Suppression of IL-12/IL-23 p40 subunit in the skin and blood of psoriasis patients by Tofacitinib is dependent on active interferon-γ signaling in dendritic cells: Implications for the treatment of psoriasis and interferon-driven diseases
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Abstract
Interleukin (IL)-12 and IL-23 are pro-inflammatory cytokines produced by dendritic cells (DCs) and associated with Psoriasis (Pso) and Psoriatic Arthritis (PsA) pathogenesis. Tofacitinib, a Janus kinase inhibitor, effectively suppresses inflammatory cascades downstream the IL-12/IL-23 axis in Pso and PsA patients. Here, we investigated whether Tofacitinib directly regulates IL-12/IL-23 production in DCs, and how this regulation reflects responses to Tofacitinib in Pso patients. We treated monocyte-derived dendritic cells and myeloid dendritic cells with Tofacitinib and stimulated cells with either lipopolysaccharide (LPS) or a combination of LPS and IFN-γ. We assessed gene expression by qPCR, obtained skin microarray and blood Olink data and clinical parameters of Pso patients treated with Tofacitinib from public data sets. Our results indicate that in DCs co-stimulated with LPS and IFN-γ, but not with LPS alone, Tofacitinib leads to the decreased expression of IL-23/IL-12 shared subunit IL12B (p40). In Tofacitinib-treated Pso patients, IL-12 expression and psoriasis area and severity index (PASI) are significantly reduced in patients with higher IFN-γ at baseline. These findings demonstrate for the first time that Tofacitinib suppresses IL-23/IL-12 shared subunit IL12B in DCs upon active IFN-γ signaling, and that Pso patients with higher IFN-γ baseline levels display improved clinical response after Tofacitinib treatment.
1 BACKGROUND
Dendritic cells (DCs) are professional antigen-presenting cells that form the bridge between innate and adaptive immunity. Myeloid dendritic cells (mDCs) are circulating DCs,1 while monocyte-derived dendritic cells (moDCs) are tissue-resident DCs that are present at the sites of inflammation.2 Activated DCs produce pro-inflammatory cytokines that prime naïve T-lymphocytes into specific effector phenotypes3, 4 and, when undergoing inappropriate chronic activation, can drive autoimmunity.5
Some of the pathways by which DCs contribute to chronic immune activation are induced by the production of IL-23 and IL-12.6 The IL-23 cytokine consists of two subunits, IL23A (IL-23p19) and IL12B (IL-12p40). The p40 subunit is shared with IL-12, while p19 is unique to IL-23. Both IL-12 and IL-23 have an important role in the differentiation of naïve T-lymphocytes into T-helper (Th) interferon (IFN)-γ-producing Th1 or IL17-producing Th17 cells, respectively.7-10
Disorders associated with deregulations of the IL-23/IL-17 and IL-12/IFN-γ immune axis include psoriasis (Pso) and psoriatic arthritis (PsA), among others.6 Pso patients display an increased presence of inflammatory DCs expressing IL-12 and IL-23 in lesional skin.11 Similarly, in PsA patients, elevated IL-12 expression is observed in serum and synovial fluid.12-14
Treatment of these diseases with Janus kinase (JAK) inhibitors has proven to be effective in recent clinical trials.15 Specifically, Tofacitinib, an oral small molecule inhibitor targeting JAK1, JAK2, JAK3 and, to a lower extent, Tyrosine Kinase 2 (TYK2)16 significantly reduces psoriatic and arthritic manifestations, while displaying comparable benefit/risk profiles with biologicals.17, 18
In vitro studies indicate that Tofacitinib suppresses the differentiation of Th1 and Th17 cells and the production of IFN-γ and IL-17.19, 20 A plausible mechanism of action consists in the suppression of JAK2/TYK2 activation mediated by IL-12 and IL-23 binding, which further prevents STAT3 and STAT4 nuclear translocation.21 However, Tofacitinib was also shown to reduce IL12B and IL23A mRNA levels in Pso lesional skin and in imiquimod-treated mice, suggesting a direct role in the regulation of the upstream cytokines leading to Th1 and Th17 differentiation.22, 23
2 QUESTIONS ADDRESSED
Current literature indicates that Tofacitinib suppresses the inflammatory cascades downstream IL-12/IL-23, key cytokines in Pso/PsA development.6 However, whether Tofacitinib is able to suppress IL-12/IL-23 production by DCs has not been documented. Here, we aimed to investigate whether Tofacitinib is able to regulate the expression of IL-12 and IL-23 in DCs, and to determine how this regulation can influence responses to Tofacitinib in Pso patients.
3 EXPERIMENTAL DESIGN
3.1 Cell isolation
Peripheral blood mononuclear cells (PBMCs) derived from either healthy control blood or buffy coats were separated with Ficoll gradient (#17-1440-02, GE Healthcare). Blood was collected following institutional ethical approval. CD1c (BDCA-1)+ mDCs and CD14+ monocytes were isolated (Miltenyi Isolation Kit #130-090–506, #130-050–201) and separated on autoMACS Pro Separator according to manufacturer's instructions. Purity was checked by flow cytometry on BD LSRFortessa™ (BD Biosciences). Before culturing, cells were washed with complete medium consisting of RPMI 1640 medium, GlutaMAX™ Supplement (#61870-036, ThermoFisher Scientific), 10% Fetal Bovine Serum (Biowest) and 1% Penicillin-Streptomycin (#15070063, ThermoFisher Scientific). mDCs were stimulated on the day of isolation, after they were rested for 1 h at 37°C.
3.2 Generation of monocyte-derived dendritic cells
To generate moDCs, monocytes were cultured at 37°C for 6 days at a density of 106 cells/ml in the presence of 500 U/ml IL-4 and 800 U/ml GM-CSF (#204-IL-50, #215-GM-500, both from R&D Systems). Cytokines and medium were refreshed on day 3. On day 6, cells were harvested, washed with fresh complete medium and replated at a cell density of 106 cells/ml. Cells were left resting overnight at 37°C.
3.3 Stimulation of moDCs and mDCs
Immature moDCs and mDCs were pre-treated or not with 1 µg/ml Tofacitinib (CP-690550, Selleckchem) or Ruxolitinib (INCB018424, Selleckchem) for 30 min. After, cells were stimulated with either 10 µg/ml Lipoteichoic acid (LTA) (#L2515, Sigma Aldrich), 100 ng/ml LPS, 1 µg/ml R848 (#tlrl-3pelps, #tlrl-r848, Invivogen), 1000 U/µl IFN-α (#CRI003B, Cell sciences) or 1000 U/ml IFN-γ (#14-8319–80, eBioscience) for 4 h.
3.4 Statistical analyses of in vitro experimental data
Statistical analyses of DCs mRNA data were performed using GraphPad Prism 8.3 Software. Non-parametric Friedman test for paired samples followed by Dunn's multiple comparisons test were computed to compare gene expression levels between 0 and 4 h of stimulation. Values with a p < 0.05 were considered significant.
Details are provided in Appendix S1.
4 RESULTS
4.1 Treatment with Tofacitinib does not reduce IL23A and IL12B mRNA expression in TLR4-activated DCs
Consistent with previous findings,24 we observed that the mRNA expression of IL-23p19 (IL23A), IL-12p40 (IL12B) and IL-12p35 (IL12A) in both mDCs and moDCs is induced by TLR2, TLR4 and TLR7/8 activation(Figure S1A–C). TLR4 was previously found upregulated in Pso PBMCs25 and skin.26, 27 Additionally, serum and epidermal expression of S100A8 and S100A9, TLR4 ligands contributing to keratinocyte hyperproliferation, were found to be significantly higher in Pso patients in comparison with healthy controls.28 Therefore, we chose TLR4 activation by LPS as a model to mimic DC activation in Pso blood (Table 1).
| Gene | Sequence (5′ → 3′) |
|---|---|
| IL12B (IL12p40) |
Forward TGCCGTTCACAAGCTCAAGT |
|
Reverse TGGGTCAGGTTTGATGATGTCC |
|
| IL23A (IL23p19) |
Forward CAACAGTCAGTTCTGCTTGC |
|
Reverse GAAGGCTCCCCTGTGAAA AT |
|
| IL12A (IL12p35) |
Forward AGGGCCGTCAGCAACATG |
|
Reverse TCTTCAGAAGTGCAAGGGTAAAATTC |
|
| CXCL10 |
Forward TGAAATTATTCCTGCAAGCCAA |
|
Reverse CAGACATCTCTTCTCACCCTTCTTT |
|
| TNF |
Forward TCTTCTCGAACCCCGAGTGA |
|
Reverse CCTCTGATGGCACCACCAG |
|
| RPL35 |
Forward CATCTGGGGAAAAGTAACTCG |
|
Reverse AGCATCACTCGGATTCTGTG |
In LPS-stimulated mDCs and moDCs pre-treated with Tofacitinib, we could not observe a reduction in the expression of IL23A, IL12B and IL12A (Figure 1A,C). Surprisingly, the expression of TNF, another key cytokine playing a role in Pso pathogenesis and suppressed by Tofacitinib in vivo,22 was also not suppressed by Tofacitinib in LPS-stimulated DCs (Figure 1B,D). We could confirm that Tofacitinib efficiently blocks the JAK/STAT signaling in these cells, as determined by the suppression of control CXCL10 expression in paired samples (Figure 1E). We further demonstrated that lack of downregulation of IL-12/IL-23 cytokines in LPS-stimulated DCs also occurs in the presence of the JAK1/JAK2 inhibitor Ruxolitinib (Figure S2), thus it is not solely attributed to Tofacitinibs mode of action.
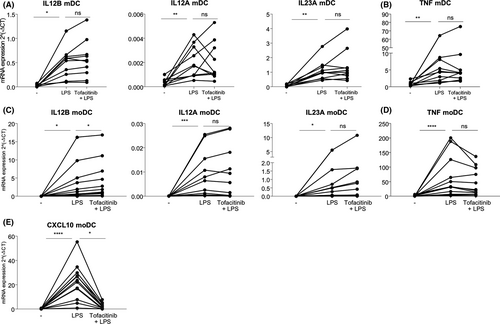
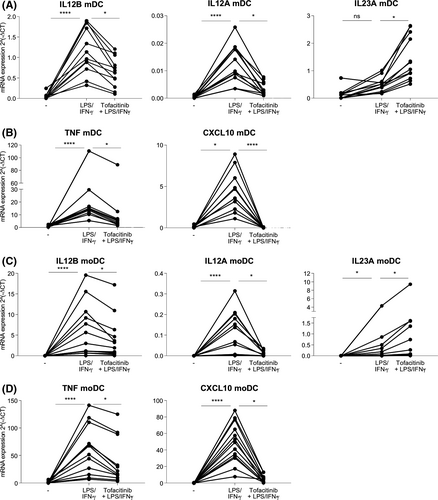
4.2 Co-stimulation with LPS and IFN-γ leads to reduced expression of IL12B mRNA in DCs treated with Tofacitinib
Previously, Bechera et al. reported that JAK1 inhibition in moDCs suppresses IL-23 and IL-12 expression when cells are stimulated with a combination of nickel sulphate, a TLR4 agonist, and IFN-γ.29 We thus investigated the effects of LPS and IFN-γ stimulation on mDCs and moDCs pre-treated with Tofacitinib. Co-stimulation of DCs with LPS and type II IFN-γ led to a significant reduction of IL12B, IL12A, TNF and CXCL10 mRNA expression upon Tofacitinib treatment (Figure 2A–D). Conversely, co-stimulation with type I IFN-α did not lead to similar modulatory effects in these cells (Figure S3). These data indicate that an active type II, but not type I, IFN signaling in DCs is required for the suppression of IL12B and other pro-inflammatory cytokines by Tofacitinib.
4.3 Psoriasis patients with higher IFN-γ levels display reduced IL-12 expression and skin severity score after Tofacitinib treatment
To test the relevance of IFN-γ stimulation in the suppression of IL-12 by Tofacitinib, we analysed publicly available data of Pso patients before and after Tofacitinib treatment to assess whether higher baseline levels of IFN-γ could predict lower IL-12 production and improved Psoriasis Area and Severity Index (PASI) score after Tofacitinib treatment.
From blood protein Olink data (GSE136435) obtained from 266 psoriasis patients, we found that IL-12 levels were significantly reduced after 4 weeks of Tofacitinib treatment, while more modestly reduced after 4 weeks of Etanercept treatment (Figure 3A). IL-23 was not determined in this assay.30 We further assessed the baseline IFN-γ levels in the two treatment groups and defined IFN-γ-high and IFN-γ-low patients (Figure 3B). We found that patients with higher IFN-γ levels at baseline also displayed higher IL-12 levels (Figure 3C). By calculating the difference in IL-12 levels between 4 week treatment and baseline (ΔIL-12), we identified that patients with higher IFN-γ levels better benefited from Tofacitinib, but not Etanercept, treatment in terms of suppression of IL-12 levels (Figure 3D). To account for the low expression of circulating IFN-γ, we performed a principal component analysis (PCA) to identify IFN-γ-clustering proteins (Table S1). We found that a higher IFN-signature at baseline was related with a better reduction in PASI scores after 12 weeks of treatment with Tofacitinib (unstandardized B = −2.701, p = 0.033, 95%CI [−5.177, −0.226]). Conversely, in Pso patients treated for 12 weeks with Etanercept, an improved PASI outcome related to higher IFN-signature was not observed (unstandardized B = 0.331, p = 0.735, 95%CI [−1.606, 2.268]) (Figure S4).
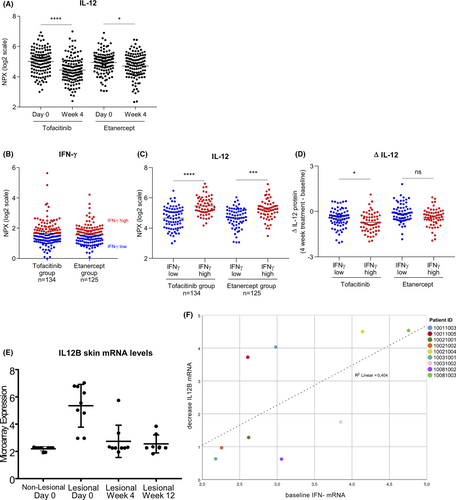
From skin microarray data (GSE69967) obtained from 9 psoriasis patients, IL12B mRNA levels were reduced in lesional skin of Tofacitinib-treated Pso patients (Figure 3E), as previously described.22 Given the low number of patients in this study, we could only observe a trend for lower ΔIL-12 expression in individuals with higher IFN-γ at baseline (Figure S5). However, higher basal IFN-γ levels correlated with a greater reduction of IL12B mRNA (decrease of 0.015 IL12B units per higher IFN-γ unit on average, p = 0.039, 95%CI [−0.029, −0.001]) (Figure 3F). Baseline characteristics are provided in Table S2.
5 CONCLUSIONS AND PERSPECTIVES
The accumulating knowledge that IL-23 and IL-12 play a key role in the initiation and maintenance of several inflammatory diseases, such as Pso and PsA,6 led to the development of multiple selective therapeutic agents that target either specific cytokines or their downstream inflammatory events.31 Tofacitinib is the first JAK-inhibitor registered for the indication of Pso and has shown promising results in clinical trials.32 Studies have shown that multiple immunoregulatory pathways, such as STAT1/STAT3 in Pso skin and NF-κB in PsA synovial fibroblasts, are targeted by Tofacitinib.22, 33, 34 However, whether Tofacitinib suppresses IL-12 and IL-23 directly, or rather indirectly through general immune suppression, has not yet thoroughly been studied.35
To answer this research question, we made use of mDCs and moDCs as representative models for systemic and localized inflammation.36 We observed that both cell types increased the expression of IL23A and IL12B after TLR4 stimulation, in line with previous studies.24, 35, 37 The stimulation with IFN-γ, a direct activator of the JAK/STAT-pathway, did not induce the expression of these cytokines, while potently inducing CXCL10, a known IFN-inducible gene (Figure S1B).
Upon LPS activation, we found that Tofacitinib was not able to suppress IL23A and IL12B mRNA expression in neither mDCs nor moDCs. A similar observation was reported earlier in a model of allergic contact dermatitis by Bechara et al.29 These results indicate that the presence of IFN-γ is needed for Tofacitinib to efficiently reduce IL12B expression in DCs.
Unlike IL12B, IL23A expression was not potentiated by LPS+IFN-γ stimulation, nor reduced by Tofacitinib. In fact, IL23A expression was decreased upon stimulation with LPS+IFN-γ, as compared with LPS alone (Figure S6). Combined LPS+IFN-γ stimulation in DCs was previously shown to promote IL23A mRNA degradation, while enhancing IL12A and IL12B mRNA stabilization.38 Thus, these findings indicate that IL-12 and IL-23 could be differentially regulated by Tofacitinib in DCs.38, 39 However, as previously documented by Bechara et al., the overall IL-23 protein production is reduced by JAK inhibition as a result of the significant suppression of the IL-23 subunit IL12p40.29 As such, Tofacitinib could still play a significant role in reducing IL-23 production, despite transiently inducing IL23A mRNA expression.
A differential response to Tofacitinib was observed in DCs stimulated with type I or type II IFN. Type II IFN-γ signals through JAK1/JAK2, which in turn promote STAT1 homodimers and interferon regulatory factor (IRF)1/IRF8 complexes.40, 41 Conversely, type I IFN-α and IFN-β signal through JAK1/TYK2, which lead to STAT1/STAT2 heterodimers associating with IRF9.42 Indeed, IRF1 and IRF8 are potently induced upon LPS+IFN-γ co-stimulation, in comparison with LPS stimulation alone.40, 43 Thus, it is plausible that Tofacitinib-dependent suppression of IL12B expression requires inflammatory events triggered by type II, but not type I IFN signaling, such as modulation of IRF1/IRF8 transcription factors. From the skin data set, we could identify a distinct expression of IRF1/IRF8 in paired lesional and non-lesional samples of Pso patients and a reduced expression in lesional skin after Tofacitinib treatment (Figure S7).
In whole lesional Pso skin, we found that higher basal levels of IFN-γ correlated with a greater reduction in IL12B after 12 weeks of Tofacitinib treatment. Additionally, from whole blood data, we observed that Pso patients with higher IFN-signature at basal levels displayed a better decrease in IL-12 after Tofacitinib, but not Etanercept, treatment which was accompanied by a significant reduction in PASI score.
Overall, our findings imply a novel role for IFN-γ in eliciting IL12B suppression by Tofacitinib in DCs. These results can be relevant for diseases characterized by IFN-γ involvement and aid to predict therapeutic responses to Tofacitinib in Pso patients.
ACKNOWLEDGEMENTS
The reviewers are thanked for critically reading the manuscript and suggesting substantial improvements.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Performed the research: NV, CA, MON, CB and EL. Designed the research study: NV, CA, SSC and AL. Contributed essential reagents or tools: NV, CA, SSC, AL and EL. Analysed the data: NV, CA and PW. Wrote the paper: NV, CA, TR and PW. All authors listed qualify for authorship according to the criteria as mentioned by the journal.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




