The geomagnetic field does not appear to influence navigation in Eastern painted turtles
Abstract
Geomagnetic cues provide important information to guide aspects of migration, from the general direction of movement to informing an animal's specific location on the planet. Although such cues are used by many long-distant migrants, its use for short- and moderate-distance migrations is less clear. We have been studying overland migratory movements in a population of Eastern painted turtles where animals use one of four precise, highly predictable routes to locate water sources during migration. Our previous work suggests a strong role of learning and spatial memory in this precise navigation, but the cues used and the context for their use are unknown. Here, we present a series of controlled field experiments in which we test the hypothesis that geomagnetic cues are utilized when Chrysemys picta engage in these intricate, overland movements. We fitted turtles with 3,309 Gauss magnets (n = 10) or aluminum disk controls (n = 5) and surveyed their ability to locate and navigate their routes during migration; turtles were similarly tested for these abilities outside of the context of migration (magnets, n = 30; control, n = 30). Strong magnets (3,309 Gauss) on their carapaces did not disrupt turtles’ ability to locate the paths or the precision with which they navigated them. Turtles, irrespective of treatment, found their paths and/or navigated their paths with the same high precision that we observed in controls and our previous work, both within and outside of the context of migration. Thus, alternative types of cues must be in use that explain the high precision and high reliability of path location over time. Future studies should examine alternative cues used in migratory navigation and the creation of complex and specific paths.
1 INTRODUCTION
Animals use a wide range of cues to facilitate navigation and orientation. For example, many species use celestial, solar, and/or olfactory cues to navigate over great distances (Chernetsov, 2016; Congdon et al., 2015; Dacke & el Jundi, 2018; Gagliardo, 2013; Krenz et al., 2018). Of the many cues employed, one of the most thoroughly studied cues, especially for long-distance migrators, is geomagnetism (Lohmann et al., 2007; Wiltschko, ).
Geomagnetic cues provide important spatial information to guide aspects of migration, from the general direction of movement to informing an animal's specific location on the planet (Lohmann et al., 2007; Wiltschko, ). For example, salmon rely on geomagnetic cues for orientation and navigation during various aspects of their migration, and can use a magnetic map to do so (Putman, 2015; Scanlan et al., 2018). Similarly, juvenile glass eels (Anguilla anguilla) can extract map-like information from geomagnetic cues, and they appear to use this information to navigate to the Gulf Stream (Naisbett-Jones et al., 2017). Many species of bird are known to use geomagnetic cues to direct orientation, navigation, and migration (Wiltschko & Wiltschko, 1996), and their use of geomagnetism may be somewhat plastic (Able & Able, 1990; Cochran et al., 2004). The importance of geomagnetic cues in migration and orientation has been particularly well-studied in sea turtles, which have been shown to use such cues for initial orientation out to sea upon hatching, maintenance of orientation when far from land navigation, and homing toward their natal beaches (Fuxjager et al., 2011; Lohmann, 1991; Lohmann & Lohmann, 1996, 2019; Salmon & Lohmann, 1989).
We study overland migratory movements in a population of Eastern painted turtles (Chrysemys picta) where animals use one of four precise (±3.5 m), intricate, highly predictable routes to locate far-off water sources during vernal migration. These routes are consistent within and among individuals and within and across years, with individuals using the same route year after year (Roth & Krochmal, 2015). Naïve turtles in our system must navigate this site prior to age 4 to be able to successfully navigate as adults. Our previous work also suggests a strong role of learning and spatial memory in this precise navigation (Roth & Krochmal, 2016, 2018), but the specific cues that are used during navigation and the context for the use of those cues are unknown.
The use of a variety of different cues could explain the high-precision movements observed at our site. For example, turtles could potentially use olfactory or visual landmarks to learn and/or recall migratory routes (Roth & Krochmal, 2015). These cues could explain the high precision of the paths used during navigation. However, turtles in our system move through a variety of different habitats (including dense woods, old fields, row crops, and mud flats) in a single migratory trip, and the landscape and habitat structure itself can change vastly from year to year depending upon weather and agricultural activity. Animals on a single migration path might find themselves at a given location in tall grass one year and row crop the next; this potentially prevents consistent access to both global and local visual or chemical cues across years. In spite of such habitat shifts, we see no loss of precision in such areas and these paths are repeatedly used across years (Roth & Krochmal, 2015).
Alternatively, some aspects of this high-precision navigation and the consistency of path use in spite of changes in habitat could be explained by the use of geomagnetic cues (direct or indirect [e.g., magnetic enhanced vision]). Although aspects of geomagnetism that inform the well-studied, long-distance migratory species (declination, inclination, and field strength) show little variation at the spatial scale across which our turtles are migrating (2 km or less), there is some support for geomagnetism driving smaller scale movements in a variety of species (Dommer et al., 2008; Fischer et al., 2001; Pereira et al., 2019).
Moreover, some terrestrial and semi-aquatic turtles might also use geomagnetic cues for some aspects of navigation. For example, Eastern box turtles (Terrapene carolina) trained to a particular movement pattern were disrupted by manipulating local geomagnetic cues (Mathis & Moore, 1988). Similarly, second-year yellow mud turtles (Kinosternon flavescens) might use a magnetic compass while making specific, directed seasonal overland movements (Iverson et al., 2009). When taken together, these and related studies imply that it may be possible that geomagnetic cues might, at least in part, explain the patterns that we observe in our system.
Here, we present a series of controlled field experiments in which we manipulated the magnetic field in C. picta engaging in intricate, moderate-distance overland migratory movements. We tested two hypotheses: First, geomagnetic cues are used by turtles to locate paths during migration. We predicted that if geomagnetic cues are used during the location of paths that turtles in the magnetic-field-manipulation treatment group will be unable to successfully begin migration (i.e., locate the historical paths). Second, we hypothesize that geomagnetic cues are used by turtles to navigate the paths irrespective of how paths are located. If navigation within our system, defined as the ability to follow the specific, intricate, high-precision migratory paths, is dependent upon geomagnetic cues, then turtles in the treatment group will demonstrate a marked loss of precision in migratory movements, relative to the historical routes.
2 METHODS
2.1 Model species
The present study focused on C. picta, a long lived pond turtle inhabiting a variety of fresh water bodies across the northeastern United States (Ernst & Lovich, 2009). This species has been studied extensively, particularly in reference to the behavior and ecology of movement (Bowne, 2008; Congdon et al., 2011; Jaeger & Cobb, 2012,), learning (Powers et al., 2009), and spatial memory (Petrillo et al., 1994; Roth & Krochmal, 2016, 2018).
2.2 Study system
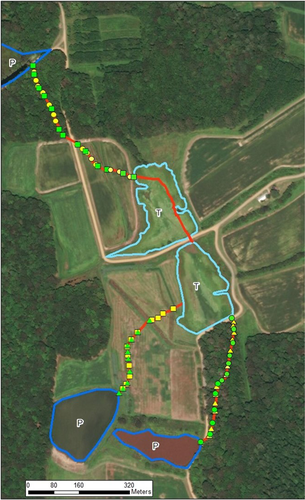
We conducted our study at Chesapeake Farms, a 1,335 ha wildlife management and agriculture research area in Kent Co., MD, USA (39.194°N, 76.187°W; Roth & Krochmal, 2015). The focal portion of the site is composed of five wetland impoundments (three permanent and two temporary). The temporary ponds (each ~ 2.5 ha in area) have experienced a rapid draining (the entire pond is drained in ~ 24 hr, a process hereafter referred to as drawdown) each summer for nearly 100 years for the purpose of wetland management. After drawdown, resident turtles leave the ponds and migrate to alternative aquatic habitats using one of four very precise, complex, and highly predictable routes described above (Roth & Krochmal, 2015).
2.3 Magnetic manipulation
We captured adult turtles (defined as 4 ≥ years old) using baited hoop traps and basking traps. We fitted turtles in the experimental group with a neodymium magnet (K&J Magnetics, Inc., Pipersville, PA; D41-N52; 0.38 g; 6.35 mm diameter, 1.59 mm thickness; surface field: 3,309 Gauss, Brmax = 14,900 Gauss; Bhmax = 52 MGOe) attached to the leading edge of the nuchal scale of the carapace (following Congdon et al., 2015). Placement of the magnets on the carapace—a fixed, rigid mineralized tissue—with epoxy assured that the positions of the magnets did not change throughout the animal's migration and was uniform across all animals in the study, thereby preventing magnet positional changes (Nimpf et al., 2019). Although the exact nature of disruption to the magnetic field can be complex, given that the magnets used were 500–1000 times greater than the strength of the Earth's magnetic field at the surface (range 0.3–0.6 Gauss) and as the magnets were < 5 cm from the center of the turtle's head, we can confidentially say that immediate magnetic field around the head of turtles was disrupted. In the control groups, we fitted turtles with aluminum disks (0.41 g; 6.35 mm diameter, 6.35 mm thickness) affixed to the same location on the carapace.
2.4 Assessing the importance of geomagnetic cue during migration to alternative habitat
We used radiotelemetry to investigate the accuracy and precision with which turtles navigate to alternative aquatic sites during migration. We fitted turtles with radiotransmitters (models RI-2B or BD-2, Holohil Systems, Ltd. <4.5% body mass). We tracked all turtles via remote triangulation, taking high precision (spatial resolution of 2.5 m) locations on all animals > 4 times per hr during the entirety of their overland movements.
To test the importance of geomagnetic cues on locating and navigating paths in the context of drawdowns, we attached radiotransmitters to the turtles with long-term epoxy adhesive in the week prior to drawdown (late June). Ten of these turtles carried magnets, while the remaining five individuals carried the control aluminum disks or comparable dimensions and mass. Both magnets and control disks were attached with epoxy as described above. We immediately returned these turtles to the ponds so that they were allowed to migrate freely with the rest of their population after drawdown. Once these turtles left their temporary ponds and began to migrate freely with the rest of the population, we took a set of bearings (at least 3 individual azimuths) on every animal > 4 times per hr from the time the animal left the drained pond until it was about to enter the permanent pond, at which point, the magnet or aluminum disk was removed and the turtle was left to complete its migration. Experiments on freely navigating turtles were conducted in early July 2015.
To test the importance of geomagnetic cues for the navigation of paths per se (i.e., navigating paths outside of the context of migration after drawdown), we attached magnets (n = 30) and aluminum disks (n = 30) to the turtles with temporary adhesive. Each of these turtles also received a radiotransmitter attached in the same manner. After capture, these turtles were tagged, placed immediately in the middle of their known paths—the paths which they have used for migration year after year for at least a decade, without fail and without switching—approximately midway between the temporary and permanent ponds, and then tracked. Based on our recent work, turtles do not need the context of the drawdown per se to navigate their paths (Krochmal et al., in Review). They can, instead, navigate with the same high precision both in and out of season and in both the forward and backward directions on the paths (i.e., they remember the path and how to travel in both directions). For animals tested outside of the context of migration, a set of bearings (at least 3 individual azimuths) was taken on every animal > 4 times per hour from the time it was placed on the path until it was about to enter a permanent pond. All turtles were returned to their capture site immediately after testing. Experiments conducted outside the context of drawdown were conducted in June and July 2015 and 2016.
2.5 Statistical analyses
We compared the movement patterns and the precision thereof between treatment groups as per our previous analyses (Roth & Krochmal, 2015, 2016, 2018). Briefly, we compared path specificity and precision by calculating the spatial variability of paths taken by each individual as the distance of each individual from the traditional route. Using LOAS (Ecological Software Solutions) and ArcGIS 10.2.1 (Esri Industries), we first documented the traditional path taken by turtles in previous years. Given that movements along these paths are historically accurate to 3.5 m, we statistically compared all turtle movements to this template by calculating the mean distance of each location for each individual (using the individual turtle as sampling unit) from that of the historical paths. We first produced a measure of the deviation of movements relative to the residents’ paths by examining the proportion of points that overlapped the traditional 3.5 m path. We then analyzed these deviations across treatment groups with a general linear model with Fisher's LSD post hoc comparison.
2.6 Ethical considerations
This work was reviewed and endorsed by the Maryland Department of Natural Resources (SCO # 51936) and the Institutional Animal Care and Use Committee of Washington College (protocol #F12-004 and SU13-001). Our field study was conducted in accordance with all local, state, and federal laws and by permission of the land owners.
3 RESULTS
The experimental addition of strong magnets on the carapace of turtles did not disrupt their ability to locate the paths at the temporary ponds (Figure 1). Of the turtles tracked during their free migration, all turtles with magnets (n = 10) located a known path and navigated that path with a precision equal to that of the aluminum controls (n = 5; F2,73 = 0.12, p = .89; Tukey, p > .99, Figure 2) and to that seen in our previous work (Tukey, p = .95); all locations fell within 3.5 m of the historical paths (Figure 2).
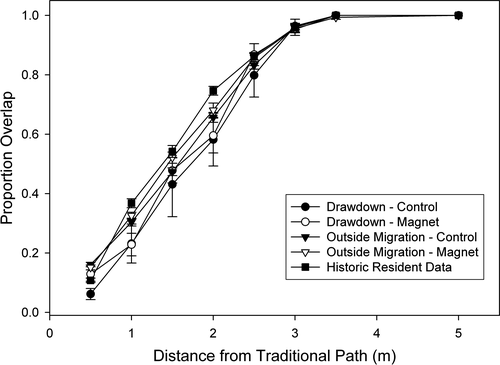
In addition, the experimental manipulations of the magnetic field surrounding the turtles did not affect the ability of the turtles to navigate their paths (follow the specific, intricate historical paths) outside of the context of migration. Of the turtles tested for navigation ability, those that were wearing magnets (n = 30) were able to navigate their paths with accuracy and precision not different from the aluminum controls (n = 30; F2,101 = 1.896, p = .16; Tukey, p = .28; Figure 2) or that of turtles on the same paths in previous years (F2,101 = 1.9, p = .16; Tukey, p > .99; Figure 2). As above, all points from all turtles in both groups were located within 3.5 m of the historical paths (Figure 2).
4 DISCUSSION
We have no apparent evidence for the role of geomagnetism for navigation in our system. The addition of strong magnets did not disrupt the turtles’ ability to locate paths during migration nor the precision or accuracy with which they navigated those paths. Every turtle in the magnet groups found their path and/or navigated on their paths with the same high precision that we observed in controls and in our previous work in this system.
Although geomagnetic cues could potentially explain the consistency of path use in spite of changing habitat types and quality migrating turtles experience in our system, we failed to find support for their use. Alternative types of cues must be in use that explain the high precision and high reliability of path location over time. To explain such specific paths, the cues must be fine scale, ground-based, local cues (Roth & Krochmal, 2015).
Alternative cues that turtles in our system might use to achieve the observed precision in navigation include solar cues and polarized light, olfactory cues, and local (ground-borne) UV visual cues. Both solar cues (DeRosa & Taylor, 1980) and polarized light (Yeomans, 1995) are used as orientation aids by a variety of animals and could, in principle, be candidates for cues used by turtles in our system. However, it unlikely that either solar cues or polarized light serves as a primary navigation cue or orientation cue for turtles in our system. First, animals in our system follow intricate, complex paths—as opposed to moving in a diffuse swarm toward water—precision not possible with solar or polarized light cues alone. Second, animals begin migration on overcast days or in rain and show no loss in movement precision (Krochmal et al., 2015) when such cues are unavailable. Finally, naïve animals brought to the site both failed to navigate the routes and failed to locate any permanent body of water over the course of three weeks, suggesting that such cues present at the site are not utilized (Roth & Krochmal, 2015).
We do, however, have preliminary evidence to suggest that turtles in our system might use local UV cues to direct their migratory movements (Roth & Krochmal, 2015), and while these results are supported in part by the literature (Arnold & Neumeyer, 1987; Jacobs, 1992; Ventura et al., 1999), the use of this cue in our system has yet to be tested in the field. Similarly, olfaction could, in principle, play a role in turtles navigating in our system, but these cues also remain untested in the field in our system.
That we failed to see any change in navigation precision when disrupting the local magnetic field does not conclusively demonstrate that animals in our system do not, in certain circumstances, use magnetic information to direct overland movements. Plainly, there are experimental variables for which we could not control—as is the nature of field-based studies. Some species are known to use a hierarchy of cues, such that if information from one cue conflicts with that of another or is temporarily unavailable, then other cues can be used. For example, some studies have shown that altering magnetic cues does not inhibit successful orientation in on sunny days, but does impact orientation when cues are not available (Wiltschko et al., 1998). However, the conditions experienced by animals in our studies were so very vast and varied, yet we saw no change in the extreme precision of their migratory movements. The data presented herein were collected over two years, with data collected on clear bright days, overcast days, and in torrential rain (potentially rendering visual landmarks, direct in indirect solar cues, and some olfactory cues less reliable). Furthermore, on their migrations, turtles in our system pass through open short grass fields, tall (~1 m) grasslands, agricultural fields, and dense deciduous woodlands. Across all of these conditions, we saw no changes in movement precision, with all animals navigating just as precisely as each other and as turtles have historically at our site. Thus, we argue that the role of geomagnetic cues in our system is unlikely.
Similarly, our results do not rule out ontogenetic or experiential effects on the role of geomagnetic cues in shaping overland movement patterns. Animals in our system appear to learn their migration routes as juveniles within a narrow critical period, and then use spatial memory to drive migratory movements as adults (Roth & Krochmal, 2015, 2016, 2018). As the present study only included adult turtles with memories of and experience on their specific migration routes, it is possible that turtles use geomagnetic cues when learning these routes during the critical period.
Though aspects of geomagnetism known to direct long-distance migratory movements in a variety of species (e.g., declination, inclination, and field strength) do not vary in biologically relevant ways at the spatial scale across which our turtles are migrating (2 km or less), geomagnetism does appear to drive, at least in part, smaller scale movements in some cases (Dommer et al., 2008; Fischer et al., 2001; Pereira et al., 2019,). More specifically, some terrestrial/semi-aquatic turtles might also use geomagnetism cues to direct aspects movement. For example, Mathis and Moore (1988) showed that orientation of Eastern box turtles (Terrapene carolina) experimentally trained to move in a particular direction were disrupted by manipulating local geomagnetic cues. Similarly, Iverson et al. (2009) speculate that second-year yellow mud turtles (Kinosternon flavescens) may rely on magnetic compass while making seasonal overland movements. On the other hand, other work, in addition to our own, fails to provide evidence to support a role of geomagnetism in directing turtle movement. Though hatchling snapping turtles (Chelydra serpentina) display specific, directed, post-hatching dispersal, geomagnetic disruption failed to alter turtle orientation (Congdon et al., 2015); the same pattern is also seen in Blanding's turtles (Emydoidea blandingii; Krenz et al., 2018)
Finally, though attaching strong magnets to animals is a common technique to study the roles of magnetic cues in a suite of behaviors (Anderson et al., 2017; Keeton, 1971; Wang et al., 2006), the approach has some potential caveats, particularly with respect to possible mechanisms underlying magnetoreception. There are two commonly accepted hypotheses addressing the mechanistic basis of magnetic compass orientation in vertebrates—iron-mineral-based and chemical-based magnetoreception. Iron-mineral-based magnetoreception hinges on magnetite (Fe3O4) crystals responding to the local geomagnetic field as the physical basis for magnetoreception (e.g., Frankel et al. 1979). As the crystals move in and align with the geomagnetic field, they potentially activate secondary receptors (e.g., hair cells, mechanoreceptors), or open ion channels (Johnsen & Lohmann, 2008). Magnetite plays a role in magnetoreception in a wide range of taxa, including turtles (Irwin and Lohmann 2005, Wiltschko & Wiltschko, 2013, Lambinet et al., 2017, Formicki et al., 2019). On the other hand, magnetoreception could be a function of chemical-based reception, which depends on magnetic field dependent biochemical pathways, involving electron transfer, pairs of free radicals as fleeting intermediates and, potentially, cryptochromes, photoreceptive proteins that some researchers believe could, in principle, serve as magnetoreceptors. (Rodgers & Hore, 2009).
Although the strong magnets we used in the present study would without question re-magnetize an iron-mineral-based compass, it is less clear if such magnets would completely disrupt the sensation of the magnetic field in animals using chemical-based magnetoreception. Though chemical magnetoreception has a strong theoretical basis and cryptochromes might exist in a variety of species, the evidence to support their role in magnetorecption is sparse and has recently been called into question (Babcock & Kattnig, 2020). Indeed, the most concrete evidence of cryptochromes being used for magnetoreception comes from fruit flies (Gegear et al., 2009), giving us no reason to expect that turtles in our system are employing this form of magnetoreception.
Overall, we have used a standard technique, although not one without caveats, to address the role of geomagnetic cues for navigation for turtles in our study. We found no role for such magnetic cues in our system. Whatever the cues used by the turtles in our system are, they are likely multimodal in nature and are probably incidental in origin (cues left by previous animals moving along the path) rather than left purposefully by turtles (i.e., probably not social in nature, Krochmal et al., 2015). Ultimately, the cues used to navigate the specific and complex paths in our system remain unknown and require additional study. Doing so will help us understand how animals migrate through complex environments.
ACKNOWLEDGMENTS
This research was funded by Washington College's Provost's Office, Middendorf Fund, Hodson Trust, and Franklin and Marshall's Hackman Fund and College of Grants. We thank E. Counihan, A. Roth, W.B. Gerwig, F. Rauh, and J. Sullivan for assistance in the field. We also thank M. Conner, R. Fleegle, D. Startt, and Chesapeake Farms / Corteva Agriscience for access to and support on the site. The authors declare no conflicts of interest.




