The immune function of dermal fibroblasts in skin defence against pathogens
Abstract
Dermal fibroblasts are the main resident cells of the dermis. They have several significant functions related to wound healing, extracellular matrix production and hair cycling. Dermal fibroblasts can also act as sentinels in defence against infection. They express pattern recognition receptors such as toll-like receptors to sense pathogen components, followed by the synthesis of pro-inflammatory cytokines (including IL-6, IFN-β and TNF-α), chemokines (such as IL-8 and CXCL1) and antimicrobial peptides. Dermal fibroblasts also secrete other molecules-like growth factors and matrix metalloproteinases to benefit tissue repair from infection. Crosstalk between dermal fibroblasts and immune cells may amplify the immune response against infection. Moreover, the transition of a certain adipogenic fibroblasts to adipocytes protects skin from bacterial infection. Together, we discuss the role of dermal fibroblasts in the war against pathogens in this review. Dermal fibroblasts have important immune functions in anti-infection immunity, which should not be overlooked.
1 INTRODUCTION
Fibroblasts are resident cells of many tissues and play a crucial role in wound healing and fibrosis.1, 2 Although fibroblasts were thought of as immune-neutral cells before, the multiple functions of fibroblasts in immunity have been observed in recent years.3 For example, fibroblasts in secondary lymphoid organs exhibit the function of maintaining the balance of inflammatory responses. They recruit lymphoid and dendritic cells (DCs) at the onset of immune responses, and suppress immune cell function when immune responses are overactive.2 In non-lymphoid tissues, fibroblasts have been shown to promote protective immunity via facilitating monocytes and secrete cytokines such as interleukin (IL)-33, which is crucial to infection control and muscle repairment.2, 4, 5 Fibroblasts can respond to various cytokines secreted by other immune cells and regulate immune responses.6 Apart from the role as a bridge that connects and facilitates the function of immune cells, recent studies have observed that fibroblasts in the skin, gut, and lung can directly sense danger.6, 7
As the outmost organ, skin exhibits the power of defending against infection. Epidermis, dermis and hypodermis are three layers composing skin. Fibroblasts are the predominant cell type in dermis. Upon microbial challenge, skin acts as a physical barrier and displays immune function as well. While how dermal fibroblasts fight infection is still less recognised, it is now known that dermal fibroblasts can directly sense microbes. They express several pattern recognition receptors (PRRs) and produce effector molecules including cytokines, chemokines, antimicrobial peptides, and matrix metalloproteinases (MMPs) to participate in anti-infection immunity.3, 8 Moreover, the potential preadipocyte–adipocyte transition of a certain dermal fibroblast lineage and crosstalk between dermal fibroblasts and immune cells augment skin immunity to infection.9-11
Fibroblast heterogeneity is extensive between organs and inside an organ, which enables fibroblast diverse functions and roles in health maintenance.3, 12 In this review, we will first discuss the clusters and distinct features of dermal fibroblasts. Then we will review dermal fibroblast immune function from pathogen recognition to inflammatory mediator production as well as fibroblast-immune cell interaction and other functions of dermal fibroblasts in infection control.
2 FIBROBLAST SUBSETS IN SKIN
Single-cell RNA sequencing and spatial transcriptional profiling of primary human dermal fibroblasts have shown that there are at least four subpopulations in skin: fibroblasts located in the upper dermis (papillary dermal cells and related to collagen expression), in the lower dermis (including preadipocytes) and the reticular dermis (containing three subpopulations including pericytes).13 Vimentin is expressed in all groups. In the papillary dermis, CD39, α5 chain of collagen VI (COL6A5) and COL23A1 are over-expressed. Genes related to secretoglobin and hair follicles (SCGB2A2, SCGB1D2, KRTAP11-1 and TCHH) are enriched in the reticular dermis. CD36 is highly expressed in the lower reticular dermis and hypodermis.13 Cell surface markers distinguishing fibroblast subclusters in human dermis are listed in Table 1. CD90 (Thy1), platelet-derived growth factor receptor (PDGFR) α and PDGFRβ are pan-fibroblast markers.13 In mouse models, papillary dermal fibroblasts (CD26 + Sca1–) are related to hair follicle formation. Fibroblasts of the reticular dermis (Delta-like homologue 1 (Dlk1) + Sca1–) and lower dermis (Dlk1+/–Sca1+) contribute to extracellular matrix (ECM) production and dermal regeneration during wound healing.14 Sca1+ or Dlk1 + Sca1– mouse dermal fibroblasts also have adipogenic activity.13, 14 Upon injury, subclusters of dermal fibroblasts can be multiple and dynamic. Several studies have uncovered the adipocyte-myofibroblast or myeloid cell-myofibroblast-adipocyte transition in murine skin wounds.15-17 Although fibroblast surface markers may differ between humans and mice, the features and functions of certain fibroblast subsets remain similar.
| Fibroblast group | Cell surface phenotype | Functional features | Location |
|---|---|---|---|
| 1 | lin–CD90 + CD39 + CD26–a | Expressing COL6A5, COL23A1 and HSPB3 | Papillary dermis |
| 2 | lin–CD90 + CD36+ | Including preadipocytes | Lower dermis |
| 3 | lin–CD90 + CD39–RGS5+ | Corresponding to pericytes | Located throughout the reticular dermis |
| 4 | lin–CD90 + CD39 + CD26+ | Unknown | |
| 5 | lin–CD90 + CD39–RGS5– | Unknown |
- a Lin–: CD31–CD45–E-cadherin–.
3 DERMAL FIBROBLASTS IN PATHOGEN RECOGNITION
A successful anti-infection response relies on accurate recognition of pathogens. Several PRRs are shown to be expressed on dermal fibroblasts, which detect pathogen-associated molecular patterns (PAMPs) of microorganisms or damage-associated molecular patterns (DAMPs), eliciting immune responses in the early stage of infection. A study has shown that human foreskin fibroblasts express Toll-like receptors (TLRs) 1–10.18 Retinoic acid-inducible gene I (RIG-I)-like receptor (RLR), inflammasome sensor absent in melanoma 2 (AIM2) and nucleotide-binding, oligomerisation domain 2 (NOD2) have been observed to be expressed on primary fibroblasts isolated from human skin.19-21
3.1 Dermal fibroblast recognition of virus
TLRs (TLR3,7,8,9), RLRs (including RIG-I and melanoma differentiation-associated gene 5 (MDA5)) and AIM2 are known to be involved in virus recognition. Those PRRs are all located in the cytoplasm.22 TLR3,7,8 and RLRs sense RNA viruses, while TLR9 and AIM2 contribute to DNA virus recognition.22, 23 As the common target for Chikungunya virus (CHIKV) and West Nile virus (WNV), human dermal fibroblasts have been observed to express elevated mRNA of TLR3,7,8,9, RIG-I and MDA5 after each virus infection in vitro. Upon CHIKV stimulation, enhanced AIM2 production and activation of the downstream inflammasome pathway contribute to infection control, as shown by inhibition of caspase-1, the central component of the inflammasome pathway, led to increased virus replication.20 RIG-I and TLR3 may act synergistically to detect dengue virus (DENV) in human primary dermal fibroblasts, as shown by increased receptor production when co-cultured with DENV in vitro.19 The infection of Zika virus (ZIKV), another virus inoculated by mosquitoes, has been shown to activate RIG-I and MDA5 in the cell line of human dermal fibroblasts by assay of co-immunoprecipitation. Both RIG-I and MDA5 combined with ZIKV NS1 protein.24 Stimulator of interferon genes (STING) has been observed to detect varicella-zoster virus (VZV) and mediates dermal fibroblast antiviral responses probably through interferon (IFN)-λ production.25 Besides, herpes viruses such as human cytomegalovirus (HCMV) and human herpesvirus 6 (HHV-6) can also be recognised by dermal fibroblasts and induce the expression of several pro-inflammatory and pro-fibrosis transcripts in primary human dermal fibroblasts.26 Integrins may act as a receptor for the herpes virus.27
3.2 Dermal fibroblast recognition of bacteria and fungi
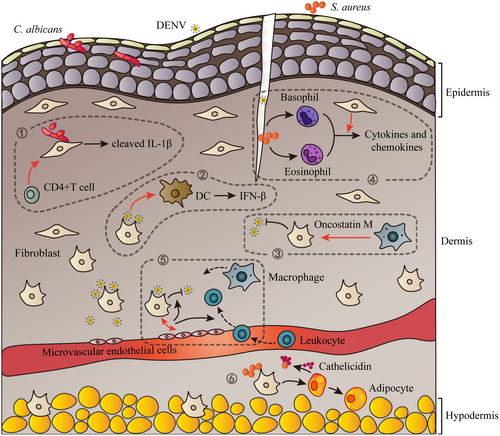
Although there is limited research on the interaction between dermal fibroblasts and bacteria or fungi, evidence has shown that dermal fibroblasts may play a pivotal role in bacteria recognition, infection control or inflammation regulation. As a transmembrane glycoprotein, TLR2 is known to recognise components from bacteria and fungi, such as lipoproteins (LPs), lipoteichoic acid (LTA), peptidoglycan (PGN), phospholipomannan and zymosan.28 It can form heterodimers with TLR1 or TLR6 on the cell membrane and act in concert with other TLRs or cytoplasmic PRRs such as NOD2 in the process of pathogen detection.28, 29 Several studies have observed that dermal fibroblasts recognise Staphylococcus aureus (S. aureus) via TLR2 and produce IL-6, tumour necrosis factor alpha (TNF-α), IL-8 and MMPs during the immune response.30-32 NOD2 may play a role in mediating the recognition of S. aureus as well.31 Furthermore, stimulation of lipopolysaccharide, an important component of gram-negative bacteria, induces primary dermal fibroblasts to secrete IL-1β, but not IL-18,33 which may rely on TLR4 (a transmembrane PRR) recognition.
Fungal infections are common in human skin, while most of them are superficial,34 which may partly explain why less studies were found between fungi and dermal fibroblasts. However, some species such as Candida albicans (C. albicans) can invade the deep dermis (containing fibroblasts) and cause severer diseases such as chronic cutaneous granulomas and even disseminated candidiasis.34, 35 TLR2 is also important in fungal recognition. A research of dermal fibroblasts in 3D tissue culture has shown that the antimicrobial function of dermal fibroblasts towards C. albicans is dependent on TLR2.11 In addition, Trichophyton benhamiae, a pathogenic dermatophyte, induces the transcription of TLR2 as well as the release of IL-1α, IL-6 and IL-8 in primary dermal fibroblasts.36
4 EFFECTOR MOLECULES PRODUCED BY DERMAL FIBROBLASTS
Following the detection of pathogens, dermal fibroblasts express multiple effector molecules. Many of them are cytokines and chemokines. In this process, several growth factors and MMPs are also produced by dermal fibroblasts, contributing to repair after tissue damage and skin wound. These effector molecules above enable the recruitment and activation of immune cells to the infection site, promoting infection control and wound healing.8, 24, 32
4.1 Cytokines and chemokines
It has been reported that IL-1α, IL-6, IL-1β, TNF-α and IFN-β cytokines are produced by dermal fibroblasts after microorganism recognition. Inflammatory cytokines exert the function of promoting anti-infection responses, as well as inducing further chemokine production.37 In dermal fibroblasts, IFN-β secretion seems to be mainly induced by viruses, as it is pivotal in antiviral immunity.19, 24, 38 IL-6 tends to be produced during bacterial or fungal infection, and IL-6 deficiency is reported to elicit severe bacterial infections in hosts.36, 39 In addition, cytokine production can be influenced by the planktonic or biofilm form of bacteria.32
Chemokines are produced by a wide range of cells, which regulates immune cell migration and positioning.37 Studies have shown that pathogen stimulation leads to the release of chemokine (C-X-C motif) ligand 8 (CXCL8), also known as IL-8, and transcriptional activation of C-C motif ligand (CCL) 3, CCL5, CXCL1, CXCL10 and CXCL11 in primary dermal fibroblasts.20, 32, 36 As chemoattractants for neutrophils, IL-8 and CXCL1 are mainly involved in the recruitment of neutrophils to the infection site.37, 40 CCL3 and CCL5 are related to leucocyte migration (such as macrophages and natural killer cells) and angiogenesis, particularly during viral infections.41 CXCL10 and CXCL11, also associated with viral infections, function in T cell responses.37 Those chemokines secreted by dermal fibroblasts unite skin resident cells with other myeloid immune cells, contributing to anti-infection responses in skin.
4.2 Antimicrobial peptides
Antimicrobial peptides are a group of cationic peptide antibiotics, widely inhibiting microorganisms such as bacteria, viruses and fungi. Human β-defensins (HBDs) and cathelicidins are two major families of antimicrobial peptides expressed in the skin.29, 42, 43 They exhibit direct microbial killing features through membrane disruption of pathogens and immunomodulatory properties.44 An in vitro study has shown that human α-defensin 5 (HD5) and HBD2 are released by cultured primary dermal fibroblasts after stimulation of DENV.19 HBDs also function in angiogenesis by promoting angiogenin production in dermal fibroblasts.45 Whether dermal fibroblasts express other antimicrobial peptides upon microbial challenge is less well understood. However, recent work has observed that a subcluster of dermal fibroblasts can proliferate and differentiate into adipocytes, releasing plenty of antimicrobial peptide cathelicidin in response to S. aureus dermal infection.46
4.3 Other molecules and related signalling pathways
In addition to cytokines, chemokines and antimicrobial peptides, dermal fibroblasts produce several functional molecules that participate in immune responses to infection. MMPs are a group of proteinases that play a crucial role in wound healing and tissue remodelling. MMPs regulate cellular environments through degrading components of ECM, contributing to cell migration, signalling transduction and cellular interaction.47 Studies have shown that several MMPs such as MMP1 and MMP3 are released or activated at the transcriptional level in dermal fibroblasts upon infection, which may accelerate the clearance of infection.30, 32, 48 Upon Cutibacterium acnes (C. acnes) stimulation, primary foreskin fibroblasts produce proMMP2 via TNF-α, which may be associated with the pathogenesis of acne.49 Furthermore, secretion of vascular endothelial growth factor (VEGF), transforming growth factor-β1 (TGFβ1) and heparin-bound epidermal growth factor (HB-EGF) induced by S. aureus in dermal fibroblasts are related to angiogenesis and wound healing.32, 50
In dermal fibroblast defence against viruses, the RLR-related signalling pathway is commonly activated. The downstream molecule IFN regulatory factor 3 (IRF-3) and IRF-7, together with nuclear factor-κB (NF-κB), activate the transcription of antiviral genes such as Type I IFN to inhibit virus infection.19, 24, 51, 52 Besides, AIM2 inflammasome and TLR3 signalling pathways also mediate antiviral immunity in dermal fibroblasts.19, 20 As a classic signalling, mitogen-activated protein kinase (MAPK) pathway is reported to be involved in innate immune responses against bacteria.53 During S. aureus infection, pathogen recognition and cytokine production in dermal fibroblasts is probably through TLR2-MAPK (Table 2).30, 31
| Pathogen | PRR | Cytokine, chemokine and other effector molecules | Related signalling | Cell origin and reference |
|---|---|---|---|---|
| Virus | ||||
| CHIKV and WNV | AIM2; RIG-I and MDA5 | IL-1β; TNF-α, IL-8, CCL3, CCL5, CXCL10 and CXCL11a | Caspase-1; NF-κB and IFN signalling | Neonatal skin20 |
| DENV | TLR3 and RIG-1 | IFN-β and TNF-α; HD5 and HBD2 | IRF3 | 19 |
| ZIKV | RIG-I and MDA5 | IFN-β | Cell line24 | |
| VZV | RIG-I and STING | IFN-β and IL-6 | IFN signalling | Cell line25, 51 |
| MCPyV | – | IL-6, IL-1β, TNF-α and IL-8a | cGAS-STING and NF-κB | Neonatal foreskin75 |
| HCMV | Integrins and TLR2 | TNF-α, IL-1β, IFN-β, CCL2, CCL11 a | NF-κB and IFN signalling | Cell line and adult skin26, 27, 76 |
| Spirochete | ||||
| B. burgdorferi ss | – | IL-6, IL-8, and CXCL1; MMP1, 3 and 12; SOD2 | NF-κB and IFN signalling | Cell line48, 77 |
| Bacterium | ||||
| S. aureus | TLR2 (and potentially NOD2) | IL-6, TNF-α and IL-8; VEGF, HB-EGF, TGF-β1; MMP1, 2, 3, 10 and 13 | MAPK | 30Cell line,31 and newborn foreskin32 |
| C. acnes | – | TNF-α and proMMP-2 | NF-κB | Foreskin49 |
| Lipopolysaccharideb | TLR4 | IL-1β | 33 | |
| Fungus | ||||
| T. benhamiae | TLR2 | IL-1α, IL-6 and IL-8 | Cell line36 | |
| C. albicans | TLR2 | IL-1βc | Caspase-1 | Immortalised cell line11 |
- Abbreviations: B. burgdorferi ss, Borrelia burgdorferi sensu stricto; C. acnes, Cutibacterium acnes; C. albicans, Candida albicans; cGAS, Cyclic GMP-AMP synthase; CHIKV, Chikungunya virus; DENV, dengue virus; HCMV, human cytomegalovirus; MCPyV, Merkel cell polyomavirus; NOD2, nucleotide-binding oligomerisation domain-containing protein 2; S. aureus, Staphylococcus aureus; SOD2, superoxide dismutase 2; T. benhamiae, Trichophyton benhamiae; VZV, varicella zoster virus; WNV, West Nile virus; ZIKV, Zika virus.
- a Transcriptional level.
- b Components of gram-negative bacteria.
- c In the presence of T cells.
5 ENHANCED ANTIMICROBIAL SIGNATURE OF DERMAL FIBROBLASTS DURING DIFFERENTIATION OR COOPERATING WITH IMMUNE CELLS
In the war against infection, dermal fibroblasts are not alone. Interactions with adjacent immune cells augment the antimicrobial function of dermal fibroblasts. In addition, the dermal fibroblast subpopulation with adipogenic properties in the lower and reticular dermis, as described above, can undergo a process named adipogenesis to inhibit bacterial infection.31, 46
5.1 The preadipocyte-adipocyte transition of dermal fibroblasts
Recent studies have illustrated enlarged dermal white adipose tissue (dWAT) during S. aureus dermal infection. A group of dermal fibroblasts highly expressing DLK1/preadipocyte factor 1 (PREF1), known as preadipocytes, differentiate into immature adipocytes upon S. aureus stimulation. This process is defined as reactive adipogenesis. During adipogenesis, immature adipocytes are able to secrete abundant cathelicidin and inhibit the growth of S. aureus.46 Moreover, perifollicular dermal preadipocytes are observed to produce cathelicidin in acne lesions, which is related to the pathogenesis of acne.54 TLR2 is the potential receptor that mediates the recognition of S. aureus and C. acnes in preadipocytes.54, 55
There are many factors that affect the adipogenic function of dermal fibroblasts. Studies on mouse and human skin have shown that dermal fibroblasts lose the capacity to become adipocytes and to produce cathelicidin during aging or in obesity, mediated by TGF-β.9, 55 Stabilisation of hyaluronan enable preadipocyte differentiation, shown by knockout of migration-inducing protein (Cemip), a gene related to hyaluronan degradation, leading to increase of adipogenesis and cathelicidin expression as well as smaller skin lesions in S. aureus-infected mouse skin.56 Moreover, application of retinoids can enhance cathelicidin expression during reactive adipogenesis in dermal infection.54, 57
5.2 Interactions with other cells during infection
Immune cells may amplify the anti-infection signature of dermal fibroblasts. A study on a 3D skin model composed of dermal fibroblasts and CD4+ T cells has shown activated dermal fibroblast antimicrobial function towards C. albicans.11 In the presence of T cells, dermal fibroblasts secret active cleaved IL-1β through caspase-1 activation, which inhibits invasion of C. albicans in 3D skin model.11 In addition, mediators produced by co-culture of DENV-infected dermal fibroblasts and microvascular endothelial cells greatly inhibit virus replication as well as induce leucocyte migration compared to dermal fibroblast monoculture.58 Oncostatin M, an IL-6 family member produced by immune cells including monocytes and macrophages, can stimulate RIG-I and MDA-5 expression as well as the antivirus responses in primary dermal fibroblasts.59 Keratinocytes, which are found in the outermost layer of the skin, also play an important role against pathogens.29 In a 3D-tissue infection model, human oral keratinocytes have been shown to protect gingival fibroblasts from the infection of S. aureus and Streptococcus oralis.60 Spatial sequencing on human leprosy skin lesions indicates the potential cell–cell interactions between keratinocytes and fibroblasts.61 The specific mechanisms remain to be studied further.
Furthermore, dermal fibroblasts could enhance the function of immune cells through direct cell–cell contacts or paracrine interactions. A study has shown that dermal fibroblasts significantly promote cytokine and chemokine secretion in basophils and eosinophils induced by NOD2 and TLR2 ligands through intercellular contact, which contribute to exacerbated inflammation in atopic dermatitis-like skin model in the presence of S. aureus.31 In DENV infection, conditioned media from infected dermal fibroblasts promote DC maturation and secretion of IFN-β by DCs, improving resistance to DENV infection.10 Together, cooperation between dermal fibroblasts and other cells is significant to anti-infection immunity in skin (Figure 1).
6 DERMAL FIBROBLASTS IN INFECTED WOUND HEALING
Skin infection is often accompanied by a wound, particularly infections in the dermis.62, 63 Wound healing is an important process in skin recovery from infection. In normal conditions, dermal fibroblasts migrate to the wound site and produce ECM (including collagen, fibrin and fibronectin), which provides a crucial micro-environment for cell migration, cell interaction and tissue remodelling.64, 65 Dermal fibroblasts can also differentiate into myofibroblasts in this process and contribute to wound contraction.62 However, pathogenic microbes such as S. aureus can impair the function of dermal fibroblasts on tissue repair, leading to delayed wound healing during infection.63, 66, 67 Therefore, besides the anti-pathogen responses mentioned above, fibroblast-mediated wound repair is also crucial to infection control.64 Methods through regulating wound healing properties of dermal fibroblasts or adding functional molecules produced by fibroblasts have been tried for the treatment of infected wounds. For example, a study has shown that a combination of fibroblast-derived ECM and antibacterial agent ciprofloxacin promotes bacteria-infected wound closure in mice.68 Platelet-derived growth factor (PDGF) which stimulates fibroblast growth and ECM production can be applied in wounds to accelerate wound closure.69 In addition, it has been reported that platelet-rich plasma (PRP) could enhance the migration and proliferation of dermal fibroblasts, as well as the production of Type I collagen, elastin and MMPs by dermal fibroblasts.70 PRP has been applied in clinical as a method for chronic wound treatment.70, 71 The composite medical coating of antimicrobial peptides and PRP is a promising treatment for infection-related wounds.72
7 CONCLUSIONS AND FUTURE PERSPECTIVES
Dermal fibroblasts participate in skin defence against pathogens through direct microbial recognition, immunological molecule production and immune regulation. It has been reported that expression of TLRs, and TLR1/2 ligand-activated transcriptional levels of IL-6 and IL-8 in dermal fibroblasts, is higher compared to keratinocytes, which shows the non-negligible function of dermal fibroblasts in pathogen detection.18 However, further research is required to better understand the role of dermal fibroblasts in antibacterial or antifungal immunity. Fibroblasts from other parts of the body that directly contact the external environment (such as cornea and gingiva) have significant protective functions as well.73, 74 Heterogeneity and plasticity of dermal fibroblasts give rise to their multiple immune functions in anti-infection immunity. To sum up, dermal fibroblasts display a pivotal role in skin defence against pathogens. Unravelling the contribution of dermal fibroblasts in skin immune responses against microorganisms will help to find out potential therapeutic targets for infectious diseases in the skin, especially for deep dermal infections.
AUTHOR CONTRIBUTIONS
JW and ML conceptualised the review idea. XC and ML supervised the work. JW involved in writing—original draft preparation. ZD and XC involved in writing—reviewing and editing. All authors have read and approved the final manuscript.
ACKNOWLEDGEMENTS
This work is supported by CAMS Innovation Fund for Medical Science (2017-I2M-1-017, 2021-I2M-1-059), National Natural Science Foundation of China (82173432, 82103749) and Nanjing Incubation Program for the National Clinical Research Center (2019060001).
CONFLICT OF INTEREST STATEMENT
The authors state no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




