A method for harvesting viable cells from wound dressings
Abstract
Wound fluid has been well studied for exploring protein biomarkers contained in it. However, cells in wound fluid have not received much attention due to the difficulty in their collection. Our study aimed to establish a method for collecting viable cells from discarded wound dressings. A protocol was designed to wash out nonadherent cells and detach adherent cells from silicone-faced foam wound dressings using trypsin–EDTA. The optimal concentration and incubation time of trypsin–EDTA for collecting equivalent proportions of different cell types to the original cell population were determined in vitro. Cell composition and gene expression changes in monocytes, lymphocytes, neutrophils, fibroblasts and keratinocytes were confirmed using immunocytochemistry and RNA-sequencing ex vivo. Full-thickness wounds were created on 9-week-old male C57BL/6J mice. Wound fluid was collected, and half of it was applied to the wound dressings. The original cell population in the wound fluid and the cell population collected from wound dressings were compared. In the in vitro study, 0.25% trypsin–EDTA and 2.5-min incubation time were considered optimal for collecting adherent cells from wound dressings. In the ex vivo study, among all cell types, only CD3+ lymphocytes showed a significantly higher cell proportion in the collected group. The relative gene expression of the five selected cells showed no significant changes (p-value >0.05, |log2 fold change| < 1.5, differential gene expression analysis). Viable nonadherent and adherent cells were collected from wound dressings without altering gene expression and could be used in future studies for cellular analysis of wound fluid.
Abbreviations
-
- CO2
-
- carbon dioxide
-
- DGE
-
- differential gene expression
-
- FBS
-
- foetal bovine serum
-
- FRSK
-
- foetal rat skin keratinocyte
-
- PBS
-
- phosphate-buffered saline
-
- Pen/Strep
-
- Penicillin–Streptomycin solution
-
- PWD
-
- postwounding day
-
- Rat-1
-
- rat fibroblast-like-1
-
- RNA-seq
-
- RNA-sequencing
-
- SD
-
- standard deviation
1 INTRODUCTION
Wound healing is a dynamic and complex biological process that involves a large number of cell types, including immune cells, fibroblasts, keratinocytes and endothelial cells, as well as growth factors and enzymes.1, 2 The interactions between wound bed cells and their microenvironment are dynamic and continuously change in response to cues within the wound fluid, such as those generated by capillary leakage and the activities of different types of local and migratory cells thought to reflect the wound condition.3 Wound fluid contains rich protein content, immune cells and a small number of other cells, such as keratinocytes and fibroblasts.4-6 The cellular activities in wound fluid vary during different phases of wound repair as a response to cell activities in wound tissue.7 Therefore, to understand the dynamic wound healing microenvironment, evaluating cell function in wound fluid could be an alternative, noninvasive method to biopsy samples.7 RNA-sequencing technology (RNA-seq) is a state-of-the-art technique for obtaining whole transcriptome information to evaluate cell function. However, bulk RNA-seq can only show the average gene expression level of pooled cell populations,8 whereas cell activities and status are heterogeneous. Therefore, to obtain more precise information, gene expression at the single-cell level using single-cell RNA-seq is desirable.9 However, wound fluid sampling can be challenging because of the requirement for high-quality viable cells.
Wound fluid sampling methods vary among studies for different purposes.10 Because the sampling technology can influence the harvested material components, such as cytokine levels,11 the suitability of the sampling methods should be carefully considered. The use of occlusive dressings to harvest accumulated fluid is the most common method for wound fluid collection.5 The transparent film dressings such as Tegaderm™ film dressing (3 M Health Care, St. Paul, MN) and Opsite dressing (Smith & Nephew, Hull, UK) are most frequently used, with a collection time period ranging from minutes to multiple days.12-15 The sampling methods are not part of the wound treatments; furthermore, the accumulated wound fluid can delay wound healing by increasing IL-1α, IL-1β and IL-8 levels.16 Another frequently used method is to use swabs to roll over the wound surface.11, 17, 18 Swabs obtain fresh wound fluid, which is used for protein and microbiota analyses. It can preserve the original fluid composition; however, the fresh wound fluid is not the same as the accumulated wound fluid composition that keeps the wound bed moisturised as a previous study showed the cytokine level (MMP9) was altered during the wound fluid accumulation.19 Another common method is an extraction from absorbent materials that are placed on the wound such as gauze, foams, polyvinyl alcohol sponges and Whatman filter paper.5 The aforementioned methods are considered suitable for use in protein analysis despite the potential effects on the wound healing process. Contrastingly, wound fluid extracted from discarded wound dressings can provide information about the real-time wound bed environment without interfering with routine treatment and can avoid the extra burden caused by sampling procedures.6
Our research group previously attempted to extract wound fluid for gene expression analysis; however, whether the extraction process isolated viable cells and whether the quality is suitable for RNA-seq remains unknown.20 A study by Fuentes et al. successfully isolated viable nonadherent cells (immune cells) from discarded dressings but found it difficult to isolate adherent cells, such as keratinocytes and fibroblasts, from the dressings.6 As wound fluid contains both adherent and nonadherent cells, whether the collection methods change gene expression remains unknown. To adapt the sampling method for future applications, a collection method that can collect both nonadherent and adherent cells without changing gene expression is needed.
The present study aimed to establish a protocol to isolate viable cells from wound dressings without altering gene expression. We modified the protocol used in our lab for extracting wound fluid from gauze used for gene expression analysis20 and further investigated whether the established protocol would change the cell composition of the wound fluid and gene expression.
2 MATERIALS AND METHODS
2.1 Animal
All mice were purchased from The Jackson Laboratory Japan (Kanagawa, Japan) and kept in the university animal building under specific pathogen-free conditions with a temperature of 23 ± 2°C, humidity of 45 ± 10%, 12:12-h light–dark cycle and ad libitum feeding and drinking.
2.2 Ethics
The experimental protocols for this study were approved by the Animal Research Committee of The University of Tokyo and were conducted in accordance with the national and international guidelines for animal welfare, including the Guide for the Care and Use of Laboratory Animals issued by the National Institutes of Health.
2.3 In vitro study: Establishment of the cell collection protocol
To establish a cell collection protocol for both nonadherent and adherent cells, we tested both cell types in vitro. For nonadherent cells, immune cells were used, while adherent cells were represented by fibroblasts and keratinocytes, as these are the predominant cell types found in wound fluid.
2.3.1 Nonadherent cell preparation
Leukocytes collected from mice whole blood were used as nonadherent cells. For the preparation, a whole blood sample (1.8 mL) was drawn from five mice (C57BL/6J, 10-week-old) by cardiac puncture under anaesthesia. Then, the whole blood was mixed with 10% 0.5 M EDTA. The mixed blood sample was carefully layered over the same amount (1.98 mL) of Polymorphprep® Density Gradient Media (Serumwerk Bernburg AG®) in a 15 mL tube and centrifuged at 470 × g for 35 min in a swing-out rotor at room temperature. The mononuclear and polymorphonuclear cells were collected following the manufacturer's instructions. Collected cell pellets were then resuspended in 1 mL RPMI-1640 medium with L-glutamine (FUJIFILM Wako Pure Chemical Corporation), supplemented with 10% (v/v) heat-inactivated foetal bovine serum (FBS) (Hyclone Laboratories Inc.) and 5% (v/v) penicillin–streptomycin solution (Pen/Strep) (Nacalai Tesque Inc.). Cells were stained with trypan blue and noncoloured viable cells were counted manually using a disposable haemocytometer (C-Chip, NanoEntek) under a microscope (Leica DMI4000B, Leica Microsystems). Then, 1 mL of the cell suspension was applied to 2.5 cm × 2.5 cm silicone-faced foam dressings for the confirmation of nonadherent cell collection (FoamLite™, ConvaTec Japan Co. Ltd.).
2.3.2 Adherent cell preparation
Cultured foetal rat skin keratinocyte (FRSK) and rat fibroblast-like (Rat-1) cell lines were used to confirm the adherent cell collection method. FRSK cells were purchased from the Japanese Collection of Research Resources Cell Bank (Osaka, Japan), and the Rat-1 cell line was purchased from the RIKEN BioResource Research Center (Ibaraki, Japan). They were cultured in DMEM (FUJIFILM Wako Pure Chemical Corporation) with high glucose supplemented with 10% (v/v) heat-inactivated FBS and 5% (v/v) Pen/Strep solution until the achievement of confluence. Confluent cells (1 mL) were harvested using 2.5 g/L-Trypsin/1 mmoL/L-EDTA Solution (Nacalai Tesque Inc.). Cell suspension (1 mL) was applied to wound dressings (2.5 cm × 2.5 cm FoamLite™ dressings).
2.3.3 Cell collection
The cell collection protocol contains four steps including adhering cells to the wound dressings, washing out the nonadherent type of cells such as immune cells, detaching adherent type cells using trypsin–EDTA and gathering all cells together. Trypsin–EDTA was used to isolate adherent cells because it is commonly used for the dissociation of adherent cells, cell aggregates and tissues into single-cell suspensions. The detailed cell collection steps were performed as follows: (1) Wound dressings with applied cell suspension were incubated for 2 h at 37°C with 5% carbon dioxide (CO2) for cells to adhere to the dressings based on our preliminary experiments. (2) The dressings were cut into small pieces and washed with 10 mL phosphate-buffered saline (PBS). (3) Wound dressing flakes were placed on top of eight pipette tips in a 50 mL centrifuge tube and centrifuged for 5 min at 300 × g. Eight 20–200 μL pipette tips were placed in the centrifuge tube to support the wound dressing during the centrifugation. (4) After centrifugation, the dressings were placed into a new tube and trypsin–EDTA (amount depending on the wound dressing size) was added to fully cover the wound dressings to detach cells from wound dressings. (5) Trypsin was inactivated using DMEM supplemented with 10% FBS and the tubes were centrifuged again. (6) All cell suspensions were pooled together and filtered using a pluriStrainer® 100 μm cell strainer (pluriSelect Life Science UG & Co. KG). (7) The cell suspension was then centrifuged again to collect cell pellets, which were resuspended in 1 mL PBS for cell counting. Cells were stained with trypan blue and noncoloured viable cells were counted using a disposable haemocytometer. The cell collection rate was calculated as collected cells dividing by the number of cells applied (Figure 1).
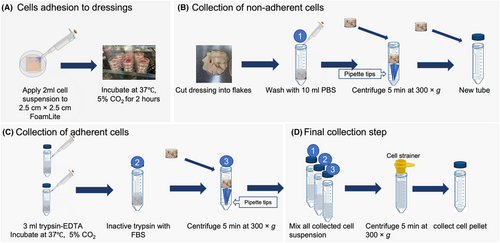
2.3.4 Confirmation of optimal trypsin–EDTA concentration and incubation time for adherent cell collection
To collect a similar amount of the different types of cells from wound dressings, different incubation times (1.25, 2.5 and 5 min) using 0.25% trypsin–EDTA were first analysed to confirm the optimal incubation time. Different concentrations of trypsin–EDTA (0.125%, 0.25% and 0.5%) with the optimal incubation time were tested, and the collected cell numbers were compared. Three millilitre of trypsin–EDTA was used to fully cover the wound dressings. The optimal time and trypsin concentration were determined by collecting an equivalent proportion of keratinocytes and fibroblasts.
2.4 Ex vivo study: Determination of the cell composition and gene expression change caused by the cell collection method
In the ex vivo study, we hypothesised that the established collection protocol would not change cell composition or gene expression. To confirm these hypotheses, we investigated the changes in the cell composition of wound fluid extracted from wound dressings, as well as the changes in gene expression caused by cell extraction. The wound fluid from the animal wound model was collected, half of which was applied to the wound dressing and then collected using the protocol that was determined in the in vitro study. The optimal trypsin–EDTA concentration and time were determined as 0.25% and 2.5 min, respectively. Detailed results are presented in the Results section. The cells in the original wound fluid and those collected from the wound dressings were compared.
2.4.1 Wound model for wound fluid collection
Twelve healthy 9-week-old male C57BL/6J mice were used as full-thickness wound models. After 7–14 days of acclimatisation, the hair on the left side of the abdomen were shaved and further removed with a hair removal cream. A 1.5 cm diameter, full-thickness wound was created on the left side of the abdomen using sterilised scissors. The centre of the wound was located on the lateral midline between the middle of the greater trochanter and the axilla. FoamLite was used to cover the wounds. On the first postwounding day (PWD 1), after rinsing the wounds with saline, the FoamLite was replaced with an extra thin hydrocolloid dressing (DuoActive ET, ConvaTec Japan Co. Ltd.) and covered with film dressing (Tegaderm™, 3 M) film for 2 days (PWD 2 and PWD 3) to accumulate wound fluid without absorbing wound fluid. Wound fluid on PWD 4 was collected with 18G syringes (Terumo Co. Ltd.) from underneath hydrocolloid dressings and diluted with 5 mL of preheated 37°C RPMI-1640 medium with L-glutamine and 1% (v/v) Pen/Strep. Six mice with wound fluid over 100 μL were used for cell collection and further analyses. Wound tissue was sampled and immersed in 10% formalin (FUJIFILM Wako Pure Chemical Corporation) for 24 h and then used for histological analysis (Haematoxylin and eosin (H&E) staining) to confirm the healing phase. The mice were sacrificed using CO2 gas (Figure 2).
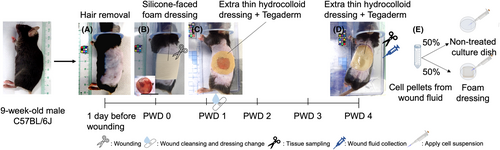
2.4.2 Experimental set-up
After the sample was brought back to the laboratory, the wound fluid in RPMI-1640 media was centrifuged for 5 min at 300 × g and the supernatant was removed with an aspirator. The cell pellet was resuspended in 2 mL RPMI-1640 medium with L-glutamine, 10% FBS and 5% (v/v) Pen/Strep. The cell number was counted using a haemocytometer as the applied cell number. After carefully and thoroughly pipetting the cell suspension, half (1 mL) of the suspension was applied to a 35-mm nontreated cell-culture dish (IWAKI Co. Ltd.) directly, and the other half (1 mL) was applied to 2.5 cm × 2.5 cm FoamLite dressings that were placed in a 35-mm nontreated cell-culture dish (Figure 2E). They were then incubated for 2 h at 37°C with 5% CO2 for cells to adhere to the dressings (2 h for cells to adhere was based on our previous pretests results). The cells were then collected from the culture dishes and wound dressings and compared. The cells that were directly applied to the culture dish were collected by rinsing them with PBS. The collected cells represented the original cell population from the wound fluid and were used as control and were defined as the original cell population through the current study. Meanwhile, the cells applied to FoamLite dressing were collected using the protocol established in the in vitro study (Figure 1B–D; 3 mL 0.25% trypsin–EDTA incubated for 2.5 min in step C) and were considered the experimental group, and were referred to as the collected cell population in the current study. Cells were isolated, and 10 μℓ of cell suspension was used for cell counting. Cells were stained with trypan blue and the number of noncoloured viable cells was counted manually using hemocytometers under a microscope. Hoechst/ Fluorescein Diacetate (FDA) staining (Invitrogen™, Gaithersburg, MD) was conducted as additional information to confirm cell viability. The remaining cells were immediately processed for protein and RNA analyses.
2.4.3 Immunocytochemistry
To determine differences in cell composition between the two groups, immunocytochemistry was performed. Cell suspension (30 μL) was applied to water-repellent APS-coated adhesive microscope slides with 106-mm diameter wells (Matsunami Glass Ind., Ltd.). The sides were incubated for 1 h at room temperature in a moist chamber and then washed with PBS for 1 min. The cells were then fixed with a 4% paraformaldehyde solution in PBS for 20 min. After fixation, the cells were carefully washed three times with PBS (1 min per wash). To block nonspecific antibody binding, the cells were incubated with 1% bovine serum albumin/PBS for 30 min at room temperature in a moist chamber. They were then incubated with primary antibodies, including anti-CD14 antibody (1:350; Rabbit, monoclonal, # ab221678, Abcam PLC) for monocytes, anti-CD3 antibody (1:20; Rat, monoclonal, # ab135372, Abcam PLC) for lymphocytes, anti-Gr-1/Ly-6G antibody (1:25; Rat, monoclonal, #MAB1037-100, R&D Systems, Inc.) for neutrophils, anti-Loricrin antibody (1:80; Rabbit, polyclonal, #ab85679, Abcam PLC) for keratinocytes and anti-S100A4 antibody (1:25; Rabbit, monoclonal, #ab197896, Abcam PLC) for fibroblasts, at 4°C overnight. After removing the primary antibodies, the cells were washed three times with PBS (10 min each). The secondary antibodies Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) (1:1000; Abcam) and Alexa Fluor® 488-AffiniPure Donkey Anti-Rat IgG (H + L) (1:1000; Jackson ImmunoResearch Laboratories, Inc.) were applied to the cells and incubated for 1 h at room temperature. The cells were then washed three times with PBS for 10 min. Finally, the cells were mounted using VECTASHIELD HardSet Mounting Medium containing DAPI (Vector Laboratories). Slides were observed under a fluorescence microscope (BZ-X810; Keyence Corporation). For each sample, three sites were randomly selected and photographed, and the number of positive cells was counted using a semi-automatic quantitative protocol in the QuPath software (version 0.3.2).21 The proportion of positive cells was calculated by dividing the number of cell numbers in the same image, and the mean proportion of the three images was used.
2.4.4 Long read RNA-sequencing
The remaining cells after immunocytochemistry were used for the gene expression analysis. Total RNA was extracted from cells using the RNeasy® Plus Mini Kit (Qiagen N.V). The purity and concentration of the RNA were assessed. Total RNA integrity was evaluated by capillary electrophoresis using Qsep1 (BiOptic Inc.), and the RNA quality number (RQN) score for RNA integrity (ranging from 0 to 10, with 10 indicating the highest integrity) was estimated. Samples with RQN scores >7 were used for RNA-seq analysis. PolyA+ RNA was enriched from total RNA using the Poly(A) RNA Selection Kit V1.5 (Lexogen GmbH). Approximately 100 ng of polyA+ RNA was used for cDNA library preparation using the Direct cDNA Sequencing Kit (SQK-DCS109; Oxford Nanopore Technologies), following the manufacturer's protocol. Primers were obtained using the Direct cDNA Sequencing Kit, and the other reagents used included the RNase Cocktail™ Enzyme Mix (Invitrogen, Gaithersburg, MD) for RNA digestion after first-strand synthesis, Maxima H Minus Reverse Transcriptase (Thermo Fisher Scientific Inc.), LongAmp Taq 2X Master Mix (New England Biolabs), NEBNext Ultra II End Repair/dA-Tailing Module (New England Biolabs) and AMPure XP magnetic beads (Beckman Coulter Inc.). cDNA was then ligated to direct cDNA sequencing adaptors (SQK-DCS109, Oxford Nanopore Technologies) using the Blunt/TA Ligase Master Mix (New England Biolabs). Final cDNA libraries were loaded into MinION flow cells (FLO-MIN106D R9.4.1, Oxford Nanopore Technologies) and sequencing was performed on MinION Mk1C sequencing device (Oxford Nanopore Technologies) for 36 h. FAST5 data were based on RNA-seq, demultiplexed, filtered for quality (QC >8), the minimum length was set to 20 bp and concatenated into individual FASTQ files.
2.5 Statistical analysis
2.5.1 In vitro study
Data are presented as mean and standard deviation (SD). The differences between groups were analysed using analysis of variance followed by the Dunnett's test, while there was a significant difference between groups. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using R 4.1.1 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
2.5.2 Ex vivo study
Cell numbers in the original cell population and the collected cell population were presented as mean and SD and compared using the Mann–Whitney U test. The positive cell proportions of the five selected cells detected using immunocytochemistry were also compared between two groups using the Mann–Whitney U test. A p-value <0.05 was considered statistically significant. Differential gene expression (DGE) analysis was performed to compare gene expression differences between the cells isolated using the established protocol and the original cell population. DGE analysis was conducted using nanoporetech pipeline-transriptome-de (https://github.com/nanoporetech/pipeline-transcriptome-de/).22 Mus musculus GRCm39.cDNA and Mus musculus.GRCm39.105 version annotations were used as references. Thresholds for calling DEGs were p-value <0.05 and log2 fold change <−1.5 or >1.5. The top 10 most often expressed genes in neutrophils, T cells, monocytes, keratinocytes and fibroblasts were selected using the ubiquitousness index provided by PanglaoDB.23 Gene expression differences between the original cells and collected cells in relation to the top 10 most often expressed genes were confirmed.
3 RESULTS
3.1 In vitro study: Establishment of the cell collection protocol
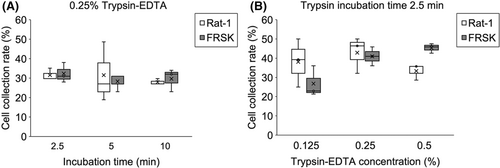
For nonadherent cell collection, 1.15 × 106 leukocytes were isolated from whole blood and applied to FoamLite (N = 5); on average, 7.3 × 105 cells (63.5 ± 4.6% of the applied cells) were successfully collected from the FoamLite. Rat-1 and FRSK were tested separately for adherent cells. Trypsin–EDTA incubation time was confirmed at a concentration of 0.25%, and the collection rate did not show a significant increase after 2.5 min in either the Rat-1 or FRSK groups (Figure 3A). Trypsin incubation time of 2.5 min was used to test different concentrations of trypsin, and Rat-1 did not show any increase in collection rate with a concentration higher than 0.25%. By contrast, the collection rate of FRSK increased with increasing trypsin concentration. However, the collection rates of Rat-1 and FRSK cells were most equal when 0.25% trypsin was used, and thus, this concentration was chosen for the cell collection protocol (Figure 3B).
3.2 Ex vivo study: Determination of the cell composition and gene expression change caused by cell collection
Wound fluid was collected from six wounds with more than 100 μL of wound fluid under the wound dressings on PWD 4. The wound site showed signs of oedema (Figure S1A,B), and many inflammatory cells were found in the upper area of the H&E-stained wound section (Figure S1C–E) showing the wound was in the inflammatory phase of the healing process. On average, 4.9 × 106 (N = 6, ranging from 0.72–8.60 × 106) cells were collected from the wound fluid from 6 mice with sufficient wound fluid and used in the following experiments.
3.2.1 Cell composition: Immunocytochemistry
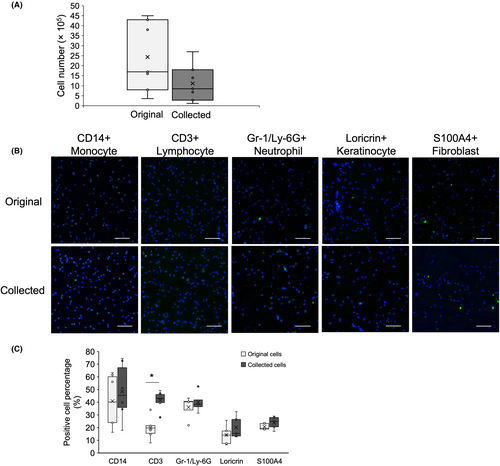
On average, in total 2.4 × 106 cells were collected from the original cell group and 1.1 × 106 cells were collected from the foam wound dressings, and there were no significant differences between two groups in cell number (Mann–Whitney U test, p > 0.05; Figure 4A). Hoechst/FDA staining images for collected cell viability can be found in Figure S2. Collected cells were suspended in 1 mL PBS, and 100 μL of the suspension was then applied to glass slides for immunocytochemistry to calculate the proportion of the five representative cell types. Representative immunocytochemistry images of the original and collected cell populations are shown in Figure 4B. Positive cell percentages of anti-CD14, anti-CD3, anti-Gr-1/Ly-6G, anti-Loricrin and anti-S100A4 were compared between the original and collected cell populations. CD3-positive lymphocytes comprised a significantly higher percentage of the collected cell population (p < 0.05) (Figure 4C).
3.2.2 Gene expression: RNA-sequencing
Fold changes in the relative gene expression of the top 10 most expressed genes in monocytes, T cells, neutrophils, keratinocytes and fibroblasts based on the ubiquitousness index are shown in Table 1 and Figure S3A. Thy1 was upregulated 1.97-fold in the collected cell populations. Figure S3B shows the combination of log2 fold changes and the statistical significance of each gene, and none of the selected genes were significantly upregulated or downregulated.
| Cell type | Gene name | Log2FC | logCPM | p-Value | FDR |
|---|---|---|---|---|---|
| Neutrophils | Cd14 | 0.10 | 10.57 | 0.91 | 1.00 |
| Mylk | 0.60 | 3.50 | 0.78 | 0.98 | |
| Hp | −0.35 | 9.40 | 0.68 | 0.98 | |
| Lcn2 | −0.60 | 13.06 | 0.58 | 0.94 | |
| Ccl6 | 0.71 | 9.31 | 0.41 | 0.87 | |
| Cxcl2 | 0.24 | 13.05 | 0.82 | 0.99 | |
| Ncf1 | −0.91 | 8.81 | 0.28 | 0.87 | |
| Slc1a5 | 0.98 | 4.05 | 0.53 | 0.92 | |
| Ccl9 | 0.99 | 8.49 | 0.23 | 0.87 | |
| Ly6g | −1.10 | 5.45 | 0.20 | 0.87 | |
| T cells | Cd81 | 0.31 | 3.74 | 0.87 | 1.00 |
| Junb | 0.39 | 9.66 | 0.65 | 0.97 | |
| Cd52 | −0.54 | 9.70 | 0.54 | 0.92 | |
| H2-Q7 | 0.14 | 5.59 | 0.87 | 0.99 | |
| Thy1 | 1.97 | 5.11 | 0.05 | 0.87 | |
| Ccl4 | −0.01 | 11.06 | 0.99 | 1.00 | |
| Trbc2 | −0.90 | 3.43 | 0.68 | 0.97 | |
| Ltb | −0.98 | 7.38 | 0.22 | 0.87 | |
| Crem | −0.33 | 4.68 | 0.76 | 0.98 | |
| Cd3d | 0.56 | 3.20 | 0.83 | 0.99 | |
| Monocytes | Psap | 0.56 | 11.92 | 0.57 | 0.93 |
| Ifitm3 | −0.59 | 8.86 | 0.48 | 0.89 | |
| Rhoc | 0.47 | 5.52 | 0.58 | 0.94 | |
| Cebpb | −0.30 | 10.43 | 0.74 | 0.98 | |
| Rgs2 | 0.50 | 6.61 | 0.53 | 0.91 | |
| Cd44 | −0.46 | 10.19 | 0.60 | 0.95 | |
| Zfp36l2 | 1.27 | 7.83 | 0.12 | 0.87 | |
| Spi1 | −0.74 | 9.14 | 0.38 | 0.87 | |
| Cd48 | −0.43 | 6.26 | 0.59 | 0.94 | |
| Cd86 | 0.01 | 5.93 | 0.99 | 1.00 | |
| Keratinocytes | Plec | −0.15 | 7.31 | 0.85 | 0.99 |
| Sfn | −0.56 | 6.23 | 0.49 | 0.90 | |
| Lcn2 | −0.60 | 13.06 | 0.58 | 0.94 | |
| Ccl2 | −0.63 | 7.27 | 0.43 | 0.87 | |
| Dmkn | 0.37 | 4.07 | 0.81 | 0.99 | |
| Cxcl1 | 0.22 | 8.01 | 0.78 | 0.98 | |
| Rel | −0.28 | 7.63 | 0.73 | 0.98 | |
| Aqp3 | −0.01 | 3.36 | 0.99 | 1.00 | |
| Sirt7 | −0.35 | 5.50 | 0.69 | 0.98 | |
| Tfap2a | 1.17 | 3.96 | 0.51 | 0.91 | |
| Fibroblasts | Vim | 0.18 | 10.67 | 0.84 | 0.99 |
| Tcf4 | 0.37 | 5.01 | 0.70 | 0.98 | |
| Klf6 | 0.03 | 10.97 | 0.97 | 1.00 | |
| Mdk | 1.04 | 3.64 | 0.62 | 0.95 | |
| Gsn | 0.31 | 8.16 | 0.70 | 0.98 | |
| Egr1 | 1.41 | 8.22 | 0.09 | 0.87 | |
| Pbx1 | 0.53 | 4.74 | 0.61 | 0.95 | |
| Klf2 | 0.39 | 8.06 | 0.63 | 0.96 | |
| S100a4 | 1.09 | 8.70 | 0.19 | 0.87 | |
| Fn1 | 0.77 | 9.23 | 0.36 | 0.87 |
- Note: edgeR analysis was conducted, log2 fold change (FC) between the original cell group and collected cells, the log-scaled counts per million measure (CPM) of abundance and the false discovery corrected p-value (FDR) were shown in this table.
4 DISCUSSION
The current study established and validated a protocol that successfully collected viable cells suitable for RNA-seq from discarded wound dressings without significantly altering gene expression ex vivo.
Wound fluid has been widely used for protein and microbiome analyses as a valuable tool for assessing the wound microenvironment.3, 5, 24 However, cells in the wound fluid have not drawn much attention to our knowledge. The current study established a novel method to collect viable cells from wound fluid, which provides a new possibility for noninvasive wound assessment. We modified the protocol from our previous study and added trypsin–EDTA, a commonly used enzyme for detaching adherent cells, to our protocol.20 We successfully isolated both nonadherent and adherent cells by adding an optimised concentration of trypsin. A concentration of 0.25% trypsin is commonly used for adherent cell passage and was confirmed to be most effective in isolating similar percentages of fibroblasts and keratinocytes with the used cell lines. Surprisingly, longer incubation time did not increase the collection rate of adherent cells from foam dressings. This may be attributed to the fact that the adhesive strength of cells varies depending on the surface material to which they adhere.25
The cell composition and gene expression changes in five types of cells (monocytes, lymphocytes, neutrophils, fibroblasts and keratinocytes) were determined through animal experiments. CD3+ lymphocytes were found to have a higher proportion in the cells that have been collected from wound dressings, indicating that the protocol can collect more nonadherent cells, such as immune cells, than adherent cells. The wound fluid was collected on PWD 4, during the inflammatory phase of wound healing, which is characterised by an increased number of T lymphocytes at the wound site as part of the immune response.26 As expected, a larger number of immune cells were collected in comparison with adherent cells. However, the relative gene expression levels of the five selected cell types were not significantly altered by the collection protocol. This result is consistent with a study that demonstrated that the use of trypsin does not change human skin cell gene and protein expression in vitro.27 Although the cell proportion can be affected by the cell collection method, depending on the original wound fluid content, the method can still provide rich and accurate information about cells in wound fluid and be used for gene expression analyses.
In the current study, although we only analysed five types of cells, a large proportion of fibroblasts and keratinocytes was confirmed in the wound fluid. In a limited number of studies that investigated cells in wound fluid, no studies have determined the proportion of fibroblasts and keratinocytes.4, 6 In addition to their role in immune defence, immune cells also produce growth factors and cytokines that affect the wound healing process.4, 5 Although the role of immune cells in wound fluid has been studied, the function of fibroblasts and keratinocytes in wound fluid has not yet been studied. With the established protocol, the function of adherent cells in wound fluid can be further investigated in the future.
The current study has a few limitations. First, only five representative cell types were investigated. However, they are the most common representative nonadherent and adherent cells in the wound healing process, and we confirmed the possibility of collecting both types of cells at a high level of consistency with the original cell population. Second, this study was conducted in vitro and ex vivo in a well-controlled environment with clean wounds. More complicated microenvironments, such as infected human wounds, wounds covered with necrotic tissue, long-time wound attachment or treatment with various wound ointments could potentially affect cell collection rate and cell viability in wound dressings and require further investigation in the future. Finally, due to the purpose of the study being to examine the effect of established collection methods on cells, the ex vivo study was conducted. Therefore, it is necessary to further validate the percentage of viable cells that can be collected directly from wound dressings attached to wounds in vivo, including human samples.
5 CONCLUSION
We established a new method for cell collection from wound dressings and successfully collected viable cells from them. The established cell collection method can be used for gene expression and cellular analyses with greater potential. Especially the possibility of harvesting viable cells enables high-resolution gene expression analysis by scRNA-sequencing. Additionally, the inclusion of this cellular analysis method, along with other wound fluid collection techniques for protein and microbiome analyses, is expected to provide further insight into the wound microenvironment in the future.
AUTHOR CONTRIBUTIONS
Q.Q. designed the study and performed the experiments, sample analyses, data analyses and manuscript writing. G.N., D.H., M.K., T.M. and H.S. were involved in designing and supervising the study. G.N., D.H., C.T. and S.T. were involved in sample analyses and interpreting the results. G.N. supervised the manuscript writing. H.S., T.M., M.K., D.H., C.T. and S.T. reviewed and revised the manuscript.
ACKNOWLEDGEMENTS
This study was funded by Fusion Oriented REsearch for disruptive Science and Technology (FOREST) from the Japan Science and Technology Agency (Grant No. JPMJFR205H).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




