Epilepsy and the risk of COVID-19-related hospitalization and death: A population study
Richard F. Chin and William O. Pickrell contributed equally as senior authors.
Abstract
Objective
People with epilepsy (PWE) may be at an increased risk of severe COVID-19. It is important to characterize this risk to inform PWE and for future health and care planning. We assessed whether PWE were at higher risk of being hospitalized with, or dying from, COVID-19.
Methods
We performed a retrospective cohort study using linked, population-scale, anonymized electronic health records from the SAIL (Secure Anonymised Information Linkage) databank. This includes hospital admission and demographic data for the complete Welsh population (3.1 million) and primary care records for 86% of the population. We identified 27 279 PWE living in Wales during the study period (March 1, 2020 to June 30, 2021). Controls were identified using exact 5:1 matching (sex, age, and socioeconomic status). We defined COVID-19 deaths as having International Classification of Diseases, 10th Revision (ICD-10) codes for COVID-19 on death certificates or occurring within 28 days of a positive SARS-CoV-2 polymerase chain reaction (PCR) test. COVID-19 hospitalizations were defined as having a COVID-19 ICD-10 code for the reason for admission or occurring within 28 days of a positive SARS-CoV-2 PCR test. We recorded COVID-19 vaccinations and comorbidities known to increase the risk of COVID-19 hospitalization and death. We used Cox proportional hazard models to calculate hazard ratios.
Results
There were 158 (.58%) COVID-19 deaths and 933 (3.4%) COVID-19 hospitalizations in PWE, and 370 (.27%) deaths and 1871 (1.4%) hospitalizations in controls. Hazard ratios for COVID-19 death and hospitalization in PWE compared to controls were 2.15 (95% confidence interval [CI] = 1.78–2.59) and 2.15 (95% CI = 1.94–2.37), respectively. Adjusted hazard ratios (adjusted for comorbidities) for death and hospitalization were 1.32 (95% CI = 1.08–1.62) and 1.60 (95% CI = 1.44–1.78).
Significance
PWE are at increased risk of being hospitalized with, and dying from, COVID-19 when compared to age-, sex-, and deprivation-matched controls, even when adjusting for comorbidities. This may have implications for prioritizing future COVID-19 treatments and vaccinations for PWE.
Key points
- We identified 27 279 people with epilepsy (136 395 controls) during the first 15 months of the COVID-19 pandemic in Wales.
- People with epilepsy have increased risk of being hospitalized with, and dying from, COVID-19 when compared to matched controls.
- This overall increased risk remained after adjusting for comorbidities associated with higher COVID-19 risk.
1 INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused significant morbidity and mortality worldwide.1-4 Epilepsy is one of the most common neurological conditions, affecting approximately 50 million people worldwide, with significant comorbidities and an increased risk of premature mortality.5
The COVID-19 pandemic caused significant changes in health care globally. Health care professionals normally providing epilepsy care were redeployed to COVID-19 work. Epilepsy consultations and investigations were also canceled, making it harder for people with epilepsy to access appropriate care.6, 7 In some countries, including Wales, people have been deterred from seeking medical attention for serious non-COVID-19 problems, including epilepsy-related issues, due to perceived COVID-19 risk.8-10
SARS-CoV-2 is known to directly affect the central nervous system, with high rates of neurological and psychiatric complications.11, 12 There is some evidence that people with epilepsy are at increased risk of COVID-19 hospitalization and COVID-19 death.13-16 This may be in part due to the increased proportion of people with epilepsy with comorbidities, such as dementia and intellectual disability, that increase the risk of severe COVID-19.13, 16
SARS-CoV-2 vaccines have significantly improved COVID-19 outcomes.17 However, given the significant morbidity and mortality that COVID-19 has caused, and the potential for further COVID-19 and other pandemics with new variants and pathogens, it is important to accurately quantify COVID-19 risks in specific populations. There have been very few population-level, epilepsy-specific studies on serious COVID-19 outcomes to date. In this study, which was part of the Coronavirus and Epilepsy in Wales project, we aimed to use detailed, population-level data to assess whether people with epilepsy were more at risk from severe COVID-19 outcomes, namely COVID-19 hospitalization and death.
2 MATERIALS AND METHODS
We used the Secure Anonymised Information Linkage (SAIL) databank, which contains anonymized individual-level, population-scale, routinely collected electronic health records from multiple different sources. These include hospital admission and demographic data for the complete Welsh population (3.1 million) and primary care records for 86% of the population.18, 19 We used the Controlling COVID-19 Through Enhanced Population Surveillance and Intervention project dataset within SAIL (Project 0911).20
We identified people with epilepsy living in Wales before or during the study period (March 1, 2020 to June 31, 2021). We used a previously validated method to define epilepsy as a primary care diagnosis of epilepsy and prescription of at least two antiseizure medications.21 To reduce confounding and the dimensionality of regression models, we created a control cohort using exact 5:1 matching on sex, age, and socioeconomic deprivation quintile measured using the Welsh Index of Multiple Deprivation 2019 (WIMD). WIMD uses weighted scores from eight domains to form a score for small geographical areas or Lower Layer Super Output Areas (LSOAs). WIMD scores for each LSOA are then grouped into quintiles, with Quintile 1 being the most deprived and Quintile 5 being the least deprived.22
We recorded SARS-CoV-2 vaccination status and comorbidities known to increase the risk of COVID-19 hospitalization and death. The comorbidities are based on those used in the QCOVID algorithm (Table 1).13, 16, 23 As a sensitivity analysis, we also created an additional control cohort (control cohort 2) using 5:1 exact matching for age, sex, and deprivation with additional propensity matching (logistic regression) based on the nearest propensity score for QCOVID comorbidities. Further to this, we created stratified models for key groups of interest including age (0–18, 18–65, 65+ years) and deprivation (individuals living in areas in the two most deprived quintiles) to highlight differences in these subgroups.
| Comorbidity | Epilepsy | Exact-matched controls | p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Prescribed immunosuppressants within 6 months | 60 | .22 | 199 | .15 | .0050 |
| Prescribed anti-leukotriene/beta2-agonists within 6 months | 1661 | 6.09 | 5133 | 3.76 | <.00001 |
| Prescribed oral steroids within 6 months | 239 | .88 | 1050 | .77 | .070 |
| Atrial fibrillation | 980 | 3.59 | 3243 | 2.38 | <.00001 |
| Heart failure | 516 | 1.89 | 1689 | 1.24 | <.00001 |
| Asthma | 5744 | 21.06 | 20 557 | 15.07 | <.00001 |
| Blood cancer | 179 | .66 | 570 | .42 | <.00001 |
| Cerebral palsy | 843 | 3.09 | 151 | .11 | <.00001 |
| Coronary heart disease | 1361 | 4.99 | 5144 | 3.77 | <.00001 |
| Cirrhosis | 151 | .55 | 293 | .21 | <.00001 |
| Congenital heart disease | 444 | 1.63 | 757 | .56 | <.00001 |
| COPD | 1153 | 4.23 | 3908 | 2.87 | <.00001 |
| Dementia | 708 | 2.6 | 972 | .71 | <.00001 |
| Previous fracture of hip, wrist, spine, or humerus | 2072 | 7.6 | 5424 | 3.98 | <.00001 |
| Motor neuron disease, multiple sclerosis, myasthenia gravis, or Huntington chorea | 160 | .59 | 384 | .28 | <.00001 |
| Parkinson disease | 152 | .56 | 265 | .19 | <.00001 |
| Pulmonary hypertension/fibrosis | 66 | .24 | 210 | .15 | .0012 |
| Cystic fibrosis, bronchiectasis, or alveolitis | 177 | .65 | 604 | .44 | <.00001 |
| Peripheral vascular disease | 343 | 1.26 | 1048 | .77 | <.00001 |
| Rheumatoid arthritis or SLE | 349 | 1.28 | 1417 | 1.04 | .00045 |
| Oral or lung cancer | 40 | .15 | 133 | .1 | .023 |
| Severe mental illness (psychosis, schizophrenia, bipolar disease, severe depression)a | 6240 | 22.87 | 18 792 | 13.78 | <.00001 |
| Sickle cell disease | 41 | .15 | 74 | .05 | <.00001 |
| Stroke | 2345 | 8.6 | 2972 | 2.18 | <.00001 |
| Diabetes type 1 | 167 | .61 | 531 | .39 | <.00001 |
| Diabetes type 2 | 2119 | 7.77 | 8549 | 6.27 | <.00001 |
| Thrombosis or pulmonary embolus | 530 | 1.94 | 1157 | .85 | <.00001 |
| Chemotherapy in the last 12 months | 107 | .39 | 476 | .35 | .27 |
| Care home | 834 | 3.06 | 549 | .4 | <.00001 |
| Homeless | 115 | .42 | 146 | .11 | <.00001 |
| Learning disability | 4299 | 15.76 | 2281 | 1.67 | <.00001 |
| Down syndrome | 24 | .09 | 29 | .02 | <.00001 |
| Bone marrow or stem cell transplant within 6 months | <5b | NAb | <5b | NAb | |
| Radiotherapy within 6 months | 39 | .14 | 105 | .08 | .0050 |
| Solid organ transplant | 18 | .07 | 47 | .03 | <.00001 |
| Chronic kidney disease stage 3 | 900 | 3.3 | 3780 | 2.77 | .070 |
| Chronic kidney disease stage 4 | 61 | .22 | 237 | .17 | <.00001 |
| Chronic kidney disease stage 5, no dialysis/transplant | 36 | .13 | 126 | .09 | <.00001 |
| Chronic kidney disease stage 5 with dialysis within 12 months | 6 | .02 | 8 | .01 | <.00001 |
| Chronic kidney disease stage 5 with transplant | 13 | .05 | 48 | .04 | <.00001 |
- Abbreviations: COPD, chronic obstructive pulmonary disease; NA, not applicable; SLE, systemic lupus erythematosus.
- a See Table S5 for more detail.
- b Small numbers are not disclosed due to potential reidentification issues.
We defined COVID-19 deaths as having International Classification of Diseases, 10th Revision (ICD-10) codes for COVID-19 on death certificates (in any position, e.g., primary or secondary cause of death) or occurring within 28 days of a positive SARS-CoV-2 polymerase chain reaction (PCR) test. In the UK, death certificates have space to record the immediate cause, underlying cause, and conditions contributing to death. COVID-19 hospitalizations were defined as having a COVID-19 ICD-10 code for the reason for admission or occurring within 28 days of a positive SARS-CoV-2 PCR test.
We used Cox proportional hazard models to calculate hazard ratios (HRs) for COVID-19 hospitalization and death. We used Schoenfield residuals to test for proportionality. For some adjusted models, comorbidities we removed due to none/very few people in one or both cohorts having the diagnosis. Details of proportionality tests are given in Table S2, and comorbidities used/removed for each model are indicated in Tables S3 and S4.
We used R (version 4.1.3) for statistical analysis.
We collaborated with Epilepsy Action, a leading UK epilepsy charity, discussing project design, results, and this article with Epilepsy Action and volunteers with epilepsy. We produced a video that showed some of the views of people living with epilepsy on their experiences of the effect of COVID-19.24
This study was approved by the SAIL independent Information Governance Review Panel (Project 0911). The Research Ethics Service has previously confirmed that SAIL projects using anonymized, routinely collected data do not require National Health Service research ethics committee approval.
3 RESULTS
There were 27 279 people in the epilepsy (case) cohort and 136 395 people in the control cohort (epilepsy prevalence of .96% at beginning of study and .95% at end of study). There were 158 (.58%) COVID-19 deaths and 933 (3.4%) COVID-19 hospitalizations in people with epilepsy, and 370 (.27%) deaths and 1871 (1.4%) hospitalizations in controls (Table 2).
| Characteristic | Epilepsy cases | Controls | ||||
|---|---|---|---|---|---|---|
| Total | C19 Hosp | C19 Deaths | Total | C19 Hosp | C19 Deaths | |
| Total | 27 279 | 565 (2.1%) | 158 (.58%) | 136 395 (100%) | 1329 (.97%) | 370 (.27%) |
| Male | 13 945 (51.1%) | 287 (2.1%) | 86 (.62%) | 69 725 (51.1%) | 666 (.96%) | 214 (.31%) |
| Female | 13 334 (48.9%) | 278 (2.1%) | 72 (.54%) | 66 670 (48.9%) | 663 (.99%) | 156 (.23%) |
| Deprivation | ||||||
| WIMD 1 [most deprived] | 7429 (27.2%) | 186 (2.5%) | 42 (.57%) | 37 145 (27.2%) | 445 (1.20%) | 112 (.30%) |
| WIMD 2 | 6369 (23.4%) | 134 (2.1%) | 38 (.60%) | 31 845 (23.4%) | 325 (1.02%) | 82 (.26%) |
| WIMD 3 | 4917 (18.0%) | 87 (1.8%) | 29 (.59%) | 24 585 (18.0%) | 197 (.80%) | 52 (.21%) |
| WIMD 4 | 4344 (15.9%) | 87 (2.0%) | 21 (.48%) | 21 720 (15.9%) | 187 (.86%) | 53 (.24%) |
| WIMD 5 [least deprived] | 4220 (15.5%) | 71 (1.7%) | 28 (.66%) | 21 100 (15.5%) | 175 (.83%) | 71 (.34%) |
| Age in years | ||||||
| 0–16 | 2562 (9.4%) | 9 (.35%) | 0 (.00%) | 12 810 (9.4%) | 15 (.12%) | 0 (.00%) |
| 16–65 | 18 754 (68.8%) | 294 (1.6%) | 38 (.20%) | 93 770 (68.8%) | 628 (.67%) | 43 (.04%) |
| 65+ | 5963 (15.8%) | 262 (4.4%) | 120 (2.01%) | 29 815 (15.8%) | 686 (2.30%) | 327 (1.1%) |
| Comorbiditiesa | ||||||
| Intellectual disability | 4299 (15.8%) | 96 (2.2%) | 24 (.56%) | 2281 (1.7%) | 30 (1.3%) | 11 (.48%) |
| Dementia | 708 (2.6%) | 72 (10.2%) | 51 (7.2%) | 972 (.7%) | 72 (7.4%) | 70 (7.2%) |
| Diabetes | 2286 (8.4%) | 117 (5.1%) | 38 (1.7%) | 9080 (6.7%) | 270 (3.0%) | 107 (1.2%) |
| Parkinson disease | 152 (.6%) | 12 (7.9%) | 5 (3.3%) | 265 (.2%) | 18 (6.8%) | 10 (3.8%) |
| Vaccinations | ||||||
| Before 1st vaccination | 532 (94.2%) | 147 (93.0%) | 1268 (95.4%) | 357 (96.5%) | ||
| After 1st vaccination | 33 (5.8%) | 11 (7.0%) | 61 (4.6%) | 13 (3.5%) | ||
| After 2nd vaccination | 10 (1.8%) | 0 (0%) | 14 (1.1%) | 0 (0%) | ||
| SARS-CoV-2 PCR tests | ||||||
| Total individuals tested | 11 823 (43.3%) | 564 (4.8%) | 144 (1.2%) | 49 945 (36.6%) | 1299 (2.6%) | 338 (.7%) |
| Total individuals with positive test | 1941 (7.1%) | 528 (27.2%) | 133 (6.9%) | 9021 (6.6%) | 1248 (13.8%) | 325 (3.6%) |
| Total tests | 47 956 | 2222 (4.6%) | 656 (1.4%) | 172 812 | 7058 (4.1%) | 1337 (.8%) |
| Total positive tests | 2377 (5.0%) | 858 (36.1%) | 177 (7.5%) | 10 011 (5.8%) | 1835 (18.3%) | 443 (4.4%) |
Comparing people with epilepsy to controls, the HRs for COVID-19 death and COVID-19 hospitalization were 2.15 (95% confidence interval [CI] = 1.78–2.59) and 2.15 (95% CI = 1.94–2.37), respectively. Adjusted HRs (aHRs; adjusted for comorbidities associated with COVID-19 hospitalization and COVID-19 death) for COVID-19 death and hospitalization were 1.32 (95% CI = 1.08–1.62) and 1.60 (95% CI = 1.44–1.78; Table 3 and Figure 1). Figure 1 shows the changes in hospitalizations and deaths with time during the study period. Steeper gradients on the graphs in Figure 1 correspond to increased rates of COVID-19 hospitalizations (Figure 1A) and deaths (Figure 1B) during the first and second COVID-19 “waves” in the UK during spring 2020 and winter 2020/2021. The alpha SARS-CoV-2 variant corresponds with the second wave. The delta variant was associated with a third wave in the UK in the summer of 2021, which is at the end of our study period and was associated with fewer deaths and hospitalizations in general.
| COVID-19 hospitalization | COVID-19 death | |||
|---|---|---|---|---|
| Crude hazard ratio | Adjusted hazard ratio | Crude hazard ratio | Adjusted hazard ratio | |
| Total | 2.15 (1.94–2.37) p < .00001 | 1.60 (1.44–1.78) p < .00001 | 2.15 (1.78–2.59) p < .00001 | 1.32 (1.08–1.62) p = .00680 |
| Age 0–18 years | 3.01 (1.32–6.89) p = .0089 | 1.72 (.64–4.58) p = .28 | — a | — a |
| Age 18–65 years | 2.35 (2.05–2.70) p < .00001 | 1.82 (1.56–2.12) p < .00001 | 4.42 (2.86–6.84) p < .00001 | 2.48 (1.49–4.14) p = .00048 |
| Age 65+ years | 1.97 (1.71–2.27) p < .00001 | 1.43 (1.22–1.66) p < .00001 | 1.87 (1.52–2.31) p < .00001 | 1.10 (.88–1.39) p = .39 |
| Intellectual disability | 1.72 (1.14–2.59) p = .0099 | 2.26 (1.45–3.52) p = .00033 | 1.14 (.56–2.32) p = .72 | 1.50 (.68–3.33) p = .32 |
| Most deprivedb | 2.10 (1.84–2.39) p < .00001 | 1.65 (1.44–1.90) p < .00001 | 2.07 (1.56–2.69) p < .00001 | 1.30 (.98–1.73) p = .067 |
- Note: Hazard ratios are presented in comparison to control in the same subgroup, for example, the hazard ratio for age 0–18 years are for people with epilepsy aged 0–18 years when compared to controls aged 0–18 years, et cetera. Significant hazard ratios are shown in bold.
- a Hazard ratios for COVID-19 death for age 0–18 years are not calculated, as there were no COVID-19 deaths in children in cases or controls.
- b Most deprived refers to two most deprived quintiles (quintile 1 and 2) as measured by the Welsh Index of Multiple Deprivation (see Section 2).
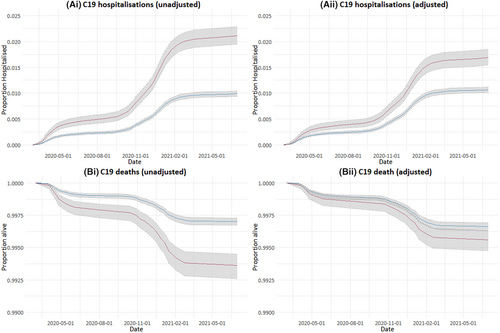
The results were similar when comparing people with epilepsy to control cohort 2 (exact-matched for age, sex, and deprivation and propensity-matched for comorbidities increasing risk of COVID-19 hospitalization and death). HRs (epilepsy compared to control cohort 2) for COVID-19 death and COVID-19 hospitalization were 1.45 (95% CI = 1.22–1.74) and 1.66 (95% CI = 1.51–1.83), respectively.
4 DISCUSSION
In this large population-level study of more than 27 000 people with epilepsy, we found that people with epilepsy were more likely to have a COVID-19-related hospitalization or death when compared to controls during the first 15 months of the pandemic in Wales (crude HRs for hospitalization and death were 2.15 [95% CI = 1.94–2.37] and 2.15 [95% CI = 1.78–2.59]). This was true (but reduced) even when considering 40 comorbidities known to be associated with COVID-19 hospitalization and death (aHRs for hospitalization and death were 1.60 [95% CI = 1.44–1.78] and 1.33 [95% CI = 1.08–1.63]). This means there was an approximately 60% increased chance of hospitalization with COVID and a 33% increased chance of dying with COVID for people with epilepsy when compared to people of the same age, sex, area-based deprivation quintile, and comorbidities during the first 15 months of the COVID-19 pandemic in Wales.
In subgroups (adults aged 18–65 years, adults aged >65 years, people with intellectual disability, and people living in the two most deprived areas), people with epilepsy were more likely to have COVID-19 hospitalizations (Table 3 and Figure 2). There was no significant increase in COVID-19 hospitalizations for children with epilepsy after adjusting for comorbidities. However, the numbers of COVID-19 hospitalizations in children were very small, with only nine hospitalizations for children with epilepsy (15 hospitalizations in the controls).
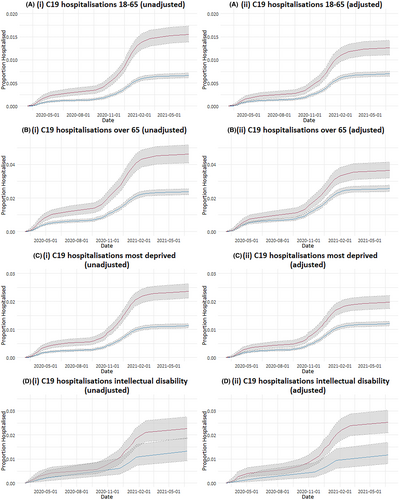
Our results showed an association between epilepsy and an increased hospitalization rate compared to the matched comparator. This emphasizes that epilepsy might cause an increased risk of severe COVID-19, as with other chronic diseases.16 The neurotropic effects of Sars-CoV-2 might explain some of this increased risk for people with epilepsy, or epilepsy might be associated with alterations in the immune system, predisposing to more severe COVID-19.11, 12, 25 Another explanation is that the increased COVID-19 hospitalization rate reflects a lower threshold for hospitalization for people with epilepsy when presenting with COVID-19 due to perceived risk. COVID-19 may have increased seizures for people with epilepsy, which would have increased the risk of hospitalization and/or death. It is also possible that we have not accounted for a confounding comorbidity in our analysis that increases COVID-19 hospitalization risk and is more common in people with epilepsy.
We have used an established definition for COVID-19 hospitalizations (a hospital admission with a COVID-19 ICD-10 code for the reason for admission, or occurring within 28 days of a positive SARS-CoV-2 PCR test).16 It is possible that this definition included hospital admissions with COVID-19 but not because of it, for example, seizure- or epilepsy-related admissions.
When considering COVID-19 deaths in subgroups, only adults with epilepsy aged 18–65 years had an increased risk of COVID-19 death when adjusting for comorbidities (Table 3 and Figure 3). There were no COVID-19 deaths in children with epilepsy or children in the control group in our study.
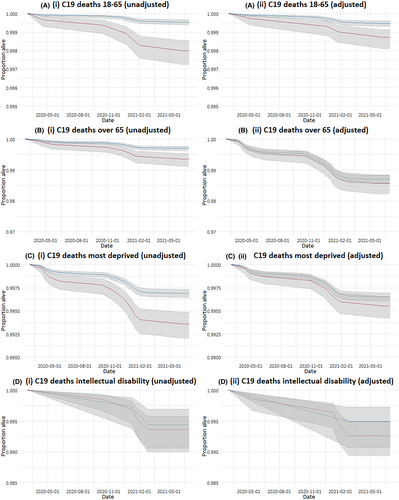
It is established that people with intellectual disability have an increased risk of epilepsy, and almost 16% (4299) of the people with epilepsy in our study had an intellectual disability. Reassuringly, there did not seem to be a significantly increased risk of COVID-19 death in this group, but the numbers of COVID-19 deaths in people with intellectual disability were small. It was also reassuring that people with epilepsy in the most deprived two quintiles were not at increased risk of death when compared to controls.
4.1 Comparison to other studies
The QCOVID study used linked routinely collected data within the QResearch database for 6 million individuals in the UK, including approximately 80 000 people with epilepsy. They found an increased risk for COVID-19 hospitalization (aHRs of 1.57 and 1.75 for women and men) and COVID-19 death (aHR of 1.58 for women and 1.6 for men) for people with epilepsy.16 A further QCOVID study with a larger population of 7 million vaccinated individuals (100 000 with epilepsy) again found an increased risk of COVID-19 hospitalization (aHR = 1.70, 95% CI = 1.32–2.20) but not death (aHR = 1.13, 95% CI = .85–1.50).13 The QCOVID studies used epilepsy diagnosis codes only, whereas we used diagnosis and prescription codes, which we have found to be more specific in identifying cases of epilepsy in routinely collected data.21
Italian, Spanish, and Korean studies (1500–4000 people with epilepsy) found an increased risk of COVID-19 hospitalization for people with epilepsy (aHR = 1.9, 95% CI = 1.4–2.7 and adjusted odds ratio = 2.05, 95% CI = 1.04–4.04) but not an increased risk of death for people with epilepsy.14, 15, 26 Scottish (4500 people with epilepsy) and Canadian studies (20 children with epilepsy from 330 hospitalized children) found an increased risk of COVID-19 hospitalization in children with epilepsy and neurological disease including epilepsy (HR and adjusted rate ratios of 2.54, 95% CI = 1.69–3.81 and 1.84, 95% CI = 1.32–2.57, respectively).27, 28 Our study had more than 27 000 people with epilepsy and more than 136 000 controls, making it larger than these studies. We were also able to study the whole Welsh population with epilepsy as opposed to other studies that have looked at people with epilepsy among the COVID-19 hospitalized populations.
4.2 Strengths
We have performed one of the largest epilepsy-specific population studies of COVID-19 outcomes. We have used a validated method of ascertaining people with epilepsy, were able to account for many comorbidities, and created a large age-, sex-, and deprivation-matched control cohort.
4.3 Limitations
Our study period was limited to the first 15 months of the COVID-19 pandemic and has not accounted for geographical and temporal variation in SARS-CoV-2 prevalence and SARS-CoV-2 variants. This variation has affected the risk of COVID-19 hospitalization and death, but it is likely that this variation would have affected the epilepsy and matched control groups in a similar way.
We have not accounted for the effect of vaccinations. However, we have previously shown that COVID-19 vaccination uptake has been higher in people with epilepsy when compared with controls, with 89.9% of people with epilepsy and 85.2% of controls having had two COVID-19 vaccines by the end of 2021.29 Also the vast majority of the COVID-19 hospitalizations and COVID-19 deaths in this study occurred in unvaccinated individuals (>93%; Table 2). We have also not accounted for prior infection with SARS-CoV-2, which reduces the risk of severe COVID-19, although the number of people with prior exposure to SARS-CoV-2 was low at the onset of the study period.30 In this study, we have not accounted for many other ways in which COVID-19 has affected people with epilepsy, including changes in seizure frequency, mental health, service provision, diagnosis rates, access to medication, and rates of long COVID. In addition, we were not able to account for changes in mental status associated with COVID-19, which may be associated with increased mortality in people with epilepsy. Further work is required to look at these and other outcomes that are important to people living with epilepsy.
5 CONCLUSIONS
People with epilepsy were at an increased risk of being hospitalized with, and dying from, COVID-19 when compared to age-, sex-, and deprivation-matched controls in Wales, even when adjusting for a large number of comorbidities. This may have implications for prioritizing future COVID-19 treatments and vaccinations for people with epilepsy.
AUTHOR CONTRIBUTIONS
Huw Strafford performed primary data and statistical analysis. William O. Pickrell and Richard F. Chin conceived, designed and coordinated the study. Michael P. Kerr, Richard F. Chin, Robert Powell, and Ronan A. Lyons provided senior clinical advice. Joe Hollinghurst and Alan Watkins provided statistical advice. Jan Paterson and Daniel Jennings provided public and patient involvement advice. All authors were part of the project team reviewing results and progress. Huw Strafford and William O. Pickrell drafted the manuscript. All authors reviewed and edited the manuscript.
ACKNOWLEDGMENTS
This study is funded by Health and Care Research Wales. The views expressed are those of the authors and not necessarily those of Health and Care Research Wales or the Welsh Government. This work was supported by Health Data Research UK, which receives its funding from HDR UK (HDR-9006) funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation, and Wellcome Trust. J.H. was supported by Health and Care Research Wales (Project SCF-18-1504). A.A., J.H., and R.A.L. were supported by the Con-COV grant funded by the Medical Research Council (grant number: MR/V028367/1); ADR Wales funded by the ADR UK (grant ES/S007393/1); and the Wales COVID-19 Evidence Centre funded by Health and Care Research Wales. This study makes use of anonymized data held in the Secure Anonymised Information Linkage (SAIL) databank. We would like to acknowledge all the data providers who make anonymized data available for research. Approval for the use of data in this study, within the SAIL databank, was granted by an independent information governance review panel (Project 0911). We acknowledge the help of Epilepsy Action volunteers who have assisted in all stages of this project; in particular we would like to thank Nigel Bennett, Sara Edwards, Carys Jones, Rebecca Longley, and Sarah Thorpe. For the purpose of open access, the author has applied a CC-BY public copyright license to any author accepted manuscript version arising from this submission.
CONFLICT OF INTEREST STATEMENT
M.P.K. has received speaker fees from UCB Pharma and Veriton and is vice chair of SUDEP ACTION. W.O.P. has received speaker fees from UCB Pharma and Angelini Pharma, travel grants from Angelini Pharma, and an unrestricted research grant from UCB Pharma. The authors confirm that they have no conflict of interests. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.




