The Free-Range Rock Cavy (Kerodon rupestris) Communicates the Urgency of a Threat Using Different Alarm Calls
Funding: This work was supported by Coordination for the Improvement of Higher Education Personnel (CAPES—Finance Code 001), Council for Scientific and Technological Development (CNPq) (Processes #303448/2019-9 and #04226/2019-0, respectively), and National Institute of Science and Technology in Interdisciplinary and Transdisciplinary Studies in Ecology and Evolution.
ABSTRACT
This study examines whether rock cavies (Kerodon rupestris), which are social rodents, modulate their alarm calls in response to various threat contexts. Conducted across four sample areas within two study sites in the Brazilian Caatinga region, alarm calls were collected using the ad libitum method. The acoustic responses of free-ranging rock cavies were then analyzed using discriminant function analysis and generalized mixed linear models to classify vocal types and assess differences in vocalization rates and acoustic parameters. The findings reveal that rock cavies produce both slow and fast alarm whistles in response to threats. Fast alarm whistles, emitted exclusively in response to nearby ocelots (Leopardus pardalis), exhibited a lower pitch (F1,16.20 = 11.41, p = 0.004), shorter duration (F1,22.59 = 14.93, p = 0.001), and shorter pulse intervals (F1,21.29 = 6.08, p = 0.022) compared to the slow alarm whistles. Slow alarm whistles were produced when rock cavies were threatened by distant ocelots, as well as by both distant and closer humans, dogs (Canis familiaris), marmosets (Callithrix spp.), tayras (Eira barbara), and birds of prey (Caracara plancus). The type of threat influenced the pulse intervals (F6,23.26 = 12.69, p < 0.001) and the high frequency (F6,18.15 = 12.08, p < 0.001) of slow alarm whistles. Rock cavies produced shorter pulse intervals when threatened by ocelots, birds of prey, or tayras compared to humans and dogs (p < 0.05) and higher-pitched slow alarm whistles when threatened by dogs, ocelots, humans, or birds of prey compared to capuchin monkeys and tayras (p < 0.05). Additionally, shorter pulse intervals (F1,25.73 = 28.87, p < 0.001) were emitted when threats were nearby compared to more distant threats. This study highlights the influence of various threats and their proximity on the modulation of rock cavy alarm calls, showcasing their behavioral adaptability. This crucial survival strategy not only enhances our understanding of rock cavies' behavior but also has the potential to inspire research in other species and ecological contexts.
1 Introduction
The alarm call is an antipredator strategy widely used by various species of mammals and birds (Hollén and Radford 2009). These calls are usually conspicuous and addressed to conspecifics as a way to announce danger (Blumstein 2007). These signs may reflect an underlying motivational state or excitement experienced by the emitter when exposed to certain predators (Morton 1977). Therefore, specific changes in the structures of the calls (e.g., number of pulses, intensity, pitch, and vocal type) are able to transmit several types of information to the receivers (Morton 1982; Shannon and Drew 2009).
Many species emit alarm calls that vary acoustically depending on the type and urgency of the encountered threat (Suzuki 2011; Gill et al. 2013; Mclachlan and Magrath 2020). By analyzing the characteristics of calls elicited by different predators, researchers can gain insights into the adaptive functionality of prey responses to predation (Macedonia and Evans 1993). For instance, white-eyed gallinules (Phylidonyris novaehollandiae) determine the reliability and urgency of an acoustic warning signal based on the number of pulses emitted. This assessment intensifies when facing more dangerous predators, as changes in both the structure and number of alarm calls quickly and reliably signal immediate danger (Mclachlan and Magrath 2020). In addition, the numerous hunting strategies employed by predators make it necessary to develop qualitatively different acoustic behaviors in prey in order to increase escape success (Blumstein and Armitage 1997; Caro 2005). Green monkeys (Chlorocebus aethiops) emit distinct alarm calls for different predators, such as carnivores, eagles, humans, and snakes (Cheney and Seyfarth 1988; Price et al. 2015). Similarly, capuchins (Cercopithecus diana) signal danger in various ways, using louder alarm calls when threatened by leopards (Panthera pardus) and quieter calls for crowned hawk-eagles (Stephanoaetus coronatus) (Zuberbuhler 2000).
These variations in alarm calls could be attributed to the different escape time constraints imposed by various predators. As a result, prey may adjust their communication to signal varying levels of danger to their conspecifics (Macedonia and Evans 1993; Blumstein 2007). In addition to predator type, this information may also pertain to the predator's location and the urgency of the response required to the threat. The large gerbil (Rhombomys opimus), for example, is a rodent that emits a rhythmic call when the predator is far away, another lower-pitched call as the predator approaches, and a short whistle when surprised by the predator (Randall and Rogovin 2002). Yellow-bellied marmots (Marmota flaviventris), on the other hand, vocalize more quickly and with a greater number of pulses as the threat approaches (Blumstein 2007). Thus, certain acoustic parameters of alarm calls can signal the immediacy of a threat by reflecting the urgency and speed needed for defensive actions (Gill et al. 2013).
Social rodents inhabiting harsh environments often emit numerous alarm calls when threatened by different predators (Blumstein and Armitage 1997). The free-range rock cavy (Kerodon rupestris) is an example of a social rodent that frequently emits alarm calls in the wild and occupies a foundational position in the food chain, serving as prey for numerous predators in Brazil's semiarid regions (Lacher 1981; Marinho et al. 2018; Penido et al. 2017; Almeida et al. 2025). K. rupestris is an endemic species found exclusively in the caatinga biome, inhabiting rocky outcrops that are challenging to access (Bonvicino et al. 2008). Classified as endangered, its population is in decline primarily due to habitat loss, fragmentation, and hunting activities (ICMBio 2018a, 2018b; MMA, Ministério do Meio Ambiente 2022). The species is organized in social groups that live in a harem structure, usually composed of one male and three to four adult females and their young. The group has a hierarchy of social dominance, and members often use the acoustic channel in their social interactions (Lacher 1981; Monticelli and Alencar-Junior 2021).
A specific type of alarm call, known as the alarm whistle, is part of the rock cavy's acoustic repertoire (Lacher 1981). This call is easily detectable in the wild during threatening situations, such as agonistic encounters involving the rock cavy (Almeida et al. 2025; Alencar-Junior 2011). The alarm whistle, when recorded in different areas and under different levels of anthropogenic impact, shows variations in the number of pulses and in acoustic parameters (high and peak frequencies), suggesting that it is a possible ecological acoustic indicator for the species in the wild (Almeida et al. 2025).
Other rodent species are known to modulate their alarm calls according to the type of predator or the urgency of the threat response required (e.g., Rhombomys opimus, Randall and Rogovin 2002; Marmota caudata and M. marmota, Blumstein 2007; Tamiasciurus hudsonicus, Digweed and Rendall 2009). Thus, in this study, we aimed to test the hypothesis that rock cavies modulate their alarm calls in response to the type of predator or the urgency of the threat response. To examine this, we compared the acoustic parameters of alarm calls across various threat contexts in the species' natural environment. Based on findings from other rodent species (Macedonia and Evans 1993; Cheney and Seyfarth 1988; Zuberbuhler 2000; Randall and Rogovin 2002; Blumstein 2007), we expect the rock cavy to produce alarm calls with a higher call rate, lower pitch and entropy, along with shorter duration, more pulses, and shorter intervals between pulses in response to greater threats (e.g., natural predators) compared to less threatening situations (e.g., exposure to nonhuman primates). Additionally, we anticipate that closer proximity to threats will result in alarm calls with more pulses and shorter intervals between pulses, consistent with observations in other rodent species (Randall and Rogovin 2002; Blumstein 2007).
2 Methods
2.1 Ethical Note
This study was approved by the Chico Mendes Institute for Biodiversity Conservation (ICMBio) (#80143-1), the Institute for the Environment and Water Resources (INEMA) (#24.230 #26.010), and the Ethics Committee for the Use of Animals in Experimentation at the State University of Santa Cruz (CEUA-UESC) (#021/21).
2.2 Study Area and Animals
The study was carried out in two conservation units, namely the State Park of the Serra dos Montes Altos (PESMA) and the Serra dos Montes Altos Wildlife Refuge (REVISMA), between the latitudes 14°16′ and 14°39′ S and longitudes 42°46′ and 43°46′ W (Figure 1). These conservation units are located in the southwestern region of the state of Bahia and are part of Brazil's semiarid region with an average annual rainfall of 694 mm (Freire and Silva 2019). The geopolitical division of these areas is between six municipalities: Candiba, Guanambi, Palmas de Monte Alto, Pindaí, Urandi, and Sebastião Laranjeiras. The PESMA and REVISMA are habitats for many rare and endemic species of caatinga and cerrado ecosystems and their transition areas (Barros and Almeida 2018). The natural landscape is characteristic of these ecotone zones, with a transition from herbaceous to arboreal caatinga vegetation, rupestrian fields, gallery forests, and relief with the presence of the sertanejo pediplain, the São Francisco River depression, and the central mountains of the Espinhaço plateau (Tasso and Chang 2013).
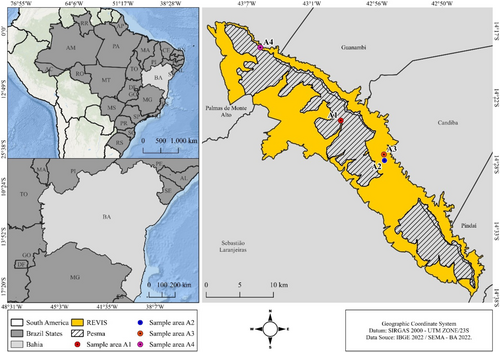
Four sampling areas were selected within the conservation units, one located on the edge of PESMA (A1: 3189 m2 central coordinate (CC) 14°24′10″ S 42°58′47″ W) and the others (A2: 3.285 m2 CC: 14°27′22″ S 42°55′08″ W; A3: 2955 m2 CC: 14°26′53″ S 42°55′11″ W; A4: 3036 m2 CC: 14°18′19″ S 43°05′30″ W) located in REVISMA. These areas have similar natural vegetation and landscape characteristics, with a predominance of shrubby and herbaceous vegetation strata typical of the caatinga, made up of dry branches and thorny plants. They are also characterized by the presence of large boulders, with no evidence of recent fires or other severe anthropogenic changes to the landscape. Two of these areas (1 and 2) are difficult to access and are located further away from human settlements (≥ 6 km) and are therefore less subject to human activity. The other areas (3 and 4) are located closer (≥ 800 m) to human habitation and are used as a source of economic resources for local populations through small-scale farming and livestock activities. The sampling areas were chosen after carrying out 12 field expeditions between the months of December (2021) and January (2022), with direct and indirect searches (traces) for the animals, as well as consulting the literature (Barros and Almeida 2018; Almeida et al. 2025) on the occurrence of K. rupestris and possible predators for the study area. We also took into account the information provided by the conservation unit guards who monitor the security of the site, visualize the groups of rock cavy and possible predators in certain areas, and informed us about accessibility. In this study, we consider individuals that inhabit a single rocky outcrop to be a rock cavy group. In this context, data was collected from 16 social groups distributed equally across each area. Each group had an average of 21 individuals (standard error = 0.34). We made this estimate based on direct observation of the rock cavies that inhabited the rocky outcrops. The rock cavies were counted by viewing the entire group of individuals with the naked eye or using binoculars (Urban gate model 80 × 80). We considered the largest number of individuals seen at one time on the rocky outcrop in each expedition.
2.3 Data Collection
During a pilot study, we estimated the period of greatest rock cavy activity (day or night) in the study area to determine the optimal collection time. This assessment was necessitated by conflicting findings concerning the peak activity period (day or night) of the species (Lacher 1981; Mares et al. 1981; Delciellos 2016; Dias et al. 2018). For this estimate, we installed six camera traps (three Bushnell model 119,436, two Suntek hc-300A and one Suntek hc-550A), which remained active in the field for 48 days (12 days for each sampling area divided into 3 days for each group investigated) in the pilot period of the study (December/2021 to mid-January/2022). The cameras were programmed in video mode (1 min of recording with a 30-s interval between recordings), triggered via a motion-temperature sensor, installed in pairs, and kept running 24 h a day. In total, we obtained 189 records of animals, with 81% of the records between 06 h00 and 18 h00, suggesting greater daytime activity by animals in the areas. In view of this result, we defined the collection period as between 06 h00 and 17 h00.
Data collection was carried out between January and December 2022, with 84 field expeditions (920 h). The alarm calls and associated behaviors were collected simultaneously using the ad libitum method (Altmann 1974) by an observer (WN) who recorded the behaviors associated with the sound emissions, and a field assistant who recorded the calls using an acoustic recorder (details below).
The acoustic signals were recorded using a portable digital recorder (mono mode, WAV format, 48 kHz sampling rate and 24-bit resolution), Tascam DR-44WL (Tokyo, Japan), coupled to a Sennheiser MKE 600 unidirectional microphone (Wedemark, Germany). To ensure the start of the animals' sound emission was not missed, the “pre-rec” function was set to 5 s. This feature guarantees that the vocalization is captured even before the recording function is fully activated. Thus, when the rock cavy vocalized, the recorder was manually triggered and kept running until the animal finished making sounds. The recorder was then put into standby mode only after the vocalization had ceased. For data collection, the observer and assistant used collection posts (on trees and high rocks), which ensured approximately 3.0–6.0 m from the animals was sampled and made it possible to spot the behaviors associated with the vocalizations according to the threat types by direct observation. The observer's posts were selected during the pilot study based on sightings of rock cavy on rocks, latrine points, and the proximity of potential collection sites to rock crevices (≥ 0.2 m apart and ≤ 1.5 m deep). This selection allowed for the observation of behaviors associated with rock cavy sound emission in these areas used for refuge and nesting. During the pilot study, the rock cavy's reactions were observed at different distances in relation to threats such as ocelots (Leopardus pardalis), humans, and dogs (Canis familiaris). When these threats were at less than 25 m from the collection points, the rock cavy emitted alarm calls that sounded more frequent and intense. Therefore, during sampling, the distance of the possible threat from the collection points was considered, with the determination of close threats (< 25 m) and distant threats (≥ 25 m). To do this, we delimited the space based on natural structures in the environment (positioning of rocks, stones, trees, and bushes). The distance in the field was measured with a tape measure. Aerial predators (birds of prey, possibly Caracara plancus) were considered a distant threat because they flew over the rocky outcrops without landing or attacking (approaching) the rock cavy.
The sex of the caller was identified by viewing the animal's genitalia at the time of the acoustic and behavioral recording. At times when the individual being sampled moved away from the observation points, its genitals were observed using binoculars. When identification was not possible or doubtful, the animal was recorded in the unidentified sex category. The classification of age group was based on observing the size of the individuals, as adults and offspring are easily distinguishable in terms of body size (Conceição and Bocchiglieri 2021). The animals were habituated to the presence of researchers during the pilot period. During this period, differences were observed and recorded between the areas in terms of the waiting time for the animals to return to their general activities after the researchers had occupied the observation posts. Therefore, before starting data collection, the average waiting time recorded in each area was presumed (A1: 50 min; SE: 3.17 A2: 52 min; SE: 2.17 A3: 37 min; SE: 1.54; A4: 30 min; SE: 1.93).
2.4 Acoustic Analysis
The aural and visual inspection of the alarm calls was carried out on a Vostro 5320 laptop computer (Dell Technologies, Texas, USA) coupled with a Tascam Lineup US-16 × 08 audio interface (Tokyo, Japan) and a pair of audio reference monitors (Yamaha Hs8, Japan) with a frequency response of 38 Hz~30KHz (−10 dB)/47 Hz~24KHz (−3 dB). We used Raven Pro software version 1.6 (Cornell Lab of Ornithology, Ithaca NY) using the settings: Hann-type window, window size 1351 samples, 90% overlap in the time domain, and DFT size 4096.
Based on this inspection, we selected the emissions with the least interference from environmental noise and the best graphic image quality. For each pulse (Figure 2) we measured the parameters of low frequency, high frequency, delta time, peak frequency, delta frequency, delta power, 1st quartile frequency 25%, 3rd quartile frequency 75%, energy, aggregate entropy, average entropy, maximum entropy, pulse intervals, and number of pulses. We chose these acoustic parameters, drawing on prior studies (Blumstein and Arnold 1995; Randall and Rogovin 2002; Digweed and Rendall 2009; Lima et al. 2022), which have demonstrated their efficacy in providing valuable insights into animal communication and adaptive responses. For subsequent analyses, we excluded erroneously classified alarm calls to prevent potential biases.
2.5 Data Analysis and Statistics
2.5.1 Call Categorization and Acoustic Parameter Selection
To identify distinct types of alarm calls emitted by rock cavies, we initially categorized the calls through aural and visual inspection, as outlined in the acoustic analysis section. To rigorously assess the accuracy of our categorization of call types and mitigate potential errors or biases inherent in these methods, we subsequently performed discriminant function analysis (DFA) on the acoustic parameters, utilizing the cross-validation test, specifically the leave-one-out cross-validation (LOOCV). This approach involves systematically omitting each observation from the dataset individually, recalculating the discriminant function using the remaining data, and predicting the group for the omitted observation.
2.5.2 Statistical Analysis
We employed generalized linear mixed models (GLMMs), with one model per acoustic parameter, to compare the selected acoustic parameters. These parameters included average entropy, 1st quartile frequency (25%), duration, high frequency, and pulse intervals, to evaluate the validity of our predictions. We incorporated the whistle call type (slow and fast alarm whistles), the sex of the rock cavy, the study area, and the interaction between the sex of the rock cavy and the study area as fixed factors within these models. Additionally, we included the identity of individuals nested within their respective groups as a random factor to appropriately account for repeated observations from the same individuals. The residuals of the models were visually inspected for normal distribution and homoscedasticity and were deemed adequate for all data.
From these previous analyses, we determined that the cavies modulated their alarm call emissions according to threat contexts, producing both slow and fast alarm whistles (see Results). However, fast alarm whistles were exclusively used in contexts involving proximity to ocelots (see Results). Consequently, we were unable to statistically evaluate whether different populations employ distinct whistle types for different threats. Therefore, all subsequent analyses focused solely on slow alarm whistles.
Subsequently, to test our hypothesis that rock cavies would emit alarm calls more frequently when confronted with natural predators and imminent threats, we employed two General Linear Models (GLMs) due to the absence of consistent threats across all areas, as indicated in Table 1. In the first GLM, we examined differences in the vocalization rate (calls/caller/h) of slow alarm whistles. In this model, we included the study area, the sex of the rock cavy, the distance from the threat, and their interactions as fixed factors. Residuals were visually inspected to verify normal distribution and homoscedasticity, both of which were deemed satisfactory. Following that, to account for variations in threat presence across different areas, we employed a separate GLM to compare vocalization rates (calls/caller/h) based on the type of threat. Given that both models were built using individual-level aggregated data and did not include repeated observations, the study design did not support incorporating individual identity nested within groups as a random effect. The residuals from both models were visually examined to assess their compliance with the assumptions of normal distribution and homoscedasticity, both of which were found to be satisfactory.
| Area | Threat | Closea | Far away |
|---|---|---|---|
| Area 1 | Tayra | 34 | 198 |
| Yellow-breasted capuchin monkey | 24 | 77 | |
| Area 2 | Bird of prey | 0 | 139 |
| Marmoset | 66 | 0 | |
| Area 3 | Dog | 241 | 42 |
| Human | 217 | 72 | |
| Ocelot | 0 | 30 | |
| Area 4 | Bird of prey | 0 | 156 |
| Dog | 292 | 84 | |
| Human | 99 | 54 | |
| Ocelot | 0 | 55 |
- a Highlighted in bold are the slow alarm whistle emissions exclusively produced by female rock cavies.
Following that, we used a set of GLMMs, with separate models for each variable, to compare the number of pulses, pulse intervals, duration, high frequency, and average entropy of slow alarm whistles. Tukey's post hoc tests were conducted when appropriate. In these models, the type of threat and distance from the threat were considered as fixed factors, while the identity of the individuals nested within their groups was included as a random factor to account for repeated observations of the same rock cavy. We excluded sex as a fixed factor in these models because slow alarm calls were exclusively produced by female rock cavies under two specific conditions: in Area 1, when capuchin monkeys were nearby (≤ 25 m), and in Area 3, when dogs were detected at a greater distance (> 25 m), as indicated in Table 1. Residuals were visually inspected for normal distribution and homoscedasticity and were deemed adequate. Statistical analyses were conducted using the Minitab program (v. 22.2.0), with a significance level set at α < 0.05.
3 Results
3.1 Types of Alarm Calls
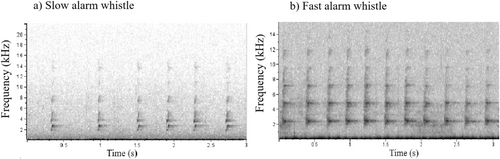
The rock cavy modulated its alarm call emissions when faced with threatening situations, producing both slow and fast alarm whistles (Figure 2; see Data S1 and S2). The most frequent call, the slow alarm whistle, was emitted 1940 times. In contrast, the fast alarm whistle occurred 197 times (details below).
A breakdown of the slow alarm whistle emissions revealed 333 occurrences in Area 1, attributed to two males, three females, and two individuals of unidentified sex (average: 47.6 calls per individual); 205 occurrences in Area 2, emitted by one male and two females, and one individual of unidentified sex (average: 51.3 calls per individual); 620 occurrences in Area 3, involving five males, five females, and three individuals of unidentified sex (average: 47.7 calls per individual); and 723 occurrences in Area 4, contributed by six males, five females, and one individual of unidentified sex (average: 60.3 calls per individual). Regarding the fast alarm whistle, an adult male in Area 3 was responsible for emitting this call 80 times, whereas in Area 4, two adult females emitted the call a total of 115 times, with an average of 57.5 calls per individual.
The fast alarm whistle call was exclusively emitted by rock cavies in Areas 3 and 4 in response to the threat of ocelots when the predator was nearby (≤ 25 m away), while slow alarm whistles were emitted by rock cavies across all sampling areas. However, while in Area 1 these calls were emitted in the presence of the tayra (Eira barbara) and yellow-breasted capuchin monkeys (Sapajus xanthosternos), in Area 4 they were emitted in the presence of birds of prey, dogs, humans, and ocelots. In Area 2, rock cavies emitted slow alarm whistles in the presence of marmosets (Callithrix spp.) and when these animals were nearby (≤ 25 m away). In Areas 3 and 4, the rock cavies emitted slow alarm whistles when threatened by ocelots that were far away (> 25 m away), while in Areas 2 and 4 the rock cavies emitted slow alarm whistles when threatened by birds of prey that were far away (> 25 m away) (Table 1).
The Discriminant Function Analysis (DFA), using the cross-validation test, promoted the accurate differentiation between the two types of alarm calls, confirming the reliability of the aural and visual classification. The analysis revealed that the slow alarm whistle was correctly classified in 1881 instances, with an accuracy rate of 97.1%, while the fast alarm whistle achieved an even higher classification accuracy of 99.0%, with 195 instances correctly identified.
3.2 Comparative Analysis of Acoustic Parameters in Slow and Fast Alarm Whistles
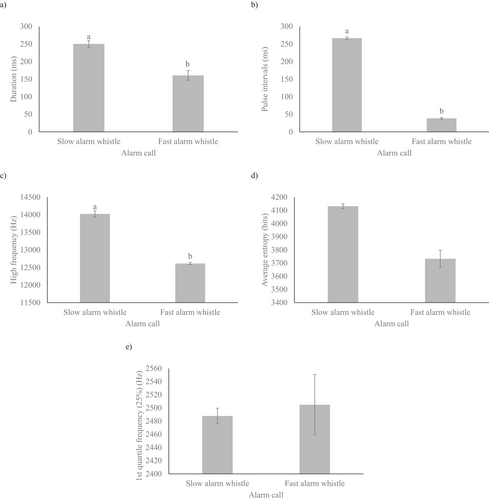
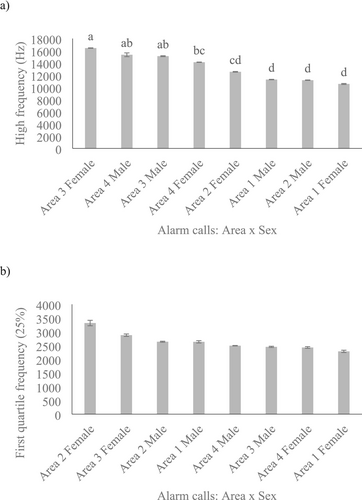
The GLMMs revealed significant differences between slow alarm whistles and fast alarm whistles in terms of duration (F1,22.59 = 14.93, p = 0.001, Figure 3a), pulse intervals (F1,21.29 = 6.08, p = 0.022, Figure 3b), and high frequency (F1,16.20 = 11.41, p = 0.004, Figure 3c). However, there were no significant differences in average entropy (F1,19.04 = 0.99, p = 0.333, Figure 3d) and 1st quartile frequency (25%) (F1,13.36 = 3.60, p = 0.080, Figure 3e) between the two types of alarm calls. The statistical model also showed significant interaction between study area and sex both for high frequency (F3,18.81 = 7.68, p = 0.001) and 1st quartile frequency (25%) (F3,17.45 = 3.54, p = 0.037). The post hoc tests revealed that, on average, both males and females in study areas 3 and 4 emitted higher-pitched alarm calls compared to those produced by males and females in study areas 1 and 2 (Figure 4a). In contrast, for the first quartile frequency (25%), the post hoc tests indicated no significant differences between the study areas and sex of the rock cavies (Figure 4b). (See Table S1 for the comprehensive description of the acoustic parameters of both slow and fast alarm whistle calls, including only correctly classified recordings).
3.3 Comparative Analysis of Slow Alarm Whistle Emissions
The results of the first General Linear Model (GLM) made to test our hypothesis that rock cavies would emit alarm calls more frequently when confronted with natural predators and imminent threats indicated that no significant differences were observed in the emission rates (calls/caller/h) of slow alarm whistles based on study area (F3,25 = 0.63, p = 0.602) (Figure 5a), sex of the rock cavies (F1,25 = 3.33, p = 0.080) (Figure 5b), distance from the threat (F1,25 = 0.04, p = 0.846) (Figure 5c), the interaction between the study area and sex of the rock cavies (F3,22 = 1.00, p = 0.410), or the interaction between the sex of the rock cavies and distance from the threat (F1,25 = 0.98, p = 0.331). The interactions involving study area and distance from the threat, as well as those between study area, sex of the rock cavies, and distance from the threat, could not be reliably estimated due to the presence of distinct threats in each area, as shown in Table 1. Consequently, these interactions were excluded from the model. The results of the second GLM indicated that there were no significant differences in the emission rates (calls per caller per hour) of slow alarm whistles based on the type of threat (F6,33 = 1.27, p = 0.297) (Figure 5d).
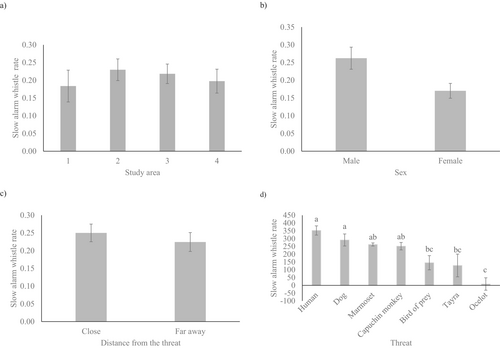
3.4 Differences in the Acoustic Parameters of Slow Alarm Whistles
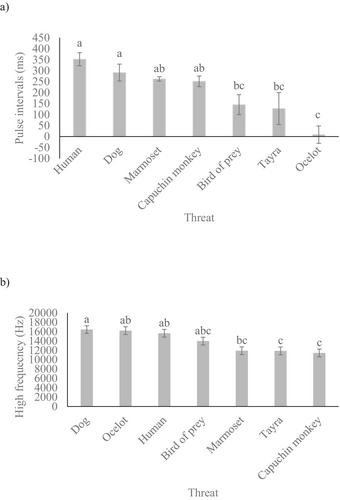
Using GLMMs used to compare the number of pulses, pulse intervals, duration, high frequency, and average entropy of slow alarm whistles, we found that the type of threat significantly influenced both the pulse intervals (F6,23.26 = 12.69, p < 0.001), and the high frequency (F6,18.15 = 12.08, p < 0.001) of the slow alarm whistles emitted by the rock cavies. The post hoc tests showed that, on average, rock cavies emitted slow alarm whistles with shorter pulse intervals when faced with threats from ocelots, birds of prey, or tayras than from humans and dogs (Figure 6a). However, the pulse intervals of the slow alarm whistles emitted by rock cavies when threatened by marmosets or capuchin monkeys remained consistent and did not differ from those observed when threatened by humans and dogs (Figure 6a). Additionally, on average, rock cavies emitted higher-pitched slow alarm whistles when faced with threats from dogs, ocelots, humans, or birds of prey than capuchin monkeys and tayras (Figure 6b). However, the high frequency in the slow alarm whistles emitted by rock cavies when threatened by ocelots, humans, or birds of prey remained consistent and did not differ from the high frequency emitted when threatened by dogs (Figure 6b). The type of threat did not influence the number of pulses (F6,21.73 = 1.39, p = 0.265), duration (F6,21.56 = 1.79, p = 0.149), or average entropy (F1,21.95 = 0.75, p = 0.614) of the slow alarm whistles.
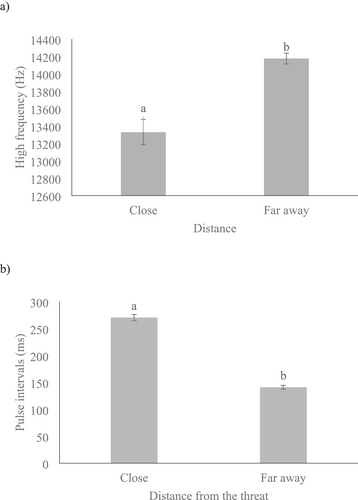
We also verified that the proximity of the threat significantly influenced the pulse intervals (F1,25.73 = 28.87, p < 0.001) of the slow alarm whistles emitted by the rock cavies. When the threat was closer (≤ 25 m away), the rock cavies emitted lower-pitched slow alarm whistles (Figure 7a) with shorter intervals between pulses (Figure 7b). Conversely, the type of threat did not affect the number of pulses (F1,26.34 = 0.92, p = 0.347), high frequency (F1,29.93 = 0.03, p = 0.864), duration (F1,17.00 = 3.68, p = 0.072), or average entropy (F1,19.36 = 0.255, p = 0.620) of the slow alarm whistles.
4 Discussion
Drawing from observations of other rodent species, we hypothesized that rock cavies modulate their alarm calls in response to threats. We anticipated that rock cavies would produce alarm calls more frequently, with lower pitch and entropy, shorter duration, more pulses, and shorter intervals between pulses. Such characteristics are likely to be more effective in alerting conspecifics to significant threats. Furthermore, we expected that closer proximity to threats would result in alarm calls with an increased number of pulses and shorter intervals between pulses, indicating a more immediate danger.
Our findings showed that rock cavies indeed modulate their alarm calls in response to threats, producing both slow and fast alarm whistles. Fast alarm whistles were emitted exclusively in response to nearby ocelots and exhibited a lower pitch, shorter duration, and shorter pulse intervals compared to slow alarm whistles. In contrast, slow alarm whistles were produced in response to distant ocelots as well as various other threats, including both distant and closer humans, dogs, marmosets, tayras, and birds of prey. The type of threat influenced the characteristics of slow alarm whistles. Additionally, shorter pulse intervals were observed when threatened by ocelots compared to humans and dogs. Furthermore, rock cavies produced lower-pitched slow alarm whistles with shorter pulse intervals when threats were nearby (≤ 25 m) compared to when threats were further away. The emission of slow alarm whistles with lower frequencies when predators are in close proximity may confer important adaptive advantages. Low-frequency sounds are generally more difficult for predators to localize, thereby reducing the likelihood of accurately detecting the source of the signal (Wiley and Richards 1978; Bradbury and Vehrencamp 2011). Additionally, lower-pitched vocalizations exhibit more efficient propagation in dense vegetative environments (Wiley and Richards 1978), which characterize the natural habitats of this species (Lacher 1981). This acoustic property may play a critical role in facilitating the rapid transmission of alarm signals to other group members in situations of imminent danger. In other social mammals, such as meerkats and marmots, variations in the frequency and tempo of alarm calls have been linked to the urgency of the threat and the predator's proximity, serving as a mechanism to modulate antipredator responses (Blumstein and Armitage 1997; Manser 2001). For rock cavies, this vocal plasticity may function as an adaptive antipredator strategy, particularly against predators such as the ocelot, whose auditory perception may be attuned to spatially distinct acoustic cues. Conversely, slow alarm whistles exhibited higher-pitched frequencies in areas 3 and 4, which are situated closer to urban centers and consequently experience greater exposure to anthropogenic disturbances. A similar pattern was documented by Almeida et al. 2025, who reported elevated alarm call frequencies in rock cavies inhabiting environments with increased human influence. These findings support the hypothesis that rock cavies modulate their alarm communication in response to both the level of threat and the ecological characteristics of their surroundings.
Contrary to our expectations, the emission rate of slow alarm whistles in rock cavies did not vary based on the type or proximity of the threat. Moreover, the similarity in the number of pulses and pulse intervals of slow alarm whistles emitted in response to marmosets and yellow-breasted capuchin monkeys, compared to those emitted when threatened by predators, may be attributed to competition for fruit or the noise generated by these nonhuman primates as they move through the Caatinga. Zuberbühler et al. (1999) reported similar findings in a study on guenons (Cercopithecus spp.), demonstrating that alarm calls were recorded not only in response to predators but also in ambiguous contexts or in reaction to non-predatory species. These instances were particularly notable when these species posed competitive challenges or generated intense environmental noise. These results suggest that, beyond predation, other forms of ecological disturbance may influence alarm call production in social species, highlighting the necessity of incorporating multiple environmental contexts into studies on antipredator communication. Given these insights, further research is warranted to deepen our understanding of the mechanisms underlying alarm signaling in varying ecological conditions.
Previously, the acoustic repertoire of this species was limited to the description of the alarm whistle call (Lacher 1981; Monticelli and Alencar-Junior 2021). Here, we describe the fast alarm whistle as a modulation of the rock cavy alarm call, adjusted in response to the type and proximity of the threat. While this call is structurally similar to the slow alarm whistle (Figure 2), they differ significantly in terms of high frequency, duration, and pulse intervals. In our records, the fast alarm whistle was emitted only in the presence and proximity of ocelots, possibly the predator with the greatest potential lethality for the rock cavy in this study area. Thus, we believe that the fast alarm whistle can be an exclusive call in the context of a high risk for the survival of rock cavies in the wild. This has also been observed in other rodents that emit specific alarm calls denoting more dangerous contexts (Otospermophilus beecheyi, Owings and Virginia 1978; Marmota flaviventris, Blumstein and Armitage 1997; Atlantoxerus getulus, Marel et al. 2019).
Referential alarm calls serve to alert conspecifics about different types or categories of predators (aerial or terrestrial) and have been extensively documented in mammals (Macedonia and Evans 1993; Furrer and Manser 2009; Zuberbuhler 2000; Chebey, 2015) and birds (Bugnyar et al. 2001; Templeton et al. 2005; Marino 2017). However, the variations we observed in the rock cavies' alarm whistles were primarily influenced by the proximity of the threat to the sender (Figure 7a,b), revealing their behavioral plasticity in relation to the type of risk. This result is consistent with findings for yellow-bellied marmots (Marmota flaviventris), which also warn of greater dangers with faster alarm calls (more units per time interval) (Blumtein, 2007). Thus, we posit that the rock cavy modulates its alarm whistle to communicate the urgency of the response needed, effectively differentiating between varying degrees of threat to its survival. This result is compatible with that shown for other rodent species (e.g., Spermophilus variegatus, Krenz 1977; Marmota caudata, Blumstein 1995; M. marmota, Blumstein and Arnold 1995; Urocitellus richardsonii, Sloan et al. 2005; U. columbianus, Harris et al. 2010), which use alarm calls to inform of threats of a greater or lesser degree without distinguishing the type of predator.
Animals that live in complex habitats, such as semiarboreal species that live in open forests, need information about the type of predator to favor escape success (Macedonia and Evans 1993; Price et al. 2015), as they are hunted by different predators that use different hunting strategies (Greene and Meagher 1998; Zuberbuhler 2000). On the other hand, for species that live in more enclosed environments and use burrows to protect themselves from predation, it is usual for them to report the urgency or degree of threat (Blumstein and Armitage 1997; Mcrae 2020). Thus, the variations we recorded in the alarm whistle of the rock cavy are compatible with the lifestyle of this rodent that lives in the rocky outcrops of the Brazilian caatinga (Dias et al. 2018), where it uses rock crevices for shelter and protection (Lacher 1981). However, being herbivorous and making use of the arboreal space to obtain food (Lacher 1981; Willig and Lacher 1991), the rock cavy may occasionally experience environments that demand referential alarm calls. From this perspective, we documented fast alarm whistles emitted exclusively in the presence and proximity of ocelots. However, given the observational nature of our study, we recommend conducting experimental tests (e.g., Blumstein 2007; Furrer and Manser 2009; Mcrae 2020) to further investigate potential referential signaling in rock cavies' alarm calls.
In our records and according to the literature (Lacher 1981; Monticelli and Alencar-Junior 2021), the alarm whistle was emitted exclusively by adults. Therefore, there is strong evidence that this call is exclusively emitted by individuals in this age group. In addition, the alarm whistle was emitted in the presence of primates (Table 1) that apparently posed no risk to the rock cavies' survival. Lacher (1981) also recorded this call in nonthreatening contexts, suggesting that it may convey complex information beyond just warning of danger. Furthermore, in other studies (Alencar-Junior 2011; Almeida et al. 2025), the alarm whistle was observed in agonistic contexts involving disputes between conspecifics. Thus, to better describe the use of this vocalization by rock cavies, we believe it is necessary to investigate this call from the perspective of experimental call function tests (e.g., Nogueira et al. 2012; Dos Santos et al. 2014).
5 Conclusion
This study provides compelling evidence that the presence of natural predators and the proximity of threats significantly influence the alarm whistles of rock cavies. The species emits alarm whistles with shorter pulse intervals when faced with immediate danger. Moreover, faster alarm whistles are produced exclusively in the proximity of predators with high hunting potential. These findings reveal that the distance and type of threat lead to adjustments in the alarm calls of rock cavies, underscoring their sophisticated communication system. Consequently, we conclude that rock cavies signal greater dangers by emitting lower-pitched and faster calls with shorter pulse intervals, highlighting the adaptive significance of their vocal responses in predator evasion.
Author Contributions
Wesley N. Almeida: investigation, writing – original draft, methodology, writing – review and editing, data curation, and formal analysis. Sérgio L.G. Nogueira-Filho: methodology, data curation, formal analysis, and writing – review and editing. Kamila S. Barros: methodology and writing – review and editing. Selene S.C. Nogueira: conceptualization, methodology, writing – review and editing, project administration, supervision, resources, and funding acquisition.
Acknowledgements
We thank park rangers João C. Santos, Edvan F. Pereira, and Antônio Marcos S. Brito for all their support in the field. We also thank the agencies that supported us, Coordination for the Improvement of Higher Education Personnel (CAPES—Finance Code 001, W.N.A. Grant, Process #88882.451299/2019-01 #8888485144/2020-00); and National Institute of Science and Technology in Interdisciplinary and Transdisciplinary Studies in Ecology and Evolution (IN-TREE—Process CNPq #465767/2014-1 and CAPES #23038.000776/2017-54), Bahia, Brazil; S.S.C.N. and S.L.G.N.F. received a grant from the Council for Scientific and Technological Development (CNPq) (Processes #303448/2019-9 and #04226/2019-0, respectively). The Article Processing Charge for the publication of this research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) (ROR identifier: 00x0ma614).
Ethics Statement
This study was approved by the Chico Mendes Institute for Biodiversity Conservation (ICMBio) (#80143-1), the Institute for the Environment and Water Resources (INEMA) (#24.230 #26.010), and the Ethics Committee for the Use of Animals in Experimentation at the State University of Santa Cruz (CEUA-UESC) (#021/21).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data for this article are available via: https://doi.org/10.6084/m9.figshare.29452655.




