Association of midazolam route of administration and need for recurrent dosing among children with seizures cared for by emergency medical services
Clinical trial registration: None.
Abstract
Objective
National guidelines in the United States recommend the intramuscular and intranasal routes for midazolam for the management of seizures in the prehospital setting. We evaluated the association of route of midazolam administration with the use of additional benzodiazepine doses for children with seizures cared for by emergency medical services (EMS).
Methods
We conducted a retrospective cohort study from a US multiagency EMS dataset for the years 2018–2022, including children transported to the hospital with a clinician impression of seizures, convulsions, or status epilepticus, and who received an initial correct weight-based dose of midazolam (.2 mg/kg intramuscular, .1 mg/kg intravenous, .2 mg/kg intranasal). We evaluated the association of route of initial midazolam administration with provision of additional benzodiazepine dose in logistic regression models adjusted for age, vital signs, pulse oximetry, level of consciousness, and time spent with the patient.
Results
We included 2923 encounters with patients who received an appropriate weight-based dose of midazolam for seizures (46.3% intramuscular, 21.8% intranasal, 31.9% intravenous). The median time to the first dose of midazolam from EMS arrival was similar between children who received intramuscular (7.3 min, interquartile range [IQR] = 4.6–12.5) and intranasal midazolam (7.8 min, IQR = 4.5–13.4) and longer for intravenous midazolam (13.1 min, IQR = 8.2–19.4). At least one additional dose of midazolam was given to 21.4%. In multivariable models, intranasal midazolam was associated with higher odds (odds ratio [OR] = 1.39, 95% confidence interval [CI] = 1.10–1.76) and intravenous midazolam was associated with similar odds (OR = 1.00, 95% CI = .80–1.26) of requiring additional doses of benzodiazepines relative to intramuscular midazolam.
Significance
Intranasal midazolam was associated with greater odds of repeated benzodiazepine dosing relative to initial intramuscular administration, but confounding factors could have affected this finding. Further study of the dosing and/or the prioritization of the intranasal route for pediatric seizures by EMS clinicians is warranted.
Key points
- Seizures are an important reason for the utilization of emergency medical services among children.
- The use of intravenous midazolam for children with seizures was associated with similar odds of repeat benzodiazepine dosing relative to initial intramuscular route.
- Use of intranasal midazolam for children with seizures was associated with higher odds of repeat benzodiazepine dosing relative to initial intramuscular route.
- Among children who received more than one dose of benzodiazepine, the interval between doses was less among those who received intranasal midazolam.
1 INTRODUCTION
Seizures are among the most common conditions among children that require prehospital care by emergency medical services (EMS), occurring in approximately 6%–9% of pediatric encounters.1, 2 The significance of timely management of pediatric seizures is emphasized in a multicenter study involving 218 hospitalized children that suggested that delayed administration of antiseizure medications for children with status epilepticus is associated with an increase in in-hospital mortality and a need for continuous infusions.3
Improving the prehospital management of seizures in children has been identified by key partners as a priority for research in pediatric prehospital treatment.4 This need partly stems from the substantial variation in EMS protocols for the management of pediatric seizures nationally.5, 6 Prospective trials have demonstrated the efficacy of intramuscular (IM) midazolam relative to intravenous (IV) lorazepam for the treatment of status epilepticus in adults.7, 8 Following from these, model EMS protocols published by the National Association of State EMS Officials (NASEMSO) promote the use of intranasal (IN) and IM seizure abortive medications, rather than the IV or intraosseous (IO) routes.9
The use of the IN route for emergency treatment of seizures has been the subject of increased interest.10-13 One recent retrospective noninferiority study suggested that the use of IN midazolam was associated with a greater need for subsequent midazolam by any route for children treated for seizures during the EMS encounter compared to the provision of IV or IM midazolam, although this study did not take into account the appropriateness of medication dosing.11 In another study, midazolam was found to be underdosed in >60% of pediatric EMS encounters,14 a finding that is corroborated by other research evaluating pediatric drug dosing.15-17 More research is therefore required to evaluate the optimal route of drug delivery of appropriately dosed benzodiazepines for pediatric seizures to inform EMS protocols and national guidelines.
In this study, we sought to evaluate the association of the route of midazolam administration with the use of additional midazolam doses for children with seizures cared for by EMS.
2 MATERIALS AND METHODS
2.1 Data source
We conducted a retrospective cohort investigation using deidentified prehospital patient care records sourced from the 2018–2022 ESO Data Collaborative (Austin, Texas). This collaborative database encompasses a collection of electronic health records drawn from roughly 2000 EMS agencies across the United States. This dataset includes information on dispatch records, demographic details, clinical presentations, vital signs, assessments, and the interventions administered by EMS professionals. An annual standardized dataset is generated for research purposes and is accessible at no cost through a formal research proposal process. Performance of the present study was approved by both ESO and the institutional review board of the Ann & Robert H. Lurie Children's Hospital of Chicago.
2.2 Study setting
In the United States, EMS agencies are most commonly staffed by any of four levels of personnel, of which the highest level, paramedic, is authorized to perform full advanced life support interventions including medical management of seizures.18 Care performed by these nonphysician personnel is generally governed by prehospital protocols that provide standing orders for medication administration or that identify the need for online medical direction from a physician for specific interventions. In the NASEMSO Model EMS Clinical Guidelines, paramedics are instructed to treat patients with seizure activity lasting >5 min, without otherwise addressing differences in the type of seizure activity. Appropriate routes for benzodiazepine administration for seizure include IN, IM, IV, or IO routes. IM or IN routes are preferred in these national model guidelines based on a previously published evidence-based guideline for prehospital seizure management,19 and it is rare for benzodiazepines to be administered by paramedics via buccal or rectal routes in the United States. It should be noted that some countries recommend buccal midazolam as first-line treatment, but this is not standard management in the United States.
2.3 Patient inclusion
We sought to identify a sample of children who were given at least one weight-appropriate dose of midazolam by EMS for the management of seizures. We selected midazolam based on previous research identifiyng this as the most commonly used benzodiazepine in prehospital pediatric seizure management.2 From the 2018–2022 ESO datasets, we excluded encounters without a listed age and limited our sample to children (<18 years old). We made the following exclusions: encounters with only a basic life support clinician (as provision of midazolam is generally out of their scope of practice), encounters not classified as an emergency telephone response from the scene, and encounters that did not result in transfer of the patient to the hospital. We elected to exclude patients not transported to the hospital, as they would have limited available outcome data, and they may not be transported to the hospital for a variety of reasons, limiting our ability to draw inferences from these. Within this subset, we identified encounters with a primary or secondary EMS clinician impression of seizures or with an emergency medical dispatch complaint of seizures, using the terms “seizures,” “convulsions,” and “status epilepticus.” We combined these terms within this analysis, as practice guidelines for the management of prehospital seizures do not differentiate between these conditions and the terms are used in the prehospital setting interchangeably.6 Consensus-based guidelines from NASEMSO, for example, recommend pharmacologically treating any seizure lasting for >5 min.9 Among encounters meeting these criteria, we excluded those for children given midazolam prior to the EMS encounter, given any other type of benzodiazepine before the EMS encounter or before the initial dose of midazolam, not given any midazolam during their encounter, given the first dose of midazolam via the IO route, whose weight-based dosing of midazolam could not be calculated (either because of a missing weight, route, or dose), or who received an inappropriate initial dose of midazolam. The appropriateness of dosing was determined based on the 2022 NASEMSO guidelines and was .2 mg/kg of midazolam if given IM or IN (up to 10 mg) and .1 mg/kg if given IV (up to 4 mg).9 A dose of midazolam was considered appropriate when it was within 80%–120% of the calculated dose range. This range has been widely used in prior research evaluating acceptable variation of dosing deviation among EMS-administered medications in the prehospital setting.2, 14, 15, 17
2.4 Outcome and exposures
Our outcome of interest was the provision of a second dose of benzodiazepine (midazolam, lorazepam, or diazepam) during the EMS encounter. We chose this outcome because the dataset did not include a variable indicating cessation of seizure and we regarded the requirement of the second dose of benzodiazepine as evidence of ongoing seizure or recurrence of seizure. Our primary exposure of interest was route of drug delivery for the initial dose of midazolam: IN, IV, or IM. It is noted that this is an indirect measure which depends on the judgment of the on-the-spot clinician. We did not incorporate buccal midazolam as (1) its use is not currently incorporated into national (or any published statewide) EMS protocol6, 9 and (2) because no patient in this dataset received midazolam via this route.
2.5 Covariables
We recorded the following demographic variables to describe the study sample: sex, race and ethnicity, and census region. We considered the following covariables for the development of a statistical model: age; initial level of consciousness; poorest level of consciousness documented during the encounter; total time EMS spent with the patient (in minutes); initial and lowest documented pulse oximetry during the encounter; and the initial, maximum, and minimum of heart rate and respiratory rate during the encounter. Vital signs of heart and respiratory rate were Z-scored for age using a previously described age-based distributional model.1 Level of consciousness was recorded within the ESO dataset as either by Glasgow Coma Scale (GCS) or using the AVPU (Awake–Verbal–Pain–Unresponsive) scale. Using prior research on the convertibility of these two schemas,20 we considered a GCS of 15 as Awake, a GCS of 9–14 as Verbal, a GCS of 4–8 as Pain, and a GCS of 3 as Unresponsive.
2.6 Analysis
Analyses was performed using the mice (v3.16.0),21 and rms (v6.7–0)22 packages in R, version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria). We presented each of the study covariables with respect to route of medication delivery. We calculated the time to initial dosing of midazolam from the start of the EMS encounter and the time from the first to the second dose of benzodiazepine (when applicable). We considered the following covariables to develop an adjusted model: level of consciousness, age, heart rate, respiratory rate, pulse oximetry reading, and time EMS spent caring for the patient. We performed univariable logistic regression for our exposure of interest and covariables with an outcome of multiple benzodiazepine dosing. We assessed missingness of data and performed multiple imputation by chained equations for missing variables. For the continuous variables of age, time spent with the patient, heart and respiratory rate, and pulse oximetry, we evaluated their association with repeated doses of midazolam using univariable splined plots to identify nonlinear associations of these predictors with our outcome. For variables that contained similar clinical information (first and lowest level of consciousness; and first, lowest, and highest of vital signs), we selected one within each category based on the lowest Akaike Information Criterion (AIC).23 We next performed multivariable logistic regression to evaluate for the association of route of midazolam with an outcome of the need for additional doses of benzodiazepine. We expressed our findings as odds ratios (ORs) with 95% confidence intervals (CIs).
We performed two subgroup analyses. First, to evaluate how our findings may be impacted during longer EMS encounters, we repeated our multivariable model within the subset of cases that exceeded 40 min of time spent with EMS (which approximated the 75th centile of this variable). We performed this subgroup analysis because a patient with a shorter EMS transport may be transferred to the emergency department (ED) sooner, where additional benzodiazepine or other antiepileptic drugs would be provided outside of the discretion of the EMS clinician. Second, to evaluate cases with greater specificity for presumed status epilepticus, we repeated our analysis in the subgroup of patients who had a specific clinician impression of this condition.
2.7 Alternative IV dosing regimen
We a priori chose to use the NASEMSO model guidelines for medication dosing, in which IV dosing is less than IM dosing. However, alternative dosing regimens for midazolam for pediatric seizure exist, including one that advises .2 mg/kg (maximum of 10 mg) for intravenous midazolam.24 As an exploratory analysis, we repeated our analysis when using this alternative definition for the correct dosing of IV midazolam.
3 RESULTS
3.1 Inclusion
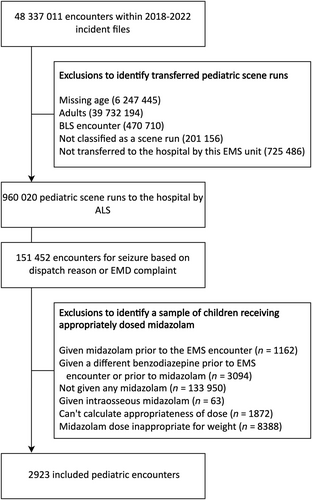
A total of 48 337 011 patients were included within the 2018–2022 ESO incident files. After initial exclusions, we identified 960 020 pediatric scene encounters where the patient was transported by EMS to a hospital. Of these, 151 452 (15.8%) were for seizures. After applying additional exclusions, we identified 2923 encounters with patients who received an appropriate weight-based dose of midazolam as first-line treatment for the present analysis (Figure 1). The median age was 3 years (interquartile range [IQR] = 2–7 years). There were a similar proportion of males (52.2%) and females. Demographics of the study population are provided in Table 1.
| Variable | n (%) or median (IQR) |
|---|---|
| N | 2923 |
| Sex | |
| Female | 1397 (47.8) |
| Male | 1525 (52.2) |
| Missing | 1 (.0) |
| Age, years | 3 (2–7) |
| Region | |
| Midwest | 743 (25.4) |
| Northeast | 85 (2.9) |
| South | 1543 (52.8) |
| West | 526 (18.0) |
| Missing | 26 (.9) |
| Race and ethnicity | |
| White non-Hispanic | 1231 (42.1) |
| Black non-Hispanic | 877 (30.0) |
| Hispanic | 536 (18.3) |
| Other or more than one | 122 (4.2) |
| Missing | 157 (5.4) |
- Abbreviation: IQR, interquartile range.
3.2 Route of midazolam dosing
Among the included sample, the initial dose of midazolam was IM in 1354 (46.3%), IN in 637 (21.8%), and IV in 932 (31.9%). Characteristics of the study sample, stratified by route of initial midazolam, are provided in Table 2. The median time to the first dose of midazolam from EMS arrival was similar between children who received IM and IN midazolam (7.3 min, IQR = 4.6–12.5 min for IM; 7.8 min, IQR = 4.5–13.4 for IN) and was longer for IV midazolam (13.1 min, IQR = 8.2–19.4). Most patients (n = 2297, 78.6%) received a single dose of midazolam. Four hundred ninety-five patients (16.9%) received a total of two doses of benzodiazepine, 101 (3.5%) received a total of three doses, and 30 (1.0%) received a total of four or more doses. The percent of patients who received more than one dose of benzodiazepine was 21.6%, 25.4%, and 18.5% of children who were respectively provided with IM, IN, and IV midazolam for their initial dose. Among patients who did receive an additional dose of benzodiazepine, the median interval between the first and second dose was 10.0 min (IQR = 6.0–14.0) for patients initially dosed with IM midazolam, 7.0 min (IQR = 5.0–11.9) for patients initially dosed with IN midazolam, and 8.0 min (IQR = 5.0–12.1) for patients initially dosed with IV midazolam.
| Characteristic | Intramuscular | Intranasal | Intravenous |
|---|---|---|---|
| n | 1354 | 637 | 932 |
| Time to first midazolam, min | 7.3 [4.6 to 12.5] | 7.8 [4.5 to 13.4] | 13.1 [8.2 to 19.4] |
| Sex | |||
| Female | 649 (47.9) | 297 (46.6) | 451 (48.4) |
| Male | 705 (52.1) | 340 (53.4) | 480 (51.5) |
| Age, years | 3 (1–7) | 2 (1–5) | 4 (2–7) |
| Total number of benzodiazepines doses | |||
| 1 | 1062 (78.4) | 475 (74.6) | 760 (81.5) |
| 2 | 228 (16.8) | 131 (20.6) | 136 (14.6) |
| 3 | 51 (3.8) | 25 (3.9) | 25 (2.7) |
| 4 or more | 13 (1.0) | 6 (.9) | 11 (1.2) |
| Pulse oximetry, % | |||
| Initial pulse oximetry | 97 [92 to 99] | 96 [91 to 99] | 96 [90 to 99] |
| Lowest pulse oximetry documented | 95 [88 to 98] | 95 [85 to 98] | 95 [87 to 98] |
| HR, Z-score | |||
| Initial HR | 1.09 [.35 to 1.73] | 1.03 [.28 to 1.56] | .97 [.29 to 1.52] |
| Highest HR | 1.45 [.80 to 2.04] | 1.35 [.73 to 1.89] | 1.33 [.68 to 1.88] |
| Lowest HR | .58 [−.34 to 1.27] | .50 [−.40 to 1.22] | .42 [−.51 to 1.04] |
| RR, Z-score | |||
| Initial RR | .66 [−.41 to 1.52] | .57 [−.54 to 1.40] | .43 [−.73 to 1.32] |
| Highest RR | 1.20 [.31 to 1.92] | 1.09 [.22 to 1.74] | .97 [.04 to 1.75] |
| Lowest RR | −.13 [−1.31 to .87] | −.23 [−1.36 to .79] | −.13 [−1.31 to .87] |
| First documented level of consciousness | |||
| Alert | 111 (8.2) | 61 (9.6) | 60 (6.4) |
| Voice | 256 (18.9) | 137 (21.5) | 207 (22.2) |
| Pain | 487 (36.0) | 207 (32.5) | 350 (37.6) |
| Unresponsive | 374 (27.6) | 157 (24.6) | 237 (25.4) |
| Lowest documented level of consciousness | |||
| Alert | 82 (6.1) | 47 (7.4) | 43 (4.6) |
| Voice | 209 (15.4) | 110 (17.3) | 147 (15.8) |
| Pain | 480 (35.5) | 226 (35.5) | 352 (37.8) |
| Unresponsive | 457 (33.8) | 179 (28.1) | 312 (33.5) |
- Note: Numbers in cells represent counts with percentages in parentheses, or medians with interquartile ranges in brackets. Time to first midazolam dosing missing in 11 (.4%), sex in one (<.1%), pulse oximetry data in 206 (7.0%), heart rate data in 133 (4.6%), respiratory rate data in 207 (7.1%), and level of consciousness in 279 (9.5%).
- Abbreviations: HR, heart rate; RR, respiratory rate.
3.3 Association of midazolam route with need for additional benzodiazepine
Complete midazolam route of administration data, outcome, and covariable data were available for 2529 encounters (86.5% of the included sample). In univariable analyses performed after multiple imputation, IN midazolam (OR = 1.23, 95% CI = .99–1.54) and IV midazolam (OR = .82, 95% CI = .67–1.02) both had similar odds of requiring additional doses of benzodiazepine relative to IM midazolam (Table S1). Univariable associations for continuous predictors are provided in Figure S1.
After reviewing the AIC for overlapping variables, we selected lowest level of consciousness, maximum heart rate, maximum respiratory rate, and initial pulse oximetry measurement within our multivariable model. In a model adjusted with these covariables, patient age, and time spent with the patient, IN midazolam was associated with higher odds of requiring additional doses of benzodiazepine (OR = 1.39, 95% CI = 1.10–1.76), and IV midazolam was associated with similar odds of requiring additional doses of benzodiazepine (OR = 1.00, 95% CI = .80–1.26) relative to IM midazolam (Table 3).
| Variable | OR (95% CI) |
|---|---|
| Route | |
| Intramuscular | Ref |
| Intranasal | 1.39 (1.10–1.76) |
| Intravenous | 1.00 (.80–1.26) |
| Lowest level of consciousness | |
| Alert | Ref |
| Voice | 1.77 (1.00–3.12) |
| Pain | 2.73 (1.61–4.64) |
| Unresponsive | 3.40 (1.99–5.80) |
| Age, Q3 vs. Q1 | .43 (.32–.58) |
| Maximum HR, Z-score; first quartile: Q3 vs. Q1 | 1.71 (1.32–2.20) |
| Maximum RR, Z-score; first quartile: Q3 vs. Q1 | 1.54 (1.18–2.00) |
| First pulse oximetry reading, Z; first quartile: Q3 vs. Q1 | .79 (.59–1.04) |
| Total time with patient, Z; first quartile: Q3 vs. Q1 | 1.18 (1.04–1.35) |
- Note: The continuous variables of age, maximum heart rate, respiratory rate, first pulse oximetry reading, and total time spent with the patient are used as continuous splined predictors, with ORs provided for demonstration purposes that compare the first quartile to the third quartile.
- Abbreviations: CI, confidence interval; HR, heart rate; OR, odds ratio; Ref, reference; RR, respiratory rate.
3.4 Longer EMS encounters
Among the subset of longer EMS transports (>40 min), we identified 778 encounters, of which the initial dose of midazolam was IM in 356 (45.8%), IV in 288 (37.0%), and IN in 134 (17.2%). More than one dose of benzodiazepine was used in 182 (23.4%) encounters, including in 85 (23.9%) patients who received an IM dose of midazolam, 60 (20.8%) patients who received an IV dose of midazolam, and 37 (27.6%) patients who received an IN dose of midazolam. Within the multivariable model, the resultant ORs demonstrated similar findings to the primary analysis but with CIs that crossed 1 relative to IM midazolam (OR = 1.04, 95% CI = .95–2.54 for IV midazolam; OR = 1.56, 95% CI = .95–2.54 for IN midazolam).
3.5 Encounters with an EMS–clinician impression of status epilepticus
In a subgroup of patients with an EMS impression of “status epilepticus” (n = 836), 410 (49.0%) received IM midazolam, 166 (19.9%) received IN midazolam, and 260 (31.1%) received IV midazolam. Point estimates from the multivariable model were similar to those obtained in the primary analysis, although with CIs that crossed unity, consistent with a smaller sample size (Table S2).
3.6 Alternative IV dosing regimen
When using a higher range to define IV dosing, we identified 292 encounters where patients were given a .2-mg/kg IV dose. In a multivariable model constructed with this sample, IV midazolam administration had lower odds of requiring additional benzodiazepine dosing compared to the initial IM route (OR = .66, 95% CI = .46–.95). IN midazolam dosing was again associated with higher odds of requiring additional benzodiazepine dosing compared to the IM route (OR = 1.41, 95% CI = 1.11–1.78).
4 DISCUSSION
Our findings from a multiagency EMS agency suggest that children who initially received IV midazolam had a similar requirement for additional dosing of benzodiazepine compared to those given IM midazolam. In contrast, provision of IN midazolam was associated with a higher odds of requiring additional benzodiazepine compared to IM midazolam. These findings were corroborated in a subgroup analysis limited to longer EMS encounters and among the subgroup of encounters with a clinician impression of status epilepticus.
Our findings compare to prior work evaluating the use of IN antiseizure medications in the prehospital setting. A retrospective noninferiority trial reported that the use of IN midazolam was associated with greater use of repeated midazolam during the EMS encounter.11 This study identified age-adjusted higher odds of requiring midazolam redosing of 2.0 (95% CI = 1.6–2.6) compared to initial administration of midazolam by the IV or IM route in a logistic regression model.11 The present study expands upon this prior work in a geographically broad patient sample within the United States, and by only including encounters given an appropriate weight-based dose of midazolam. Another study of 124 children with seizure activity witnessed by EMS (of any type) compared IN midazolam with per-rectal diazepam and found that children who were treated with the IN regimen had shorter seizure durations and were less likely to be seizing upon ED arrival.10 Concerns with respect to using the IN route, reported in one qualitative study of paramedics, included those related to using IN equipment in smaller children, airway compromise, and lack of effectiveness relative to the IM route. Some respondents in that study stated a preference for the IN route, citing greater perceived safety, specifically with challenges regarding using a needle to administer medication to an actively seizing patient.25 A number of prospective studies have suggested similar efficacy between IN midazolam relative to other rectal or IV benzodiazepines, but the applicability of these findings to the present study are limited, given differences in study setting, comparison to the per rectal route (which was not evaluated in this study), and comparisons to the use of multiple benzodiazepines, including lorazepam and diazepam.26 Additional considerations beyond the scope of this study include the concentration of midazolam administration. The use of a higher concentration of midazolam may allow for improved efficacy when given by the IN route, a finding that may be comparable to dosing recommendations for naloxone in patients with opioid overdoses.27 However, there are no data to support the use of higher concentrations of midazolam in improving the efficacy of this medication when administered via the IN route for pediatric seizures. Another important avenue of research lies in exploring the use of the buccal route of medication dosing for children. Although this is not currently common practice in US prehospital systems, prior work has demonstrated that buccal midazolam is as28 or more29, 30 effective compared to rectal diazepam for children with acute seizures. Another study among 150 children with active seizures in the ED, however, reported that a higher proportion of children given IM midazolam (61%) had seizure cessation within 5 min compared to those given buccal midazolam (46%), although this did not reach statistical significance.31
Our findings are generally consistent with national guidelines for the treatment of seizures in the prehospital setting.9 These results are not only informative for the management of pediatric seizures by EMS clinicians, but are relevant for caregivers of children with seizures. Although the higher dose of midazolam through the IV route demonstrated a lower odds of requiring repeat benzodiazepine dosing compared to the IM route in our exploratory analysis, this improved performance must be considered with the challenges faced by EMS clinicians in attempting to obtain IV access in a seizing child.32 In addition to its recommendation within national protocols, IM administration is a commonly identified route within existing prehospital protocols for the management of pediatric seizures.5, 6 The IM route carries the advantages of being reliable and being rapid to administer and does not require IV access, although the exact time to medication administration depends on a multitude of variables, including patient age and weight, and medication packaging. A purported benefit of IN administration is its ease of access and rapid administration, yet we demonstrated that the median time to administration of the first IM or IN midazolam dose were similar. Prior work has demonstrated that when midazolam is given IM, it is more frequently dosed appropriately than when given by the IN or IV routes.14 This is of relevance because a low dose of benzodiazepine to abort seizure is associated with the need for additional benzodiazepine dosing.2 We found that the IV route, which demonstrated similar odds of redosing of additional benzodiazepines to the IM route, took nearly twice as long to administer within this patient sample.
Our findings are subject to limitations. This was a retrospective study that used previously charted medical record data and thus may be subject to errors in data abstraction and coding. Specifically, there may have been errors in documentation of the timing of events, including timing of the initial dose of medication. Time to seizure cessation is commonly used in prospective studies and refers to the duration of clinically apparent seizure activity.33 We used administration of additional doses of benzodiazepines instead of time to seizure cessation as our outcome measure, although decisions regarding the need for additional dosing were based on individual clinician assessments. Although this outcome carries face validity and has been used in other prehospital research,11 it may be subject to variability and assumes that additional doses of benzodiazepines are indicative of ongoing, clinically apparent seizure activity. We relied on EMS impressions to identify children with seizures. Prior work has suggested that EMS clinicians have moderate sensitivity, but high specificity, for the identification of pediatric seizures in practice.34 We elected to exclude patients who were not transported to the hospital from the present study, as limited data would be available for these encounters. Although this may represent a cause of confounding, the number of additional records that would be included without this specific exclusion criterion was low (n = 151), and a repeat analysis incorporating these encounters did not change the overall study findings (results not shown). Our findings are from North American EMS agencies, where most responses are addressed by nonphysician clinicians. Results may have been different in EMS systems that involve physicians, with better differentiation between different types of seizure conditions. We were unable to identify whether certain routes were preferred by EMS clinicians within specific clinical contexts (e.g., provision of IV midazolam in patients with status epilepticus, versus IN medications for patients with serial seizures). However, our findings within the subset of patients having status epilepticus are similar to the results of our primary analysis, supporting the use of our case definition. We were unable to ascertain adherence to seizure management protocols in this multiagency study, which has been reported to occur frequently.35 We could not evaluate the effectiveness of buccal midazolam, which is not currently incorporated into US EMS guidelines. Despite these limitations, our findings demonstrate the need for further evaluation of IN midazolam as a first-line medication of choice for children requiring prehospital care for seizures.
5 CONCLUSIONS
In this multiagency retrospective study, we evaluated the association between the route of the first appropriately dosed midazolam dose administered to children with seizures and the need for additional benzodiazepines administration during the EMS encounter, although these findings are limited by potential confounders. Compared to IM midazolam, IN midazolam was associated with a greater need for additional benzodiazepines dosing, and IV midazolam performed similarly. Our findings suggest a need to further evaluate the dosing and/or the prioritization of the IN route for the management of pediatric seizures by EMS clinicians.
AUTHOR CONTRIBUTIONS
Sriram Ramgopal participated in participation, formal analysis, investigation, methodology, visualization, and writing of the original draft. Sylvia Owusu-Ansah participated in conceptualization, and review and editing. Remle P. Crowe participated in investigation, methodology, data curation, and review and editing. Masashi Okubu participated in investigation, methodology, and review and editing. Christian Martin-Gill participated in conceptualization, methodology, investigation, supervision, and review and editing.
ACKNOWLEDGMENTS
None.
FUNDING INFORMATION
This publication was supported by Pediatric Pandemic Network resources. The Pediatric Pandemic Network is supported by the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services (HHS) as part of grant awards U1IMC43532 and U1IMC45814 with 0% financed with nongovernmental sources. The content presented here is that of the authors and does not necessarily represent the official views of, nor an endorsement by HRSA, HHS, or the US Government. For more Information, visit HRSA.gov.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ETHICAL STATEMENT
Performance of this study was approved by the Ann & Robert H. Lurie Children's Hospital Institutional Review Board (IRB #2024-6522).
PATIENT CONSENT STATEMENT
A waiver from the requirement of informed consent has been obtained for this study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from ESO. Restrictions apply to the availability of these data, which were used under license for this study.




