Strategic Deployment of Secondary Defences in the Springbok Mantis (Miomantis Caffra)
Funding: This work was partially funded through a Marie Skłodowska-Curie Postdoctoral Research Fellowship to NWB (Project ID: SCISM 101060650).
ABSTRACT
Deimatic displays are visually conspicuous behaviours designed to prevent predation by scaring off predators. Animals that exhibit deimatic behaviours often show other secondary defences as well, but the factors that influence which defences are expressed are poorly understood. Because prey are expected to deploy multiple defences strategically, conspicuous deimatic displays could be performed later in the predation sequence after primary or other secondary defences have failed. Their expression could additionally depend on the state of the performer, especially if deimatic behaviours are costly to produce or involved in a functional trade-off. Here, we investigate female defensive responses to escalating attacks involving non-tactile and tactile stimuli by a human model predator using the springbok mantis, Miomantis caffra. We found that all defensive behaviours were expressed only after mantises were physically provoked, indicating a counter defence rather than a pre-emptive one. Most females responded to initial tactile contact with non-deimatic behaviours—most commonly fleeing, but also striking with raptorial forelegs and freezing. When predator attacks escalated and tactile contact increased, females mostly produced deimatic behaviours, including raising forelegs, flaring wings, exposing jaws and/or swaying from side to side. Females that were heavier for their size and therefore closer to oviposition were also less likely to flee and more likely to strike and display, although this pattern depended on the level of predator threat. Our results suggest that the expression of deimatic displays is prompted by the repeated failure of non-deimatic defences to ward off escalating predator attacks, which may be why deimatism has gone unnoticed in M. caffra until now. Our findings also suggest an important role for fight-or-flight trade-offs in the expression of antipredator behaviours in this species: heavier egg-loads appear to compromise females' ability to flee, triggering more aggressive and deimatic responses instead.
1 Introduction
Avoiding predation is key to survival (Creel and Christianson 2008). The evolutionary drive to avoid being eaten has generated a dazzling diversity of antipredator tactics, including mechanical, visual, chemical and behavioural defences (Caro 2005; Ruxton et al. 2019). But prey often use multiple defensive traits that together comprise a ‘defence portfolio’—a subset of available defences deployed at the prey's discretion during a predator–prey interaction (Britton et al. 2007; Kikuchi et al. 2023). However, despite the expectation that defence portfolios should be widespread, the factors that influence their evolution and deployment are poorly understood (Kikuchi et al. 2023).
Multiple defences could evolve if each phase of the predation sequence (i.e., detection, identification, approach, subjugation, consumption) selects for different defences (Endler 1991; Ruxton et al. 2019). There is some support for this idea since primary defences like crypsis, masquerade, and aposematism tend to prevent predation by interfering with the ability of predators to detect, identify or approach prey, whereas secondary defences like spines, toxic sprays or fleeing typically become useful only when an approaching predator is attempting to subjugate or consume their prey (Ruxton et al. 2019). A striking example of a secondary behavioural defence is deimatic displays—also known as startle displays—which are conspicuous, suddenly produced but sustained behaviours triggered by the perception of an approaching or subjugating predator, designed to dissuade further pursuit of the displaying individual (Umbers et al. 2017). While many examples of deimatic behaviour involve the production of visual displays comprising hidden colours (Kang et al. 2017), mimicking eyespots (Edmunds 1972, 1976) and/or startling movements (Vidal-García et al. 2020), other behavioural modes are also known, including the production of defensive sounds (Edmunds 1972). As secondary defences, deimatic behaviours are expected to be deployed after prey have already been detected by a predator either as a pre-emptive defence during predator approach or as a counter defence during attempted subjugation (Drinkwater et al. 2022). Although much research has focused on the expression of deimatic displays, prey typically have more secondary defensive behaviours at their disposal than just deimatism (Drinkwater et al. 2022; Kikuchi et al. 2023). For example, prey can interrupt the approach of predators and prevent subjugation by fleeing or freezing (Eilam 2005). More aggressive responses can also be deployed to interfere with a predator's ability to subjugate or consume their victim, such as retaliatory blows, kicks or bites (Mukherjee and Heithaus 2013). When multiple secondary defences are available—whether deimatic or non-deimatic—prey face an important decision: which defences should they utilise, and when? Understanding the factors that shape this decision is crucial for explaining how multiple defences evolve (Kikuchi et al. 2023).
How a prey deploys its multiple secondary defences should be a function of the relative costs and benefits of those defences (Kikuchi et al. 2023). Prey might express different defensive behaviours at different phases of the predation sequence to optimally match the level of risk or need (Nonacs and Blumstein 2010; Kang et al. 2017). For example, if the threat of death by predation increases with each escalating phase of the predation sequence, then prey might engage their least costly defences in earlier phases when the risk is lowest and save their most costly defences for the most dangerous later phases. Differential costs could also determine who expresses which defences: higher condition individuals might be more likely to perform expensive defences because they have sufficient resources to do so (e.g., Nazareth et al. 2016). But when should multiple defences be deployed simultaneously? Prey might engage two or more defences at the same time if the protection they provide in combination is greater than their benefit individually (Penney et al. 2014; Kikuchi et al. 2023). But simultaneous deployment can be constrained if defences are involved in a functional trade-off (Kikuchi et al. 2023). For example, if the capacity to flee from a predator is hampered by a prey's size or weight, then larger or heavier individuals—such as gravid females close to oviposition or live birth—might be forced to rely on more stationary, hostile or conspicuous defences instead (Stankowich 2009; De Bona et al. 2020). The extent to which relationships between defences within a portfolio are synergistic versus antagonistic is not well understood. However, exploring whether trade-offs affect the expression of anti-predator defences in different contexts should help to enhance understanding of the strategic deployment of multiple defences.
Praying mantises are a group of insects that use crypsis or masquerade as a primary defence against predators (de Alcantara Viana et al. 2023). But they are also well-known for their secondary defences, which can include deimatic behaviours such as conspicuous displaying of weapons, flaring of wings or forelegs to reveal hidden colours or eyespots, exposure of jaws and even the production of clicking sounds (reviewed in Vidal-García et al. 2020). Secondary defences can also include a range of non-deimatic behaviours, including retaliatory behaviours like striking with forelegs and biting, and more avoidant behaviours like fleeing and thanatosis (Edmunds 1972; O'Hanlon et al. 2018). The presence of multiple defences makes mantises excellent subjects for investigating the potential for functional trade-offs to underlie optimal defence deployment. However, defence expression is also likely to be costly and could therefore be condition dependent. It may be possible to distinguish between functional trade-offs and condition-dependent defence portfolios by assessing which deimatic and non-deimatic behaviours are and are not deployed together and what effect gravidity or relative body weight has on these patterns of expression.
The springbok mantis, Miomantis caffra, is native to South Africa but was introduced accidentally to New Zealand around 50 years ago and is now naturalised there (Ramsay 1984), with invasive populations more recently observed in other parts of the world (Marabuto 2014; Connors et al. 2022). Females are highly fecund, with some individuals investing more than half their body weight in the production of a single ootheca (personal observation). Because body weight increases through time as females get closer to ovipositing, body condition and gravidity covary considerably with age (personal observation). Similar to other mantises, this species' primary defence against predators is crypsis, which it achieves through plastic background colour matching (Burke and Holwell 2024). Nothing is known about its secondary defences. However, females are extremely aggressive towards males and regularly attack and cannibalise them without mating (Burke and Holwell 2021). Whether similar aggression is deployed against predators is unknown. Deimatic displays have not been reported for M. caffra before but are suspected based on their presence in closely related species (see Vidal-García et al. 2020).
To investigate the contexts in which deimatic and non-deimatic anti-predator behaviours are expressed in M. caffra, we ran a series of trials of escalating predator attacks on female individuals using a human model predator. Attacks were performed in sequential order (i.e., approach with no contact, followed by initial physical contact, then repeated physical contact) to simulate a naturalistic predation sequence. We predicted that anti-predator behaviours—whether deimatic or non-deimatic—would be deployed only when the primary defence of camouflage fails (i.e., when the model predator makes physically contact with the mantis at the approach phase). Given that deimatic displays have not been reported in this species before, we additionally predicted that anti-predator responses would involve mostly non-deimatic behaviours like fleeing, freezing or retaliating and if deimatic behaviours are present at all, they would be deployed in response to severe threats as a last resort. Finally, we anticipated that female responses might be involved in a fight-or-flight trade-off, such that females that weigh more for their body size might be more likely to engage in dangerous retaliatory behaviours (like striking) and conspicuous deimatic behaviours (like wing flaring and foreleg raising) and less likely to avoid attacks by fleeing. Alternatively, the absence of any fight-or-flight trade-off but greater deimatism in heavier-per-unit-size females would suggest condition-dependent deployment of deimatic defences.
2 Methods
The M. caffra females used in this study were F2 individuals obtained from laboratory stocks originally established from oothecae collected from public parks and private gardens in Auckland, New Zealand. Mantises were raised in the laboratory at 24°C and 60% humidity in upturned plastic cups that increased in size as the mantises developed (1st to 3rd instar: 50 mL cup; 3rd instar to adulthood: 400 mL cup). To facilitate easy perching, small cups were lightly abraded on the inside and had a hole in the ceiling stuffed with cottonwool, whereas large cups had a ceiling of fine mesh and a strip of the same material attached to the wall. Feeding occurred twice a week with size-appropriate prey: 3–5 fruit flies (Drosophila sp.) during the first two to three instars, 1–2 green bottle flies (Lucilia sp.) during the 3rd to 5th instars, and 1–2 blowflies (Calliphora sp.) during the final instars. Throughout adulthood, mantises were fed 2–3 blowflies. Cups were watered at feeding time for mantises to drink.
To examine anti-predator responses, we conducted a series of behavioural trials in which 138 M. caffra females obtained from 87 oothecae of unknown parentage were each exposed to a sequence of predator attacks of increasing threat intensity that mimicked a real-life predation sequence. Because birds are significant predators of mantises, we designed the trials to imitate an avian predation sequence. For standardisation, we started each of the four trials with females standing upright on the laboratory bench facing away from the experimenter, with the lid of a 5-cm diameter plastic petri dish placed over the top of the females for ~10 s to hold them in position. Each trial began by removing the petri dish and performing the predator threats, as described below. The first trial (trial 1) involved slowly moving a pair of pointed metal tweezers towards the abdomen of females from above without any resulting physical contact, stopping approximately 1 cm away. This trial represented the lowest threat level, equivalent to a looming or approaching avian predator. The second trial (trial 2), which involved a single tap of the tweezers on the dorsal side of the abdomen, aimed to replicate a scenario where an avian predator makes initial physical contact after identifying its victim. The third trial (trial 3) involved repeatedly tapping the abdomen with tweezers for a period of 10 s and was intended to represent an avian predator attempting to subjugate its prey. The fourth trial (trial 4) was a repeat of the third trial and represented an escalation of attempted subjugation. To approximate a real-life predation sequence, we performed the trials in sequential order, waiting approximately 10 s between each one. Thus, the trials represented an increase in cumulative threat with each progressive phase of the predation sequence, simulating how predation by birds and other predators occurs in real-life conditions (Endler 1991). Trials were performed on a single afternoon under artificial white light and under the same constant temperature and humidity that the mantises were raised (see above).
During the trials, we recorded the presence/absence of several non-deimatic and deimatic defensive behaviours. The non-deimatic behaviours were: (1) striking (i.e., rapidly attacking the tweezers with raptorial forelegs), (2) fleeing (i.e., walking or running away from the tweezers), and (3) freezing (i.e., remaining completely still, either upright or up-side-down). Based on previous observations in mantises (Vidal-García et al. 2020), the deimatic behaviours we identified and recorded were: (1) raising forelegs (i.e., facing towards the tweezers and stretching forelegs out sideways to reveal the pointed claws at the ends of the tibia), (2) flaring wings (i.e., raising both pairs of wings for a sustained duration), (3) swaying (i.e., rocking from side to side), and (4) opening the mouth (i.e., extending the mouth parts to expose the black jaws). We considered a response to be deimatic if at least one of the above-listed display behaviours was performed. While adult females were our primary focus, we also conducted a small pilot study on 11 males using the same methodology described above for trials 3 and 4 to get a sense of the similarities and differences in behaviour between the sexes (for detailed methods of the male trials, see Supporting Information—S1). However, because of the small number of males used, male behaviour was not formally analysed. Also, since the experimental design of the male trials was modified compared to that of the female trials, formal analysis of sex differences was not possible.
To assess the effect of threat escalation on anti-predator responses, we used a generalised linear mixed effects model (GLMM) with a binomial error structure to analyse the presence/absence of deimatism, such that the presence of a deimatic behaviour was coded as 1, and the absence of any deimatic behaviour was coded as 0. Trial number (i.e., trials 2, 3 and 4) was fitted as the categorical fixed effect to test whether females changed their behaviour as the threat escalated. Trial 1 was not included as no behaviours occurred in this treatment (see Figures 1 and 2). To account for the potential effect of gravidity on behavioural responses, we included a metric for gravidity as a covariate in the model. This metric was calculated as the residuals of the linear regression of the natural logarithm of body weight in g versus the natural logarithm of prothorax length in mm (a proxy for body size)—a calculation commonly used for scoring body condition (Jakob et al. 1996). Females also varied in age, but since age was very strongly positively correlated with gravidity (Pearson's product–moment correlation: = 0.44, 95% CI = 0.30 to 0.57, < 0.0001), we included only the latter variable as a covariate, which was scaled and centred to a mean of 0 and standard deviation of 1. To account for the possibility that behavioural changes across the phases of the predation sequence were dependent on gravidity, we allowed the gravidity term to interact with the fixed effect of trial number. Female identity was included as a random effect to account for the fact that the behavioural responses of each female was measured four times. Since a number of females originated from the same oothecae and were therefore sisters, we also included family identity (i.e., ootheca identity) as an additional random effect.
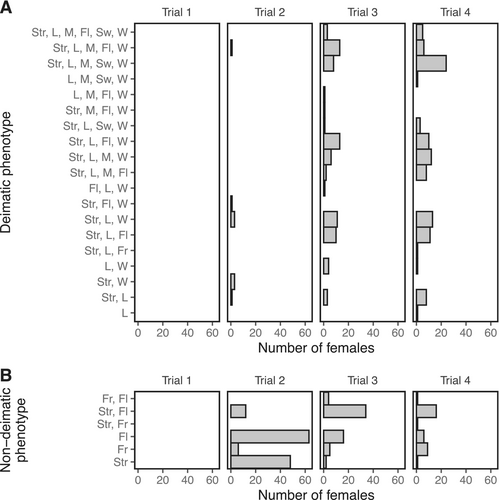
We also analysed the presence/absence of each deimatic and non-deimatic behaviour in separate binomial GLMMs. The binary response variables were striking, fleeing, raising forelegs, and flaring wings. Variation in freezing, swaying, and mouth opening was unable to be modelled because the incidence of these behaviours was too low for models to converge. The low incidence of raising forelegs and flaring wings in trial 2 meant that only trials 3 and 4 could be compared for these behaviours. In all models, the categorical predictor was trial number, which was allowed to interact with gravidity (which was scaled and centred), with female identity and family identity as the random effects.
All models were fitted using the lme4 package (Bates et al. 2015) in R version 4.4.1 (R Core Team 2024). A bobyqa optimiser was used to improve model convergence where necessary. We used a model reduction approach to obtain maximally reduced models containing only significant effects. The significance of each term was assessed by sequentially removing the most nonsignificant terms one at a time, starting with the interaction effect, and comparing models with and without these terms using likelihood ratio tests (LRTs). Variables involved in significant interactions were always retained. Trial 2 was fixed as the reference level, but for models where trial 2 had to be excluded, trial 3 was the reference level instead. Using the emmeans package (Lenth 2024), we conducted pairwise comparisons when trial number was significant, and compared the marginal means of the linear trends when trial number and gravidity interacted significantly. We report the beta coefficients () and standard errors () for all significant effects from maximally reduced models, Chi-square statistics () and p-values () for all LRTs, and estimates (), standard errors (), z-ratios (), and p-values () for pairwise linear trend comparisons. Random effect variances and standard deviations are reported in Table S1. The unit of replication was female individual.
3 Results
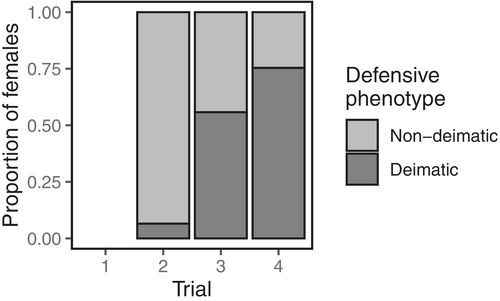
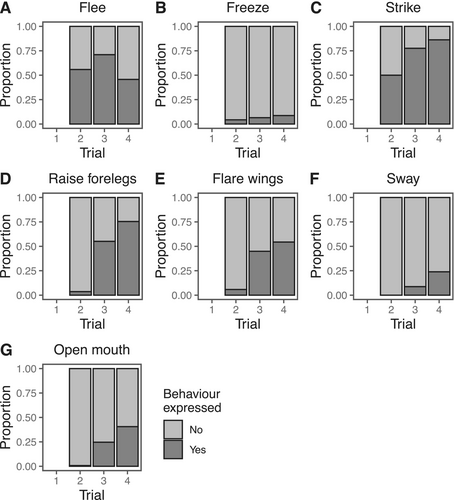
Females performed a range of defensive behaviours in response to physical contact (see trials 2, 3 and 4 in Figures 1 and 2), but showed no reaction at all when approached without contact (see trial 1 in Figures 1 and 2). Deimatic displays were mostly absent from trial 2 when physical contact with the predator was short and brief (deimatism presence: trial 2: 9/138 = 7%; Figures 1 and 2). Defences in this context were mostly non-deimatic, including striking (69/138 = 50%), freezing (6/138 = 4%), and fleeing (77/138 = 56%) (see Figure 3). However, as the level of predation threat increased with increasing trial number, females were more likely to respond with deimatic displays (deimatism presence: trial 3: 77/138 = 56%, trial 4: 104/138 = 75%; GLMM: trial 3: = 4.81, = 0.68; trial 4: = 6.43, = 0.95; LRT: = 193.30, < 0.0001; Figures 1, 2 and 4; Video S1), including behaviours such as raising forelegs (trial 2: 5/138 = 4%, trial 3: 76/138 = 55%, trial 4: 104/138 = 75%), flaring wings (trial 2: 8/138 = 6%, trial 3: 62/138 = 45%, trial 4: 75/138 = 54%), mouth opening (trial 2: 1/138 = 7%, trial 3: 34/138 = 25%, trial 4: 56/138 = 41%), and/or body swaying (trial 2: 0/138 = 0%, trial 3: 12/138 = 9%, trial 4: 33/138 = 24%) (see Figure 3). These deimatic behaviours were also prolonged in their duration, although the exact length of time was not recorded.

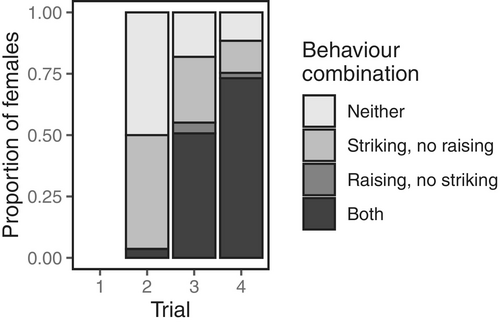
No stridulating sounds, eyespots or bright colours accompanied the deimatic displays. But raised forelegs and striking occurred together very frequently during the subjugation phase (Figure 5), with females often observed alternating between the two behaviours repeatedly. Pairwise comparisons indicated that many more females responded with a deimatic display in the third and fourth trials compared to the second trial (trial 2—trial 3: = − 4.81, = 0.77, = −6.27, < 0.0001; trial 2—trial 4: = − 6.43, = 0.95, = −6.76, < 0.0001), and in the fourth trial compared to the third (trial 3—trial 4: = − 1.63, = 0.42, = −3.92, = 0.0003). However, deimatic behaviours were most often performed in conjunction with non-deimatic behaviours—especially striking—and in a range of different behavioural combinations (Figure 1). Interestingly, we also found that deimatic displays were strongly correlated with gravidity: females that were more gravid and closer to oviposition were universally more likely to perform deimatic behaviours, regardless of the threat level (GLMM: = 0.68, = 0.28; LRT: = 6.79, = 0.009; Figure 6).
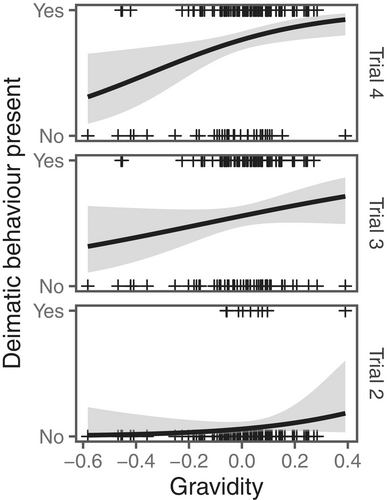
Assessing each deimatic and non-deimatic behaviour separately revealed that trial number or female gravidity (or both) affected the expression of most behaviours. Of the non-deimatic behaviours representative of the fight-flight dichotomy, fleeing and striking were both influenced by the interaction between trial number and gravidity, but in opposite directions (fleeing: trial 3 x gravidity: = 1.18, = 0.36; trial 4 × gravidity: = 0.79, = 0.35; LRT: = 12.85, = 0.002; striking: trial 3 × gravidity: = −1.11, = 0.41; trial 4 × gravidity: = −0.65, = 0.43; LRT: = 8.94, = 0.01). Post hoc tests indicated a stronger negative relationship between fleeing and gravidity in trial 2 than trial 3 (trial 2—trial 3: = − 7.62, = 2.32, = −3.29, = 0.003) and a stronger positive relationship between striking and gravidity in trial 2 than trial 3 (trial 2—trial 3: = 7.12, = 2.63, = 2.71, = 0.02). Of the deimatic behaviours, raising forelegs and flaring wings both increased from trial 3 to trial 4 (raising forelegs: = 1.96, = 0.50; LRT: = 23.83, < 0.0001; flaring wings: = 1.05, = 0.32; LRT: = 6.70, = 0.01; Figure 3), with more gravid females more likely to raise their forelegs ( = 0.83, = 0.38; LRT: = 6.04, = 0.01).
In our pilot study, males responded to escalated attacks with the same non-deimatic defensive behaviours as females, but with additional male-specific behaviours (for more detailed male results, see Supporting Information–S2). Males showed no behaviours that were discernibly deimatic (see Figure S1).
4 Discussion
Females produced defensive responses only after physical contact with the predator, indicative of a strategy of counter defence. Initial contact induced mostly non-deimatic secondary defences, with fleeing being the most common, whereas prolonged and repeated contact mostly triggered deimatic behaviours together with striking, suggesting that M. caffra females deploy deimatism and aggression synergistically to counter subjugation attempts after initial non-deimatic defences—especially fleeing—fail. We additionally found strong evidence that behavioural expression was dependent on female gravidity since deimatism was universally more likely among more gravid females. But we also found clear evidence of a fight–flight trade-off: striking was more likely and fleeing less likely during initial contact compared to initial subjugation among more gravid females, suggesting that defensive behaviours that help females to stand their ground are used preferentially when mobility is constrained by heavy body weight. Our results suggest that both deimatic and non-deimatic antipredator behaviours are deployed strategically to match both the level of the threat and the ability of individuals to flee.
Prey are expected to utilise deimatic displays either to pre-emptively ward off approaching predators or to prevent further escalation of attempted subjugation (Drinkwater et al. 2022). Our results unequivocally support the latter prediction, since M. caffra females produced deimatic behaviours only after physical contact. This conforms with the expectation that prey should be unlikely to engage in conspicuous displays during the approach phase of the predation sequence if crypsis is already an effective primary defence (Drinkwater et al. 2022). Similar patterns of postattack deimatism have been observed in other cryptic prey (Umbers and Mappes 2015; O'Hanlon et al. 2018). While a single poke with a paintbrush appears to be sufficient to elicit deimatic behaviour in females of three other mantis species (O'Hanlon et al. 2018), our results suggest that M. caffra females are much more tolerant of initial contact and require a greater degree of threat escalation to induce their deimatic display. Indeed, the more we escalated the simulated subjugation, the more females engaged in deimatism. Other prey are known to respond similarly by increasing their display intensity in response to escalation (Brown et al. 2007; O'Hanlon et al. 2018), while others reduce it (Glaudas et al. 2006), suggesting that deimatic defence deployment is likely to be dependent on the specific predation pressures experienced by a species.
Resource investment and functional trade-offs are increasingly recognised as important factors in the evolution and deployment of antipredator defence portfolios (Poitrineau et al. 2003; Britton et al. 2007; Cressler et al. 2010; Wang et al. 2019; Kikuchi et al. 2023). Our observation that gravidity mediates the expression of both deimatic and non-deimatic behaviours provides a new empirical example of how functional trade-offs can underlie defence expression. Although a recent meta-analysis suggests that body size is not a significant predictor of the presence of deimatism in mantises at the species level (Vidal-García et al. 2020), our results suggest that the ratio of body weight to size (i.e., the level of gravidity) greatly influences how females deploy their secondary defences at the individual level. Our finding that gravidity was positively associated with the presence of deimatism could be interpreted as evidence for deimatic behaviours being more costly than non-deimatic ones, since our gravidity metric is also a score of condition, and heavier females per unit body size might have more resources to invest and therefore be better positioned to break crypsis sooner with conspicuous deimatism. However, we additionally found that gravidity was more negatively correlated with fleeing but more positively correlated with striking in trial 2 (initial contact) compared to trial 3 (first escalation/subjugation). This contrasting effect, in combination with the general greater likelihood of deimatism in more gravid females, provides strong evidence for a functional trade-off between fleeing and standing ground mediated by body weight, such that heavier females that are closer to oviposition are forced to fight back and implement deimatic displays because their heaviness handicaps their ability to escape. Suggestive evidence of a similar fight-or-flight trade-off has been reported for the deimatic displaying mountain katydid (De Bona et al. 2020). Numerous mammals also show fight-or-flight trade-offs (Carrier 2016). An alternative explanation of our results could be that heavier females are visually more conspicuous and easier for predators to detect because of their engorged abdomens, as has been suggested for larger-bodied individuals in other insect systems (e.g., Remmel and Tammaru 2009), and adopting a more aggressive strategy under these conditions might offer greater protection. A similar pattern has been observed in a polymorphic poison-dart frog where males that avoid predation through conspicuous aposematism show higher levels of aggression than males that rely on cryptic colouration (Rudh et al. 2013). Whether M. caffra females become more conspicuous to predators with increasing weight is yet to be determined, however.
Either way, the fact that deimatic behaviours were almost always performed with retaliatory strikes and not consistently as an exclusive set of display behaviours indicates that deimatism in this species does not have a purely stereotyped presentation. Rather, both deimatic and non-deimatic behaviours appear to be used dynamically and synergistically in a ‘pick-and-choose’ manner that reflects the nature of the threat and the state of the individual. Such behavioural flexibility could help prey avoid different types of predators and/or attacks in different settings or habitats (Kikuchi et al. 2023). But our results additionally suggest that plastic switching between multiple defences may be an effective strategy for victims in varying states of gravidity. Defensive behaviours may need to be versatile and flexible so that prey can adaptively respond to the full breadth of ecological conditions and physiological states experienced in nature.
Deimatic displays in mantises are well-known to incorporate elements of mimicry. Some species have eyespots on their wings or forelegs that mimic the eyes of larger animals and which only become apparent to predators once the startle display is performed (reviewed in Vidal-García et al. 2020). Other mantises produce clicking sounds that are hypothesized to mimic extant or extinct predators to scare off approaching threats (reviewed in Vidal-García et al. 2020). Given that M. caffra females do not exhibit hidden eyespots suggests that they do not incorporate a component of mimicry in their deimatism. Instead, their foreleg raising and wing flaring are likely to startle predators by exaggerating the displaying individual's size and threat level. In this vein, female foreleg raising may function to draw attention to the intimidating weaponry of the raptorial legs. The fact that foreleg raising and striking often co-occurred in the same trial in repeated alternation suggests that females might display their weapons to threaten predators and then use them in retaliation if their threat is ignored. Whether foreleg raising and striking provide greater protection from predators when deployed together than separately is yet to be determined, but the strength of their correlated expression suggests that the co-occurrence of these behaviours is likely synergistic and adaptive.
Empirical studies of antipredator behaviour commonly use human models as the predatory stimulus (Umbers and Mappes 2015; Kang et al. 2017; O'Hanlon et al. 2018; Troscianko et al. 2018; Vidal-García et al. 2020). Similar to previous studies (e.g., Kang et al. 2017; Mourmourakis et al. 2022), our approach was to simulate consecutive phases of the predation sequence by manipulating the number of times mantises were tapped on the abdomen. Other researchers have used different stimuli, including pinching and blowing air onto prey (Adamo and McKee 2017; O'Hanlon et al. 2018). Whether such artificial threats are representative of the kind of stimuli that prey experience in interactions with real-life predators is unclear. If prey responses to human models are weaker or stronger than those elicited by natural predators, then assessments of antipredator behaviour may be systematically biased. Using real-life predators—but especially ecologically relevant ones—would allow for more accurate assessments. An alternative to the idea that multiple defences are selected for different phases of the predation sequence is the hypothesis that multiple behaviours are selected for different predators (Kikuchi et al. 2023). Testing both hypotheses simultaneously would be possible in field experiments that use multiple real-life predators. Such an experimental approach could also help to elucidate the fitness value of antipredator behaviours, which is crucial for understanding the effectiveness of defence portfolios (Kikuchi et al. 2023).
The experimental nature of our study meant that the full range of ecological conditions and individual states that this species experiences in the wild could not be fully captured. In nature, M. caffra inhabits a variety of substrates—from bushy shrubs to tree trunks—but the extent to which antipredator behaviours are influenced by such habitat heterogeneity is unknown. Moreover, the mantises in our study were obtained from laboratory populations originating from the species' introduced range in New Zealand. Since introduced populations can diverge from native populations due to different selection pressures (e.g., Pintor and Sih 2009), additional assessment of mantises from the native range of South Africa would be required to ascertain whether the patterns in behaviour we report here are representative of the species as a whole. Although we did not formally assess sex differences in defensive behaviour, our pilot study on a small number of males found both similar and different non-deimatic behaviours in response to exaggerated threats compared to females, but no deimatic displays at all, indicating that males and females may be selected to avoid predators in different ways, as has been suggested for other mantises (O'Hanlon et al. 2018). We also did not account for whether deimatic or non-deimatic behaviours occur at developmental stages other than adulthood, which may be an important consideration (see Robinson 1969; Watanabe and Yano 2010). Understanding the effects of population, microhabitat, sex, and ontogeny will help to provide a more complete picture of the evolution and expression of multiple defences in this species.
In summary, we found that M. caffra exhibits a diversity of deimatic and non-deimatic secondary defensive behaviours. Our results suggest that both types of defences are state dependent: less gravid females were more likely to flee initial contacts, while more gravid females were more likely to strike with their weaponised forelegs in response to such contacts, indicative of a fight-or-flight trade-off. Deimatic behaviours were also more common in more gravid females and at later phases of the predation sequence, but were not always produced in a ritualised display. Instead, females mixed and matched deimatic and non-deimatic behaviours dynamically. Our results show how multiple defences can manifest at different phases of the predation sequence to match the level of the threat, with behaviours deployed either separately or together, both synergistically and antagonistically. Our study is also important in showing how gravidity can mediate functional trade-offs in behavioural defences.
Author Contributions
Nathan W. Burke: conceptualization, investigation, funding acquisition, writing – original draft, methodology, validation, visualization, writing – review and editing, formal analysis, project administration, data curation, resources. Laura Knapwerth: conceptualization, investigation, writing – review and editing, validation.
Ethics Statement
The authors have nothing to report.
Acknowledgements
Open Access funding enabled and organized by Projekt DEAL.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data will be made available through the public data repository Data Dryad upon publication.




