Limited association between Wolbachia and Plasmodium falciparum infections in natural populations of the major malaria mosquito Anopheles moucheti
Abstract
Since the discovery of natural malaria vector populations infected by the endosymbiont bacterium Wolbachia, a renewed interest has arisen for using this bacterium as an alternative for malaria control. Among naturally infected mosquitoes, Anopheles moucheti, a major malaria mosquito in Central Africa, exhibits one of the highest prevalences of Wolbachia infection. To better understand whether this maternally inherited bacterium could be used for malaria control, we investigated Wolbachia influence in An. moucheti populations naturally infected by the malaria parasite Plasmodium falciparum. To this end, we collected mosquitoes in a village from Cameroon, Central Africa, where this mosquito is the main malaria vector. We found that the prevalence of Wolbachia bacterium was almost fixed in the studied mosquito population, and was higher than previously recorded. We also quantified Wolbachia in whole mosquitoes and dissected abdomens, confirming that the bacterium is also elsewhere than in the abdomen, but at lower density. Finally, we analyzed the association of Wolbachia presence and density on P. falciparum infection. Wolbachia density was slightly higher in mosquitoes infected with the malaria parasite than in uninfected mosquitoes. However, we observed no correlation between the P. falciparum and Wolbachia densities. In conclusion, our study indicates that naturally occurring Wolbachia infection is not associated to P. falciparum development within An. moucheti mosquitoes.
1 INTRODUCTION
In the last decades, Wolbachia has emerged as a promising tool against vector-borne diseases in addition to the classical control measures, such as the use of insecticides (Bourtzis et al., 2014; Caragata et al., 2021; Ferguson, 2018; Iturbe-Ormaetxe et al., 2011; Ross et al., 2019). This endosymbiont bacterium presents two major characteristics that can be exploited as an alternative vector control strategy. First, Wolbachia bacteria can induce cytoplasmic incompatibility in their hosts to enhance their maternal transmission. Specifically, the host sperm-egg compatibility is altered when infected males mate with uninfected females (Werren et al., 2008). Usually, this reproductive phenotype exhibits high prevalence in their hosts (Caragata et al., 2021; Ross et al., 2019). Therefore, releasing infected males in a non-infected population should drastically reduce the vector density, called population suppression. This approach has been successfully employed to control dengue transmission by the mosquito Aedes aegypti (Crawford et al., 2020; Utarini et al., 2021). Second, this endosymbiont bacterium can mediate and protect their hosts from infection by a pathogen. This protective phenotype has been observed for a large variety of pathogens and hosts (Braquart-Varnier et al., 2015; Cattel et al., 2016; Gupta et al., 2017; Martinez et al., 2014). Nevertheless, the underlying molecular mechanisms are still unknown. Some studies suggest a competition for the host cell resources that limits the parasite development (Paredes et al., 2016). However, other studies hypothesized the pre-activation of the host innate immune system (Iturbe-Ormaetxe et al., 2011; Pan et al., 2018). This implies that Wolbachia infection stimulates the host active defenses, and consequently has a protective effect against pathogens. This phenotype has been observed mainly when Wolbachia is artificially transferred into a new host (Ross et al., 2019). In natural systems, the prevalence of an endosymbiont with a protective phenotype infection can occur at intermediate or low frequencies if the intensity of reproductive manipulation is low (Hilgenboecker et al., 2008; Ross et al., 2019). The two phenotypes are not exclusive, and they have been concomitantly observed upon the release of A. aegypti infected with the Wolbachia strain wMel (Ross et al., 2019, 2022).
In Anopheles mosquitoes, the discovery of Wolbachia infection in natural conditions has been controversial. For decades, it was thought that Anopheles vectors were not infected by this bacterium by multiple factors, including the presence of Asaia as antagonist to Wolbachia (Hughes, Dodson, et al., 2014; Hughes, Rivero, et al., 2014; Rossi et al., 2015). Few years ago, Wolbachia was detected in Anopheles gambiae, the major malaria vector in Africa (Baldini et al., 2014). However, other researchers have failed to detect Wolbachia in this species and raised doubts about this finding (Chrostek & Gerth, 2018; Sawadogo et al., 2022). Wolbachia was already studied in Anopheles by transfer of an exogenous strain into An. gambiae and Anopheles stephensi (Bian et al., 2013; Hughes et al., 2011; Kambris et al., 2010). Despite the promising effect on blocking malaria transmission, only one mosquito line of self-sustainable infection by maternal inheritance has been established in An. stephensi (Bian et al., 2013; Hughes, Dodson, et al., 2014; Hughes, Rivero, et al., 2014), limiting its applicability. After the potential discovery of naturally occurring infected An. gambiae and Anopheles coluzzii populations, some authors investigated Wolbachia role in Plasmodium falciparum infection and revealed a strong negative correlation between Wolbachia presence and P. falciparum infection (Gomes et al., 2017; Shaw et al., 2016). Although most Anopheles species exhibit a low Wolbachia infection rate, between 0% and 20% (Ayala et al., 2019), few species show higher rates, particularly Anopheles moucheti (50%–70% in independent populations), in which vertical transmission was confirmed (Ayala et al., 2019; Walker et al., 2021). Anopheles moucheti sensu stricto (hereafter An. moucheti) is considered one of the major malaria vectors in Africa (Antonio-Nkondjio et al., 2006; Fontenille & Simard, 2004). It belongs to a group of 3 species, among which An. moucheti is the main malaria vector (Antonio-Nkondjio & Simard, 2013). This malaria mosquito is considered to be at the origin of human malaria from the transfer of the non-human malaria parasites from primates to humans (Paupy et al., 2013). Moreover, it is broadly present in the forested areas of Central Africa (Ayala et al., 2009), where it contributes significantly to malaria transmission. Therefore, the high Wolbachia prevalence in this mosquito species offers a compelling opportunity to study how this endosymbiont bacterium modulates Plasmodium infection in its host. Unfortunately, one of the major drawbacks to experimental approaches is the absence of established An. moucheti laboratory colonies, limiting current investigations to mosquitoes sampled from natural populations.
In this study, we investigated the effect of Wolbachia on P. falciparum infection in a natural An. moucheti population in Cameroon, Central Africa. We screened mosquitoes to detect the presence of Wolbachia and P. falciparum using a newly developed qPCR assay. We further quantified the density of both microorganisms in An. moucheti specimens. With this approach, we empirically assessed the Wolbachia effect on P. falciparum infection in this mosquito under natural conditions. Our results contribute to the study of Wolbachia in Anopheles and its potential value to the development of new strategies for malaria control.
2 MATERIALS AND METHODS
2.1 Sample collection, mosquito identification, and DNA extraction
Mosquitoes were collected in Ndangueng, Cameroon, in February 2020 and July 2021. Adult females were sampled using the human landing catch method (HLC), following the recommendations of the National Ethical Committee in Cameroon (CNERSH N° 2020/07/1259/CE/CNERSH/SP). Mosquitoes were morphologically identified on the basis of taxonomical identification keys (Gillies & Coetzee, 1987; Gillies & De Meillon, 1968). Specimens belonging to the An. moucheti group were transported and kept alive for up to 10 days at the Malaria research Service of OCEAC, Yaoundé, Cameroon. For mosquitoes that died within the first 3 days after capture (gonotrophic cycle), abdomens were dissected and kept separately. All dead specimens were individually preserved in tubes containing a desiccant (silica gel, Sigma-Aldrich) and stored at −20°C for molecular studies. DNA was extracted using the CTAB method, as described in Morlais et al. (2004). Briefly, samples were ground in 200 μL of 2% CTAB solution (1 M Tris HCl pH 8.0, 0.5 M EDTA, 1.4 M NaCl, 2% cetyltrimethylammonium bromide) and incubated at 65°C for 5 min. Total DNA was extracted in chloroform, precipitated in isopropanol, washed in 70% ethanol before resuspension in Qiagen TE buffer and storage at −20°C. Then, a sample subset was analyzed to identify members of the An. moucheti group using the species-specific PCR assays developed by Kengne et al. (2007).
2.2 Wolbachia and P. falciparum detection and quantification
The detection and quantification of Wolbachia and P. falciparum in An. moucheti mosquitoes were assessed using quantitative PCR assays (here after qPCR). To this end, a plasmid carrying a specific fragment of gene from both targeted organisms was designed. All primers were designed with Primer3plus (Untergasser et al., 2012), targeting mono-copy housekeeping genes to avoid over-estimations: An. moucheti GPDG gene (F: 5′-AAGTTGTTTCCGGACGTTTG-3′; R: 5′-CGTCGGATAGATTAATGGTG-3′) (Nsango et al., 2023), Wolbachia coxA gene (GenBank accession number: MK755519.1) (F: 5′-GGTGCTATAACTATGCTGCT-3′; R: 5′-TATGTAAACTTCTGGATGACC-3′), and P. falciparum Cox1 gene (GenBank accession number: LR605957.1) (F: 5′-TTACATCAGGAATGTTATTGC-3′; R: 5′-ATATTGGATCTCCTGCAAAT-3′) (Boissiere et al., 2013). To construct the plasmid, the three amplicons (An. moucheti, 111 bp; Wolbachia, 126 bp; P. falciparum, 120 bp) were cloned together (total length of 357 bp) (Appendix S1) in the pEX-A128 vector. The primers, amplicons and plasmid were prepared by Eurofins.
The molecular quantification of P. falciparum and Wolbachia gene copies was carried out by qPCR. 1 μL of total DNA was added to a mixture (final volume of 10 μL) that contained 0.6 μL of each primer (see above) at 10 μM, 0.5 μL of 2X Mix SensiFast Sybr NO-ROX Kit (Bioline), and 2.8 μL of sterile molecular biology grade water (Hyclone Hypure, Cytiva). Each plasmid contains a gene copy for each organism, therefore, calculating the number of plasmid copies, we can estimate the gene copies for both Wolbachia and P. falciparum in each mosquito. To construct the standard curve, the plasmid was diluted seven times from 10−2 to 10−7 (7.45 × 107 to 7.45 × 102 plasmid copies/μL). For each PCR plate, one separate mixture for each of the three targeted genes per sample and per plasmid dilution was prepared. In total, 24 samples were tested in each 96 PCR plate. Cycling conditions included an initial denaturation step at 95°C for 10 min, followed by 40 cycles of denaturation (95°C for 10 s), annealing (57°C for 5 s), and elongation (72°C for 20 s), followed by a melting step (95°C for 2 min, 68°C for 2 min, and then up to 97°C, holding for 15 s once at 97°C) with a continuous fluorescence detection to construct the melting curves, and a final cooling cycle at 40°C for 10 s. All qPCR assays were carried out on a LightCycler 480 (Roche Diagnostics) using the LightCycler 480 software version 1.5.1.
2.3 Statistical analysis and data visualization
Statistical analyses were carried out with R (R Core Team, 2022) and data modeling with the package “tidyverse” (Wickham et al., 2019). To estimate the repeatability of our quantitative measures, the mixed effects model framework implemented by the package “rptR” (Stoffel et al., 2020) was used with the “species” variable (i.e. P. falciparum or Wolbachia) as random-effect predictors. Groups (i.e. infected vs. non-infected mosquitoes) were compared with the Mann–Whitney test and the package “stats”. Correlation between ratios (i.e. P. falciparum and Wolbachia) were computed using the Pearson correlation coefficient (r) in R. Data, including all figures, were visualized using the package “ggplot” (Wickham, 2016) and associated packages, such as “patchwork” (Pedersen, 2020) and “ggpubr” (Kassambara & Kassambara, 2020).
3 RESULTS
3.1 Prevalence of malaria parasites and Wolbachia in An. moucheti
In total, we collected 2042 mosquitoes belonging to the An. moucheti group (Table S1). Although, An. moucheti is considered the predominant species of the group in this region (Ayala et al., 2009), we molecularly analyzed a subset of 179 mosquitoes to determine the species (Kengne et al., 2007) and attest that they were all assigned to the An. moucheti species. Therefore, we considered that An. moucheti was the only species of the group present in our sampling. To analyze their role in malaria transmission, the prevalence of P. falciparum-infected mosquitoes was determined through qPCR assays. We found that 64/2042 females (3%) were infected with P. falciparum, a slightly higher rate than what previously reported (~1.4%) in the same geographic zone (Christophe Antonio-Nkondjio et al., 2002). We then assessed Wolbachia presence in the infected mosquitoes and equal number of non-infected to Plasmodium. In total, we analyzed 130 specimens. We removed specimens showing elevated cycle threshold (ct > 26) to avoid any potential false positive amplification. Among the 113 specimens retained, 107 (95%) were infected by Wolbachia. This rate was higher than what previously observed in Gabon (71%; Ayala et al., 2019) and Cameroon (56.6%; Walker et al., 2021). We used these 113 mosquitoes for the subsequent analysis.
To estimate the reliability of our quantifications using the plasmid, we performed a repeatability analysis (Stoffel et al., 2020). We randomly re-analyzed 21 mosquitoes from the 64 infected mosquitoes both organisms in this study. In our mixed effects model, we used the ratio of Wolbachia and P. falciparum value as explanatory variables, while the sample was used as random variable. The number of parametric bootstraps was 1000 for Gaussian data. The repeatability index (R) values were 0.853 for Wolbachia (p < 0.001) and 0.701 P. falciparum (p < 0.001).
3.2 Effect of Wolbachia on P. falciparum infection
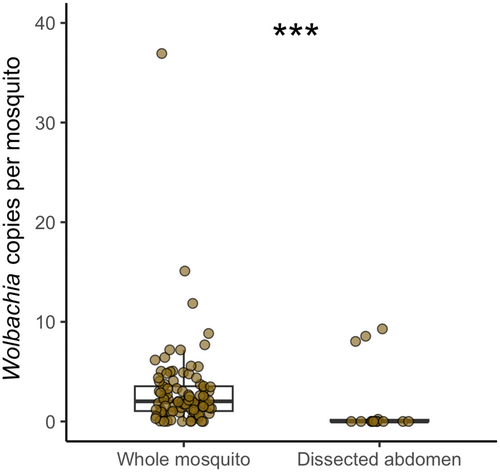
Higher Wolbachia densities are usually observed in the ovaries of its hosts (Werren et al., 2008). To confirm that Wolbachia was present also in other An. moucheti tissues/organs, we compared the ratio of Wolbachia coxA gene copies to mosquito GPDG gene copies in whole mosquitoes (n = 93) and in abdomen alone (n = 14) (Figure 1). The ratio was significantly higher in whole mosquitoes (Mann–Whitney, W = 1387, p < 0.001). Specifically, the median ratios of Wolbachia gene copies to mosquito gene copies were 2.02 (maximum ratio = 36.93) in whole mosquitoes and 0.0006 (maximum ratio = 9.2) in dissected mosquitoes. When looking at P. falciparum infection, we found that only two dissected mosquitoes were infected. Conversely, the median ratio of P. falciparum Cox1 gene copies to mosquito gene copies in whole mosquitoes was 0.17, with a maximum of 8.62, which was much lower than the ratio for Wolbachia gene copies.
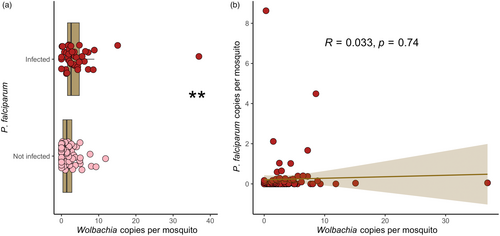
Prior studies suggested a potential role of Wolbachia in protecting An. gambiae from P. falciparum infection in natural populations (Gomes et al., 2017; Shaw et al., 2016). We first carried out a quantitative analysis to compare Wolbachia density in mosquitoes infected (n = 38) and uninfected with P. falciparum (n = 55) (Figure 2a). We found that Wolbachia density was slightly, but significantly higher in infected (mean gene copies = 4.21) than in uninfected (mean gene copies = 2.23) An. moucheti (Mann–Whitney, W = 996, p = 0.004). We then asked whether the Wolbachia-P. falciparum relation was density-dependent. Using the ratio of Wolbachia and P. falciparum copies relative to their host (An. moucheti), we observed a negative, but not significant correlation (Pearson, R = –0.11, p = 0.49, Figure 2b). These results suggest that Wolbachia does not hinder P. falciparum infection or development in this malaria vector, in agreement with its prominent role as major malaria vector in Central Africa (Fontenille & Simard, 2004).
4 DISCUSSION
Wolbachia is a maternally inherited endosymbiont proposed to reduce the malaria burden (Ross et al., 2019). In this study, we evaluated Wolbachia protective effect in An. moucheti, one of the major malaria vectors in Africa (Antonio-Nkondjio et al., 2002; Fontenille & Simard, 2004). First, we estimated the number of mosquitoes infected with Wolbachia and P. falciparum under natural conditions. Second, we quantified Wolbachia and P. falciparum densities in An. moucheti and found that 3% (64/2042) of mosquitoes were infected by P. falciparum and 95% (107/113) by Wolbachia. We found that Wolbachia is not correlated with P. falciparum infection in An. moucheti. Unfortunately, we could not either control Plasmodium development, nor Wolbachia density in the mosquitoes, which could affect the observed phenotype (Gomes et al., 2017). Overall, this study allows better understanding Wolbachia place as a biological tool for malaria control in An. moucheti.
An. moucheti presents one of the highest Wolbachia prevalence rates in Anopheles (Ayala et al., 2019; Jeffries et al., 2018). Wolbachia is common across its geographical range (i.e. Cameroon, Gabon, Democratic Republic of Congo), ranging from more than 50% in Cameroon or Democratic Republic of Congo to almost fixed (95%). Differences in prevalence can be due to geographical heterogeneities, previously observed in other species (Ahmed et al., 2015), or technical issues. For instance, we used coxA instead of 16S for PCR-based detection (Gomes et al., 2017; Jeffries et al., 2018). Nonetheless, our high prevalence is in agreement with results on maternal transmission in Gabon (from 90% to 100%) (Ayala et al., 2019), and with the theoretical prevalence of Wolbachia across its hosts (Zug & Hammerstein, 2012). We then quantified Wolbachia density in its mosquito host. We observed a median density of 2.02 gene copies of Wolbachia in An. moucheti. This number is similar to what observed in Culex pipiens, a mosquito with high infection rate (Berticat et al., 2002). Walker et al. (2021), also quantified Wolbachia density in An. moucheti. Unfortunately, they used a different method (i.e., Wolbachia 16S copies/ng DNA), making impossible the comparison between studies. Another study in An. gambiae proposed to normalize the host genome level using the S7 rRNA gene and two independent RT-qPCR assays (Gomes et al., 2017), with similar results. We observed that Wolbachia densities varied greatly in our sample. The host age and the physiological status can greatly influence its density, as usually observed in other insect species (Duron et al., 2007; Tortosa et al., 2010; Unckless et al., 2009). Our An. moucheti mosquitoes were from a natural population, therefore, without control of age or physiological cycle, and this may explain the variation of Wolbachia densities we observed, which could affect Plasmodium infection (see below). Moreover, as mosquitoes were kept alive for at most 10 days, some of them could have developed eggs, possibly increasing Wolbachia density. All these factors need to be considered to accurate the association between Wolbachia and Plasmodium infection.
In Central Africa, An. moucheti plays a key role in malaria transmission (Christophe Antonio-Nkondjio & Simard, 2013). Therefore, our first question was how this mosquito can be a major malaria vector despite this high Wolbachia prevalence if this endosymbiont exerts a negative impact on P. falciparum development as previously observed in An. gambiae (Gomes et al., 2017; Shaw et al., 2016). The P. falciparum infection rate (3%) in our An. moucheti population was relatively higher compared with other studies (Christophe Antonio-Nkondjio et al., 2002). This could be explained by our experimental protocol: the probability of developing the infection would increase in mosquitoes that live longer (Dawes et al., 2009). Moreover, we quantified P. falciparum density in mosquitoes, and confirmed that in natural conditions, it is very low (Medley et al., 1993). When we studied Wolbachia effect on P. falciparum infection, we first quantitively analyzed P. falciparum and Wolbachia infection (Figure 2a). Our data suggests that Wolbachia density is not associated with malaria parasites development in this mosquito. Rather, our results revealed that Wolbachia density was higher in P. falciparum-infected mosquitoes. Besides hindering Plasmodium infection, Wolbachia might also affect its development. Gomes et al. (2017), showed a significant association between Wolbachia, even at low density, with the malaria parasite in An. gambiae and An. coluzzii. This was particularly true in the artificially infected An. coluzzii colony with the development of sporozoites (Gomes et al., 2017). Unfortunately, we could not differentiate between sporozoite and oocyst infections. In our sampling, only one dissected mosquito (only sporozoites, Table S1) was positive for both Wolbachia and Plasmodium infection. On the other hand, our experimental design allowed the development of the parasite in the mosquito. Therefore, we could consider that an important proportion of mosquitoes could have produced sporozoites (Guissou et al., 2021). Moreover, only two P. falciparum-positive mosquitoes were not infected with Wolbachia. Although this represents 33% of the 6 non-Wolbachia-infected mosquitoes (Table S1), it is much higher than the expected probability (0.15% expected vs. 33% observed) to find a non-infected Wolbachia mosquito (5%) with Plasmodium parasites (3%) (expected probability = 0.05 × 0.03 = 0.0015). Unfortunately, due to the low number of uninfected Wolbachia specimens, we cannot exclude a random effect. Moreover, the Plasmodium density was similar between the non-infected and infected Wolbachia mosquitoes (Figure 2a,b, Table S1). This interesting result need to be controlled with a larger sample size. In our study the Wolbachia-P. falciparum density correlation was not significant (Figure 2b). In An. gambiae, two independent studies revealed a blocking role of Wolbachia in P. falciparum transmission in natural conditions (Gomes et al., 2017; Shaw et al., 2016). Wolbachia protective phenotype is very common when an exogenous Wolbachia strain invades a new host (Ross et al., 2019). It has been observed in An. stephensi and An. gambiae, where transfection of the Wolbachia strain (wAlbB) drastically reduced the ability to transmit the malaria parasites (Bian et al., 2013; Hughes et al., 2011). For instance, this phenotype has boosted the use of the Wolbachia strain wMel, coming from the fruit fly, in A. aegypti (Schmidt et al., 2017; Utarini et al., 2021). On the other hand, in longtime established and high prevalence Wolbachia-host associations, it is more unusual, and when it occurs, the bacterium prevalence is low, as it has been observed in the transmission of avian malaria parasites by Culex (Rivero & Gandon, 2018; Zele et al., 2014). Altogether, our results indicate that in An. moucheti, Wolbachia does not have a protective role against P. falciparum infection. These results are in agreement with its main malaria vector role in Central Africa. Although, future studies should study more carefully the Wolbachia density in salivary glands and the effect on sporozoite development.
Our finding may suggest that natural infections of Wolbachia are not useful for malaria control in An. moucheti, unlike what proposed by previous studies in An. gambiae (Ross & Hoffmann, 2021). Multiple factors could explain these discrepancies. First, while An. gambiae has been rarely found infected by Wolbachia with prevalence rates higher than 20%, An. moucheti exhibits one of the highest prevalence rates among the studied Anopheles species (Ayala et al., 2019; Jeffries et al., 2018). Therefore, we could hypothesize that An. moucheti immune system has been adapted to Wolbachia presence for long time as occur. Actually, the bacterium may increase the transmission in a long-term association as it has been observed in the avian malaria system, where Wolbachia-infected Culex pipiens mosquitoes transmit better than non-infected (Hughes, Rivero, et al., 2014; Zele et al., 2014). Moreover, high Wolbachia prevalence is rather associated with cytoplasmic incompatibility than with protection against vector-borne pathogens (Werren et al., 2008). Unfortunately, crossing experiments cannot be performed in An. moucheti because of the absence of laboratory colonies. The results of experiments using other Anopheles species with established laboratory colonies showed that colonies were infected with slightly different Wolbachia strains (Ayala et al., 2019; Jeffries et al., 2018; Walker et al., 2021), which could indicate an unidirectional CI (i.e., Wolbachia-infected male mated with Wolbachia uninfected female), as evidenced in other systems (Werren et al., 2008). On the other hand, in hosts with low Wolbachia prevalence, this endosymbiont can be associated more with a protective phenotype (Oliver et al., 2010). This is the case in An. gambiae (Gomes et al., 2017). Despite the protective phenotype of the transinfected or naturally occurring Wolbachia strains against Plasmodium infection, currently, no program has been implemented in which Wolbachia-infected Anopheles are used to reduce the malaria burden. The main challenge is to find a Wolbachia strain that can invade and exhibit a protective phenotype against malaria infection. The highly prevalent and Anopheles-adapted wAnmo Wolbachia strain could represent an opportunity to carry out transfers to Anopheles gambiae cells, for instance. More studies controlling physiological parameters and parasite development on An. moucheti and Wolbachia are needed to determine the potential value of Wolbachia as a biological tool for malaria control.
5 CONCLUSIONS
The use of Wolbachia as vector control strategy is a reality. In the last decade, hundreds of thousands of Wolbachia-infected A. aegypti specimens have been released in many different countries. As result, several dengue epidemics have been reduced and the wild vector population replaced by Wolbachia-infected mosquito line (Utarini et al., 2021). In Anopheles, many studies have been conducted to determine Wolbachia place as a vector control strategy. Here, we characterized Wolbachia role in malaria transmission by An. moucheti, one of the major malaria vectors in Africa. Although, Wolbachia presence and density were not associated with protection against P. falciparum in An. moucheti, in agreement with its role in malaria transmission, this strategy could be proposed for other malaria vectors, such as An. gambiae.
ACKNOWLEDGEMENTS
We would like to thanks the volunteers and authorities from Ndangueng, Cameroon, for their assistance and compliance during the field investigations. We also thank the IRD Representation in Yaoundé for their logistic assistance during this study. This work was funded by ANR, France (ANR-18-CE35-0002-01–WILDING), grant awarded to DA. This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Grant Number U19AI110818 to the Broad Institute (DEN).
CONFLICT OF INTEREST STATEMENT
None declared.
Open Research
DATA AVAILABILITY STATEMENT
Additional supporting information may be found online in the Supporting Information section at the end of the article.




