Keratoacanthoma: a distinct entity?
Abstract
Keratoacanthoma (KA) are common but exceptional benign tumors, often appearing on sun-exposed areas of light skinned people and showing spontaneous resolution. The goal of this study was to review existing literature, to point out the etiological complexity of KA biology and to answer the controversial debate if or not KA is a distinct entity or a variant of squamous cell carcinoma (SCC). Relying on recent results, we highlight that KA is an individual lesion with a unique molecular signature caused by alterations in the TGFβ signalling pathway. These recent findings will help to understand the nature of KA and to develop new reliable diagnostic tools, simplifying the discrimination of the histologically similar KA and SCC.
Introduction
The first description of the ‘crateriform ulcer of the face’ was given by Jonathan Hutchinson in 1889 1, and since then many different synonyms like molluscum sebaceum, self healing-epithelioma and keratocarcinoma have been used 2-4. In the 1940′s Freudenthal proposed the name Keratoacanthoma (KA) to highlight acanthosis and keratosis as major histological changes of the tumor 4-7. KA however is a benign tumor, characterized by rapid growth over 4–5 weeks followed by spontaneous regression 5, 8. KA occurs mainly as a solitary lesion on sun-exposed locations 9-11, but multiple tumors appear in several syndromes such as familial self-healing epithelioma of Ferguson-Smith, Xeroderma pigmentosum or Muir-Torre syndrome 12, 13. Morphologically similar to squamous cell carcinoma (SCC), a better biological understanding of KA as defined as a self-healing benign tumor is needed to develop reliable diagnostic tools. Since recent findings showed that altered TGFβ signalling is the main cause of familial KA, a detailed view on this pathway should allow new insights.
Etiology
Sun exposure and thus UV light was known for a long time to be necessary for KA development 14 as suggested by preferential localization on sun exposed skin of elderly patients with light complexions, an increased appearance in summer and autumn, and their frequent emergence in Xeroderma pigmentosum 10, 11, 15-19. Solitary and multiple KA have also been observed in PUVA-treated patients 20, 21.
Similarly, KA emergence was reported after cutaneous X-ray or Megavolt radiation therapy 13, 22, 23. Further etiological factors are immune-compromised or immune-suppressed patients 24, skin trauma when pretreated with chemicals 18, carbondioxide ablation therapy, likely by induction of a wound healing response 13, 22, 23, and most importantly, genetic predisposition in multiple KA of Ferguson Smith type 25. Drugs as Imiquimod and BRAF-inhibitors induce KA as a side effect 26-31. Contradictory results were published on the implication of human papilloma viruses (HPV) 5, 32-34.
Classification
Several forms of KA exist, with solitary KA as the most common variant. Multiple KA have been described by Ferguson Smith, Grzybowski and Witten-Zak.
Solitary KA
- The typical solitary KA is rapidly growing up to 2 cm, appearing as a rose nodule with a stretched shiny epidermis and a keratotic centre. After this mature phase with a duration around 6 months, the lesions becomes smaller until complete regression 5.
- Giant KA, usually grow larger than 2 cm, predominantly appears on eyelids and nose 35-37. Unusual vertical growth invading dermal and underlying tissue is observed 38-40.
- Subungual KA is a rare KA variant, appearing under nails, show rarely spontaneous regression and may destroy the terminal phalanx 10, 41, 42.
- Mucosal KA another extremely rare KA variant, has also no tendency to regress 43 and is observed mostly intraorally 44. Since intraoral mucous membranes bear no follicles it was suggested, that these KA may arise from ectopic sebaceous glands 45.
- Keratoacanthoma centrifugum is a rare exoendophytic type, characterized by multifollicular origin, progressive peripheral expansion (2–40 cm) and atrophic central healing 46-48. Resolving of the tumor occurs spontaneously and involution is mostly complete within week 12 5, 49.
Multiple KA
- Ferguson Smith type, known as multiple self-healing squamous epithelioma (MSSE), is the most common form of multiple KA. This autosomal dominant genodermatosis is characterized by sudden appearance and rapid growth, slow regression and periodical recurrence 25, 50. The number of tumors varies from a few to hundreds, mostly leaving deep scars after involution. With a mean onset in young adolescence, appearance of KA is much earlier than the typically reported in sporadic cases 10. In 2011, Goudie et al. 51 identified 11 monoallelic mutations in transforming growth factor beta receptor 1 (Tgfβr1) revealing the cause of the MSSE phenotype.
- Generalized eruptive keratoacanthomas of Grzybowski appear spontaneously in 6–8 months represented by hundreds to thousands follicular papules 52, 53. Skin involvement may be severe with masked facies and ectropion. There is neither preference in sex nor in a familial pattern 52, 54. Fusing tumor nodules can form larger lesions rather involving the mucosa than palms and soles. The tumors may be indistinguishable from solitary KA 52.
- Multiple KA of Witten and Zak type arise in a range of 1–30 papules which stay either small or increase their size enormously. The lesions are showing central healing and the life cycle with resolution is completed after several months. A familial predisposition is assumed as well as sunlight as an etiological factor 55.
- KA in Muir-Torre syndrome appear solitary or multiple 10. Muir-Torre syndrome is an autosomal dominant genodermatosis combining visceral malignancy, notably colon cancer with sebaceous neoplasms 56. The syndrome is caused by germline mutations of hMSH2 and hMLH1 genes, involved in the mismatch repair system. KA appearing within this syndrome is explained by the common pilosebaceous gland origin of sebaceous neoplasms and KA 10.
- KA have been observed in patients suffering xeroderma pigmentosum, an autosomal recessive disorder with 100% penetrance, caused by defects in genes coding for DNA repair enzymes 57. Compared to normal cells, cells of XP patients fail to repair UV-induced DNA lesions, thereby linking sun exposure to a hypermutable genotype 58. This failure of the repair system causes development of melanoma, basal cell carcinoma, SCC and KA in early childhood on sun exposed areas of the body 15.
- Non-familial multiple persistent KA have been observed on conjunctivae, palms and soles. In one special case, the tumor continued to grow over a time period of 35 years 10. Affleck 59 described this distinct variant as sporadic, idiopathic without spontaneous resolution.
- Reactive KA is induced by treatment of melanoma patients with the BRAF kinase inhibitors Vemurafenib or Sorafenib 29, 30. These patients often develop multiple disseminated solitary KA on sun-exposed and non-sun-exposed areas 60. The iatrogenic tumor formation usually occurs 8 weeks after inhibitor therapy initiation 29, by paradoxical activation of the ERK MAPkinase pathway 61.
Histopathology
Three different stages are determined by histological examination during evolution of KA: (1) a proliferative phase, (2) the resting fully developed mature stage and (3) regression 10. The majority of tumors arises over the skin surface 62 embedded in a well-differentiated squamous cell epithelium 63, 64. A keratin-filled epidermal invagination, eventually reaching into the dermis, emerges from an adjacent hair follicle 65 13, 66. Atypical squamous cells with multiple mitotic figures have been observed in dermal extensions 13.
Proliferation
The initial, rapidly proliferating immature KA appears as a skin-collared papule with an acanthotic hyperproliferating epidermis characterized by hypergranulosis with large keratohyaline granules and hyperkeratosis with premature keratinization. Enlarged keratinocytes are noticed within the stratum basale and spinosum 4. Eosin staining highlights keratinized areas with a pale cytoplasm giving the impression of a ‘glassy’ appearance 13, 66. Later in proliferation, a central depression and inflammatory infiltrates consisting primarily of neutrophils and less eosinophils are present 4, 10. Perineural invasion is rarely observed. Invasion beyond the level of eccrine glands is an ominous sign since KA usually not extend deeper than sweat glands 10, 67.
Maturity
The typical prominent dome shaped symmetric nodule with a keratin plug in its centre is the prerequisite for correct diagnosis. Epidermal lips rise around both sides and cover partially the top of the crater (Fig. 1). Thereby, both elongated thin tumor lips are covered on the surface and towards the crater by two epidermal sheets which are in parallel with the skin surface. The keratin filled crater plonges into multilocular spaces of epithelial tumor lobules in the periphery in a semi-star shaped fashion 68, 69. These are composed of well-differentiated squamous cells at the dermal interface 9. Thereby the tumor does not appear to grow vertically into the dermis like SCC but into all directions below the skin surface. Atypical basal keratinocytes are decreased in number as are keratinocytes with pale and glassy cytoplasm 13. However, apoptotic keratinocytes and intraepithelial abscesses primarily composed of neutrophils and less eosinophils become prominent 9, 10. Horn pearls arranged in concentric keratinocyte layers with a central eosinophilic keratin plug, also observed in SCC, are common 4, 10, 13. Increased dermal inflammatory infiltrates composed of eosinophils, neutrophils, plasma cells and histiocytes are observed 4, 9.
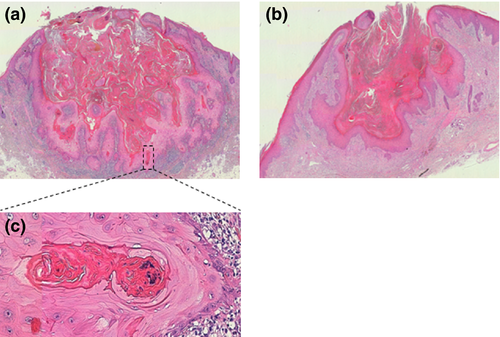
Regression
The third stage occurs relatively slowly. Most of the lesions involute completely by week 12 5. Involution is initiated by disappearance of mitotic activity at the border of the lesion 4 and loss of glassy, enlarged squamous cells. KA retain the crateriform architecture but decrease in height, followed by a reduction in horizontal size 70 (Fig. 1). Around the tumor on the dermoepidermal interface a lichenoid inflammation reaction may be seen. The regression is likely based on immunological mechanisms exhibited among others by activated CD4+ T lymphocytes 71. Mitotic fibroblasts beneath the lesion forcing fibrosis and a dermal infiltrate with multinucleated histiocytes may be observed 10. Recent finding suggested that increased differentiation and loss of proliferating epithelial cells together with inhibition of the Wnt-β-catenin pathway are key steps in KA regression 72. Similarly, SOX2 deletion in SCC led to decreased proliferation and increased apoptosis and consequently to tumor regression in mice 73. After complete resolution, the tumor leaves a fibrous scar without adnexal structures 74.
Discrimination: KA versus SCC
Classification controversy
Clinical and morphological distinction between KA and SCC may cause difficulties in certain cases (Tables 1, 2 and 4). Also the question whether KA is a distinct entity 75-77 or a variant of SCC 43, 78 was discussed controversially until molecular identification of TGFβR1 mutations in Ferguson Smith syndrome and genetic studies clarified that at least familial KA have bona fide an own pathogenetic cause 51, 77, 79, 80. Indeed, the mutation rate of TGFBR1 for supposed MSSE is about 85–90% (B. Lane, personal communication). A recent study analysing a large number of SCC by exome and targeted sequencing reported a comparable frequency of ‘gatekeeper’ mutations in NOTCH1/2 (82%) while TGFBR1 mutations were much less frequent 81. Further studies are needed to confirm that this is a consistent fundamental distinction between all KA and SCC. Furthermore, the topic if KA is essentially benign is lively debated and was forced by occasionally malignant behaviour of KA 82. If KA are truly able to form metastasis is still a matter of debate, in contrast with SCC which metastasize in up to 5% 83, 84. As reported, these metastasizing KA could have been misdiagnosed as KA but are true SCC 5, 8, 69, 76, 78, 85-87. In fact 10% as KA diagnosed tumors are malignant and progressing SCC 9, 69. Hallmarks of malignant tumors such as, prominent mitoses or cytological atypia, are not observed in KA. Spontaneous regression and missing features characterizing a malignant phenotype, prompted us and others to the conclusion that KA is a benign neoplasm 8, 51, 85. Subungual and mucosal KA appear under the global name of KA and arise on non-follicle bearing parts of the body. These borderline tumors are histologically different from KA and SCC and show regression only in a few cases (Table 3). Even if subungual KA show rather progressive growth than spontaneous involution, cellular atypia and the mitotic index are reduced compared to SCC 88, 89 and several authors consider it to be benign 41, 90, 91.
| Common biological features |
| Lateral growth predominant |
| Metastatic potential |
| Local tissue destruction (if untreated) |
| Similar etiologic factors (UV exposure) |
| Common histopatological features |
| Intraepithelial polymorphonuclear abscesses |
| Parakeratosis |
| Dyskeratosis |
| Cup shaped |
| Atypical squamous cells |
| Glassy keratinocytes |
| KA | SCC |
|---|---|
| Biological differences | |
| Rapid growth till 1–2 cm | Slowly growing |
| Involution | No involution |
| Exoendophytic | Predominantly endophytic |
| Histologpathologic differences | |
| Epithelial lips | – |
| – | Stromal desmoplasia |
| Intraepithelial abscesses in the lesion | Rarely observed |
| Rare ulceration | Common |
| Distinct edge between tumor and stroma | Indistinct |
| – | Anaplasia |
| Intraepithelial abscesses with acantholytic cells | No association between eosinophils and acantholytic cells |
| Flask shaped | – |
| Epithelial collarette | Rarely observed |
| Symmetric | Asymmetric |
| – | Melanocytes present |
| Absence of stromal desmoplasia | Present |
| Variants of KA | Differences from solitary KA | Differences to corresponding squamous cell carcinoma (SCC) | Similarities to corresponding SCC |
|---|---|---|---|
| Ferguson Smith |
|
||
| Grzybowski |
|
||
| Giant |
|
||
| Mucosal |
|
|
|
| KA centrifugum marginatum |
|
||
| Subungual |
|
|
|
- Comparison of the rare subungual and mucosal KA with its SCC counterparts.
In sum, the recently found TGFβR1 driver mutations in familial KA, Comparative Genomic Hybridization studies, micro array experiments and missing clinical features of a malignant phenotype in KA are in line with our view that KA and SCC are two distinct lesions 51, 77, 79, 80. Unfortunately, there is still a notable lack of a reliable molecular marker, allowing for the unequivocal distinction of KA from SCC in the laboratory.
Histological criteria
The requirement to distinguish KA from SCC is complete excision of the lesion reaching to the subcutis including the crater, both lips and the surrounding of uninvolved epidermis 10. KA are exoendophytic whereas the majority of cutaneous SCC show interior proliferation 13. For reliable diagnosis, dermatolopathologists have to include several criteria. Architectural attributes are essential since both tumors possess nearly identical cytological features (Tables 1-3) 10.
Cribier et al. examined 14 criteria on 262 previously diagnosed samples and defined five significant criteria: (1) epithelial lips, (2) sharp outline between tumor and stroma favouring KA- (3), and ulceration, (4) abundant mitotic cells, (5) pleomorphism/anaplasia SCC-favouring. These features allowed a consensual diagnosis in 88% of cases, although they did not increase the sensitivity in difficult cases 92. Other studies claimed inflammation or pattern of keratinization as reliable distinctive features (Table 4) 10, 75.
| KA | SCC |
|---|---|
| Histopathology | |
| Cribier et al.: | Cribier et al.: |
• Epithelial lips |
• Ulceration |
• Sharp outline between tumor and stroma |
• Numerous mitosis |
• Pleomorphism/anaplasia |
|
| Schwartz et al.: | Schwartz et al.: |
• Intratumoral abscesses with granulocytes and acantholytic cells |
• Intratumoral abscesses contain few or no inflammatory cells |
| Weedon et al.: | Weedon et al.: |
• Pattern of keratinization |
• Pattern of keratinization |
| Genetics | |
| Li et al.: | Li et al.: |
• Aberrations on chromosome: 17, 19, 20, X |
• Aberrations on chromosome: 7, 8, 10 |
Cytological markers
Cytokines
PCR screening showed elevated levels of interleukin 10 (IL10) and a decrease of granulocyte macrophage colony-stimulating factor (GM-CSF) in KA compared with SCC. IL10 usually exhibits anti-tumoral but also immunosuppressive functions 93, 94 thereby, inhibiting eosinophile GM-CSF and Th1 cytokine production 95. Low GM-CSF serum concentration leading to an immunosuppressive state probably enables rapid tumor growth on one hand and allows involution on the other hand 96.
Some studies reported diffuse distribution of transforming growth factor-a (TGFa) in the majority of KA. Forty per cent of SCC exhibit a focal enrichment of TGFa whereas 90% of KA exhibit no staining in this area 97.
Proteins functioning in cellular adhesion processes
Differences in VCAM1 and ICAM1 expression in KA compared to SCC were not sufficient for a reliable marker 98. Significantly increased expression of stromelysin (ST3) was found in SCC and even more in metastatic SCC allowing to predict their biological behaviour 99.
Cell surface receptors
EGFR is a receptor tyrosine kinase affecting epithelial and keratinocyte differentiation, proliferation and tumorgenesis 100. Canonical EGFR signalling occurs through mitogen activated kinase pathway (MAPK), and thus through Ras and Raf proteins. Comparative genetic hybridization identified different chromosomal aberration patterns in KA compared with SCC providing evidence that KA and SCC are two distinct entities 80. Consequently, EGFR copy numbers were altered more often in SCC but amplification was not significant, and no increased EGFR protein expression could be observed 101-103. However, this is controversial, as increased expression of EGFR was detected in the majority of primary and metastatic SCC but not in KA 100, 104. This may indicate that EGFR expression in skin carcinogenesis is rather facilitating progression 104.
As in many tumors, alteration of Ras and Raf proteins are also important for SCC and KA formation. Both can develop as secondary tumors upon melanoma treatment with BRAF inhibitors 29, 105.
As shown recently, TGFβ signalling is implicated in KA formation. Eleven monoallelic mutations in the Tgfβr1 gene were specified in Ferguson Smith disease 51. The role of TGFβ signalling in KA and SCC formation will be discussed in detail in this report.
Cell cycle and apoptosis regulators
Ki-67 expression varies in KA since proliferative KA display higher immunostaining frequency than mature KA. Staining of p53 was strong and mainly localized to expanding tumor islands in KA and a diffuse nuclear staining throughout the whole lesion in SCC. Expression of p53 in KA and SCC is not sufficiently discriminatory to use it as a diagnostic tool but may be supporting the differential diagnosis of subungual KA versus subungual SCC 88, 89, 106, 107. Cyclin A and B exhibited predominantly basal and parabasal staining in KA, whereas it was diffuse in SCC 108.
In sum, these and many other targets have been tested in the last decades, but no reliable marker was identified exhibiting sufficient sensitivity and specificity.
Genetics
Array CGH
Array comparative genomic hybridization (array CGH) allows the discrimination of KA and SCC in 85% of the cases. Recurrent aberrations on chromosome 7, 8 and 10 have been the best predictors for SCC, suggesting a role in prevention of apoptosis or activation of proliferation and infiltration for genes located in these areas. In contrast chromosomes 17, 19, 20 and X seemed to be aberrant mainly in KA, indicating crucial genes for KA development. These and other differences in the aberration pattern between the two tumors were proven to be significant, leading to the assumption that KA and SCC are two distinct entities 79. A recent study investigating the relationship between KA and SCC by DNA microarray technique concludes that KA and SCC are distinct, further defining KA as a distinct regressing neoplasm 77.
Molecular pathways contributing to SCC and KA formation
TGFβ signalling in KA and SCC
The implication of TGFβ signaling in KA formation was demonstrated recently by Goudie et al. They ascertain a clear correlation between loss of function mutations in the Tgβr1 gene and a MSSE phenotype. With high-throughput genomic sequencing they identified the disease locus on chromosome 9 and by exome sequencing they localized in three unrelated families several distinct mutations in Tgfβr1. In total, 11 monoallelic mutations in 18 families have been described, occurring in the cytoplasmic kinase domain or in the extracellular ligand-binding domain. In the cytoplasmatic region of the receptor nonsense or frameshift mutations occurred, resulting in a null mutation. In the extracellular ligand-binding domain frameshift, nonsense or missense mutations have been demonstrated, affecting the binding of TGFβ or the interaction with TGFβR2. These mutations were shown to be null supported by the fact that the bases of three of four of the missense mutations are highly conserved across vertebrates. These heterozygous MSSE mutations exhibit a loss of function, which is covered by the functional protein transcribed by the wildtype allele 51. The paradoxical role of TGFβ signalling could explain the rapid evolution and involution of KA after ablation of TGFβ mediated signal transduction. Additionally, a discontinuous membranous expression was observed in spontaneous KA but rarely in SCC. If this was due to mutation in the receptor could not be answered definitively 109.
Transforming growth factor beta receptor 1 and 2 (TGFβR1 and TGFβR2) are transmembrane serine/threonine kinases, transducing signals of the TGFβ protein superfamily from the cell surface to the cytoplasm. Downstream signalling occurs mostly through SMAD-proteins but also affects Ras, PI3K/Akt- or MAPK-pathways 110, 111. TGFβ signalling is found in a large number of tissues where it controls differentiation and growth 112 and alterations are implicated in malignant transformation of keratinocytes and tumorgenesis 113, 114.
Transgenic mice overexpressing TGFβ, were protected against an early development of tumors but promoted tumor metastasis later in tumorgenesis 111. A widely accepted hypothesis is that an effect favouring tumor development is mediated by paracrine signalling, stimulating angiogenesis and inflammation at late stages whereas autocrine signalling seems to function as tumor inhibitor at least in early stages 115. Secondary effects as onset of inflammation or angiogenesis upon TGFβ overexpression seem to be responsible for keratinocyte hyperproliferation of head and neck epithelium in vivo 116.
TGFβR1 deletion leads in a small number of animals to spontaneous formation of HNSCC, thus indicating that loss of the receptor is not an initiation event in tumor formation 117. Concomitant loss of TGFβR1 and of PTEN provoked the formation of spontaneous tumors 118. Combining TGFβR2 ablation with either overexpression of oncogenic K-ras or DMBA treatment led rapidly to the formation of aggressive growing SCC 119, 120. Glick et al. 113 reported significantly augmented tumor formation after skin graft experiments of TGFβ1 negative keratinocytes expressing virally transduced oncogenic v-ras.
Haploinsufficient TGFβ1+/− mice developed fewer benign tumors with reduced incidence and size compared with TGFβ1+/+ mice. Even if TGFβ+/+ mice had a higher onset of benign tumors, no increased numbers of SCC were observed 121.
The fact that KA and SCC appearance is linked to TGFβ alteration is not sufficient to conclude that they are one entity. Since the molecular background of SCC and KA differs strongly it is very probable that the outcome of TGFβ signalling is also altered 77. Consequently, stem cells within the same SCC tumor, exhibit opposite phenotypes depending on if they respond to TGFβ stimuli or not 122. Furthermore, rather than Tgfbr1 mutations, recently identified NOTCH1/2 receptor mutations are considered as early gatekeeper mutations in SCC development 81.
Par3 signalling in KA formation
The Par3 pathway is implicated in KA formation and affects downstream targets of TGFβR1 such as PI3K/Akt and MAPK. Specifically, Par3 is involved in apico-basal cell polarity and asymmetric cell division. Loss of cell polarity appears to be a prerequisite in tumor formation and progression. In DMBA/TPA tumor models, Par3 overexpression facilitates papilloma formation but reduces KA development. Opposite effects were observed in the same tumor model with Par3 ablation, what may be explained by its different intracellular localizations 123. Whether staining of Par3 may be helpful in histopathology needs to be investigated.
RAF inhibitors favours KA and SCC development
A specific category of KA appears in melanoma patients treated with BRAF inhibitors 29, 61, 105. Ninety per cent of all BRAF mutations are V600E changes, causing a higher BRAF activity thereby activating MAPK signalling pathway independently of RAS 124, 125. A side effect of the mutation-specific BRAF V600E drug Vemurafenib in melanoma patients is the appearance of SCC or KA in 15–20% of the patients. Sixty per cent of these epidermal lesions have a mutated RAS leading to BRAF inhibitor mediated paradoxical MAPK pathway activation in BRAF wild-type cells 29. Melanoma patients treated with Sorafenib also developed SCC or KA lesions in up to 7% of the cases 30, 31.
Interferon treatment
Imiquimod, a nucleoside analogue of the imidazoquinolone family, is usually applied against actinic keratoses and basal cell carcinoma. It leads to upregulation of pro-inflammatory cytokines due to activation of the NFkB pathway. Recently, there is a growing evidence of Imiquimod induced KA as a secondary effect of the treatment 26, 28.
Treatment of KA and SCC
Treatment of patients with diagnosed KA include excision (standard or Mohs), radiotherapy, systemic retinoids and intralesional methotrexate 19, 126. Mohs surgery should be used in the applicable case 19. Application of a Imiquimod 5% cream led to fast regression of KA 127. Regarding the patients interest, conservative handling or Mohs surgery are the treatments of choice.
Conclusion
The etiology of KA involves many different factors, of which some are seemingly more evident than others giving rise to a broad spectrum of KA variants 10, 11, 13-30, 32, 33, 69, 128. Genetic predisposition or spontaneous formation adds another level to the complex classification system of KA. Today we conclude that SCC and the benign neoplasm KA are two distinct entities, prototypically evidenced by alterations in the TGFβ pathway in Ferguson Smith Disease, chromosomal aberration differences and pronounced alterations in gene expression 51, 77, 79, 80. Since TGFβR1 mutations were identified as driver mutations in Ferguson Smith Disease, it would be important to identify mutations leading to spontaneous KA and other KA variants e.g. by identifying more disease loci followed by high-throughput genomic sequencing. Additionally, the identification of pathways implicated in the spontaneous involution of KA would be of serious interest. With regard to the development of new therapeutics it would be interesting to investigate the immune response in KA during different stages, leading to new insights concerning presented tumor antigens or identification of specific reactive T-cells. Up to now, dermatopathologists have a difficult task to distinguish KA and SCC on histopathological grounds. For this purpose a fully excised lesion is helpful, but to discriminate both tumors in an early stage remains challenging. Consequently, it is essential to find morphological, biological and/or molecular markers which are showing reliable differences between these two lesions to prove that a given KA is not a SCC. Taken together, KA is a rapidly growing, spontaneously regressing tumor. Recently published data lead us to assume that KA is distinct from SCC and caused by early gatekeeper alterations in the TGFβ pathway.
Acknowledgements
This work has been supported by grants to M.H. and D.H. by the Swiss National Science Foundation and the Dind Cottier Foundation.
Author contribution
T.G. wrote and conceptualized this review. E.C. and M.H. revised the paper. D.H. conceptualized, revised and coordinated the work.
Conflict of interests
The authors have declared no conflicting interests.




