The role of host environment in cancer evolution
Correction added on 6 September 2020, after first online publication: Author name ''Lapene'' has been corrected to ''Laplane'' in this version.
Abstract
Somatic mutations in oncogene and tumor suppressor genes accumulate in healthy tissues throughout life and delineate clones with limited expansion. Lifestyle-related toxic insults increase the size and number of these clones that participate to tissue aging. Their identification has blurred the boundaries between clonal expansion and malignant tumor and has drawn more attention to the role of the host environment in tumor emergence and progression. Local tissue factors such as disrupted cell interactions and stromal cell senescence combine with systemic and distant alterations to initiate the reiterative process of clonal expansion, multilayer intrinsic diversification and clonal selection that eventually characterize overt tumor evolution. In turn, tumors remodel their close and distant environments, establishing positive feedback loops that contribute to disease progression. Strategies emerge to preserve the tumor suppressive properties of healthy tissue landscapes and delay age-induced changes that eventually lead to cancer.
1 WHY ARE CANCERS NOT JUST GENETIC MUTATIONS?
Mutagenic forces introducing 1–10 somatic mutations per cell division throughout life lead to the gradual accumulation of point mutations in otherwise healthy tissues. Most of these mutations are selectively neutral passenger events, including single nucleotide substitutions that result in no change in the amino-acid composition of the encoded protein (synonymous mutations) and a majority of nonsynonymous mutations that have no impact on cell behavior. Some rare events are potentially advantageous driver mutations in oncogenes and tumor suppressors. Contrary to germ cells that efficiently eliminate deleterious mutations, somatic cells could use a variety of subterfuges to tolerate potentially toxic genetic alterations with a near complete absence of negative selection (Martincorena et al., 2017; Williams, Werner, Barnes, Graham, & Sottoriva, 2016).
Cancer is commonly seen as a consequence of this process of somatic evolution in which driver mutations accumulate in a cell, typically in a stem cell or a progenitor. Then, the forces that shape the malignant genome and promote tumor growth, progression, and relapse are viewed as the results of an evolutionary process mainly fueled by genetic (Gerstung et al., 2020) and epigenetic diversification (Gaiti et al., 2019). These forces include endogenous reorganization of the genome (Alexandrov et al., 2020; Cortés-Ciriano et al., 2020; Rodriguez-Martin et al., 2020) and the epigenome (Pastore et al., 2019) as well as integration of external pathogen genomes (Zapatka et al., 2020). This evolution precludes cancer cure with cytotoxic agents and targeted drugs as resistant clones expand under the additional selection pressure exerted by these treatments, needing sophisticated measurements and algorithms to optimize the use of these agents (Bolan et al., 2020; Siravegna et al., 2015). While the diverse genetic and epigenetic alterations and their evolution along the progression of established tumors are well studied and increasingly understood, the boundary between normal evolution and malignant progression remains blurred.
Alterations are not restricted to cancer cells. Analyses of adjacent tissues that appear histologically normal have shown genetic, epigenetic, and transcriptomic alterations, sometimes referred to “field cancerization” (Slaughter, Southwick, & Smejkal, 1953). Whether these alterations predate or follow the emergence of cancer is still a matter of debate (Abdalla et al., 2017; Aran et al., 2017; Teschendorff et al., 2016). More strikingly, an increasing number of studies show the presence of mutated clones in tissues of healthy individuals. Initial detection of clonal derivation of cells in healthy tissues was based on analysis of random X-chromosome inactivation in females. At the beginning of the 60s, each X-chromosome in excess of one was shown to be randomly inactivated in cells during the early development of female embryo (Lyon, 1962). All the female tissues are a mosaic pattern of two cell populations, one expressing maternal and the other paternal X-linked genes with a theoretical ratio of 1:1. This process was used to demonstrate the clonality of established tumors; that is, all the tumor cells express the same X-linked alleles (Linder & Gartler, 1965). Subsequently, a skewing of random X-chromosome inactivation was detected in healthy tissues and was shown (a) to increase with age and (b) to differ among tissues, with blood cells being more skewed than any other tissue. Several mechanisms could explain random X-chromosome inactivation skewing and its prevalence in hematopoietic cells (Ayachi, Buscarlet, & Busque, 2020). For example, such a skewing could be due to a selective growth advantage conferred by an X-gene allele, inducing a polyclonal expansion of cells with a skewed ratio of inactivated X-chromosomes. Alternatively, a stem cell could have acquired a mutation in a gene conferring a growth advantage, leading to clonal expansion. Such a situation was identified in 2012 when an acquired clonal mutation in TET2, a tumor suppressor gene commonly mutated in myeloid malignancies, was shown to be compatible with normal hematopoiesis (Busque et al., 2012).
In the following years, clonally restricted hematopoiesis was identified as a common aging-associated biological state (designated either as ARCH for age-related clonal hematopoiesis or CHIP for clonal hematopoiesis of indeterminate significance) that not only predisposes to subsequent development of a hematological malignancy but also to cardiovascular death (Genovese et al., 2014; Jaiswal et al., 2014; Lee-Six et al., 2018; Xie et al., 2014). Somatic mutations leading to limited clonal expansion were subsequently detected in a variety of other healthy tissues, including skin (Martincorena et al., 2015), esophagus (Martincorena et al., 2018; Yokoyama et al., 2019), liver (Brunner et al., 2019; Zhu et al., 2019), colon (Kakiuchi et al., 2020; Lee-Six et al., 2019; Nanki et al., 2020), lung (Yoshida et al., 2020), endometrium (Moore et al., 2020), and many others (Yizhak et al., 2019).
Widespread positive selection of these mutant clones may contribute to tissue aging by negatively affecting tissue function. Toxic exposures further increase mutational burden, cell-to-cell heterogeneity and driver mutations, as observed in the bronchial epithelium of tobacco smokers (Yoshida et al., 2020) and in hepatocytes of cirrhotic patients (Brunner et al., 2019). Toxic insults expand the size of pre-existing clones while generating new mutational signatures. In the normal liver, stem cells have a low mutational burden and limited diversity of signatures. When progressing from health to disease, including hepatic inflammation, cirrhosis, liver failure and finally hepatocellular carcinoma, mutational signatures chronicle the exposures, toxicity, regeneration and clonal structure of the tissue (Brunner et al., 2019).
While this accumulative process looks simple, explaining why one of the clones that emerge in a given tissue becomes an overt malignant tumor while the others do not remains challenging. For example, positive selection of a driver gene mutation can be decoupled from the risk of malignant transformation. In the esophagus, a strong positive selection of clones carrying mutations in cancer genes was identified. With aging, these clones cover much of the epithelium, with inactivating NOTCH1 mutations affecting up to 80% of cells (Martincorena et al., 2018). NOTCH1 mutations are much less prevalent in esophageal carcinomas. This could indicate that the presence of epithelial progenitors with inactivated NOTCH1 could be less likely to accumulate additional cancer-promoting mutations such as TP53 mutation (Laconi, Marongiu, & DeGregori, 2020). In accordance with this hypothesis, the ability of clonal expansion to exert a protective effect toward malignancies was observed in a cirrhotic liver (Zhu et al., 2019) and NFKBIZ-mutant clones, which are highly prevalent in inflamed intestine of patients with ulcerative colitis, are selected against during colorectal carcinogenesis (Kakiuchi et al., 2020).
These rapidly expanding observations indicate how little we know about somatic evolution that is likely to take place in every normal tissue. Genetic alterations lead to small, nonmalignant clones. Their persistence indicates that they do not activate negative selection processes but also that some mechanisms might limit their expansion. Their accumulation in an aging, yet nonmalignant tissue, may promote cancer development through diverse trajectories; that is, either one of the clone toggles to a malignant tumor or a new clone with cancer properties independently emerges. Both events could take advantage of tissue malfunction induced by the accumulation of nonmalignant clones or by external factors. Whatever the mechanism, detection of a clonal mutation in a driver oncogene or a tumor suppressor is no more sufficient to define a malignant clone and these observations create new challenges to detect or prevent cancer emergence.
When a cancer has evolved, the lineage history is traditionally charted by single-cell integration of genetic, epigenetic and transcriptional information (Gaiti et al., 2019; Rodriguez-Meira et al., 2019). The dynamic regulation that contributes to cancer initiation and progression includes pre-mRNA splicing, RNA binding protein activity and the so-called epitranscriptome (Jiang, Crews, Holm, & Jamieson, 2017). However, before going through the reiterative process of clonal expansion, multilayer intrinsic diversification and clonal selection that characterizes its evolution, a malignant tumor must emerge, meaning that clonal cells must compete with the normal microenvironment and overcome antitumorigenic pressures. Tissue homeostasis and architecture may inhibit cancer emergence and development, and changes in the microenvironment are required to shift the balance of these signals to a procancerous state. Many components located near a clone or abroad then combine with intrinsic, cell-autonomous evolution to move from clonal expansion to malignancy. The following part of this paper focuses on how tissue and host environments affect cancer emergence and evolution.
2 WHY DON'T WE GET MORE CANCER?
Most of the individuals that cancer kills are postreproductive; that is, more than 90% of cancers are diagnosed in humans older than 50 years (Laconi et al., 2020). The low rate of cancer diagnosis during reproductive age in multicellular organisms suggests a long evolutionary history of adaptation and natural selection against life-disrupting cancer growths (Cairns, 1975; Frank, 2003, 2007; Nowak, Michor, & Iwasa, 2003). Most of the typically rare pediatric cancers do not affect the epithelia, as adult cancers, but develop in the lymphoid tissue and the central nervous system, which have both undergone more recent evolutionary change (Leroi, Koufopanou, & Burt, 2003). The capacity of the other tissues in the human body to restrain the aberrant growth of precancerous cells is an exciting feat of evolutionary biology.
A major transition in evolution to multicellular organisms relies on the repression of cell over-proliferation (Maynard Smith & Szathmary, 1995). Normal cells and tissues have developed multiple adaptive mechanisms that prevent or delay tumorigenesis, including cell-autonomous mechanisms such as DNA repair, cell cycle control and cell death programs, and non-cell-autonomous processes that include immune surveillance and tissue structure maintenance (Bhat & Bissell, 2014; Bissell & Radisky, 2001; Ewald & Swain Ewald, 2013). To develop, a cancer must overcome these diverse protective mechanisms, which generates a breakdown in multicellular cooperation (Aktipis & Nesse, 2013; Buss, 1987; Godfrey-Smith, 2009; Nunney, 2013; Pradeu, 2019).
Not only do we accumulate clones of mutated cells throughout life (Tomasetti, 2019), but some malignant tumors at early stages may emerge, yet remain under control of the neighboring cells for a long time (Bissell & Hines, 2011). Almost one century ago (Rich, 1935), routine histological examination of prostate tissues from autopsied young men who had died from unrelated causes already identified in situ intraepithelial neoplasms with a surprisingly high frequency, for example, in 9% of men in their twenties (Sakr, Haas, Cassin, Pontes, & Crissman, 1993). This observation was extended to many other tissues, suggesting that overt tumors may appear several decades before clinically detected carcinomas and arguing for normal contextual cues refraining malignant tumor emergence and expansion (Bissell & Hines, 2011; Greaves, 2014). Pushing the concept to its climax, the clinically defined entity “cancer of unknown primary site” describes a disease revealed by a metastasis, while the anatomical site of the primary tumor remains occult after detailed investigations, indicating that some tissue control on primary tumor development had persisted partially, even though metastatic migration has become effective (Varadhachary & Raber, 2014).
Initial studies on carcinogenesis demonstrated that, to generate a cancer, mutagenic chemicals such as benzo(a)pyrene derivatives required to be combined with another agent acting as a tumor promoter by overcoming the protective role of the microenvironment (Slaga, 1983). Wounding was identified as a highly effective tumor promoter (Sieweke & Bissell, 1994). Seminal experiments performed by Mina Bissell's group provided strong support to these concepts. In a virus-induced sarcoma model, virus injection to hatch birds was shown to cause a rapidly growing tumor at the site of injection (Dolberg & Bissell, 1984). The same virus replicated but was not tumorigenic in bird embryos, while the cells derived from infected tissues expressed a transformed phenotype in culture. While the virus was present in the blood of tumor bearing animals, no other tumors were found distant from the site of inoculation during its life span, except if a wound was made away from the primary tumor (Martins-Green, Boudreau, & Bissell, 1994). Together, these experiments established that virus infection could initiate a tumor whose development was dependent on the tissue environment. In the following years, using a variety of sophisticated models and techniques, it was shown that the microenvironment could revert a malignant (mammary cancer cells, melanoma cells) into a normal phenotype whose genotype remained unchanged (Bussard, Boulanger, Booth, Bruno, & Smith, 2010; Hendrix et al., 2007; Weaver et al., 1997), building on the early observation that teratocarcinoma cells transplanted in normal mouse embryo blastocysts participate to the healthy development of these animals (Illmensee & Mintz, 1976; Mintz & Illmensee, 1975). Normalization of altered cells in healthy environment could result from cell–cell contact with normal cells (Rubin, 2006, 2008), or from soluble proteins released by surrounding cells (Rubin, 2003). Thus, most probably, the tissue environment has a stabilizing influence on clones and occult tumors that accumulate with age.
Some of the cancer cells that disseminate from an established primary tumor reside in patient organs for years before their outgrowth, entering a long-lasting dormant state with occasional cell divisions (Giancotti, 2013). Mechanistically, these cancer cells enter a reversible and programmed growth arrest known as quiescence for periods that, in estrogen-positive breast cancer and prostate cancer, can be longer than a decade, overriding the driver genetics that may fuel their growth. These cells have also to evade the immune system, which involves a variety of sophisticated mechanisms (Pommier et al., 2018; Saudemont & Quesnel, 2004). Inflammation can reactivate dormant cancer cells, for example, activated neutrophils release nucleases that alter the host tissue matrix, releasing basement membrane components that activate kinases in dormant cancer cells and induce their proliferation (Albrengues, Meneguzzi, & Gaggioli, 2014), leading to clinically detectable metastases.
It remains unclear to which extend a normal cell progeny spreads in a given tissue and whether the clones identified by the presence of a somatic mutation extend beyond the size of a normal cell progeny. Some of the clones detected in the peripheral blood are defined by the presence of a passenger mutation that may not confer any fitness advantage to its progeny (Genovese et al., 2014; Zink et al., 2017). Although still controversial (Watson et al., 2020), these observations suggest that the formation of a mutated clone is compatible with neutral evolution. When barriers to oncogenesis, that is, mechanisms that prevent essential steps in cell transformation such as the occurrence of an oncogenic mutation, are crossed, then restraints to oncogenesis; that is, mechanisms that inhibit the exacerbating effect of various events, may operate (Ewald & Swain Ewald, 2013). Examples of such restraints include the capacity of adaptive immunity to hold occult cancers in equilibrium (Koebel et al., 2007) and resource or space limitations (Aktipis, Boddy, Gatenby, Brown, & Maley, 2013).
3 WHY DO CANCERS EVENTUALLY EMERGE?
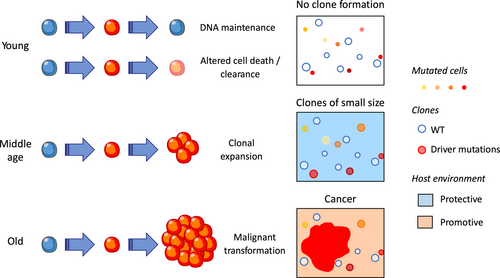
According to the current representation of cancer, accumulation of mutations inducing a fitness advantage is the rate-limiting factor of disease development. An alternative model suggests that aged-related alterations of the microenvironment may be the major rate-limiting factor (Rozhok, Salstrom, & DeGregori, 2014). The components of the host environment playing active roles in cancer development can span from the local cells surrounding an emerging clone to systemic and distant elements (Laplane, Duluc, Larmonier, Pradeu, & Bikfalvi, 2018; Figure 1).
3.1 Cell competition
To emerge, a clone must outcompete its neighboring cells. Somatic cell competition is a process that is conserved from Drosophila to mammals and corrects developmental errors, maintains adult tissue health, and delays aging effects by eliminating less fit cells (Merino et al., 2015). In Drosophila, cells that harbor mutations in a gene called “Minute” survive in a homogenous population of cells, while they are eliminated as soon as they are mixed with wild-type cells (Mello & Bohmann, 2020; Morata & Ripoll, 1975). Following a similar principle, cells of the wing disks that overexpress the oncogene Myc induce the death of neighboring wild-type cells (de la Cova, Abril, Bellosta, Gallant, & Johnston, 2004; Moreno & Basler, 2004). In this cell competition via short-range cell–cell interaction, eliminated cells are called “losers,” while the others are called “winners” (Nagata & Igaki, 2018). Flower, a membrane protein that exists in different isoforms, is a fingerprint of winner and loser cells; that is, some isoforms (called Flower-lose) mark cells to eliminate while others (called Flower-win) are expressed at the surface of winner cells (Rhiner et al., 2010). Flower-lose and Flower-win isoforms were recently identified in humans. For a clone of mutated cells to persist in an otherwise healthy tissue, wild-type and mutated neighboring cells should express similar levels of Flower-lose and Flower-win. Disruption of this equilibrium, for example, if healthy cells increasingly express Flower-lose as they age and/or if cancer cells overexpress Flower-win, could favor the emergence of a tumor by activating a nonimmune cell recognition and selection mechanism. Accordingly, the expression of the two human Flower-win isoforms was observed to be higher in cancer cells of expanding tumors than in surrounding tissues (Madan et al., 2019). Thus, deregulation of Flower gene splicing in a population, either tumor cells or surrounding healthy cells, could permit a clone to escape the control of its immediate environment. Whether this mechanism may be sufficient to initiate the shift from a clone to a malignancy remains unclear.
Another way by which mutated clones can compete is related to their differential resistance to external insults. Therapy-related myeloid neoplasms, including acute myeloid leukemias and myelodysplastic syndromes, provide a unique example of how external perturbations can promote cancer evolution. This clinical entity typically occurs as a late complication of chemotherapy and radiotherapy administered for a primary cancer. Next-generation sequencing of peripheral blood cells collected at the time of a primary tumor identified clonal hematopoiesis of indeterminate potential in 25% of cancer patients, with ~5% harboring presumptive leukemia driver mutations. In multiple cases, the exact TP53 or PPM1D mutation found at diagnosis of a therapy-related hematological malignancy was also present at a very low frequency (<1%) in blood leukocytes before any chemotherapy was given for treating the primary tumor (Schulz et al., 2015; Wong et al., 2015). Accordingly, TP53 or PPM1D gene mutations can be detected in small populations of peripheral blood cells of some healthy, chemotherapy-naïve, elderly individuals. In mouse, bone marrow chimaera models containing both wild-type and Tp53- or Ppm1d-mutated hematopoietic stem cells, these mutated preferentially expand after exposure to chemotherapy (Coombs et al., 2017; Gibson et al., 2017; Hsu et al., 2018). These data support a model in which rare stem cells carrying age-related TP53 or PPM1D mutations have resisted to initial cytotoxic therapy, then expanded preferentially. Rather than inducing a leukemia-specific mutation by itself, cytotoxic treatment facilitated the expansion of a pre-existing clone. Mechanistically, mutant p53 interacts with the epigenetic regulator EZH2 to alter the expression of genes regulating stem cell self-renewal and differentiation, establishing EZH2 as a potential therapeutic target for TP53-mutated clones and malignancies (Chen et al., 2019). Importantly, a similar selection of TP53-mutated clones could be observed in the skin exposed to UV light exposure (Klein, Brash, Jones, & Simons, 2010) and in chronically inflamed intestine (Vermeulen et al., 2013). Similar mechanisms apply to other DNA repair pathway gene mutations such as RAD21 and BRCC3 (Husby et al., 2020). However, all pre-existing clones do not similarly expand upon therapy exposure, indicating that the risk of leukemic evolution depends on the mutations they harbor.
3.2 Alteration of local tissue environment
Some years ago, a debate emerged regarding the respective role of intrinsic factors—the number of stem cell divisions and inherited genetic factors—and extrinsic factors—exposure to toxic insults—in the accumulation of somatic mutations leading to cancer (Tomasetti & Vogelstein, 2015; Wu, Powers, Zhu, & Hannun, 2015). The assumption was that selective advantage is a fixed attribute of genetic changes; that is, this debate did not consider the dynamic properties arising at the interface of the mutated cells and their environment (Rozhok & DeGregori, 2019). Actually, the normal tissue landscape plays a role in maintaining structural organization, inhibiting cell growth and clonal expansion, eliminating the cells with damaging alterations and reversing malignant phenotypes, indicating that any alteration of these repressive forces may contribute to the emergence and growth of malignant tumors.
Within tissues across the body, fibroblasts are the most common component of the stroma, synthesize the extracellular matrix that regulates tissue structure, and secrete a variety of soluble factors, providing a growth-restricted microenvironment for premalignant cells (Krtolica, Parrinello, Lockett, Desprez, & Campisi, 2001; Sahai et al., 2020). Other stromal cells include endothelial cells, adipocytes, immune cells, and nerves (Boilly, Faulkner, Jobling, & Hondermarck, 2017; Quail & Dannenberg, 2019). Correlations have been established in the breast and the liver between an altered tissue stroma; for example, an increased density of the breast stroma measured by imaging methods (Boyd et al., 2010) and the risk of cancer. Experimental settings supporting the role of the tissue stroma in tumor emergence include expression of stromelysin-1 in mouse normal epithelial cells that promotes malignant conversion in mammary glands (Sternlicht et al., 1999), and inactivation of the TGF-beta type II receptor gene in mouse fibroblasts that modulates the growth and oncogenic potential of adjacent epithelia (Bhowmick et al., 2004). These experiments supported the idea that, when tissue homeostasis declines, adaptive phenotypes are selected, of which some defined by somatic mutations and epimutations evolve toward an overt cancer.
Natural selection has invested into tissue maintenance to maximize organismal reproductive fitness, whereas physiological aging along the postreproductive lifetime progressively degrades tissue maintenance, which can be accelerated by toxic environmental insults. Aging promotes both degenerative pathologies through debilitating loss of tissue or cellular functions and hyperplastic pathologies of which the deadliest is cancer. The cellular stress response common to these pathologies is cellular senescence, a potent tumor suppressive mechanism that also generates toxic inflammation (Campisi, 2013). Senescence is a trait that elicits a beneficial phenotype early in life and becomes detrimental with aging, which has been proposed to define a so-called “antagonistic pleiotropy” (Fane & Weeraratna, 2020). Cells undergoing senescence secrete a variety of soluble factors (typically ~75 cytokines, chemokines, growth factors and proteases) that form the senescence-associated secretory phenotype (SASP; Faget, Ren, & Stewart, 2019).
In young tissue, the SASP is mostly beneficial by attracting immune cells that clear altered cells following an injury. In aging tissue, the SASP becomes detrimental and drives tumor progression as well as many other aging-related diseases. Recent evidence indicates that aging modifies the secretome of fibroblasts and other stromal cells to a state that is more permissive for the growth and invasion of malignant cells (Kaur et al., 2016; Krtolica et al., 2001; Liu & Hornsby, 2007). Several mechanisms may account for age-related stromal cell senescence, including DNA damage, epigenetic changes, and retrotransposable element derepression (Coppé, Desprez, Krtolica, & Campisi, 2010; De Cecco et al., 2019; Liu et al., 2019). Their permissive effect on tumor emergence involves a great diversity of secreted factors (Fane & Weeraratna, 2020). As an example, genetic deletion of the senescence-inducing factor SIN3B (a histone deacetylase-associated protein) in a KrasG12D-driven mouse model of pancreatic intraepithelial neoplasia significantly reduces the initiation and progression of the pancreatic lesions by decreasing IL-1β secretion (Rielland et al., 2014). Osteoblasts, the cells that build the bone, generate an extracellular matrix that is mineralized and their senescence increases bone remodeling by releasing the cytokine IL-6 to activate osteoclasts, the cells that destroy the bone, and promote the growth of tumor metastasis in mouse models (Luo et al., 2016). Together, these data indicate that senescence of fibroblasts and other stromal cell populations in an aging tissue generates a microenvironment that is mostly protumorigenic (Faget et al., 2019).
3.3 Systemic and distant alterations
Systemic alterations could complement the effects of tissue microenvironment aging in the promotion of cancer emergence. One of them, which is shared by the causes and effects of aging, is low level of chronic inflammation. The so-called “inflammaging” includes elevated levels of pro-inflammatory cytokines and chemokines, both within the tissue microenvironment and the systemic milieu. It also provokes a low-level persistent infiltration of tissues with immune cells, primarily but not exclusively cells of the innate immune system (Balkwill & Mantovani, 2001; Cevenini, Monti, & Franceschi, 2013; Pinti et al., 2016). Interleukin-6 (IL-6) and C-reactive protein are commonly used as indicators of this inflammation and increase in an age-dependent manner, even in subjects never diagnosed with diseases commonly associated with age. The origin of inflammation includes the already mentioned accumulation of senescent cells that become maladaptive and promote diseases through their secretome or SASP (Faget et al., 2019). Two other factors that may account for low-grade chronic inflammation associated with aging include the adipose tissue and the microbiota.
Among the lifestyle choices that affect cancer risk, obesity, whose prevalence is increasing rapidly, now competes with tobacco as the leading preventable risk factor for cancer. Obesity is associated with both increased cancer incidence and mortality. It induces substantial and complex metabolic and inflammatory changes in the adipose tissue that disrupt physiological homeostasis within local tissues and systemically (Lengyel, Makowski, DiGiovanni, & Kolonin, 2018). Not only the volume but also the quality of adipose tissue (inflammation, hypoxia, adipocyte hypertrophy) and its distribution may affect the risk of disease through driving metabolic aberrancies that include insulin resistance and metabolic syndrome (Quail & Dannenberg, 2019). The normal aging process also modifies the adipose tissue, that is, increases levels of visceral and subcutaneous adipose tissue. The role of adipose tissue in the progression of established cancer is well documented. Whether it could also promote cancer initiation remains debated but it creates an inflammatory milieu in which radical oxygen species production is elevated to a level at which genomic instability may ensue.
Independently of overt inflammation and obesity, consumption of a high-fat diet can enhance adenocarcinoma development and metastasis formation in K-rasG12Dint mice. In this model, high-fat diet promotes the progression from low-level dysplasia in normally fed mice to small bowel carcinogenesis and metastasis by inducing a dysbiosis. Importantly, the carcinogenic phenotype could be reproduced by fecal transplants, demonstrating that cancer arises from an interaction between genes and the bacteria (Schulz et al., 2014). This model suggests that microbiota could play a central role in absorbing the impact of various dietary insults and passing the consequences on to the host.
Initial relationships between the human microbiome composition and cancer development were more correlative than causative. For example, Fusobacterium, predominantly F. nucleatum, was observed to preferentially colonize colorectal cancer over normal colon tissue and, accompanied by other microorganisms, to persist during metastatic tumor growth, yet is unlikely a colorectal cancer driver by itself (Bullman et al., 2017). Very recently, unique microbial signatures were detected in the tissues and the peripheral blood of the major types of cancers, suggesting that identification of cell-free microbial DNA circulating in the peripheral blood plasma might be tested as an early diagnostic tool (Poore et al., 2020). Finally, metagenomics of patient stool samples established a correlative link between microbiome composition and clinical tumor response to chemotherapeutic drugs (Geller et al., 2017) as well as immune checkpoint inhibitors (Gopalakrishnan et al., 2018; Matson et al., 2018; Routy et al., 2018).
A more causative relationship was detected in mouse models in which dysfunction of the small-intestinal barrier allows for bacterial translocation that promotes an amplification of Tet2-deficient hematopoietic cells (Meisel et al., 2018). Some members of the microbiome may be directly damaging to human cell DNA and shape a tumor genome (Barrett, Hand, Shanahan, Murphy, & O'Toole, 2020). For example, a certain strain of the gut-dwelling bacterium Escherichia coli encodes a genotoxin called colibactin that, in human colon cell derived organoids cultured in vitro, generates a typical mutational signature that is detected also in a fraction (4%–8%) of human primary colorectal cancers and their metastases (Pleguezuelos-Manzano et al., 2020). Still uncertain, the causal role of this bacterial strain, which is enriched in gut microbiota of a large fraction of people who have colorectal cancer, may depend on the local context and involve crosstalks with immune cells (Jin et al., 2019; Ma et al., 2018) and with other microbes (Barrett et al., 2020). All these observations raise important questions with clinical implications. A first one is whether routine sequencing of the microbiome and analysis of microbial signatures in tumors or the peripheral blood plasma will provide much-needed predictive biomarkers and early diagnostic tools. Another question is whether cautious manipulation of factors that modify the microbiome composition, including diet and probiotics, could prevent or delay cancer development. Finally, the use of some medications, such as antibiotics, may need careful control in patients with overt cancer receiving certain cytotoxic or immune therapies.
3.4 Can the environment cause cancer?
In light of the important role played by the host environment in cancer development, one open question is whether its alteration could induce malignant transformation (Sonnenschein & Soto, 1999, 2020). Myeloid neoplasms, whose origin is the transformation of a hematopoietic stem or progenitor cell, illustrate this current interrogation. The bone contains a heterogeneous population of stromal cells organized into anatomically defined regulatory environments or niches, some of these niches supporting the survival and differentiation of hematopoietic stem and progenitor cells (Wei & Frenette, 2018). Recent experimental evidence supports the concept of bone marrow niche-driven malignant transformation in which primary alterations of niche cells drive the malignant transformation of hematopoietic cells and disease progression (Pronk & Raaijmakers, 2019).
Initial studies in mice showed that deletion of RARγ (Walkley et al., 2007) or IkBa (Rupec et al., 2005) genes from the microenvironment resulted in neoplasms with a myeloproliferative component. Subsequent studies targeted the deletion of Dicer1, which encodes a microRNA-processing endonuclease (Raaijmakers et al., 2010), and that of Sbds, which is mutated in Shwachman-Diamond syndrome (SDS; Zambetti et al., 2016) in osteoblast precursors, generating a phenotype that mimics myelodysplastic syndromes. Specific mutations targeted to bone marrow mesenchymal stromal cells also triggered a myeloid phenotype, that is, an activating mutation of β-catenin resulted in a myelodysplastic syndrome through Notch signaling activation in hematopoietic stem and progenitor cells (Kode et al., 2014), whereas Ptpn11 mutation promoted a transplantable myeloproliferation (Dong et al., 2016). Deletion of Sipa1 (signal-induced proliferation associated-1) in the microenvironment also drove a myeloid phenotype in mice (Xiao et al., 2018). Finally, mesenchymal stromal cells, expanded ex vivo, could promote the propagation of human myelodysplastic stem cells in xenograft models (Medyouf et al., 2014).
Mechanistically, genetic alterations in bone marrow stromal cells may activate the WNT/β-catenin signaling pathway and induce an oxidative stress. They also induce the release of soluble inflammatory factors such as CCL3 and the alarmins S100A8 and S100A9, which, either directly or through recruiting inflammatory cells, may accumulate at very high concentration in the local environment that forms the niche and promote genetic alterations and subsequent transformation of a HSPC. Similar abnormalities could be observed in the bone marrow niches as an effect of aging, making it conceivable, though difficult to demonstrate in humans, that primary alterations in the mesenchymal niche are inducing myeloid malignancies (Pronk & Raaijmakers, 2019).
Alterations of the host environment could also contribute to the emergence of solid tumors. For example, in the rat, healthy mammary epithelial cells injected in a fat pad previously cleared from mammary tissue, then exposed to a carcinogen, can transform into an epithelial tumor (Maffini, Soto, Calabro, Ucci, & Sonnenschein, 2004). Another example was obtained in mice in which obesity, through increasing tissue stiffness, promotes the malignant transformation of premalignant human breast epithelial cells (Seo et al., 2015). Overexpression of some matrix metalloproteases in mouse mammary glands also promotes malignant transformation of mammary epithelial cells (Ha et al., 2001; Sternlicht et al., 1999), which explains why these important components of the extracellular matrix were compared to carcinogens (Radisky & Bissell, 2006).
Altogether, accumulating evidence has shown that we eventually get a cancer as quality control mechanisms that take place in the tissue to prevent clonal expansion and tumor development during our reproductive life are disrupted by external stimuli or progressively slacken with age. In addition to a better control of lifestyle risks, aging increasingly appears as a modifiable risk factor. Efforts to target the main biological drivers of aging include caloric restriction, free radical inhibition and cellular senescence control. These efforts may restore or prolong bodily functions by decreasing inflammatory responses, proteostasis and epigenetic changes, with the hope that it may decrease the risk of cancer and multiple other chronic diseases.
4 HOW DO TUMORS GET THE UPPER HAND ON THEIR ENVIRONMENT?
When a tumor grows, cancer cells receive from their microenvironment a complex array of cues that regulate their survival and biological behavior. Surrounding healthy cells, which are in competition with tumor cells for survival factors or mechanical stress, have a decreased fitness. To optimize their interactions, cancer cells remodel a number of cell components in their environment; that is, they promote angiogenesis, compromise the functions of stromal cells, damage nerve fibers, neutralize immune cells, and generate immunosuppressive cells (Costa et al., 2018).
Such a symbiotic interaction between cancer cells and their environment has been depicted in myeloid neoplasms in which a crosstalk between leukemic cells and their bone marrow environment likely drives disease evolution. In mouse models and human diseases, leukemic cells affect, either directly or indirectly, the cell components of the bone marrow niche, including mesenchymal stromal cells (Arranz et al., 2014; Baryawno et al., 2019; Hanoun et al., 2014; Mead et al., 2017; Schepers et al., 2013), sympathetic nerve fibers (Arranz et al., 2014), bone marrow adipocytes (Boyd et al., 2017), and endothelial cells (Duarte et al., 2018). This reshuffling of the environment includes the reprogramming of mesenchymal stromal cells by clonal megakaryocytes into myofibroblasts that induce myelofibrosis (Schneider et al., 2017). Leukemic cells thereby create a local environment in which an intricate network of interactions promotes mesenchymal cell senescence or differentiation into myofibroblasts, functional repression of normal hematopoietic stem cells, modification of the extracellular matrix, leukemic cell expansion, and disease progression (Pronk & Raaijmakers, 2019). In chronic myeloid neoplasms in which cell differentiation is preserved, mature cells of the clone contribute to the bone marrow niche in which normal and transformed hematopoietic stem and progenitor cells accommodate. These mature cells synthesize and secrete cytokines such as IL-6 that promotes the expansion of leukemic stem cells in a positive feedback loop (Reynaud et al., 2011). In a preclinical model, therapeutic inhibition of this cytokine could prevent disease installation and progression (Welner et al., 2015).
Similar crosstalks between tumor cells and neighboring cells are observed in other tissues where cancer cells orchestrate a tumor microenvironment comprised of fibroblasts, immune cells, and endothelial cells embedded in a robust extracellular matrix. Normal fibroblasts are the major producers of connective tissue extracellular matrix, a function that changes with age (Kaur et al., 2016). Typically, they become activated as myofibroblasts during wound healing, expressing alpha smooth muscle actin and producing transforming growth factor-β (Sahai et al., 2020). Fibroblast expansion may precede the conversion to malignancy as they often circumscribe early or premalignant lesions, suggesting an initial tumor-suppressive function (Lockwood et al., 2003). In overt tumors, cancer-associated fibroblasts (CAFs) result from the activation of local tissue-resident fibroblasts by multiple cues received from tumor cells or their tissue microenvironment. They modulate cancer growth and metastasis with both protumorigenic and antitumorigenic effects, remodel the extracellular matrix, modify tissue stiffness, and secrete soluble factors that modulate anticancer immune response, influence angiogenesis, and affect therapy responses. Single-cell analyses recently pointed to the heterogeneity and plasticity of CAFs, some of them (myoCAFs) producing matrix while others (iCAFs) regulate inflammatory and immune responses (Costa et al., 2018; Pelon et al., 2020; Puram et al., 2017). Various strategies are currently developed and tested, including clinically, to therapeutically manipulate these CAFs in order to improve the tumor response to other therapeutic approaches (Sahai et al., 2020).
This microenvironment reshuffled by cancer cells is overtly immunosuppressive, leading to cytotoxic T-cell dysfunction (Anderson, Stromnes, & Greenberg, 2017). Some key players in immune surveillance escape are tumor-associated macrophages (Cassetta et al., 2019), myeloid-derived suppressor cells (Kumar, Patel, Tcyganov, & Gabrilovich, 2016), and regulatory T cells (Najafi, Farhood, & Mortezaee, 2019) that release cytokines and growth factors and regulating cancer cell plasticity. To hijack the host immune response and generate such an immunoprivileged microenvironment, cancer cells induce an angiogenic switch, which is an essential step to go beyond a microscopic size and disseminate. The growth of microvessel is orchestrated by a range of angiogenic factors and inhibitors that have become conventional therapeutic targets in cancer therapy (De Sanctis, Ugel, Facciponte, & Facciabene, 2018). Finally, as in myeloid neoplasms, cancer cells could interact with other cells of the malignant clone; for example, minor cell populations could support the growth of bigger clones (Aceto et al., 2014; Marusyk et al., 2014), which was shown to involve Wnt (Tammela et al., 2017) and Notch (Lim et al., 2017) pathways in lung cancers.
Precluding the dormancy of metastatic cells, primary tumors actively and selectively modify target tissues before metastatic spread has even started. Tumor released factors and exosomes that provoke vascular leakiness, alter local resident cells, remodel the extracellular matrix, and recruit nonresident cells such as bone marrow-derived cells, which induces the stepwise formation of a conducive tissue stroma called a “premetastatic niche” (Peinado et al., 2017). Adjuvant epigenetic therapy could disrupt this niche by inhibiting the trafficking of myeloid-derived suppressive cells or promoting their differentiation (Lu et al., 2020). The organotropism of metastasis formation illustrates the diversity of tissue environments (Gao et al., 2019; Müller et al., 2001) and the specificity of their interaction with cancer cells (Hoshino et al., 2015; Wortzel, Dror, Kenific, & Lyden, 2019).
As mentioned above, the tumor environment extends beyond the tissue microenvironment to tumor–host interactions, which consists of metabolites, cytokines, and chemokines that circulate in the blood, connecting systemic metabolism and cancer cell proliferation. The term “adaptive homeostasis” was proposed to describe short-term adaptations of biological systems, that is, transient expansion or contraction of the homeostatic range, in response to mild changes in conditions such as exercise training or subtoxic, nondamaging events (Davies, 2016). This concept could apply to cancer cells. Typically, these cells consume more glucose than normal cells. Activation of intrinsic pathways contributes to adapt this requirement but may not be sufficient to provide the quantity of glucose that is needed to drive robust cancer cell growth. Therefore, malignant cells reduce the glucose utilization of normal tissues, such as muscle and adipose tissue. Thus, analogous to parasites, cancer cells compete with the host for essential systemic resources. For example, leukemic cells induce high-level production of IGFBP1 (Insulin growth factor binding protein 1) from adipose tissue to mediate insulin sensitivity. In a mouse model of leukemia, disease-induced gut dysbiosis, serotonin loss, and incretin inactivation were shown to combine to suppress insulin secretion. Importantly, attenuated disease progression and prolonged survival could be achieved through disruption of this leukemia-induced adaptive homeostasis (Ye et al., 2018). Tumor-derived waste could be also repurposed and subsequently utilized as fuel for tumor growth. For example, lactate, a waste product of hypoxic cancer cells, can serve as a substrate for oxidative metabolism of oxygenated tumor cells (Sonveaux et al., 2008).
In this respect, specific components of the circadian clock machinery may be highly susceptible to factors secreted by the tumor. In the KrasLSL-G12D/+;p53fl/fl mouse model, lung adenocarcinoma rewires the circadian transcriptome and metabolome in the liver through tumor-dependent inflammation that releases IL-6, which dampens insulin and glucose sensitivity and alters hepatic circadian lipid metabolism (Masri et al., 2016). A similar effect was detected in a mouse model of triple-negative breast cancer, resulting in increased oxidative stress (Hojo et al., 2017), suggesting that circadian oscillations of metabolism are highly susceptible to systemic cues that reorganize physiological homeostasis.
Clonal hematopoiesis is associated also with a substantial increase in the risk of cardiovascular and other diseases of aging (Jaiswal et al., 2017). Mechanistically, blood cells that harbor the mutations give rise to immune cells that reside in nearly all tissues. Somatic mutations that generate the clone may amplify the inflammatory response of immune cells. The best example of damage induced by cancers to distal tissues is paraneoplastic syndromes, which are due to cancer but not to the local presence of cancer cells. These indirect effects of tumors on distal tissues are thought to be generated by circulating tumor-derived soluble factors, including cytokines and hormones, as well as by immune and inflammatory cells. The generated symptoms such as neurological defects, thrombosis, anemia, or glomerulonephritis partially overlap with phenotypes that are common among nondiseased elderly. Thus, cancers drive the development of noncancer pathologies associated with aging. This effect is amplified by cancer treatments that frequently result in a long-term burden of senescent cells and their proinflammatory SASP.
5 CONCLUDING REMARKS
Enforcing former identification of small overt cancers in young people died from other causes, recent improvement of genomic technique have identified a number of cell clones, as defined by somatic mutations in driver oncogenes or tumor suppressor genes, in healthy tissues. These events may appear decades before the clinical diagnostic of cancers, blurring the frontier between premalignant and malignant tumors while indicating that contextual cues may long refrain malignant tumor expansion. The fitness of cancer cells is relative to their environment whose impact on clonal evolution largely depends on age. Typically, young healthy tissues are tumor suppressive; that is, they eliminate or limit the expansion of cells that have acquired somatic mutations, whereas aging or damaged tissues become tumor tolerant and even promoting; they are progressively unable to eliminate these cells and counteract their selection (Laconi et al., 2020; Figure 1).
Parsing the causes of the switch of a tissue toward a protumorigenic behavior identifies a number of potential mechanisms that could be schematically classified into (a) general—all tissues may become protumorigenic while aging— versus specific—for example, some tissues become protumorigenic as a consequence of a local insult such as lung tissue exposed to tobacco smoke; (b) direct—the tissue environment actively promotes tumor growth—versus indirect—changes in the environment becomes deleterious to normal cells; (c) active—for example, the tissue recruits protumorigenic immunosuppressive cells— versus passive—for example, the tissue fails to recruit antitumor immune cells; (d) mandatory—for example, changes in the microenvironment are required for a cancer to grow— versus contributive— these changes favor cancer progression.
In addition to avoiding well-identified lifestyle insults, accumulating evidence indicates that healthy tissue landscape endowed with tumor suppressive properties could be actively preserved by adopting favorable behaviors such as caloric restriction or balanced diet and exercise. Although efficient in animal models, the long-term use of anti-inflammatory and senolytic drugs to promote tissue rejuvenation must be balanced with potential side effects. In established cancers, it has become crucial to distinguish diseases that may be cured through therapeutic intervention focusing on cancer cells from those who will benefit from stimulation of surrounding cells, as illustrated by the transformative results obtained with targeting T-cell immune checkpoints in some cancers. Although more speculative, by improving our ability to measure cell fitness in specific contexts, we could develop adaptive therapy to protect the less aggressive clones that, in a heterogeneous tumor, limit the dominance of the most aggressive cells (Acar et al., 2020; Gatenby, Silva, Gillies, & Frieden, 2009). Finally, early diagnostic of cancers, which may provide the best chance of cure, will integrate an appropriate evaluation of the surrounding tissue in order to spare patients from unnecessary treatments.
ACKNOWLEDGMENTS
The team “Hematopoietic stem cell aging” is supported by the Ligue Nationale Contre le Cancer (Equipe Labellisée Françoise Porteu) and grants from the Institut National du Cancer, the Fondation pour la Recherche Médicale, Fondation de France, Fondation ARC, and James S. McDonnell Foundation.
CONFLICT OF INTEREST
None declared.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing was not applicable to this article as no datasets were generated or analyzed during the current study.




