Cold adaptation in the Asian tiger mosquito's native range precedes its invasion success in temperate regions
Abstract
Adaptation to environmental conditions within the native range of exotic species can condition the invasion success of these species outside their range. The striking success of the Asian tiger mosquito, Aedes albopictus, to invade temperate regions has been attributed to the winter survival of diapause eggs in cold environments. In this study, we evaluate genetic polymorphisms (SNPs) and wing morphometric variation among three biogeographical regions of the native range of A. albopictus. Reconstructed demographic histories of populations show an initial expansion in Southeast Asia and suggest that marine regression during late Pleistocene and climate warming after the last glacial period favored expansion of populations in southern and northern regions, respectively. Searching for genomic signatures of selection, we identified significantly differentiated SNPs among which several are located in or within 20 kb distance from candidate genes for cold adaptation. These genes involve cellular and metabolic processes and several of them have been shown to be differentially expressed under diapausing conditions. The three biogeographical regions also differ for wing size and shape, and wing size increases with latitude supporting Bergmann's rule. Adaptive genetic and morphometric variation observed along the climatic gradient of A. albopictus native range suggests that colonization of northern latitudes promoted adaptation to cold environments prior to its worldwide invasion.
Biological invasions are one of the main drivers of environmental change, causing significant biodiversity loss and ecosystem alteration (Simberloff et al. 2013). The risk of biological invasions is expected to increase with the ongoing human-induced global change (Vitousek et al. 1997) and the exponential increase in transportation networks (Hulme 2009). Yet, only a small proportion of introduced species became established, pointing the need to improve our understanding of processes determining invasion success.
The facilitating role of adaptive evolution in invasions is now well recognized (Lee 2002; Prentis et al. 2008; Bock et al. 2015). Three adaptation scenarios have been proposed for explaining that introduced species establish naturalized populations and spread. The genetic shift hypothesis states that invasive phenotypes arise after introduction from rapid evolutionary responses to new selective pressures encountered in the invaded area (Sakai et al. 2001). It has long been viewed that genetic shift is unlikely to contribute to the invasion process due to an expected low adaptive potential of genetically reduced introduced populations, but a growing number of studies report such de novo changes in invasive populations (Lee 2002; Prentis et al. 2008; Whitney and Gabler 2008; Colautti and Barrett 2013; Colautti and Lau 2015). The two alternative hypotheses are two-step scenarios. The bridgehead scenario, whereby a primary site of invasion promotes new introductions (Lombaert et al. 2010), may facilitate further establishment in areas with similar environmental pressures. However, there is currently no empirical support for adaptive changes taking place at bridgeheads (Bertelsmeier and Keller 2018). The last hypothesis is a preadaptation scenario (i.e., prior adaptation sensu; Hufbauer et al. 2012), which denotes the case in which key evolutionary changes for invasion occurred within the native range prior to introduction into a novel range. The similarity of selective regimes in native and introduced ranges may, thus, facilitates establishment success due to low environmental filtering, and species with a broad geographical or climatic native range are more likely to become invasive (Bossdorf et al. 2008; Lee and Gelembiuk 2008; Schlaepfer et al. 2010; Rey et al. 2012; Foucaud et al. 2013).
The Asian tiger mosquito, Aedes albopictus, is currently one of the most invasive species (Global Invasive Species Database, http://www.issg.org/database/). In its native Asian range, A. albopictus occurs across a broad latitudinal range and a contrasted environmental gradient that encompasses tropical regions of Southeast Asia to temperate regions of Japan (Hawley 1988). Reconstructed historical distribution of A. albopictus during late Pleistocene suggests that the species originated in Southeast Asia (Poretta et al. 2012). In this region, marine regression from the Last Interglacial (LIG, 120,000–140,000 years ago) to Last Glacial Maximum (LGM, 18,000–21,000 years ago) resulted in the formation of the Sunda shelf (Voris 2000). The occurrence of climatically suitable areas across the Sunda shelf, the Indochinese Peninsula, and Southeast China, would have favored the expansion of the species approximately 70,000 years ago in this region (Poretta et al. 2012). Due to the extension of ice sheets in northern latitudes, these regions would have been colonized only after the LGM, which may have promoted adaptations to cold environments.
As in many insects, the winter survivorship of A. albopictus in cold environments is mainly determined by the photoperiodic-induced diapause of eggs (Hawley 1988; Hanson and Craig 1994; Urbanski et al. 2010). Studies analyzing the ecology and molecular physiology of diapause in A. albopictus support the occurrence of two adaptive phenotypes within its native range: a tropical phenotype that does not undergo diapause and a temperate phenotype with a plastic diapause response (Hawley 1988). This response is programmed by environmental factors perceived prior to entering diapause that induce metabolic changes (Delinger 1991). Concordantly, transcriptomic studies have shown that a few thousand genes, involved in the perception of external factors and several metabolic pathways, were differentially expressed between diapausing and nondiapausing conditions in A. albopictus (Poelchau et al. 2013a, b; Huang et al. 2015). A recent study based on transposable element insertions also reported genomic signatures of selection between temperate invasive (Europe) and tropical native (Vietnam) populations (Goubert et al. 2017). However, this later study does not indicate whether adaptations arose in invasive populations (genetic shift) and/or in the native population from which invasive ones were introduced (preadaptation). Evidence that most temperate invasive populations originated from near-temperate regions within the native range (Battaglia et al. 2016; Kotsakiozi et al. 2017; Manni et al. 2017; Sherpa et al. 2019) suggests that the source populations were already adapted to winter diapause and ready to become invasive in temperate regions. Furthermore, there is no clear indication for niche expansion that would have required adaptive evolution in temperate invaded areas (Cunze et al. 2018). Demonstrating that adaptation occurred within the native range would shed light into the contribution of preadaptation in the invasion process (Hufbauer et al. 2012). So far, no study focused on identifying genetic polymorphisms related to the cold-stress response within the native range of A. albopictus.
In this study, we aim at better understanding the factors and processes implicated in the evolutionary divergence of A. albopictus native populations. We analyze natural populations from three biogeographical regions (Malaysia, China, and Japan) that contrast in climatic conditions, with nondiapausing populations located in southern latitudes and diapausing populations in northern latitudes. We use published genetic data obtained from high-throughput sequencing, showing that geographically isolated populations are genetically differentiated (Sherpa et al. 2019). We also aim to assess if the factors that shaped genetic variation within the native range of A. albopictus influenced morphological variation. For this purpose, we analyze wing size and shape using lab-raised individuals from the same collected populations. We first refine the previously proposed biogeographic scenario of A. albopictus native populations (Poretta et al. 2012). If changes in habitat distribution due to climatic fluctuations during late Pleistocene impacted the dynamics of populations, the demographic histories of populations are expected to vary according to their biogeographical origin. However, this has not been demonstrated and the hypothesis of northward expansion only stands on species distribution models (Poretta et al. 2012). To test this hypothesis, we reconstruct changes in effective population size over time. Then, we evaluate if cold adaptation has occurred within the native range of A. albopictus. To detect genes potentially involved in the diapause response and/or cold tolerance, we search for genomic signatures of selection using methods based on population differentiation. We then evaluate the role of climatic conditions in shaping adaptive genetic and morphometric variation by testing whether the variation of these traits correlates with climatic variation. In light with the recent literature describing the ecology, physiology, and genetics of A. albopictus in both native and invaded ranges, we discuss how our results support one or several adaptation scenarios.
Materials and Methods
DATA COLLECTION
The genetic and morphometric data were collected from six natural populations sampled in Malaysia, China, and Japan (Fig. 1, Supporting Information Table S1). The genetic dataset comprising 67 individuals was obtained from previously published data available at the European Nucleotide Archive (Supporting Information Table S1; Sherpa et al. 2019). Raw fastq sequences, obtained from double-digest restriction associated DNA sequencing (ddRADseq), were processed using the bioinformatics pipeline described in Sherpa et al. (2019). Briefly, reads were trimmed to equal length of 110 bp using BBmap version 37.33 (Bushnell 2014) and mapped to the A. albopictus reference genome (Chen et al. 2015) using BWA-MEM version 0.7.5 (Li and Durbin 2009). Uniquely aligned reads with MapQ ≥30 were retained, resulting in 432,000 reads per individual on average (Supporting Information Table S2). STACKS version 2.0 (Catchen et al. 2013) was used to merge paired-end reads. We obtained 16,600 loci per individual on average (Supporting Information Table S2) and retained only loci with read depth ≥5 that were present in at least 80% of the individuals. The median coverage among individuals for filtered loci was 26 ± 8X. We included all polymorphic positions, resulting in 36,512 SNPs with 10% missing data on average (Supporting Information Table S2). Missing data were imputed via matrix completion (Chi et al. 2013) from allele frequencies and ancestry coefficients estimated using the sNMF algorithm implemented in the LEA R package version 1.4.0 (Frichot et al. 2014; Frichot and François 2015) and considering three genetic groups (Supporting Information Fig. S1). This SNP dataset was used for demographic inferences and then filtered on minor allele frequency, removing SNPs with allele count <5. The remaining 10,483 SNPs were used for detecting genomic signatures of selection. Individuals used for genetic analyses were either field-collected adults or adults reared from eggs for China and Malaysia, and only field-collected adults for Japan (Supporting Information Tables S1 and S2). As field- and lab-raised adults from the same sampling locality were not genetically different (Supporting Information Table S3), they were analyzed together as a single population.
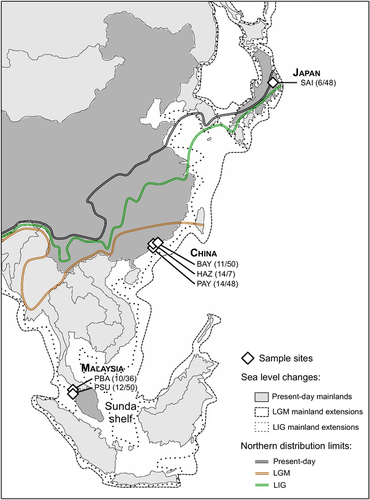
The collection for morphometric analyses comprises 239 adult mosquitoes from the same six populations (Fig. 1, Supporting Information Table S1). This dataset was newly collected for the purpose of this study. The characterization of morphometric variation was performed on lab-raised individuals to report morphometric differences that reflect genetic differences among populations, thus, avoiding variations that could result from natural environmental conditions. We measured the first generation so that the phenotypic characteristics of populations are not altered. Eggs were reared in standard laboratory conditions (27°C, 70% relative humidity, and day length cycles of 14:10h light:dark), with <1 larvae per milliliters.
CHANGES IN EFFECTIVE POPULATION SIZE OVER TIME
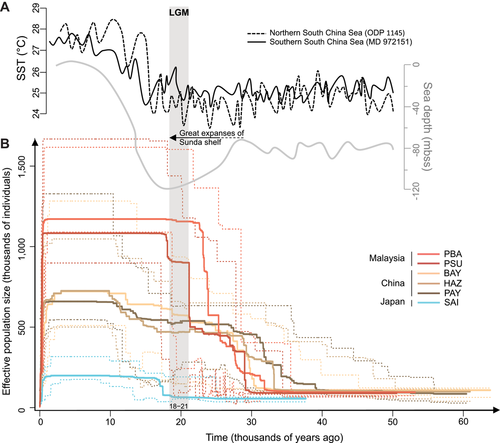
To reconstruct the demographic history of populations, we used the stairway plot method to infer changes in effective population size over time (Liu and Fu 2015). The six populations were analyzed separately. After removing nonpolymorphic loci for each population, the number of SNPs used for computing the Site Frequency Spectrum (SFS) was 14,662 for PBA, 15,605 for PSU, 12,462 for BAY, 14,269 for PAY, 13,464 for HAZ, and 7,395 for SAI. We used a mutation rate per site per generation of 10−8 (Rašić et al. 2014; Bennett et al. 2018) and the generation time was approximated from the reproductive period. The reproductive period was defined as the number of months for which photoperiod was above 11.25 h of light (average monthly day length obtained from the geosphere R package version 1.5.7; Hijmans 2017) and temperature above 10.5°C (average monthly temperatures obtained from the WorldClim database at 30 s resolution; Fick and Hijmans 2017). Based on reproductive period length and considering a generation time of one month (Liu et al. 1985), we used generation times of 0.083 year for Malaysia, 0.111 year for China, and 0.143 year for Japan.
DETECTION OF OUTLIER SNPS
We performed three analyses to detect genomic signatures of selection: two FST-based methods, contrasting populations from the three biogeographical regions, and one model-free method based on principal component (PC) analysis. The first FST-based method is the Bayesian algorithm implemented in BayeScan version 2.1 (Foll and Gaggiotti 2008) that decomposes population FST coefficients (divergence among populations) into locus- and population-specific regression terms. The MCMC algorithm was run for 500,000 iterations following a burn-in period of 100,000 with the proposal distributions for parameters adjusted by 20 short pilot runs of 2000 iterations. We assumed the chains converged because acceptance rates ranged between 0.25 and 0.40. The final sample size was 10,000 iterations (one sampled every 50). The second FST-based method is the likelihood approach implemented in the OutFLANK R package version 0.1 (Whitlock and Lotterhos 2015). This procedure uses a measure of FST uncorrected for sample size but as we imputed missing data, all loci have the same sample size. We removed low heterozygosity variants (<10%). Model fitting after trimming the highest FST values suggests that as much as 40% of loci are affected by spatially heterogeneous selection (Supporting Information Fig. S2A). For the model-free method, we used the pcadapt R package version 4.04.3 (Luu et al. 2018). The genetic variation among populations on the two first PCs accounting for 48% of total variation is shown in Supporting Information Figure S2B. We evaluated the correlation of each SNP with the two first PCs and the P-values were estimated using Mahalanobis distances.
We considered the SNPs detected as outliers following several criteria. We used a Q-value framework to control for false discovery rate (FDR) (Storey and Tibshirani 2003; Storey et al. 2004). We assumed a FDR threshold of Q-value ≤ 0.05 for all tests of neutrality of SNPs (Supporting Information Table S4). BayeScan directly calculates Q-values for each SNP. A Q-value threshold of 5% corresponds to BayeScan posterior probabilities ranging from 0.76 to 1. For the two R packages, Q-values were computed using the qvalue R package 2.4.2 (Storey et al. 2015). To further reduce FDR, we assumed that only SNPs detected by at least two of the three methods were outliers. Outlier SNPs were searched against the VectorBase biomart online tool (https://biomart.vectorbase.org/biomart/martview/) to screen genes in or within 20 kb distance from the SNPs (AaloF1.2 annotations). When genes were not annotated, we either evaluated orthologous genes in the VectorBase database or homologous proteins using the NCBI's BLASTp tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins). We synthetized the molecular and biological functions involved by these genes using Gene Ontology annotations of the Universal Protein Knowledgebase (UniProt, http://www.uniprot.org). Finally, we compared the genes identified to those showing differential expression profiles between diapausing and nondiapausing conditions (Poelchau et al. 2013a, b; Huang et al. 2015).
WING GEOMETRIC MORPHOMETRICS
Wing morphometrics was performed on adults stored in 75% EtOH at −20°C. The left wing was detached from the mosquito thorax and mounted on a glass microscopic slide. All slides were photographed using an Olympus DP70 digital camera (3.1 Megapixels) connected to an Olympus SZX12 stereomicroscope (DF PLAPO 1.2xPF2 objective). Wing geometric morphometrics was performed using 20 landmarks (LMs) located at vein intersections and termini that were digitalized for each specimen (Supporting Information Fig. S3) using tpsUtil version 1.76 (Rohlf 2006) and tpsDig2 version 2.31 (Rohlf 2008). Variation due to scale, orientation, and position was removed by applying a Procrustes superimposition to landmarks coordinates using the IMP CoordGen8 program (Sheets 2008). The resulting Procrustes shape coordinates and log-transformed centroid size were used in morphometric analyses. We applied a PCA on Procrustes coordinates to evaluate wing shape variation and determined the confidence with which a priori sample classification was supported by shape data by performing a Canonical Variate Analysis (CVA) on Procrustes coordinates using the Morpho R package version 2.6 (Schlager 2017). Finally, morphometric differentiation between males and females, countries, and populations were assessed using Type II multivariate (MANOVA, Procrustes coordinates) and univariate (ANOVA, wing size) analyses of variance using the car R package version 3.0-2 (Fox and Weisberg 2011).
CLIMATIC VARIABLES AND ENVIRONMENTAL CORRELATIONS
To identify environmental variables that explain adaptive genetic variation, which correspond to genetic variation restricted to outlier SNPs, we performed redundancy analyses (RDA) (Steane et al. 2014; Capblancq et al. 2018). Climatic data were obtained from the WorldClim database (http://www.worldclim.org, Fick and Hijmans 2017) at 30 arc seconds resolution. We considered the 19 BIOCLIM variables, mean annual water vapor pressure, mean annual wind speed, and annual solar radiation. The elevation was obtained from the raster R package version 2.4–8 (Hijmans 2018). We also used RDA to test the influence of environmental variables on morphometric variation. We analyzed males and females separately and tested the effect of climatic variables on wing shape, wing shape after removing allometric effect, and wing shape and size (log-transformed centroid size) as responses variables. RDA were performed using the vegan R package version 2.4–5 (Oksanen et al. 2017).
Results
DEMOGRAPHIC HISTORY
Aedes albopictus native populations show large proportion of singletons (37–44%) with an excess of rare variants compared to neutral expectations (Supporting Information Fig. S4). The strongest excesses are recorded for Malaysian (10–11%) and Chinese (7–10%) populations whereas the Japanese population shows only slight excess (2%). The reconstruction of past changes in effective population size shows similar demographic patterns for populations within a biogeographical region but different between the biogeographical regions (Fig. 2B). Populations in China started to expand first around 60 kya (BAY: 66 kya; HAZ: 60 kya; PAY: 58 kya), followed by Malaysian populations around 50 kya (PBA: 53 kya; PSU: 50 kya), and the Japanese population started to expand later around 37 kya. Population sizes were constant for several thousand years with equivalent population sizes in China (BAY: 108,000, HAZ: 99,000, PAY: 85,000) and Malaysia (PBA: 95,000, PSU: 89,000), and lower in Japan (SAI: 55,000). Then, populations from Malaysia and China strongly expanded between 30 and 40 kya. Median values obtained from 50 replicate analyses also suggest that populations from China started to expand first but 95% confidence intervals do not differentiate the expansion events in Malaysia and China. Populations from Malaysia show stronger and fast population growth between 18 and 28 kya, reaching up to 1,170,000 individuals. The effective population sizes remain near constant over time for Chinese populations, comprised between 500,000 and 660,000 but another slight expansion event is detected between 10 and 17 kya. For the population sampled in Japan, the only expansion event detected is more recent (around 17 kya) and reached a lower size than those recorded in populations from continental Asia (∼200,000 individuals).
GENOMIC POLYMORPHISMS SHOWING SIGNATURE OF SELECTION
We detected 128 SNPs as being more differentiated than expected by chance among the three biogeographical regions (Fig. 3). The FST-based methods detected 92 and 117 outlier SNPs, respectively for BayeScan and OutFLANK analyses. The PCA-based method identified 637 outlier SNPs, of which 87 and 114 were also detected by the two FST-based methods, respectively. Most outliers show strong allelic change between Malaysia and Japan, whereas China shows both Japan- and Malaysian-like allele frequencies (Fig. 3, Supporting Information Table S5). Among them, 15 are fixed for the reference allele (REF) in China and Malaysia whereas Japan shows a decrease in REF frequencies, suggesting de novo mutation of the alternative allele (ALT) in Japan. We also found 31 SNPs with low ALT frequencies in Malaysia and China while they are high in Japan, suggesting genetic changes from standing genetic variation, with 12 SNPs reaching fixation for ALT in Japan. Likewise, 76 SNPs show the inverse pattern, with low REF frequencies in Malaysia and high in China and Japan, including 49 SNPs fixed for REF in Japan. Overall, half the outliers (57/128) displays a progressive shift in allele frequencies from South to North.
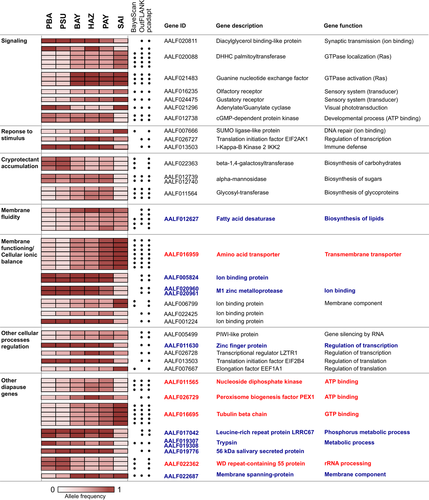
Among the 128 outliers, 89 SNPs are located in or within 20 kb distance from genes (Supporting Information Table S5). Based on VectorBase and UniProt annotations, the main molecular functions encoded by the 61 genes identified in the vicinity of outlier SNPs comprise binding (e.g., ATP, nucleic acids, ions, and lipids, carbohydrates, or proteins) and catalytic activity (e.g., hydrolase, peptidase, transferase, kinase, oxidoreductase, and cyclase). The main biological functions represented are cellular and metabolic processes (Supporting Information Fig. S5, Table S5). Cellular processes include proteins involved in cell cycle, gene silencing, signal transduction and response to stimulus, and metabolic processes include the biosynthesis of organic substances (glutathione, mannose, carbohydrates), aromatic compounds (cAMP, GTP, UTP, CTP), and membrane lipids (sphingolipids), as well as protein catabolism (Supporting Information Fig. S4, Table S5). Among these genes, five and 10 genes respectively were previously found up- and downregulated in diapausing conditions in A. albopictus (Supporting Information Table S5, Fig. S6).
WING MORPHOMETRIC VARIATION
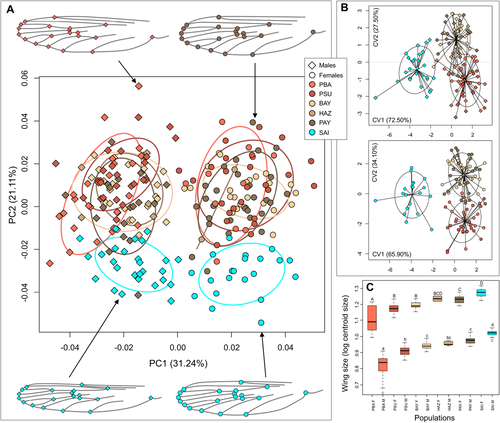
PCA on Procrustes shape coordinates shows differentiation between males and females on the first PC, accounting for 31% of total variation (Fig. 4A). Wing shape deformations reveal that males have flattened wings compared to females. Consequently, we found wing shape between males and females significantly differentiated (MANOVA, F = 52.11, P-value < 2 × 10−16). Wing size was also significantly different between males and females (ANOVA, F = 1045.20, P-value < 2 × 10−16). Females have larger wings than males and the difference between sexes is equivalent for all populations, with an average increase of 0.78 ± 0.04 log centroid size (Fig. 4C). The second PC of PCA, accounting for 21% of wing shape variation, reveals differentiation between Japanese population and those of continental Asia, but not between Chinese and Malaysian populations (Fig. 4A). Nonetheless, both males and females show significant differences in wing shape between countries (MANOVA, males: F = 4.51, P-value < 2 × 10−16; females: F = 5.15, P-value < 2 × 10−16) and populations (MANOVA, males: F = 2.60, P-value < 2 × 10−16; females: F = 2.20, P-value < 2 × 10−16), and the CVA based on countries further discriminates Chinese and Malaysian populations (Fig. 4B). The percentage of correct assignment of males was 90% for Malaysia, 86% for China, and 100% for Japan, and 67% for Malaysia, 78% for China, and 91% for Japan for females. In support with PCA results, the largest part of total variance explained by CVA (CV1) ranges between 66 (females) and 72% (males), and show differentiation between continental Asia and Japan (Fig. 4B). For both males and females, wing size was also significantly different between countries (ANOVA, males: F = 129.91, P-value < 2 × 10−16; females: F = 63.05, P-value < 2 × 10−16) and populations (ANOVA, males: F = 104.59, P-value < 2 × 10−16; females: F = 39.64, P-value < 21 × 10−16) (Fig. 4C). For males, mean wing size was 2.37 ± 0.16 mm in Malaysia, 2.60 ± 0.07 mm in China, and 2.77 ± 0.05 mm in Japan. For females, mean wing size was 3.18 ± 0.18 mm in Malaysia, 3.36 ± 0.10 mm in China, and 3.57 ± 0.09 mm in Japan.
ENVIRONMENTAL CORRELATIONS
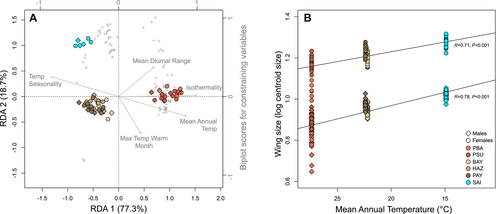
We investigated the extent to which adaptive genetic variation (outlier SNPs) and morphometric variation can be attributed to climatic variation. Only five of the climatic variables were enough to represent the total amount of climatic variations among populations. Thus, only these variables were taken into account in RDA (mean annual temperature, maximum temperature of the warmest month, mean diurnal range, isothermality, and temperature seasonality). RDA on outlier SNPs revealed that 61% of adaptive genetic variation is constrained by climatic conditions (Fig. 5A). The correlation of SNPs with RDA axes allows explaining the variation of allele frequencies among populations (Fig. 3, Supporting Information Table S5). Outlier SNPs showing different allele frequencies between Malaysian and Chinese-Japanese populations are associated with isothermality, mean annual temperature, and temperature seasonality (RDA 1), and those showing different allele frequencies between Malaysian-Chinese and Japanese populations are associated with mean diurnal range and the maximum temperature of the warmest month (RDA 2). Wing size is strongly correlated to mean annual temperatures in both males and females, with larger wings in colder regions (Fig. 5B). RDA on morphometric variables reveals that 81 (males) and 65% (females) of wing variation (including size and shape) is constrained by climatic conditions, but only 20 (males) to 22% (females) when considering wing shape only (Supporting Information Fig. S7).
Discussion
BIOGEOGRAPHICAL HISTORY OF Aedes albopictus NATIVE POPULATIONS
Recent studies on genetic variation among A. albopictus native populations revealed geographic pattern of differentiation across large Southeast Asian Islands (Indonesia, Philippines), the Malaysian Peninsula (Malaysia, Thailand, Singapore), the western (Myanmar), and the eastern (Vietnam) Indochinese Peninsula, China, and Japan (Supporting Information Fig. S8; Battaglia et al. 2016; Kotsakiozi et al. 2017; Maynard et al. 2017; Sherpa et al. 2019). Here, we show that populations genetically differentiated at neutral loci (Fig. S2B) also differ in morphometric traits (Fig. 4, Supporting Information Fig. S7). As wing shape is generally heritable (Henry et al. 2010; Klingenberg 2010) and often reflects the demographic history of populations (Demari-Silva et al. 2014; Lorenz et al. 2017 and references therein), our results suggest that historical factors shaped genetic and morphometric variation similarly. Among historical events affecting species diversity during the Pleistocene, climatic and associated changes in the distribution of habitats have likely contributed to the diversification of forest species (Haffer and Prance 2001), promoting geographical isolation of populations in allopatric refugia during the LGM in several mosquito forest species within Southeast Asia (O'Loughlin et al. 2008; Chen et al. 2011; Morgan et al. 2011). Genetic differentiation of A. albopictus continental populations (Malaysian Peninsula, Vietnam, Myanmar, and China) supports the habitat fragmentation hypothesis during the LGM while the differentiation of island populations may be due to postglacial isolation following marine transgression (Indonesian Islands, Japan). Morphometric variation also supports postglacial isolation of Japanese population, which shows strong differentiation in wing shape as compared to continental populations (Malaysia, China) where gene flow could have occurred (Fig. 4).
Changes in effective population size over time reveal an earlier expansion of Chinese and Malaysian populations, around 60 and 50 kya, respectively (Fig. 2B). Similar to studies showing larger genetic diversities in the Indochinese Peninsula and South China (Poretta et al. 2012; Sherpa et al. 2019), this result supports an initial expansion in Southeast Asia and is congruent with the previous phylogeographic reconstruction showing that A. albopictus started to expand between the LIG and the LGM (∼70 kya) (Poretta et al. 2012). Reconstructed historical distributions suggest that climatic conditions during the LGM in northern latitudes of Asia would have impeded the persistence of A. albopictus in these regions (Poretta et al. 2012). However, we reveal an expansion of the Japanese population around 37 kya, suggesting the species were present in Japan before the end of the LGM. Land bridges that continuously emerged from 60 to 8 kya between continental Asia and Japan (Soya Strait) or more briefly during the LGM (Tsugaru and Tsushima Straits) would have created connections with Japan (Ono 1990; Koizumi et al. 2012). The constant low population size we recorded for the Japanese population until the end of the LGM suggests northern LGM refuges for A. albopictus, as shown for other insect species (Aoki et al. 2011). Malaysian and Chinese populations also show constant population sizes for thousands of years followed by a simultaneous expansion between 30 and 40 kya that could reflect the increase of emerged land in southernmost regions of Asia (Voris 2000; Yokoyama et al. 2007). Large amounts of habitats provided by suitable climatic conditions (Poretta et al. 2012) and the persistence of rainforests on the Sunda shelf (Cannon 2012 and references therein) have promoted expansion of populations. The Sunda shelf extended between large Indonesian islands and the Indochinese Peninsula (Fig. 1; Voris 2000), which may explain the stronger population growth of Malaysian populations. Chinese populations may have also benefited from this increased availability of suitable areas as they are located below the northernmost distribution limit of A. albopictus during the LGM (Fig. 1; Poretta et al. 2012). The post-LGM expansion of the Japanese (17 kya) and Chinese (from 17 to 10 kya) populations reflects the amelioration of climatic conditions after the end of the LGM (Fig. 2; Oppo and Sun 2005; Wang et al. 2014). Our results support a scenario of northward colonization from southern regions of Asia during the last glacial period but a post-LGM expansion rather than a post-LGM colonization of northern latitudes (Poretta et al. 2012). However, a large-scale phylogeographic reconstruction, including populations from Indonesia, the Indochinese Peninsula, and temperate regions of China, is needed to confirm this putative scenario.
ADAPTIVE EVOLUTION WITHIN THE NATIVE RANGE OF AEDES ALBOPICTUS
Genetic and morphometric variation could also result from adaptive processes. We identified a set of significantly differentiated SNPs of which most show a progressive change in allele frequencies from South to North (Fig. 3, Supporting Information Table S5). About 70% of them were located in or within 20 kb distance from genes encoding proteins whose main biological functions are cellular and metabolic processes (Supporting Information Fig. S6, Table S5). Because ddRADseq is a random sampling of whole genome, none of the thousands of SNPs analyzed were located within 20 kb of any of the diapause genes or signaling pathways involved in diapause regulation (e.g., biological clocks, hormones, and signaling cascades) previously described in A. albopictus and other insects (Bradshaw and Holzapfel 2007; Meuti and Denlinger 2013; Sim and Delinger 2013; Huang et al. 2015; Armbruster 2016; Denlinger and Armbruster 2016; Koštál et al. 2017). Nonetheless, we detected signatures of selection in several genes implicated in signal transduction or stimuli response (Fig. 3), including a modulator of visual phototransduction (Sokal et al. 2003), and olfactory and gustatory receptors. This suggests that the perception of external signals differs between temperate and tropical environments, and supports previous studies showing changes affecting the olfactory system at low temperatures in Drosophila species (Dalton 2000; Riveron et al., 2009, 2013; Parker et al. 2018).
Changes in metabolic activities are classically documented in cold adaptation, with the accumulation of molecules acting as cryoprotectants (Holden and Storey 1994; Qin et al. 2005; Denlinger and Lee 2010; Koštál et al. 2011). In support, we identified genes involved in the biosynthesis of carbohydrates, sugars, and glycoproteins (Fig. 3). Other mechanisms include changes in membrane fluidity, pump, ion or membrane permeability (Hochachka and Somero 2002; Koštál et al. 2003; Purac et al. 2011). Congruently, we detected genes implicated in the maintenance of cellular homeostasis. We identified an enzyme of fatty acid metabolism responsible for changes in the composition of membrane lipids and regulation of membrane fluidity (Williams 1998) potentially involved in cold adaptation (Hazel 1995; Koštál et al. 2003; Cooper et al. 2014; Garba et al. 2017), as well as genes involved in the functioning of cell membranes, cellular ionic balance and membrane components, which may prevent metabolic perturbations and cell damage (Fig. 3).
This is the first study reporting genomic signatures of selection within the native range of A. albopictus. To reduce the FDR, we used a Q-value framework and considered SNPs as outliers when detected by at least two methods (de Villemereuil et al. 2014; Nadeau et al. 2016). More than half of these SNPs show a progressive change in allele frequencies from South to North that is correlated to climatic variation (Figs 3 and 5A, Supporting Information Table S5). We suggest that the regulation of cellular processes, such as stimuli responses, cryoprotection, and membrane adjustments, may be implicated in the cold-stress response in A. albopictus. Confirming the functional and adaptive significance of these loci would require rigorous gene expression or selection experiments (Pardo-Diaz et al. 2015) but we were further able to validate 15 of these genes based on previous transcriptomic studies (Supporting Information Fig. S6; Poelchau et al. 2013a, b; Huang et al. 2015). Concerning morphometric traits, the correlation between wing shape and climatic variables was low but we show that wing size increases with mean annual temperatures (Fig. 5B). This result supports the ecogeographical rule of Bergmann with larger individuals found in cold environments, as wing size can be used as an indicator of adult body size (Dujardin 2008). Larger wings in cold environment could be related to longer developmental stages (Armbruster and Hutchinson 2002; DeLatte et al. 2009).
These results suggest that the colonization of northern latitudes promoted adaptation to cold environments. Our sampling design however precludes strong conclusions on environmental effects as we only sampled three regions. Furthermore, about 30 outlier SNPs suggest that de novo mutations arose either in China (one allele absent in Malaysia) or in Japan (one allele absent in Malaysia and China). Although FST-based methods take into account populations’ demographic history (average neutral differentiation), this pattern could be the result of nonadaptive processes, such as genetic drift, as we sampled islands (Japan and Penang, Malaysia). A comprehensive analysis including climatically intermediate populations is, thus, required to conclude on the respective roles of neutral and adaptive processes.
PRE-EXISTING COLD ADAPTATION AND SUCCESSFUL INVASION
Selective pressures encountered by founders during introduction represent strong environmental filters, and insects’ phenotypic adjustments already existing in the source populations are likely key for successful invasions (Renault et al. 2018). Documenting the preadaptation scenario requires both ecological and genetic approaches (Hufbauer et al. 2012). The evidence points needed for supporting this preadaptation scenario, and patterns observed in A. albopictus supporting that cold adaptation within the native range promoted successful invasion in introduced temperate regions are presented in Table 1. Concerning the habitat, the climatic niches should at least partly overlap between native and introduced ranges and population genetic analyses should provide evidence that introduced populations originated from populations in similar habitat within the native range (points 1 to 4). In A. albopictus, temperate invasive populations show niche conservatism relative to Asian native populations and originated from near-temperate regions of the native range (Table 1). From an evolutionary perspective, it should be demonstrated that adaptive evolution has occurred within the native range and that introduced populations show adaptations similar to those already found in native populations (points 5 to 7). Aedes albopictus native populations show two distinct phenotypes with a cold-induced diapause only in the temperate phenotype that confer higher survival in cold environments, and there is evidence that invasive temperate populations possess phenotypic traits similar to northern Asian populations (Table 1).
| Evidence points | Method | Pattern in A. albopictus | References |
|---|---|---|---|
| (1) The species is present in different environments within the native range | The native range enclose tropical and temperate regions | Hawley (1988) | |
| Distribution modeling | Changes in populations’ historical distribution during Pleistocene | Poretta et al. (2012) | |
| (2) Native and introduced ranges climatic niches are similar | Niche comparison | Niche conservatism for invasive populations | Cunze et al. (2018) |
| (3) Native populations are structured at neutral loci | Population genetics | Native populations show geographical structuration of neutral genetic variation | Battaglia et al. (2016); Kotsakiozi et al. (2017); Maynard et al. (2017); Sherpa et al. (2019) |
| Demographic inferences | Evidence that Pleistocene climatic fluctuations shaped populations' demographic history | Present study | |
| Morphometrics | Native populations differ in wing morphometric traits | Present study | |
| (4) Introduced populations originated from populations located in similar habitat within the native range | Population genetics | Temperate invasive populations originated from populations located in near-temperate regions | Battaglia et al. (2016); Kotsakiozi et al. (2017); Sherpa et al. (2019) |
| (5) Adaptive evolution to specific environments has occurred within the native range | Two distinct diapause phenotypes within the native range | Hawley (1988) | |
| Transcriptomics | A few thousand genes are differentially expressed in diapausing conditions | Poelchau et al. (2013a, b); Huang et al. (2015) | |
| Detection of outlier SNPs | Outlier SNPs in candidate genes for cold adaptation | Present study | |
| Environmental correlations | Adaptive genetic and morphometric variation correlate with climatic variation | Present study | |
| (6) Adaptive evolution of native populations leads to differences in fitness | Physiological experiments | Cold-adapted diapause eggs have higher survival chance than nondiapause eggs in cold environments | Sota and Mogi (1992); Lounibos et al. (2011) |
| (7) Introduced populations show similar adaptations to those found in native populations | Physiological experiments | Similar diapause response in temperate invasive and native populations | Hawley et al. (1989) |
| Physiological experiments | No variation in size-based morphological traits in temperate invasive and native populations | Urbanski et al. (2012) |
The present study provides insight into the evolutionary history of native populations (points 3 and 5). Analyzing high resolution nuclear SNPs and wing morphometric traits, we provide evidence that climatic fluctuations during late Pleistocene shaped genetic and morphometric variation, by either promoting neutral genetic differentiation of populations due to geographical isolation (point 3) and local adaptation to environmental conditions (point 5). Regardless of the environmental conditions, adaptation of native populations may more generally confer higher invasive potential (point 4; Hufbuaer et al. 2012). Cold adaptation could confer higher establishment probabilities of temperate phenotypes outside the native range relative to tropical phenotypes. Indeed, diapause eggs are also tolerant to desiccation (Urbanski et al. 2010), conferring a greater ability to be transported for a long time. Since pupal mass and wing length are indicators of fecundity in A. albopictus (Armbruster and Hutchinson 2002), larger cold-adapted individuals could also have a better reproductive potential.
Although invaders originating from cold environments were probably preadapted for invading temperate regions, some studies suggest the two other adaptation scenarios (genetic shift and bridgehead scenario) might still occur. The only other study investigating genomic signatures of selection in A. albopictus compared temperate invasive and tropical native populations (Goubert et al. 2017). None of the potentially adaptive genes detected by the later study match those we detected within the native range, possibly because different genomic regions were targeted in the two studies (transposable elements insertion polymorphisms and ddRADseq SNPs). An alternative hypothesis is that genetic changes detected by transposable elements occurred after introduction (genetic shift). In support of this hypothesis, a rapid evolution of the diapause response has been documented during the American invasive range expansion of A. albopictus (Urbanski et al. 2012; Armbruster 2016; Kreß et al. 2016; Kreß et al. 2017). Because North American invasive populations acted as a source for introduction in other temperate regions (e.g., Europe; Sherpa et al. 2019), the bridgehead adaptation scenario might be a component of A. albopictus invasion success if adaptive evolution occurred in North America. Demonstrating postintroduction adaptations would require reciprocal transplant experiments to demonstrate differences in genetically determined phenotypic traits between invasive populations and their sources, and to show that these traits confer invaders higher fitness relative to their source and promoted niche expansion in the invaded area (Bertelsmeier and Keller 2018). Nevertheless, the three adaptation scenarios are not mutually exclusive. While the preadaptation of invaders to environmental conditions in the novel range may result in the rapid establishment of introduced populations, postintroduction adaptations may favor the expansion of these populations.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
SS, LD, and MB designed the study. SS collected genetic and morphometric data and performed genetic and geometric morphometrics analyses. SS wrote the manuscript and all authors contributed to revising the manuscript.
ACKNOWLEDGMENTS
We thank I.H. Ishak, X. Zhou, X.-G. Chen, and S. Kasai for mosquito collections. We also thank the support of T. Gaude and F. Laporte for help in mosquito rearing. This research was supported by grant from Labex OSUG@2020 (Investissements d'avenir—ANR10 LABX56).
DATA ARCHIVING
The raw sequences analyzed in the present study are available in the European Nucleotide Archive repository (http://www.ebi.ac.uk/ena) and accessible under study accession number PRJEB31109. The full ddRADseq SNP dataset and wing morphometric data, in the form of landmark Procrustes coordinates, are available at Dryad Digital Repository (doi:10.5061/dryad.rb0qk13). Digitalized pictures of wings are freely available upon request from S. Sherpa.
LITERATURE CITED
Associate Editor: M. Matos
Handling Editor: D. W. Hall




