Effect of human skin explants on the neurite growth of the PC12 cell line
Abstract
The skin is a densely innervated organ. After a traumatic injury, such as an amputation, burn or skin graft, nerve growth and the recovery of sensitivity take a long time and are often incomplete. The roles played by growth factors and the process of neuronal growth are crucial. We developed an in vitro model of human skin explants co-cultured with a rat pheochromocytoma cell line differentiated in neuron in presence of nerve growth factor (NGF). This model allowed the study of the influence of skin explants on nerve cells and nerve fibre growth, probably through mediators produced by the explant, in a simplified manner. The neurite length of differentiated PC12 cells co-cultured with skin explants increased after 6 days. These observations demonstrated the influence of trophic factors produced by skin explants on PC12 cells.
Nerve growth following a traumatic injury, such as an amputation, burns or skin graft, takes a long time and is often incomplete. Neurotrophins and semaphorins have been implicated in the guidance of neuronal growth 1, 2 through interactions with each other to regulate the motility of the sensory neuronal growth cone. However, the mechanisms of the interactions between the peripheral neurons and the keratinocytes/epidermis/skin remain unclear. To study these interactions under normal physiological and injured conditions, the development of in vitro co-culture models is important. At present, models enabling the study of neuron–skin interactions are rare 3, with the majority involving either primary sensory neurons or a neuronal cell line co-cultured with keratinocytes 4-6, reconstructed skin 7, a skin explant 8 or lesional skin 9. Furthermore, the neurons used in these models are limited due to ethical problems involved in harvesting primary neurons and by the necessity to select an appropriate cell line. Today, adult stem cells could be small sources of neurons or other cellular types for regenerative medicine and tissue engineering, but they are not co-cultured 10, 11.
PC12 is a versatile rat pheochromocytoma cell line that can be differentiated into neurons with sensory or autonomic characteristics using NGF 12, 13. These differentiated PC12 cells have been used as a model for sensory or autonomic neurons 14-16. We developed an in vitro model of human skin explant, which can be considered as an equivalent of injured skin, co-cultured with PC12 cells differentiated into neurons.
PC12 cells were co-cultured with human skin explants (E) until 10 days. Skin explants (three donors, one for each experiment) were cut using a 6-mm diameter biopsy punch and placed in 12-well culture plate with one skin explant per well. The skin explants were co-cultured with PC12 cells for more than 10 days at an air–liquid interface, and the medium was changed every 2 days. The PC12 cells, which were obtained from the ATCC (ATCC, CRL-1721), were grown in DMEM-F12 media (Lonza, BE12-719F) supplemented with 10% calf serum (PAA, A11-101). The cells were dissociated with a non-enzymatic solution of DPBS containing 0.7 mm EDTA for five to ten minutes. To differentiate the PC12 cells, the cells were plated at a density of 50,000 per well on a plate precoated with 40 μg/ml poly-l-lysine. The skin explants were placed into the centre of the wells a few hours after the initial seeding of the PC12 cells. The maintenance medium comprised a DMEM-F12 3:1 mixture (Lonza, BE12-719F and BE12-604F/U1) supplemented with insulin at 5 μg/ml (Sigma, I6634; St Louis, MO, USA), hydrocortisone at 10 ng/ml (Sigma, H0135) and NGF at 25 ng/ml (Sigma, N1408). The medium was changed every 2 days.
At 10 days, the PC12 cells cultured with skin expressed SP, CGRP (4.8 pg/ml by ELISA), peripherin, PGP9.5, neurofilaments and react with IB4 (e-supplement). To determine the effects of skin explants on the growth of PC12 neurites, the cells were cultured with or without the skin explant for 10 days (Fig. 1 and e-supplement), and neurite length was measured every 2 days using ImageJ software by previously published method 4, 17. After 8 days of culture, the neurites in the presence of the skin explant were too long and mingled to measure the neurite length (e-supplement). Because these results could not be correctly interpreted, they were not included in the analysis. For PC12 cells cultured in the absence of skin explants, the neurite lengths (n = 3) were 45.9 ± 3.4, 47.3 ± 12.8, 59.4 ± 5.5 and 49.5 ± 5.6 μm on days 2, 4, 6 and 8, respectively. In the presence of the skin explant, the neurite lengths (n = 3) were 28.6 ± 6.1, 54.1 ± 15.3, 106.7 ± 11.1 and 99.5 ± 3.4 μm on days 2, 4, 6 and 8, respectively. On days 6 and 8, the lengths of the neurites in the presence of the skin explant were significantly increased (P < 0.01) by factors of 1.79- and 2.01-fold, respectively, when compared with the control condition (Fig. 2).
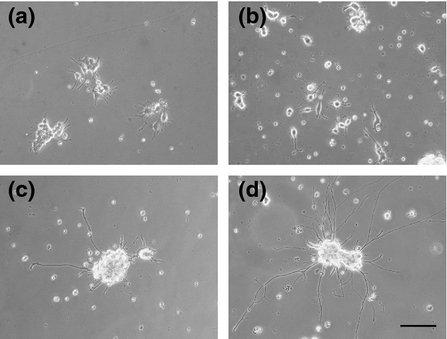
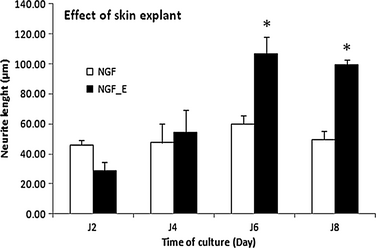
Hence, this model represents a simplified method to observe the growth of nerve fibres in response to mediators producedby the skin explant. The length of nerve fibres sprouted by cells cultivated alone or in the presence of skin explants was measured every 2 days for a total of 8 days. The neurites were longer in the presence of the skin explant and were significantly longer on culture days 6 and 8. These data demonstrated the direct influence of the skin explant on the neurite outgrowth of the differentiated PC12 cells with NGF. NGF could be also implicated in the neurite growth. However, the addition of NGF is necessary in the control condition (without explant) because NGF is necessary to induce the differentiation of PC12 cells into neurons and prevent cell death 18. The skin explants likely affected the growth of nerve cells by the secretion/release of a number of distinct factors, maybe produced by injured tissue or the surrounding areas, constitutively produced by keratinocytes and/or produced by keratinocytes in response to a loss of epidermal innervation. All these different factors still need to be identified. Under different conditions, the roles of NGF, NT3, BDNF and GDNF have been investigated in trophic models with or without keratinocytes 4, 19-22, but these factors have been rarely studied in a skin organotypic model. Another hypothesis is that the skin explant may be considered as lesional skin because the skin in our model was progressively degraded because the level of apoptotic cells was increased with time of culture (e-supplement), as this was already described in the work of Paus and colleagues 23. In wounded or lesional skin, NGF does not appear to be the unique factor that stimulates sensory neurons 9, 24. Further studies of this in vitro model will be performed to identify the soluble factors involved in the repulsive or trophic effects of neurite outgrowth.
In conclusion, we developed a simple model to study interactions between nerves and skin using PC12 cells to understand the trophic mechanism involved in neurite outgrowth in reference to skin released molecules. The presence of skin explants was sufficient to induce a trophic response of differentiated PC12 cells to skin. Although co-cultures of skin and cells from other species have been previously performed 7, 17, these different origins (humans and rats) of cells could be a limitation in interpretation of data. In our model, glial cells, which play an important role in reparation of nerve injury, were absent; consequently, their implication was not evaluated. Some interesting applications of this model could include the study of the effects of skin from different origins, (i.e. gender of the skin donor, original skin location or a disease, such as diabetes, psoriasis, atopic eczema, neuropathies…) on PC12 cell growth.
Acknowledgements
We thank Dr S. Valentin, Dr P. De Vries and Pr W. Hu for providing skin samples. The authors declared no conflict of interest.
Authors' contribution
NL, JPP performed the research; NL, LM, CJ, LD designed the research study; UP, CL, JC, JPP contributed essential reagents or tools; NL, NB, CJ, LD analysed the data; NL wrote the paper. LM, CJ, GP, JC, CL rewrote and corrected the paper.
Conflict of interests
The authors have declared no conflicting interests.




