The Relevance of Flash Coloration Against Avian Predation in a Morpho Butterfly: A Field Experiment in a Tropical Rainforest
Editor: Wolfgang Goymann
Funding: AV-S received financial support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Finance Code 001) and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2020/06756-3). GBE was funded by CAPES (Finance Code 001). AVLF was supported by a research grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 304291/2020-0), FAPESP (2021/03868-8). PSO was supported by research grants from the CNPq (302219/2017-0, 303730/2021-8) and FAPESP (BIOTA Program, 2017/16645-1, 2022/06529-2).
ABSTRACT
The flash coloration hypothesis postulates that otherwise cryptically colored animals suddenly displaying conspicuous colors during movement confuse predators, reducing capture. Morpho helenor butterflies have contrasting colors on dorsal (iridescent blue) and ventral (brown) wing surfaces, resulting in sequential blue “flashes” during flight. We tested whether this flashing pattern reduces avian predation on M. helenor in Atlantic rainforest by changing the flashing effect in three experiments. In Experiment 1, we added a blue band to the ventral wing. In Experiment 2, we covered the dorsal wing's blue band with a brown band. Control groups in each experiment were painted such that wing color patterns remained unaltered. Survivorship was evaluated through mark-recapture censuses and beak marks on the wings. Results show that survivorship of treated butterflies in Experiment 1 decrease markedly compared to unaltered control individuals, while survivorship of treated butterflies in Experiment 2 did not differ compared to control individuals. In Experiment 3, we detected scant predation on treated (blue band added to ventral wing) and control butterflies (brown band added to ventral wing) on the forest floor (wings closed), corroborating that flash coloration is an important protective mechanism during flight. Our field experiments provide the first evidence, to our knowledge, that flash coloration in bright blue Morpho butterflies is an effective defense mechanism against avian predators in a tropical rainforest.
1 Introduction
Predation is a major threat for flying insects, especially in the tropics where insectivorous birds are diverse and abundant (Haffer 1985; Stotz et al. 1996). While pursuing a given flying prey, both coloration and flight patterns are important factors defining whether the bird will attack (Cott 1940; Kassarov 2003). Therefore, defense mechanisms emerged in several groups where the prey's behavior and coloration operate together to avoid predation (Ruxton, Sherratt, and Speed 2004; Stevens and Ruxton 2019). This is the case of the defense mechanism known as flash coloration.
Flash coloration was described in detail by Cott (1940), who pointed out that some prey animals exhibit a predominantly cryptic pattern when motionless, suddenly revealing a body part of conspicuous color as they move. In essence, crypsis and conspicuousness occur sequentially as the animal alternates between motionless and movement, creating a succession of flashes of conspicuous coloration that may confuse or disorient predators while chasing the prey. According to Edmunds (1974), the appearance of a conspicuous color could make the predator seek the color itself during pursuit, and with the sudden disappearance of the color as the prey becomes motionless, the predator would assume that the prey itself had also disappeared.
Both vertebrates and invertebrates may exhibit contrasting patterns that alternate between cryptic and conspicuous colors during movement (Edmunds 1974). Among insects, numerous examples are found in Lepidoptera, Orthoptera and Phasmatodea, which display conspicuously colored hindwings during movement (Cott 1940; Edmunds 1974).
The defensive value of flash coloration against predation, however, is yet to be demonstrated in nature (Edmunds 2008). Recent experiments using computer simulations and humans as predators have provided important data and insights on the flash coloration hypothesis (Loeffler-Henry et al. 2018; Murali 2018; Bae et al. 2019; Murali and Kodandaramaiah 2020; Loeffler-Henry, Kang, and Sherratt 2021; Sherratt and Loeffler-Henry 2022; Silvasti et al. 2024). However, demonstration of the selective value of flash coloration in nature awaits further experimental manipulation using real predators and real prey.
Due to the great diversity of color patterns in their wings and the ease with which they may be observed and manipulated; butterflies have been extensively used as model organisms in studies of defensive coloration (Edmunds 1974; Brakefield and French 1993). Indeed, part of the huge variation in butterfly color patterns is thought to have evolved as a form of defense against predators, mostly birds (Carpenter 1939; Chai 1986; Pinheiro and Cintra 2017; Willmott et al. 2017).
Tropical Morpho butterflies are well-known for their iridescent structural blue color, with most species presenting contrasting color patterns during flight (Debat et al. 2018). This iridescent color produces very bright, dynamic, directionally reflected signals that change appearance with lighting and viewing angle (Pegram, Han, and Rutowski 2015), which allows these structural colors to carry additional signals than colors based on pigments (Kemp and Rutowski 2007). Widely distributed in Neotropical forests, Morpho helenor (Cramer) alternates dramatically the brightness and spectral properties of their radiance during flight: the butterflies show an iridescent blue band on the dorsal wing surface and a brown pattern with marginal eyespots on the ventral surface, that makes the butterfly cryptic during flight in the dark forest understory (Figure 1). Combined with its erratic flight pattern (Debat et al. 2018), the flashing blue on the dorsal wing surface of M. helenor would make it difficult for a predator to know where the butterfly will move next, as predicted by the flash coloration hypothesis.

Here, we test the flash coloration hypothesis in M. helenor through field experiments in the Brazilian Atlantic rainforest. During its irregular flight in the dark forest understory, we hypothesize that the blue flashes from M. helenor's dorsal wing surface would make it harder for avian predators to predict the insect's flightpath, and thus to successfully capture the butterfly. Specifically, we evaluated butterfly survivorship through time on treated (altered color pattern) versus control (unaltered color pattern) individuals via mark-recapture censuses. Additionally, we manipulated the color of the wing ventral surface to evaluate predation by birds on motionless butterflies pinned on the leaflitter (altered vs. unaltered color pattern), simulating their resting posture (wings closed) on the forest floor.
By experimentally change the dynamic flashing pattern during flight, we were able to demonstrate decreased survival in altered compared to unaltered butterflies. Moreover, butterfly predation on the forest floor proved scant and did not differ between experimental butterfly groups. Our field study with M. helenor provides the first, to our knowledge, experimental evidence in nature supporting flash coloration during flight as a defense mechanism against avian predation in tropical butterflies.
2 Material and Methods
2.1 The Study Species
Morpho helenor commonly flies in the understory of tropical forests (DeVries, Penz, and Hill 2010). Adults are palatable (Chai 1986; Pinheiro and Campos 2019) and feed mainly on fallen rotten fruits, showing marked annual abundance peaks in the study area in February–March and in September–October (Nascimento et al. 2020; Freitas et al. unpublished). The butterfly does not present marked sexual dimorphism, with males and females showing an iridescent blue band on the dorsal surface of the wings and a predominantly brown color on the ventral surface (Figure 1). Adults fly close to the ground (about 1 m high; DeVries, Penz, and Hill 2010) and are most active in the study area from 10:00 to 16:30 h (personal observation). The butterflies frequently land on the ground to rest and feed (Figure S2). Occasionally, they land on leaves for thermoregulation in the early morning sun (personal observation; Clench 1966).
2.2 Fieldwork
Fieldwork was carried out in an Atlantic rainforest reserve at Serra do Japi, state of São Paulo, southeast Brazil (23°14'S 46°58'W). Previous studies have shown that Morpho helenor is locally abundant (Santos et al. 2017), consistently recorded through standardized samplings for the past 11 years (Freitas et al. unpublished). Several birds at Serra do Japi are potential predators of M. helenor, particularly insectivorous species in the family Tyrannidae such as the Boat-billed Flycatcher (Megarynchus pitangua Linnaeus), the Great Kiskadee (Pitangus sulphuratus Linnaeus), and the Streaked Flycatcher (Myiodynastes maculatus Müller) (Pinheiro and Cintra 2017). Other common species in the region are the thrushes (Turdus rufiventris, Turdus leucomelas), which have been recorded attacking butterflies in flight in the early morning and late afternoon (Pinheiro and Cintra 2017). No species of insectivorous Galbulidae occurs in our study site, including the Rufous-tailed Jacamar (Galbula ruficauda Cuvier), which can capture large butterflies and other insects in the air (Pinheiro and Cintra 2017).
Individually marked butterflies were monitored using a capture-mark-recapture method (Nichols 1992), and collections were carried out using baits and nets. Twenty Van Someren-Rydon bait traps were placed approximately 40 m apart from one another and 1.5 m above the ground, along a forest trail ~5 m wide (following the protocol of Freitas et al. 2014). Baits consisted of a mixture of banana and sugar cane juice, fermented for at least 48 h before the sampling, and were replaced every 3 days. Traps were checked daily in the morning (between 10:00 h and 12:30 h) and in the afternoon (between 14:00 h and 16:30 h). All captured individuals were numbered on the underside of the medial hindwing with a black felt-tipped pen. Then, the butterflies were painted in accordance with its experimental group and released. Collections with entomological nets were made along the same forest trail used for the bait traps; both sampling methods were carried out simultaneously.
2.3 Experimental Procedures
Color pattern manipulation was performed with non-toxic enamel paint (Testors Co., Rockford, Illinois), which has proven an effective marking method. The paint is waterproof, dries quickly, and has no apparent effect on flight behavior (Simon and Bissinger 1983; Cakmak 2009; Young 2017). To test whether the color of the paints used in the experiments resembled the original colors of the butterfly wing, we measured the reflectance spectrum of individuals with painted wings (10 individuals, 5 for each paint color) and of unpainted individuals (5 individuals to test the blue color and 5 to test the brown color; Figure S3). The reflectance spectrum was measured between 300 and 700 mm (including ultraviolet light [UV]), using a Jaz-S spectrometer (Ocean Optics, Jaz Modular Optical Sensing Suite, Dunedin, FL, USA). A pulsed xenon light source was used with a spectral range between 190 and 1100 nM. A black fiber-optic holder was used to block any external light. Reflectance was evaluated using a PTFE disc (WS-1 Diffuse Reflectance Standard, PTFE; Ocean Optics) as the white standard and a black suede paper as the black standard. The measures were obtain using a standardized region of each individual forewing, which were positioned in a black suede paper horizontally. The spectral analyses were performed using the Pavo package (Maia et al. 2019) in R software (R Core Team 2020).
Because the blue coloration on the dorsal surface is structural and iridescent, the predator's perception of the color changes depending on the butterfly's position and the light conditions. It would be impossible to replicate this pattern on the ventral surface of the butterflies using only paint, as the physical properties are different. However, by adding this metallic blue paint, we guarantee that the butterfly will not display the low reflectance typical of brown coloration during flight (Figure S3). By increasing the reflectance on the ventral surface with blue paint, the experiment reduces the contrast between the ventral and dorsal face of the butterfly, thereby diminishing the confusion effect caused by dynamic coloration. In all experiments, butterflies were assigned to treatment or control group through the flip of a coin (see below). The experiments were carried out during the population peaks of M. helenor at the study site. Experiment 1 lasted 30 days between September and October 2021 and Experiment 2 lasted 30 days between February and March 2021. Experiment 3 was carried out in February/March 2022 and September/October 2023; butterflies were placed on the ground in successive days in each period, totaling 24 days of exposure to predation.
2.3.1 Experiment 1
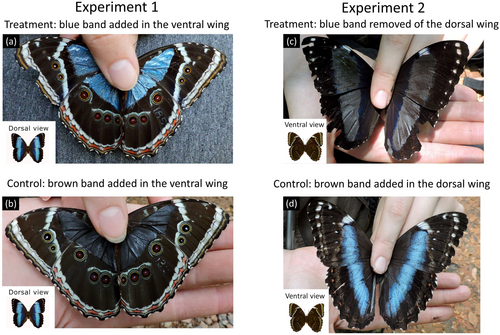
The manipulation consisted of adding a blue band (color code 1539TT) to the ventral wing surface of each wing, such that flying butterflies in the treatment group would exhibit a bright blue color on both sides of their wings (Figure 2a), thus decreasing the amplitude of the spectral and brightness changes in the butterfly's appearance that occur in flight. Butterflies in the control group were painted with a dark brown band (color code 1121TT) on the same region of each ventral wing, as done in treated individuals (Figure 2b). The eyespot region present on the ventral surface of the wings was kept unpainted in both groups. Because eyespots on the wing can act as a visual signal (Stevens 2005), their removal could affect the experiment. Painted butterflies were kept with their wings open for at least 2 min to ensure the paint dried, and to prevent smudging and sticking.
2.3.2 Experiment 2
Removal of the bright blue band on the dorsal wing surface such that flying butterflies would always be cryptic for a chasing bird (Figure 2c) and, therefore, less vulnerable to predation in the shaded forest understory. In the treatment group, the bright blue band of the dorsal wing surface was covered by a dark brown band (color “Rubber”, code 1183TT, Testors Co., Rockford, Illinois) (Figure 2c). In the control group, a similar dark brown band was painted on the dorsal wing surface of the butterflies next to the bright blue band, thus keeping the dorsal flashing blue during flight (Figure 2d). As in Experiment 1, painted butterflies were kept with their wings open for at least 2 min before release.
2.3.3 Experiment 3
The brown coloration of the ventral wing surface makes M. helenor cryptic when resting on the leaflitter with the wings closed (Figure S2). Therefore, by changing the color pattern of the ventral wing surface by adding a blue band (as in Experiment 1) resting butterflies would become more conspicuous (Figure 3). Experiment 3 was designed to evaluate how the ventral blue bands of blue paint affected the predatory attacks on landed butterflies. Butterflies were manipulated in the same way as in Experiment 1, that is, treatment individuals were painted a blue band on the ventral wing surface whereas control individuals were painted a brown band on the same region of the ventral wing (Figure 2). Butterflies were killed by pinching their thorax using the thumb and forefinger. Individuals were then painted, attached to a wooden stick, and positioned close to the ground at the edge of a forest trail (Figure 3; method follows Willmott et al. 2017, to test predation by birds). We used instant glue (Tekbond) to keep the butterfly wings closed, simulating a resting posture (Figure S2). We applied Tanglefoot resin (Grand Rapids, MI, USA) in the base of the sticks to prevent ground foraging ants from accessing the butterflies. Experimental individuals were positioned at least 10 m from one another, along a 4 km trail in the forest. The direction in which each individual was positioned (North, South, East, or West) was randomly selected. Butterflies in the treatment and control groups were checked for beak marks on their wings every 24 h (around 16:00 h). After checking, experimental butterflies were repositioned along the trail. Beak marks on butterfly wings have a typical triangular shape (bird beak) and have previously been used extensively as indicators of predation attempts by birds (Figure S1; Benson 1972; Shapiro 1974; Bowers and Wiernasz 1979; Ohsaki 1995; Ide 2005).

2.4 Data Analysis
To compare the proportion of recaptured individuals in each group in Experiments 1 and 2, and to evaluate differences between the number of individuals attacked in the groups of Experiment 3, we used a chi-squared test. Data collected monthly through 13 years at the study site (Freitas et al. 2024) show that Morpho helenor is highly sedentary, being rarely recaptured far from the site of first capture. Therefore, recapture rate can be confidently used as a proxy of survival.
2.5 Ethical Note
This study followed to ethical standards and was conducted under permits issued by the Biodiversity Authorization and Information System (SISBIO, Brazil) with license numbers 10438-6 and 73086.
In Experiments 1 and 2, individuals collected with active capture were immediately extracted from the nets and handled with utmost brevity. This process ensured that each butterfly spent no more than 3 min in human hands before being released. Passive capture involved checking traps in both the morning and afternoon, minimizing the duration of entrapment for the butterflies. Experiment 3 necessitated the sacrifice of Morpho helenor individuals for predation trials. Butterflies were euthanized via thoracic compression immediately upon collection and then stored in envelopes for later painting. Real specimens were chosen over paper models to accurately record bird beak marks on the wings. The number of individuals sacrificed was carefully calibrated to maintain consistency in sample sizes across all three experiments, facilitating meaningful comparisons of results.
3 Results
3.1 Experiment 1
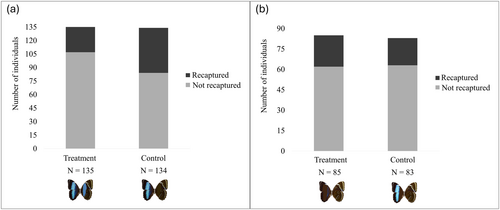
The number of recaptured individuals differed significantly between experimental groups of Morpho helenor (Figure 4a). Out of 135 individuals marked in the treatment group (color pattern altered), 20.7% were recaptured. In the control group (color pattern unaltered), out of 134 marked individuals, 37.3% were recaptured. The sex ratio of marked individuals did not differ between experimental groups (χ2 = 0.141; df = 1; p = 0.7078).
3.2 Experiment 2
Out of 85 individuals marked in the treatment group (color pattern altered), 27% were recaptured. In the control group (color pattern unaltered), out of 83 marked individuals, 24% were recaptured (Figure 4b). The number of recaptured individuals did not differ between experimental groups of Morpho helenor (Figure 4b); as well as the sex ratio (χ2 = 0.017; df = 1; p = 0.895).
3.3 Experiment 3
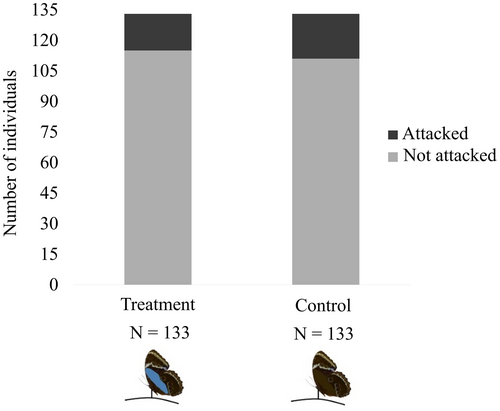
Experimental manipulation used dead butterflies pinned on the leaflitter. We evaluate if treatment butterflies painted with a bright blue band on the ventral wing surface would be more attacked by birds, when landed on the forest floor (wings closed), than control butterflies with unaltered color pattern (brown band added to the ventral wing surface). Avian predation on Morpho helenor butterflies pinned on the forest floor, as expressed by beak marks on their wings, was scant and did not differ between treatment (13.5%, altered individuals) and control (16.5%, unaltered individuals) groups (Figure 5; χ2 = 0.47; df = 1; p = 0.49).
4 Discussion
Our study shows that individuals painted with a blue band on the ventral wing surface do not suffer increased predation when landed on the forest floor, which is an indication that this extra blue band does not make the landed butterflies more attractive to predators. Therefore, the decreased survival of treatment compared to control butterflies in Experiment 1 is probably due to predation during flight. Birds are among the main visually oriented predators of butterflies (Théry and Gomez 2010; Carpenter 1939; Chai 1986; Curio 2012), and most species chase and capture butterflies during flight, not when they are landed (Carpenter 1939; Carpenter and Hale 1942; Chai 1986; Pinheiro and Cintra 2017). In-flight predation by birds likely accounts for the outcome of Experiment 1, since beak marks in Experiment 3 indicated scant predation attempts on both treatment and control butterflies landed on the forest floor. Given that treatment and control butterflies were manipulated exactly in the same way in both experiments (same wing area), external factors potentially altering their survival or likelihood of being recaptured should affect both groups equally. Thus, different recapture rates between treatment and control M. helenor can reasonably be attributed to the color used in each group, that is, altering or not the dynamic flashing pattern during flight.
The decrease in the recapture probability of Morpho helenor, when the flash was changed during flight, suggests that color manipulation might have resulted in a higher incidence of successful predation of treated butterflies in the air, which became more vulnerable visual targets to chasing predators. Our results provide the first experimental evidence, to our knowledge, supporting dynamic flash coloration as a protective mechanism in Morpho butterflies against predation. Although previous studies have suggested that variation between cryptic and conspicuous colors during movement can decrease the chance of a given prey being captured, the supporting evidence is based only on virtual experiments using artificial prey and humans as predators (Loeffler-Henry et al. 2018; Murali 2018; Bae et al. 2019; Murali and Kodandaramaiah 2020; Loeffler-Henry, Kang, and Sherratt 2021; Sherratt and Loeffler-Henry 2022; Silvasti et al. 2024). Our experimental study is unique by using real prey and real predators, in their natural habitat, to corroborate flash coloration as a defense mechanism that potentially decreases the predator's ability to track the butterfly during flight.
Equal survival between treatment and control butterflies in Experiment 2 is likely due to the removal of the blue band in treatment individuals, which became cryptic to visually hunting birds during flight in the forest understory. There are different forms of crypsis that evolved in butterflies in their adult stage. In background matching, detection by predators is avoided due to the similarity of the animal's appearance—whether by color, brightness, contrast, or pattern—with one or more types of background (Stevens and Merilaita 2009). Many Satyrinae butterflies, including some Morpho species, are brown on the dorsal and ventral wing surfaces and are considered cryptic during flight (Schwanwitsch 1948). Thus, by removing the bright blue color from the dorsal face of M. helenor, we made the color pattern of treated butterflies cryptically colored during flight (as reported for brown satyrines), which may explain the outcome of Experiment 2. Indeed, a uniform color pattern resembling the background can be advantageous for moving prey. Murali (2018) has shown that artificial prey with background matching pattern were less caught by humans than prey with white coloration when in motion, suggesting that crypsis may have some advantage over more conspicuous coloration for moving prey. Furthermore, other studies have demonstrated that targets exhibiting a uniform color that resembles the average background color can be difficult to capture (Stevens, Yule, and Ruxton 2008; Hughes, Troscianko, and Stevens 2014; Hughes, Magor-Elliott, and Stevens 2015), and can be just as effective at escaping from humans as dynamically changing color targets (Murali and Kodandaramaiah 2020).
Therefore, one may ask why M. helenor has not evolved cryptic coloration during flight, as many other Satyrinae. Indeed, the adaptive value of the iridescent blue color in Morpho butterflies, and the factors that might have shaped the evolution of this color pattern, have been asked for a long time. This blue color was already present in males of the lineages that first diverged in the group, Morpho marcus and Morpho eugenia, suggesting that blue iridescence was present in the ancestor of Morpho and this taxonomic character has been maintained in a large part of the group (Chazot et al. 2016; Debat et al. 2018). The evolution of coloration patterns is under various selective pressures, which are often highly interconnected (Caro, Sherratt, and Stevens 2016; Cuthill et al. 2017), such as intraspecific for communication, thermoregulation, and defense against predation (Caro, Sherratt, and Stevens 2016). For M. helenor, intraspecific communication appears to have played no important role in the evolution of the blue pattern, given that there is no marked sexual dimorphism in this species, and males do not have any kind of territorial behavior towards other males (Blandin and Morris 2007). The importance of wing color for thermoregulation in Morpho is unclear. Berthier (2010) claims that the iridescent blue and its high reflectivity do not seem to play such an important role in absorption capacity. More recently, Thomé, Richalot, and Berthier (2020) suggests that the structure of the wing scales is related to a phenomenon that helps thermoregulation. As far as we know, no work has assessed whether the scale structure and iridescence of M. helenor wings could have any advantage for thermoregulation. Our field experiments with M. helenor provide evidence that defense against predation via flash coloration during flight might have been an important factor shaping the evolution of the iridescent blue coloration in this species. Indeed, Le Roy et al. (2021) recently suggested that similar wing color patterns of sympatric Morpho species with erratic flight abilities may function as escape mimicry and discourage further predatory attacks. Further investigation integrating multiple selective forces should help clarify the evolution of the flashy, iridescent coloration pattern in Morpho butterflies.
For 160 years, naturalists (e.g., Bates 1863) have marveled at the flashing and intense blue color of Morpho butterflies, however, tests of adaptive explanations of flash coloration in these butterflies have hitherto been absent (Debat et al. 2018). Here we demonstrate experimentally that the flashy blue color of M. helenor is an effective defense mechanism against visually oriented predators, mostly birds, in Atlantic rainforest. We suggest that the dynamic flash coloration during flight makes it difficult for predators to chase and capture the butterflies in the shady forest understory. The phenomenon of dynamic flash coloration is known to occur in many Morpho species (Chazot et al. 2016), as well as in other butterfly genera, such as Doxocopa and Myscelia. Our experimental study is a first step to understand the adaptive value of such a widely distributed trait that combines color and behavior, and which is so commonly observed among insects in tropical and temperate environments.
Author Contributions
Aline Vieira-Silva: conceptualization, investigation, methodology, validation, formal analysis, data curation, visualization, writing – original draft, software. Gabriel B. Evora: formal analysis, investigation, writing – review and editing, software. André V. L. Freitas: conceptualization, methodology, visualization, writing – review and editing. Paulo S. Oliveira: conceptualization, data curation, funding acquisition, methodology, supervision, visualization, writing – review and editing, project administration, resources.
Acknowledgements
We are grateful to R. L. Rutowski, D. H. Janzen, G. Machado, M. Elias, R. J. Marquis, P. R. Guimarães for discussions and helpful comments on the manuscript. We also thank L. Murari and G. Sonoda for the help in the field work. The Fundação Serra do Japi provided logistic support for field work. L. P. Morellato and A. Martins helped with the spectral analyses. AV-S received financial support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Finance Code 001) and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2020/06756-3). GBE was funded by CAPES (Finance Code 001). AVLF was supported by a research grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 304291/2020-0), FAPESP (2021/03868-8). PSO was supported by research grants from the CNPq (302219/2017-0, 303730/2021-8) and FAPESP (BIOTA Program, 2017/16645-1, 2022/06529-2).
Ethics Statement
This study followed ethical standards and was conducted under permits issued by the Biodiversity Authorization and Information System (SISBIO, Brazil) with license numbers 10438-6 and 73086.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data available from Zenodo Repository: https://doi.org/10.5281/zenodo.13880901.




