An Easy Method to Test Odour Recognition in Songbird Hatchlings
Abstract
We describe an easy method to test odour detection and recognition in 1-d-old zebra finch hatchlings (Taeniopygia guttata). Day-old chicks beg in a stereotypical posture, which can be induced by directing gentle puffs of air from a plastic wash bottle near the face. We used this method to experimentally test whether begging duration of chicks was indicative of nest odour recognition. We manipulated the olfactory environment of 12 nests throughout incubation and hatching with either an artificial odour (orange oil) or with a neutral control (tap water). We then presented these two stimulus odours to 25 day-old chicks and measured the duration of the first begging bout exhibited for each odour. Zebra finches hatched in a nest environment enriched with orange oil scent begged significantly longer when exposed to orange oil odour, compared to control hatchlings. Our simple testing procedure can be used to efficiently quantify odour recognition and/or preference in altricial songbirds at a very early developmental stage.
Introduction
Testing odour recognition or detection abilities are essential to understand the functions and mechanisms of olfactory communication (Eisenberg & Kleiman 1972; Albone 1984; Hildebrand & Shepherd 1997). Birds and especially songbirds have traditionally been considered ansomic or microsmatic (Bang & Cobb 1968). However, evidence suggests that even songbirds use their sense of smell in important and adaptive ways, including navigation (Holland et al. 2009), foraging (Mennerat et al. 2005) and social communication (e.g. Amo et al. 2012a,b; Whittaker et al. 2013; Krause et al. 2014). Thus far, every study investigating the chemical senses of songbirds has focused on fledglings (Caspers & Krause 2011; Krause et al. 2012; Caspers et al. 2013) or adults (e.g. Mennerat 2008; Amo et al. 2012a,b; Krause & Caspers 2012; Gwinner 2013; Whittaker et al. 2013; Krause et al. 2014). Almost nothing, however, is known about the songbird olfaction at earlier developmental stages.
Porter et al. (1999) developed a method to test odour recognition of day-old precocial chicks. Domestic chicks (Gallus gallus domesticus) held on their backs, underneath a heat lamp, in a ‘sleep-like’ state, exhibited significantly different reactions to a series of volatile odours. For example, individuals exhibited a relatively stronger response towards mint compared to lavender or orange odour (Porter et al. 1999).
Here, we asked whether day-old altricial nestlings recognize the odour of their home nest, and we quantified their responses to test odours by adapting Porter's et al. (1999) protocol. Birds appear generally capable of learning odours during embryonic development (in ovo; Bertin et al. 2010, 2012; Hagelin et al. 2013) and early post-hatch (Caspers et al. 2013). Compared to precocial chicks, however, young altricial hatchlings typically have their eyes closed, produce little if any sound, and have a very limited behavioural repertoire, apart from stereotypical begging (Starck & Ricklefs 1998).
Previous investigations have demonstrated that zebra finch fledglings prefer the odour of the nest in which they hatch (Caspers & Krause 2011; Krause et al. 2012; Caspers et al. 2013). We specifically tested whether day-old zebra finches incubated in experimental nests scented with orange oil would exhibit a differential response towards orange oil, compared to control chicks. We measured the duration of an altricial chick's begging response to quantify differences in odour recognition.
Methods
Experimental Design and Nesting Environment
Experiments were performed from April to May 2013 at the Department of Animal Behaviour, Bielefeld University, Germany. Adult zebra finch males and females from the university's domesticated laboratory stock (Forstmeier et al. 2007; Hoffman et al. 2014) were randomly assigned to create 12 breeding pairs. Pairs were divided equally into two-odour treatment groups (orange oil and water control; described below). Each pair was housed in a two-compartment cage (83 × 40 × 30 cm) with a wooden nest box attached (15 × 15 × 15 cm; Fig. 1a).
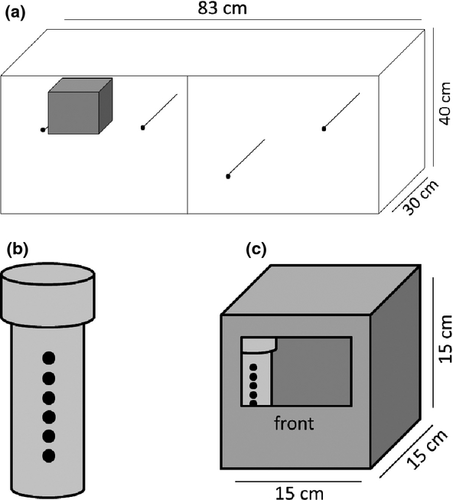
Prior to nest building, we added a perforated 50-ml Falcon Tube™ containing a cotton swab with odour treatment into the corner of each empty nest box (Fig. 1b,c). Nest boxes of the orange oil group (n = 6 pairs) received cotton swabs treated with two droplets of Florida orange oil (Roth, #6728.1) and nine droplets of tap water. Nest boxes of the control group (n = 6 pairs) received cotton swabs treated with nine droplets of tap water only. Each tube contained eight 2 mm perforations (Fig. 1b), which allowed volatiles from the swab to diffuse into the nest box (Fig. 1c). Cotton swabs were renewed twice a week to maintain the olfactory environment. Any visual stimuli of odoured swabs was eliminated, as all tubes had been covered in a layer of brown tape prior to drilling perforations; nest boxes were also enclosed, dark environments. To avoid odour contamination, cages of both treatment groups were kept in separate rooms, but under the same standardized laboratory conditions (below), with the same animal caretaker.
All birds were kept under a 14-h dark: 10-h light cycle, constant temperature (24.5–25.5°C), and received water and seed ad libitum. Three times a week pairs received germinated feed and a nutritional supplement (Egg Food for Tropical Finches, CéDé, Evergern, Belgium). Coconut fibres were provided as nest material. All nests were checked in a daily routine for new eggs or new hatchlings. Handling of nest, egg or hatchling was done with fresh nitrile gloves.
Behavioural testing protocol
Prior to removing a chick from the nest for testing, we prepared two 500-ml wash bottles, each with a test scent, for odour delivery (Carl Roth, T401.1). One bottle contained orange oil (cotton swab with two droplets of orange oil and nine droplets of water), and the other contained the tap water control (cotton swab with nine droplets of water only). Fresh test bottles were used for each chick.
Day-old altricial chicks beg in a stereotypical posture, which can be induced by directing gentle puffs of air from a plastic wash bottle near the face (Figs 2 and 3). To test for odour recognition, a hatchling was laid on the experimenter's palm and exposed to the two test scents in a manner adapted from Porter et al. (1999). Namely, each chick was exposed to the scent of orange oil or tap water by gently puffing the 500-ml wash bottle ten times (approx. 1 puff/s) within 1 cm of the chick's nares. Begging behaviour (Figs 2b and 3c) was recorded via video. The experimenter waited until the chick was quiet again (<10 s), before administering and recording the chick's response to the second test odour (Figs 2a and 3d). Presentation order of the two odours was randomised across chicks, and the experimenter was blind with respect to the odour stimulus. That is the experimenter did not know during the behavioural test which bottle contained which odour. A complete two-odour test sequence typically lasted 30–60 s. All chicks were tested on the day of hatching during the early afternoon (1200–1400H). The experimenter wore a fresh pair of nitrile gloves for each test sequence.
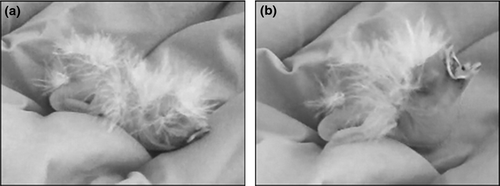
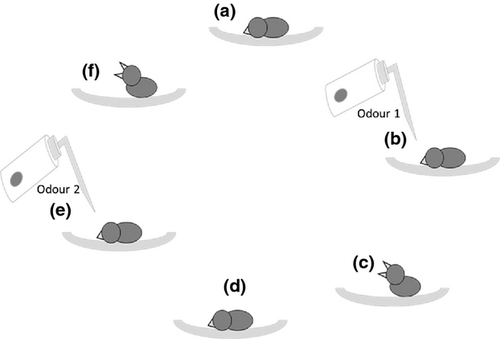
Video footage recorded the entire two-odour test of each chick and was later scored by a double-blind observer. The observer recorded the duration (in seconds) of the first bout of begging that was initiated while the odour stimulus was administered. We ensured responses specifically coincided with odour presentation, as many different tactile and acoustic cues can initiate stereotypical begging in altricial nestlings (Bischof & Lassek 1985). If a chick began begging immediately when placed into the palm, we waited until it lay motionless before initiating a trail (approx. 10–20 s; Fig. 2a). Unlike Porter et al. (1999), we quantified chick responses as a continuous variable (in seconds), rather than a ranked category.
Statistical Analysis
We first verified that exposure to orange oil scent during incubation and hatching did not adversely affect behaviour by comparing begging duration of chicks from the two treatments to the control stimulus (tap water) using a Mann–Whitney U-test. To test whether the treatment influenced chick responses to the two-odour stimuli, we analysed relative differences in the duration of a chick's first begging bout (orange oil – water control) using a linear mixed effect (LME) model. The LME included nest treatment (orange oil/control) as a factor and brood ID (cage ID) as a random factor. Residuals of data were controlled for a normal distribution. To test for evidence of odour recognition, we also ran a Wilcoxon signed-rank test on each treatment group which examined the difference in response to the two stimuli.
Ethical Statement
Housing and breeding of the birds were conducted under permission of the Veterinäramt Bielefeld, Germany (Az: 530.421630-1, 18.04.2002). All birds remained in the laboratory stock after the experiment was completed.
Results
In total, all but one pair of birds successfully hatched young. Odour treatment had no effect on brood size, or hatching success (Table 1). Of the 39 chicks produced, 25 responded to the two test odours and were included in analyses (Table 1). Begging response to the control (tap water) was similar in both treatments (Mann–Whitney U-test: z = 1.24; p = 0.215 (Table 1)).
| Orange oil treatment | Tap water control | |
|---|---|---|
| Breeding pairs (pairs that had hatchlings) | 6 (6) | 6 (5) |
| Mean brood size ± SD | 3.5 ± 0.95 | 3.67 ± 0.47 |
| Mean hatching success ± SD | 74.2 ± 22.8% | 75.3 ± 4.8% |
| Number of chicks that showed a begging response (chicks that did not beg)a | 16, (5) | 9, (7) |
| Begging response to the tap water control odour (Median, Min.-Max.) | 10.5, 0–26 s | 6, 0–19 s |
- a In each treatment group 1 chick died shortly after hatching.
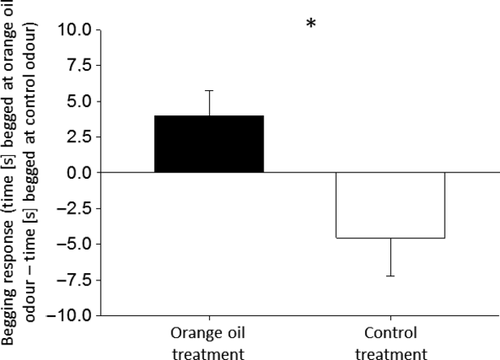
Odour treatment was significantly associated with relative differences in begging duration (LME: treatment F1,23 = 7.93, p = 0.01). Specifically, chicks hatched in odour enriched nests begged significantly longer, on average 13 s, when exposed to orange oil (Fig. 4). Chicks from the orange oil treatment responded differently to the two-odour treatments (Wilcoxon signed-rank test: N = 16; z = 2.13; p = 0.033), whereas chicks from the control group did not (Wilcoxon signed-rank test: N = 9; z = −1.36; p > 0.05).
Discussion
Here we show that experimental manipulation of odours in the nest box environment of an altricial songbird can alter olfactory responses of day-old chicks, as measured by the duration of stereotypic begging (Fig. 4). Day-old hatchlings from nest boxes enriched with orange oil exhibited relatively longer begging responses when exposed to orange oil than the control group (Fig. 4). A relatively greater response to experimentally altered nest odour is consistent with studies of older zebra finch chicks, in which fledglings preferred the natural scent of their natal nest (Caspers & Krause 2011; Krause et al. 2012; Caspers et al. 2013). Our results now suggest that recognition of nest-associated odours in songbirds develop in ovo and/or within 24 h of hatching. Although it is presently unclear why control chicks lacked any odour preference, responses were generally in the opposite (predicted) direction from the orange oil group (Fig. 4), which warrants further study.
We know relatively little about early olfactory development of altricial songbirds. However, there is growing evidence indicating that avian odour learning not only occurs post-hatch, but also in ovo (Sneddon et al. 1998; Bertin et al. 2010, 2012; Hagelin et al. 2013). Such developmental sensitivity raises the tantalising possibility that birds imprint to scents they experience early in life (e.g. Sneddon et al. 1998; Bonadonna et al. 2006; Cunningham & Nevitt 2011; Caspers et al. 2013). Chicks, for example, can learn the odours of food (Bonadonna et al. 2006), or aromatic plants used during nest construction (Gwinner & Berger 2008; Mennerat 2008; Gwinner 2013). It is also possible that body odours provide an early chemosensory basis for chicks to imprint to parents, kin or self, and thereby avoid maladaptive breeding decisions later in life (e.g. Bonadonna & Nevitt 2004; Mardon & Bonadonna 2009; Bonadonna & Sanz-Aguilar 2012; Krause et al. 2012). Aside from zebra finches, only one other study has addressed early odour exposure in a songbird. Adult European Starlings (Sturnis vulgaris) exhibited preferences for experimentally manipulated nest odours they had experienced as chicks (Gwinner & Berger 2008).
Our test protocol lays the groundwork for what we hope will result in further studies of olfactory development in young of altricial species. The simple, hand-held method focuses on the duration of stereotyped begging as the key criterion for quantifying odour perception. Newly hatched altricial species exhibit relatively few behaviours except for stereotyped begging, which is energetically costly (Bachman & Chappell 1998). Hence, we chose duration of begging as the proxy for magnitude of response. Our focus on a single, continuous response variable (begging) differs from the ranked scale that Porter et al. (1999) assigned to precocial chicks. We believe our method is readily adaptable to laboratory or field uses, enabling scientists to quantify sense of smell of hatchling songbirds at a very early developmental stage.
Acknowledgements
BAC is currently funded by a Freigeist Fellow of the Volkswagen Foundation. ETK is funded by a fellowship of the Volkswagen Foundation under its Evolutionary Biology Initiative (85994). We also thank Ulla Kodytek for taking care of the animals. We are also grateful to Sam Slowinski and an anonymous referee for their helpful comments on an earlier version of the manuscript.




