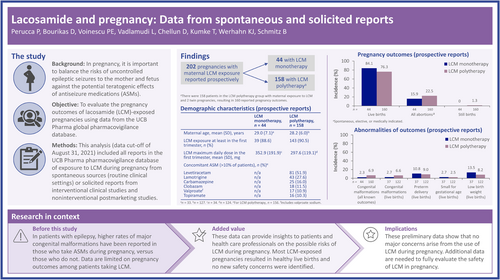
Lacosamide and pregnancy: Data from spontaneous and solicited reports
Abstract
Objective
In pregnancy, it is important to balance the risks of uncontrolled epileptic seizures to the mother and fetus against the potential teratogenic effects of antiseizure medications. Data are limited on pregnancy outcomes among patients taking lacosamide (LCM), particularly when taken as monotherapy. The objective of this analysis was to evaluate the pregnancy outcomes of LCM-exposed pregnancies.
Methods
This analysis included all reports in the UCB Pharma pharmacovigilance database of exposure to LCM during pregnancy from spontaneous sources (routine clinical settings) or solicited reports from interventional clinical studies and noninterventional postmarketing studies. Prospective and retrospective reports were analyzed separately.
Results
At the data cutoff (August 31, 2021), there were 202 prospective pregnancy cases with maternal exposure to LCM and known outcomes. Among these cases, 44 (21.8%) patients received LCM monotherapy and 158 (78.2%) received LCM polytherapy. Most patients received LCM during the first trimester (LCM monotherapy: 39 [88.6%]; LCM polytherapy: 143 [90.5%]). From the prospective pregnancy cases with maternal LCM exposure, there were 204 reported outcomes (two twin pregnancies occurred in the polytherapy group). The proportion of live births was 84.1% (37/44) in patients who received LCM as monotherapy, and 76.3% (122/160) for LCM polytherapy. The overall proportion of abortions (for any reason) was 15.9% (7/44) with LCM monotherapy, and 22.5% (36/160) with LCM polytherapy. Congenital malformations were reported in 2.3% (1/44) of known pregnancy outcomes with maternal exposure to LCM monotherapy, and 6.9% (11/160) with polytherapy.
Significance
Our preliminary data do not raise major concerns on the use of LCM during pregnancy. Most pregnancies with LCM exposure resulted in healthy live births, and no new safety issues were identified. These findings should be interpreted with caution, as additional data are needed to fully evaluate the safety profile of LCM in pregnancy.
Graphical Abstract
Key points
- This is the first analysis of prospective and retrospective reports in the UCB Pharma global pharmacovigilance database of LCM exposure during pregnancy
- Among prospective reports, the proportion of live births was 84.1% in patients who received LCM monotherapy and 76.3% for LCM polytherapy
- Among prospective reports, congenital malformations were reported in 2.3% of known pregnancy outcomes with maternal exposure to LCM monotherapy and 6.9% with polytherapy
- Among prospective reports, low birth weight was reported in 13.5% of live births with maternal exposure to LCM monotherapy and 8.2% with polytherapy
- Additional data are needed to fully evaluate the safety profile of LCM in pregnancy
1 INTRODUCTION
In pregnancy, the risks of uncontrolled epileptic seizures to the mother and fetus need to be balanced against the potential teratogenic effects of antiseizure medications (ASMs).1 Patients with epilepsy who do not take ASMs during pregnancy show similar rates of major congenital malformation as the general population (from 2% to 4%).2 The rates are two- to threefold higher (from 4% to 8%) when ASMs are taken during pregnancy,2 particularly in the first trimester.3 Higher rates of malformations have generally been reported with ASM polytherapy compared with monotherapy.3-5 However, this is dependent on the individual ASM used, as different monotherapies carry different risks6; on the doses used, as some ASMs carry dose-dependent risks; and on the particular combination of ASMs used for patients on polytherapy.
Some of the common major congenital malformations from ASM exposure during pregnancy include cleft lip and palate, heart defects, urogenital abnormalities, and neural tube defects.2, 3 For ASM monotherapies used during pregnancy, statistically significant associations have been reported between valproate and spina bifida, lamotrigine and cardiac defects, and carbamazepine and urogenital anomalies.4 Less is known about teratogenicity risks with newer ASMs. Prenatal exposure to certain ASMs has also been linked with poorer child neurodevelopment and other adverse outcomes, including low birth weight and being small for gestational age.3, 6 An observational study of ASM-exposed pregnancies showed that lacosamide (LCM) was the most commonly used newer ASM7; however, data are limited on pregnancy outcomes among patients taking LCM, particularly when taken as monotherapy.
LCM is a functionalized amino acid that selectively enhances slow inactivation of voltage-gated sodium channels.8 LCM has been licensed for clinical use in the United States and European Union since 2008,7 and is currently indicated as adjunctive therapy and monotherapy for focal seizures in patients 1 month of age and older in the United States, and in patients 2 years of age and older in the European Union.9, 10 LCM is also indicated as adjunctive therapy for the treatment of primary generalized tonic–clonic seizures in patients 4 years of age and older in the United States, European Union, Australia, and Japan.9-12 LCM is also approved in several countries across North and South America and the Asia Pacific region. The objective of this analysis was to evaluate the fetal outcomes of LCM-exposed pregnancies using data from the UCB Pharma global pharmacovigilance database.
2 MATERIALS AND METHODS
2.1 Data sources
This analysis (cutoff August 31, 2021) included all reports available in the UCB Pharma global pharmacovigilance database of exposure to LCM during pregnancy from spontaneous sources (routine clinical settings) or solicited reports from interventional clinical studies and noninterventional postmarketing studies. Unpublished reports received from the International Registry of Antiepileptic Drugs and Pregnancy (EURAP) or North American AED (antiepileptic drug) Pregnancy Registry (NAAPR) as well as published data from noninterventional studies and non-UCB Pharma studies were excluded, which is a similar approach to that taken in an analysis of another ASM (perampanel) used during pregnancy.13 These reports were excluded because the UCB Pharma pharmacovigilance database only includes data for pregnancies involving occurrence of adverse drug reactions with LCM. Reports of pregnancy cases with no adverse effects of LCM are not entered into the database, introducing a potential bias.
Reports were classified as prospective if the pregnancy was ongoing at the time that they were recorded (irrespective of stage) and the pregnancy outcome was unknown, or if no abnormality regarding the fetal health was reported at the time of initial notification. Cases were also considered to be prospective if the initial contact date (including enrollment date in a clinical study) was before or after normal fetal test results and before infant outcome was known; a case remained prospective even if the pregnancy outcome was provided at the follow-up. Attempts were made to follow up prospective pregnancy outcomes within 30 calendar days after the estimated date of delivery.
Reports were classified as retrospective in a current ongoing pregnancy if the initial contact date (including enrollment date in a clinical study) was after abnormal fetal test results (including false positives) and/or after infant outcome was known/should have been known. Because retrospective reports are biased toward adverse pregnancy outcomes, this analysis focused on the pregnancy outcomes of prospective cases; however, results of retrospective reports have also been included for completeness.
2.2 Analyses
As recommended by the European Medicines Agency14 and the US Food and Drug Administration,15 data from prospective and retrospective cases were analyzed separately. This analysis included pregnancies with LCM exposure irrespective of the indication (epilepsy or not). LCM monotherapy was reported if the patient had no reported suspect or concomitant ASMs at any time during the period of the pregnancy. A case was coded as preterm birth or preterm delivery if reported as such, or if the reported information revealed that the delivery occurred before the 37th week (i.e., before day 259) as calculated from the due date, if provided, or otherwise 259 days after the first day of the last menstrual period. A case was coded as small for gestational age if reported as such by the reporter. A case was coded as low birth weight if reported as such by the reporter; a case could also have been coded as low birth weight by UCB Pharma if the actual birth weight was provided and was <2500 g.
Demographic characteristics and reported outcomes and abnormalities from reports of patients with maternal exposure to LCM were assessed. Data are presented overall and by data source (data from spontaneous reports and data from clinical studies or solicited reports). All analyses were descriptive in nature; n values and percentages were analyzed for binary data, and mean and SD were analyzed for continuous data.
3 RESULTS
3.1 Findings from prospective reports
3.1.1 Demographic characteristics
At the data cutoff of August 31, 2021, there were 213 cases of pregnancy with known outcomes identified from spontaneous and solicited prospective reports; 202 of these were maternal exposure pregnancies (Figure 1). There were 11 pregnancies identified from prospective reports with known outcomes with paternal exposure to LCM, which will not be described further. Overall, there were 285 pregnancy cases where the outcome was unknown (of which 36 were ongoing at the time they were recorded). Pregnancies with maternal exposure to LCM and known outcomes included 159 spontaneous reports from routine clinical settings, and 43 from patients enrolled in clinical studies or solicited reports.
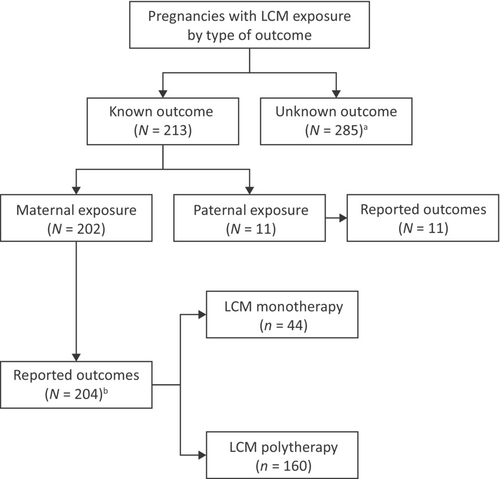
Among maternal exposure pregnancies with known outcomes (N = 202), 44 (21.8%) patients received LCM as monotherapy during pregnancy and 158 (78.2%) received LCM as polytherapy. At the start of pregnancy, the mean (SD) age of patients was 29.0 (7.1) years for those on LCM monotherapy, and 28.2 (6.0) years for those on LCM polytherapy (Table 1). Most patients (39 [88.6%] on LCM monotherapy and 143 [90.5%] on polytherapy) received LCM during the first trimester (Table 1). The mean (SD) maximum dose of LCM during the first trimester was 352.9 (191.9) mg/day for patients on LCM monotherapy and 297.6 (119.1) mg/day for patients on polytherapy. Overall, 90 (57.0%) patients on LCM polytherapy had one concomitant ASM and 66 (41.8%) had ≥2 concomitant ASMs (two patients had an unknown number of concomitant ASMs; Table 1). The most common concomitant ASMs (taken by >20% of all patients) were levetiracetam (51.9% [81/156]) and lamotrigine (27.6% [43/156]; Table 1). There were 10.9% (17/156) of patients on concomitant valproate, and 10.3% (16/156) of patients on concomitant topiramate.
| Total, N = 202 | Spontaneous reports, n = 159 | Clinical studies or solicited reports, n = 43 | ||||
|---|---|---|---|---|---|---|
| LCM monotherapy, n = 44 | LCM polytherapy, n = 158b | LCM monotherapy, n = 37 | LCM polytherapy, n = 122 | LCM monotherapy, n = 7 | LCM polytherapy, n = 36 | |
| Regions, n (%) | ||||||
| Americas | 20 (45.5) | 33 (20.9) | 16 (43.2) | 25 (20.5) | 4 (57.1) | 8 (22.2) |
| Europe | 19 (43.2) | 98 (62.0) | 18 (48.6) | 86 (70.5) | 1 (14.3) | 12 (33.3) |
| Western Pacific | 5 (11.4) | 27 (17.1) | 3 (8.1) | 11 (9.0) | 2 (28.6) | 16 (44.4) |
| Maternal age, years, mean (SD) | 29.0 (7.1)c | 28.2 (6.0)d | 28.8 (7.0)e | 28.3 (5.9)f | 29.6 (8.1) | 27.9 (6.1)c |
| Maternal age, years, n (%) | ||||||
| <18 | 1 (2.3) | 0 | 1 (2.7) | 0 | 0 | 0 |
| ≥18 to ≤35 | 26 (59.1) | 113 (71.5) | 21 (56.8) | 84 (68.9) | 5 (71.4) | 29 (80.6) |
| >35 | 6 (13.6) | 14 (8.9) | 4 (10.8) | 10 (8.2) | 2 (28.6) | 4 (11.1) |
| Missing | 11 (25.0) | 31 (19.6) | 11 (29.7) | 28 (23.0) | 0 | 3 (8.3) |
| LCM exposure, n (%) | ||||||
| At least first trimester | 39 (88.6) | 143 (90.5) | 32 (86.5) | 108 (88.5) | 7 (100.0) | 35 (97.2) |
| All trimesters | 19 (43.2) | 79 (50.0) | 19 (51.4) | 73 (59.8) | 0 | 6 (16.7) |
| Other | 4 (9.1) | 11 (7.0) | 4 (10.8) | 10 (8.2) | 0 | 1 (2.8) |
| Missing | 1 (2.3) | 4 (2.5) | 1 (2.7) | 4 (3.3) | 0 | 0 |
| LCM maximum daily dose, mg, mean (SD) | ||||||
| Overall | 345.8 (191.0)g | 294.3 (119.9)h | 325.0 (186.0)i | 287.9 (115.2)j | 450.0 (197.5)k | 313.6 (133.0)c |
| First trimester | 352.9 (191.9)l | 297.6 (119.1)m | 332.1 (187.7)n | 290.8 (113.6)o | 450.0 (197.5)k | 317.2 (133.6)p |
| Concomitant ASMs, n (%) | ||||||
| 1 ASM | n/a | 90 (57.0) | n/a | 76 (62.3) | n/a | 14 (38.9) |
| 2 ASMs | n/a | 42 (26.6) | n/a | 26 (21.3) | n/a | 16 (44.4) |
| >2 ASMs | n/a | 24 (15.2) | n/a | 19 (15.6) | n/a | 5 (13.9) |
| Unknown | n/a | 2 (1.3) | n/a | 1 (.8) | n/a | 1 (2.8) |
| Concomitant ASM [>10% of patients in any group], n (%)q | ||||||
| Levetiracetam | n/a | 81 (51.9) | n/a | 62 (51.2) | n/a | 19 (54.3) |
| Lamotrigine | n/a | 43 (27.6) | n/a | 31 (25.6) | n/a | 12 (34.3) |
| Carbamazepine | n/a | 25 (16.0) | n/a | 18 (14.9) | n/a | 7 (20.0) |
| Clobazam | n/a | 18 (11.5) | n/a | 15 (12.4) | n/a | 3 (8.6) |
| Valproater | n/a | 17 (10.9) | n/a | 12 (9.9) | n/a | 5 (14.3) |
| Topiramate | n/a | 16 (10.3) | n/a | 13 (10.7) | n/a | 3 (8.6) |
- Note: Trimesters were defined as follows: first trimester: week 0 to week 13 and 6 days; second trimester: week 14 to week 27 and 6 days; third trimester: ≥week 28.
- Abbreviations: ASM, antiseizure medication; LCM, lacosamide; n/a, not applicable.
- a Indications included epileptic seizures/epilepsy, other indications, and cases where the indication was missing.
- b There were 158 patients in the LCM polytherapy group with maternal exposure to LCM and two twin pregnancies, resulting in 160 reported pregnancy outcomes.
- c n = 33, d n = 127, e n = 26, f n = 94, g n = 36, h n = 132, i n = 30, j n = 99, k n = 6, l n = 34, m n = 124, n n = 28, o n = 92, p n = 32.
- q For LCM polytherapy, n = 156 for total patients, n = 121 for spontaneous reports, and n = 35 for clinical studies or solicited reports.
- r Includes valproate sodium.
3.1.2 Reported outcomes and abnormalities
Among pregnancies with maternal exposure to LCM, there were 204 reported outcomes (two twin pregnancies occurred in the polytherapy group). The proportion of live births was 84.1% (37/44) in patients who received LCM as monotherapy, and 76.3% (122/160) in those who received LCM as polytherapy (Table 2). The overall proportion of abortions (due to any reason) was 15.9% (7/44) with LCM as monotherapy and 22.5% (36/160) with LCM as polytherapy (Table 2). No ectopic pregnancies were reported.
| Total, N = 204 | Spontaneous reports, n = 161 | Clinical studies or solicited reports, n = 43 | ||||
|---|---|---|---|---|---|---|
| LCM monotherapy, n = 44 | LCM polytherapy, n = 160b | LCM monotherapy, n = 37 | LCM polytherapy, n = 124 | LCM monotherapy, n = 7 | LCM polytherapy, n = 36 | |
| Pregnancy outcomes, n (%) | ||||||
| Live births | 37 (84.1) | 122 (76.3) | 31 (83.8) | 101 (81.5) | 6 (85.7) | 21 (58.3) |
| Ectopic pregnancies | 0 | 0 | 0 | 0 | 0 | 0 |
| All abortions | 7 (15.9) | 36 (22.5) | 6 (16.2) | 21 (16.9) | 1 (14.3) | 15 (41.7) |
| Spontaneous | 2 (4.5) | 17 (10.6) | 1 (2.7) | 13 (10.5) | 1 (14.3) | 4 (11.1) |
| Elective | 3 (6.8) | 18 (11.3) | 3 (8.1) | 7 (5.6) | 0 | 11 (30.6) |
| Medically indicated | 2 (4.5) | 1 (.6) | 2 (5.4) | 1 (.8) | 0 | 0 |
| Still births | 0 | 2 (1.3) | 0 | 2 (1.6) | 0 | 0 |
| Abnormalities of outcomes, n/Nsub (%)c | ||||||
| Congenital malformations based on all known outcomes | 1/44 (2.3) | 11/160 (6.9)d | 1/37 (2.7) | 9/124 (7.3)d | 0/7 | 2/36 (5.6) |
| Genetic cause chromosomal abnormalities | 0/44 | 2/160 (1.3)d | 0/37 | 2/124 (1.6)d | 0/7 | 0/36 |
| Congenital malformations based on live births | 1/37 (2.7) | 8/122 (6.6) | 1/31 (3.2) | 7/101 (6.9) | 0/6 | 1/21 (4.8) |
| Genetic cause chromosomal abnormalities | 0/37 | 1/122 (.8) | 0/31 | 1/101 (1.0) | 0/6 | 0/21 |
| Preterm delivery based on live births | 4/37 (10.8) | 11/122 (9.0) | 2/31 (6.5) | 6/101 (5.9) | 2/6 (33.3) | 5/21 (23.8) |
| Single births | 4/37 (10.8) | 11/122 (9.0) | 2/31 (6.5) | 6/101 (5.9) | 2/6 (33.3) | 5/21 (23.8) |
| Multiple births | 0/37 | 0/122 | 0/31 | 0/101 | 0/6 | 0/21 |
| Small for gestational agee based on live births | 1/37 (2.7) | 3/122 (2.5) | 1/31 (3.2) | 3/101 (3.0) | 0/6 | 0/21 |
| Low birth weightf based on live births | 5/37 (13.5) | 10/122 (8.2) | 5/31 (16.1) | 8/101 (7.9) | 0/6 | 2/21 (9.5) |
- Note: N refers to pregnancy outcomes, e.g., twins are counted twice.
- Abbreviation: LCM, lacosamide.
- a Indications included epileptic seizures/epilepsy, other indications, and cases where the indication was missing.
- b There were 158 patients in the LCM polytherapy group with maternal exposure to LCM and two twin pregnancies based on spontaneous reports, resulting in 160 reported pregnancy outcomes.
- c Nsub is the number of live births, live singleton births, or known outcomes (used as denominator for percentages).
- d There was one case of tuberous sclerosis complex for which two outcomes were reported; one was an unspecified congenital malformation related to tuberous sclerosis complex, and the other was tuberous sclerosis complex. Therefore, this case was included in both categories of congenital malformations and genetic cause chromosomal abnormalities.
- e A case was coded as small for gestational age if reported as such by the reporter.
- f A case was coded as low birth weight if reported by the reporter and could be additionally coded by UCB Pharma if the birth weight was provided and <2500 g.
Congenital malformations were reported in 2.3% (1/44) of known pregnancy outcomes with maternal exposure to LCM monotherapy and 6.9% (11/160) with polytherapy (Table 2). Exposure to LCM occurred during the first trimester in all cases (Table S1). The single case of congenital malformation reported in a patient taking LCM monotherapy was an ear malformation with no associated hearing disorders; the daily dose of LCM was 100 mg/day, and the indication was not reported. Of the cases of congenital malformation on LCM polytherapy, three were exposed to ≥3 ASMs. Of the eight pregnancies exposed to one concomitant ASM in addition to LCM, there were two on concomitant lamotrigine, two on concomitant levetiracetam, and one each on concomitant topiramate, valproate, carbamazepine, and perampanel (Table S1). Preterm delivery was reported in 10.8% (4/37) of live births with maternal exposure to LCM monotherapy and 9.0% (11/122) with polytherapy. Small for gestational age was reported in 2.7% (1/37) of live births with maternal exposure to LCM monotherapy and 2.5% (3/122) of live births with maternal exposure to LCM polytherapy. Low birth weight (<2500 g or if reported as such) was reported in 13.5% (5/37) of live births with maternal exposure to LCM monotherapy and 8.2% (10/122) with polytherapy.
3.2 Findings from retrospective reports
3.2.1 Demographic characteristics
Data collected from retrospective reports were limited, with only a fraction of the patient population providing all the requested information. At the data cutoff, there were 243 cases of pregnancy with known outcomes identified from retrospective reports; 235 of these were maternal exposure pregnancies (Figure S1). There were eight pregnancies identified from retrospective reports with known outcomes with paternal exposure to LCM, which will not be described further. Overall, there were 19 pregnancy cases where the outcome was unknown. The pregnancies with maternal exposure to LCM and known outcomes included 226 spontaneous reports from routine clinical settings, and nine from patients enrolled in clinical studies or solicited reports.
Among maternal exposure pregnancies with known outcomes (N = 235), 76 (32.3%) patients received LCM as monotherapy during pregnancy, and 159 (67.7%) received LCM as polytherapy (Table S2). At the start of pregnancy, the mean (SD) age of patients was 33.3 (5.9) years for those on LCM monotherapy and 29.1 (6.0) years for those on LCM polytherapy. Overall, 55.3% (88/159) of patients on LCM polytherapy were on one concomitant ASM and 44.7% (71/159) were on ≥2 concomitant ASMs. Forty-three (56.6%) patients on LCM monotherapy and 107 (67.3%) on LCM polytherapy received LCM during the first trimester, at a mean (SD) maximum dose of 362.0 (194.3) mg/day with monotherapy and 349.4 (339.7) mg/day with polytherapy. The most common concomitant ASMs (taken by >20% of all patients) were levetiracetam (52.8% [84/159]) and lamotrigine (24.5% [39/159]). There were 11.9% (19/159) of patients on concomitant topiramate, and 8.8% (14/159) of patients on concomitant valproate.
3.2.2 Reported outcomes and abnormalities
Among pregnancies with maternal exposure to LCM, there were 238 reported pregnancy outcomes (as there was one twin pregnancy and one triplet pregnancy in the polytherapy group). The proportion of live births was similar with LCM as monotherapy (77.6% [59/76]) versus polytherapy (74.1% [120/162]; Table S3). The proportion of abortions (due to any reason) was also similar between LCM monotherapy (22.4% [17/76]) and polytherapy (24.1% [39/162]).
Congenital malformations were reported in a numerically lower proportion of pregnancy outcomes with maternal exposure to LCM monotherapy (6.6% [5/76]) compared with LCM polytherapy (19.8% [32/162]; Table S3). Preterm delivery was reported in 5.1% (3/59) of live births with maternal exposure to LCM monotherapy and 15.0% (18/120) with polytherapy; small for gestational age was reported in 1.7% (1/59) of live births with monotherapy and 4.2% (5/120) of live births with polytherapy. Low birth weight was not reported in any live births in patients on LCM monotherapy, and was reported in 5.8% (7/120) of live births with maternal exposure to LCM polytherapy.
4 DISCUSSION
Most patients with epilepsy need to continue their ASMs throughout pregnancy, as seizures can be detrimental to both mothers and their unborn children. However, there are limited data on the risks of congenital malformations and other adverse pregnancy outcomes for several ASMs—particularly newer ASMs such as LCM—used during pregnancy. There are also limited data available on the pharmacokinetics of LCM during pregnancy in the plasma or breast milk. The current analysis aimed to present preliminary data on the risk of congenital malformations with LCM during pregnancy. A prospective, observational clinical study is currently ongoing to assess the pharmacokinetic changes of LCM in blood and breast milk during pregnancy and postpartum as part of the Raoul Wallenberg Australian Pregnancy Register.16
Preclinical studies and studies in perfused human placentas have shown that LCM can cross the placental barrier.10, 17 Malformations and embryonic lethality were observed in mice with LCM exposure during embryonic development, although most of them were born without any clear malformations.18 According to the label, there was no indication of teratogenic effects in studies of LCM treatment in rats and rabbits; however, embryotoxicity was observed at maternal toxic doses of LCM.10 Notably, teratogenic effects have been observed in animal studies of lamotrigine, levetiracetam, and oxcarbazepine, even though these are currently considered to be some of the safest ASMs in human pregnancy.3, 13 This highlights the importance of analyzing fetal outcomes with LCM exposure in human pregnancies.
Prospective pregnancy registries, including EURAP, NAAPR, the UK and Ireland Epilepsy and Pregnancy Registers, and the Raoul Wallenberg Australian Pregnancy Register of Antiepileptic Drugs, have an important role in assessing the teratogenic risk of ASMs.19 Currently, there are no substantial data available from these registries on outcomes of LCM-exposed pregnancies.3, 4, 20-22 According to their latest online report (October 2023), limited data have been published on LCM by the NAAPR, reporting a 0% prevalence of major malformations (95% confidence interval = 0%–5.21%) from LCM monotherapy during the first trimester, based on 88 cases.23
Pharmacovigilance and safety databases can also provide important information on pregnancy outcomes and risks of congenital malformations for ASM-exposed pregnancies. In an observational study of ASM-exposed pregnancies reported to the German Embryotox Center of Clinical Teratology and Drug Safety database, LCM was the most frequently reported ASM among those with marketing authorization between 2005 and 2019.7 Of the 55 prospectively ascertained cases of LCM-exposed pregnancies in their observational study (six of which were LCM monotherapy at conception), eight were electively terminated, three ended as spontaneous abortion, one case was stillbirth, and nongenetic major birth defects were detected in two pregnancies.7 Of the 36 neonates who were exposed to LCM until delivery, four had functional cardiac disorders.7 Overall, the authors concluded that there was no indication of substantial teratogenic or embryotoxic effects of LCM.7
By interrogating the UCB Pharma global pharmacovigilance database, we found 202 prospective cases of pregnancy with maternal exposure to LCM and known outcomes (44 as monotherapy and 158 as polytherapy), most of which resulted in live births. The rate of spontaneous abortions was 4.5% for LCM monotherapy and 10.6% for polytherapy, which is no higher than the rate reported in the general population (15.3%).24 Congenital malformations were reported in 2.3% of pregnancies exposed to LCM monotherapy, a rate not dissimilar to that of major congenital malformations (2.6%) reported in a network meta-analysis of women with epilepsy not exposed to ASMs during pregnancy.25 A numerically higher rate of 6.9% was observed in pregnancies with exposure to polytherapy including LCM. Of the 12 cases of congenital malformations that were identified (based on all known outcomes), LCM was used as monotherapy in a single case. Of the remaining 11 cases on polytherapy, eight pregnancies were exposed to 1 concomitant ASM and three were exposed to ≥3 concomitant ASMs. Two patients received dual therapy with LCM in combination with an ASM shown to increase the risk of malformations when used in polytherapy (valproate or topiramate).4 In a longitudinal, prospective cohort study, the risk of major congenital malformations associated with eight commonly used ASM monotherapies ranged from 2.5% (levetiracetam) to 9.9% (valproate).26 Despite the inherent bias associated with retrospective reports, monotherapy data from the retrospective outcomes are also reassuring, with most pregnancies resulting in healthy live births.
Exposure to ASMs during the first trimester is typically associated with an increased risk of malformations, because this is a critical time for organogenesis.27 In our analysis, most patients received LCM during the first trimester. The overall rate of congenital malformations based on all known outcomes was numerically higher for LCM polytherapy versus monotherapy, which is similar to that reported for other ASMs where malformation rates were higher with polytherapy versus monotherapy.4, 5
Prenatal exposure to ASMs has been associated with an increased risk of infants being born small for gestational age and with low birth weight compared to unexposed ones.28, 29 In our study, small for gestational age was reported in 2.7% (1/37) of live births with maternal exposure to LCM monotherapy and 2.5% (3/122) with polytherapy, and low birth weight (<2500 g or if reported as such) was reported in 13.5% (5/37) of live births with LCM monotherapy and 8.2% (10/122) with polytherapy. According to data collected prospectively in the NAAPR, among women with epilepsy receiving ASMs during pregnancy, small for gestational age was reported in 9.8% (494/5020) of those with ASM monotherapy and 14.2% (218/1539) with ASM polytherapy; low birth weight was reported in 5.6% (281/5020) with ASM monotherapy and 8.9% (137/1539) with ASM polytherapy.30 Given the differences in the study designs and sample sizes assessed, it is not possible to make direct comparisons with the reported rates of adverse fetal growth outcomes across these studies.
Studies using data from safety databases have several important limitations, especially when evaluating drug risks in pregnancy. For many of the prospective reports, the pregnancy outcome was unknown despite follow-up attempts, limiting the sample size for assessment of pregnancy outcomes and abnormalities. Highlighting the importance of collecting pregnancy outcomes in assessing the risk of congenital malformations for different ASMs and allowing physicians and patients to make informed decisions might increase the gathering of pregnancy outcome reports. The interpretation of results of the congenital malformations is confounded by the use of concomitant ASMs in a subset of pregnancies and limited by the lack of a comparator group. Results of analyses on retrospective cases have been provided for completeness. However, retrospective reports have an inherent bias, because abnormal outcomes are more likely to be reported than normal outcomes. The risk for possible LCM-related neurodevelopmental effects also needs to be assessed, given that fetal exposure to some ASMs, particularly valproate and topiramate, has been associated with increased risks of cognitive and behavioral problems.31, 32 Finally, due to the nature of safety reporting, direct comparisons with the reported rate of spontaneous abortions (15.3%)24 or major congenital malformations (3.5%)33 in the general population or other populations cannot be made.
5 CONCLUSIONS
These data provide preliminary information on LCM use during pregnancy. According to the current limited data, no major concerns arise from the use of LCM during pregnancy. Most LCM-exposed pregnancies resulted in healthy live births, and no new safety issues were identified. These findings should be interpreted with caution, as additional data are needed to fully evaluate the safety of LCM in pregnancy. It is important that more prospective data from the use of LCM during pregnancy—especially as monotherapy—are collected and published to help patients and treating physicians to make more informed decisions on pregnancy planning.
AUTHOR CONTRIBUTIONS
Piero Perucca: Supervision (equal); writing—review and editing (equal). P. Emanuela Voinescu: Writing—review and editing (equal). Lata Vadlamudi: Writing—review and editing (equal). Daya Chellun: Writing—review and editing (equal). Konrad J. Werhahn: Writing—review and editing (equal). Thomas Kumke: Formal analysis (lead); writing—review and editing (equal). Dimitrios Bourikas: Conceptualization (lead); data curation (equal); writing—original draft preparation (lead); writing—review and editing (equal). Bettina Schmitz: Writing—review and editing (equal).
ACKNOWLEDGMENTS
The authors would like to acknowledge Ciara Duffy, PhD, CMPP (Evidence Scientific Solutions Ltd., Horsham, UK) for writing assistance, which was funded by UCB Pharma. Publication coordination was provided by Tom Grant, PhD (UCB Pharma, Slough, UK). Part of this work was presented as a poster at the 77th Annual Meeting of the American Epilepsy Society on December 1–5, 2023. Open access publishing facilitated by The University of Melbourne, as part of the Wiley - The University of Melbourne agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
This study was funded by UCB Pharma. UCB Pharma was responsible for the study design, and collection and analysis of the data. The authors, some of whom are UCB Pharma employees, were responsible for data interpretation, revising the manuscript for intellectual content, and approving of the manuscript for submission.
CONFLICT OF INTEREST STATEMENT
P.P. is supported by an Emerging Leadership Investigator Grant from the Australian National Health and Medical Research Council (APP2017651), the University of Melbourne, Monash University, the Weary Dunlop Medical Research Foundation, Brain Australia, and the Norman Beischer Medical Research Foundation. He has received speaker honoraria or consultancy fees to his institution from Chiesi, Eisai, LivaNova, Novartis, Sun Pharma, Supernus, and UCB Pharma, outside the submitted work. He is an Associate Editor for Epilepsia Open. P.E.V. has received speaker honoraria from Neurodiem and Physicians' Education Resource. B.S. has received speaker honoraria or consultancy fees from Angelini Pharma, Desitin, Eisai, Precisis, Sanofi, and UCB Pharma. D.B., D.C., K.J.W., and T.K. are paid employees of UCB Pharma and receive stock or stock options from their employment. L.V. has no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
Data from noninterventional studies are outside of UCB Pharma's data-sharing policy and are unavailable for sharing.