Light-independent pro-inflammatory and pro-oxidant effects of purified human hair melanins on keratinocyte cell cultures
1 Background
Epidermal melanins, whose production is regulated by a multidimensional hormone-related network, are commonly believed to provide protection against sunlight-induced burns, DNA damage and skin cancer, thanks to the broad absorption spectrum and the free radical scavenging properties1, 2 (s1–s4). Yet, evidences accumulating over the last decades have highlighted a much more controversial role of melanins in human pigmentation (s5). This holds particularly for redheads, who have a higher ratio of yellow pheomelanin to brown eumelanin (s6) and are at a greater risk for melanoma (s7, s8). Two UV-dependent pathways for the induction of melanoma have been identified, which revealed an unexpected and significant role for melanin in melanomagenesis.3 Nonetheless, direct relationship between sun exposure and melanoma is still missing, and issues have been raised of why melanoma is not restricted to sun-exposed areas of the body, and UV radiation signature mutations are infrequently oncogenic drivers.4 Recently, the occurrence of UV-independent pathways of carcinogenesis was demonstrated by multiple experiments showing that an activating mutation of BRAF into red hair mice resulted in a high incidence of invasive melanomas in UV absence. In addition, pheomelanic mice's skin contained higher levels of oxidative DNA and lipid damage than albino-Mc1re/e mice.5 These data suggested that the pheomelanin pathway produces UV-independent carcinogenic contributions to melanomagenesis, probably by a mechanism of oxidative damage.5 Although pheomelanin is not located in the nucleus, it might cause damage by promoting the formation of ROS, which could overwhelm cellular antioxidant reserves and cause oxidative damage to biomolecules.6 Indeed, in vitro studies with synthetic pigments indicated that both eumelanin and pheomelanin are able to promote DNA strand breaks in the dark (s9) and even act as direct mutagenic agents by generation of CPD in the absence of UV.7 The molecular basis to interpret the role of melanins in these processes was provided by in vitro experiments showing that, in the dark, natural and synthetic pheomelanins can sustain autoxidation of cellular antioxidants (GSH and NADH) with ROS production,8 while both eumelanin and pheomelanin were shown to be redox-active by an electrochemically based methodology, with pheomelanin exhibiting a more oxidative redox potential (s10). However, neither the effects of melanins at cellular level, nor the possibility that the pigment may display a pro-inflammatory activity, have been investigated.
2 Question Addressed
Aim of our study was to test the ability of natural melanins to induce an inflammatory response on cultured keratinocytes and to affect the levels of endogenous antioxidants, independently from light exposure.
3 Experimental Design
Immortalized keratinocytes were incubated in the dark with increasing doses of RHP, BHE and WHP, purified from untreated hair of healthy volunteers (s11). Integrative experiments with the three compounds (RHP*, BEH* and WHP*) previously washed with EDTA, a strong chelating agent, have been performed to ensure removal of possible contaminants. Thereafter the direct pro-inflammatory ability was investigated through IL-1β, TNF-α and IL-6 gene (RT-PCR) and protein expression (ELISA). In addition, to explore the direct pro-oxidant ability of RHP, BHE and WHP, the levels of cellular antioxidants (GSH and NADPH) and lipid peroxidation markers (TBARS) were determined by HPLC with electrochemical/UV detection and by spectrophotometry, respectively. Materials and methods are described under supplementary material 1 (S1).
4 Results
Exposure to RHP or BHE, but not to WHP, moderately decreased keratinocyte viability (Fig. 1a). Proliferation/apoptosis assessment showed not significant differences (Fig. S1). RHP, and to a minor extent BHE, promoted expression of pro-inflammatory interleukins and oxidative damage, whereas WHP was ineffective. EDTA-treated compounds (RHP*, BHE* and WHP*) showed similar toxic or pro-inflammatory effects (Fig. S2).
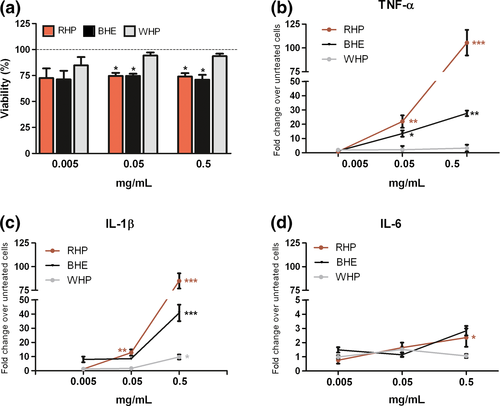
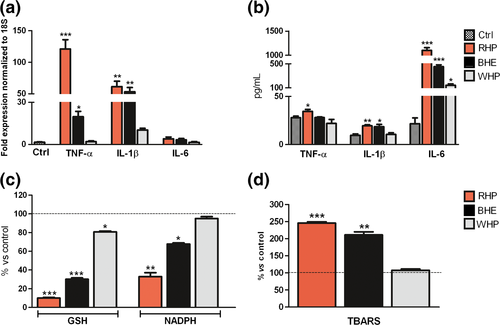
More in detail, after 24 hours, gene expression increase was dose related (Fig. 1b–d) and highly significant for TNF-α and IL-1β (Fig. 2a), whereas protein secretion of IL-6 resulted more enhanced than that of TNF-α or IL-1 β (Fig. 2b). No significant differences in protein expression emerged at 48 hours (data not shown). The discrepancy between IL-6 gene expression and protein secretion is likely due to an early rise of mRNA, which has already decayed after 24 hours, when protein has accumulated, also inducing TNF-α negative regulation. TNF-α, IL-1β and IL-6 were studied as pivotal mediators of chronic inflammation, implicated in most stages of tumorigenesis. Although their role is still controversial, upregulation has been reported in melanoma, also in relation to inflammasome activation, occurring in response to PAMPs or DAMPs (s12–s14). As to the assessment of the pro-oxidant activity, depletion of crucial cellular antioxidants such as GSH and NADPH (Fig. 2c) and an increase in lipid peroxidation products (TBARS) (Fig. 2d) were registered after exposure to RHP and, in a lower degree, to BHE. These results are in line with the effects of RHP on GSH and NADPH levels, in air-equilibrated metal-free buffer.8 As a cross-point between the IL-1β inflammatory pathway and the direct oxidative damage (s15), the expression of COX-2 gene was then determined. A moderate but significant increase was observed after exposure to RHP (Fig. S3).
5 Conclusions
The results of our study indicate that RHP, and to a minor extent BHE, works as a direct pro-inflammatory and pro-oxidant agent in keratinocyte cultures, independently from light exposure.
The mechanism of melanin internalization, involving endocytosis by keratinocytes,9 supports the effects observed for RHP and BHE in our experiments. The fact that chronic oxidative damage and reiterated inflammation can lead to carcinogenesis has been widely demonstrated (s15). Indeed, elevated levels of ROS, perceived as harmful stimuli, should activate defensive inflammatory processes to limit or remove the damage, but failure of such mechanisms may results in cancers. In this set, RHP could play a crucial role in worsening inflammatory skin conditions by lowering endogenous antioxidant defenses as well as by producing ROS. On the other hand, BHE, in accord with its lower redox potential (s10), may also contribute to ROS generation.
Acknowledgements
Monfrecola, Lembo, Balato, Napolitano and Panzella designed the research study; Lembo and Di Caprio performed cellular experiments. Micillo and Panzella purified melanins and performed pro-oxidant assays; Lembo, Di Caprio, Balato, Napolitano and Panzella analysed the data and wrote the manuscript.
Funding
None.
Conflicts of interests
None declared.
Abbreviations
-
- BHE
-
- black hair eumelanin
-
- COX-2
-
- cyclooxygenase-2
-
- CPD
-
- cyclobutane pyrimidine dimers
-
- DAMPs
-
- damage-associated molecular patterns
-
- EDTA
-
- ethylene diamine tetraacetic acid
-
- GSH
-
- glutathione
-
- MED
-
- minimum erythemal dose
-
- NADPH
-
- nicotinamide adenine dinucleotide phosphate
-
- PAMPs
-
- pathogen-associated molecular patterns
-
- RHP
-
- red hair pheomelanin
-
- ROS
-
- reactive oxygen species
-
- TBARS
-
- thiobarbituric acid-reactive substances
-
- UV
-
- ultraviolet
-
- WHP
-
- white hair protein




