2F,G).21
2.4.2 Mutations of the pathogenic gene of DUH4
We subjected the exomes of five affected and five unaffected family members to whole-exome sequencing in the mild DUH family.21 The SASH1 variants including the c.1761C>G (p.Ser587Arg) variant,9 the c.1553A>C (p.Q518P) one,10 and the c.1556G->A(p. S519N)18 one of SASH1 which had been reported previously to associate with genodermatosis or DUH were not indicated in the Exome sequencing results. Sanger sequencing analysis indicated that a SASH1 variant (c. 1574C>G (p.T525R)) was only identified in the proband but not in affected and unaffected individuals in the DUH family. Sanger sequencing analyses also suggested that, a SNP, which is a missense SNV (c. 517C>T (p.P173S)), was detected in seven affected members of the family, but not in any of the 17 unaffected individuals. All of these results indicated that this SNP PER3 rs772027021 cosegregated with the pigmented phenotype.21
2.4.3 Mosaic-like melanin distribution in basal and horny layers in the hyperpigmented macules also appeared in the affected epithelial layers of PER3rs772027021 SNP DUH
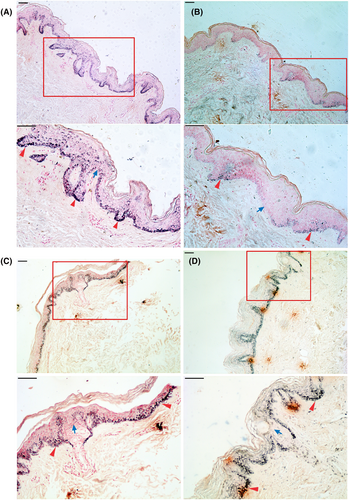
In general, DUH should be considered in the differential diagnosis of all cases manifesting as mixed hyper and hypopigmented macules, and biopsy specimens should be obtained to confirm the diagnosis.22 Phenotypes of increased melanin pigmentation were demonstrated in the affected skin epithelial tissues of two affected individuals and the proband of this PER3rs772027021 SNP-related DUH family using melanin staining. These melanin staining analyses also revealed excessive melanin pigmentation and a mosaic-like distribution of melanin were shown in both the basal and suprabasal layers of the proband's hyperpigmented and hypopigmented macules (Figure 3A). Less melanin and a mosaic-like melanin distribution were observed in the hypopigmented macules of the proband (Figure 3B). Similar to the proband, increased melanin pigmentation and a mosaic-like melanin distribution were found for the basal and suprabasal layers of the hyperpigmented macules of two affected individuals in this DUH family (Figure 3C,D).
2.4.4 Differential diagnosis and molecular and pathological diagnosis of DUH
Although only pigmentary abnormalities in the skin, and rarely other organs and systems were affected by the DUH, these unsightly diseases pose a severe psychological burden on affected individuals and their families. Thus, a correct diagnosis and/or prenatal diagnosis are necessary for clinical and genetic counselling and precision medicine in the future. Here, we propose a diagnostic strategy for this kind of genodermatosis. DUH mostly overlaps with the SASH1-mutated phenotypes with diffuse lentigines and hypo- and hyperpigmented macules. TRS, also known as target region sequencing, is a technical means to capture and enrich genome regions of interest by PCR or probe hybridization, and to carry out high-throughput sequencing. It can detect genetic variation heterotopies in target genome regions, and obtain variation information in specified target regions.23, 24 To make an accurate diagnosis, Sanger sequencing or Target Region Sequencing of the causative genes is recommended for the clinical diagnosis and/or prenatal diagnosis of DUH and DUH-like patients.
The affected epidermis from an inherited lentiginosis patient with a SASH1 variant (c.1556G->A, p. S519N) was double-stained with the melanocyte marker, MART1, and the proliferation antigen, Ki67. Unlike the proliferative melanocytes induced by SNP PER3 rs772027021 in the DUH4-affected epithelial layers,21 the proliferative melanocytes which were both positive for MART1 and Ki67 in the affected epithelial layers did not show the mosaic-like distribution in the affected epithelial layers.18 Therefore, the mosaic-like distribution of melanin, proteins encoded by causative genes and melanogenesis-related molecules may be an important pathological feature of DUH, which can be utilized for pathological diagnosis.
3 THE MOLECULAR MECHANISM OF MOSAIC-LIKE MELANIN IN THE AFFECTED LAYERS OF HYPER- AND/OR HYPO-PIGMENTED MACULES FROM DUH1 AND DUH3 PATIENTS
3.1 Upregulation of melanogenesis associated partners was induced by the upregulation of causative genes
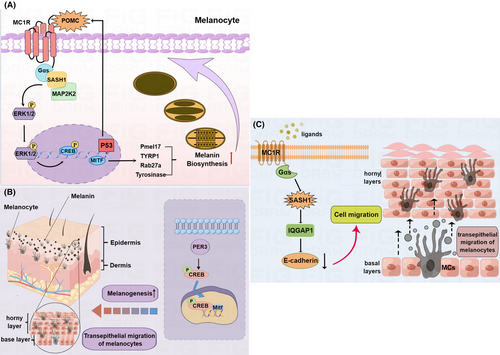
Heterogeneous expression or mosaic-like expression of the SASH1 protein that is, upregulated SASH1 in some regions in different epithelial layers and downregulated SASH1 in the other regions in different epithelial layers of the same hyperpigmented macules and upregulated SASH1 were observed in all of the epithelial layers in the epidermal tissues from the Y551D-SASH1 DUH1-affected individuals.13, 14 Pmel17 is a mature melanosome resident protein, which is responsible for melanin and melanin polymerization and TYRP1 is mature melanosome resident protein, which is responsible for melanin biosynthesis and tyrosinase stabilization in vitro and in vivo.13, 25 Rab27a plays a pivotal role in the transport of melanosomes to the dendrite tips of melanocytes.26 Myosin VIIa and Rab27a synergistically transport and constrain melanosomes within a region of filamentous actin, which is particularly important for the motility and localization of melanosomes.27 Our previous reports revealed that heterogeneous expression of Pmel17, TYRP1, Rab27a and tyrosinase, and upregulated Pmel17, TYRP1, Rab27a and tyrosinase were detected in all of the epithelial layers of the epidermal tissues from the DUH-affected individuals, which eventually resulted in mosaic-like melanin and excessive synthesis of melanin in the affected epithelial layers.13, 14 Microphthalmia transcription factor (Mitf) which is a melanocyte master transcription factor, is responsible for pigment cell-specific transcription of the melanogenesis enzyme genes.28 Recently, we performed multi-colour IHC (mIHC) to investigate the effects of PER3 rs772027021 SNP or SASH1T525R variant on melanocyte proliferation in the affected epithelial layers of DUH4. The Ki67-positive cells in the epithelial tissues can be identified as the proliferative cells, the Mitf-positive cells can be suggested to be melanocytes were quantified and the melanocytes both with Ki67 and Mitf both being positive were suggested as the proliferative melanocytes. Immunofluorescence analyses indicated that increased absolute numbers of Ki67- positive or Mitf- positive cells or enhanced ratio of proliferative melanocytes in the proliferative cells in the hyperpigmented macules were induced by PER3 rs772027021 SNP and/or SASH1 T525Rvariant, respectively.21 Oppositely, increased absolute numbers of Ki67- positive or Mitf- positive cells or enhanced ratio of proliferative melanocytes in the hypopigmented macules were not induced. Additionally, mosaic-like expression and distribution of PER3 was detected in basal and superbasal layers of hyperpigmented macules from the proband and her aunt (Figure S1A,B).21
3.2 Novel melanogenesis associated pathways were activated by the upregulation of causative genes
We further investigated the cascades that DUH associated-causative genes were involved in melanogenesis and identified the potential molecular mechanism of DUH associated-causative genes engage in. Melanogenesis in the affected epithelial layers from was mediated by mutated SASH1s (E509K-SASH1, L515P-SASH1 and Y551D-SASH1) through the p53/α-MSH/POMC/Gαs/SASH1 cascade,13 which indicated that mutated SASH1 was mediated by p53 pathway to promote melanogenesis (Figure 4A). With the help of novel p53/α-MSH/POMC/Gαs cascade, mutated SASH1s crosstalk with the Extracellular signal-regulated kinase 1 (ERK1/2)-cAMP-response element binding protein (CREB) cascade through MAP2K2 to promote melanogenesis (Figure 4A).26 Additionally, in a heterozygous mouse model in which the SASH1 c.1654T>G (p.Tyr551Asp, Y551D) mutation was knocked in, we pointed out that SASH1 regulates the expression of Mitf in the nucleus by acting as a scaffold molecule that participates in the assembly of the SASH1-MITF molecular complex and promotes the hyperpigmentation phenotype in the pathogenesis of DUH and other dermatoses.29 Although an increase in phosphorylated ERK1/2 and CREB levels is caused by mutated SASH1 alleles,26 enhanced phosphorylation level of CREB, and decreased phosphorylation levels of ERK1/2 was induced the SNP PER3rs770720021 (Figure 4B), which indicated a novel PER3-CREB phosphorylation cascade could mediate melanogenesis.21 Up to now, at least the canonical CREB/ERK cascade which mediate melanogenesis is revealed to be regulated by two causative genes that is, SASH1 and PER3. However, S587R-SASH1 exhibited reduced SASH1 expression and the downregulation of S587R-SASH1 may be caused by TGF-β treatment. So, Cui et al. pointed out that S587R-SASH1 may be negatively mediated by the TGF-β1 pathway.9 However, this work could only give some bioinformatic predication and lacked further investigation to illustrate how the TGF-β1 pathway regulate S587R-SASH1 expression.
3.3 Increased translocation of melanocytes from basal layers to horny layers may be a major reason to mosaic-like melanin in the affected skin epithelial layers
Mutated SASH1s was found to increase migration in melanoma cells through a Gαs–SASH1–IQGAP1–E-Cadherin dependent pathway,11 which to a certain degree explains the underlying mechanism of the phenotypes that melanocytes not only located in the basal layers but also horny one in the affected epithelium of the skin. The recent study revealed heterogeneous distribution of melanocytes was detected in some regions of the affected epidermis and SASH1-positive cells were wildly distributed in multiple affected epithelial layers of DUH individuals.9 Our latest study shows that mosaic-like melanin is observed in multiple epithelial layers in the DUH4-affected individual, which suggests that the suprabasal layers translocation of proliferative melanocytes from basal layers may result from PER3 rs772027021 SNP.21
Cui group's study revealed that SASH1-positive cells were wildly distributed and S587A-SASH1 was upregulated in multiple epithelial affected layer compared that in the unaffected epidermis, and their further experiments suggested that the invasion and migration of melanocyte (PIG1 cells) with transfected with S587R-SASH1 were triggered by upregulated S587A-SASH1, which suggested upregulated mutated SASH1 promoted melanocyte migration in the affected epidermis; however, their immunoblot results showed S587A-SASH1 was downregulated compared to wild-type SASH1 in PIG1 cells. The results detected in the affected epidermis are inconsistent with the results of the cell experiments.
Taken above, we believe that enhanced expression of melanogenesis related proteins is induced by upregulated causative genes (SASH1 and PER3) through the activation of these signal pathways including the p53/α-MSH/POMC/Gαs/SASH1 cascade, the p53/α-MSH/POMC/Gα/SASH1/MAP2K2/CREB/ERK cascade and the PER3/CREB cascade. Enhanced transferring of melanocytes from basal layers to horny layers may be promoted by mutated SASH1 through the Gαs–SASH1–IQGAP1–E-Cadherin cascade (Figure 4C) and SNP PER3rs772027021 (Figure 4B). Upregulated melanogenesis partners including melanosome resident/transport proteins, tyrosinase and Mitf and increased translocation of melanocytes from basal layers to horny layers could synergistically contribute to the formation of mosaic-like melanin in the affected epidermis. The molecular characteristics of four DUH subtypes were summarized Table 1. The molecular characteristics includes the causative genes, mosaic-like distribution of melanin in the hyperpigmented and hypopigmented macules, transferred melanocytes from basal layers to horny layers, upregulated melanogenesis associated partners and canonical melanogenesis pathway involved in Table 1.
| DUH types | ||||
|---|---|---|---|---|
| Molecular characteristics | DUH1 | DUH2 | DUH3 | DUH4 |
| Causative gene | SASH1 | A homozygous interval between the SNP markers rs1921045 and rs2373584 on chromosome 12 | ABCB6 | PER3rs772027021 SNP |
| Mosaic-like distribution of melanin | Hyperpigmented macules and/or hypopigmented macules | Not showed | Not showed | Hyperpigmented macules and/or hypopigmented macules |
| Transferred melanocytes | Hyperpigmented macules | Not showed | Not showed | Hyperpigmented macules |
| Upregulation of melanogenesis associated partners | Pmel17, YRP1, Rab27a, tyrosinase etal. | Not reported | Not reported | Mitf |
| Canonical melanogenesis pathway involved in | MEK/ERK/CREB pathway, p53/POMC cascade | Not reported | Not reported | CREB pathway |
4 CONCLUSIONS
Although only pigmentary abnormalities in the skin, and rarely other organs and systems were affected by the genetic reticulate pigmentary disorders, these unsightly diseases pose a severe psychological burden on affected individuals and their families. Thus, a correct diagnosis or/and prenatal diagnosis are necessary for clinical and genetic counselling and precision medicine in the future. DUH mostly overlaps with the SASH1-mutated phenotypes with diffuse lentigines and hypo- and hyperpigmented macules. To make an accurate diagnosis, Sanger sequencing or TRS of causative genes is recommended for the clinical diagnosis and/or prenatal diagnosis of DUH and DUH-like patients. Additionally, the mosaic-like distribution of melanin, causative genes encoding proteins and melanogenesis associated partners is an important pathological feature of DUH, which can be utilized for pathological diagnosis. TRS detection technology to the candidate causative genes (SASH1, ABCB6 and SNP PER3) and the mutation regions (chromosome 12q21-q23) and new identified causative genes of DUH has being developed in collaboration with Shanghai WeHealth Biomedical Technology Co, Ltd utilizing our group's sequencing experience and their sequencing technology, which will promote rapid differential diagnosis and prenatal diagnosis of DUH and other pigmentation anomalies. The latest study shows that CREB-IN-1 TFA is a potent, orally active CREB inhibitor, which inhibits breast cancer cell growth.30 Our previous study suggested CREB phosphorylation was triggered by SASH1 variants and PER3 rs772027021 SNP.14, 21 Therefore, targeted intervention of the melanogenesis associated pathways that CREB phosphorylation was activated by SASH1, PER3 and other identified causative genes of DUH may be an alternative strategy to treat DUH.
AUTHOR CONTRIBUTIONS
Ding'an Zhou conceived the article and drafted the manuscript. Pingping Yang and Hongyu Chen performed extensive literature review and contributed to image drawing.
ACKNOWLEDGEMENTS
This work was supported partly by the following funds: National Natural Science Foundation Project (31860319 and 32060165 to Ding'an Zhou), Guizhou Provincial Science and Technology Department Project (grant no: qian ke he zhicheng [2020]4Y125 and qian ke he jichu-ZK[2021] zhongdian 031 to Ding'an Zhou and grant no: qian ke he jichu-ZK[2022]yiban448 to Miao Zhang).
CONFLICT OF INTEREST STATEMENT
The authors have declared that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




