Magnetic resonance imaging fingerprints of status epilepticus: A case–control study
Abstract
Objective
Status epilepticus (SE) is frequently associated with peri-ictal magnetic resonance imaging (MRI) abnormalities (PMA). However, the anatomical distribution of these alterations has not been systematically studied. The aim of this study was to assess the localization patterns of PMA in patients with SE.
Methods
In this prospective case–control study, we compared the distribution and combinations of diffusion-restricted PMA to diffusion-restricted lesions caused by other neurological conditions. All patients of the SE group and the control group underwent MRI including a diffusion-weighted imaging sequence. Patients with SE were imaged within 48 h after its onset.
Results
We enrolled 201 patients (51 with SE and 150 controls). The most frequent locations of PMA in SE were cortex (25/51, 49%), followed by hippocampus (20/51, 39%) and pulvinar of thalamus (10/51, 20%). In the control group, the cortex was involved in 80 of 150 (53%), white matter in 53 of 150 (35%), and basal ganglia in 33 of 150 (22%). In the control group, the pulvinar of thalamus was never affected and hippocampal structures were rarely involved (7/150, 5%). Involvement of the pulvinar of thalamus and the hippocampus had high specificity for SE at 100% (95% confidence interval [CI] = 98–100) and 95% (95% CI = 91–98), respectively. The sensitivity, however, was low for both locations (pulvinar of thalamus: 20%, 95% CI = 10–33; hippocampus: 39%, 95% CI = 26–54).
Significance
Diffusion-restricted MRI lesions observed in the pulvinar of thalamus and hippocampus are strongly associated with SE. These changes may help physicians in diagnosing SE-related changes on MRI in an acute setting, especially in cases of equivocal clinical and electroencephalographic manifestations of SE.
Key points
- Patients with SE show particular locations for PMA in diffusion-weighted imaging. The most prevalent locations are cortex, hippocampus, and pulvinar of thalamus.
- Hippocampus and pulvinar of thalamus emerged as highly specific locations for SE, when compared to the control group.
- PMA affecting cortex, hippocampus, and pulvinar of thalamus could aid in diagnosing SE when uncertainties occur.
1 INTRODUCTION
Status epilepticus (SE) is a common neurological emergency1, 2 frequently associated with peri-ictal magnetic resonance imaging (MRI) abnormalities (PMA).3, 4 They occur in the ictal or early postictal period, and may resume completely in a matter of days or may persist, leading to structural changes such as hippocampal atrophy or laminar cortical necrosis.5
PMA are seen in different MRI sequences including diffusion-weighted imaging (DWI), fluid-attenuated inversion recovery, perfusion sequences such as arterial spin labeling (ASL), or perfusion with contrast substance. Diffusion restriction is frequently observed in SE,3 making it sometimes difficult to differentiate SE-related MRI changes from those associated with other conditions such as acute ischemic stroke.6
PMA, including diffusion-restricted lesions, appear in brain areas such as cortex, hippocampus, and pulvinar of thalamus.3, 4, 7-9 However, the specificity of these locations for PMA has never been systematically investigated. PMA may involve different parts of the brain simultaneously as a consequence of network aberration. Understanding these aspects may provide a possibility of developing additional diagnostic biomarkers in cases of SE with ambiguous presentations.
The main purpose of this MRI study was to investigate whether specific brain areas are affected in SE.
2 MATERIALS AND METHODS
2.1 Study design and participants
This is a case–control, monocentric study on an adult population (≥18 years old) with diffusion-restricted lesions on cranial MRI.
2.1.1 Index group
The patients with a definite diagnosis of SE at the discharge from the hospital who underwent MRI in the first 48 h after its onset were recruited prospectively at the Department of Neurology, Christian Doppler University Hospital, Paracelsus Medical University, Salzburg, Austria between February 2019 and August 2022. The diagnosis of SE was based on the criteria of the International League Against Epilepsy.10 Ictal electroencephalographic (EEG) recordings were available in all SE patients with the exception of 10 with generalized convulsive SE. Salzburg EEG criteria were employed for diagnosing nonconvulsive SE (NCSE).11
In the index group, we prospectively enrolled 476 patients with an electroclinical diagnosis of SE. Among them, 284 of 476 (60%) underwent MRI, which was performed in the first 48 h of SE onset in 246 of 284 (87%) patients. Eight patients with SE due to global cerebral hypoxia after cardiac arrest were excluded from the analysis. Of the remaining 238 patients, 51 (21%) had diffusion-restricted lesions related to the SE with a hyperintensity in a DWI sequence and a hypointensity in an apparent diffusion coefficient (ADC) map. The median time to MRI in these patients was 10 h (interquartile range [IQR] = 2.2–24).
2.1.2 Control group
Patients with different neurological conditions (stroke, brain tumor, brain trauma, etc.), who had an MRI in an acute setting between February 2019 and July 2019, were identified retrospectively. We limited the analysis to these 6 months because the spectrum of patients with neurological conditions who underwent MRI in this period of time was representative of the 3.5 years when patients of the index group were recruited. Patients with previous history of seizures, seizures in the setting of a present acute condition, SE, or epilepsy were excluded from the control group.
In the control group, we identified 1631 potential patients matched with patients of the index group in terms of sex and age. Patients were excluded from the analysis if MRI was performed on a different machine than patients of the index group (n = 372), if a DWI b-1000 sequence was not performed (n = 168), if a patient had a history of SE, seizures, or epilepsy (n = 43), or if the clinical data could not be retrieved (n = 13). Duplicate files were seen in 27 patients. Of the remaining 1008 patients, 150 (15%) patients had diffusion-restricted lesions with the hyperintensity in the DWI and the hypointensity in the ADC map.
In both groups, only patients with MRI performed on a 3-T machine, Achieva dStream (Philips Medical Systems), at the Department of Neuroradiology, Christian Doppler University Hospital, Paracelsus Medical University, Salzburg, Austria were included in the analysis. In patients who underwent multiple MRIs during the recruitment period, only the first MRI was analyzed. Patients with MRIs of scarce quality (i.e., movement artifacts) were excluded.
2.2 Magnetic resonance imaging
Patients with SE underwent MRI with a standardized protocol described in the supplementary material.
We chose to compare locations of diffusion-restricted lesions in two groups because DWI sequence is included in most MRI protocols of our institution.
The following parameters of the DWI sequence were employed in both groups of patients. Spin-echo echo-planar diffusion imaging was used to acquire 28 slices with echo time = 47 ms, repetition time = 3051 ms, field of view = 230 × 230 mm, and voxel size = 2.05 × 2.56 mm, with a slice thickness of 4 mm and a gap between the slices of 1 mm. The diffusion sequence was acquired with four b-values of 0, 333, 666, and 1000 s/mm.2 Diffusion gradients were applied in three directions. A brain lesion was considered diffusion restricted if its signal was hyperintense in DWI (b-value of 1000) and hypointense in the ADC map.
Diffusion-restricted lesions in the SE group were attributed to PMA if (1) there was a nonvascular distribution in case of multiple lesions, (2) the diffusion-restricted lesion showed hyperperfusion on ASL, or (3) the quantification of diffusion-restricted lesions showed signal intensity ratios of <1.495 for DWI and >.735 for ADC.6
To determine signal intensity ratios for DWI and ADC, we compared intensities of gray values of diffusion-restricted lesions to the healthy mirror side in DWI slices with a b-value of 1000 and in ADC maps. The quantification of the diffusion-restricted lesions was done by drawing manually circular regions of interest using IntelliSpacePortal version 10.1.6
Locations of diffusion-restricted lesions in patients of both groups were categorized as follows: cortex, hippocampus, thalamus, pulvinar of thalamus alone, corpus callosum, basal ganglia, white matter, brainstem, and cerebellum. In the first step of the analysis, lesion locations were determined for each group. In the second step, combinations of lesions were assessed and compared between the two groups.
Two independent reviewers blinded to the clinical data (L.M. and P.T.D.) defined the locations of diffusion-restricted lesions in the SE group. The same process was done for the control group by two reviewers (P.B.V. and P.T.D.). Disagreements were resolved after consulting a third reviewer (G.K.), and the interrater agreement was calculated using Cohen kappa coefficient.
2.3 Statistical analysis
Data were extracted and tabulated, then analyzed with descriptive and inferential statistics using R version 4.1.3 and SPSS. Categorical variables were summarized with frequencies and percentages. Continuous variables were summarized with median and interquartile range. Groups were compared using Fisher exact test, and the resulting p-values were adjusted using Bonferroni–Holm method. Additionally, sensitivities and specificities were calculated for all locations in the SE group compared to the control group. Sample odds ratios (ORs) and their 95% confidence interval (CI) for the most frequently affected locations were calculated to assess associations with cortical involvement, which was defined as presence of either diffusion-restricted lesion or hyperperfusion in a cortical area.
For comparing signal intensity ratios for DWI and ADC between the SE and control groups, Mann–Whitney–Wilcoxon test was utilized.
2.4 Standard protocol approvals, registrations, and patient consents
The Ethics Committee of the Region of Salzburg approved this study on human subjects (approval number 415-E/2422). All patients gave informed consent for MRI, which was performed in the framework of diagnostic workup and a current study.
This study was conducted based on the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.12
3 RESULTS
3.1 Demographic and clinical features
The main demographic and clinical data of patients with SE and the control group are detailed in Tables 1 and 2. In the SE group, 26 of 51 (51%) patients had an NCSE and 17 of 51 (33%) had a convulsive SE (CSE). In eight of 51 (16%) patients, CSE progressed to comatose NCSE.10, 13
| Age, years, median (IQR) | 69 (59–78) |
|---|---|
| Sex, W/M | 26 (51%)/25 (49%) |
| Etiology | |
| Cerebrovascular disease | 17 (33%) |
| Acute/subacute ischemic stroke | 4 (8%) |
| Chronic ischemic stroke | 6 (12%) |
| PRES | 2 (4%) |
| Acute intracerebral bleeding | 3 (6%) |
| Acute subdural hemorrhage | 2 (4%) |
| Intracranial tumor | 8 (16%) |
| Head trauma | 7 (14%) |
| Metabolic disturbance | 5 (10%) |
| Cryptogenic | 3 (6%) |
| Neurodegenerative disease | 2 (4%) |
| Autoimmune disease | 2 (4%) |
| Withdrawal of ASM | 2 (4%) |
| Alcohol related | 2 (4%) |
| CNS infection | 2 (4%) |
| Kikuchi–Fujimoto disease | 1 (2%) |
| DWI ratio, median (IQR) | 1.39 (1.27–1.46) |
| ADC ratio, median (IQR) | .83 (.76–.92) |
- Abbreviations: ADC, apparent diffusion coefficient; ASM, antiseizure medication; CNS, central nervous system; DWI, diffusion-weighted imaging; IQR, interquartile range; M, men; PRES, posterior reversible encephalopathy syndrome; W, women.
| Age, years, median (IQR) | 70 (58–78) |
|---|---|
| Sex, W/M | 62 (41%)/88 (59%) |
| Etiology | |
| Stroke | 122 (81%) |
| Hemorrhage | 12 (8%) |
| Brain neoplasm | 9 (6%) |
| Hypoxic encephalopathy | 3 (2%) |
| Multiple sclerosis | 1 (.7%) |
| ADEM | 1 (.7%) |
| PRES | 1 (.7%) |
| TGA | 1 (.7%) |
| DWI ratio, median (IQR) | 1.70 (1.43–1.92) |
| ADC ratio, median (IQR) | .67 (.56–.79) |
- Abbreviations: ADC, apparent diffusion coefficient; ADEM, acute disseminated encephalomyelitis; DWI, diffusion-weighted imaging; IQR, interquartile range; M, men; PRES, posterior reversible encephalopathy syndrome; TGA, transient global amnesia; W, women.
In the index group, SE had the following etiologies: cerebrovascular disease in 17 of 51 (33%), intracranial tumors in eight of 51 (16%), head trauma in seven of 51 (14%), metabolic disturbance in five of 51 (10%), and cryptogenic (i.e., unknown etiology) in three of 51 (6%). Other etiologies accounted for 11 of 51 (21%) of the cases.
More than half of the patients (30/51, 59%) responded well to treatment (median duration of SE = 3 h, IQR = 1.5–24), whereas 20 of 51 (39%) had refractory SE and one of 51 (2%) had superrefractory SE (median duration of SE in these 21 patients = 24 h, IQR = 3.8–48). Refractoriness of SE was not influenced by time lapse between the first and second line treatments. In 41 of 51 (80%) patients, an ictal or ictal/interictal continuum (IIC) pattern was recorded. In 10 of 51 (20%) who had a CSE, an EEG was recorded after cessation of a CSE and it did not show an ictal pattern. The most frequent patterns on EEG were lateralized periodic discharges seen in 24 of 41 (59%) patients, followed by lateralized rhythmic delta activity, seen in 17 of 41 (41%). In 19 of 51 (37%) patients, an IIC pattern was recorded on EEG. EEG patterns in relation to locations of diffusion-restricted lesions are presented in Table S1.
Patients with SE and diffusion-restricted lesions had longer duration of SE (4.6 h, IQR = 2.44–48 vs. 2 h, IQR = .5–6.38) and greater need of sedation (17/51, 33% vs. 34/187, 18%) when compared to those without diffusion-restricted lesions. The former had a lower rate of SE due to withdrawal of antiseizure medications or cryptogenic SE as compared to the latter (2/51, 4% vs. 20/187, 11% and 3/51, 6% vs. 27/187, 14%; Table S2). Approximately half of patients with SE and diffusion-restricted lesions underwent MRI during ongoing SE (24/51, 47%).
In the control group, acute ischemic stroke was the most frequent diagnosis (122/150, 81%). Other diagnoses were intracerebral hemorrhage (12/150, 8%) and intracranial brain neoplasm (9/150, 6%). Three patients had a hypoxic encephalopathy, one had multiple sclerosis, one had acute disseminated encephalomyelitis, one had a posterior reversible encephalopathy syndrome, and one had transient global amnesia.
We calculated ratios of signal intensity values in the DWI sequence and in the ADC map of the lesion side to the healthy mirror side in both groups. In the DWI sequence, the median of ratios in the SE group was significantly lower as compared to the control group (1.39 vs. 1.70 respectively, p < .001), suggesting less intensity of diffusion restriction in the SE group compared to the control group. In the ADC map, the median of ratios was significantly higher in the SE group as opposed to the control group (.83 vs. .67, respectively, p < .001), suggesting higher signal intensity of the lesions in the SE group compared to the control group.
3.2 Locations of diffusion-restricted lesions
Locations of diffusion-restricted lesions in both groups are shown in Table 3. The interrater agreement regarding locations of diffusion-restricted lesions in the SE group was 92.7%, with a Cohen kappa of .791 indicating a substantial agreement. In the control group, interrater agreement was 99.5%, with a Cohen kappa of .97 indicating almost perfect agreement.
| Locations | SE group, n = 51 | Control group, n = 150 | p | Adj. p | Odds ratio (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|
| Cortex, overall | 25 (49%) | 80 (53%) | .6 | 1.0 | .84 (.45–1.59) | 49% (35–63) | 47% (39–55) |
| Frontal | 11 (22%) | 46 (31%) | .3 | 1.0 | .62 (.29–1.32) | 22% (11–35) | 69% (61–77) |
| Temporal | 4 (8%) | 15 (10%) | .8 | 1.0 | .77 (.24–2.42) | 8% (2–19) | 90% (84–94) |
| Parietal | 11 (22%) | 47 (31%) | .2 | 1.0 | .60 (.28–1.28) | 22% (11–35) | 69% (61–76) |
| Occipital | 9 (18%) | 10 (7%) | .03 | .4 | 3.00 (1.14–7.87) | 18% (8–31) | 93% (88–97) |
| Insular | 4 (8%) | 14 (9%) | 1.0 | 1.0 | .83 (.26–2.64) | 8% (2–19) | 91% (85–95) |
| Hippocampus | 20 (39%) | 7 (5%) | <.0001 | <.0001 | 13.18 (5.13–33.88) | 39% (26–54) | 95% (91–98) |
| Thalamus | 5 (10%) | 17 (11%) | 1.0 | 1.0 | .85 (.30–2.43) | 10% (3–21) | 89% (83–93) |
| Pulvinar alone | 10 (20%) | 0 (0%) | <.0001 | <.0001 | – | 20% (10–33) | 100% (98–100) |
| Basal ganglia | 2 (4%) | 33 (22%) | .002 | .04 | .15 (.03–.63) | 4% (.5–13) | 78% (71–84) |
| White mattera | 1 (2%) | 53 (35%) | <.0001 | <.0001 | .037 (.005–.27) | 0% (0–7) | 65% (57–72) |
| Cerebellum | 1 (2%) | 26 (17%) | .004 | .05 | .09 (.013–.72) | 2% (.1–10) | 83% (76–88) |
| Corpus callosum: splenium | 1 (2%) | 1 (.7%) | .4 | 1.0 | 2.98 (.18–48.53) | 2% (.1–10) | 99% (96–100) |
| Corpus callosum: not splenium | 0 (0%) | 2 (1%) | .6 | 1.0 | – | 0% (0–7) | 98% (94–99) |
| Brainstem | 0 (0%) | 17 (11%) | .008 | .1 | – | 0% (0–7) | 89% (83–93) |
| Unilateral | 43 (84%) | 127 (85%) | 1.0 | 1.0 | .97 (.41–2.34) | 84% (71–93) | 15% (10 – 22) |
| Right | 23 (45%) | 53 (35%) | .2 | 1.0 | 1.50 (.79–2.87) | 45% (31–60) | 65% (57–72) |
| Left | 20 (39%) | 74 (49%) | .3 | 1.0 | .66 (.35–1.27) | 39% (26–54) | 51% (42–59) |
| Bilateral | 8 (16%) | 22 (15%) | .8 | 1.0 | 1.08 (.45–2.61) | 16% (7–29) | 85% (79–91) |
- Note: All p-values were calculated using Fisher exact test and further adjusted using Bonferroni–Holm method. Abbreviations: Adj., adjusted; CI, confidence interval; SE, status epilepticus.
- a White matter comprises internal capsule, external capsule, corona radiata, and centrum semiovale.
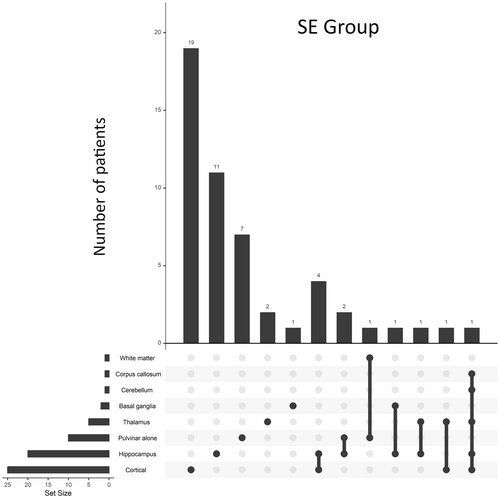
The most frequent locations in the SE group were cortical areas (25/51, 49%), followed by the hippocampus (20/51, 39%) and the pulvinar of thalamus (10/51, 20%). Most lesions were unilateral (43/51, 84%). In eight of 51 (16%) patients, bilateral lesions were observed.
In the control group, the most frequently affected brain structures were cortex (80/150, 53%), white matter (53/150, 35%), and basal ganglia (33/150, 22%). In 127 of 150 (85%) patients, lesions were unilateral, whereas in 22 of 150 (15%) they affected both hemispheres.
DWI alterations of the brainstem and the corpus callosum (other than splenium) were observed only in the control group.
Regarding the most affected locations in the SE group, involvement of the pulvinar of thalamus and the hippocampus had high specificity for SE, at 100% (95% CI = 98–100) and 95% (95% CI = 91–98), respectively. The sensitivity, however, was low for both locations: for the pulvinar of thalamus, 20% (95% CI = 10–33) and for the hippocampus, 39% (95% CI = 26–54). Cortical involvement had both low specificity and sensitivity (Table 3).
Combinations of locations affected by DWI restricted lesions are reported in Figures 1 and 2 for the SE and control groups, respectively. A spectrum of different combinations of PMA was observed in the SE group, without a clear predominance of one combination over the others. Combinations of alterations that were seen only in the SE group were the pulvinar of thalamus + hippocampus (2/51, 4%), pulvinar of thalamus + white matter (1/51, 2%), thalamus + hippocampus (1/51, 2%), and corpus callosum + cerebellum + thalamus + hippocampus + cortex (1/51, 2%).
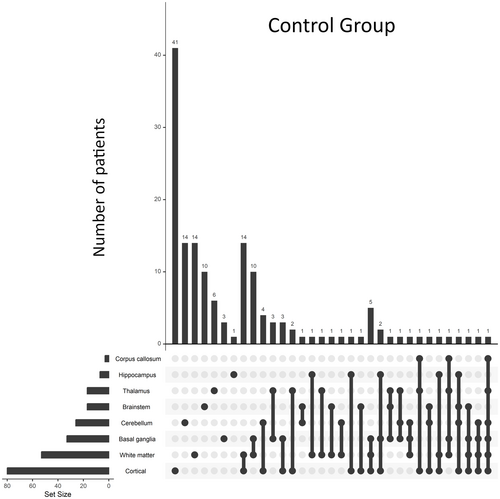
Combinations that were present only in the control group were white matter + cortex (14/150, 9%), white matter + basal ganglia (10/150, 7%), white matter + basal ganglia + cortex (5/150, 3%), thalamus + basal ganglia (3/150, 2%), basal ganglia + cortex (3/150, 2%), thalamus + basal ganglia + cortex (2/150, 1%), thalamus + basal ganglia + cerebellum (1/150, .5%), and all those involving white matter or brainstem.
3.3 Association of diffusion-restricted lesions and peri-ictal hyperperfusion in SE patients
In patients with SE, ASL was performed in 40 of 51 (78%) cases (Figure 3). Among patients who showed diffusion restriction in the pulvinar of thalamus, nine of 10 (90%) underwent ASL. Peri-ictal hyperperfusion in the same location as diffusion restriction was observed in one patient of nine (11%), and an additional ipsilateral cortical hyperperfusion (without diffusion restriction) was seen in three of nine (33%). No significant associations were seen between different cortical areas (affected either in DWI or ASL) and the pulvinar of thalamus when compared to those patients without MRI alterations in the pulvinar of thalamus.
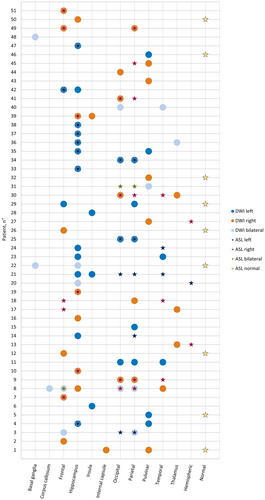
Within the subset of patients displaying DWI alterations in hippocampus, 18 of 20 (90%) underwent ASL as well. In this subgroup of patients, hyperperfusion in the same location was seen in 10 of 18 (56%), and an additional ipsilateral cortical hyperperfusion (without diffusion restriction) was seen in five of 18 (28%). There was a negative association between diffusion restriction in hippocampus and the presence of cortical involvement (either in DWI or ASL) in the frontal (OR = .15, 95% CI = .03–.78), parietal (OR = .17, 95% CI = .04–.68), and occipital regions (OR = .18, 95% CI = .03–.90). No associations were seen between diffusion restriction in hippocampus and diffusion restriction or hyperperfusion in temporal or insular cortices.
4 DISCUSSION
In this case–control study, we compared the locations of diffusion-restricted lesions due to SE to those due to other neurological conditions. We demonstrated that diffusion-restricted lesions of the pulvinar of thalamus and of hippocampus are frequently seen in patients with SE as compared to those with other neurological conditions. In our cohort of patients, diffusion-restricted lesions associated with SE manifested in a variety of combinations of locations, without a clear emerging pattern. In light of the infrequent occurrence of these combinations, their utility in aiding clinicians with the differential diagnosis of various neurological conditions remains limited. However, singularly affected brain regions can hold distinctive value in identification of PMA, as certain individual locations, such as the pulvinar of thalamus and hippocampus, exhibit a heightened specificity for SE.
By assessing peri-ictal hyperperfusion in cortical regions, we could obtain additional information about combinations of PMA locations. No significant association between diffusion restriction in the pulvinar of thalamus and concomitant peri-ictal cortical hyperperfusion was observed. This could be explained by the pulvinar of thalamus being concurrently interconnected with different parts of the cortex14 and only few patients in our cohort (n = 10) having pulvinar involvement. As for the hippocampus, if PMA occurred in the frontal, parietal, or occipital cortices, it was less probable to see a simultaneous involvement of the hippocampus. Conversely, no significant associations were found between PMA in hippocampus and temporal/insular cortices, two brain areas that are closely interconnected.15, 16 This does not exclude; however, a potential positive association were the numbers of patients with these lesions high. A larger sample size would allow clarification of whether combinations of PMA follow the propagation pathways of ictal activity.
4.1 Hippocampus and its connectivity in SE
The role of hippocampus in epilepsy is well documented, with evidence showing both a causal and a consequential relationship with SE.17 Numerous studies have highlighted the vulnerability of hippocampus to prolonged seizures, leading to neuronal loss and atrophy.18-20 Despite the amount of evidence, the exact mechanisms underlying the transfer of information between the hippocampus and other areas of brain during SE remain uncertain. Recent animal studies explored the connectivity between the hippocampus, thalamus, and cortical regions during SE. Altered functional connectivity between frontal cortical regions, the hippocampus, and the thalamus has been reported.21 This study highlighted the critical role of the hippocampus in initiating SE discharges, whereas the thalamus seemed to be crucial in the propagation of the ictal activity, as evidenced by the high connectivity between these structures during the pre-ictal stage. Furthermore, during the ictal and postictal periods, an effective connection was observed between the frontal cortex, the thalamus, and the hippocampus.22 In this study, the wiring between the hippocampus and the ipsilateral thalamus in patients with temporal lobe epilepsy has been studied, revealing enhanced connectivity ipsilateral to the side of the hippocampal sclerosis. This finding suggests structural remodeling along common pathways of seizure propagation. Moreover, in another study,23 superresolution diffusion MRI was employed to study connections of white matter fibers from the hippocampus to other brain areas. The strongest single connection in terms of number of tracks was found between the hippocampus and the ipsilateral thalamus; however, strong connections were also found with adjacent temporal structures (medial parahippocampal gyrus, temporal pole, and anterior transverse collateral sulcus), the medial lingual sulcus, and other limbic and subcortical regions (amygdalae, mammillary bodies, putamen). Not surprisingly, diffusion-restricted lesions involving the hippocampus seemed to follow hippocampal connectivity in our cohort, with the thalamus and the cortex being the most frequently affected locations in combination.
4.2 Pulvinar of thalamus and its connectivity in SE
The corticothalamic excitatory loop has been identified as playing a crucial role in sustaining ongoing seizure activity during SE.24 Notably, the medial part of the pulvinar, which is the largest nucleus of thalamus, appears to be particularly vulnerable during epileptic activity.25, 26 Despite these findings, the precise role of the pulvinar of thalamus in the pathophysiology of SE remains unclear.
The relation between cortex and pulvinar has been highlighted by studies utilizing causal manipulations, which have shown that the corticocortical information transmission is compromised without the influence of the pulvinar of thalamus. Additionally, functional connectivity between cortical areas decreases when the pulvinar is inactivated. Input from pulvinar to cortex has a relevant role in controlling the excitability of neurons in the superficial and/or granular layers. This facilitative role in cortical function may occur through excitatory control of interneurons, as evidenced by observed increases in baseline firing during pulvinar inactivation.27, 28
Neuroimaging and electrophysiology studies provided evidence of extensive connections of pulvinar of thalamus with various cortical and subcortical regions during epileptic activity. In a tractography study, connections between the pulvinar and several cortical regions such as prefrontal/frontal, temporal, parietal, and occipital as well as caudate were illustrated.14 Interestingly, pulvinar involvement seemed to have a predilection for patients with seizures originating in the temporal lobe.29 This is in line with our study, where half of patients with diffusion restriction in pulvinar had focal SE originating from a temporal lobe. Another study showed that ictal changes in the medial pulvinar activity are frequently observed (in 80% of the patients) during temporal lobe seizures.30 This activity is characterized by rhythmic slow waves or rhythmic spikes in seizures arising from mesiotemporal structures, and low-voltage fast activity in seizures of neocortical origin. The reason for this dichotomy is not clear, but it could be explained by two different pathways connecting the temporal lobe to the medial pulvinar; a monosynaptic corticothalamic connection arises mainly from the lateral temporal neocortex, whereas disynaptic or polysynaptic pathways connect the hippocampus to the medial part.31 Of interest, in the same study, propagation between mesiotemporal structures and lateral temporal neocortex did not occur in seizures that did not exhibit medial pulvinar ictal involvement, suggesting a role of pulvinar in the spreading of seizures.30
The pulvinar of thalamus has been significantly less involved in SE originating in the frontal or parietal lobes.26 Beyond its cortical connections, evidence suggests that the pulvinar is also interconnected with hippocampal structures. For instance, a study using structural MRI with tractography evaluated thalamic connectivity in healthy volunteers and patients with mesial temporal lobe epilepsy, revealing high-density connections between the medial part of the pulvinar and the hippocampus.32 More so, based on the strong connectivity between neocortical regions and pulvinar, recently conducted research advocates for using pulvinar as a potential therapeutic target in patients with refractory epilepsy.33
4.3 Limitations
Although our study identified specific locations of diffusion-restricted lesions associated with SE, it is essential to acknowledge that similar imaging features may be seen in other neurological conditions that were not included in the control group, such as Creutzfeldt–Jakob disease.34 The differential diagnosis in cases with similar MRI presentations should be carefully considered, and additional investigations may be necessary to accurately differentiate SE from other neurological conditions. Another limitation is related to the majority (81%) of patients in the control group having an acute stroke and some conditions that could potentially cause SE not being represented in the control group (e.g., infection, mitochondrial disease). Uneven distribution of different etiologies in two groups of patients represents another limitation. Furthermore, our study relied on conventional MRI techniques, which provided valuable insights. However, the absence of advanced imaging modalities, such as tractography, limits a comprehensive understanding of the structural connectivity between these identified regions. Future studies incorporating these techniques could provide deeper knowledge of the underlying network involved in SE.
5 CONCLUSIONS
Diffusion-restricted MRI lesions observed in pulvinar of thalamus and hippocampus are strongly associated with SE. These changes may help physicians in diagnosing SE-related changes on MRI in an acute setting, especially in cases of equivocal clinical and EEG manifestations of SE.
AUTHOR CONTRIBUTIONS
Pilar Bosque Varela, Payam Tabaee Damavandi, Lukas Machegger, Tanja Prüwasser, and Andreas Oellerer contributed significantly to conception and design of the presented paper; acquisition, analysis, and interpretation of the data; and drafting of the paper. Georg Zimmermann, Johannes Pfaff, Mark McCoy, Georg Zimmermann, and Jürgen Steinbacher contributed to acquisition and analysis of data and revising the paper for intellectual content. Giorgi Kuchukhidze and Eugen Trinka contributed significantly to conception of the study and interpretation of the results, and gave final approval of the submitted version of the manuscript.
ACKNOWLEDGMENTS
This study was supported by Fonds zur Förderung der Wissenschaftlichen Forschung, Austrian Science Fund (project number KLI 969-B).
FUNDING INFORMATION
The presented research was funded by Fonds zur Förderung der Wissenschaftlichen Forschung, Austrian Science Fund (project number KLI 969-B).
CONFLICT OF INTEREST STATEMENT
E.T. reports personal fees from EVER Pharma, Marinus, Argenx, Arvelle/Angelini, Medtronic, Bial–Portela & Cª, NewBridge, GL Pharma, GlaxoSmithKline, Hikma, Boehringer Ingelheim, LivaNova, Eisai, UCB, Biogen, Genzyme Sanofi, GW Pharmaceuticals/Jazz, and Actavis outside the submitted work; his institution has received grants from Biogen, UCB Pharma, Eisai, Red Bull, Merck, Bayer, the European Union, FWF Osterreichischer Fond zur Wissenschaftsforderung, Bundesministerium für Wissenschaft und Forschung, and Jubilaumsfond der Österreichischen Nationalbank outside the submitted work. G.Z. gratefully acknowledges the support of the WISS 2025 project IDA-Lab Salzburg (20204-WISS/225/197-2019 and 20 102-F1901166-KZP). The remaining authors have no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The corresponding author takes full responsibility for the data, the analyses and interpretation, and the conduct of the research; the corresponding author has full access to all of the data, and has the right to publish any and all data separate and apart from any sponsor.




