Sperm metabolic rate predicts female mating frequency across Drosophila species
Abstract
Female mating rates vary widely, even among closely related species, but the reasons for this variation are not fully understood. Across Drosophila species, female mating frequencies are positively associated with sperm length. This association may be due in part to sperm limitation, with longer-spermed species transferring fewer sperm, or to cryptic female choice. However, a previously overlooked factor is sperm metabolic rate, which may correlate with sperm length. If faster-metabolizing sperm accumulate age-related cellular damage more quickly, then females should remate sooner to obtain fresh sperm. Alternatively, frequent female mating may select for increased sperm competitiveness via increased metabolism. Here, we measure sperm metabolism across 13 Drosophila species and compare these measures to published data on female mating rate and on sperm length. Using fluorescent lifetime imaging microscopy, we quantify NAD(P)H metabolism ex vivo, in intact organs. Phylogenetically controlled regression reveals that sperm metabolic rate is positively associated with sperm length and with female mating frequency. Path analysis shows sperm length driving sperm metabolism and sperm metabolism either driving or being driven by female mating rate. While the causal directionality of these relationships remains to be fully resolved, and the effect of sperm metabolism on sperm aging and/or sperm competitiveness remains to be established, our results demonstrate the importance of sperm metabolism in sexual selection.
Female mating rate varies widely across taxa, often differing substantially even among closely related species. The degree of multiple mating by females has broad ecological and evolutionary implications, affecting not only sexual selection and sexual conflict, but also fields as diverse as population genetics, epidemiology, altruism and cooperation, speciation, and conservation (Pizzari and Wedell 2013; Taylor et al. 2014). Despite a wealth of theoretical and empirical research on the costs and benefits of polyandry (Prokop et al. 2012; Slatyer et al. 2012), the causes underlying the observed variation in female mating frequency often remain unclear.
One taxonomic group in which female mating rate shows particularly strong diversification is Drosophila, where the number of matings per female ranges from once in a lifetime to several times per day (Markow 1996). This variation has been linked to a similarly wide variation in sperm length, which ranges from less than a third to, famously, over 20 times the length of the male's body (Pitnick et al. 1995). These two variables are positively associated, with females mating more frequently in species with longer sperm (Markow 2002). This association has been explained by two pathways. First, because there is a tradeoff between sperm length and the number of sperm produced and transferred (Pitnick 1996; Bjork and Pitnick 2006), females in species with longer and therefore fewer sperm may use up their sperm stores more quickly, remating sooner to avoid sperm limitation (Fig. 1a-c). Second, frequent female mating may drive the elaboration of sperm length through postcopulatory sexual selection, with heightened sperm competition intensifying the advantage held by long sperm in long seminal receptacles (Miller and Pitnick 2002; Pattarini et al. 2006; Lüpold et al. 2012) (Fig. 1d-g; see also Discussion).
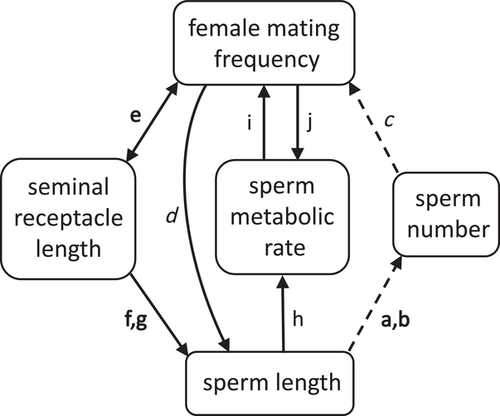
A previously unconsidered explanation for the association in Drosophila between longer sperm and faster female remating is that longer sperm cells may metabolize faster, and sperm metabolism may itself affect or be affected by female mating frequency. Sperm metabolic rate and female mating rate may be linked in two ways. First, frequent female mating may select for increased sperm metabolism in males. Such selection may occur if faster-metabolizing sperm are inherently more competitive (Fig. 1j), possibly due to increased motility via higher ATP production (Tourmente et al. 2013, Tourmente et al. 2015); or if longer sperm, which as noted above have an inherent competitive advantage, require higher metabolic rates (Fig. 1d and h).
Second, higher sperm metabolism may select for increased female mating rate, if faster-metabolizing sperm use up energy reserves or accumulate age-related damage more quickly (Fig. 1i). From the time of production to the moment of fertilization, sperm cells undergo a process of aging that involves an accumulation of damage to a range of cellular components (Reinhardt 2007; Pizzari et al. 2008), with corresponding decreases in various aspects of sperm function, including motility and velocity (Gasparini et al. [2017]; Cattelan and Gasparini [2021]); though see Vega-Trejo et al. [2019)]), fertilization capacity (White et al. 2008), and competitive success (Gasparini et al. 2017; Gasparini et al. 2018). This decline in sperm function with increasing sperm age represents a potential cost for both males and females, as offspring resulting from old sperm have reduced fitness in a variety of taxa ([White et al. 2008; Gasparini et al. 2017]; reviewed in Tarín et al. [2000]; Reinhardt [2007]; Pizzari et al. [2008]), including Drosophila (Tan et al. 2013). One evolutionary strategy for mitigating this cost is through frequent mating, which ensures a constant supply of young, undamaged sperm in female storage. Females in species with higher rates of sperm metabolism should be under stronger pressure to avoid using old sperm by mating more often.
The hypothesis that sperm aging via cellular metabolism drives frequent copulation was proposed some time ago (Siva-Jothy 2000; Reinhardt 2007). However, the challenges of determining sperm metabolism under natural conditions have up to now prevented a rigorous test of this hypothesis, and of the alternative hypothesis that female mating rate drives sperm metabolic rate via increased competitiveness. Recently, a method has been developed to measure sperm metabolism ex vivo, in intact organs: fluorescent lifetime imaging microscopy, or FLIM (Wetzker and Reinhardt 2019; Turnell and Reinhardt 2020). This method, widely used in cancer and stem cell research, enables the quantification of two autofluorescent forms of NAD(P)H inside cells: free versus protein-bound (Skala et al. 2007; Stringari et al. 2011). Compared to NAD(P)H that is free in solution, protein-bound NAD(P)H has a distinctly longer lifetime when excited (∼1−8 vs. ∼0.2−0.4 ns [Blinova et al. 2005]). Because free NAD(P)H is preferentially used as an electron carrier and is consumed at a disproportionately faster rate than the protein-bound form as ATP production increases, the rate of aerobic cellular metabolism corresponds to the fraction of bound NAD(P)H existing in the cell (Blinova et al. 2008). This fraction is represented by the relative amplitude of the autofluorescence signal at the longer lifetime (a2%).
Here, we apply this technique to measure, in 13 species of Drosophila (Fig. 2), the metabolic rate of sperm that is stored in the male's seminal vesicle. We chose to measure the metabolic rate in male-stored sperm because of the technical difficulties associated with measuring sperm stored in the female storage organs, and because metabolic rate in male-stored sperm predicts metabolic rate in female-stored sperm (Wetzker & Reinhardt, unpubl. data; see also Discussion). We compare our measures of sperm metabolic rate, controlling for phylogeny, to previously published data on female mating rate and on sperm length.
Methods
FLY LINES AND HUSBANDRY
Fly lines were obtained from the University of California, San Diego Drosophila Stock Center (D. arizonae [15081-1271.21], D. melanogaster [14021-0231.38], D. pseudoobscura [14011-0121.00]), and from the National Drosophila Species Stock Center at Cornell University (D. affinis [14012-0141.13], D. funebris [15120-1911.03], D. hydei [15085-1641.74], D. littoralis [15010-1001.04], D. mojavensis [15081-1352.48], D. montana [15010-1021.14], D. persimilis [14011-0111.02], D. subobscura [14011-0131.16]). Drosophila recens and D. simulans were provided by, respectively, Kelly Dyer (University of Georgia) and Pavel Tomancak (Max Planck Institute of Molecular Cell Biology and Genetics). All flies were maintained at approximately 20°C on a 12:12 light:dark cycle and fed on a standard yeast-corn-sugar medium (corn 90 g/L, agar 12 g/L, sugar 100 g/L, yeast 40 g/L, nipagin 20 mL/L, propionic acid 3 mL/L), except for D. affinis and D. hydei, which were fed on a banana-malt medium (bananas 138 g/L, agar 14 g/L, sugar beet syrup 95 g/L, liquid malt extract 30 g/L, yeast 42 g/L, methylparaben 2 g/L, ethanol 22 mL/L), and D. recens, which was fed on a standard mushroom medium (mushrooms 270 g/L, agar 7 g/L, sugar beet syrup 48 g/L, liquid malt extract 30 g/L, yeast 28 g/L, methylparaben 6 g/L). Virgin males were isolated from females upon eclosion and kept in groups of three to eight individuals. They were used for sperm metabolism analysis one to seven days (mean ± SD = 3.08 ± 1.59) after reaching sexual maturity, as reported for each species in Table 7.3 of Markow and O'Grady (2006), between June 2018 and May 2019.
DATA SOURCES
Data on female mating rate, collected in laboratory settings, were obtained from the following sources: D. affinis, D. persimilis, D. pseudoobscura (Snook and Markow 2001); D. arizonae, D. hydei, D. mojavensis (Markow 1982); D. funebris and D. simulans (Markow 1996); D. littoralis, D. montana (Aspi 1992); D. melanogaster (Singh and Singh 2004); D. recens (Markow 2002); D. subobscura (Maynard Smith 1956). Female mating rate, defined as the number of matings per day, was calculated where necessary by taking the reciprocal of the average remating latency. Other data sources are as follows: Sperm length: D. affinis, D. persimilis, D. subobscura (Snook and Markow 2001); D. funebris (Joly and Bressac 1994); D. hydei (Pitnick and Markow 1994); D. melanogaster, D. mojavensis (Pitnick 1996); D. pseudoobscura (Snook et al. 1994); all other species (Pitnick et al. 1995). Seminal receptacle length: D. affinis, D. persimilis, D. subobscura (Holman et al. 2008); D. funebris (Joly and Bressac 1994); all other species (Pitnick et al. 1999). Thorax length: D. funebris (Atkinson 1979); D. subobscura (Misra and Reeve 1964); all other species (Pitnick et al. 1995).
MEASUREMENT OF SPERM METABOLIC RATE VIA TWO-PHOTON FLUORESCENCE LIFETIME IMAGING MICROSCOPY (FLIM)
Intracellular NAD(P)H lifetimes were measured using time-correlated single photon counting FLIM. The microscope setup is comprised of an upright AxioExaminer.Z1 (Carl Zeiss, Jena, Germany) with an xy-motorized stage, a Chameleon Ultra II two-photon titanium:sapphire laser (tunable range of 690−1080 nm, 80 MHz repetition rate, 140 fs pulse width, Coherent, USA), and two hybrid GaAsP photon detectors (HPM-100-40, Becker & Hickl GmbH, Berlin, Germany). Laser intensity was controlled using an internal acusto-optical modulator (AOM, Becker&Hickl GmbH) and measured with a powermeter (PM100A, ThorLabs, Dachau, Germany).
Seminal vesicles (one from each male) were dissected in PBS. A representative area of sperm cells within the seminal vesicle was localized prior to imaging by transmission light illumination. Areas were chosen to maximize coverage by sperm cells; any portions of the chosen areas not covered by sperm were later excluded manually (see below). Light was focused on the selected planes using a water immersion objective (LD C-Apochromat 40 × /1.1 W, 421867-9970-000, Carl Zeiss). Images were acquired in a 512 × 512 pixel format with a pixel dwell time of approximately 5 μs and 100 frames, yielding a total scan time of approximately 140 s. Samples were excited at a wavelength of 740 nm and emission was collected using a beam splitter of 505 nm and a bandpass filter of 450/525 nm (AHF analysentechnik AG, Tübingen, Germany). Images were exported to SPCImage software version 6.5 (Becker&Hickl GmbH). Regions of interest were manually selected to include only sperm cells. The lifetimes and amplitudes of free and protein-bound intracellular NAD(P)H in the regions of interest were calculated with a scatter of 0 and shift values fixed for each image.
STATISTICAL ANALYSIS
All statistics were performed in R version 3.6.1 (R Core Team 2020). Divergence times were obtained from the TimeTree database (Kumar et al. 2017). To account for phylogeny (Felsenstein 1985; Harvey and Pagel 1991), phylogenetic generalized least squares (PGLS) regressions were performed using the gls() function of the nlme package with a phylogenetic correlation matrix specified using the ape package (Paradis et al. 2004). For all models, the phylogenetic variance-covariance matrix was transformed using the parameter lambda (Pagel 1999), which ranges from 0 to 1. This transformation allows for a variable degree of phylogenetic correlation, with 1 representing evolution under a Brownian motion model, where all branches of the tree are evolving at a constant rate, and 0 representing complete statistical independence between the species. Lambda was estimated by maximum likelihood. The residuals of all models were verified to be normal and homoscedastic. To calculate standardized regression coefficients, variables in all models were standardized to have a mean of 0 and a standard deviation of 1.
Because of the positive relationship between male body size and sperm length,1 male thorax length was included as a cofactor in all models where sperm length was a predictor unless otherwise noted (both variables were log-transformed). Because no relationship exists between male body size and sperm metabolic rate, the regression of female mating frequency on sperm metabolic rate (Fig. 4) did not control for male thorax length2; however, including male thorax length in this model does not qualitatively change the results (Supporting information Table S1). Variance inflation factors were calculated for the regression of female mating frequency on sperm metabolic rate, sperm length, and male thorax length to ensure that multicollinearity was not present.
For sperm metabolic rate, a target sample size of at least 10 individuals per species was chosen to balance representativeness and feasibility. Final sample sizes were as follows: D. affinis, 2; D. arizonae, 7; D. funebris, 11; D. hydei, 12; D. littoralis, 21; D. melanogaster, 11; D. mojavensis, 12; D. montana, 13; D. persimilis, 17; D. pseudoobscura, 8; D. recens, 15; D. simulans, 11; D. subobscura, 12. For three species, the final sample size was <10; however, there was no relationship between the within-species sample size and the within-species coefficient of variation in sperm metabolic rate (Spearman's ρ = 0.183, p = 0.55; Supporting information Fig. S3). In addition, excluding D. affinis (n = 2) did not qualitatively change any of the regression or path analysis results, including or excluding D. hydei (Supporting information Tables S3–S6).
Within-species variance in sperm metabolic rate was, however, unequal across species (asymptotic test for the equality of coefficients of variation, p < 1e−13 [Feltz and Miller 1996; Marwick and Krishnamoorthy 2019]). We therefore reanalyzed the regression of female mating frequency on sperm metabolic rate while accounting for both phylogeny and the within-species variance structure (Ives et al. 2007). Because of the relatively small sample sizes for a few of the species as well as the unequal means, a pooled coefficient of variance was used to estimate species-specific variances (Garamszegi 2014). Regression results changed minimally when within-species variance was considered (Supporting information Table S1).
Path analysis was conducted using the R package phylopath (van der Bijl 2018), which is based on the d-separation method (Shipley 2009), controlling for phylogeny as described in von Hardenberg and Gonzales-Voyer (2013). The six path models represent the following scenarios: (1) More frequent female mating is driven by more quickly metabolizing sperm, with metabolism driven by sperm length. (2,3) More frequent female mating is driven by more quickly metabolizing sperm, independent of sperm length, which either (2) drives or (3) is driven by female mating frequency. (4,5) Higher sperm metabolism, regardless of sperm length, is driven by frequent female mating; sperm length either (4) drives sperm metabolism or (5) is causally associated with female mating frequency. (6) Longer sperm metabolize more quickly, but this does not affect female mating frequency independently of the relationship between mating frequency and sperm length.
Note that while models 1−3 are consistent with the sperm aging hypothesis, in which more quickly metabolizing sperm age faster and thus select for frequent female mating, they do not test this hypothesis directly as we have no data on sperm aging. Similarly, while models 4 and 5 are consistent with the hypothesis that higher sperm metabolism confers an advantage during sperm competition and is thus selected for in species with frequent female mating, they do not test this hypothesis directly as we have no data on sperm competitiveness. Model 6 is consistent with the null hypothesis, in which sperm metabolic rate and female mating frequency are linked only indirectly through sperm length.
To account for male body size, the residuals of the phylogenetically controlled regression of log-adjusted sperm length on log-adjusted male thorax length were used. The C-statistic information criterion (CIC, or CICc when adjusted for small sample sizes) was calculated for all models. CICc is analogous to the Akaike information criterion and is based on Fisher's C-statistic, which estimates the goodness of fit of the model (Shipley 2013). CICc weights provide an estimate of the likelihood of each model. Models with ΔCICc ≤2 were averaged to obtain the best-fitting model (Burnham and Anderson 2002).
Results
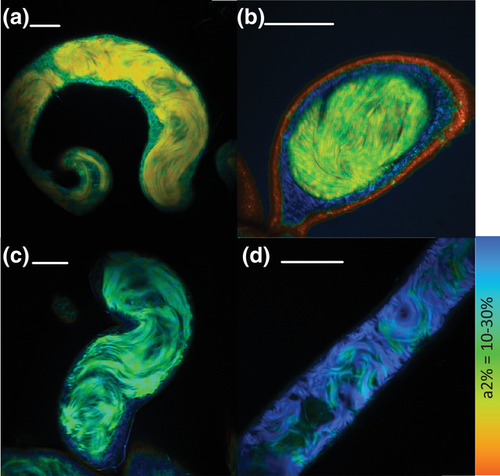
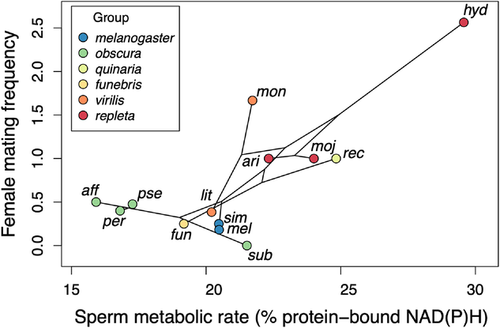
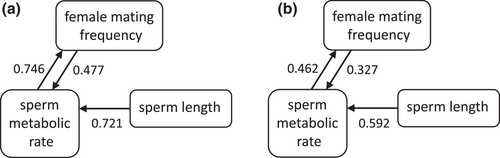
Ex vivo sperm metabolic rates in males varied across species (representative images, Fig. 3a-d; raw data, Supporting information Fig. S1) and significantly predicted female mating frequency, with females in species with higher sperm metabolism mating more frequently (Fig. 4; all PGLS regression results are shown in Table 1). Sperm metabolic rate was significantly predicted by sperm length, both in absolute terms (Supporting information Table S1) and when controlling for male body size by including it as a covariate in the model (Freckleton 2002) (Fig. 5). This result raised the possibility that the association between female mating frequency and sperm metabolic rate could simply be a consequence of sperm number and/or seminal receptacle length, both of which are correlated with sperm length (Pitnick 1996; Miller and Pitnick 2002; Bjork and Pitnick 2006; Lüpold et al. 2016). Reanalyzing the effect of sperm metabolic rate on female mating frequency while controlling for sperm length, however, revealed an even stronger association between quickly metabolizing sperm and rapid female remating. In this model, sperm length had a negative effect on female mating rate, while male body size had a positive effect. When analyzed alone, neither sperm length nor seminal receptacle length, which were strongly correlated, had an effect on female mating rate (see Supporting information Table S2 for seminal receptacle results).
| D. hydei | Models | Estimate | Std. error | t | p | λ |
|---|---|---|---|---|---|---|
| Female mating frequency (fig. 4) | ||||||
| Included | Sperm metabolic rate | 0.746 | 0.201 | 3.710 | 0.003* | 0 |
| Excluded | Sperm metabolic rate | 0.462 | 0.281 | 1.646 | 0.13 | 0 |
| Female mating frequency | ||||||
| Included | Sperm metabolic rate | 1.167 | 0.219 | 5.322 | < 0.001* | 0 |
| Sperm length | −0.890 | 0.312 | −2.948 | 0.016* | ||
| Male thorax length | 0.901 | 0.219 | 4.117 | 0.003* | ||
| Excluded | Sperm metabolic rate | 1.094 | 0.310 | 3.532 | 0.008* | 0 |
| Sperm length | −1.048 | 0.405 | −2.588 | 0.032* | ||
| Male thorax length | 1.151 | 0.338 | 3.405 | 0.009* | ||
| Female mating frequency | ||||||
| Included | Sperm length | 0.382 | 0.356 | 1.074 | 0.31 | 0 |
| Male thorax length | 0.272 | 0.356 | 0.765 | 0.46 | ||
| Excluded | Sperm length | 0.043 | 0.395 | 0.109 | 0.92 | 0 |
| Male thorax length | 0.396 | 0.395 | 1.002 | 0.34 | ||
| Sperm metabolic rate (fig. 5) | ||||||
| Included | Sperm length | 1.090 | 0.266 | 4.103 | 0.002* | 0 |
| Male thorax length | −0.539 | 0.266 | −2.029 | 0.07 | ||
| Excluded | Sperm length | 0.870 | 0.320 | 2.723 | 0.024* | 0.353 |
| Male thorax length | −0.737 | 0.279 | −2.638 | 0.027* |
- Sperm length and thorax lengths were log-transformed. All variables were standardized to have a mean of 0 and a standard deviation of 1.
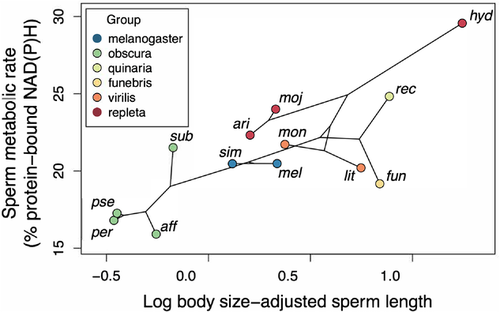
To check whether the relationships we found between sperm metabolic rate, sperm length, and female mating frequency were driven by D. hydei, which had the highest values for all three variables, we reran all regression models while excluding this species. The effect of sperm metabolic rate on female mating frequency, controlling for sperm length and male body size, remained significant, as did the effect of sperm length on sperm metabolic rate, controlling for male body size (Fig. 5). The effect of sperm metabolic rate alone on female mating frequency (Fig. 4), and of absolute sperm length on sperm metabolic rate (Supporting information Table S1), were no longer significant.
To further investigate the positive relationships between sperm metabolic rate, sperm length, and female mating frequency, we used path analysis. Our path analysis compared a total of six models (Supporting information Fig. S2; see Methods for details). Regardless of whether D. hydei was included, two models fit the data equally well, based on CICc scores: model 1, in which sperm metabolic rate drives female mating frequency and is driven by sperm length; and model 4, in which female mating frequency and sperm length both drive sperm metabolic rate (Table 2). These two models were averaged to determine the best-fitting model (Fig. 6).
| D. hydei | Models | Pathways | CICc | ΔCICc | Likelihood | Weights | p |
|---|---|---|---|---|---|---|---|
| Included | 1 | SL → SMR → FMR | 20.89 | 0.00 | 1.000 | 0.614 | 0.313 |
| 4 | SL → SMR ← FMR | 22.15 | 1.25 | 0.534 | 0.328 | 0.167 | |
| 3 | SMR → FMR → SL | 27.14 | 6.25 | 0.044 | 0.027 | 0.014* | |
| 6 | FMR ↔ SL → SMR | 27.53 | 6.64 | 0.036 | 0.022 | 0.011* | |
| 5 | SL ↔ FMR → SMR | 29.69 | 8.80 | 0.012 | 0.008 | 0.004* | |
| 2 | SMR → FMR ← SL | 32.25 | 11.36 | 0.003 | 0.002 | 0.001* | |
| Excluded | 4 | SL → SMR ← FMR | 20.07 | 0.00 | 1.000 | 0.421 | 0.966 |
| 1 | SL → SMR → FMR | 20.38 | 0.31 | 0.856 | 0.360 | 0.827 | |
| 3 | SMR → FMR → SL | 23.02 | 2.95 | 0.299 | 0.096 | 0.221 | |
| 6 | FMR ↔ SL → SMR | 23.13 | 3.06 | 0.217 | 0.091 | 0.210 | |
| 5 | SL ↔ FMR → SMR | 26.10 | 6.03 | 0.049 | 0.021 | 0.047* | |
| 2 | SMR → FMR ← SL | 27.25 | 7.18 | 0.028 | 0.012 | 0.027* |
- Models are ranked according to ΔCICc; bold indicates ΔCICc ≤ 2 (see Methods for details).
- Significant p-values indicate rejection of a model. FMR = female mating rate; SL, residual sperm length; SMR, sperm metabolic rate.
Discussion
Our results provide cross-species evidence that frequent female mating is associated with fast sperm metabolism in males. This finding is consistent with the hypothesis that rapid cellular aging in quickly metabolizing sperm selects for higher female mating rates. As our path analysis results underscore, it is also consistent with the reverse hypothesis, that high female mating rates select for increased sperm competitiveness via faster sperm metabolism. Further work is required to disentangle these two, non-mutually exclusive possibilities.
FREQUENT MATING MAY HELP FEMALES AVOID THE COSTS OF SPERM AGING
Sperm cells are particularly vulnerable to a major component of age-related cellular damage: oxidative stress caused by reactive oxygen species (ROS), which are generated by the mitochondrial electron transport chain during aerobic metabolism. This vulnerability (Aitken 2020; Friesen et al. 2020; Turnell and Reinhardt 2020) is due both to their limited antioxidant reserves and to their reliance on oxidation-prone polyunsaturated fatty acids in their membranes for motility (Am-in et al. 2011; DelBarco-Trillo and Roldan 2014; Khoshvaght et al. 2016). By regularly obtaining fresh sperm, Drosophila females in species with high sperm metabolism may restrict the average chronological age of their sperm stores and therefore the average level of those sperm stores’ oxidative stress.
A different strategy for avoiding the costs of sperm aging is to limit not the actual age, but rather the aging rate, of sperm in male or female storage by slowing their metabolism (Reinhardt 2007; Hettyey et al. 2012). Limiting sperm cells’ rates of aerobic metabolism and hence their production of ROS may be particularly beneficial in species with long-term female sperm storage. For example, honeybee sperm undergo a metabolic shift from oxidative phosphorylation to the less-damaging glycolysis upon entering the female sperm storage organ (Paynter et al. 2017), where they may remain for up to eight years (Baer et al. 2016). Similarly, in crickets (Ribou and Reinhardt 2012) and bedbugs (Reinhardt and Ribou 2013), in which females can store sperm for several weeks, both metabolic rate and the rate of ROS production are reduced after sperm are transferred to the female.
Drosophila melanogaster sperm, in contrast, actually show an increased rate of aerobic metabolism in the female's seminal receptacle (Wetzker and Reinhardt 2019), despite remaining there for up to two weeks (Stewart et al. 2007). This surprising finding suggests either that sperm aging is not as costly for Drosophila as it is for other taxa; or that Drosophila females have arrived at a different solution to the problem of sperm aging, relying more on the frequent uptake of new sperm than on the delayed aging of sperm already in storage. These new sperm may outcompete the older sperm (Gasparini et al. 2017, Gasparini et al. 2018), or females may have some means of actively favoring younger sperm, such as differential storage (Reinhardt and Siva-Jothy 2005; Manier et al. 2013; Ala-Honkola and Manier 2016; Hemmings and Birkhead 2017) or differential effects of female reproductive tract secretions (Gasparini et al. 2020; Pitnick et al. 2020). If Drosophila avoid the costs of sperm aging by modulating their mating frequency rather than the metabolism of their stored sperm, why might they have arrived at this evolutionary solution?
One possibility is that sperm metabolism may be constrained by sperm length, a trait that in Drosophila is under strong sexual selection (Miller and Pitnick 2002; Lüpold et al. 2016). If long sperm require a high metabolic rate to function properly, as is suggested by the association we found across species between these two traits, then any selection for decreased sperm metabolism may be counteracted by selection for increased sperm length. In D. melanogaster, longer sperm are favored by long seminal receptacles, where they are better able than shorter sperm to displace rival sperm and to resist displacement (Miller and Pitnick 2002; Pattarini et al. 2006; Lüpold et al. 2012). Frequent female remating, which promotes the competition of ejaculates in female storage, should increase the overall fertilization bias toward these long sperm by giving them more opportunity to exercise their superior competitive abilities (Fig. 1d). Indeed, female mating rate and seminal receptacle length are genetically linked, highlighting the likely fitness advantages of this combination of traits (Lüpold et al. 2016) (Fig. 1e).
There are at least two, non-mutually exclusive explanations for the postcopulatory sexual selective pressures on Drosophila sperm length. First, female Drosophila may obtain a good genes benefit by favoring long-spermed males: long sperm have been proposed to be the gametic equivalent of a peacock's tail, with only males in good condition being able to produce a sufficient number of them (Miller and Pitnick 2002). In support of this idea, the condition-dependence of the number of sperm produced by a given male increases, across species, with the species-average sperm length (Lüpold et al. 2016). Second, sperm length may evolve by Fisherian runaway sexual selection, given that seminal receptacle length in D. melanogaster is genetically linked not only to female mating frequency but also to sperm length (Lüpold et al. 2016) (Fig. 1f). Regardless of the exact processes driving the strong selective pressures on Drosophila sperm length, if longer sperm require higher metabolic rates, then delaying sperm aging by slowing sperm metabolism in female storage may not be a viable evolutionary strategy.
FREQUENT FEMALE MATING MAY SELECT FOR HIGHER SPERM METABOLISM
The key prediction of the sperm aging hypothesis is that the female mating rate, given the other factors affecting this parameter, is positively affected by sperm metabolic rate. While the regression of female mating frequency on sperm metabolic rate alone was significant only when D. hydei was included in the analysis, the same regression controlling for sperm length and male body size was highly significant whether or not D. hydei was included. Path analysis, both with and without D. hydei, also indicated that female mating rate is driven by sperm metabolic rate, which in turn is driven by body size-adjusted sperm length.
Path analysis also supported a third path from female mating rate to sperm metabolic rate, suggesting that higher sperm metabolism confers an advantage during sperm competition and is thus selected for in species with frequent female mating (Fig. 1j). Evidence for a positive effect of female mating frequency on sperm metabolism has been found in rodents, where species with higher levels of sperm competition have more ATP in their sperm, both absolutely and per unit length (Tourmente et al. 2013, Tourmente et al. 2015, Tourmente et al. 2019). These ATP-rich sperm are able to sustain the faster swimming speeds that confer a fertilization advantage in mammals. However, this advantage is not without a cost: sperm competition is also associated in rodents with increased sperm DNA fragmentation, suggesting that quickly metabolizing sperm not only swim faster but may also accumulate cellular damage more rapidly ([DelBarco-Trillo et al. 2016]; though DNA fragmentation may also result from faster spermatogenesis). It would be interesting to test whether such accelerated sperm aging in promiscuous species exerts positive feedback on female mating frequency in this group.
While faster-metabolizing rodent sperm may be more competitive because of their increased speed, this is almost certainly not the case in Drosophila, where slower sperm are in fact better at displacing rival sperm and at resisting displacement (Lüpold et al. 2012). Rather, any link between sperm metabolism and competitiveness in this taxon is more likely to be mediated by sperm length, which is both correlated with metabolic rate (as shown in this study) and under strong postcopulatory sexual selection (as noted above). Surprisingly, however, our path analysis found that female mating frequency, and therefore sperm competition levels, had a direct impact not on sperm length but rather on sperm metabolic rate, suggesting that sperm metabolism itself is under postcopulatory sexual selection. The reasons for this are unclear, but combined with our recent finding, noted above, that sperm metabolic rate increases in female storage, it raises the intriguing possibility that faster-metabolizing sperm, regardless of length, are somehow able to outcompete rival sperm, perhaps by displacing them more effectively via higher motility (that is, the proportion of sperm that are actively moving, regardless of forward progression or speed [World Health Organization 2010]). As in rodents, this increased competitiveness may come at the cost of increased cellular damage, potentially creating a positive feedback loop in which high female mating frequencies favor higher rates of sperm metabolism, which in turn drive more frequent female mating.
THE ROLE OF SPERM LENGTH IN SPERM METABOLISM AND FEMALE MATING FREQUENCY
The positive effect we found of absolute sperm length on sperm metabolic rate did not hold up when D. hydei was removed (Supporting information Table S1). However, when controlling for male body size, the effect of length on metabolic rate was both twice as great as in the uncontrolled model and robust to the removal of D. hydei. A possible explanation for this discrepancy is that body size in Drosophila is positively correlated with sperm length ([Lüpold et al. 2016], this study3). If the negatively allometric relationship between body mass and cellular metabolic rate (West et al. 2002; Savage et al. 2007) applies to sperm as well as to somatic cells (as has been shown in mammals [Tourmente and Roldan 2015]), then sperm from larger Drosophila species should have lower metabolic rates than their longer dimensions would predict.
We did not find evidence for a positive effect of sperm length, independent of sperm metabolic rate, on female mating frequency (Fig. 1a-c), or vice versa (Fig. 1d-g). In our phylogenetically controlled regression analyses, sperm length, controlling for body size, either had no effect on female mating frequency or, when sperm metabolic rate was included in the model, in fact had a negative effect. Similarly, our path analysis did not favor any models including a direct link between length and mating rate. It is important to note, however, that the d-separation method of path analysis we used is based on testing whether connections absent from a given model are in fact supported by the data (Shipley 2009), and therefore cannot evaluate models where each variable is connected to all others. Thus, our failure to find a relationship between sperm length and female mating frequency is likely due to the strong links each of these two variables has to sperm metabolic rate, rather than to an actual absence of such a relationship.
Adding seminal receptacle length and sperm number as variables to the path analysis would solve this problem, and would also allow for a more nuanced evaluation of the possible mechanisms underlying the relationship between female mating frequency and sperm length (Fig. 1a-g). An analysis of more variables would also require a larger sample size. Unfortunately, while data on sperm and seminal receptacle lengths are available in dozens of Drosophila species (e.g., Pitnick et al. [1999]), corresponding data on mating rates and on the number of sperm stored by females are comparatively limited. While our results are robust to the removal of D. hydei and/or D. affinis, determining these values in more species will be necessary to clarify the relative strengths of the various relationships diagrammed in Figure 1.
SPERM METABOLISM IN MALE VERSUS FEMALE STORAGE
As discussed above, sperm metabolism can change markedly between male and female storage. Testing the hypotheses advanced here, that female mating frequency drives sperm metabolic rate or vice versa, therefore ultimately requires the measurement of sperm metabolism in the female storage organs. However, our study provides an important baseline for future work examining the extent to which sperm metabolic rates in males are altered in females. The few data that exist suggest that cross-sex differences are likely to be smaller than cross-species differences. While species identity explained 43% of the variance in sperm metabolic rate in the present study, storage location (male vs. female) explained 13% (Wetzker and Reinhardt, unpubl. data) and 23% (Wetzker and Reinhardt 2019) of the variation in D. melanogaster; 15% in the cricket Gryllus bimaculatus (Ribou and Reinhardt 2012); and 8% in the bedbug Cimex lectularius (Reinhardt and Ribou 2013).
Furthermore, within D. melanogaster, the metabolic rate of a given male's sperm is correlated between the male and the female storage environments (Spearman's ρ = 0.31, p = 0.04; Wetzker and Reinhardt, unpubl. data). If individual-specific metabolic signatures are retained across storage environments within a given species, then at least some species-specific metabolic signatures are also likely to be retained between male and female storage. Finally, sperm metabolism in the male may itself affect mating rate, as sperm also age in male storage (Gasparini et al. 2017; Cattelan and Gasparini 2021), and males may mate more often in order to avoid transferring such aged sperm (Reinhardt and Siva-Jothy 2005; Hettyey et al. 2012).
Fully testing the hypothesis that sperm aging via cellular metabolism drives female mating frequency also requires an experimental manipulation of sperm metabolic rate, along with a simultaneous measurement of cellular damage markers and of any subsequent declines in sperm function. Regardless of the precise mechanism underlying the association we show between sperm metabolism and mating rate, however, our results establish sperm metabolism as an important trait that should in the future be considered alongside other sperm characteristics.
Within species, sperm aging via cellular metabolism may account for much of the across-male variation in paternity success, the majority of which is unexplained by genetic factors (Reinhardt 2007). Sperm metabolism may also affect other sperm traits influencing fertilization success, or mediate the relationship between several of these traits. For example, sperm metabolic rate may be the key to explaining the relationships found in various taxa between sperm length and speed, which depending on the species may be positive or negative (Humphries et al. 2008; Simpson et al. 2014). Measuring sperm metabolic rate alongside sperm viability and paternity outcomes would also help to clarify the shape of the sperm aging curve over time, a pattern that determines how costly this aging is likely to be in a given species (Reinhardt 2007; Dobler and Reinhardt 2016). Finally, adaptive shifts in sperm length across species (Kahrl et al. 2021) are likely accompanied by corresponding shifts in sperm metabolic rate; how these two variables interact over the course of evolution remains to be determined.
Conclusions
As our results demonstrate, metabolic rate can play as influential an evolutionary role as other sperm traits, and has the potential to resolve many of the current mysteries of sexual selection. This study also demonstrates the power of combining cell biological methods with evolutionary approaches to hypothesis testing. In particular, fluorescence lifetime imaging microscopy, already well established in the biomedical field, has the potential to revolutionize metabolic analyses in ecology and evolution.
AUTHOR CONTRIBUTIONS
BRT and KR conceived and designed the study, BRT collected and analyzed the data and drafted the initial version of the manuscript, and BRT and KR contributed to later versions of the manuscript.
ACKNOWLEDGMENTS
BRT was supported by a National Science Foundation Postdoctoral Research Fellowship in Biology (1612234). KR was supported by the Deutsche Forschungsgemeinschaft through the Excellence Initiative Award to TU Dresden. We thank Cornelia Wetzker for comments on an earlier version of this manuscript.
DATA ARCHIVING
The data and analysis code used in this study are archived at the Dryad Digital Repository (https://doi.org/10.5061/dryad.sj3tx966c).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
LITERATURE CITED
Associate Editor: M. Dean
Handling Editor: T. Chapman
- 1 Log sperm length ∼ Log male thorax length: PGLS: β = 0.393 ± 0.218, t = 1.802, p = 0.100, λ = 1; linear regression: R2 = 0.501, p = 0.007
- 2 Sperm metabolic rate ∼ Log male thorax length: PGLS: β = -0.120 ± 0.316, t = −0.381, p = 0.71, λ = 1; linear regression: R2 = 0.054, p = 0.45
- 3 PGLS: β = 0.393 ± 0.218, t = 1.802, p = 0.099, λ = 1; GLS: β = 0.708 ± 0.213, t = 1.802, p = 0.007, R2 = 0.501; both variables log-adjusted.




