Quiescent melanocytes form primary cilia
Abstract
We show, for the first time, that melanocytes can form a primary cilium in vitro, corresponding to an immotile or sensory cilium. Such cilia are observed when melanocytes reach confluence or when medium nutrient levels are insufficient. This observation should greatly improve our understanding of the signal transduction processes potentially occurring in these cells during embryonic development, homeostasis in adulthood and melanomagenesis.
Background
Primary cilia are microtubule-based protrusions that can act as cellular antennae and play a role in signal processing 1, 2. The assembly and disassembly of cilia is synchronised with the cell cycle. Cilia assemble in cells in a non-proliferative state and disassemble in proliferative cells. In cell culture, quiescence is induced by growing cells to confluence or in suboptimal conditions to prevent proliferation. Cilium grows from basal body (centrosome mother centriole) and consists of nine parallel, polarised microtubule doublets, forming cylindrical structures, axonemes, wrapped in ciliary membrane. Axoneme microtubules are stabilised by tubulin acetylation. Primary cilium controls key signalling pathways during development and tissue homeostasis (hedgehog, Notch and Wnt/PCP). Primary cilia have been detected in almost all vertebrate cell types other than immune cells. However, it is unknown whether pigmented melanocytes in vitro can assemble cilia. It has been shown that one-third of human melanocytes have cilia, and it has been suggested that this apparatus is associated with the lack of mitotic activity 3. Moreover, most melanocytes in naevi possess cilia, and very few melanocytes of melanoma possess a primary cilium 4. As naevi cells possess cilia and did not bypass senescence, and as tumor cells possess very few cilia and bypassed senescence, one may suggest that melanocytes that have bypassed senescence are refractory to cilium growth
Question addressed
The aim of this study was to investigate the presence of primary cilia in cultured melanocytes.
Experimental design
Exponentially growing wild-type Melan-a and 9v cells, and β-catenin mutant 10d cells were maintained as previously described 5-7. Further information is provided in supplemental information.
Results
Melan-a cell growth was stopped, by culture to stationary phase in complete medium (growth culture conditions, GCC) or by culture in medium lacking serum and TPA (non-growth culture conditions, NGCC). No cilium formation was observed in exponentially growing cells after seeding and cell attachment (6 h), in either set of conditions (Fig. 1a,c,e). Staining for pericentrin (basal body) and acetylated tubulin (axonemes) revealed the presence of cilia in cells that had stopped growing. After 48 h, such single cilia were more frequently observed in NGCC than in GCC (Fig. 1b,d,e). After 3 days of culture, 60% of melanocytes cultured in NGCC and 20% of those cultured in GCC had cilia. In GCC, the cells continued to grow until confluence, whereas NGCC did not provide the melanocytes with ideal conditions for proliferation. Similar results were obtained with an independent wild-type melanocyte cell line, 9v (not shown). Non-dividing cells including senescent cells have been reported to be more likely to develop cilia 4. We have shown that the expression of a mutated form of β-catenin leads to the bypassing of senescence. We therefore hypothesised that wt- and bcat-immortalised melanocytes would generate cilia with similar efficiency under the same conditions. Our results (not shown) suggest that once the cells have bypassed senescence, expression of the mutated form of bcat does not affect cilium growth. A single primary cilium appeared in the presence or absence of appropriate conditions for growth, and the key factor underlying cilium formation appeared to be the proliferative status of the melanocytes.

Cilia are assembled in quiescent cells. We evaluated the percentages of cells expressing the proliferative marker Ki-67 and of melanocytes presenting cilia, after 24, 48 and 72 h in NGCC and GCC (Fig. 2a,b). The percentage of proliferating cells was very low in NGCC, in which a large proportion of cells presented cilia. Similar results were obtained in GCC, but the proportion of proliferating cells was much higher in these conditions, with a correspondingly lower proportion of melanocytes presenting cilia. These results are consistent with our previous finding that ciliated RPE1 and MEF cells are indeed negative for Ki-67 8. In a minor proportion of cells, a cilium was also present in starved or confluent cells retaining Ki-67 staining. These cells are likely corresponding to either cells in which Ki-67 remains present or cells which are reinterring the cell cycle and therefore in the process of cilia disassembly.
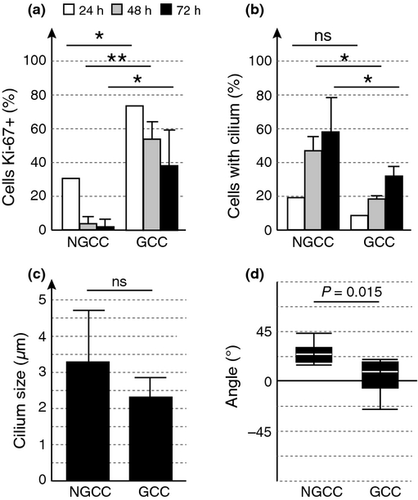
Cilia were of similar length in both sets of conditions (Fig. 2c), but angles between cilia and substratum were significantly different (Fig. 2d). Cilia were almost horizontal in GCC, as previously shown in RPE1 cells 9, but grew out at an angle of 20° in NGCC. This difference in angle may reflect differences in the type of quiescence, resulting from cell–cell contact in GCC and a lack of nutrients in NGCC.
Conclusions
Culture melanocytes form cilia when quiescent. Quiescence occurs when the growth of the cells is inhibited, either at confluence or in the absence of essential factors (FCS or TPA, which is known to induce the PKC pathway). The function of these cilia in quiescent melanocytes remains unknown. By analogy to other cellular systems, they may be involved in various processes, including mechanotransduction, and they may act as cellular antennae for soluble factors, in the control of signal pathways (GPCRs, Hedgehog, Wnt) or in polarised secretion, as suggested in chondrocytes 1, 10. Moreover, cilium growth is not random and has been shown to be parallel to the substratum to which the cells adhere 9. In our experimental conditions, cilia grew parallel to adherent substratum at confluence or at an angle of 20° in the absence of FCS and TPA. Further investigations are required to decipher and evaluate the biological importance of this difference. Melanoma heterogeneity is an important issue, because it may be an obstacle to effective therapy. Within any tumor, some cells may be proliferating, others slowly cycling and still others may be quiescent. Classical therapies are effective against proliferative cells, but not against quiescent cells. Improvements in our understanding of these quiescent cells and their cilia are therefore required, to improve treatment and prognosis.
Acknowledgements
We thank Dot Bennett for Melan-a cells and Fabrice Cordelières of the imaging facility of Institut Curie. This work was supported by LNCC and INCa. MLC performed the research; LL designed the research study; MLC and LL analysed the data; MLC, AB and LL wrote the paper.
Conflict of interest
The authors have no conflict of interest to declare.




