Dynamic evaluation of pathological changes in a mouse acne model by optical imaging technology
Abstract
Acne vulgaris is a disorder of the pilosebaceous unit that is primarily caused by hyperseborrhoea, colonization with Propionibacterium acnes, hyperkeratosis and an inflammatory response. Existing pharmacodynamic assessment methods primarily focus on a single causative factor at a certain time point, making it difficult to assess multiple factors simultaneously in real time. Therefore, it is crucial to establish a dynamic and nondestructive method for the assessment of acne in vivo. This study utilized four-dimensional optical imaging techniques to assess the pathogenic factors and pathological progression of acne. LSCI was employed to measure blood flow; TPEF was used to observe inflammatory changes (NAD(P)H) in epidermal granular layer cells and structural changes in collagen fibres in the dermal layer. Additionally, the dermatoscope was used to investigate the micro-characterization of the lesions. We observed that the epidermis in the lesion area was thickened, hair follicles were keratinized, and there was obvious inflammation and blood flow aggregation by optical imaging technology. Based on these findings, the pathological progression of this acne model could be divided into the inflammation phase, accompanied by bacterial colonization, and the reparative phase. These results provide a new perspective for the assessment of acne and offer an experimental basis for the selection of precise drugs for clinical use.
1 INTRODUCTION
Acne vulgaris is the eighth most prevalent disease worldwide, affecting 85% of individuals between the ages of 14 and 25 years, with some cases perpetuate to adulthood. Epidemiological surveys indicate that the prevalence of acne ranges from 20% to 95%. Acne vulgaris is often considered a self-limited disease and thus receives less attention compared with other diseases. However, repeated inflammatory attacks and facial lesions can result in permanent scars, facial pigmentation and other long-term sequelae, which cause considerable burden on patients' physiology and psychology, and even cause despondent, anxiety, inferiority, social disorders, etc.1, 2 Therefore, an accurate early diagnosis and targeted treatment based on the individual differences in pathogenic factors of each patient are essential.
Currently, the mainstream animal models utilized for preclinical research or pharmacodynamic screening for acne involve ear models of rabbits, rats and mice that are subcutaneously/intradermally injected with Propionibacterium acnes. These models primarily focus on the inflammatory reaction and colony-forming units (CFU) of bacteria among the four factors (hyperseborrhoea, abnormal follicular keratinization, bacterial colonization and inflammation), the pharmacodynamics are evaluated using CFU, inflammatory cytokines, HE staining and other testing indexes at a fixed time point.3-6 Pathological changes in acne involve a dynamic multiparameter evolutionary process, and a single-point evaluation may overlook some physio-pathological information, leading to misjudgement of disease development and pharmacodynamic action. In recent years, optical imaging technology has become a research hotspot due to its ability to penetrate the epidermis and dermis of the skin, allowing for a more comprehensive evaluation of acne pathology. The skin contains various endogenous auto-fluorescent species, including collagens, elastins, nicotinamide adenine dinucleotides (NAD(P)H), melanins, porphyrins and flavin adenine dinucleotides (FAD) that generate strong endogenous fluorescence in a certain spectral range without the need for additional labelling or staining. This fluorescence can be monitored noninvasively and in vivo.7
In our preliminary studies, we used two-photon excitation laser scanning fluorescence microscopy (TPEF) to observe that NAD(P)H gathered around the nucleus of the epidermal granular layer in mouse auricle swelling. We also found that TPEF could dynamically monitor the thickness of the epidermis in vivo, which was highly consistent with results obtained from HE staining.8 We also employed optical coherence tomography (OCT), laser speckle contrast imaging (LSCI), dermatoscope and other optical imaging techniques to assess skin diseases such as burns and wound healing.9, 10 The previous researches we conducted have established a foundation for the application of optical imaging technology in the diagnosis and efficacy evaluation of skin diseases. In this study, we utilized a mouse back acne model that closely resembles clinical acne lesions and employed multiple nondestructive optical imaging techniques to reveal the occurrence, development and changes of acne in vivo. By doing so, we provide a new approach to screen and evaluate the pharmacodynamics of anti-acne drugs.
2 MATERIALS AND METHODS
2.1 Bacterial strains and culture conditions
Propionibacterium acnes (P. acnes, ATCC 6919) was grown in BHI medium at 37°C anaerobically until it reached the stationary phase. The cultures were centrifuged at 3500 rpm for 10 min, washed in BHI three times and the resulting pellets were resuspended in medium. For murine experiments, cultures were grown in fresh BHI until an OD600 of 0.5–0.6 (≈5 × 108 CFU) was reached.
2.2 Murine model of acne
Six eight-week-old BALB/c male mice weighing 19–21 g were obtained from Beijing Weitong Lihua Laboratory Animal Technology Co., Ltd. China. All animals were housed individually at 22 ± 2°C with a relative humidity of 50 ± 10% and a 12-h light/12-h dark cycle. Six mice were kept in each cage. Randomly divided into no treat group (NT), P. acnes + Artificial sebum group (PA). The animals had free access to food and water. The mice were shaved prior to epilation and injected intradermally with approximately 5 × 108 CFU/mL of P. acnes in 50 μL BHI medium the next day; then, 20 μL freshly made synthetic sebum (17% oleic acid, Macklin, 45% triolein, Macklin, 25% jojoba oil Macklin and 13% squalene, Macklin) was applied to the skin and reapplied daily; the acne induction period was given for 3 days. The lesions were harvested aseptically at specified time points for indicator detection and the CFU was determined on agar plates incubated anaerobically, in addition to the daily application of optical imaging detection.
2.3 Two-photon excitation laser scanning fluorescence microscopy (TPEF)
The Olympus MPE FV1000 confocal microscope system was utilized with the Mai Tai DeepSee laser (100 fs, 80 MHz; Mai Tai HP DS-OL, Spectra-Physics, Inc). The acquisition conditions for the lesion signal were the following: spontaneous fluorescence of NAD (P) H, EX = 750 nm, spontaneous collagen fibre, EX = 850 nm, exception DM 690 nm, BF 420–460 nm, ×25 objective lens with aperture of 1.05 was used for imaging and the scanning speed was 2 μm/pixel. Graphics were achieved from the epidermis to the dermis as a z-stack image sequence (step size 1 μm on the Z-axis) with 1024 × 1024 pixels.10, 11
2.4 Optical coherence tomography
The vertical section of the mouse back pustules was visualized using optical coherence tomography (OCT) (QSLF-1500, Shenzhen MOPTIM Imaging Technology Co., Ltd.) with linear scanning at a central wavelength λ = 850 nm and a resolution 320 × 240.12, 13 The height and direction of the lens were adjusted, and the centre of the pustule was scanned to capture the image when the presentation was clear and the signal was stable. Finally, the image parameters were analysed using ImageJ software (V1.8.0).
2.5 Medical electronic dermoscopy
Medical electronic dermoscopy (MED) (BN-WG-1001, Nanjing Beining Medical Device Co., Ltd.) was used to observe micro-characterization of acne lesions. After the model was created, the mice were anaesthetised and placed on the imaging platform, and the magnified image was captured using the 160X objective lens.14
2.6 Laser speckle contrast imaging
Laser speckle contrast imaging (SIM BFI HR Pro, Wuhan XunWei Optoelectronic Technology Co., Ltd.) was used to monitor blood perfusion in the pustule area.15, 16 After anaesthesia, mice were placed 20 ~ 30 cm below the stereo microscope until clear images were obtained. Real-time blood flow images were then collected with an acquisition time of 15 s. The LED intensity was set at 4000 times, the optical multiple at 12 times and the brightness at level 5.5. Image analysis was performed using a laser speckle blood flow imaging system (SIM BFI-WF) with the quantitative index unit expressed in perfusion units (PU).
2.7 H&E and gram staining
For histology analysis, the skin tissue samples of acne lesions were fixed in 4% paraformaldehyde at 0, 1, 3 and 7 days after modelling. The fixed tissues were then embedded in paraffin, and 6 μm thick sections were obtained for H&E and Gram staining. The tissue sections were observed under an upright microscope (BX51, Olympus) for further analysis.
2.8 ELISA
Mouse IL1α (Cat. NO: RK00103, ABclonal Technology Co., Ltd.) and IL1-β (Cat. No: RK00006, ABclonal Technology Co., Ltd.), and KC/CXCL1 (Cat. No: RK00038, ABclonal Technology Co., Ltd.), and TNF-α (Cat. NO: RK00027, ABclonal Technology Co., Ltd.) ELISAs were performed according to the manufacturer's instructions.
2.9 Statistical analysis
All data were expressed as mean ± SEM and analysed by two-way ANOVA or one-way ANOVA tests. Statistical analyses were conducted using GraphPad Prism v.8.0 (GraphPad Software, Inc.) with Bonferroni post-tests or Tukey's post hoc test to identify differences between groups. ImageJ software (V1.8.0) was used to analyse the image parameters. A p-value of less than 0.05 had statistical difference.
3 RESULTS
3.1 Analysis of typical manifestations and contents in the evolutionary process of acne
Propionibacterium acnes is a normal part of the skin microbiota and is commonly found in the pilosebaceous unit, where it utilizes lipid-rich sebum as a source of nutrients. With excessive sebum secretion, P. acnes rapidly proliferate in an anaerobic environment, leading to inflammation through the release of multiple inflammatory mediators. When comedones rupture, the content of the sebaceous gland unit seeps into the surrounding dermal tissue, producing inflammatory lesions such as papules, pustules, nodules or cysts (Figure 1A).17 We slight modified the acne animal model established by Stacey L. Kolar,18 briefly, prepared the mouse acne model by injecting P. acnes intradermally and applying freshly synthetic sebum to the skin, with daily reapplication (Figure S1). This allowed us to simulate the formation process of abscesses and inflammation that is similar to the clinical manifestation of acne lesions.
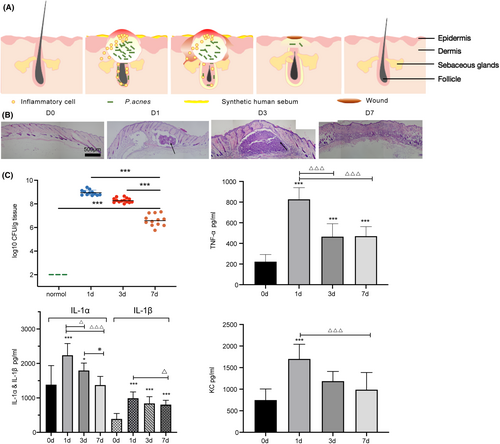
The model shows that P. acnes colonizes under lipid and anaerobic environment, the CFU of P. acnes in pustular tissue was its peak on the first day after modelling and continued until the third day, and then gradually decreased, from the initial 108 ~ 109 to 105 ~ 106 by the seventh day. The trend in cytokine levels was consistent with those of P. acnes. Specifically, TNF-αlevels decreased from 828.02 pg/mL (1 day) to 466.47 pg/mL (7 days), while IL-1β levels decreased from 991.86 pg/mL (1 day) to 840.52 pg/mL (7 days), with no significant difference between the seventh day and the third day, but it was still significantly higher than pre-modelling. IL-1 α levels decreased from 2239.06 to 1797.75 pg/mL (3 days), while KC levels from 1703.12 to 1108.83 pg/mL (3 days). However, by the seventh day, IL-1α and KC levels gradually recovered to pre-modelling levels (Figure 1C). H&E staining results revealed that inflammatory cells infiltrated the dermis and subcutaneous tissues from 1 to 3 days after modelling, the area of the pustule was obvious, the pustule began to subside after 7 days, and the inflammatory cells gradually cleared (Figure 1B). These findings suggested that the acne model successfully demonstrated colony colonization accompanied by inflammation. The CFU showed no significant difference from 1 to 3 days after modelling, the colony gradually cleared between Days 3 to 7, with a corresponding gradual fading of inflammation.
3.2 Optical evaluation indicators of acne pathogenic factors
Inflammation, bacterial proliferation, sebum hypersecretion and follicular hyperkeratosis are the main pathogenic factors of acne. However, due to the lack of sebum secretion in mice, we mainly used optical imaging to observe the other three factors in vivo. H&E staining found that the follicles began to expand on the third day after modelling, and abnormal translucent plug follicle morphogenesis were formed near the opening of the skin surface (Figure 2A). Sebaceous gland hyperplasia and typical obstructed follicles with inflammatory infiltration around the hair follicles were observed (Figure 2B).
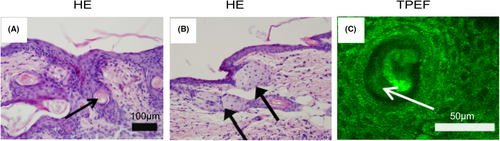
In 2010, Hans George Breunig et al.19 studied the relationship between the spontaneous fluorescence intensity and excitation wavelength of the main endogenous fluorescent groups in the skin. They found that using an excitation wavelength in the range λ = 720 to λ = 760 nm mainly excites the self-fluorescence of keratin on the skin surface. At a depth of 22 μm with the same excitation wavelength, the main fluorophores in the stratum spinosum were NADH and NAD(P)H, which also showed the distribution of mitochondria in cells. This research provides a foundation for using fluorescence imaging to study the skin's properties and cellular structure. In our research, we used an excitation wavelength of λ = 750 nm to observe the spinous layer cells and hair morphology. Generally, the excessive content of keratin in hair appears as bright dots or lines under the same laser power, after modelling, the hair follicle grew abnormally, which could be seen to have a high fluorescence signal and no fixed shape of the filling of the corneum plug (Figure 2C).
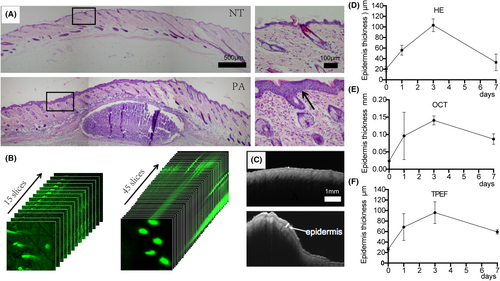
In this study, animal models were used to observe not only abnormal keratinization of hair follicle, but also the thickening of epidermal tissue remarkably (Figure 3A). H&E staining found that the epidermis thickness of NT group was 25 ± 5 μm and epidermis thickness of abscess in PA group increased to 110 ± 10 μm. TPEF observation and z-stack scanning provided a more detailed measurement of the entire epidermis layer, from the top to the bottom, the horny layer, the granular layer, the stratum spinosum and the basal layer cells. The epidermis thickness of the NT group was 28 μm and the thickness of the abscess epidermis of the PA group was 90 ± 4 μm (Figure 3B). OCT observation of the entire skin layer revealed that the reflective signal of the corneum layer on the skin surface was bright, while the signal of the epidermis layer was obviously dark (low signal reflection). The signal at the lower boundary of the epidermis represented the dermal–epidermal junction, and the strong signals in the dermis originated from collagen fibres. On the third day after modelling, it was observed that the signal in the corneum was significantly enhanced, and the low signal area in the epidermis of the abscess area was significantly thickened to 120 ± 5 μm (Figure 3C), TPEF techniques were used to measure the thickness of the epidermis dynamically in vivo, and the results were consistent with H&E staining (Figure 3D,F).
The excessive keratinization in hair follicles can lead to the formation of an anaerobic environment that favours the colonization of P. acnes. It produces antigens that are taken up by dendritic cells, leading to the activation of T cells and the release of inflammatory mediators. Therefore, the immune response plays a key role in explaining the pathogenesis of acne, rather than the damage caused by bacteria. Therefore, the evaluation of inflammation by in vivo imaging is crucial to reveal the occurrence and development of acne lesions.
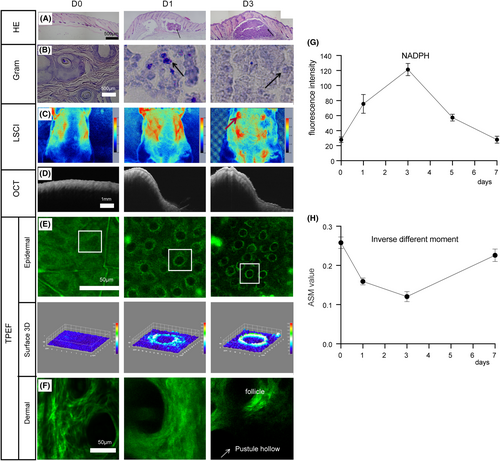
A study of the histology of acne lesions revealed that from 1 to 3 days after modelling, there was an acute inflammatory response located between the dermis and the subcutaneous tissue, presenting a lump-like (abscess) pattern with slight spillage into the subcutaneous layer, with no inflammation accumulated in the hair follicles or sebaceous glands (Figure 4A). Gram staining showed bacteria co-existing with inflammatory cells in the abscess area, with P. acnes aggregated in the inflammatory cells (Figure 4B). The blood flow in the pustular area can be observed by using LSCI. At the initial stage(1–2 days) of abscess formation, no significant blood flow aggregation was found in the lesion area and there was no significant difference with the normal area. As the formation of abscess, the blood flow in the pustular area accumulated to 40PU (Figure 4C). OCT was used to determine the depth and diffusion area of the abscess (Figure 4D). Three days after modelling, the depth could reach 2 mm. The NAD(P)H signal was uniform distribution in the cytoplasm of normal epidermal living cells.20 After abscess formation, the NAD (P) H fluorescence signal was enhanced and converged from scattered distribution to nucleus (Figure 4E). On the third day, the fluorescence intensity was the strongest (Figure 4G). Meanwhile, it was observed that the dermis was damaged,21 the structure of collagen fibres around the abscess area was blurred and lost at the pustule (Figure 4F). By calculating the characteristic parameter of grey-level co-occurrence matrix, inverse difference moment, which represented the measurement of local changes in image texture, the more regular the texture, the greater the inverse difference moment, it was found that the collagen fibres become blurred during inflammation, and the texture morphology was irregular, so the inverse difference moment on the third day was the minimum (Figure 4H).
Using optical imaging techniques, we conducted a detailed assessment of the pathogenic factors associated with acne in this model. We induced an inflammatory response by intradermally injecting P. acnes and observed the formation of abscesses, as well as a significant aggregation of blood flow, indicating the close association between P. acnes proliferation and acne. The synthetic sebum was applied and reapplied daily, in the presence of both sebum and P. acnes, we also observed the epidermal thickening and follicular hyperkeratosis. These acne-related causative factors interacted with each other and contribute to the development of this skin lesion.
3.3 Multidimensional optical imaging observation on the evolution of acne in mice
We had characterized the evolution of acne inflammation in mice using an established multidimensional optical imaging evaluation method (Figure 5) and observed the entire process of pustule formation, development and regression. From a horizontal perspective, a dermatoscope and LSCI were used to assess the changes in the vascular morphology and blood flow of acne lesions. At 1–2 days after bacterial inoculation, the superficial blood vessels in the abscess area were significantly angiectatic and congested without significant aggregation. At 3–4 days after moulding, the epidermal scabbed and the superficial vasodilation decreased and regional blood flow increased. On Day 4, the blood flow increased to the peak, and on Day 5, the crust began to fall off, the dilated blood vessels subside from the central area and extended like the surrounding skin. The local blood flow gradually decreased; on Day 7, pustules subsided and blood flow recovered to normal levels (Figure 5A,B,F). From the vertical section, we used OCT to evaluate changes in epidermis thickness epidermis and abscess depth throughout the inflammation period. On the first day, the abscess began to form, and on the third day, the deepest of abscess reached 2 mm, while the epidermis thickened to 120 ± 5 μm, and returned to normal on the 7th day (Figure 5C,G).
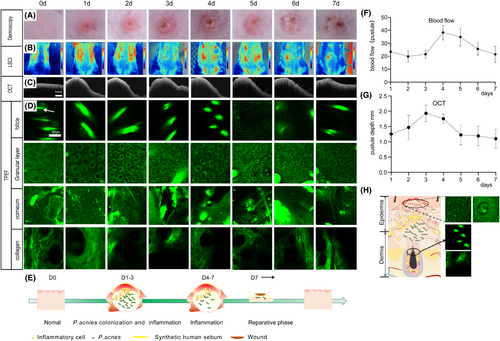
We scanned the skin tissue of mice in a z-stack mode using TPEF to monitor the morphological changes at different depths during the evolution of acne (Figure 5D,H). The typical changes were the alterations in the NAD(P)H signal in the epidermal granular layer and the collagen fibres in the dermis. The typical changes were the changes in NAD(P)H signal in the epidermal granular layer and collagen fibres in the dermis; on 1 day after inoculation, the NAD(P)H signal in the granular layer began to enhance, and the morphology of collagen fibres became blurred. From 2 to 5 days, the NAD(P)H signal in the granular layer cells gathered around the nucleus, and the collagen fibres blurred and were damaged at the inflammatory site, on the 6th day, the NAD(P)H signal gradually dispersed uniformly in the granular layer cells, and the morphology of collagen fibres became clear, the defect area was gradually filled with collagen fibres as the abscess subsided.
We also observed the changes in the sebaceous gland and stratum corneum using TPEF. The sebaceous gland image gradually disappeared from Day 1 to Day 5 and began to recovered from Day 6 to Day 7. The boundary of stratum corneum cells blurred from Day 1 to Day 2. On Day 3 ~ 4, a scab formed where the morphology of keratinocytes was damaged and incomplete. On Day 5, the scab fell off and there were no obvious keratinocytes, which were then regenerated on Day 6 ~ 7. Based on the above results, the model in this study had an inflammation period of 1–7 days and there was no difference in colony counting with 1–3 days (Figure 1C), indicating that this was the main period of bacterial colonization. The bacteria were slowly swallowed from Day 3 to Day 7 and the CFU decreased (Figure 5E).
3.4 Typical manifestations and dynamic evolution of acne scab during the repair stage
To evaluate the wound healing and repair process of the acne skin lesions, a previously published evaluation method by our group was used.9 H&E staining was performed, and on the 7th day, the wound of the lesions and new hair follicles nearby were observed (Figure S2a). From the xy plane, the dermoscope observed that the lesion site was scabbed after the acne abscess subsided (Figure S2b). From the vertical section, OCT was used to evaluate changes in the thickness of the low signal area between the epidermis and dermis, and it was found that the area was locally expanded (Figure S2c). TPEF imaging observed that the scabbed tissue was accompanied by a newborn hair follicle (Figure S2d).
Wound healing after skin injury is a complex process that involves interactions between various skin cells. Any deviation from the normal repair process can result in skin defects such as ulcers and scars.22 After the acne inflammation subsided, the crust appeared on the skin lesions and the evolution process of the acne scab repair stage was dynamically observed using various optical imaging technologies. The micro-characterization process of wound recovery was observed by dermoscope (Figure S3a), and vertical section observation was conducted by OCT and TPEF. By the 8th to 9th day, the wound edge was clear, and the scabbed area was covered by new epidermal cells on the 10th day, and the skin morphology tended to be normal on the 13th day (Figure S3b). TPEF could observe the dynamic recovery process of hair follicle, stratum corneum, granular layer and dermis (Figure S3c). On the 8th day, there were still cavities in the dermis collagen in the epidermis covering area around the skin lesion, on the 9th and 10th days, loose granular layer cells and new hair follicles were visible, on the 12th day, the sebaceous gland structure could be seen, and the collagen fibre structure was clear, and the new granular layer cells were relatively complete, on the 13th to 14th days, the restored intact keratinocyte morphology could be imaged.
4 DISCUSSION
Acne vulgaris is a chronic inflammatory condition of the pilosebaceous unit, that occurs due to various factors such as increased androgen levels, excessive sebum secretion, hyperkeratinization of the follicular, hypertrophy of the sebaceous gland and accumulation of clusters of keratinocytes, resulting in the obstruction of the pilosebaceous canal. A microcomedo formation occurs when the normal flow of sebum on the skin surface is obstructed by follicular hyperkeratosis. This leads to the accumulation of sebum, which eventually results in the formation of a visible comedo.
In the sebaceous gland, P. acnes produces lipase which hydrolyses triglycerides into free fatty acids and glycerol. Additionally, P. acnes can produce porphyrin and other inflammatory substances that can be released to the skin, causing inflammation, forming inflammatory papules and pustules, and even cysts and nodules in severe cases.23 Acne vulgaris attacks repeatedly and is difficult to cure due to various factors such as the patient's age, genetics, diet, lifestyle and the severity of their acne. Furthermore, irrational use of medication during treatment, incomplete treatment24 and antibiotic-induced drug resistance can also contribute to the difficulty in finding a cure for acne.25
Acne vulgaris is a complex disease with multiple interacting pathogenic factors, and its pathogenesis is still not completely understood.26 The current animal models for acne pharmacodynamic research, such as the ear swelling model and the rabbit ear anti-keratinization model, these models primarily focus on a single pathogenic factor and are limited in their ability to fully reflect the pathological characteristics of the disease, particularly the interaction of multiple pathogenic factors, which is similar to that observed in human acne. To gain a more comprehensive understanding of the pathogenesis of acne and to better assess the effectiveness of anti-acne drugs, it is imperative to develop an animal model that more closely reflects the pathogenesis of human acne. The mouse acne model used in this study was optimized based on Stacey L Kolar,18 the bacteria CFU was adjusted to 5 × 108 and continuously injected for 3 days, and acne was induced by intradermal injection of P. acnes and topical application of synthetic sebum. The model comprehensively encompasses all the pathogenic factors that are closely linked to acne, and thus provides a more accurate reflection of the development and changes of each pathogenic factor involved in acne. This model possesses clinical characteristics that are similar to the pathogenesis of human acne, making it a useful tool in studying this condition.
Optical imaging is a rapid, non-invasive, in vivo evaluation method that can be detected in real time. Unlike ionizing radiation, which poses significant health risks, optical imaging has been successfully applied in both basic experimental and clinical research. Furthermore, it plays a crucial role in the early diagnosis of disease.7 Moreover, optical imaging offers the added advantage of significantly reducing the number of experimental animals required. For instance, in this study, the classical anti-acne efficacy assessment method required 24 animals from both the model and blank control groups at different time points to obtain all the necessary data. In contrast, by utilizing the optical imaging method, only six animals were required, saving experimental animals and experimental cost.
Our group has conducted research on optical imaging technology, utilizing various cellular and animal models and others in vivo and ex vivo assays,27, 28 such as ear swelling in mice,8 back trauma healing in rats9 and skin burns in mice.27 Based on this, this research had established four dimensions of optical imaging using the dermatoscope, OCT, TPEF and the LSCI technique to assess the pathological progression of the mouse acne model in the dorsum (Figure 6). Our observations revealed that multiple pathogenic factors contribute to acne, such as follicular keratinization, epidermal thickening and inflammation. We also examined the three-dimensional structure of the lesions in both the horizontal and vertical planes to observe the dynamic evolution of the lesions in the temporal dimension.
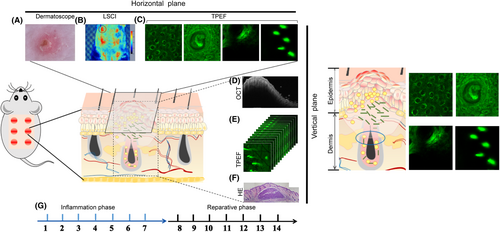
From the horizontal perspective, firstly, we established Laser speckle contrast imaging (LSCI) to measure the changes of blood flow in the acne inflammatory area (Figure 6B). LSCI is an optical imaging technique that provides a wide-field view of blood flow without the need for scanning. When a coherent light source illuminates biological tissue, backscattered light can be focused through a limiting aperture that gives rise to diffraction and is termed speckles, which is widely used in fundamental and clinical research for imaging vascular structure and related haemodynamics.29 Second, TPEF was used to observe inflammatory changes in epidermal granular layer cells with NAD(P)H change as an indication and structural changes in dermal collagen fibres within a depth of 280 μm (Figure 6C). TPEF works by using nonlinear photoexcitation of molecules, in which two low-energy photons are almost simultaneously absorbed in the same focal point, resulting in fluorescence emission. This process involves imaging from the excited state to the ground state, and short pulse lasers can keep the average power of the sample at a low level, reducing tissue damage and allowing for long-term imaging.30, 31 Finally, the dermatoscope was used to investigate the micro-characterization changes of the lesions (Figure 6A).
From the vertical perspective, observing changes in abscess depth and area size can be achieved using OCT (Figure 6D). OCT detects the backscattered light signals from tissue features, and its imaging contrast arises from the intensity variation caused by differences in refractive index distribution within heterogeneous tissue. OCT has gained more and more attention in dermatology due to its relatively high resolution (up to 1 mm), deep imaging depth (1–3 mm) and real-time image acquisition capabilities.32 Meanwhile, TPEF could longitudinally scan the abscesses by z-stack scanning to investigate the thickness of epidermis and the morphology of epidermal cells and collagen fibres at various depths (Figure 6C,E).
From the time dimension, the model was divided into two phases: the inflammation period and the recovery period based on the observation results. The inflammation period typically lasts from Day 1 to Day 7, whereas the recovery period lasts from Day 7 to Day 14 (Figure 6G). During the first 3 days of the inflammation period, there is a significant colonization of bacteria, which coexist with inflammation, and the epidermis began to thicken. After 3 days, the bacteria were gradually swallowed, the inflammation was severe from the third to the fifth days and gradually subsides by the 7th day. On the third day, follicular hyperkeratinization was observed, all dynamically revealed the evolution of the acne model in vivo.
This research examined the process of acne evolution in animals model for the first time. The study revealed that inflammation and bacterial colonization occur simultaneously, and as bacteria were engulfed, the bacterial abundance decreased, and inflammation slowly subsided, and then entered the recovery period. We also investigated the interaction and influence of various pathogenic factors for the first time, which showed that the epidermis thickened in the lesions, and blood flow gathered in the lesions during the inflammation period. As the inflammation regressed, blood flow returned to normal. Moreover, we observed that the hyperkeratosis of hair follicle and bacterial colonization caused the inflammation coincidentally, which resembled the pathogenesis of human acne. This finding could provide further insight into the pathogenesis of acne.
The use of optical imaging techniques in clinical settings has the potential to improve the accuracy of acne diagnosis and facilitate more effective treatment strategies. By providing objective measurements of key acne-related factors, such as whether the inflammation has subsided and whether there is follicular hyperkeratosis creating an anaerobic environment that allows P. acnes to proliferate and cause inflammation. Additionally, this assessment method may aid in the screening of anti-acne drugs that target multiple pathways, including keratinization, inflammation and bacterial proliferation, as well as promote cell regeneration and collagen synthesis. Moreover, this approach may provide experimental evidence to treat acne and facilitate the selection of precise drugs for clinical use.
AUTHOR CONTRIBUTIONS
DL and YS contributed equally to this work and should be considered co-first authors. YW contributed to the conceptualization. DL contributed to the writing–original draft. DL, YS, NZ, XR, GH, LL and SM contributed to the research investigations. DL and YS contributed to the statistical analysis and methodology. DL, YW and YS contributed to the writing—review and editing. YW and YS contributed to the supervision. Each author listed on the manuscript has seen and approved the submission of this version of the manuscript and takes full responsibility for the manuscript.
ACKNOWLEDGEMENTS
The authors acknowledge the Fundamental Research Funds for the Central Public Welfare Research Institutes (XTCX2021002, RXRC2022004, JBGS2021007 and ZZ13-YQ-077) and CACMS Innovation Fund (CI2021A00601).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




