New insights into the roles of myofibroblasts and innervation during skin healing and innovative therapies to improve scar innervation
Abstract
During the resolution phase of normal skin wound healing, there is a considerable loss of various cell types, including myofibroblasts by apoptosis. Inappropriate delay of apoptosis, and thus increased survival of myofibroblasts, may be a factor leading to pathologies and excessive scarring. Considerable data now clearly suggest that innervation plays a major role in wound healing, including the modulation of fibroblast cellular activity. An abnormal level of neuromediators is implicated not only in the development of chronic wounds but also in excessive scar formation. Understanding interactions between neuromediators and myofibroblasts, allowing normal reinnervation and having adequate levels of neuromediators during the healing process are clearly important to avoid the appearance of pathological healing or fibrosis/scarring. The aim of this review was first to discuss the mechanisms leading to normal or excessive scarring and then to present the roles of innervation during wound healing. Finally, the latest therapeutic strategies to help wound repair and reinnervation after skin damage will be introduced. Advantages and limitations in the use of neuropeptides, growth factors and biomaterials will be discussed as well as the most recent studies on electrostimulation and the potential of targeting resident skin mesenchymal stem cells.
Abbreviations
-
- BDNF
-
- brain-derived neurotrophic factor
-
- CGRP
-
- calcitonin gene-related peptide
-
- DRG
-
- dorsal root ganglion
-
- ECM
-
- extracellular matrix
-
- GAG
-
- glycosaminoglycans
-
- GRP
-
- gastrin-releasing peptide
-
- iPS
-
- induced pluripotent stem cell
-
- MMP
-
- matrix metalloproteinases
-
- MSC
-
- mesenchymal stem cell
-
- NGF
-
- nerve growth factor
-
- NT
-
- neurotrophin
-
- SKP
-
- skin-derived precursor
-
- SP
-
- substance P
-
- TGF-β1
-
- transforming growth factor-β1
-
- TIMPs
-
- tissue inhibitor of metalloproteinases
-
- VIP
-
- vasoactive intestinal polypeptide
1 INTRODUCTION
In view of the important functions of the skin as a barrier against foreign pathogens and water loss, and in the regulation of body temperature and sensory perception, normal wound healing can be considered as a process that is critical for survival. In current medical practice, severe scarring disorders can affect patient quality of life at a functional level, but also have aesthetic and psychosocial effects. Although several therapeutic strategies have been studied, scarless regeneration of injured skin remains out of reach. Understanding the mechanisms involved in normal and pathological healing processes is therefore of fundamental importance. Many different cell types play important roles after skin damage and a dialogue between these cells is essential. Among these cells, myofibroblasts, due to their contractile properties and their capacities to both synthesize and remodel the extracellular matrix (ECM), play a pivotal role.1 More recently, the roles of nerve fibre endings and neuromediators in the different phases of the cutaneous healing process have been highlighted.2 This review will discuss the possible role of peripheral nerves during skin scarring and the evidence for an interplay with myofibroblasts in orchestrating skin repair. The latest therapeutic strategies allowing functional reinnervation within the healing tissue will also be summarised.
2 GENERAL MECHANISMS OF SCAR FORMATION
Following skin injury and blood vessel damage, a provisional matrix composed mainly of fibrin and fibronectin is established and contributes to the initiation of the inflammatory response. This provisional matrix allows the migration of fibroblasts into the wound bed where they acquire a myofibroblastic phenotype and contribute to the formation of the granulation tissue. These myofibroblastic cells synthesize and deposit ECM components which progressively replace the provisional matrix. Myofibroblasts also exhibit contractile properties, due to the expression of α-smooth muscle actin in microfilament bundles or stress fibres, playing a major role in contraction and in maturation of the granulation tissue.3 Myofibroblasts can, depending on the experimental or clinical situation, express other smooth muscle related contractile proteins, such as desmin or smooth muscle-myosin heavy chain; however, the expression of α-smooth muscle actin represents the most reliable marker of the myofibroblastic phenotype.4 The last phase of healing, scar formation, involves a progressive remodelling of the granulation tissue. During this remodelling process, proteolytic enzymes, essentially matrix metalloproteinases (MMPs), and their inhibitors (tissue inhibitor of metalloproteinases, TIMPs) play a major role.5 The synthesis of ECM is at this point considerably reduced. In addition, the synthesized components are modified as the matrix is remodelled. Progressively, type I collagen, the main structural component of the dermis, replaces type III collagen which is the major collagenous component of granulation tissue. Finally, elastin which is absent in the granulation tissue and normally contributes to skin elasticity also reappears. During the resolution phase of healing, generally, when the tissue integrity has been sufficiently restored to be mechanically coherent, cellularity is drastically reduced by apoptosis of both myofibroblasts and vascular cells.6 The signal triggering this cell death is currently unknown but may be related to modifications in the concentrations of local trophic factors, including neuromediators, or in changes in myofibroblast adhesion to the ECM. Interestingly, smooth muscle cell contraction is rapid and short, whereas myofibroblast contraction is in contrast long-lasting, resulting in a more permanent tissue retraction.7 Interactions between myofibroblasts and the ECM are certain to be involved in this specific situation. This long-duration type of contraction that is present in myofibroblasts explains, at least in part, the roles played by these cells during formation and remodelling of excessive scarring such as that seen in hypertrophic scars and fibrotic tissues.
3 ROLES OF INNERVATION AND NEUROPEPTIDES IN NORMAL SKIN AND DURING HEALING
The nervous system, essentially in the form of sensory nerve fibre endings, is present within the skin and influences a variety of physiological and pathophysiological cutaneous functions. Cutaneous sensory nerve fibres are endings of dorsal root ganglia (DRG) neurones that carry signals from sensory organs towards the appropriate integration centre of the brain via the spinal cord. The skin is a highly sensitive organ which is densely innervated both in the dermis and the epidermis, discriminating between pain, pruritus, thermal and tactile sensations.8 When deep skin damage occurs, cutaneous nerves and sensory receptors are destroyed while the neurone cell bodies persist in the DRG. Mechanical stimuli are detected via mechanoreceptors associated with sensory corpuscles through Aβ fibres or with Aδ free nerve fibre endings, temperature via the thermoreceptors and pain via the nociceptors both through Aδ and C free nerve fibre endings.9 These fibres are accompanied by Schwann cells in the dermis but not in the epidermis. The larger nerve fibres, Aβ and free nerve fibre endings Aδ, are associated with Schwann cells, which produce myelin sheaths. C free nerve fibre endings are the termination of unmyelinated fibres and are associated in the dermis with Schwann cells which do not produce myelin. In addition to mechanical, thermal and pain stimuli, C and Aδ free nerve fibre endings respond to a range of physical stimuli (osmotic changes, ultraviolet light), chemical and biochemical agents (toxic agents, allergens, proteases).10
In unstimulated nerves, neuromediators are barely detectable within the skin. Upon direct stimulation or during pathological situations such as inflammation or trauma, a significant increase in neuromediator levels is observed. Skin lesions and peripheral nerve damage cause resident and infiltrating immune cells, and sensory nerve terminals themselves, to release neuro-inflammatory mediators including interleukin-1α, tumor necrosis factor-α, bradykinin, substance P (SP), calcitonin gene-related peptide (CGRP), nerve growth factor (NGF), and prostaglandins, thus contributing to the “inflammatory soup.”11 Studies using capsaicin-mediated denervation have further highlighted the role of neuromediators in wound healing.12, 13 Other experimental studies carried out by Hsieh et al in a rodent model with denervated skin and Lebonvallet et al using re-innervated cultured skin explants have also described the impact of innervation on keratinocyte proliferation and epidermal thickness.14-16 Thus, neuromediators derived from sensory nerves may play an important regulatory role in the skin under many physiological and pathophysiological conditions. Furthermore, neuromediators such as neuropeptides, neurohormones, cytokines and growth factors are involved in different steps of wound healing, implicating the participation in the healing process of all skin tissues and cells, including epidermis, dermis, vessels and immune cells.2, 17-19
4 PATHOLOGICAL SCARRING
Myofibroblasts are involved in many pathological scarring and fibrotic diseases where they are responsible for the important process, after initial injury, of providing mechanical support and integrity to the tissue. It is now accepted that, in many scarring and fibrotic conditions, as well as in the stromal response to epithelial tumors, myofibroblasts fail to undergo cell death (as occurs in physiological situations), persist and thus in turn lead to ongoing pathology and excessive scarring (Figure 1). In clinical research, it has been reported that α-smooth muscle actin is detected in hypertrophic scars but not in keloids.20 Contractures develop in hypertrophic scars due to the abundant presence of α-smooth muscle actin-expressing myofibroblasts but these do not occur in keloids. An example of reduced or inhibited apoptosis leading to excessive scarring can be observed in a model where mechanical loading increases the survival of myofibroblasts in turn inducing excessive scar formation.21, 22 A better understanding of the control and signalling that governs apoptosis (or autophagy) in myofibroblasts may lead to more targeted approaches avoiding both fibrosis and excessive scarring. Apoptosis in myofibroblasts is thought to be regulated by a reduction in the local growth factors that drive and sustain myofibroblast differentiation, in particular local concentrations of transforming growth factor-β1 (TGF-β1) and endothelin-1 which play a role in stimulating myofibroblast survival via AKT activation.23 However, changes in mechanical signalling such as unloading of mechanical force, likely also play a role.24 Recently, it has been shown that stretch induces the activation of latent TGF-β1 in a model of pulmonary fibrosis and maintains a vicious circle involving myofibroblast contraction and ECM deposition.25 Obviously, it is not possible to stop breathing to avoid mechanical TGF-β1 activation in established fibrotic tissue. However, therapeutic interventions may be possible, particularly during hypertrophic scar development, by targeting the cellular side of mechanical TGF-β1 activation. The importance of myofibroblasts in causing fibrosis in internal organs and in the skin (hypertrophic scars), and the role that persistence of stromal myofibroblasts appear to play in tumor growth and spread, makes the (down)regulation of myofibroblasts and the potential regulation of myofibroblast disappearance through apoptosis of increasing interest.26

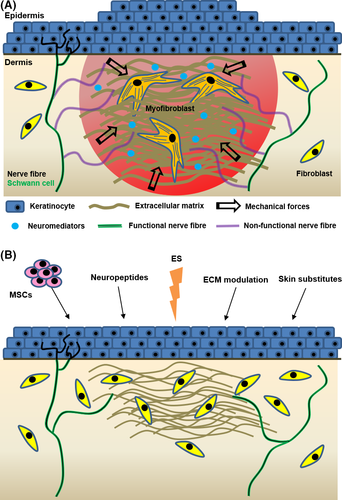
Little is known about the role of sensory fibres in modulating myofibroblast differentiation and activity. Interestingly, altered SP levels have been suggested to contribute to impaired cutaneous healing responses associated with chronic wounds or hypertrophic scar formation.27 Topical application of exogenous SP enhances wound closure kinetics in diabetic rats, suggesting that diabetic wounds may have insufficient SP levels to promote a neuro-inflammatory response necessary for normal wound repair (Table 1).28 In contrast, increased nerve fibre numbers and neuropeptide levels with reduced neutral endopeptidase (a membrane-bound metallopeptidase that degrades SP at the cell membrane) levels are found in hypertrophic scar samples.29 This suggests that excessive neuropeptide activity can induce exuberant inflammation and ECM deposition with persistent activation of myofibroblasts in hypertrophic scars (Figure 2A). Other neuropeptides of interest, such as vasoactive intestinal peptide (VIP), CGRP, neurotrophic factors such as NGF and brain-derived neurotrophic factor (BDNF), as well as neurotrophins (NTs) such as NT-3 and NT-4 have been shown to enhance fibroblast cellular activity either in vitro, in skin explants or in in vivo healing studies (Table 1). These neuropeptides are secreted not only by nerve endings but as well by invading immune cells and maybe as well by epithelial cells (keratinocytes, hair epithelial cells) and stem cells present in the area.2
| Neuromediators | Experimental model | Effect(s) on fibroblasts | Reference | |
|---|---|---|---|---|
| Neuropeptide(s) | SP | Cultured human fibroblasts | ↗ Migration | 30 |
| SP, VIP, CGRP | Human skin explants | ↗ Adhesion and differentiation | 17 | |
| SP | Excisional skin wound in diabetic rats | ↗ Proliferation | 28 | |
| Neurotrophin(s) | NGF | Excisional skin wound in rats | ↗ Migration | 31 |
| NGF, BDNF, NT-3, NT-4 | Cultured human fibroblasts | ↗ Migration and differentiation | 32 | |
| NGF, BNDF | ↗ Contractile activity | |||
- BDNF, brain-derived neurotrophic factor; CGRP, calcitonin gene-related peptide; NGF, nerve growth factor; NT, neurotrophins; SP, substance P; VIP, vasoactive intestinal polypeptide.
Fujiwara et al have demonstrated that direct contacts between fibroblasts and neurones are necessary for differentiation into myofibroblasts and for the induction of collagen gel contraction, both important processes that are able to promote wound healing. However, the molecular mechanism driving fibroblast differentiation through direct contact with neuronal processes was not identified in this study.33 Another study has also shown that both keratinocytes, and to a lesser extent fibroblasts, display trophic effects on sensory neurones via the secretion of glial cell-line-derived neurotrophic factor and NGF. Unfortunately, the establishment of direct cellular contacts was not investigated in this study.34 More recently, Krishnan-Kutty et al35 have reported that keratinocytes promoted cellular contact with neural crest cells differentiated into sensory neurones, whereas fibroblasts did not. These contradictory results can, in part, be explained by differences in cellular models that need to be better characterized. Interactions between neurones and fibroblasts have been clearly demonstrated but more investigations are needed to shed light on the nature of specific cross-talk and the cellular contacts involved.
Nerve regeneration also occurs during the wound healing process.36 Interestingly, symptoms of itch and pain, and abnormal thermo-sensory thresholds, are present in excessive scarring in the skin, suggesting that these pathological situations are closely associated with small nerve fibres. Moreover, the density of neuropeptide-containing nerve fibres was found to be higher in tissue with excessive scarring compared to normal skin samples.37, 38 Recently, we have demonstrated the importance of sensory innervation in the process of cutaneous healing associated with neuropathies.39 Specifically, damage to skin innervation leads to delayed healing and to the development of abnormal innervation in the scar tissue.
5 THERAPEUTIC STRATEGIES TO IMPROVE WOUND HEALING AND REINNERVATION AFTER SKIN LESIONS
5.1 Neuropeptides
Two distinct but complementary therapeutic approaches may be used to accelerate the resolution of wound healing. One approach using neuropeptides normally produced by neuronal cells influencing skin cells (eg, SP, CGRP) and the other using growth factors (eg, NGF, BDNF) permitting neuronal regrowth. SP and CGRP are widely studied neuropeptides with interesting results in skin healing while others, such as VIP, galanin, neurotensin and gastrin-releasing peptide (GRP), are also promising candidates. However, SP is known to induce secondary effects such as pain or pruritus.40-42 The use of a modified SP formulation keeping the beneficial effects without causing unpleasant sensations is of interest. Recently, SP has been used in combination with curcumin. This study showed that wound closure associated with neuronal and endothelial recovery was accelerated and associated with a modulation of inflammatory mediators.43
Strategies using neuropeptides to stimulate skin cells and trophic factors to stimulate neurite growth can be either combined or provided sequentially. A possible source of human neuropeptides, ideally from sensory neurones, could be generated using skin-derived precursors (SKPs) or induced pluripotent stem cells (iPS) differentiated into neurones.44, 45 Due to the distinct role of different neuropeptides and NTs, the use of a cocktail would be interesting with delivery either by single administration or ideally by delayed delivery into the wound.46 Thus, different delivery systems are possible via topical application or using vehicles such as microspheres, hydrogel or gelatin.47, 48 A recent study using a specific ECM to capture and deliver platelet-derived growth factor into the wound could also be an interesting alternative as a carrier of neuropeptides and to concentrate their positive effects within the wound.49
5.2 Extracellular matrix modulation
The ECM is important for scar formation and neurite growth. Hence, another interesting strategy is to modulate the ECM composition either by degradation using MMPs, addition of ECM, or by influencing the production of TIMPs, to then permit normal neuronal regrowth or are even able to accelerate it.50, 51 Among inhibitory factors of neurite growth, glycosaminoglycans (GAGs) have been widely documented. Chondroitin sulfate and dermatan sulfate proteoglycans are known to have an inhibitory effect on neuronal regrowth52, 53 and the degradation of GAGs by chondroitinase permits axonal regeneration.54-56 However, the role of GAGs in skin repair still needs to be further studied as they possess important functions that favour wound healing through actions on keratinocytes and fibroblasts. Using natural or artificial ECM molecules delivered into the wound could be another interesting approach to promote nerve recovery. Santos et al have shown that a combination of laminin and NGF/NT3 induces a synergistic and beneficial effect on neurite growth and density in rat DRG explants. The same combination of factors promotes sensory function recovery after injury associated with nerve fibre regeneration in the epidermis and skin appendages.57
5.3 Biomaterials and skin substitutes
Another therapeutic strategy to improve reinnervation during the wound healing process is the use of biomaterials (for review, see 36). These biomaterials must be biocompatible, flexible and porous. Nerve conduits currently used in the clinic are either natural or synthetic, but none allows complete functional repair. Among synthetic biomaterials, we can distinguish between materials consisting of polylactic acid, poly-ε-caprolactone, polylacticoglycolic acid, polyamidoamin, polyethylene glycol and silicone. The principal advantage of using synthetic biomaterials is their mechanical integrity; however, they are not degradable. Natural materials used to improve nerve regeneration include chitosan, collagen, hyaluronic acid, keratin, silk fibroin, fibronectin, keratin and gelatin. These biomaterials are biocompatible and biodegradable, but their main drawback is their low mechanical resistance. To overcome this limitation, most of these biomaterials are used in gel form. Given their promising properties, several research teams have developed composite materials combining synthetic and natural materials. For example, Kijeńska et al58 have developed a material with poly (l-lactic-acid)-copoly (ε-caprolactone) (P(LLA-CL)) and collagen and they showed that this composite material more efficiently stimulates nerve growth compared to P(LLA-CL) alone. Currently, new materials are designed with a synthetic external layer and a natural flexible material internal layer to facilitate cell migration through the conduit.59, 60 Some of these materials used to improve nerve regeneration have been approved by the Food and Drug Administration or by Conformit Europe. Seven are currently used by surgeons. For example, NeuraGen®, Neurotube® and Neurolac® have shown good results in terms of nerve regrowth and in the restoration of sensitivity and mobility.61-63 Neurolac®, however, shows a lack of degradability as fragments have been shown to persist in the duct 2 years after grafting.64
Currently, a number of skin substitutes are available to accelerate wound closure and enhance wound healing.36, 65 Unfortunately, none of them are designed to promote wound reinnervation and it has also been suggested that promoting fast wound closure may be deleterious on nerve regrowth. New skin substitutes combining biomaterials and cells or substances able both to stimulate nerve regrowth and to promote skin healing are thus needed to offer a more suitable and complete therapeutic option.
5.4 Skin mesenchymal stem cells
Adult mesenchymal stem cells (MSCs) are present in various organs and possess capacities of self-renewal and multipotency. They have been intensively studied and represent promising tools in the field of regenerative medicine, notably due to their immunotolerance as well as immunomodulation properties. MSCs can be isolated and cultured ex-vivo to then be transplanted as a cell-mediated treatment. Upon differentiation, they can generate various cell lineages including neuronal and glial cells. Sources of easily accessible and intensively studied autologous MSCs originate from the adipose tissue, bone marrow and dental tissue. The umbilical cord, as well as the amniotic membrane, are also potential sources of allogenic MSCs.66
The skin also possesses its own reservoir of dermal MSCs termed SKPs.67 For more information, see “Appendix S1” with Figure S1. SKPs form a neural crest-related stem cell niche that arises in the skin during embryogenesis and persists, although in lower numbers, into adulthood.68 Little is known regarding the role of SKPs in wound healing, but several studies have shown that SKP-derived Schwann cells may help in promoting peripheral nerve regeneration.69-72 SKP-derived Schwann cells or SKPs themselves are, in a model of nerve injury, capable of inducing remyelination and regeneration of peripheral nerves.73, 74 Indeed, without predifferentiation, grafted proliferative SKPs induce positive effects on cutaneous nerve regeneration. Using a Lac-Z reporter mouse model, Lac-Z positive SKPs were shown to spontaneously migrate to nerve fibres and differentiate into myelinating Schwann cells to promote nerve repair.75 Nonmyelinating Schwann cells are also certainly involved in unmyelinated fibre repair. In another study, Stratton et al have also shown that SKP-derived Schwann cells assist peripheral nerve injury repair through their immunomodulatory properties. They further demonstrated that these cells can produce cytokines and their conditioned medium could induce increased tumor necrosis factor-α release by macrophages. After transplantation into injured sciatic nerve, the number of SKP-derived Schwann cells correlated with a higher macrophage density and clearance of myelin debris.76 These data suggest that SKPs and SKP-derived Schwann cells are fully-functional in promoting immune cell chemotaxis and efficient axonal regrowth. Sato et al77 have also shown that intradermal injections of SKPs around full-thickness excisional cutaneous wounds in diabetic mice mediated faster wound closure and re-epithelization, induced earlier angiogenesis and may thus promote wound reinnervation. Likewise, transplantation of SKPs in a collagen sponge facilitated wound healing in diabetic mice, this improvement being probably mediated by increased efficiency of angiogenesis.78 Interestingly, another study has also shown that SKP transplantation, in denervated cutaneous wounds in nude mice, promoted wound closure and the local secretion of neuromediators such as SP, as well as NGF.79 More studies are needed to determine if local or transplanted SKPs can either directly differentiate into Schwann cells following skin injury or if they in some way help to mediate the migration of local Schwann cells and/or axonal regrowth of nerve fibres during scar formation. SKPs represent an easily accessible and very promising source of autologous MSCs. Thus, the most straightforward therapeutic strategy would be direct targeting of resident dermal SKPs to promote wound healing and/or reinnervation within the wounded area.80 The study of SKPs’ secretome upon chemical or physical stimulation is of foremost interest in this context and could shed light on efficient therapeutic SKP “cell priming” strategies.
5.5 Electrostimulation
Electrical stimulation (ES) or electrotherapy is a noninvasive and pain-free treatment, known to promote skin healing, that is of growing interest. This method has beneficial effects, especially in chronic wounds and ulcers, as it stimulates cell proliferation and migration thus accelerating the healing process.81 It has also been shown to stimulate dermal angiogenesis and keratinocyte proliferation.82, 83 The principle of ES is to mimic the endogenous electric current that occurs during wound healing and for that purpose, it is essential to establish ideal and standardized protocol parameters such as: modality, method of delivery and duration of treatment.84 ES has also been used to manage chronic neuropathic pain and improve nerve function in diabetic patients.85, 86
The impact of ES on wound reinnervation has been recently reported. Sebastian et al have shown that ES contributes to increased expression of PGP9.5 and TUBB3, neural differentiation biomarkers, and SP neuropeptide within the wound. They also describe an expansion of melanocytes and TUBB3+ Merkel cells (specialized epidermal mechanoreceptors) during healing.87 Considering the role of innervation in wound healing and the beneficial impact of ES treatment, it can be suggested that ES could directly stimulate local nerve fibre endings to promote repair and/or encourage the reinnervation process within the wound. The underlying biomolecular and cellular mechanisms involved in this therapy remain to be precisely elucidated, but ES represents a promising therapeutic option for future wound treatment.
5.6 Mechanical stimulation and physical therapies
Several studies have shown the importance of the mechanical environment in pathological scarring.88-91 Chin et al92 have reported that in a mouse stretched skin model, the expression of neuropeptides and growth factors is increased, whereas their respective receptors are down-regulated. In another study, vacuum assisted wound closure was reported to be associated with increased SP, CGRP and NGF expression, higher dermal and epidermal nerve fibre densities and improved wound healing kinetics.93 Moreover, it has been demonstrated that tension applied to human dermal fibroblasts increased the production of NGF. However, the effect on wound healing has not been investigated.94 These studies suggest that a contained or controlled mechanical stimulation induced by various systems (eg, strap, vacuum, or any other mechanical stimuli) may help to improve wound healing by stimulating skin nerve fibre endings and resident skin cells to release neuromediators.
Currently, the effect of physical therapies including manual techniques, physical activities, hydrotherapy or thermotherapy, have not been thoroughly investigated on wound healing. However, several studies have shown that physical activities appear to have a beneficial effect, for very different reasons, on nerve fibre regeneration after peripheral nerve injury or neuropathies.95-99 Considering that mechanical and electrical stimuli, discussed above, seem to provide beneficial outcomes on wound healing, physical therapies could also represent promising alternative approaches that are worth testing.
6 MAJOR OUTSTANDING QUESTIONS AND HOW TO ANSWER THEM
Understanding the processes involved in excessive scar formation, particularly the roles played by the mechanical environment, is an important question that remains to be answered. Certainly, peripheral innervation and neuro-inflammatory mediators that are released are important factors contributing to skin healing and future studies will, it is hoped, shed light on new mechanisms involved in normal and pathological scarring. Current research using human primary cells and skin explants remain the gold standard, but a strong limitation of this approach arises from the limited access to human primary sensory neurones. In vitro and in vivo experimental models are useful in providing alternative sources of sensory neurones. They range from the culture of murine DRGs, generation of human iPS or MSCs-derived neurones and in vivo studies using murine or porcine models.
Several other questions remain to be answered concerning the mechanisms involved in the reinnervation process during healing and how it is possible to drive correct nerve fibre growth within the wound to both avoid neuropathic symptoms and improve scar quality. Among other approaches, the intricate link between reinnervation and angiogenesis is of foremost interest considering that the vascular tree could be used as a support to guide nerve fibre regrowth. It has also been shown that, during healing, mainly C free nerve fibre sprouting is observed.38, 100 More studies are required to promote efficient C, Aδ and Aβ nerve fibre sprouting and, in some cases, normal Aβ corpuscle reconstruction, to provide efficient sensory recovery. Although autologous cell-based therapy appears to be the most promising therapeutic strategy, this approach remains expensive and time-consuming. Currently, research is focused on cell priming and biomaterials to improve cell survival and engraftment at the wound site and the study of the secretome may also lead to more efficient alternative therapies.
7 CONCLUSIONS AND TRANSLATIONAL PERSPECTIVES
It is now established that the absence of innervation impairs skin healing. However, pathological scarring such as hypertrophic and inflammatory scars can be associated with either excessive innervation, reduced innervation or even sometimes normal nerve density thus leading to contradictory conclusions.101 Several studies have started to shed light on the complex cellular dialogue between fibroblasts and innervation, but more research is still needed to better understand how innervation regulates skin scarring and how the reinnervation process occurs during healing. Promising tissue engineering strategies and cellular-based therapies, including the use of biomaterials, skin substitutes, stem cells and mechanical- or electrostimulation, could provide novel alternative treatments to help avoid the establishment of pathological scars and improve sensory recovery in patients (Figure 2B).
ACKNOWLEDGEMENTS
This work was supported in part by the French Armaments Procurement Agency (DGA, No 2013 94 0903). Betty Laverdet and Dorothée Girard were supported by fellowships (doctoral and postdoctoral, respectively) from the French Armaments Procurement Agency.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
NL, BL, LM, AD and DG wrote and critically revised the review, and approved the submitted and final versions; NL, DG and AD designed and prepared the figures.




