Assessing the diagnostic performance of investigations in pediatric myoclonic epilepsies: A retrospective cohort study
Abstract
Objective
The primary purpose was to assess the diagnostic performance of investigations in children with myoclonic epilepsy. The secondary objectives were to examine the definitive syndromic diagnoses and report the outcomes of pediatric myoclonic epilepsies.
Methods
We conducted a retrospective monocentric study from a pediatric center for rare epilepsies. We included pediatric patients investigated for myoclonic epilepsy at our center from 2009 to 2022. Data were collected from their medical records.
Results
Forty-one children were included; 32 (78%) underwent untargeted etiological investigations, including brain magnetic resonance imaging and diverse laboratory tests to rule out an underlying etiology for progressive myoclonus epilepsy (PME). These investigations led to an etiological diagnosis of epilepsy for two patients, exclusively based on genetic investigations. At the final follow-up, an underlying etiology for epilepsy was established for nine patients (22%). The definitive syndromic diagnoses were diverse, comprising myoclonic epilepsy in infancy, epilepsy with myoclonic absences, Rasmussen syndrome, and PME. Some patients were diagnosed with nonsyndromic developmental and epileptic encephalopathy or unclassified nonsyndromic myoclonic epilepsy. Developmental delay or regression at the initial evaluation was found to be significantly associated with an unfavorable neurological outcome, the total number of antiseizure medications (ASMs) prescribed, and the unlikelihood of achieving ASM freedom. No patients with an abnormal head circumference or born of a consanguineous union were in the favorable neurological outcome group, although this finding did not reach statistical significance.
Significance
Except for the need to promptly identify diseases for which precision medicine treatments are available, a first-line genetic approach seems reasonable to investigate children diagnosed with epileptic myoclonus.
Key points
- Myoclonic epilepsies with myoclonic seizures only have a better prognosis than myoclonic epilepsies with both myoclonic seizures and other seizure types.
- Developmental abnormalities are linked to a higher total number of antiseizure medications in pediatric myoclonic epilepsies.
- Nonsyndromic myoclonic epilepsy is frequently observed in children.
1 INTRODUCTION
Epileptic myoclonus or myoclonic seizure is defined as a very short electromyographic burst or silent period (<50 ms for positive myoclonus, up to 500 ms for negative myoclonus) preceded by an epileptiform discharge on the electroencephalogram (EEG).1, 2 Myoclonic seizures are a key feature in various pediatric epilepsy syndromes, such as myoclonic epilepsy in infancy (MEI),3 juvenile myoclonic epilepsy (JME), and progressive myoclonus epilepsy (PME).4 Myoclonic seizures could also be the predominant seizure type in epilepsy with myoclonic atonic seizures (EMAtS). There are also a large number of patients who have a myoclonic seizure as the predominant seizure type without fulfilling the criteria for any syndrome; they are usually called myoclonic epilepsies (MEs). The most common etiologies are structural, metabolic, and genetic, particularly chromosomal disorders.
The entity of PME, first described by Herman Lundborg in 1903,5 is currently defined by the presence of myoclonus, progressive motor and cognitive impairment, sensory and cerebellar signs, and abnormal background slowing on EEG that appear in an individual with prior normal development and cognition.6 PME is a rare syndrome encompassing various underlying etiologies, such as neuronal ceroid lipofuscinosis (NCL) and mitochondrial disorders in infants and children.6, 7 Due to the progressive nature of these conditions, PMEs might be challenging to diagnose in the early stages.8
However, early etiological diagnosis is crucial for prognosis and genetic counseling, and it can influence the choice of antiseizure medication (ASM).9 This becomes even more critical when an etiology-specific treatment is available and needs to be initiated without delay, as in NCL type 2 (NCL2).10 Furthermore, new therapeutic strategies are emerging with advances in gene therapy.11
For pediatric neurologists in charge of a patient with an unidentified epileptic syndrome, MEs are associated with suspicion of PME, including NCL2, or some treatable underlying etiologies, particularly glucose transporter type 1 deficiency syndrome. Various investigations are prescribed in this context, including laboratory tests such as enzymatic activity or glycorrhachia measurements.11, 12 However, daily practice does not investigate most patients with JME and EMAtS without alerting signs.13, 14
This retrospective longitudinal study aimed to assess the diagnostic performance of investigations in a cohort of patients with MEs diagnosed from 2009 to 2022 at a tertiary care center for rare epilepsies. Furthermore, we described the definitive syndromic diagnoses and the outcomes of these patients.
2 MATERIALS AND METHODS
2.1 Study design
This is a monocentric retrospective study. We identified patients in the French pediatric neurology department database of Robert Debré Hospital, Paris, who were admitted to our daily care center for ME investigations. We reviewed each electronic medical record to collect data from January 1, 2004 to December 31, 2023.
2.2 Outcomes
2.2.1 Primary outcome
The primary outcome was to determine whether investigations, including the untargeted investigations from our department's written protocol (see below), contribute to the diagnosis of underlying causes for children with ME.
2.2.2 Secondary outcomes
This study had secondary outcomes: (1) to characterize the patients followed for ME in terms of electroclinical phenotype, imaging characteristics, and treatment; (2) to describe the syndromic and etiological diagnoses made during the follow-up of these patients; and (3) to describe the outcome of the patients with at least 2 years of follow-up.
Patients with an unfavorable neurological outcome were defined as those fulfilling at least one of the following criteria: drug-resistant (>2 ASMs used), non-seizure-free, unable to attend regular school (which comprised regular school with special needs and a unit for school inclusion), no independent walking, or inability to form sentences.
Patients with an unfavorable epileptic outcome were defined as those fulfilling at least one of the following criteria: drug-resistant (>2 ASMs used) or non-seizure-free.
Patients with an unfavorable developmental outcome were defined as those who fulfilled the following criterion: they could not attend regular school (which comprised regular school with special needs and a unit for school inclusion).
2.3 Investigations
A homogeneous set of investigations has been implemented at our center since 2017 for patients exhibiting ME, except for patients with a precise syndromic diagnosis of JME or EMAtS or when phenotype strongly suggests a specific etiology. We performed awake and asleep video-EEG (with Fp1, Fp2, C3, C4, F7, F8, T5, T6, P3, P4, and O1 electrodes positioned according to the 10/20 system and electrodes to record deltoid electromyographic activities, electrocardiogram, and respiratory movements) including intermittent photic stimulation (IPS) at .5, (1 for video-EEG recorded after January 1, 2022,) 2, 4, 6, 8, 12, 14, 16, 20, 30, 50, and 60 Hz. Most patients had at least one video-EEG with a prolonged IPS at slow frequencies (.5, 1, 2, and 4 Hz). Brain electric activity was recorded on a Nihon-Khoden device up to December 2021 and a Compumedics (Graël or Neuvo amplifier) from January 2022. The investigations included brain magnetic resonance imaging (MRI) performed with a dedicated epilepsy protocol in accordance with the standard care protocol in use at the time of the investigation, ophthalmological fundus examination, visual evoked potential test and electroretinogram (VEP-ERG), and blood lactate, pyruvate, ketone bodies, glucose, and free fatty acids at fasting and after a meal. Other recommended investigations are not mandatory (according to clinical assessment), including white blood cell enzymatic activity of palmitoyl-protein thioesterase 1 and tripeptidyl peptidase 1, lysosomal enzyme activity, comparative genomic hybridization (CGH) array or single nucleotide polymorphism (SNP) array, lumbar puncture with cerebrospinal fluid (CSF) glucose for calculation of the blood/CSF, CSF lactate and pyruvate, urinary oligosaccharides, and a skin biopsy (electron microscopy and functional mitochondrial respiratory complexes analysis).
Other investigations, not explicitly recommended for patients exhibiting epileptic myoclonus, were often prescribed by practicians as part of a general approach to screening for metabolic disorders: plasma chromatography of amino acids (CAA), blood ammonia, plasma carnitine, and acylcarnitine profile, urinary CAA, urinary chromatography of organic acids (COA), urinary carnitine, and lactate.
2.4 Syndromic diagnoses
For the syndromic diagnoses, we used the 2017 International League Against Epilepsy (ILAE) classification.15 We reviewed the files and adapted previous terminologies to this current classification. When no syndrome was identified, we categorized the patients into the following: (1) developmental and epileptic encephalopathy (DEE), defined as severe early onset epilepsy and neurodevelopmental comorbidity that may be attributable to the underlying cause or uncontrolled epileptic activity; or (2) ME that did not meet the criteria for any syndrome or DEE and was not associated with slowing or regression after the onset of epilepsy, which was labeled as nonsyndromic ME.
2.5 Statistics
All statistics were performed using R (Core Team [2024], R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, https://www.R-project.org/) with the interface RStudio v2024.4.2.764 (Posit team [2024], RStudio: Integrated Development Environment for R, Posit PBC, http://www.posit.co/).
Associations between outcome and the initial characteristics of patients and between initial development and outcome characteristics were analyzed for continuous variables with the Wilcoxon rank-sum exact test and categorical variables with Fisher exact test or Pearson chi-squared test (according to the sample size). We defined statistical significance as p < .05. Missing data were excluded from the analysis to maintain the integrity of the statistical results.
2.6 Ethics
The research ethics committee of Robert Debré Hospital (Comité d'Evaluation de l'Ethique des Projets de Recherche de Robert Debré) approved the study (2023-660bis). According to French law, all legal guardians of patients in the study received written notice, including a procedure for expressing their opposition.
3 RESULTS
Fifty-nine patients were identified in our database. Nine patients were excluded because they were referred from another hospital for a second opinion. Nine patients were excluded after reviewing their files because they did not exhibit any epileptic myoclonus. A total of 41 patients were included in the study.
Nine patients (9/41) did not undergo untargeted investigations. One had a family history of ceroid lipofuscinosis type 2 (CLN2), one had consecutive brain MRIs diagnosing Rasmussen syndrome (RS), and one had a history and brain MRI consistent with hypoxic–ischemic encephalopathy. Two patients had prior investigations for other symptoms, another patient with a predominant phenotype of autism spectrum disorder was prescribed only genetic testing, and one had focal seizures and underwent a restrained set of investigations. A patient with multiple seizure types did not undergo additional tests to determine the cause of the ME. One patient had parents who refused the investigation during the admission.
The study population consisted of 41 patients, 24 of whom were male (58.5%). Table 1 presents their characteristics based on data collected during ME investigations.
| Characteristic | Valuea | NA |
|---|---|---|
| Sex | ||
| M | 24 (58.5%) | |
| F | 17 (41.5%) | |
| Age at first seizure, years | 2.1 (1.0, 4.0) | 2 |
| Age at first myoclonic seizure, years | 2.8 (1.2, 4.7) | 3 |
| Age at first neurology consultation, years | 3.3 (2.0, 5.7) | 11 |
| Delay from first seizure to first neurology consultation, months | 7.3 (2.6, 22.8) | 11 |
| Reported type of the first seizure [except febrile seizure] | ||
| Myoclonic | 25 (61.0%) | |
| Focal | 7 (17.1%) | |
| Generalized unclassified | 4 (9.76%) | |
| Absence | 1 (2.44%) | |
| Atonic | 1 (2.44%) | |
| Head dropping | 1 (2.44%) | |
| Spasm | 1 (2.44%) | |
| Tonic–clonic | 1 (2.44%) | |
| Febrile seizures | 8 (19.5%) | |
| Other seizure types | 23 (56.1%) | |
| Absence seizures | 12 (29.3%) | |
| Focal seizures | 9 (22.0%) | |
| Tonic–clonic seizures | 5 (12.2%) | |
| Generalized seizures without specification | 5 (12.2%) | |
| Atonic seizures | 3 (7.32%) | |
| Drop head seizures | 3 (7.32%) | |
| Spasms | 1 (2.44%) | |
| Tonic seizures | 1 (2.44%) | |
| Myoclonia-induced falls | 8 (19.5%) | |
| Unrelated parents | 28 (82.4%) | 7 |
| Family or neonatal history | ||
| None | 37 (90.2%) | |
| Family diagnosis | 1 (2.44%) | |
| Hypoxic–ischemic encephalopathy | 1 (2.44%) | |
| Premature birth [28 weeks of pregnancy] | 1 (2.44%) | |
| Relevant family history | 1 (2.44%) | |
| Developmental delay | 22 (53.7%) | |
| Developmental regression | 2 (4.88%) | |
| Head circumference | 9 | |
| Normal [2 SD] | 26 (81.3%) | |
| Congenital megalencephaly | 2 (6.25%) | |
| Progressive microcephaly | 2 (6.25%) | |
| Congenital microcephaly | 1 (3.13%) | |
| Progressive megalencephaly | 1 (3.13%) | |
| Clinical exam | ||
| Normal | 24 (58.5%) | |
| Neurological finding | 9 (22.0%) | |
| Dermatologic finding | 5 (12.2%) | |
| Dysmorphic features | 3 (7.32%) | |
| MRI | 1 | |
| Normal | 17 (42.5%) | |
| Nonspecific white matter abnormality | 9 (22.5%) | |
| Other nonspecific abnormality | 4 (10.0%) | |
| Nonspecific cortical atrophy and white matter abnormality | 2 (5.00%) | |
| Periventricular leukomalacia | 2 (5.00%) | |
| Ventricular enlargement without hydrocephalus | 2 (5.00%) | |
| No MRI | 1 (2.50%) | |
| Nonspecific cortical atrophy | 1 (2.50%) | |
| Nonspecific perfusion abnormality | 1 (2.50%) | |
| Structural etiological finding | 1 (2.50%) | |
| EEG background | 2 | |
| Normal with sleep recording | 14 (35.9%) | |
| Abnormal organization | 11 (28.2%) | |
| Normal without sleep | 6 (15.4%) | |
| Organized but too sharp | 5 (12.8%) | |
| Slow | 2 (5.13%) | |
| Asymmetric | 1 (2.56%) | |
| Interictal generalized spike or polyspike-and-wave | 13 (33.3%) | 2 |
- Note: Data were collected at the time of myoclonic epilepsy investigations or first neurological consultation.
- Abbreviations: EEG, electroencephalographic; F, female; M, male; MRI, magnetic resonance imaging; NA, not available.
- a n (%) or median (Q1, Q3).
The first seizure (excluding febrile seizures) occurred at a median age of 2.1 years (interquartile range [IQR] = 1.0–4.0) and was a myoclonic seizure for 25 patients (61%). Eight patients (19.5%) experienced at least one febrile seizure. The median delay from the first seizure to the first neurology evaluation was 7.3 months (IQR = 2.6–22.8). More than half of the patients (56.1%) had other seizure types, such as absences (29.3%) and focal (22%) seizures.
Most patients had no family or neonatal history (90.2%) and were not born from consanguineous unions (82.4%).
Developmental delay was present in 22 patients (53.7%) and regression in two (4.88%). More than half of the patients' clinical examinations were normal (58.5%); the abnormal findings were mainly neurological (nine patients, 22%). The head circumference was in the normal range for 81.3% of the patients.
MRI results showed nonspecific abnormalities, with only 17 (42.5%) having normal MRI.
The first EEG report leading to the diagnosis of epileptic myoclonus was available for 39 of 41 patients exhibiting normal background (n = 20), with sleep recording for 14 patients (35.9%). An abnormal organization was found for 11 patients (28.2%). Interictal generalized spikes or polyspike-and-wave were seen in one third of patients (13, 33.3%). For four patients, this first EEG showed nonepileptic myoclonus.
3.1 Characteristics of patients who underwent pediatric ME investigations
Characteristics of patients at the time of the ME investigation prescription are presented in Table 2. Thirty-two of 41 patients (78%) underwent untargeted ME investigations.
| Characteristic | Valuea | NA |
|---|---|---|
| Treatment | 2 | |
| No treatment | 17 (56.7%) | |
| First monotherapy | 8 (26.7%) | |
| Second monotherapy | 3 (10.0%) | |
| First bitherapy [after monotherapy failure] | 1 (3.33%) | |
| Tritherapy [after bitherapy failure] | 1 (3.33%) | |
| Myoclonic seizure frequency | 1 | |
| Several per day | 20 (64.5%) | |
| Not specified | 8 (25.8%) | |
| None or less than annually | 2 (6.45%) | |
| Daily | 1 (3.23%) | |
| Other seizure type frequency | 3 | |
| None or less than annually | 21 (72.4%) | |
| Several per day | 4 (13.8%) | |
| Not specified | 3 (10.3%) | |
| Weekly | 1 (3.45%) | |
| Standard intermittent photic stimulation | 1 | |
| No photosensitivity | 26 (83.9%) | |
| Photosensitivity | 5 (16.1%) | |
| Slow intermittent photic stimulation | 1 | |
| No photosensitivity | 31 (100%) | |
| Time from first neurology consultation to prescription of PME investigations | 10 | |
| First consultation | 14 (63.6%) | |
| <6 months | 5 (22.7%) | |
| From 6 months to 1 year | 2 (9.09%) | |
| >1 year | 1 (4.55%) | |
| ME investigations finished in >1 month | 18 (56.3%) | |
| Diagnosis made by ME investigations | 2 (6.25%) | |
| Diagnostic method, n = 2 | ||
| CGH array or SNP array | 2 (100.0%) | |
- Abbreviations: CGH, comparative genomic hybridization; ME, myoclonic epilepsy; NA, not available; PME, progressive myoclonus epilepsy; SNP, single nucleotide polymorphism.
- a n (%).
ME investigations were prescribed at the first neurology consultation for 14 patients (63.6%). More than half (17, 56.7%) had no ASM at the time of the ME investigation prescription, and eight patients (26.7%) had only a first monotherapy. The majority of patients (20, 64.5%) experienced multiple daily myoclonic seizures, whereas other types of seizures were infrequent; 21 patients (72.4%) either did not have any other types of seizures or experienced them less than once per year. Of the 31 patients tested, no patients showed photosensitivity to slow IPS during EEG recordings. The time to complete the investigations was >1 month for 18 patients (56.3%).
3.2 Investigations performed for ME
The percentage of patients who underwent each test composing the ME investigations is shown in Figure 1. Investigations completed at >80% were CAA, measurements of lactate, pyruvate, ketone bodies, glucose, and free fatty acids, fasting and after a meal (redox), and CSF glucose measurement. At least half of the patients underwent CGH array or SNP array, urinary COA and CAA, CSF lactate measurement, and VEP-ERG.
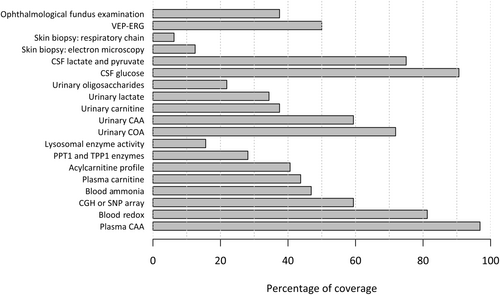
Supplementary Material 1 displays, for each year, the number of patients who underwent ME investigations; 12 patients completed their investigations before 2017 (when a protocol for ME investigations was initiated in our department).
3.3 Etiological diagnosis
Table 2 shows that ME investigations, exclusively by CGH or SNP array, made an etiological diagnosis for two patients. RS was diagnosed in one patient by repeated MRI showing progressive hemispheric atrophy. One patient had a clinical history and brain MRI findings consistent with hypoxic–ischemic encephalopathy. Some patients underwent genetic tests at different times during follow-up and independently from the initial set of investigations carried out to diagnose ME.
Table 3 displays the number of patients who received each genetic test and their results. More than half (21 patients, 51.2%) underwent CGH array or SNP array, depending on the local preference at the prescription time. More than one quarter (11 patients, 26.8%) underwent a panel of genes. Only two patients (4.88%) had whole exome sequencing, whole genome sequencing, or mitochondrial genome sequencing.
| Patients who received each genetic test, n (%) | |
| CGH array or SNP array | 21 (51.2%) |
| Panel of genes | 11 (26.8%) |
| Whole exome sequencing | 2 (4.88%) |
| Whole genome sequencing | 2 (4.88%) |
| Mitochondrial genome sequencing | 2 (4.88%) |
| Genetic diagnosis and method | |
| SCN1A pathogenic variant (2 patients) | Whole genome sequencing, gene panel |
| EEF1A2 pathogenic variant | Whole genome sequencing |
| Duplication (3 patients) | CGH array or SNP array |
| 2q32.1q32.4 | |
| 5p15.33p14.1 | |
| 28-kb interstitial duplication in 4q.31.23 including PRMT9 gene | |
| CLN2 gene pathogenic variants | Single gene sequencing |
| Time from genetic sample to diagnosis (2 not reported), n | |
| <6 months | 2 |
| From 6 months to 1 year | 2 |
| >1 year | 1 |
| Impact of genetic diagnosis | |
| Change of antiseizure medication | 2 |
| Organ-specific screening test | 1 |
- Note: Data are shown for the global population (N = 41).
- Abbreviations: CGH, comparative genomic hybridization; SNP, single nucleotide polymorphism.
Seven genetic diagnoses of an underlying etiology for ME were made, comprising two patients with SCN1A pathogenic variant, one with EEF1A2 pathogenic variant, and three with chromosomic duplication (see details in Table 3). The patient exhibiting pathogenic variants in the CLN2 gene had a family history of CLN2 and underwent single-gene sequencing to confirm enzymatic activity assessment, so the etiological diagnosis was already made.
The interval between blood sampling and genetic diagnosis exceeded 6 months for four patients.
The genetic diagnosis led to a change of ASM for the two patients with SCN1A pathogenic variant without Dravet syndrome phenotype.
We analyzed the study population at diagnosis and prognosis, distinguishing between patients presenting with myoclonic seizures only and those experiencing both myoclonic seizures and additional seizure types (Tables 4 and 5; Supplementary Material 2). At diagnosis, patients with multiple seizure types more frequently exhibited developmental delay (Table 4). Furthermore, those experiencing both myoclonic seizures and additional seizure types had a worse prognosis, not only in terms of epilepsy severity but also regarding non-epilepsy-related outcomes (Table 5).
| Characteristic | Myoclonic only, n = 9a | Other types of seizures, n = 23a | p b | NA |
|---|---|---|---|---|
| Sex | .7 | |||
| M | 5 (55.6%) | 15 (65.2%) | ||
| F | 4 (44.4%) | 8 (34.8%) | ||
| Age at first seizure, years | 1.2 (.7, 2.9) | 2.1 (1.0, 3.7) | .3 | 2 |
| Age at first myoclonic seizure, years | 1.2 (.7, 2.9) | 2.8 (1.4, 5.7) | .13 | 3 |
| Febrile seizures | 3 (33.3%) | 5 (21.7%) | .7 | |
| Other seizure type already present | 0 (0%) | 17 (73.9%) | <.001 | |
| Absences seizures | 0 (0%) | 7 (30.4%) | ||
| Focal seizures | 0 (0%) | 8 (34.8%) | ||
| Tonic–clonic seizures | 0 (0%) | 4 (17.4%) | ||
| Generalized seizures without specification | 0 (0%) | 5 (21.7%) | ||
| Atonic seizures | 0 (0%) | 3 (13.0%) | ||
| Drop head seizures | 0 (0%) | 2 (8.70%) | ||
| Spasms | 0 (0%) | 1 (4.35%) | ||
| Tonic seizures | 0 (0%) | 1 (4.35%) | ||
| Unrelated parents | 6 (85.7%) | 17 (81.0%) | .5 | 4 |
| Family or neonatal history | .8 | |||
| None | 8 (88.9%) | 20 (87.0%) | ||
| Family diagnosis | 0 (0%) | 1 (4.35%) | ||
| Neonatal hypoxic encephalopathy | 0 (0%) | 1 (4.35%) | ||
| Prematurity | 1 (11.1%) | 0 (0%) | ||
| Relevant family history | 0 (0%) | 1 (4.35%) | ||
| Developmental delay | 3 (33.3%) | 17 (73.9%) | .049 | |
| Developmental regression | 0 (0%) | 2 (8.70%) | >.9 | |
| Head circumference | .6 | 6 | ||
| Normal [2 SD] | 8 (88.9%) | 13 (76.5%) | ||
| Congenital megalencephaly | 0 (0%) | 2 (11.8%) | ||
| Congenital microcephaly | 1 (11.1%) | 0 (0%) | ||
| Progressive megalencephaly | 0 (0%) | 1 (5.88%) | ||
| Progressive microcephaly | 0 (0%) | 1 (5.88%) | ||
| Clinical exam | .069 | |||
| Normal | 8 (88.9%) | 10 (43.5%) | ||
| Neurological finding | 0 (0%) | 9 (39.1%) | ||
| Morphological finding | 1 (11.1%) | 2 (8.70%) | ||
| Dermatological finding | 0 (0%) | 2 (8.70%) | ||
| EEG background | .6 | 2 | ||
| Abnormal organization | 2 (22.2%) | 8 (38.1%) | ||
| Normal with sleep recording | 3 (33.3%) | 7 (33.3%) | ||
| Normal without sleep recording | 2 (22.2%) | 2 (9.52%) | ||
| Organized but too sharp | 2 (22.2%) | 1 (4.76%) | ||
| Slow | 0 (0%) | 2 (9.52%) | ||
| Asymmetric | 0 (0%) | 1 (4.76%) | ||
| Interictal generalized spike or polyspike-and-wave | 2 (25.0%) | 10 (45.5%) | .4 | 2 |
- Abbreviations: EEG, electroencephalographic; F, female; M, male; NA, not available.
- Note: Bold value indicates highlight of the significant difference.
- a n (%) or median (Q1, Q3).
- b Fisher exact test or Wilcoxon rank-sum exact test.
| Characteristic | Myoclonic only, n = 9a | Other types of seizures, n = 23a | p b | NA |
|---|---|---|---|---|
| Total number of antiseizure medications used | 2.0 (1.0, 3.0) | 4.0 (2.0, 7.0) | .009 | |
| Drug-resistant epilepsy | 3 (33.3%) | 17 (73.9%) | .049 | |
| Number of antiseizure medications at last follow-up | .14 | |||
| 0 | 6 (66.7%) | 8 (34.8%) | ||
| 1 | 3 (33.3%) | 5 (21.7%) | ||
| 2 | 0 (0%) | 7 (30.4%) | ||
| 4 | 0 (0%) | 3 (13.0%) | ||
| Frequency of myoclonus seizures | .7 | |||
| None or less than annually | 7 (77.8%) | 15 (65.2%) | ||
| Still occurring, unknown frequency | 0 (0%) | 4 (17.4%) | ||
| Several per day | 1 (11.1%) | 1 (4.35%) | ||
| Weekly | 1 (11.1%) | 1 (4.35%) | ||
| Annually | 0 (0%) | 1 (4.35%) | ||
| Daily | 0 (0%) | 1 (4.35%) | ||
| Frequency of other seizures | .5 | |||
| None or less than annually | 9 (100.0%) | 14 (60.9%) | ||
| Monthly | 0 (0%) | 3 (13.0%) | ||
| Annually | 0 (0%) | 2 (8.70%) | ||
| Still occurring, unknown frequency | 0 (0%) | 2 (8.70%) | ||
| Weekly | 0 (0%) | 2 (8.70%) | ||
| Unfavorable developmental outcome | 2 (22.2%) | 16 (69.6%) | .022 | |
| Language milestone achieved | .4 | |||
| Normal | 7 (77.8%) | 11 (47.8%) | ||
| Sentences | 2 (22.2%) | 6 (26.1%) | ||
| Nonverbal communication | 0 (0%) | 4 (17.4%) | ||
| Words | 0 (0%) | 2 (8.70%) | ||
| Motor milestone achieved | .6 | |||
| Normal | 9 (100.0%) | 17 (73.9%) | ||
| Walking | 0 (0%) | 4 (17.4%) | ||
| Independent moving on knees | 0 (0%) | 1 (4.35%) | ||
| No voluntary movement | 0 (0%) | 1 (4.35%) |
- Abbreviation: NA, not available.
- Note: Bold values indicate highlight of the significant differences.
- a Median (Q1, Q3) or n (%).
- b Wilcoxon rank-sum test or Fisher exact test.
3.4 Syndromic diagnosis
Figure 2 exhibits the proportion of patients classified into each diagnostic group (syndromes and nonsyndromic epilepsies). An equal number of six patients (14.6%) were classified as MEI (detailed characteristics in Supplementary Material 3) and epilepsy with myoclonic absence (EMA; detailed characteristics in Supplementary Material 4). Eleven patients (26.8%) were diagnosed with DEE, and an etiological diagnosis was established for six of them during follow-up.
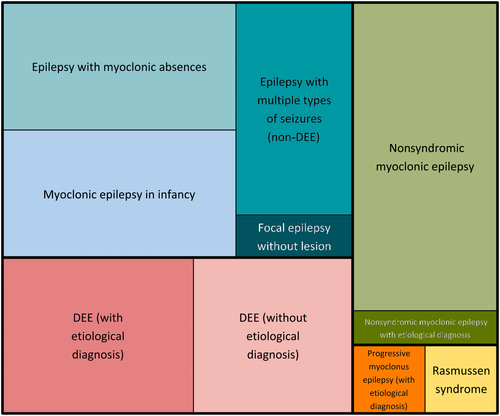
Ten patients (24.4%) were labeled nonsyndromic ME, as they had generalized epilepsy with predominantly myoclonic seizures, which did not match the diagnostic criteria for a syndrome or DEE. Five patients (12.2%) had unclassified epilepsy with multiple types of seizures not matching the criteria for DEE. One patient had focal epilepsy without lesion, another had RS, and a third had progressive myoclonus epilepsy due to CLN2.
3.5 Clinical outcome
We restricted the outcome assessment to the 36 patients who received an extended follow-up of at least 2 years at the time of data collection. For these patients, the median duration of follow-up was 6.1 years (IQR = 4.4–8.8).
To assess which individual characteristics were associated with the outcome at the beginning of the follow-up, we classified patients into favorable or unfavorable neurological outcomes (see Materials and Methods).
The detailed features of the 25 patients classified into the unfavorable neurological outcome group are available in Supplementary Material 4. In this group, institutions were the more prominent type of schooling (12 patients, 48.0%). Six patients could not talk in sentences, but only two patients did not achieve independent walking. Only six patients (24.0%) were free of ASM, but the proportion of patients having no seizures or seizures less than annually was 60.0% (15/25) for myoclonic seizures and 64.0% (16/25) for other seizure types.
To evaluate factors associated with the neurological outcome, we compared the main characteristics of the patients according to their outcomes (Table 6). A statistically significant association was found between the unfavorable neurological outcome and developmental delay or regression outcome at initial evaluation (p = .025) and an abnormal clinical examination (p = .030). Although it was not statistically significant, all patients with related parents and those with abnormal head circumferences were in the unfavorable neurological outcome group.
| Characteristic | Favorable, n = 11a | Unfavorable, n = 25a | p b | NA |
|---|---|---|---|---|
| Age at first seizure, years | 2.9 (1.2, 3.5) | 1.7 (.8, 4.3) | .7 | 2 |
| Age at first myoclonic seizure, years | 2.9 (1.3, 3.0) | 2.4 (1.0, 5.7) | >.9 | 3 |
| Type of the first seizure [except febrile seizure] | >.9 | |||
| Myoclonic | 7 (63.6%) | 14 (56.0%) | ||
| Focal | 2 (18.2%) | 4 (16.0%) | ||
| Generalized unclassified | 1 (9.09%) | 3 (12.0%) | ||
| Absence | 1 (9.09%) | 0 (0%) | ||
| Atonic | 0 (0%) | 1 (4.00%) | ||
| Head dropping | 0 (0%) | 1 (4.00%) | ||
| Spasm | 0 (0%) | 1 (4.00%) | ||
| Tonic–clonic | 0 (0%) | 1 (4.00%) | ||
| Febrile seizures | 3 (27.3%) | 5 (20.0%) | .7 | |
| Other seizure types | 6 (54.5%) | 14 (56.0%) | >.9 | |
| Unrelated parents | 9 (100.0%) | 18 (78.3%) | .3 | 4 |
| Developmental delay or regression | 3 (27.3%) | 18 (72.0%) | .025 | |
| Normal head circumference | 9 (100.0%) | 14 (73.7%) | .14 | 8 |
| Clinical exam | .030 | |||
| Normal | 10 (90.9%) | 11 (44.0%) | ||
| Neurological finding | 0 (0%) | 9 (36.0%) | ||
| Dermatologic finding | 0 (0%) | 3 (12.0%) | ||
| Dysmorphic features | 1 (9.09%) | 2 (8.00%) | ||
| MRI | .2 | 1 | ||
| Normal | 6 (60.0%) | 8 (32.0%) | ||
| Nonspecific white matter abnormality | 3 (30.0%) | 4 (16.0%) | ||
| Nonspecific cortical atrophy or ventricular enlargement | 0 (0%) | 5 (20.0%) | ||
| Other nonspecific abnormality | 0 (0%) | 4 (16.0%) | ||
| Periventricular leukomalacia | 0 (0%) | 2 (8.00%) | ||
| Nonspecific perfusion abnormality | 1 (10.0%) | 0 (0%) | ||
| Structural etiological finding | 0 (0%) | 1 (4.00%) | ||
| EEG background | .073 | 2 | ||
| Normal [with or without sleep recording] | 8 (72.7%) | 9 (39.1%) | ||
| Abnormal organization or slow | 1 (9.09%) | 11 (47.8%) | ||
| Organized but too sharp | 2 (18.2%) | 2 (8.70%) | ||
| Asymmetric | 0 (0%) | 1 (4.35%) | ||
| Interictal generalized spike or polyspike-and-wave | 1 (10.0%) | 12 (50.0%) | .051 | 2 |
- Note: For continuous variables, we performed Wilcoxon rank-sum exact test. For categorical variables, we performed Fisher exact test. For nonspecific cortical atrophy or ventricular enlargement without hydrocephalus, two had nonspecific white matter abnormality, one had cortical atrophy, and two had ventricular enlargement without hydrocephalus.
- Abbreviations: EEG, electroencephalographic; MRI, magnetic resonance imaging; NA, not available.
- Note: Bold values indicate highlight of the significant differences.
- a Median (Q1, Q3) or n (%).
- b Wilcoxon rank-sum exact test or Fisher exact test.
Interictal generalized spike or polyspike-and-wave (on the first EEG with epileptic myoclonus) was a feature observed for 12 patients (50.0%) in the unfavorable group but for one patient (10.0%) in the favorable group (p = .051). Of note, patients exhibiting interictal generalized spike or polyspike-and-wave were diagnosed as diverse syndromes (DEE for six of them, 46.1%). EEG background organization was not significantly related to the neurological outcome (p = .073).
The comparison based on epileptic outcomes (see Materials and Methods) revealed a statistically significant association between the initial EEG background and the epileptic outcome (p = .042). However, all patients with related parents and those with abnormal head circumferences were in the unfavorable epileptic outcome group. We did not find any predictive factor for the developmental outcome.
Supplementary Materials 6 and 7 present the statistical analyses for the unfavorable epileptic and developmental outcome groups (see Materials and Methods).
3.6 Developmental delay or regression and outcomes
To better understand the correlation between development assessed at the beginning of follow-up and various outcomes, we compared the 21 patients with developmental delay or regression to the 15 patients with initial normal development (Table 7).
| Characteristic | Delay or regression, n = 21a | Normal development, n = 15a | p b | NA |
|---|---|---|---|---|
| Total number of ASMs used | 4.0 (2.0, 6.0) | 2.0 (2.0, 3.0) | .032 | |
| Free of ASM | 6 (28.6%) | 10 (66.7%) | .023 | |
| Frequency of myoclonic seizures | .13 | |||
| None or less than annually | 12 (57.1%) | 14 (93.3%) | ||
| Not specified | 4 (19.0%) | 0 (0%) | ||
| Several per day | 1 (4.76%) | 1 (6.67%) | ||
| Weekly | 2 (9.52%) | 0 (0%) | ||
| Annually | 1 (4.76%) | 0 (0%) | ||
| Daily | 1 (4.76%) | 0 (0%) | ||
| Emergence of other seizure types during follow-up | .083 | 1 | ||
| None | 11 (52.4%) | 11 (78.6%) | ||
| Clonic–tonic | 5 (23.8%) | 0 (0%) | ||
| Generalized unclassified | 2 (9.52%) | 0 (0%) | ||
| Myoclonic absence | 1 (4.76%) | 1 (7.14%) | ||
| Absence | 0 (0%) | 1 (7.14%) | ||
| Focal | 1 (4.76%) | 0 (0%) | ||
| Spasm | 0 (0%) | 1 (7.14%) | ||
| Tonic | 1 (4.76%) | 0 (0%) | ||
| Frequency of other seizures | .5 | |||
| None or less than annually | 14 (66.7%) | 13 (86.7%) | ||
| Monthly | 3 (14.3%) | 0 (0%) | ||
| Annually | 2 (9.52%) | 0 (0%) | ||
| Not specified | 1 (4.76%) | 1 (6.67%) | ||
| Weekly | 1 (4.76%) | 1 (6.67%) | ||
| Schooling | <.001 | |||
| Medicosocial institution | 11 (52.4%) | 1 (6.67%) | ||
| Standard school | 1 (4.76%) | 11 (73.3%) | ||
| Standard school with special needs assistant | 4 (19.0%) | 1 (6.67%) | ||
| Unit for school inclusion | 4 (19.0%) | 1 (6.67%) | ||
| Childcare | 0 (0%) | 1 (6.67%) | ||
| No schooling | 1 (4.76%) | 0 (0%) | ||
| Motor milestone achieved | .9 | |||
| Normal | 16 (76.2%) | 14 (93.3%) | ||
| Walking | 3 (14.3%) | 1 (6.67%) | ||
| Independent moving on knees | 1 (4.76%) | 0 (0%) | ||
| No voluntary movement | 1 (4.76%) | 0 (0%) | ||
| Language milestone achieved | .2 | |||
| Normal | 10 (47.6%) | 12 (80.0%) | ||
| Sentences | 5 (23.8%) | 3 (20.0%) | ||
| Nonverbal communication | 4 (19.0%) | 0 (0%) | ||
| Words | 2 (9.52%) | 0 (0%) | ||
| Diagnosis | .048 | |||
| Nonsyndromic myoclonic epilepsy | 3 (14.3%) | 5 (33.3%) | ||
| Epilepsy with myoclonic absences | 2 (9.52%) | 4 (26.7%) | ||
| DEE [without etiological diagnosis] | 5 (23.8%) | 0 (0%) | ||
| DEE [with etiological diagnosis] | 5 (23.8%) | 0 (0%) | ||
| Epilepsy with multiple types of seizures [non-DEE] | 2 (9.52%) | 2 (13.3%) | ||
| Myoclonic epilepsy in infancy | 2 (9.52%) | 2 (13.3%) | ||
| Focal epilepsy without lesion | 0 (0%) | 1 (6.67%) | ||
| Nonsyndromic myoclonic epilepsy with etiological diagnosis | 1 (4.76%) | 0 (0%) | ||
| Progressive myoclonus epilepsy [with etiological diagnosis] | 1 (4.76%) | 0 (0%) | ||
| Rasmussen syndrome | 0 (0%) | 1 (6.67%) | ||
| Etiological diagnosis | 7 (33.3%) | 1 (6.67%) | .10 |
- Note: For continuous variables, use the Wilcoxon rank-sum exact test. We performed Fisher exact test or Pearson chi-squared test (according to sample size) for categorical variables. For the positive etiological diagnosis, the one patient in the normal development group is the patient with Rasmussen syndrome.
- Abbreviations: ASM, antiseizure medication; DEE, developmental and epileptic encephalopathy; NA, not available.
- Note: Bold values indicate highlight of the significant differences.
- a Median (Q1, Q3) or n (%).
- b Wilcoxon rank-sum test, Pearson chi-squared test, Fisher exact test.
An initial delay or regression was associated with a higher total number of ASMs (p = .032) and the unlikelihood of achieving ASM freedom at the end of follow-up (p = .023).
Expectedly, the initial development was associated with the schooling type at the end of follow-up (p < .001). Because developmental characteristics are part of the syndromic definitions, the association between the two (p = .048) was not surprising.
The patient with RS was the one patient in the normal initial development group who reached an etiological diagnosis. Seven of 21 patients (33.3%) in the delay or regression group had an etiological diagnosis determined (see Table 3).
4 DISCUSSION
Conducting investigations to identify etiology in pediatric onset ME results in a limited number of final diagnoses (9/41). However, our study showed that nonsyndromic ME is frequently observed. We show that some features in pediatric onset MEs are linked with unfavorable neurological or epileptic outcomes. Initial developmental delay or regression and initial abnormal clinical examination were associated with a worse neurological outcome, and the initial EEG background was associated with a worse epilepsy outcome. Moreover, initial developmental delay or regression was significantly associated with a higher total number of ASMs and the likelihood of remaining on ASM at the last follow-up point. Some trends were also observed in our cohort, with no patient with an abnormal head circumference or born of a consanguineous union being in the favorable neurological outcome group.
Investigations, even conducted for each patient at diagnosis, resulted in a single diagnosis of PME by untargeted investigations during the 15 years of our study. It was a patient with CLN2, but the diagnosis was made due to the family history. In the meantime, no patient had a syndromic diagnosis of PME with an unknown etiology, suggesting the unlikelihood of undiagnosed disorders responsible for PME. The incidence of PME, mainly very low and with various ages at onset, explained the absence of PME diagnoses in our cohort. In a large cohort of 147 patients with PME from India published in 2010,16 the mean age at onset of pediatric onset PME was 14.6 ± 5.8 years for ME with ragged-red fibers and 5.9 ± 9.1 years for NCL. Studies have shown that the average delay for etiological diagnosis of PME remains as long as 1.4 years, even for patients treated in the past decade,17 emphasizing the ongoing need to enhance diagnostic methods and effectiveness. The importance of achieving a rapid etiological diagnosis in pediatric ME may become more pronounced with the upcoming rise of disease-modifying therapies beyond its current role in facilitating genetic counseling.
Genetic investigation has significantly transformed the field of epilepsy, with a growing focus on its clinical utility.18 In cases of epilepsies and DEE in infancy and early childhood, early targeted whole exome sequencing has already proven to be a cost-effective approach.19 For PME, next generation sequencing in a large Italian cohort of 204 patients increased the overall genetic diagnostic yield to >80%, underscoring the value of a genetic approach for diagnosis.20 In the most recent report of the ILAE Genetics Commission,21 the group recommends early genetic testing as the primary diagnostic tool to diagnose PMEs, given the association of nearly 50 genes with the condition, stating that laboratory tests and tissue biopsies should be used to confirm genetic findings rather than serving as primary diagnostic methods. In our study, genetic tests provided an etiological diagnosis for six patients, resulting in significant changes in the management of three of them.
Approximately half of children younger than 3 years of age are classified with epilepsy syndromes.3, 22 In our study, seven patients met the diagnostic criteria for an ILAE syndrome comprising myoclonic seizures, namely MEI or PME. We also observed six patients with epilepsy with EMA and one patient with RS. The latter had a particular phenotype that included myoclonic seizures. Based on our inclusion criteria, we found that patients with epilepsy with myoclonic absences were investigated similarly to patients with ME. A group of 11 patients had DEEs that could not be classified into another syndrome. Among them, two patients had SCN1A pathogenic variants with a non-Dravet syndrome as previously described.23, 24 Another group consists of the 10 patients with nonsyndromic ME. This group was defined in the first edition of “Guide bleu” (Epileptic Syndromes in Infancy, Childhood, and Adolescence).25 In a more recent study, the term myoclonic encephalopathy in a nonprogressive disorder designates children with myoclonic status who do not have a progressive neurological disorder, regardless of age at onset.26 Despite the meaningful clinical information conveyed by these terms, no such group is recognized in the current ILAE classification.15
We identified a group of nonsyndromic ME without an identification of an etiology. In our cohort of patients with ME, unfavorable neurological outcome was significantly associated with the initial developmental delay or regression. This abnormal development was significantly associated with the total number of ASMs used and the likelihood of remaining on ASM. These results, although based on univariable analysis, are consistent with the existing literature studying predictors of drug-resistant epilepsy27, 28 and recurrence risk after withdrawal.29 Strikingly, a retrospective study of 127 young children with drug-resistant epilepsy demonstrated through multivariable analysis that developmental delay at the time of diagnosis was one of four independent variables.30 Some of the factors with a statistical trend, such as abnormal head circumference or family history of consanguineous union, indirectly point to the genetic origin of ME.
Our study has limitations, including its retrospective real-life design and the inclusion criteria. In real-life studies, routine practice descriptions often face variability in clinician practices, as evidenced by imperfect adherence to local investigation guidelines. The inclusion criteria focused on admission for ME investigations do not allow the identification of all patients with ME but do allow the identification of similar investigations in other syndromes, such as epilepsy with myoclonic absences. The inclusion of patients investigated at daycare centers prevented us from studying patients requiring an initial hospital stay, for example, for myoclonic status epilepticus or neonatal onset epilepsies. The limited number of patients might also have restricted reaching statistical significance in our comparisons by epilepsy. Our findings provide valuable insights, although larger, multicenter studies are needed to confirm these results. In particular, it would be of interest to explore EEG findings as a predictive biomarker of the outcome. Finally, our patients were not all investigated by whole genome sequencing due to the study period. We can hypothesize that a few additional patients might have a final etiological diagnosis.
In conclusion, our study highlights key findings regarding pediatric onset ME. Although our research did not show a high diagnostic rate with early etiological investigation, the treatable nature of some causes of ME makes this evaluation essential and unavoidable. We suggest investigating ME with brain MRI, CSF glucose, targeted tripeptidyl peptidase 1 dosage or molecular biology, and genetic investigations. Currently, genetic testing with whole genome sequencing should be offered early in this context with a possible impact on early diagnosis rate and adequate management. We found some predictive factors on the outcome that could be particularly helpful in informing families on the prognosis in the case of nonsyndromic ME. ME with myoclonic seizures only has a better prognosis than ME with both myoclonic seizures and other seizure types. Our data demonstrate that early developmental abnormalities are significantly linked to a higher total number of ASMs and an increased likelihood of continued ASM use at the end of follow-up. Moreover, patients with abnormal initial EEG patterns, particularly interictal generalized spike or polyspike-and-wave, also tended to have worse outcomes, raising the question of their role as part of epileptic encephalopathy, as recently reported.31 Further research with larger cohorts and more standardized diagnostic protocols will be critical to refining the management of ME in children.
ACKNOWLEDGMENTS
None.
FUNDING INFORMATION
This work benefited from a government grant managed by the Agence Nationale de la Recherche under the France 2030 program (ANR-23-IAIIU-0010).
CONFLICT OF INTEREST STATEMENT
S.A. is deputy editor of Epilepsia. He has received personal fees for lectures or advice from Angelini, Biocodex, Eisai, Encoded, GRIN Therapeutics, Jazz Pharmaceuticals, Longboard, Neuraxpharm, Nutricia, Orion, Proveca, Servier, Stoke, UCB Pharma, and Xenon. He has been an investigator for Eisai, Marinus, Proveca, Takeda, and UCB Pharma. B.D.-P. has received personal fees for lectures or advice from Biocodex, Jazz Pharmaceuticals, and UCB Pharma. None of the other authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




