Cooperation-mediated plasticity in dispersal and colonization
Abstract
Kin selection theory predicts that costly cooperative behaviors evolve most readily when directed toward kin. Dispersal plays a controversial role in the evolution of cooperation: dispersal decreases local population relatedness and thus opposes the evolution of cooperation, but limited dispersal increases kin competition and can negate the benefits of cooperation. Theoretical work has suggested that plasticity of dispersal, where individuals can adjust their dispersal decisions according to the social context, might help resolve this paradox and promote the evolution of cooperation. Here, we experimentally tested the hypothesis that conditional dispersal decisions are mediated by a cooperative strategy: we quantified the density-dependent dispersal decisions and subsequent colonization efficiency from single cells or groups of cells among six genetic strains of the unicellular Tetrahymena thermophila that differ in their aggregation level (high, medium, and low), a behavior associated with cooperation strategy. We found that the plastic reaction norms of dispersal rate relative to density differed according to aggregation level: highly aggregative genotypes showed negative density-dependent dispersal, whereas low-aggregation genotypes showed maximum dispersal rates at intermediate density, and medium-aggregation genotypes showed density-independent dispersal with intermediate dispersal rate. Dispersers from highly aggregative genotypes had specialized long-distance dispersal phenotypes, contrary to low-aggregation genotypes; medium-aggregation genotypes showing intermediate dispersal phenotype. Moreover, highly aggregation genotypes showed evidence for beneficial kin-cooperation during dispersal. Our experimental results should help to resolve the evolutionary conflict between cooperation and dispersal: cooperative individuals are expected to avoid kin-competition by dispersing long distances, but maintain the benefits of cooperation by dispersing in small groups.
Cooperation exists in most taxonomic groups (West et al. 2007a) and lies at the heart of major transitions from unicellular live forms to multicellularity and societies (Maynard Smith and Szathmary 1995b; Crespi 2001). Explaining why organisms carry out costly cooperation is thus fundamental to our understanding of the evolution of various biological functions (Maynard Smith and Szathmary 1995a; Crespi 2001). The main hypothesis to explain the emergence and maintenance of cooperation is kin selection, which states that for cooperation to evolve, costly cooperative behaviors should be directed toward kin, thus indirectly increasing gene transmission (Hamilton 1964; West et al. 2007a). Genetic relatedness and population genetic structure consequently play a major role in the evolution of cooperation (Hamilton 1964; Griffin and West 2002, p. 200; West et al. 2002), since the maintenance of kin-based groups allows cooperative behaviors to be directed toward kin, providing a simple mechanism to control invasion by cheaters (Hamilton 1964; West et al. 2007a).
Dispersal, the ability of individuals to move from one place to another during their life, is expected to decrease relatedness in local populations (i.e., average relatedness of neighborhoods; Clobert et al. 2012). Since kin selection theory predicts that stable groups of kin should maintain cooperation, cooperation should favor low dispersal (Hamilton 1964; West et al. 2002). As a corollary, dispersal has long been seen as an opposing force to cooperation (Hamilton 1964; West et al. 2002; Lion and Baalen 2008). However, while group stability among kin provides benefits of kin cooperation, it also increases kin competition, which can negate these benefits (Hamilton and May 1977; Taylor 1992; Queller 1994). This contradiction is even more intriguing given ample empirical evidence of cooperative species performing dispersal movements (Bourke and Franks 1995; O'Riain et al. 1996; Sinervo and Clobert 2003), which has led to several theoretical studies showing that cooperation and dispersal can coevolve (Le Galliard et al. 2005; Hochberg et al. 2008; Parvinen 2013). Substantial theoretical work has demonstrated that various factors could allow cooperation to evolve and be maintained despite kin competition, including overlapping generations (Irwin and Taylor 2000), population structure and environmental context [e.g., range expansion (Datta et al. 2013), empty patches (Alizon and Taylor 2008), patch quality (Rodrigues and Gardner 2012); see also (Lion and Baalen 2008)], budding dispersal (Gardner and West 2006; Kümmerli et al. 2009), and dispersal-dependent cooperation (El Mouden and Gardner 2008).
However, most theoretical work on cooperation and dispersal assumes that dispersal is independent of ecological or social context (Le Galliard et al. 2005; Hochberg et al. 2008; Patterson et al. 2008). By contrast, a large body of empirical work shows that dispersal is a plastic trait that depends on the internal state of organisms and their environmental context (Bowler and Benton 2005a; Clobert et al. 2009), including in cooperative species (O'Riain et al. 1996; Sinervo and Clobert 2003). Individuals are expected to adjust dispersal decisions to stay in habitats that match their phenotype, or to leave poor quality patches to find a better place to live (Bowler and Benton 2005b; Edelaar et al. 2008; Clobert et al. 2012; Jacob et al. 2015a). For cooperative species, habitat quality is dependent upon the balance between kin cooperation and kin competition, and we might thus expect dispersal decisions to vary accordingly. Dispersal decisions that vary according to the balance between the costs and benefits of cooperative opportunities in a given social environmental context (i.e., plastic dispersal behavior) could resolve the cooperation-dispersal paradox and promote the evolution of cooperation (Pepper and Smuts 2002; Aktipis 2004, 2011; Pepper 2007). Although these models have pointed out the potential importance of conditional movements for the evolution of cooperation, experimental evidence for the existence of such cooperation-mediated plasticity of dispersal is still lacking.
Here, we experimentally tested the hypothesis that conditional dispersal decisions are mediated by the cooperative strategy in the ciliated protozoa Tetrahymena thermophila. As for many microorganisms, the survival and growth of T. thermophila depends on a quorum sensing mechanism: extracellular emission of chemical signals drives population growth, especially by preventing mortality and favoring growth at low density (Christensen and Rasmussen 1992; Christensen et al. 1995, 1996, 2003; Rasmussen et al. 1996; Straarup et al. 1997; Witzany and Nowacki 2016). According to the classical definition of cooperation (i.e., a behavior that provides a benefit to another individual and which is selected because of this beneficial effect; West et al. 2007b), cells thus cooperate through the emission of different molecules for example insulin, endorphins, “growth factors” (TPAFs; reviewed in Rasmussen and Wheatley 2007; Csaba 2012). Furthermore, cells can produce growth inhibition factors reducing cell division at high densities (Christensen et al. 1998), as expected for kin cooperation among clones (Hamilton 1964).
In addition to cooperative behavior, Tetrahymena cells can form aggregative groups among kin, a behavior frequently associated with cooperative behavior in other taxa (e.g., Keller and Ross 1998; Queller et al. 2003; Griffin et al. 2004; Sinervo et al. 2006; Cornwallis et al. 2010) and which is likely to facilitate the exchange of growth factors in this species. Importantly, there is evidence for genetic variation in this aggregative behavior and it is associated with specific behavioral and life-history traits that allowed the classification of six Tetrahymena genotypes into a gradient of cooperation levels (Schtickzelle et al. 2009; Chaine et al. 2010; Jacob et al. 2016). First, the expression of this aggregative behavior is modulated by population relatedness: decreasing relatedness in populations lead to decreased levels of aggregation as expected in cooperative species (Chaine et al. 2010). Second, genotypes defined as highly aggregative move toward habitats that contain traces of a kin population, whereas those defined as low aggregative move away from kin, and medium aggregative ones show no preference (i.e., kin recognition and orientation; Chaine et al. 2010). Third, highly aggregative genotypes show reduced growth rate much like cooperative lines in other taxa, but live longer on a finite resource compared to low aggregative genotypes (i.e., growth rate is negatively correlated to carrying capacity in this species (Fjerdingstad et al. 2007) and time to reach 1/2 of the carrying capacity increases with aggregation level (Chaine et al. 2010)), which is a classic resolution of the “tragedy of the commons.” Globally, variation in aggregative behavior is consistently linked to variation in traits or behaviors predicted by cooperation such that aggregation level can be used as a proxy for variation in cooperation. Future work should confirm this link, but our present analysis focuses on variation in aggregation level as a metric for cooperation. Tetrahymena thermophila therefore provides an excellent opportunity to investigate how cooperation shapes plastic dispersal decisions since genetically distinct clonal strains differ in their cooperation strategy (Schtickzelle et al. 2009; Chaine et al. 2010; Jacob et al. 2016).
According to kin selection theory, although an increase of population density generally intensifies competition between individuals, it can also increase the benefits of cooperation in cooperative species (Darch et al. 2012). Compared to low densities where competition is weak but cooperative interactions are limited, higher densities should increase efficiency in the use of extracellular chemicals for cooperative organisms (positive density-dependent benefits of quorum-sensing; Darch et al. 2012). Accordingly, the negative effects of increasing density in T. thermophila should be weaker in highly cooperative genotypes than in low cooperative ones (Chaine et al. 2010). Consequently, we expect that organisms differing in their tendency to engage in cooperative interactions (i.e., cooperation strategy) should react differently to the balance between kin cooperation and competition. Under the conditional movement hypothesis, we therefore expect that the cooperation strategy should interact with the social environment, especially population density, to drive dispersal decisions.
We manipulated density in clonal populations of six genetic strains of T. thermophila that have previously shown consistently contrasted aggregation levels and thus cooperation strategies (two highly, two medium, and two low aggregation strains; Schtickzelle et al. 2009; Chaine et al. 2010), and tested the consequences of the interaction between aggregation strategy and social context (density) on three key aspects of dispersal: emigration rate, the phenotype of dispersers, and colonization success by immigrants. First, we expected the interplay between aggregation strategy and social environment to result in the modification of dispersal rate (emigration) as a result of changes in the balance between the costs and benefits of cooperation. Specifically, low-aggregation genotypes should experience increased competition when density rises, whereas highly aggregative ones should also benefit from kin interactions at high densities. In aggregative genotypes, low competition but limited cooperative interactions at lower densities and increased benefits of cooperation as density rises should lead to a quadratic relationship between density and dispersal rate, with dispersal rate first decreasing and then increasing when density reaches higher levels. Second, dispersers and residents are often found to show different phenotypes, with dispersers being phenotypically specialized for reduced dispersal costs during transience and/or colonization (phenotype-dependent dispersal; Clobert et al. 2009; Le Galliard et al. 2012). While all dispersers should be selected for reduced dispersal costs during transience, dispersers from cooperative species should also be specifically selected to escape kin competition, and thus be specialized for long-distance dispersal (Rousset and Gandon 2002; Schtickzelle et al. 2009; Bitume et al. 2013). We therefore expect aggregative genotypes to show dispersers specialized in long-distance movement (i.e., more elongated cells in the case of T. thermophila; Fjerdingstad et al. 2007; Schtickzelle et al. 2009) more often than for low-aggregative genotypes. Finally, the key immigration step, influencing gene flow and changes in a species distribution, depends on an organism's ability to colonize new environments (Clobert et al. 2012). We thus tested whether aggregation strategy affects colonization efficiency, and compared colonization efficiency from single founder cells with colonization efficiency of several founder cells (akin to group dispersal). Since dispersing in groups is expected to favor the evolution of cooperation (“budding dispersal”; Gardner and West 2006; Kümmerli et al. 2009), we expect aggregative genotypes to perform better in colonizing a new environment when colonization occurred from a group of individuals rather than from a single founder cell.
Methods
We performed all experiments in microcosms using experimental populations of six genetically distinct T. thermophila strains (i.e., isolated genotypes kept as clonal populations) that differ in their aggregation behavior, indicative of differences in cooperation strategy (low = 4A, 7; medium = D3, P; high = E, Q; 5 replicates per experimental design level, see below; Schtickzelle et al. 2009; Chaine et al. 2010). In a first experiment, we measured the density dependence of dispersal rate as well as disperser phenotype using standard connected microcosms: two habitat patches consisting of 1.5 ml microtubes, connected by a corridor made of 4 mm internal diameter, 2.5-cm long silicone tube. Cells were maintained at 22°C and fed with growth media (2% Difco proteose peptone, 0.2% yeast extract) as described in previous studies (Fjerdingstad et al. 2007; Schtickzelle et al. 2009; Chaine et al. 2010; Pennekamp et al. 2014; Jacob et al. 2015b). Cells were placed in the start patch at each of three different densities (100,000; 250,000, and 400,000 cells/ml) that correspond to the range of densities commonly observed in our culture conditions, from the minimum density required to reliably quantify dispersal through to near carrying capacity (Chaine et al. 2010; Pennekamp et al. 2014) and allowed to disperse toward the target patch for 17 hours (about ½ to 1 generation time at 23°C; Chaine et al. 2010; Jacob et al. 2015b). There was no difference in carrying capacity between aggregation strategies on the basis of previously published data (F2,3 = 1.63; P = 0.33; 3 replicates per genotype; data from Fjerdingstad et al. 2007), meaning that the population densities we tested reflect a similar environmental context for the six different genetic lines. Furthermore, cell condition in this experiment should be similar given that all six genetic lines were raised under standard conditions immediately prior to the experiment. We created five replicates of the two-patch systems for each density by genotype combination, for a total of 90 two-patch microcosms.
We measured (1) dispersal rate as the proportion of cells in the target patch compared to the start patch, and (2) phenotype of dispersers as cell size and shape (measured as cell area and cell major/minor axis ratio of a fitted ellipse; Fjerdingstad et al. 2007) using automated digital analysis of pictures taken under dark field microscopy (Pennekamp and Schtickzelle 2013) using ImageJ software (version 1.47, National Institutes of Health, USA, http://imagej.nih.gov/ij). The measure of dispersal rate, used in several previous studies (Fjerdingstad et al. 2007; Schtickzelle et al. 2009; Chaine et al. 2010; Pennekamp et al. 2014; Jacob et al. 2015b), has been found insensitive to density-dependent effects on growth (Pennekamp et al. 2014). We summarized disperser phenotype through the first axis of a principal component analysis on cell size and shape (loading factors: cell size = –0.833; cell shape = 0.833; variance explained = 69.4%). Disperser phenotype increases when cells became small and elongated, and decreases when dispersers became bigger and less elongated. In this species, elongated cells are specialized for long-distance movement and show greater swim speed and straighter movements (Nelsen 1978; Fjerdingstad et al. 2007; Schtickzelle et al. 2009; Pennekamp et al. 2014). The induction of this phenotype is especially common when escaping starvation conditions (Nelsen 1978).
In a second experiment, we tested whether cooperation strategy affects the efficiency of dispersing cells to colonize a new environment. To this end, we transferred disperser cells from the above experiment into new empty patches (single unconnected 1.5 ml standard microtubes), either as a single disperser cell (individuals isolated using a 10 μl pipetteman) or as a group of disperser cells transferred as a fixed volume from the target patches of the dispersal systems [1.4 μl resulting in 175.57 ± 8.39 cells; no significant difference of number of cells transferred between density treatments (F1,83 = 2.92; P = 0.09) and aggregation strategies (F2,3 = 1.62; P = 0.33)]. For each of the 90 two-patch dispersal microcosms, we created 10 single cell replicates and one group dispersal replicate. We defined colonization efficiency of a new patch as population growth after a standard period of time (i.e., four days for the single disperser case, 24 hours for the group dispersal case). For colonization from several cells, colonization efficiency was measured as the final population size (Nfinal) relative to initial (Ninitial), and calculated as follows: (Nfinal – Ninitial)/Ninitial. This measure is not confounded by colonization failure, which did not occur in our experiments.
To investigate how cooperation strategy affects density-dependent dispersal rate, dispersal phenotype, and colonization efficiency from a single cell or several cells, we tested for the interaction between aggregation strategy and density using linear-mixed models (lme, nlme R-package; Pinheiro and Bates 1996). We used dispersal rate, disperser phenotype, and colonization efficiency from a single cell or from several cells as dependent variables in four separate models. The population density treatment was used as a continuous factor (in linear and quadratic form), aggregation strategy as a fixed categorical factor (i.e., three levels of aggregation—low, medium, high—containing two strains each), and genotype as a random categorical factor to account for our nested design (two genotypes per aggregation level). We computed linear models with a Gaussian distribution, and in all cases residuals of the models followed a normal distribution. We estimated the variance explained by the models using r.squaredGLMM (MuMIn R-package; Nakagawa and Schielzeth 2013). Following a backward selection procedure, we removed cooperation, density, and their interaction from the models when nonsignificant at the 0.05 level.
Results
First, we tested whether emigration decisions depend on the interplay between cooperation strategy and social environment. We found that the effect of density on dispersal rate differed depending on the aggregation strategy of genotypes (Table 1; Fig. 1A). In highly aggregative genotypes, the dispersal rate was the highest at low density (Density2: estimate ± SE = 0.10 ± 0.05; F1,26 = 4.33; P = 0.04; Density: –0.47 ± 0.20; F1,26 = 5.48; P = 0.03; Fig. 1A), while the dispersal rate in low-aggregation genotypes was the highest at intermediate densities (Density2: –0.18 ± 0.06; F1,26 = 10.09; P = 0.004; Density: 0.63 ± 0.23; F1,26 = 7.59; P = 0.01; Fig. 1A). Finally, medium aggregation strains showed an intermediate level of dispersal rate not significantly affected by density (Density2: 0.04 ± 0.04; F1,26 = 1.38; P = 0.25; Density: –0.22 ± 0.15; F1,26 = 2.09; P = 0.16).
| R2m = 0.26 | R2c = 0.53 | ||
|---|---|---|---|
| Dispersal rate | Df | F | P |
| Aggregation | 2.3 | 3.41 | 0.17 |
| Density | 1.78 | 4.07 | 0.05 |
| Density^2 | 1.78 | 4.52 | 0.04 |
| Aggregation x density | 2.78 | 0.76 | 0.47 |
| Aggregation x density∧2 | 2.78 | 9.53 | <0.001 |
| R2m = 0.77 | R2c = 0.85 | ||
|---|---|---|---|
| Dispersal phenotype | Df | F | P |
| Aggregation | 2.3 | 10.06 | 0.05 |
| Density | 1.78 | 14.75 | <0.001 |
| Density^2 | 1.78 | 10.85 | 0.002 |
| Aggregation x density | 2.78 | 6.39 | 0.003 |
| Aggregation x density∧2 | 2.78 | 5.70 | 0.005 |
| Colonization from a | R2m = 0.78 | R2c = 0.82 | |
|---|---|---|---|
| single cell | Df | F | P |
| Aggregation | 2.3 | 24.43 | 0.01 |
| Density | 1.81 | 0.48 | 0.49 |
| Aggregation x density | 2.81 | 15.89 | <0.001 |
| Colonization from a | R2m = 0.25 | R2c = 0.55 | |
|---|---|---|---|
| group | Df | F | P |
| Aggregation | 2.3 | 1.98 | 0.28 |
| Density | 1.83 | 2.55 | 0.11 |
- Final models after backward elimination are shown (nonsignificant fixed effects are shown for colonization from a group for clarity). Marginal (R²m, fixed effects alone) and conditional [R²c, combined fixed and random effects (i.e., genotypes)] estimates of model fit are shown.
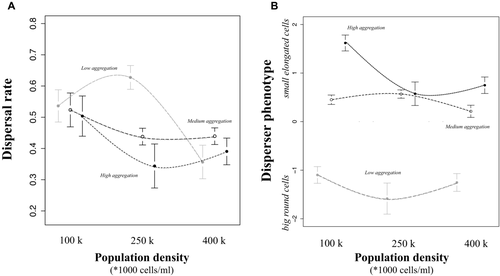
Cooperation governs dispersal decisions in Tetrahymena thermophila. (A) Cooperation strategy mediates reaction norms of dispersal rate to congener density (aggregation × density2 interaction: F2,78 = 9.53; P < 0.001). (B) The phenotype of dispersers differed depending on their cooperation strategy and population density (aggregation × density2 interaction: F2,78 = 5.70; P = 0.005). Highly aggregative genotypes produced specialized disperser phenotypes that were small and elongated, while low-aggregative genotypes produced dispersers that were bigger but less elongated. Each curve, fitted using individual replicates as data points, corresponds to one aggregation strategy and contains two genetic strains with five replicates each [low aggregation strategy (7, 4A); medium aggregation strategy (D3, P); high aggregation strategy (E, Q)]. Mean ± SE for the two genotypes of each density per aggregation combination are shown. Points are staggered on the x-axis for clarity.
Second, we investigated whether disperser phenotype differed according to cooperation strategy, and especially whether aggregative genotypes had dispersers that were morphologically specialized for long-distance dispersal. We found a significant effect of the aggregation × density interaction on disperser phenotype (Table 1; Fig. 1B). First, dispersers were small and elongated in aggregative genotypes and in contrast big and less elongated in low-aggregation genotypes (Fig. 1B), while dispersers from medium-aggregation strains showed an intermediate dispersal phenotype between low- and high-aggregation strains. Importantly, this difference is not simply a result of among genotype phenotypic variation. Dispersers of highly aggregative genotypes were indeed more elongated than residents (disperser minus resident phenotype: mean ± SE = 0.75 ± 0.15), whereas low-aggregation dispersers were bigger and less elongated than residents (–0.57 ± 0.14), and medium-aggregation dispersers were similar to residents (0.01 ± 0.09; aggregation level effect on relative disperser phenotype: F2,3 = 11.57; P = 0.039). Second, apart from medium-aggregation strains in which density has no significant effect on disperser phenotype (Density2: –0.24 ± 0.12; F1,26 = 3.85; P = 0.06; Density: 0.82 ± 0.49; F1,26 = 2.88; P = 0.10), dispersers from low and high-aggregation strains were the biggest and least elongated at medium density (Low: Density2: 0.41 ± 0.17; F1,26 = 5.49; P = 0.03; Density: –1.72 ± 0.71; F1,26 = 5.90; P = 0.02; High: Density2: 0.61 ± 0.24; F1,26 = 6.50; P = 0.02; Density: –2.88 ± 0.49; F1,26 = 2.88; P = 0.10).
Finally, because colonization efficiency after dispersal is a key step if dispersal is to produce gene flow and changes in species distribution, we tested whether cooperation strategy affects colonization efficiency, both from single founder cells and from several cells. In the single founder cell experiments, the effect of density on colonization efficiency depended on the aggregation strategy (Table 1; Fig. 2A). First, dispersers from highly aggregative genotypes showed significantly reduced colonization efficiency from a single cell compared to low and medium aggregation genotypes whatever initial population density (contrasts after Tukey adjustment: high vs. low: –139.85 ± 16.87; t1,27 = 8.29; P = 0.008; high vs. medium: –109.65 ± 16.87; t1,27 = 6.50; P = 0.01; low vs. medium: 30.20 ± 16.87; t1,27 = 1.79; P = 0.31). In contrast to the specialized long distance dispersers of aggregative genotypes, dispersers from low and medium aggregation genotypes were bigger, a phenotype that we may hypothesize to be responsible for this increased colonization efficiency (Fig. 3). Furthermore, predispersal density had a significant positive effect on single cell colonization of low aggregation genotypes (13.84 ± 5.20; F1,27 = 7.08; P = 0.01), a negative effect in medium aggregation genotypes (–41.82 ± 9.79; F1,27 = 18.24; P < 0.001), and no significant effect in highly aggregative genotypes (–4.91 ± 5.17; F1,27 = 0.90; P = 0.35).
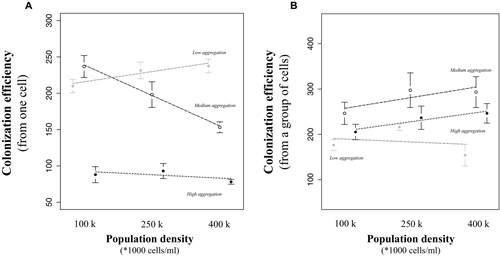

Furthermore, we found that aggregative genotypes were as efficient as low and medium aggregation genotypes in colonization from a group of cells (Table 1; Fig. 2B), and we found no significant effects of density × aggregation interaction on colonization from several cells (P > 0.05).
Discussion
Plasticity in dispersal decisions that balance the costs and benefits of cooperative behaviors among kin might resolve the cooperation-dispersal paradox, hence potentially promoting the evolution of cooperation (conditional movement; Pepper and Smuts 2002; Aktipis 2004, 2011; Pepper 2007). Here, we demonstrated that the density-dependent reaction norms of dispersal rate and the phenotype of dispersers depended on the aggregation level, a behavior linked to cooperation strategy in the ciliated protozoa T. thermophila. Furthermore, we found that the colonization efficiency of single founder cells differed depending on their aggregation strategy and its associated disperser phenotype. Interestingly, when colonization occurred from a group of cells, colonization success was equivalent among all three aggregation levels.
COOPERATION MEDIATES DENSITY-DEPENDENT DISPERSAL REACTION NORMS
We found that T. thermophila genotypes of different aggregation strategies show contrasted reaction norms of density-dependent dispersal decisions (Fig. 1A). The benefits of cooperation between unicellular organisms have been predicted to increase with density, as seen in the bacteria Pseudomonas aeruginosa (Darch et al. 2012). However, when density reaches high levels, competition between kin is expected to negate the benefits of cooperative acts, leading to negative effects of high densities (Hamilton and May 1977; Queller 1992; Taylor 1992). In our study, highly aggregative genotypes showed a quadratic relationship between density and dispersal rate, dispersal rate being the highest at low density, as found in other studies (Kim et al. 2009; Matthysen 2012; Fronhofer et al. 2015). When density increased, dispersal rate decreased as expected if the benefits of cooperation show positive density-dependence (Darch et al. 2012). Highly aggregative genotypes have previously been found to live longer at high density than genotypes with lower levels of aggregation, thereby suggesting positive density dependence of cooperation (Chaine et al. 2010). This suggests that highly cooperative genotypes may try to avoid habitats where density is either not high enough to generate benefits of kin cooperation or so high that negative effects of kin competition negates the benefits of cooperation, and instead will disperse to habitats with medium congener densities. Interestingly, the balance between kin cooperation and competition occurring in this clonally reproducing species led to a U-shape density-dependent dispersal comparable to that found in sexually reproducing colonial species, such as the Blue-footed Booby (Sula nebouxii) where benefits of population density result from opportunities for pair formation (Kim et al. 2009; see also Fronhofer et al. 2015).
Contrary to highly aggregative genotypes, nonaggregative genotypes should not benefit from cooperative behaviors when density increases, but rather will experience higher competition. While dispersal rate in medium-aggregative genotypes was not significantly affected by density, dispersal of low-aggregative genotypes was higher at intermediate density. Then when density reached high levels, these low-aggregative genotypes sharply decreased their dispersal rate. This decrease in dispersal at high density is unexpected since these genotypes show very low levels of cooperative behaviors even at high density and preferentially orient toward nonkin during dispersal (Chaine et al. 2010). One possible explanation for this decrease in dispersal rate could be that crowded habitats might hinder cell movements or lead to poorer cell condition, consequently reducing dispersal. For example, a recent study on a similar species (Tetrahymena pyriformis) found that increasing density in populations while keeping resources and chemical compounds exchanged among cells constant resulted in a decreased distance traveled by cells (Fronhofer et al. 2015). However, except for medium aggregation genotypes from a single cell, predispersal density has no significant effect on colonization efficiency in our study (Fig. 2), making density effects on cell condition unlikely. Alternatively, high population density could be a good predictor of high habitat quality: negative density-dependent dispersal is expected to occur when congener density provides individuals with more reliable information about habitat quality than direct estimation of the quantity of resources available (Clobert et al. 2009; Baguette et al. 2011; Rodrigues and Johnstone 2014). While T. thermophila does use social information in dispersal decisions (Jacob et al. 2015b), further experiments that decouple the effects of crowding on movement ability and cell condition from kin cooperation and competition, for instance by manipulating cooperative molecules in the culture media, are required to fully understand these patterns.
Overall, our results demonstrate the existence of conditional dispersal decisions that depend on the interaction between aggregation strategy and social context. This result is in agreement with theoretical work showing that this process can resolve the cooperation-dispersal paradox and favor the evolution of cooperation (Pepper and Smuts 2002; Pepper 2007; Aktipis 2011).
COOPERATION-DEPENDENT DISPERSER PHENOTYPE
Specialized dispersal behaviors and morphs have been found in cooperative species (Bourke and Franks 1995; O'Riain et al. 1996; Sinervo and Clobert 2003), which could help to resolve the cooperation-dispersal paradox among kin (Schtickzelle et al. 2009). Here, we found that the phenotypes of dispersing cells depend on the aggregation strategy of the genetic strains (Fig. 1B). Dispersers from highly aggregative genotypes were small and elongated, dispersers from low-aggregation genotypes were big and round, and medium-aggregation genotypes showed an intermediate phenotype. In T. thermophila, small and elongated cells show greater swim speed and straighter movements, and are thought to be more efficient dispersers because of reduced resistance during movement (Stein and Bronner 1989; Fjerdingstad et al. 2007; Schtickzelle et al. 2009; Pennekamp et al. 2014). Our results suggest that highly aggregative genotypes develop specialized dispersal morphs and thus would be able to disperse longer distance, as expected for cooperators to escape regionally structured kin-based populations (Rousset and Gandon 2002; Lion and Baalen 2008; Hatchwell 2009; Schtickzelle et al. 2009; Bitume et al. 2013). In contrast, low-aggregation genotypes produced dispersers that were bigger and less elongated, with likely reduced long distance dispersal ability but probably greater energetic reserves and competitive ability for establishment in a new population (Fjerdingstad et al. 2007). Indeed, selection should favor competitive ability during colonization in low cooperative individuals, with no real advantage of longer distance dispersal. Furthermore, we found that in both low and high-aggregative genotypes, dispersers were the biggest and least elongated at medium density (Fig. 1B), showing that disperser phenotype is also a density-dependent plastic trait in this species.
COOPERATION AND COLONIZATION EFFICIENCY
While emigration is the most frequent focus of empirical work, the fitness consequences of dispersal critically depend on the ability of dispersers to settle and reproduce in the colonized habitat (Clobert et al. 2012). Since dispersing in groups of relatives has been suggested to favor the evolution of cooperation (“budding dispersal”; Gardner and West 2006; Kümmerli et al. 2009), we measured colonization efficiency both from single founder cells and from a group of disperser cells. We found that highly aggregative disperser cells showed significantly reduced colonization efficiency compared to low- and medium-aggregation genotypes when colonization occurred from a single cell (Fig. 2A). The larger cell size of dispersers from low-aggregation genotypes relative to highly aggregative ones may explain this difference in single cell colonization success (Fig. 3), by providing advantages during colonization because of higher nutrient reserves or improved competitive abilities (Stein and Bronner 1989; Fjerdingstad et al. 2007). The low colonization efficiency of a single cell in highly aggregative genotypes might also result from a stronger need for cooperation during colonization (Christensen et al. 1996, 2001; Gardner and West 2006; Kümmerli et al. 2009; Schtickzelle et al. 2009).
When colonization occurred from a group of cells, highly aggregative genotypes were indeed as efficient as low-aggregation genotypes (Fig. 2B), as would be expected if group dispersal evolved with cooperation, allowing an escape from kin competition while maintaining the benefits of kin cooperation during dispersal (Gardner and West 2006; Kümmerli et al. 2009; Schtickzelle et al. 2009). Our results suggest that cooperation between kin might be required during transience and/or settlement for cooperative individuals to efficiently colonize a new environment. The maintenance of cooperation in this species could result from an increase in the colonization efficiency of highly cooperative genotypes through group dispersal to levels similar to genotypes with lower cooperation levels.
Kin competition has been found to positively affect colonization success in lizards (Cote et al. 2007). Here, we found that, as in lizards, higher predispersal density increased colonization efficiency from a single founder cell in low-aggregation genotypes, while it negatively affects colonization efficiency in medium-aggregation genotypes. Furthermore, we found no significant effect of predispersal density on colonization efficiency from a group of cells, suggesting that the potential costs and benefits of predispersal density on colonization from one cell did not affect colonization efficiency when several cells arrived simultaneously in a new patch.
Conclusion
The interactions and potential coevolution between cooperation and dispersal have been the focus of considerable theoretical work since dispersal should reduce the value of cooperation among kin, yet alleviate kin competition (Hamilton 1964; West et al. 2002; Lion and Baalen 2008; Aktipis 2011; Parvinen 2013). Our results provide experimental support for the hypothesis that dispersal decisions vary according to an interaction between cooperation strategy and social environment, a process that may help resolve the conflicting pressures of kin-cooperation and kin-competition (Pepper and Smuts 2002; Aktipis 2004, 2011; Pepper 2007). Cooperation strategy governed how population density affected emigration and subsequently influenced the efficiency of dispersers to colonize new environments. Furthermore, our results suggest that cooperation between kin during transience and/or settlement might be required for cooperative genotypes to be able to efficiently colonize new habitats after dispersal. Overall, the results from these highly controlled experiments expand our knowledge about the relationship between cooperation and dispersal, highlighting the role of cooperation in shaping nonrandom movements of organisms across the landscape.
ACKNOWLEDGMENTS
This study was supported by the ANR EVO-INF-ECOL and INDHET to S.J. and J.C., Royal Society of New Zealand ISAT grant to P.W., ANR Netselect to A.S.C., and ARC 10–15/031, F.R.S.-FNRS, and UCL-FSR to N.S., D.L., and S.J. N.S. is Research Associate and D.L. is Postdoctoral Researcher of the F.R.S.-FNRS. This work is part of the Laboratoire d'Excellence (LABEX) entitled TULIP (ANR-10-LABX-41) for S.J., A.S.C., M.H., and J.C., and is contribution BRC373 of the Biodiversity Research Centre to which N.S., D.L., and S.J. are affiliated. A.S.C., P.W., S.J., and J.C. defined the research theme; A.S.C., P.W., and M.H. designed and performed the experiments. S.J., P.W., and N.S. performed picture analyses, and S.J., P.W., and D.L. analyzed the data. S.J. wrote the manuscript, and P.W., A.S.C., D.L., N.S., and J.C. contributed substantially to revisions.
DATA ARCHIVING
The doi for our data is 10.5061/dryad.n0d03.




