Skin maturation from birth to 10 years of age: Structure, function, composition and microbiome
Georgios N. Stamatas and Pierre-Francois Roux are cofirst authors.
Abstract
Infant and adult skin physiology differ in many ways; however, limited data exist for older children. To further investigate the maturation processes of healthy skin during childhood. Skin parameters were recorded in 80 participants of four age groups: babies (0–2 years), young children (3–6 years), older children (7–<10 years) and adults (25–40 years). Overall, skin barrier function continues to mature, reaching adult levels of transepidermal water loss (TEWL), lipid compactness, stratum corneum (SC) thickness and corneocyte size by the age of about 6 years. Higher levels of lactic acid and lower levels of total amino acids in the SC of babies and young children further indicate higher cell turnover rates. In all age groups, TEWL and skin surface hydration values remain higher on the face compared with the arm. Skin becomes darker and contains higher levels of melanin with increasing age. The composition of skin microbiome of the dorsal forearm in all children groups is distinct from that in adults, with Firmicutes predominating in the former and Proteobacteria in the latter. Skin physiology, along with the skin microbiome, continues to mature during early childhood in a site-specific manner.
1 INTRODUCTION
The infant skin barrier is competent at birth, but several skin properties continue to mature in the first years of life. Numerous investigations over the years have determined that infant skin is different from that of adults in several ways, including in structure, composition and function (Table 1). The stratum corneum (SC) is about 20%–30% thinner in babies (aged 3–24 months) compared with adults1, 2 and corneocytes are smaller in babies compared with adults.2 Studies in babies (aged 3–12 months) and in young children (aged 3–49 months) showed that transepidermal water loss (TEWL) and skin conductance are higher in babies and children and decrease with age, indicating differential water holding and transport properties of the SC.1, 3-8 These data were confirmed in different ethnic groups and in different geographical locations.1, 4, 9, 10
| Parameter | Babies 0–24 months | Children 25–48 months | ||
|---|---|---|---|---|
| Structure | Surface | Microrelief grooves | Denser than in adults2 | |
| Intrarelief structures | More rounded and plump than in adults2 | |||
| Thickness | Stratum corneum | Thinner than in adults2, 7, 18 | Thinner than in adults7, 18 | |
| Epidermis | Thinner than in adults1, 2, 18 | |||
| Cell size | Corneocytes | Smaller than in adults2 | ||
| Granular cells | Smaller than in adults2 | |||
| Composition | Water content | Water holding (skin conductance, Raman confocal spectroscopy) | Highest compared with children and adults1, 4, 5, 10 | Lower than in infants but higher than in adults4, 10 |
| Natural moisturizing factor | Significantly lower than in adults5 | |||
| Surface lipids | Less total and sebaceous lipids than adults37 | |||
| Melanin | Facultative pigmentation | Indistinguishable from constitutive17 | ||
| Erythema | Cross-polarized imaging | Less prevalent trend than in adults4 | ||
| Function | Water handling properties | Absorption/desorption | Faster absorption/desorption than adults5 | |
| Barrier function | TEWL | Highest TEWL compared with children and adults1, 4, 5, 7, 9, 10 | TEWL lower than in infants but higher than in adults4, 10 | |
| pH | More alkaline than in adults3, 8 | |||
| Epidermal cell proliferation | Higher rate than in adults2 | |||
| Microbiome | Relative abundance (evenness) | Lower but increases with age (over the first year of life)10 | ||
| Predominant phyla | Firmicutes3, 10 | |||
| Predominant genera | Staphylococci; Streptococci10 | |||
- Abbreviation: TEWL, transepidermal water loss.
- Note: Data are from the forearm unless otherwise indicated.
In addition, a few studies have characterized and compared the skin microbiome in infants and adults.11-13 Like the microbiome in other organs such as the gut, accumulating evidence indicates that the commensal microbial species interact with the host immune system and play a role in excluding pathogens by competing for metabolites.14 Thus, it is important to characterize the microbiome of healthy skin across a range of ages, which can be used as reference data to be compared with diseased states.
However, prior work in characterizing the differences in skin properties and the microbiome among children compared with adults was limited in the investigated age ranges.1, 4, 11, 15-17 A few studies included children up to age 515 or 8 years,18 but were limited in the number of recorded parameters in these age groups. Due to this lack of published data, it remains uncertain at what age a child's skin becomes functionally equivalent to that of an adult. The objective of the present study is to extend the knowledge of the skin maturation processes regarding skin structure, function, composition and microbiome in children up to 10 years of age compared with adults.
2 METHODS
2.1 Study design
The study was conducted in May 2019 in Schenefeld/Hamburg, Germany and complied with the Declaration of Helsinki and Good Clinical Practice. Each participant or their legal guardian provided informed consent and the study was approved by an Institutional Review Board (study 19.0198). The trial was not registered because it was a single-visit skin measurement study to establish baseline healthy skin conditions and no product was used.
Participants for each of the four age groups were children aged 0–2, 3–6 or 7–<10 years, and their mothers aged 25–40 years. All were self-reported as healthy and had Fitzpatrick skin type I–III. Exclusion criteria included pregnancy (in adults); conditions that might influence the test/evaluation; serious illness such as cancer or rheumatic disease that might require regular systemic medication; unmedicated cardiovascular disease, thyroid hyperfunction, asthma or hypertension; diabetes; and body mass index >30. In addition, participants were excluded if they had any skin-related conditions including atopic dermatitis, seborrheic dermatitis, psoriasis or a skin infection; had visibly dry skin, active skin disease, moles, tattoos, scars or irritated skin at the test area; had received systemic therapy with immunosuppressive drugs and/or antihistamines in the last 7 days or with antiphlogistic agents or analgesics (excepting minor pain relief) in the last 3 days; had applied any topical medication in the test areas in the last 3 days; or had long sun exposure, UV therapy, artificial tanning or sunburn in the test areas in the last 6 weeks.
Participants were instructed to not apply any leave-on cosmetics (e.g. creams, lotions and oily cleansing products) in the test areas within 3 days of study start, not to smoke (adults only) or drink any caffeinated beverages in the 2 h prior to instrumental assessments and not to apply any detergents or decorative cosmetics in the test areas on the day of study start. Participants were also instructed to avoid contact of the test areas with water in the 2 h prior to instrumental assessments.
Skin barrier function (water loss), skin surface hydration and skin colour were assessed, skin tapes were collected, and images were acquired on the cheek, dorsal forearm and upper inner arm on one side (left or right, randomly assigned). Raman spectroscopy and confocal imaging were performed on the volar forearm, and skin swabs for microbiome analysis were collected from the dorsal forearm of the opposite side.
2.2 Methods
Water loss was assessed by TEWL using a closed-chamber system (Aquaflux AF200, Biox Systems Ltd, London, UK). Skin surface hydration was measured as electrical capacitance using a corneometer (CM 825, Courage & Khazaka, Cologne, Germany). Skin colour was assessed by spectrophotometer (CM 700d, Minolta, Langenhagen, Germany), and images of skin were acquired using a digital camera (Canon EOS Mark III, Canon, Tokyo, Japan) equipped with a dermatoscope (DermLite, San Juan Capistrano, CA, USA). The apparent concentration of melanin (skin pigmentation) was calculated from the diffuse reflectance spectra recorded by the same spectrophotometer and based on a previously published algorithm.19 Lipid compactness20 and the amounts of lactic acid, total amino acids21 and DNA on the skin surface were calculated from the Raman spectra acquired in the high wave number and in the fingerprint region using in vivo Raman microspectroscopy (Skin Analyser model 3510, River Diagnostics, Rotterdam, the Netherlands) and the SkinTools software provided by the manufacturer. SC thickness was measured from confocal microscopy images (Vivascope 1500, Lucid, Inc, Rochester, NY, USA). To evaluate corneocyte size, D-Squame Standard Sampling Discs (CuDerm Corporation, Dallas, TX, USA) were applied for about 5 s to non-invasively collect skin samples, which were frozen at −20°C until shipment and evaluation. Sample images were acquired using epiluminescence microscopy (KH-1300 HiScope digital microscope system, HiROX Co Ltd, Tokyo, Japan) under 35× magnification. Cell size was calculated from the images using ImageJ software (US National Institutes of Health, Bethesda, MD, USA). Samples for microbiome analysis of skin on the dorsal forearm were obtained by swabbing back and forth about 50 times on a 4 × 4-cm area of skin with a flocked swab (COPAN 502cs01) soaked in molecular-grade, DNA-free water (Sigma-Aldrich). The head of the swab was aseptically cut and stored in a sterile tube at −80°C until shipment and analysis. The swab samples were sent for DNA extraction and 16S ribosomal RNA sequencing using primers encompassing variable regions V1–V2 (CosmosID, Rockville, MD, USA). Raw data were analysed as relative abundances of amplicon sequence variants as previously described.12
2.3 Statistical analysis
The study population with sufficient collected data comprised the full set for statistical analysis. All statistical analyses were performed in R version 4.1.122 or Minitab (version 21.3.1, Minitab LLC, State College, PA, USA). Linear regression coefficients (R2) and p values were calculated on children's data as a function of age. For all age groups (including adult), data were aggregated per group and differences were assessed by one-way analysis of variance (ANOVA) with 95% confidence level followed by post hoc Tuckey's test. Statistical comparison between two given groups was performed using Student's t-test following the Anderson–Darling normality test. All statistical tests were two-sided at a significance level α = 0.05. For the skin microbiome data, overall richness was expressed as the number of amplicon sequence variants and quantified using the Chao1 richness estimator, and overall diversity was expressed using the Shannon Diversity index.
3 RESULTS
Of the 86 participants enrolled in the study, 80 comprised the full analysis set. Participants in the study were grouped (20 per group) according to age: babies (0–2 years), young children (3–6 years), older children (7–<10 years) and adults (25–40 years) (Table S1). Most participants had Fitzpatrick skin type II.
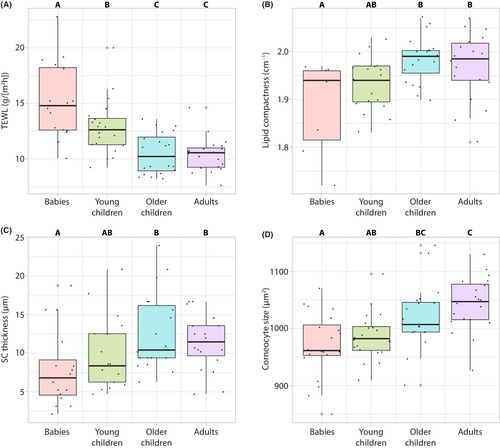
Maturation of the skin barrier function was evident by the age-dependent decrease in TEWL values in children for all three sites measured, with faster rates of change on the upper inner arm site, followed by the cheek and the dorsal forearm (Table 2 and Figure S1A–C). Mean TEWL values were higher on the cheek compared with the arm, which was statistically significant for all age groups except babies (Table S2). Age group analysis showed that TEWL gradually decreased from babies to young children and older children, with the values of older children statistically similar to those of adults (Figure 1A), indicating a full maturation of the skin barrier function by the age of 6 years. In examining skin attributes that contribute to barrier function, both lipid compactness and SC thickness increased with age in children (Table 2 and Figure S1D,E), with values in the older children group statistically similar to those in adults (Figure 1B,C). Corneocyte size, another parameter directly involved in skin barrier function, gradually increased with age in children, but only on the cheek site (Table 2 and Figure S1F) and reached adult levels in the older children group (Figure 1D).
| Parameter, units | Site | R 2 | Regression p value | Rate of change, units/year |
|---|---|---|---|---|
| TEWL, g/m2h | Cheek | 0.103 | 0.017 | −0.385 |
| Dorsal forearm | 0.123 | 0.007 | −0.354 | |
| Upper inner arm | 0.288 | <0.001 | −0.566 | |
| Lipid compactness, AU | Volar forearm | 0.266 | <0.001 | 0.012 |
| SC thickness, μm | Volar forearm | 0.208 | 0.001 | 0.768 |
| Corneocyte size, μm2 | Cheek | 0.107 | 0.011 | 0.533 |
| Dorsal forearm | <0.001 | 0.979 | 0.005 | |
| Upper inner arm | 0.001 | 0.809 | −0.040 | |
| Skin surface hydration, AU | Cheek | 0.017 | 0.327 | −0.410 |
| Dorsal forearm | 0.410 | <0.001 | −1.812 | |
| Upper inner arm | 0.271 | <0.001 | −1.488 | |
| Lactic acid, AU | Volar forearm | 0.157 | 0.007 | −63.84 |
| Total amino acids, AU | Volar forearm | 0.055 | 0.121 | 219.6 |
| Skin pigmentation, AU | Cheek | 0.005 | 0.585 | −0.005 |
| Dorsal forearm | 0.167 | 0.001 | 0.040 | |
| Upper inner arm | 0.094 | 0.017 | 0.024 | |
| Skin lightness, L* | Cheek | 0.103 | 0.012 | −0.356 |
| Dorsal forearm | 0.381 | <0.001 | −1.021 | |
| Upper inner arm | 0.086 | 0.023 | −0.294 | |
| DNA amount, AU | Volar forearm | 0.257 | <0.001 | 0.0125 |
- Note: Boldface indicates statistical significance (p < 0.05).
- L* is the name of the measured skin lightness parameter.
- Abbreviations: AU, arbitrary units; SC, stratum corneum; TEWL, transepidermal water loss.
Skin surface hydration values showed an age-dependent decrease for the arm sites but remained statistically unchanged on the cheek (Table 2 and Figure S2). In all age groups, facial skin maintained higher skin surface hydration levels than the arm sites. Age group analysis showed only the baby group was statistically different from adults (Figure 2A,B).
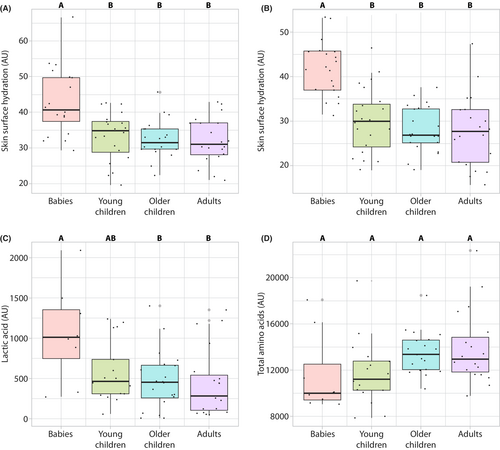
The SC concentrations of lactic acid and total amino acids on the volar forearm were assessed by Raman microspectroscopy. While lactic acid concentration in the SC decreased with age, the SC concentration of total amino acids showed an increasing trend but did not reach statistical significance between the groups (Figure 2C,D Table 2, Figure S3).
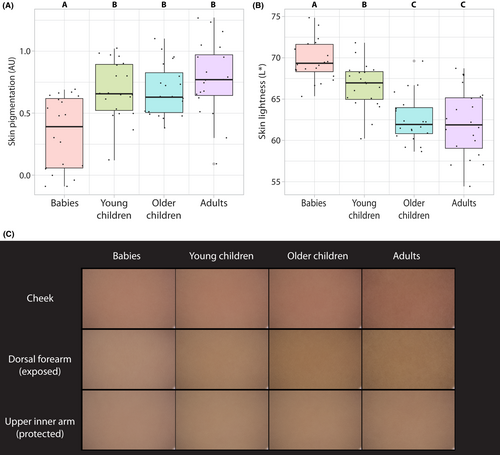
Skin pigmentation levels (melanin content) as measured by reflectance spectrophotometry increased in an age-dependent manner in children in both arm sites but not on the cheek, and correspondingly, skin lightness values decreased (Figure 3A,B, Table 2, Figure S4). On the arm, the rate of change for both parameters was higher on the exposed (dorsal) compared with the protected (upper inner) site (Table 2). Facial skin appeared to be redder compared with the arm for all age groups. These effects can be observed in digitally averaged clinical photographs of the cheek, dorsal forearm and upper inner arm (Figure 3C).
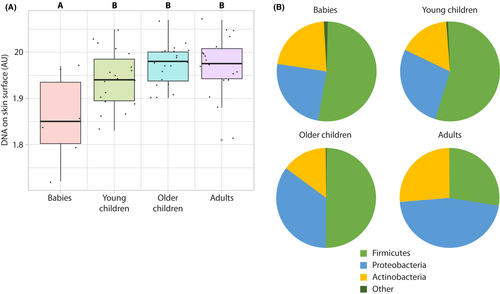
Age dependence was observed in the skin microbiome sampled on the volar forearm. The skin surface DNA signal measured by in vivo Raman microspectroscopy increased in children in an age-dependent manner (Table 2 and Figure S5), indicating an increase in microbial load with age. The value of this parameter for children older than 2 years was statistically similar to that measured in the adult group (Figure 4A). Firmicutes was the predominant phylum in all baby/children groups, while Proteobacteria was most predominant in adults (Figure 4B). At the genus level, the skin microbial composition in children was found to dynamically change, with Cutibacterium of greater abundance on the dorsal forearms of adults compared with babies and children (Figure S6). Staphylococcus and Streptococcus (both in the Firmicutes phylum) were of greater abundance in babies and decreased with age. Other genera such as Bacillus increased with age in children but were almost totally absent in adults. No differences were observed between the age groups in microbiome diversity as assessed via the Shannon index (babies, 2.18 ± 0.84; young children, 2.15 ± 0.96; older children, 2.33 ± 1.21; adults, 1.74 ± 0.81; p = 0.257 by ANOVA).
Male versus female comparisons were performed in the children data. There was no statistical difference in any of the measured parameters on the cheek, the dorsal and the upper inner arm. However, on the volar forearm (where the Raman measurements were performed) there are three parameters that showed statistical differences between the sexes: Female children have slightly more compact lipid organization, slightly more DNA content on the skin surface and less lactic acid content (Table S3).
4 DISCUSSION
Results from this study demonstrate that the skin barrier is more permeable during childhood compared with adults due to a thinner SC, less compact lipids and smaller corneocyte sizes. This work extends our prior knowledge, in part by including older children (those aged 5 to <10 years). As the child grows, the skin barrier quality begins to differentiate from that of young children, becoming more mature (less permeable). This can be seen in the similarities between older children and adults in the values of TEWL rates, lipid compactness, SC thickness, total amino acids and skin lightness. To our knowledge, lipid compactness had only been previously studied in adults.23 In the present study, lipid compactness was lowest in babies and increased with age, with older children and adults showing similar values.
Lactic acid production is indicative of increased anaerobic metabolism, which typically accompanies proliferating cells.24, 25 Amino acids are end products of enzymatic protein breakdown that occurs in the SC.26 Both the higher levels of lactic acid and the lower levels of total amino acids observed in babies and young children indicate higher cell turnover rates compared with adults. The insight about higher cell turnover rates in children is also supported by the smaller corneocyte size compared with adults2, 27 and further explains the differences in the skin barrier parameters mentioned above.28
The results of this study are consistent with prior work on babies and adults. TEWL was higher in infants compared with adults, as seen previously.1, 4, 5, 7 In addition, SC thickness values in infants (6–24 months) and adults compare well with the dorsal forearm measurements in this study.1, 2 Corneocyte size increases with age,2 and the data here indicate that corneocytes on the cheeks of children up to 6 years of age remain smaller compared with adults. Free amino acid content in the SC showed an age-dependent increasing trend, consistent with prior work.29 Skin pigmentation increased with age, as previously seen in a study of babies aged 6–12 or 16–24 months and their mothers (aged 30–40 years).17, 30
TEWL rates measured on either the face or the arm of children decreased with age, indicating the ongoing maturation of the barrier function. At all ages, facial skin exhibited a more permeable barrier than on the arm. The higher TEWL rates and skin surface hydration values for adult cheeks compared with those of babies and children may reflect long-term use of facial skincare products such as antiaging creams that stimulate cell turnover rather than a physiological difference. Another explanation could be the chronic exposure of adult faces to solar UV radiation, which is known to reduce the skin barrier function.31
In addition to the instrumental assessments, results observed here with the skin microbiome are consistent with prior work. Previous findings also showed that Firmicutes was the predominant phylum on infant skin.11, 12 Data from the present study now show that Firmicutes also predominate on the skin of young and older child groups. Proteobacteria, Firmicutes and Actinobacteria were the most predominant phyla on the forearm in adults, consistent with prior work.32-34 At the genus level, Staphylococcus and Streptococcus have previously been shown to decrease with age,11, 13 as seen here. In a study of the microbiome of the volar forearm in 28 healthy participants in Washington, DC, USA, Streptococcaceae were significantly higher in participants in Tanner stage 1 (prepubescent) versus those at Tanner stage 5 (puberty complete).35
Some data have indicated that the mode of birth (i.e. vaginal versus caesarean section) influences the skin microbiome, particularly in the first days of life.36 However, there is evidence that the mode of delivery does not affect the skin microbiome at 6 weeks of age.37 In line with those findings, the data here were analysed by mode of birth, and no effect was observed (data not shown). Finally, it has been suspected that there are associations between the intestinal and skin microbiomes.38 Although sampling the gut microbiome was beyond the scope of this study, the changes reported here relating to the skin microbiome are not necessarily associated with any potential changes in the gut as related to skin-specific taxa.
In this study, the population was limited to Whites with mainly Fitzpatrick skin type II. Previous studies on infant skin maturation have reported similar processes in babies of different ethnic backgrounds and skin types.1, 4, 9, 10 It would be valuable to extend the findings of these studies to children of older ages. One further limitation is that while the children were from both sexes, the adult group consisted only of their mothers (as is often the case with such studies), and therefore there was no representation of males in the adult group. Given this limitation, data analysis was repeated excluding male children and the results followed closely the patterns of the original analysis when all children were included.
In summary, skin maturation processes previously observed only in infants and very young children were investigated in children aged up to 10 years in this study. Compared with adults, the skin of infants and young children displays a more permeable barrier, higher skin surface hydration and lighter colour compared with adults. These differences disappear in children above 6 years of age. Interestingly, the composition of microbial communities remains different than that of adults even in older but preadolescent children. Due to the hitherto limited evidence on physiological data throughout various age groups, the results presented in this study can serve as reference data to compare with data in diseased conditions.
AUTHOR CONTRIBUTIONS
GNS was involved in conceptualization, investigation, methodology, data analysis, writing and revision; P-FR was involved in methodology, data analysis, writing and revision; EB-A was involved in data analysis and revision; IL was involved in data analysis and revision; TO was involved in supervision and revision. All authors have read and approved the final manuscript.
ACKNOWLEDGEMENTS
Writing assistance was provided to the authors by Jennifer L. Giel, PhD, on behalf of Evidence Scientific Solutions, Inc, and was funded by Johnson & Johnson Consumer Health. The authors would like to acknowledge the help of Dr Thomas Ondet for the image acquisition of tapes and analysis of corneocyte sizes. This study was fully funded by Johnson & Johnson Santé Beauté France.
CONFLICT OF INTEREST STATEMENT
At the time this work was performed, authors were employees of Johnson & Johnson Santé Beauté France.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




