Ancestral environment determines the current reaction to ultraviolet radiation in Daphnia magna
Abstract
An individual's phenotype can be altered by direct contact with its present environment but also by environmental features experienced by previous generations, that is, parental or grandparental effects. However, the strength and direction of these transgenerational effects may be highly variable according to the ecological conditions experienced by ancestral generations. Here, we performed a reciprocal split-brood experiment to compare transgenerational responses to the threat of ultraviolet radiation (UVR) in the zooplankter Daphnia magna, which had, or had not, been exposed to UVR for more than 150 generations. We found that the environment at which parents and grandparents were reared significantly influenced both behavior and life-history traits of their descendants. However, such transgenerational responses differed between D. magna individuals with contrasting ancestral stress history, that is, when exposed to UVR previously unexposed individuals rapidly changed their behavior and life-history traits, whereas individuals previously exposed to UVR showed less pronounced response when the UVR threat level relaxed. Hence, we here demonstrate an asymmetric transgenerational plasticity in response to UVR threat. The findings advance our understanding on the evolutionary ecology of such transgenerational effects and their potential role in response to changes in the local environment.
An organism's phenotype is the product of its genotype, environmental factors, and random noise. One genotype may produce a range of phenotypes at different environmental conditions, which is known as phenotypic plasticity that can be expressed either within a generation or across generations (Agrawal et al. 1999; Pigliucci 2001). Environmental variability will result in evolution of within-generation plasticity (WGP) when the environmental changes occur fast relative to the generation time (Pigliucci 2001), so that individuals may buffer against negative impacts of their immediate environment through changing their behavior, morphology, or other phenotypic traits within their lifetime. However, transgenerational plasticity (TGP) would be favored when there is environmental heterogeneity across generations and offspring environmental conditions can be predicted from their parental environmental conditions (Leimar and McNamara 2015; McNamara et al. 2016). In this case, not only the parental phenotypes but also the parental experiences or actions can be passed onto the offspring and therefore profoundly shape the expression of offspring phenotypic traits without altering genotypes (Agrawal et al. 1999; Salinas and Munch 2012; Walsh et al. 2014, 2015; Tariel et al. 2020b). TGP may have the potential to enable offspring to better cope with environmental variation when parental environments are reliable predictors of the offspring's environments so that parents may refine offspring phenotypes in anticipation of the environmental conditions they are likely to experience (Herman and Sultan 2011; Donelson et al. 2018; Yin et al. 2019). For example, parents experienced with predation risk may produce offspring that exhibit stronger antipredator defensive traits to reduce their vulnerability to predation (Agrawal et al. 1999; Storm and Lima 2010; Luquet and Tariel 2016). TGP may be described as a generalization of parental effects in which parental environment drives the transgenerational response and therefore modifies the offspring phenotype. To date, parental, especially maternal, effects are well studied and ample evidence has been found for many organisms in response to a variety of environmental perturbations such as toxins (Beyer and Hambright 2017; Radersma et al. 2018), food availability (Harney et al. 2017; Coakley et al. 2018; Zhou and Declerck 2020), ultraviolet radiation (UVR) (Huebner et al. 2013; Ghanizadeh Kazerouni et al. 2017), or predation risk (Storm and Lima 2010; Bestion et al. 2014; Donelan and Trussell 2015; Freinschlag and Schausberger 2016; Donelan and Trussell 2018; Sharda et al. 2021). However, under certain circumstances, phenotypic changes induced by environmental stressors may be broader than just a parental effect and instead persist over multiple offspring generations, leading to a strong and long-lasting transgenerational effect on the offspring survival and performance (e.g., Painter et al. 2008; Remy 2010; Groot et al. 2016; DeCourten et al. 2020). Therefore, in natural populations, an organism may gain information about its environment from its grandparents (ancestors), its parents, or its own personal experience and then decide how and whether to adhere to this information from different sources. Compared to the widely studied parental effects on offspring phenotype, insights into the combined effects of grandparental (ancestral) and parental experiences together with personal experience to produce adaptive transgenerational outcomes in organisms are still limited (but see Hafer et al. 2011; Walsh et al. 2014; Tariel et al. 2020b). Thus, further exploration of the long-term effects of stressors over several generations that consider different combinations of multigenerational environments from ancestors to offspring is urgently needed.
The relative strength and direction of TGP may not be consistently expressed across different environmental conditions but instead highly variable and dynamic. Recent studies have shown that these transgenerational effects are likely to be context dependent according to the offspring environment (Plaistow et al. 2006; Groot et al. 2016; Luquet and Tariel 2016; Harney et al. 2017; Stein et al. 2018; Schwanz et al. 2020). For example, Plaistow et al. (2006) found that the effects of past food environment persisted across at least three generations in the soil mite Sancassania berlesei; however, such effects were context dependent and varied between high and low food level environments where offspring were reared. Similarly, when exposed to thermal stress, jacky lizard Amphibolurus muricatus also expressed a context-dependent pattern of TGP, which varied with the offspring thermal environment (Schwanz et al. 2020). Moreover, Groot et al. (2016) proved that the context dependency of transgenerational effects also exists in plant Arabidopsis thaliana. Transgenerational effects represent communication of information from ancestors to offspring (Bell and Hellmann 2019) and therefore, in addition to the offspring environment described above, the stress history experienced by the ancestral generations may also potentially affect the expression and direction of these effects. In a pioneering study, Etter (1988) suggested asymmetric plasticity in an intertidal snail (Nucella lapillus), where snails from protected areas produced a larger foot when exposed to stronger wave action, whereas negligible changes occurred in exposed morphs transplanted to a protected shore. Although this study only considered WGP, it may imply that plastic responses across generations can also be different due to the contrasting past environmental history of populations. Accordingly, Walsh et al. (2016) studied transgenerational responses of Daphnia ambigua in populations that experienced different fish predation for many generations (consistently strong, consistently weak, or variable predation risk). They found that Daphnia populations experienced with consistently strong (or weak) predation risk displayed stronger TGP in response to predator cues than those with variable risk. In contrast, Goeppner et al. (2020) did not find differential patterns of predator-induced TGP between two Physa acuta populations that had or had not been exposed to predators for many generations. Sentis et al. (2018) reported similar results for two pea aphid (Acyrthosiphon pisum) populations after being reared with or without predators for 16 generations, although predator-exposed populations evolved a significantly weaker plastic response after 25 generations of exposure compared to those never exposed to predators. In general, these pioneering studies indicate that the presence of TGP can evolve but may vary in strength and direction according to the past environments experienced by the population for many generations. Thus, measuring transgenerational effects (i.e., TGP) in the context of a single population with certain evolutionary history may limit insights of its evolutionary ecology and its role in response to environmental change. Although there are numerous studies documenting the existence of TGP in many plant and animal taxa (Donelson et al. 2018; Bell and Hellmann 2019; Tariel et al. 2020a), our understanding of the evolutionary potential of such transgenerational responses remains elusive. Moreover, it needs to be tested whether the contrasting ecological conditions experienced by ancestral generations have the ability to drive the evolutionary changes in TGP when animals exposed to other abiotic stressors that are not mentioned above, such as UVR.
UVR is a ubiquitous environmental stressor, which is biologically damaging to both terrestrial and aquatic organisms, such as algae, zooplankton, and fish, owing to its highly energetic short wavelength (Rautio and Tartarotti 2010). Several previous studies have shown that exposure to UVR can cause DNA damages, impaired reproduction, and higher mortality rates among zooplankton (Hansson and Hylander 2009; Huebner et al. 2009, 2013; Oexle et al. 2016). To handle this threat, many zooplankton taxa have developed various strategies, including alterations in behavior, accumulation of photoprotective compounds, or shifts in life-history patterns (Rhode et al. 2001; Hansson and Hylander 2009; Fernández et al. 2018; Sha et al. 2020). Vertical migration has been observed as a behavioral response among many zooplankton taxa to cope with UVR threat in natural systems (Leech and Williamson 2001; Ekvall et al. 2015), as well as under laboratory conditions (Hylander et al. 2014; Hansson et al. 2016; Ekvall et al. 2020; Sha et al. 2020). By moving down to deep waters during day, zooplankton avoid the high exposure to the dangerous radiation and therefore reduce the detrimental effects of UVR (Rhode et al. 2001; Hansson and Hylander 2009). Zooplankton may also respond to UVR by acquiring or producing photoprotective pigments, such as melanin, that can either be consistently present throughout the individual's lifetime or induced when needed (Rhode et al. 2001; Hansson et al. 2007; Fernández et al. 2020). Such abovementioned plastic responses have been well documented in many zooplankton species and taxa (e.g., Hansson et al. 2007; Hylander et al. 2014; Overholt et al. 2016; Fernández et al. 2018, 2020), but most studies have mainly focused on acute or short-term exposure within a single generation (e.g., WGP). However, this plasticity may also be transgenerational as levels of UVR varies considerably both temporally over the season and spatially within and between water bodies, resulting in fluctuating exposure within as well as among generations. This transgenerational effects of UVR have been investigated by a few studies (Huebner et al. 2009, 2013; Sha et al. 2020). For example, in our recent study, we investigated patterns of TGP in Daphnia and found that naïve Daphnia individuals gained UVR tolerance rapidly and became locally adapted to the threat after three generations of exposure (Sha et al. 2020). Interestingly, it has also been reported that UVR tolerance can be different and likely be controlled by the evolutionary history of populations (Fernández et al. 2020). Based on these results, we predict that contrasting ecological conditions, that is, stress history, may drive divergent patterns of TGP in Daphnia when exposed to fluctuating levels of UVR.
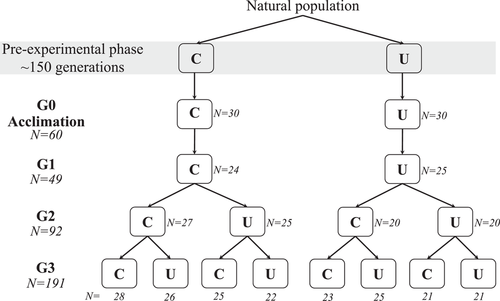
Here, we performed a reciprocal split-brood experiment to compare phenotypic responses in Daphnia magna when exposed or not exposed to UVR across three parthenogenetic generations (Fig. 1). Individuals were randomly chosen from two groups with contrasting UVR environments, consisting of a control group (C) that had never experienced UVR, and a treatment group (U) reared under UVR. Both groups were reared under the respective conditions for more than 150 generations, ensuring a stable environment without or with UVR threat, respectively. From these strains, we then reared D. magna over three generations using a full factorial design of exposure to only visible light or to UVR, resulting in two treatments in generation 1, four, and eight treatments in generations G2 and G3, respectively (Fig. 1). For each generation, we quantified the UVR avoidance behavior and two specific life-history traits (age at maturation and number of offspring born from the first four clutches) for each treatment. We aimed to (1) elucidate whether the patterns of ecologically driven divergence in TGP as a result of the evolution of stress history in past environments, that is, to specifically focus on if the plastic response depends on the specific stress history of the ancestral generations, and (2) investigate the persistence of transgenerational effects over two generations and reveal how the effects of multigenerational environments in grandparent, parent, and offspring together influence the UVR avoidance behavior and two specific life-history traits of D. magna. Based on previous results that TGP can evolve in experimental settings (Sikkink et al. 2014; Dey et al. 2016; Sentis et al. 2018), we predicted that contrasting past environmental history in the two experimental groups, that is, C-group exposed to non-UVR and U-group exposed to UVR for many generations, may drive differential patterns of transgenerational effects. Therefore, the phenotypes of offspring should be affected by the environment of the previous generations (grandparents and parents) as observed in several previous studies (Hafer et al. 2011; Walsh et al. 2014; Tariel et al. 2020b), but such effects may vary between the two D. magna groups due to the evolutionary history of stress. Specifically, naïve individuals from the C-group were hypothesized to rapidly establish new plastic traits in response to UVR. Because individuals from the U-group had been exposed to UVR for many generations, they may have had the opportunity to establish phenotypes with increased resistance to high UVR exposure and may not need to rely on plasticity to respond to the threat of UVR (Sikkink et al. 2014). Therefore, the transgenerational response of individuals from the U-group may be hypothesized to be less pronounced and more robust to environmental variation between generations than naïve individuals from the C-group. Hence, our overall hypothesis was that the plastic responses to UVR across generations are asymmetric and depend on the evolutionary history of ancestral generations.
Materials and Methods
A natural population of D. magna originating from Lake Bysjön, southern Sweden (55.67° N, 13.54° E) was divided into two groups exposed to only visible light and to the combination of visible light and UVR (denoted as C- and U-group, respectively). Daphnia magna were kept in this non-UVR and UVR treatment for several years so that at least 150 generations would pass prior to the start of the experiment. During these pre-experimental generations, D. magna from each group were reared in six replicate 1-L jars at 20°C with a light:dark cycle of 12:12 h. The approximate density in each jar was maintained at 15–20 individuals L−1 throughout the pre-experimental phase. We used four UVA fluorescent tubes (UVA-340 Q-panel) and four cool white fluorescent lamps (Aura Ultimate Long Life 36 W) to provide the UVR and photosynthetically active radiation (PAR, visible light). Individuals unexposed to UVR (C-group) were covered by a UVR-screening Plexiglass (Röhm GS 233; Röhm, Darmstadt, Germany) that can effectively remove radiation below 370 nm, whereas a UVR-transparent Plexiglass (Röhm GS 2458; Röhm, Darmstadt, Germany) was used for the UVR-exposed individuals (U-group). Both the two types of Plexiglass allowed the visible light to pass through, resulting in a light intensity of 25.5 μmol m−2 s−1 for all individuals from both C- and U-group. Additionally, individuals kept in the UVR treatment (U-group) were exposed to a dose of UVA radiation of 132 μW cm−2, resembling the solar UVA radiation at noon on a day with some overcast. All animals were fed ad libitum three times a week with Scenedesmus sp. suspension and were transferred to clean jars with new medium once a month. For more details about the D. magna culture, please consider Hylander et al. (2014).
EXPERIMENTAL DESIGN
To initiate the experiment, we collected five female adults with eggs from each of the six jars as mentioned above that held the initial stock populations so that a total of 30 individuals were isolated from each of the two groups (C-group: non-UVR exposed vs. U-group: UVR exposed). Animals were then placed under the same light conditions as their original environments, constituting the initial G0 generation (G0: 2 treatments × 30 replicates = 60 individuals; Fig. 1). We used the same type of Plexiglass to provide either non-UVR-exposed or UVR-exposed treatments throughout the experiment as described above for the pre-experimental phase. Specifically, G0 individuals coming from the C-group were only exposed to the visible light at a light intensity of 20 μmol m−2 s−1 (treatment: C) by covering the experimental jars with a UVR-screening Plexiglass, whereas we used a UVR-transparent Plexiglass for the U-group individuals, leading to an additional exposure of UVA radiation with an intensity of 150 μW cm−2 (treatment: U). During the experiment, maternal lines for the experimental animals were raised individually in 100-mL jars filled with 80 mL tap water, corresponding to a density of 12.5 individuals L−1, which was similar to the density of the initial stock populations. All the daphniids in the G0 generation were checked daily and fed with 1 mL (66.68 μg carbon L−1) of Scenedesmus sp. every second day.
The first experimental generation of D. magna (G1) was initiated by collecting one neonate (<12-h old) from the second clutch per female per treatment (N total = 60 individuals) and individually transferring them into 100-mL jars containing 80 mL tap water and specified quantities of Scenedesmus sp. (66.68 μg carbon L−1). For each group (C- and U-group), the remaining neonates from each female were pooled and evenly divided into five replicate jars (250 mL) for later pigmentation analysis. All individuals were reared under the same treatments as their mothers so that neonates from G0 generation in the C-group were reared under non-UVR conditions and neonates from G0 generation in the U-group were reared under UVR, constituting two treatments: C and U. Six individuals in treatment C and five individuals in treatment U died during the experiment, giving 24 and 25 replicates of treatments C and U, respectively (Fig. 1).
The second experimental generation (G2) was obtained from the second clutch from G1 animals. Reciprocal transplants between C and U treatments were then performed. Specifically, we collected two newly born offspring (<12-h old) per female from the C treatments and then equally split them into two treatments, that is, one sibling was raised in non-UVR conditions (treatment CC) and one under UVR exposure (treatment CU) (G2: 27 and 25 replicates, respectively). The leftover individuals from each female were pooled and also equally distributed into the C and U treatments for later pigmentation analysis, resulting in five replicate jars for each of the two treatments. Similarly, two newly born offspring per female from the U treatments were equally divided between C and U treatments in the G2 generation (treatments UC and UU; G2: 20 replicates × 2 treatments = 40 individuals) and all the other individuals from each female were equally distributed into the C and U treatments for later pigmentation analysis. Finally, we got four treatments with a total of 92 individuals in the G2 generation, including treatments of CC, CU, UC, and UU (Fig. 1). The same procedure was repeated for the G3 generation when the G2 D. magna delivered their second clutch, resulting in eight treatments, CCC, CCU, CUC, CUU, UCC, UCU, UUC, and UUU (G3: 21–28 replicates × 8 treatments = 191 individuals; Fig. 1). It should be noted that we cannot completely exclude effects of offspring early exposure, or no exposure, to UVR (0–12 h after birth), which is a general problem with all studies of TGP, irrespective of taxa. During this experimental phase, we fed individuals with 1 mL (66.68 μg carbon L–1) of Scenedesmus sp. every second day and transferred them to clean jars with new medium once a week. Because multiple individuals were kept in a single jar for the pigmentation analysis, we fed them with 3 mL of Scenedesmus sp. every second day and refreshed the medium every 2 weeks.
After the D. magna was born, individuals at each of the three experimental generations were checked every second day for the presence of eggs (defined as day of maturation) and the production of offspring. We monitored the animals for approximately 40 days when all of them had released their fourth clutch. The number of neonates born in the first four clutches was therefore recorded to estimate the reproductive output. A total of 332 individuals were monitored for their life-history data throughout the experiment. At the end of the life-history analysis, animals were collected and assayed individually for their behavioral response to UVR.
SWIMMING BEHAVIOR
After 40 days of exposure to the respective treatment (C or U) since they were born, female adults from each generation and each treatment (20–28 replicates × 3 generations × 14 treatments = 332 individuals; Fig. 1) were assayed individually for their behavioral response to UVR in a three-dimensional tracking setup, composed by four synchronized digital cameras (Pike F-210C, Allied Vision Technologies GmbH) and a 0.2 m × 0.2 m × 0.85 m Plexiglas aquarium. Thirty liters of tap water was filled in the aquarium, resulting in a 0.75-m water column (Ekvall et al. 2020). Prior to the recording, individuals were labeled with yellow fluorescent nanoparticles (585 ITK Carboxyl Quantum dot, Life technologies, Prod. Nr: Q21311MP) following an adapted protocol from Ekvall et al. (2013). The labeling process involved incubating each individual with 8 μL of the poly-l-lysine-conjugated Quantum dot solution (Qdots) in the absence of light at room temperature for 1 h, and then removing the excess Qdots by washing the organism with filtered tap water. Each labeled D. magna was then individually introduced into the tracking arena using a 3-mL plastic Pasteur pipette and was allowed to acclimatize to the water and light conditions for 10 min before tracking the swimming behavior of individual D. magna. A 2-min video, built up by two distinct phases, with six frames per second, was recorded for each individual. The first 1 min was recorded under only excitation light, which can be considered as the acclimation phase. The second minute was the UVR threat phase when the UVR LED was turned on to mimic the presence of solar radiation (100 mA, corresponding to a UVR intensity of 150 μW cm−2). The video recording was started when the individual had reached the top 10 cm of the water column to ensure that all individuals were exposed to a similar level of UVR before their behavior response was recorded. The specific individual was discarded if it failed to reach the top 10 cm within 1 h.
The Daphnia’s three-dimensional positions shown as the list of XYZ coordinates were extracted from the recordings according to the method described in Palmér et al. (2016) in MATLAB R2017b (Mathworks 2017). We then extracted the swimming depth as the Z coordinates for each individual using R version 4.2.0 (R Core Team 2022). We calculated the individual mean values of depth position in the water column for each recording phase. Here, we only focus on the mean depth during the exposure to UVR (UVR threat phase), that is, during the second minute of each trial, to assess the differences in the UVR avoidance behavior between treatments. Therefore, large values of mean depth are associated with individuals that behaviorally avoid UVR, whereas a small value of mean depth shows that individuals are UVR tolerant and remain high up in the water column when exposed to UVR.
After the behavioral assay, each D. magna was photographed with a camera (Infinity 1–2CB) mounted on a microscope (Olympus SZX7). We then measured body size (from the top of the head to the origin of the tail spine) using the software ImageJ (version 1.52a, National Institutes of Health, Bethesda, Maryland, USA).
PIGMENTATION
Analysis of photoprotective pigmentation was performed on Daphnia individuals from both treatments and the methods are described in the Supporting Information.
STATISTICAL ANALYSES
All data were statistically analyzed in R version 4.2.0 (R Core Team 2022). During the experiment, all D. magna with a total of 332 individuals from the two groups with contrasting past environmental history (C-group: exposed to non-UVR vs. U-group: exposed to UVR) were measured individually for its UVR avoidance behavior (mean depth), life-history traits (age at maturation and the total number of offspring born from the first four clutches), and body size for three generations (Table S1). Therefore, we performed a multivariate analysis of variance (MANOVA) first to evaluate whether past environmental history (C-group vs. U-group) and generation (G1, G2, G3) influenced the phenotypic responses of D. magna (behavior, two life-history traits, and body size). Linear mixed models were then used to investigate how past and present environments affect each of the above-observed offspring phenotypic traits using the package nlme (Pinheiro et al. 2022). We analyzed the three experimental generations separately. Jar identity was entered as a random effect to account for unexplained variation among jars that contained the initial stock populations. Maternal line was also included as a random effect for each of our model analyses. In the G1 generation, we compared differences in the above variables between the C versus U treatments by including the offspring environment as the fixed factor. In the G2 generation, the parental (G1), offspring (G2) environments, and the parent × offspring environment interaction were entered as fixed effects, whereas grandparental (G1), parental (G2), offspring (G3) environments, and possible interactions were included as fixed effects for the G3 generation. For each test, we included all the possible interactions between fixed effects in the initial models but sequentially removed highly nonsignificant interaction terms (P > 0.2, fixed effects only for the G2 and G3 generations) until the final model yielded the lowest AIC score (final models for each analysis are shown in Table 1). We tested the significance of the random effects with likelihood ratio tests for all models. We used ANOVA to compare differences in melanin concentration between treatments for each of the three generations.
| Mean Depth | Age at Maturation | Number of Offspring | ||
|---|---|---|---|---|
| df | F (df) | F (df) | F (df) | |
| Generation G1 | ||||
| Fixed effects | ||||
| Treatment | 1 | 7.326* (10) | 17.459** (10) | 6.703* (10) |
| Random effects | ||||
| Jar ID | 1 | 0.490NS | 1.427NS | 1.063NS |
| Maternal line | 1 | 0.000NS | 0.000NS | 0.000NS |
| Generation G2 | ||||
| Fixed effects | ||||
| Parental treatment | 1 | 7.093* (10) | 9.792* (10) | 46.977*** (10) |
| Offspring treatment | 1 | 7.483** (33) | 0.800NS (34) | 0.783NS (34) |
| Parental × Offspring | 1 | 1.739NS (33) | - | - |
| Random effects | ||||
| Jar ID | 1 | 0.000NS | 0.000NS | 0.000NS |
| Maternal line | 1 | 0.000NS | 7.770** | 6.806** |
| Generation G3 | ||||
| Fixed effects | ||||
| Grandparental treatment | 1 | 5.947* (10) | 6.152* (10) | 30.045*** (10) |
| Parental treatment | 1 | 3.523NS (129) | 5.665* (129) | 19.563*** (129) |
| Offspring treatment | 1 | 6.243* (129) | 0.106NS (129) | 7.442** (129) |
| Grandparental × Parental | 1 | - | - | - |
| Grandparental × Offspring | 1 | - | - | - |
| Parental × Offspring | 1 | - | - | - |
| Grandparental × Parental × Offspring | 1 | - | - | - |
| Random effects | ||||
| Jar ID | 1 | 0.027NS | 0.000NS | 0.691NS |
| Maternal line | 1 | 2.201NS | 1.347NS | 31.096*** |
- Note: The denominator degrees of freedom are displayed after each F value. Nonsignificant interaction terms that were removed from the models are hyphenated. The random effect of “Maternal line” had a significant effect on the two life-history traits of individuals from G2. This effect was also significant for the number of offspring at G3 generation. However, removing this random effect from the analysis did not change the patterns of significance for the main fixed effects, and thus such random effect was considered to be marginal. Bold values indicate significant results (P < 0.05). *P < 0.05; **P < 0.01; ***P < 0.001; NSP > 0.05.
Besides, we also ran separate ANOVA and Tukey's test to assess how the contrasting past environmental history (C-group vs. U-group) influenced the expression of environment-induced transgenerational effects. We found a significant positive relationship between the body size and the total number of offspring born from the first four clutches for most of treatments, indicating that larger individuals have the potential to produce more offspring (Fig. S1). However, there were no significant body size differences between treatments for any of the three generations (Table S2). Therefore, here we only focused on behavior and the two life-history traits to perform the ANOVA analyses. Specifically, for the C-group, we compared differences in trait responses when animals were transplanted from non-UVR conditions to UVR exposure for one and two consecutive generations (C, CC, CCC vs. CU, CCU vs. CUU), whereas for U-group, we tested how animals recovered when released from the threat for one or two generations (U, UU, UUU vs. UC, UUC vs. UCC). All data on life-history traits were square root transformed to better meet assumptions of normality.
Results
There was a significant interaction between the past environmental history and generation on the behavior (mean depth), life-history traits (age at maturation and the total number of offspring born from the first four clutches), and body size measured in this experiment (MANOVA; Table S3), suggesting that there were different transgenerational effects between the two groups exposed to contrasting past environmental history (C-group vs. U-group).
We found that exposure to UVR caused significant transgenerational effects on both behavior and the two life-history traits in D. magna (Table 1; Figs. 2, 3). In the G1 generation, D. magna reared under UVR showed significantly less behavioral responses when again exposed to the UVR threat than animals reared without UVR (Table 1). We found that UVR-induced individuals swam downward to a mean depth of 330 mm (± 33 SE) when exposed to the UVR during the behavioral recording, which was 26% shallower than the estimated mean depth chosen by the control individuals (Fig. 2a; Table S1). Besides, exposure to UVR also caused individuals to mature later and produce less offspring (Table 1). The average time to maturation was 10.4 days (± 0.4 SE) for the individuals reared under UVR, which was ∼2.6 days later than the individuals from the C treatment (Fig. 3a; Table S1). Similarly, individuals reared under UVR produced 35% less offspring than did Daphnia grown at control light conditions (Fig. 3d; Table S1).
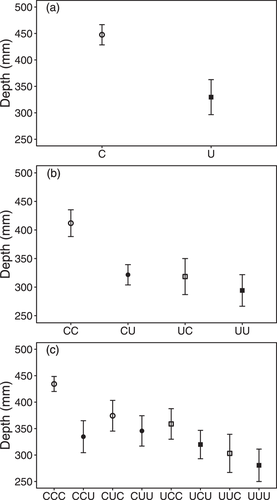
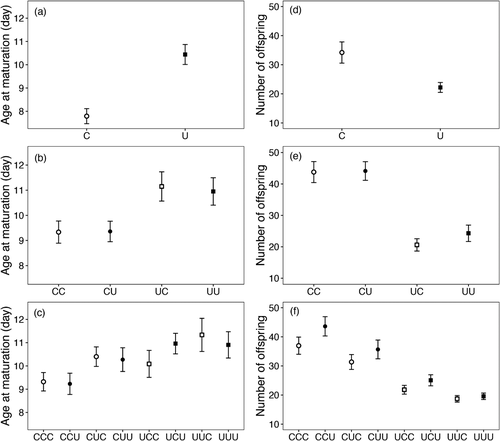
In the G2 generation, the parental treatment significantly influenced both behavior and life-history traits (P < 0.05; Table 1). We found that D. magna that had parents reared under UVR showed significantly less of behavioral response to the UVR threat, later maturation, and less reproductive output compared to individuals whose parents were reared under non-UVR (Figs. 2, 3). Specifically, the offspring of parents reared under UVR dove downward to a mean depth of 306 mm (± 21 SE) when again exposed to UVR, which was 17% shallower than the mean depth chosen by the offspring that had parents grown at non-UVR conditions (Fig. 2b). Individuals born from UVR-induced parents also matured ∼1.7 days later and produced 49% less offspring compared to the individuals whose parents were reared under non-UVR (Figs. 3b, 3e). In addition, we also found a significant effect of offspring environment on the mean depth of D. magna (Table 1). Offspring that had always been reared under non-UVR (CC) selected a mean refuge depth of 412 mm (± 23 SE) when exposed to UVR, whereas their sisters that were reared under UVR (CU) showed significantly less behavioral response in their mean depth (322 mm ± 18 SE) (Fig. 2b; Table S1). No such difference was recorded for offspring from U-parents (UC vs. UU).
In the G3 generation, the mean depth was significantly influenced by the grandparental treatment (G1; Table 1). Hence, G3 individuals whose grandparents received UVR exposure responded significantly less in their mean depth (317 mm ± 15 SE) than those from non-UVR-exposed grandparents (375 mm ± 13 SE; Fig. 2c). The offspring rearing environment, that is, exposure to UVR, also significantly reduced the mean depth chosen by the individuals in the G3 generation (Table 1), similar to that observed in the G1 and G2 generations (Fig. 2c). For the age at maturation, we found that grandparental (G1) and parental (G2) exposure to UVR significantly delayed the maturation of G3 individuals by 10% and 8%, respectively (Table 1), compared to the individuals whose grandparents or parents had never experienced UVR (Fig. 3c). Similarly, the grandparental (G1) and parental (G2) treatments also significantly influenced the number of offspring in the first four clutches (Table 1). Granddaughters with UVR-exposed grandparents produced 42% fewer offspring than those with non-UVR-exposed grandparents. Moreover, G3 individuals whose parents received UVR exposure also showed a 18% reduction in the reproductive output compared to those with non-UVR-exposed parents (Fig. 3f). The offspring treatment also influenced the reproduction of G3 individuals, but here individuals reared under UVR produced 12% more offspring than those reared under non-UVR conditions (Fig. 3f). Information of past environments may have been actively transmitted from generation to generation via physiological pathways, but parents and grandparents may also have transferred their poor condition resulting from UVR exposure to their offspring. However, our study was not designed to assess potential pathways for information flow, but such issues indeed deserve further research.
We did not detect any changes or differences in the melanin concentration in any of the three generations or treatments (Table S4).
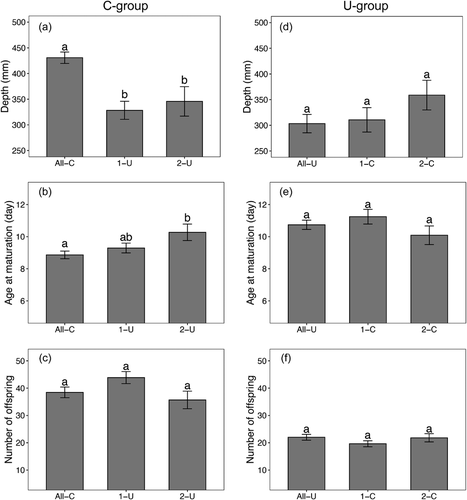
To evaluate the impact of ancestors on the transgenerational effects of UVR, we compared differences in trait responses across generations for both C-group and U-group separately. For the C-group, we found that exposure to UVR significantly influenced the behavioral responses in the mean depth of D. magna through generations (F2,149 = 14.040, P < 0.0001). A Tukey post hoc test showed that D. magna reared under UVR for one generation showed significantly shallower depth than those reared under non-UVR conditions, but two consecutive generations exposure to UVR did not further reduce the mean depth (Fig. 4a). Similarly, we also found a significant effect of UVR on the age at maturation of D. magna through generations (F2,149 = 3.636, P = 0.029), but D. magna delayed the maturation after two consecutive generations of exposure to UVR (Fig. 4b). However, we found no changes in the number of offspring through generations (F2,149 = 2.825, P = 0.062; Fig. 4c). For the U-group, D. magna did not change their behavior or life-history traits after being released from UVR exposure for either one or two generations (P > 0.05; Figs. 4d–f). Hence, the overall result is that a sudden exposure to a threat (UVR) gives a fast response in behavior and life-history traits, whereas the transgenerational response to a sudden relaxation of the same threat is slower and less pronounced.
Discussion
In a constantly changing environment, it may be beneficial, and faster, to show a plastic response to threats than expressing a genetically fixed response. However, it may also be beneficial to show a somewhat reduced response to a declining threat level, whereas exposure to an increasing level of a threat should instead induce a rapid plastic response. Although few studies have addressed this issue (but see, e.g., Sikkink et al. 2014; Sentis et al. 2018), we may predict that when many generations have experienced a relatively relaxed threat level that suddenly, within the lifetime of a generation, increases, the response may be predicted to be fast, whereas if the threat decreases after many generations of severe exposure, the response may be predicted to be slower because a return to severe exposure will be detrimental to the individual. In line with this, we show here that exposure to a UVR threat induced plastic phenotypic changes in Daphnia manga across generations, including alterations in behavior and shifts in life-history traits. However, those transgenerational effects from UVR were different due to the evolutionary history of stress in the ancestral generations, as well as to the traits considered. Context dependency of transgenerational effects has been found in several previous studies addressing environmental stressors, such as food availability (Plaistow et al. 2006; Harney et al. 2017), predation risk (Luquet and Tariel 2016; Stein et al. 2018), or other abiotic factors (Groot et al. 2016; Schwanz et al. 2020), which influenced the behavior, morphology, or life-history traits in a way that depended on the offspring environment. However, such context-dependent patterns were not found in our study, but instead we show that the presence and strength of transgenerational effects differed between D. magna individuals exposed, or not exposed, to UVR for more than 150 generations (i.e., contrasting ancestral stress history). Below, we interpret these results in greater detail.
Environmental factors, such as temperature (Salinas and Munch 2012; Walsh et al. 2014), cyanobacterial toxins (Gustafsson et al. 2005; Beyer and Hambright 2017; Gillis and Walsh 2019), and predation (Walsh et al. 2015; Luquet and Tariel 2016; Walsh et al. 2016; Tariel et al. 2020b), have been shown to influence the expression of traits among offspring via TGP, although similar studies related to UVR are rare. For example, Huebner et al. (2009) observed that UVR exposure consistently reduced survival and reproduction in both parental and offspring generations and two consecutive generations of exposure significantly decreased the reproductive output of Daphnia, suggesting a long-term cumulative adverse effect of UVR exposure. However, Ghanizadeh Kazerouni et al. (2017) found the opposite result, that is, when exposed to UVR, offspring born from UVR-exposed parents had greater sustained swimming performance as well as increased antioxidant enzyme activities compared to controls, suggesting that parental exposure to UVR increased the resilience of their offspring to counteract the negative effects of UVR threat in guppies (Poecilia reticulata). In our study, we found that individuals previously experienced with UVR showed a tolerant behavior; however, such UVR tolerance is associated with a cost in reproduction as UVR-exposed individuals matured later and produced less offspring compared to unexposed individuals in the G1 generation. Similar results have also been found in a previous study of D. magna where individuals reared under UVR showed a more tolerant behavioral response, but also a reduced clutch size (Sha et al. 2020). Unlike the present study, Sha et al. (2020) found that UVR-exposed individuals gradually increased their clutch size and were able to reproduce in a similar way as unexposed siblings after three generations of exposure. In contrast, Fernández et al. (2018) found the opposite response that D. pulex populations historically exposed to high levels of UVR reproduced at an earlier age with a higher fecundity compared to those historically exposed to low UVR. The differences in Daphnia clones or cladoceran species may account for these disparate results, as studies have shown marked differences in threat response across clones within several species of Daphnia (Connelly et al. 2016; Gillis and Walsh 2019; Langer et al. 2019) as well as across cladoceran species (Hansson et al. 2016; Ekvall et al. 2020; Liu et al. 2022). Interestingly, in the G3 generation, we found that individuals reared under UVR (treatments of CCU, CUU, UCU, UUU) produced more offspring compared to unexposed conspecifics (treatments of CCC, CUC, UCC, UUC). As demonstrated by Fernández et al. (2018), more offspring produced under UVR may compensate for the high mortality caused by the radiation to maintain the stable population fitness. But more studies are needed to explain why this pattern only appeared in the G3 generation.
In contrast to the phenotypic responses in the G1 generation, the association between behavior and life-history traits became unclear for individuals from the G2 and G3 generations as we found that their phenotypes were significantly affected by previous generations. These effects of parental and grandparental generations ultimately yielded patterns of variation and changes in traits that are not easily predicted from the trends revealed in the G1 generation, illustrating a complexity in TGP that may go beyond the parental generation and that the offspring phenotype can result from a combination of multigenerational effects. This is consistent with several previous studies on both Daphnia and other animal taxa (Hafer et al. 2011; Walsh et al. 2014; Tariel et al. 2020b), which show complex patterns of phenotypic responses in offspring due to different combinations of grandparental, parental, and developmental effects. For example, Hafer et al. (2011) and Walsh et al. (2014) demonstrated that the age at maturation of Folsomia candida and Daphnia ambigua was influenced by an interactive effect between grandparental and parental environments. However, in the snail Physa acuta, grandparental and parental exposure to predation risk did not interact with each other to influence offspring antipredator defenses, but instead these effects acted in opposite directions depending on the offspring environment (Tariel et al. 2020b). In our study, we did not find any significant interactive effects between grandparental, parental, or offspring environments, suggesting that the effects from multiple generations contributed additively to shape the offspring phenotype.
We also found that D. magna naïve to UVR (treatments of C, CC, and CCC) showed strong behavioral responses to this radiation threat by swimming down to deeper depths during the UVR exposure. However, these individuals quickly changed their behavior when they had previously experienced UVR within their lifetime (treatments of CU, CCU), that is, individuals showed an UVR-tolerant behavior by selecting a shallower refuge depth when again exposed to UVR. These results are in accordance with several previous studies, showing that zooplankton, including copepods and Daphnia, reduce their behavioral response to UVR after previously being exposed to this radiation threat for 12 or 30–40 days, or longer than 8 months (Hylander et al. 2014; Overholt et al. 2016; Sha et al. 2020). Interestingly, we also found that naïve Daphnia individuals quickly acquired the behavioral tolerance to UVR right after one generation of exposure to the threat, whereas two or more consecutive generations of exposure did not further increase their behavioral tolerance. In contrast to the naïve population, D. magna that had been reared under UVR for more than 150 generations (U-group) kept the tolerant behavior through generations even when the threat level declined (transferred to the C treatment), that is, individuals exhibited similar behavioral responses as their mothers or grandmothers irrespective of their own rearing environment. Hence, UVR-induced plastic responses in behavior varied between the two experimental groups, suggesting the evolutionary potential of transgenerational effects on D. magna resulting from different stress history in the ancestral generations. Several previous studies have also demonstrated experimental evolution of transgenerational effects (TGP) on phenotypic responses of offspring survival, morphology, or life-history traits for nematodes (Sikkink et al. 2014; Dey et al. 2016) and pea aphids (Sentis et al. 2018). However, our study extends our understanding on how contrasting ecological conditions in past generations drive evolutionary changes also in behavioral traits.
Behavioral traits are very labile and can be changed instantly after exposure to the threat. Besides, selection seems to favor faster plastic responses within a generation to reduce the risk of a mismatch between phenotype and the environment (Padilla and Adolph 1996). We therefore predicted that the offspring environment rather than the environment experienced by the past generations would determine the individual's behavior, so that WGP would be more common in the behavioral decision-making. This has also been shown in a previous study showing that antipredator behavior in the snail P. acuta was only expressed as WGP and individuals reared with predator cues responded less to the predation threat compared to the control individuals (Beaty et al. 2016). Similarly, Freinschlag and Schausberger (2016) showed in the two-spotted spider mite (Tetranychus urticae) that offspring increased their moving activity substantially when predator cues were present in their own environment. Tariel et al. (2020b) also found that offspring exposure to predator cues significantly increased the escape behavior in a freshwater snail (P. acuta) but meanwhile such antipredator behavior was also influenced by the grandparental environment. Several other studies have compared WGP and TGP on behavior, showing that parental and offspring environments can interact to shape the behavioral reaction norms in offspring (Bestion et al. 2014; Donelan and Trussell 2015, 2018; Luquet and Tariel 2016; Ghanizadeh Kazerouni et al. 2017; Stein et al. 2018), although these studies reported no clear trend for how behavioral WGP was altered by TGP among different systems. Therefore, both WGP and TGP have the potential to affect the individual behavioral traits and they may act independently or interactively. Interestingly, in the present study, we found that individuals from the naïve population (C-group) quickly changed their behavioral responses to UVR according to their own environment, which was consistent with our prediction mentioned above and the two previous studies performed by Beaty et al. (2016) and Freinschlag and Schausberger (2016). However, parental or grandparental environment seemed to have a pronounced effect on the behavioral responses of individuals from the UVR-raised population (U-group), although no significant interaction was found between parental and grandparental environmental effects. Notably, these individuals from the U-group kept the similar behavior as their parents or grandparents even when the threat had been removed for two generations, which suggests that long-lasting transgenerational effects from UVR can span across several generations. Hence, our results are partly consistent with previous studies on D. magna (Walsh et al. 2015), the nematode Caenorhabditis elegans (Remy 2010), fish (DeCourten et al. 2020), or humans (Painter et al. 2008), where environment-induced phenotypes persisted for several offspring generations after the stimulus was ceased. Such persistence of acquired behavior could reach more than 40 offspring generations in C. elegans (Remy 2010). However, as noted above, we here provide novel understanding regarding that at increasing threat levels, the response was rapid, suggesting that environment-induced phenotypic changes may be asymmetrical depending on experiences from previous generations. Such asymmetrical adjustments are in line with the error management theory (Sheriff et al. 2018), that is, a result of that the cost of not responding rapidly to an increasing threat level is likely far higher than the cost of not responding to an environment with relaxed stress level, leading to an asymmetrical response to the same threat depending on ancestral experiences.
When considering individuals from G2 and G3 generations, we found that in contrast to behavior, life-history traits, including age at maturation and number of offspring, were mainly influenced by the parental and even grandparental environments. When mothers or grandmothers had been exposed to UVR, offspring generally responded with a delayed maturation and reduction in reproductive output, which was not associated with the offspring rearing environment (except for number of offspring in the G3 generation). A possible reason for such negative effects of UVR on rate of development and reproduction may be UVR-induced damage on gut tissue (Huebner et al. 2013), which leads to reduced nutrient uptake and therefore a reduction in energy reserves available for growth and reproduction. Another reason may be due to the energy allocation that D. magna invest more energy to the UVR defensive strategies. In addition to the avoidance behavior, D. magna can also accumulate photoprotective compounds, such as melanin, to reduce the UVR damage, which can act as the complementary strategy to the behavior (Hansson et al. 2007, 2016; Hansson and Hylander 2009). However, this was not the case in our study as we did not find any changes in the melanin concentration among individuals from different treatments. Apart from pigmentation, other mechanisms beyond the scope of our study, such as increased photoenzymatic repair (Hansson and Hylander 2009) and the induction of internal antioxidants (Borgeraas and Hessen 2002; Oexle et al. 2016), may have provided protection against UVR. Therefore, D. magna reared under UVR may have allocated energy from reproduction to such photoprotective mechanisms to compensate for UVR damage, leading to a slower growth and less offspring production. Moreover, grandparents or parents may also have delivered the information of their poor growing conditions (exposure to UVR) to their offspring, so that their descendants would continue to mature later and produce less offspring even though they have been reared under non-UVR conditions for one or two generations (Figs. 4e,f). Such information transmission from previous generations to the offspring has been reported for zooplankton when exposed to food with limited phosphorus content (Zhou and Declerck 2020) and toxic cyanobacteria (Beyer and Hambright 2017). Furthermore, stress, such as exposure to certain chemicals, has previously been shown to be epigenetic and transgenerational in, for example, rats (Crews et al. 2012) and fish (Kelley et al. 2021). Although it is beyond the scope of our study to speculate about physiological mechanisms, epigenetic transfer of information between generations is a likely process involved.
Although previous studies have shown that TGP can evolve in experimental settings (Sikkink et al. 2014; Dey et al. 2016; Sentis et al. 2018), we here provide novel insights on that the intensity and direction of TGP can evolve, that is, TGP can be under natural selection and participate to adaptation. Hence, our data disentangle the features of TGP and add novel understanding by showing that plasticity is rapidly (within one generation) exposed in situations where the threat level increases, but slower (more than two generations) when a population is exposed to a declining threat. Hence, we here demonstrate an asymmetric plasticity across generations in response to UVR. Specifically, when exposed to UVR, naïve individuals quickly changed their behavior according to the offspring rearing environment, whereas changing maturation age may need two generations to be established, suggesting that behavioral traits are more plastic than life-history traits. However, previously UVR-exposed individuals exhibited the similar behavioral and life-history responses as their mothers or grandmothers irrespective of their own rearing environment. Hence, we here show that exposure to UVR has persistent, transgenerational consequences for offspring phenotypes that span at least three generations. Evolutionary history of stress in the ancestral generations may affect the expression of such effects leading to an asymmetric TGP, which may serve as an adaptive way of handling changes in the local environment.
AUTHOR CONTRIBUTIONS
YS and LAH conceived and designed the study. YS conducted the experiment. YS wrote the first version of the manuscript, and both provided comments on the manuscript.
ACKNOWLEDGMENTS
We would like to thank C. Solano Udina for her help in the pigmentation analysis. This study was funded by the China Scholarship Council and the Swedish Research Council (VR; grant #2016-03552).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA ARCHIVING
Data associated with this study are available in the Dryad Digital Repository at https://doi.org/10.5061/dryad.4j0zpc8fb (Sha and Hansson 2022).
REFERENCES
Associate Editor: R. Stelkens
Handling Editor: A. McAdam




