Therapeutic drug monitoring in pregnancy: Levetiracetam
Abstract
Objective
Levetiracetam (LEV) is an antiseizure medication that is mainly excreted by the kidneys. Due to its low teratogenic risk, LEV is frequently prescribed for women with epilepsy (WWE). Physiological changes during gestation affect the pharmacokinetic characteristics of LEV. The goal of our study was to characterize the changes in LEV clearance during pregnancy and the postpartum period, to better plan an LEV dosing paradigm for pregnant women.
Methods
This retrospective observational study incorporated a cohort of women who were followed up at the epilepsy in pregnancy clinic at Tel Aviv Sourasky Medical Center during the years 2020–2023. Individualized target concentrations of LEV and an empirical postpartum taper were used for seizure control and to reduce toxicity likelihood. Patient visits took place every 1–2 months and included a review of medication dosage, trough LEV blood levels, week of gestation and LEV dose at the time of level measurement, and seizure diaries. Total LEV concentration/dose was calculated based on LEV levels and dose as an estimation of LEV clearance.
Results
A total of 263 samples were collected from 38 pregnant patients. We observed a decrease in LEV concentration/dose (C/D) as the pregnancy progressed, followed by an abrupt postpartum increase. Compared to the 3rd trimester, the most significant C/D decrease was observed at the 1st trimester (slope = .85), with no significant change in the 2nd trimester (slope = .11). A significant increase in C/D occurred postpartum (slope = 5.23). LEV dose was gradually increased by 75% during pregnancy compared to preconception. Average serum levels (μg/mL) decreased during pregnancy. During the postpartum period, serum levels increased, whereas the LEV dose was decreased by 24%, compared to the 3rd trimester.
Significance
LEV serum level monitoring is essential for WWE prior to and during pregnancy as well as postpartum. Our data contribute to determining a rational treatment and dosing paradigm for LEV use during both pregnancy and the postpartum period.
Key points
- LEV clearance is significantly increased throughout pregnancy and abruptly declines postpartum; this pattern correlates with renal function during pregnancy as expected for ASMs that are mostly eliminated by the kidneys
- To maintain stable LEV blood level, increases of dose during pregnancy and decrease of dose postpartum are required
- Increase in dosing should start as early as the 1st trimester
- LEV serum level monitoring is essential for all women with epilepsy upon planning pregnancy, monthly during pregnancy especially during the 1st trimester, and following delivery
- Considering an empirical titration regime where therapeutic drug monitoring is not available routinely, we recommend a gradual increase by 50% during the 1st trimester and up to 75% by the end of the 3rd trimester compared to baseline dosage before pregnancy
1 INTRODUCTION
Seizures during pregnancy, and tonic–clonic seizures in particular, are associated with significant risks to both mother and fetus. Consequently, continued treatment with antiseizure medications (ASMs) during pregnancy is required for most women with epilepsy (WWE).1 Approximately one third of individuals treated with ASMs are women of childbearing age.2 The incidence of newly diagnosed epilepsy in women of reproductive age stands at 20–30/100 000/year.3 The fraction of WWE among all pregnant women is .3%–.5%,3 and 3–4 women per 1000 pregnancies take ASM.2
Physiological changes during the different stages of gestation, such as increase in volume of distribution, total body water, and hepatic and renal clearance, affect the pharmacokinetic characteristics of ASMs.2, 4, 5 This effect varies depending on the type of ASM and its route of elimination.6 These alterations in serum concentrations are of clinical relevance. There is a known association between declining serum concentrations of ASMs during pregnancy and deterioration in seizure control.6-9 Levetiracetam (LEV) is an ASM indicated for the treatment of generalized and focal seizures.5 It is frequently used in WWE of reproductive age due to its low teratogenic potential.10-14 Approximately one third of administered LEV is hydrolyzed in the blood, and the rest is excreted, unchanged, by the kidneys.2, 15, 16 Elevated LEV clearance during pregnancy results in a decrease in serum drug levels unless the dose is increased.1, 2, 17-19 Previous studies have found different patterns of changes in LEV clearance throughout pregnancy.2, 18-24
The LEV reference range is typically defined as between 12 and 46 μg/mL,25 with the currently accepted management policy being to titrate to the lowest effective ASM dose prior to conception.7 A recent study has suggested the range of 3.80–13.60 μg/mL as optimal for pregnant women with epilepsy (PWWE),2 This recommendation rests on the assumption that the risks associated with exposure to higher doses of LEV outweigh the seizure-related risks to mother and fetus. Nevertheless, a breakthrough seizure can impact the patient's social life, mood, and quality of life and restrict her driving for a prolonged period.26, 27
There are large interindividual differences in LEV serum concentrations during pregnancy as well as significant variability in concentration changes.2 Therapeutic drug monitoring (TDM) and dose adjustments offer useful tools in the treatment of PWWE for maintaining stable serum concentrations, preventing seizure aggravation, and improving clinical outcomes.7, 28 To date, decisions on dose adjustments are made on an individual basis.7
Although the pharmacokinetic changes of ASMs during pregnancy were studied before, the timing and frequency of drug concentration measurements and the optimal dose adjustments are not well established. The goal of our study was to characterize the magnitude and course of changes in LEV clearance during pregnancy and the postpartum period, and to establish a rational treatment plan and dosing paradigm to minimize fetal exposure without compromising seizure control.
2 MATERIALS AND METHODS
2.1 Study design
This retrospective observational study comprised a cohort of women who were under the care of a specialized epilepsy in pregnancy clinic at Tel Aviv Sourasky Medical Center (TASMC). The local TDM protocol includes individualized targeted LEV concentrations during gestation to minimize the risk of seizures, as well as a semiempirical postpartum taper applied over roughly 3 weeks to reduce the likelihood of maternal toxicity. All participants were treated with LEV during pregnancy. The collected samples were divided into six time periods with respect to pregnancy: “preconception” “1st trimester” (1–13 weeks of gestation), “2nd trimester” (14–26 weeks of gestation), “3rd trimester” (27–40 weeks of gestation), “postpartum” (12 weeks postdelivery), and “after pregnancy” (over 12 weeks postpartum). Office visits during pregnancy usually took place every 1–2 months, with self-reports of seizure frequency routinely documented at each visit.
Week of gestation, the LEV dose at the time of blood collection, and LEV serum levels were obtained from the medical records for all samples. All LEV serum levels results were included, starting 70 weeks prior to pregnancy and up to 66 weeks postpartum.
2.2 Study population
PWWE who were being treated with LEV and had undergone at least one LEV blood level measurement during pregnancy during the years 2020–2023 were included in the study. Both patients receiving LEV monotherapy and patients taking LEV as part of polytherapy were included.
2.3 Sample preparation
All measurements were conducted at the same laboratory at TASMC. Venous blood was drawn before the morning dose as trough level. A VACUETTE TUBE 5 mL CAT Serum Separator Clot Activator 13 × 100 gold cap-gold ring, black label, nonridged by Greiner Bio-One (reference number 456018) was used. The laboratory used an ARK Levetiracetam Assay, which is a homogeneous enzyme immunoassay used for quantitative determination of LEV in human serum or plasma on automated clinical chemistry analyzers. The assay range is 2.0–100.0 μg/mL. Below or above this range, results are reported as <2.0 μg/mL or >100.0 μg/mL.
2.4 Data analysis
Total LEV concentration/dose (mL/day) ratio (C/D) was calculated as an estimation of LEV clearance during the different weeks of pregnancy. The ratio was calculated as LEV serum level (μg/mL) divided by the LEV daily dose (mg/day) and multiplied by 1000.
2.5 Statistical methods
The distribution of three variables—namely, LEV serum level, LEV daily dose, and LEV C/D—was assessed using the Shapiro–Wilk test. As a nonnormal distribution was observed for all three variables, the results were summarized by means of the median and interquartile range. For categorical measurements, percentages and counts were used to summarize the findings.
To visually portray the LEV measurements across the different pregnancy periods, boxplots were employed.
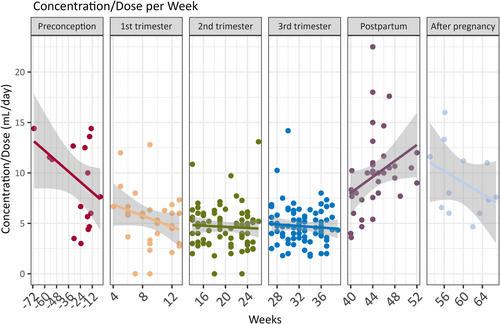
Furthermore, scatterplots accompanied by linear regression lines were generated for each pregnancy period. These plots illustrated the trends in LEV C/D over the weeks within each pregnancy period.
Our study comprised 38 participants, each undergoing measurements across six distinct pregnancy periods. This comprehensive investigation resulted in a total of 263 measurements, with each participant contributing a total of 3–10 measurements based on the number of visits. This variability in the number of measurements per participant reflects the inherent longitudinal nature of our dataset. To account for the repeated measurements within each participant across the different periods, we applied a generalized estimating equation (GEE) model with an exchangeable correlation matrix. This model was utilized to assess the impact of the stage of gestation on the concentration dose as compared to the 3rd trimester while considering the variation within each trimester. This approach enabled us to effectively analyze and interpret the slope for each specific period. The comparison was made to the 3rd trimester because it had contained the highest number of samples (n = 84) and the most patients (n = 35).
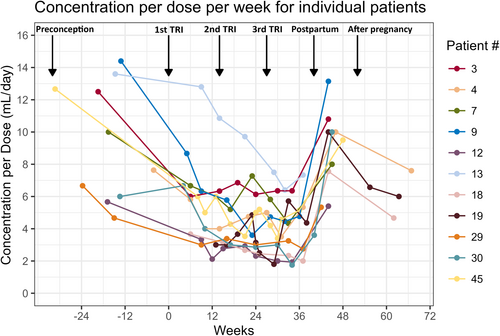
We opted for a time trend plot to illustrate the distinct patterns and variations in measurements for 11 participants, particularly those with visits across five or six pregnancy stages. This concise visual representation facilitates the quick identification of unique trends within this subset.
Statistical significance was determined according to a two-sided p-value threshold of <.05. All analyses were carried out using R-4.1.3, as provided by the R Foundation for Statistical Computing in Vienna, Austria.
2.6 Ethics
We confirm that the patient's medical records and research data were handled anonymously. The study was approved by the local ethics committee (institutional review board).
3 RESULTS
3.1 Patient characteristics
A total of 263 samples from 38 pregnant patients were collected between the years 2020 and 2023, with an average of 6.9 samples per patient. The average patient age at the time of the first sample collection while pregnant was 32.3 years. Forty-seven percent of patients had generalized epilepsy and 52% focal epilepsy. LEV monotherapy was used by 81% of patients (Table 1).
| Characteristic | Total group |
|---|---|
| Total number of patients | 38 |
| Average age at pregnancy, years, mean ± SD | 32.3 ± 5.2 |
| Generalized epilepsy, n (%) | 18 (47.3%) |
| Focal epilepsy, n (%) | 20 (52.7%) |
| ASM monotherapy (LEV) | 31a |
| ASM polytherapy | |
| 2 ASMs (LEV, LTG); (LEV, VPA); (LEV, CLB); (LEV, OXC [+VNS]) | 8a |
| 3 ASMs (LEV, LCM, CLB) | 1 |
| Samples | |
| Total number of samples | 263 |
| Preconceptionb | 11, 18 (7%) |
| 1st trimesterb | 20, 33 (13%) |
| 2nd trimesterb | 33, 78 (30%) |
| 3rd trimesterb | 35, 84 (32%) |
| Postpartumb | 30, 38 (14%) |
| After pregnancyb | 9, 12 (5%) |
| Samples per patient, mean ± SD | 6.9 ± 2.7 |
- Abbreviations: ASM, antiseizure medication; CLB, clobazam; LCM, lacosamide; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; VNS, vagal nerve stimulation; VPA, valproic Acid.
- a One patient began pregnancy with 2 ASMs (LEV, VPA) and continued with 1 ASM (LEV).
- b Displayed as number of patients in each group, number of samples in the group (% of total samples).
3.2 LEV dose and concentration trends per trimester
The median LEV dose was significantly higher during the 1st, 2nd, and 3rd trimesters and in the postpartum group (2750, 3000, 3500, and 3000 mg [Q1 = 1500, 2000, 2750, and 2000, Q3 = 3000, 4000, 4500, and 3187]) compared to the preconception dose, 2000 mg (Q1 = 1500, Q3 = 2500; Figure 1A). This observed outcome shows that LEV dose gradually increased by 75% during pregnancy compared to the preconception dose, and there was a 24% decrease in LEV dose postpartum compared to that of the 3rd trimester. In addition, a 33% rise was observed postpartum compared to preconception dose.

Observation of LEV serum levels showed that the median LEV serum level in the preconception group was 17 μg/mL (Q1 = 14.2, Q3 = 19.8). The median serum levels decreased to 11 μg/mL during the 1st trimester (Q1 = 9, Q3 = 16). The median serum levels were 13 and 15 μg/mL (Q1 = 9.2 and 18, Q3 = 18 and 19.2) during the 2nd and 3rd trimesters, respectively. Postpartum, LEV median levels increased to 24 μg/mL (Q1 = 20.2, Q3 = 29.5; Figure 1B).
The C/D decreased during pregnancy. The most significant decrease (54%) in C/D was apparent during the 1st trimester, from a median C/D ratio of 10.7 (Q1 = 5.8, Q3 = 13.4) prior to conception to 5.8 (Q1 = 3.7, Q3 = 6.7) during the 1st trimester. The ratio further decreased slightly during the 2nd trimester to 4.8 (Q1 = 3.1, Q3 = 5.8). The decrease in C/D ratio between the 2nd and 3rd trimester, 4.5 (Q1 = 3.6, Q3 = 5.6), was very mild. A significant increase of 140% was observed postpartum, from 4.5 during the 3rd trimester to 9.8 postpartum (Q1 = 7.7, Q3 = 10.8; Figure 1C).
3.3 C/D trends per week within a trimester
The C/D trend throughout the pregnancy showed a gradual decrease per week (Figure 2). Postpartum, an abrupt increase in LEV C/D was observed, even as soon as a couple of days following parturition in certain cases. Table 2 presents the GEE models compared to the 3rd trimester. The most significant C/D decrease was that of the 1st trimester (slope estimate = .85). There was no significant change in the 2nd trimester (slope estimate = .11) in comparison to the 3rd trimester. Moreover, a significant increase in C/D can be seen postpartum, with a positive slope (slope estimate = 5.23).
| Group | Slope | p | Lowest CI | Highest CI |
|---|---|---|---|---|
| Preconception | 3.84 | <.001 | 2.18 | 5.51 |
| 1st trimester | .85 | .042 | .03 | 1.68 |
| 2nd trimester | .11 | .685 | −.42 | .64 |
| Postpartum | 5.22 | <.001 | 4.31 | 6.13 |
| After pregnancy | 4.14 | <.001 | 2.97 | 5.31 |
- Abbreviation: CI, confidence interval. Bold values statistically significant p<.05
3.4 Trends per week per individual patient
Eleven patients had at least one sample in five of six defined time periods, including at least one nonpregnant sample (preconception or after pregnancy). As can be seen in Figure 3, most of the decline in the C/D was evident by the end of the 1st trimester, except for Patient 13, for whom the most evident decline in C/D was at the 2nd trimester. An abrupt increase was observed postpartum.
3.5 Seizure frequency
Twenty-eight of the total of 38 patients had experienced no seizure for at least 1 year prior to conception (73.6%). Of these, five patients had experienced a seizure during the pregnancy period (17%). Two of the five suffered from hyperemesis gravidarum that resulted in decreased absorption of the drug. Hence, their LEV serum levels tested immediately following the time of the seizure were below the laboratory's threshold (<2 μg/mL). Another one of the five patients presented to our clinic early in her pregnancy while being treated with valproic acid (VPA). She suffered a seizure during transition to treatment with LEV, 1 week after VPA cessation. Her LEV serum level was 9 μg/mL at the time of the seizure. She experienced no additional seizures when LEV dose was increased, and her blood levels reached the reference range (12–46 μg/mL). The two other patients who relapsed during pregnancy experienced a decrease in their LEV blood level. One of them had two seizures during pregnancy, with a decrease in LEV serum level around the time of each seizure of 37% and 19% (17 and 22 μg/mL, respectively) from prepregnancy level (27 μg/mL). The other patient showed a decrease in LEV serum level of 37% around the time of her seizure (10 μg/mL compared to 16 μg/mL). Of the 10 patients who had not been seizure-free prior to conception, nine (90%) continued to have seizures during their pregnancy period. One patient suffered from a 75% increase in seizure frequency, but the other nine patients did not show a significant change from baseline in seizure frequency during pregnancy.
4 DISCUSSION
Our data demonstrate that LEV clearance significantly increases in the 1st trimester of pregnancy and abruptly declines postpartum. This pattern correlates with renal function during pregnancy, as expected for an ASM that is mostly eliminated by the kidneys.4, 15 Renal clearance is elevated during the 1st trimester up to 1.7-fold in comparison with the pregestational period, and it remains high throughout the entire pregnancy due to elevated renal blood flow and increased glomerular filtration rate.6 Our findings are in line with those of other studies that showed that the greatest increase in LEV clearance took place in the 1st trimester, with smaller changes throughout the 2nd and 3rd trimesters and a decrease postpartum.2, 19, 20, 22-24 This trend contradicts the findings from a study that observed an increase in LEV clearance during the 1st trimester but a decrease during the 3rd trimester,18 and from another study that found that the most significant decrease occurred during the 3rd trimester.21 In the current study, a gradual change in the C/D was observed throughout the 1st and 2nd trimesters, as shown using the GEE model. The comparison to the 3rd trimester was chosen because we had the highest number of samples (n = 84) for it and the most patients (n = 35). To maintain a stable LEV blood level, an increased dose during pregnancy and a decreased dose postpartum are required. According to our findings, the increase in dosing should start as early as the beginning of the 1st trimester and a LEV dose reduction plan should be ready to implement immediately following parturition. There was a high variability in C/D in the nonpregnant women (preconception and after pregnancy), representing high interpatient variability.
This study was conducted at a single center, TASMC, and the data collection and serum blood levels were all checked in the same laboratory, thereby contributing to reducing different management bias and laboratory result variations. In contrast to other studies,24 the serum LEV level sample collection was carried out only at trough levels. Furthermore, our data per sample depicts the changes in LEV daily dose, LEV serum levels, and LEV C/D per week and not per trimester or per month, in contrast to previous studies.2, 18-24 As discussed previously, there are contradictory studies regarding the changes in LEV clearance in each trimester. Having measurements per week gives a better resolution of LEV clearance change. Furthermore, for each patient several measurements are included, adding to the value of the study and enabling the following of individual changes.
Our data revealed that the changes taking place in LEV clearance are gradual and continuous. We concluded that monthly serum level monitoring and LEV dose adjustments are therefore essential. This is especially true for women who are seizure-free prior to their pregnancy. A breakthrough seizure can have a great impact with regard to quality of life, as well as in regard to legal issues such as driving restrictions. Furthermore, it can increase psychiatric comorbidities such as anxiety and depression.26, 27 In addition, although rare, a single tonic–clonic seizure can even be fatal.7
Patients with hyperemesis gravidarum (excessive vomiting) should be monitored more frequently for LEV serum changes, as levels can drop significantly. TDM could also assist in determining the appropriate LEV dose if the patient switches from another ASM to LEV during pregnancy. In these cases, because there are no baseline LEV serum levels, it is advised to target the reference range (12–46 μg/mL). By using TDM to adjust LEV dose, a specific LEV serum level can be achieved for PWWE. The ideal target concentration for maintaining seizure control while minimizing perinatal fetal exposure is different for each individual PWWE. A recent study by Schelhaas et al.18 had calculated an estimation of the recommended ratio of target concentration concerning LEV (RTC-LEV), which is a calculation of LEV concentration during pregnancy divided by LEV concentration prior to pregnancy. The authors showed an RTC-LEV of .462 for seizure-free patients (n = 14 in their study) prior to pregnancy,18 indicating that they consider an LEV serum reduction of up to 54% as safe. Our own observations, however, found that even a reduction of as little as 19% could result in a breakthrough seizure. Moreover, ASMs are frequently downtitrated to the lowest effective dose prior to conception. In these cases, any percentage of LEV level drop could result in a breakthrough seizure. In addition, the absolute levels should also be considered, especially when the maintenance levels of a patient are on the lower end of the reference range.
Our study nonetheless has several limitations, related primarily to it being retrospective. Although we used a large, computerized database that contributed to reducing inconsistencies, there are missing data, because not all the patients had their LEV levels checked at every stage of their pregnancy, for instance, at earlier stages (1st and 2nd trimester), as defined for the purpose of the study. In addition, for some patients there were multiple samples from the same stage. Hence, not all patients are included in all the six groups representing the different stages, and some patients are included more than once in the same group. To address the complexities arising from the varying measurement counts per participant and the longitudinal design of our study, we employed a GEE model. This model accommodates correlated observations within individuals with irregular numbers of measurements over different periods. The use of an exchangeable correlation matrix allowed robust examination of the impact of gestational stage on concentration dose, accounting for within-participant variations across the different trimesters. In addition, having several measurements included for each patient enabled us to follow individual trends per patient in a subgroup of patients who had at least one sample in five of six defined time periods. Moreover, sample size was insufficient to establish a correlation between the C/D and seizure frequency.
In conclusion, LEV serum level monitoring is essential for all women with epilepsy upon planning pregnancy, during pregnancy, and following delivery. We recommend a monthly monitoring of LEV serum level throughout pregnancy and especially during the 1st trimester. Nevertheless, our data indicate that when TDM is not routinely available, an empirical titration regime is appropriate. We recommend a gradual increase up to 50% during the 1st trimester and up to 75% by the end of the 3rd trimester compared to the preconception dose. Further large prospective studies are warranted to evaluate and recommend an empirical LEV dose regime throughout the pregnancy and postpartum.
AUTHOR CONTRIBUTIONS
Noam Fallik: Investigation (equal); writing–original draft (lead); writing–review and editing (equal). Ilia Trakhtenbroit: Investigation (supporting). Firas Fahoum: Writing–original draft (supporting); writing–review and editing (equal). Lilach Goldstein: Conceptualization (lead); methodology (lead); investigation (equal); writing–original draft (supporting); writing–review and editing (equal); project administration (lead).
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




