Effect of levodopa on pathological gait in Dravet syndrome: A randomized crossover trial using three-dimensional gait analysis
Abstract
Objective
Individuals with Dravet syndrome (DS) exhibit progressive gait disturbance. No quantitative studies have been conducted to evaluate the effectiveness of medication for gait disturbance. Therefore, the aim of this study was to evaluate the effectiveness of levodopa for pathological gait in people with DS using three-dimensional gait analysis (3DGA).
Methods
Nine individuals with DS, ages 6–20 years, participated in a crossover study of levodopa and were randomly assigned to the levodopa precedence or no levodopa precedence group. Levodopa/carbidopa hydrate was prescribed at a dose of 5 mg/kg/day (body weight <60 kg) or 300 mg/day (body weight ≥60 kg). The medication was taken for 4–6 weeks (4-week washout period). 3DGA was performed three times before the study, with and without levodopa. A mixed-effects model was used to evaluate the effectiveness of levodopa. The primary outcome was the change in the Gait Deviation Index (GDI). In addition, spatiotemporal gait parameters, 6-minute walking distance (6MD), and balance were evaluated. The correlation between the effectiveness of levodopa and age or gait performance before starting levodopa was analyzed.
Results
Levodopa improved the GDI by 4.2 points, (p = .029), 6MD by 52 m (p = .002), and balance test result by 4.1 mm (p = .011) in participants with DS. No severe adverse events were observed, with the exception of one participant, who exhibited fever and consequently stopped taking levodopa. Levodopa was more effective in younger participants with a higher baseline gait performance.
Significance
Our randomized crossover trial showed that levodopa has the potential to improve gait disturbance in people with DS.
Key points
- Levodopa improved Gait Deviation Index (GDI; 4.2 points), 6-minute walking distance (52 m), and balance test (4.1 mm) results in participants with Dravet syndrome.
- The GDI was significantly improved by levodopa in younger participants with a higher baseline gait performance.
- Although one participant developed fever, the other eight showed no or mild adverse events such as somnolence, menoxenia, or excitability.
1 INTRODUCTION
Dravet syndrome (DS) is a rare developmental and epileptic encephalopathy characterized by the onset of febrile or afebrile seizures, often of long duration, occurring during the first year of life, and followed by multiple additional seizures and slowing of developmental and cognitive skills.1, 2 Most people with DS have a mutation in the SCN1A gene, which encodes the alpha-subunit of voltage-gated sodium channels.1, 2 The main focus of clinical studies on DS had been seizure control. However, over the last decade, motor disabilities have come to the forefront.3-5 Gait disturbance is one of the most significant problems for people with DS and caregivers because the ability to walk is important for activities of daily living and quality of life.3-5 A recent study reported progressive deterioration of gait and motor function with age in people with DS.6
Progressive gait abnormalities including ataxic gait, crouch gait, and parkinsonian gait have been reported in people with DS.5, 7-9 Currently there are few established treatments for pathological gait in people with DS. One case report pointed to the possible effectiveness of levodopa for parkinsonian gait in an adult woman with DS.10 However, randomized controlled trials have not been conducted to assess the effectiveness of levodopa for gait abnormalities in DS. The lack of objective, quantitative gait assessment methods may in turn explain the lack of randomized controlled trials.
Three-dimensional gait analysis (3DGA) of spatiotemporal data, kinematics, and kinetics is a reliable way to evaluate gait quantitatively.11 The Gait Deviation Index (GDI) is a comprehensive index used to detect gait pathology based on the kinematics of the lower extremities. The GDI has made it easier to assess gait quantitatively.12-14 Recently, 3DGA was used to assess specific gait characteristics and improve therapeutic outcomes in pediatric neurological diseases and cerebral palsy.15-18
Based on this background, we hypothesized that levodopa could improve gait ability in people with DS, and that 3DGA has the capacity to quantify gait changes objectively. The aim of the present randomized, crossover, unblinded study was to evaluate quantitatively the improvement in pathological gait associated with the administration of levodopa using 3DGA.
2 MATERIALS AND METHODS
This study was approved by Nagoya University Graduate School of Medicine's Clinical Research Review Board (approval number: N0028). Written informed consent was obtained from the parents of all participants. The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
2.1 Study population and overview of the study
We prospectively enrolled ambulatory people with DS ≥6 years of age from June 2020 to November 2021. All individuals exhibited the clinical phenotype of DS, and the SCN1A gene mutation was confirmed in all participants. All participants underwent a clinical evaluation, which included a physical examination, conducted by pediatric neurologists and orthopedists. If both the individual with DS and the parents agreed to participate, the participant was subsequently enrolled in the randomized, crossover, unblinded study to validate the effectiveness of levodopa. We excluded people who had ever been treated with levodopa, had a contraindication for levodopa administration, had serious concomitant medical problems (such as severe liver or kidney dysfunction), who were unable to complete the 3DGA without support, or had a history of acute encephalopathy. We also excluded people whose parents did not provide written informed consent.
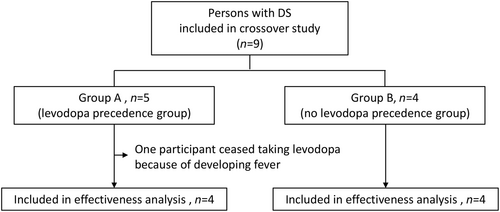
Participants were randomized in a 1:1 ratio to group A (levodopa precedence group) or group B (no levodopa precedence group) using a minimization algorithm (Figure 1). Randomization was performed using the online system of Nagoya University Hospital. Stratification was performed according to age (<15 vs ≥15 years). A schematic of the crossover, randomization process is shown in Figure 2. Participants in group A took levodopa orally for 4–6 weeks and then underwent a second 3DGA. Then, levodopa was tapered to two-thirds of the initial dose over 3 days and to one-third of the initial dose over a further 3 days, after which it was terminated. A third 3DGA was performed 4–6 weeks after the cessation of levodopa. Participants in group B underwent a second 3GDA at 4–6 weeks after entering the study without taking levodopa. Four weeks after the second 3DGA, these participants started to take levodopa and underwent a third 3DGA 4–6 weeks later.

Levodopa/carbidopa hydrate was prescribed at a dose of 5 mg/kg/day for participants with a body weight <60 kg, or 300 mg/day for those weighing ≥60 kg. These doses were determined based on a study by Fasano et al., in which 300 mg/day levodopa was administered to adults with DS.10 The dosing schedule was three times per day (30 min after main meals).
2.2 3DGA procedure
The MX-T 20S 3DGA system (Vicon Motion Systems Ltd., Oxford, UK), which includes eight optical cameras, was used to measure spatiotemporal gait variables. The sampling frequency was set at 100 Hz. Twenty-four retro-reflective markers were attached to the lower extremities and pelvis of the participants according to Conventional Gait Model 2.3.19 Participants were instructed to walk barefoot for 2 m on a flat surface at their preferred speed. Depending on the level of cooperation, 5–20 trials were completed by each participant, and the mean values from three trials representative of their usual gait were used in the final analysis. Before performing the 3DGA, physical measurements including body height, body weight, and lower limb lengths were obtained. We also evaluated the 6-minute walking distance (6MD) and postural stability using eight force plates (AMTI OPT; Advanced Mechanical Technology, Inc., Watertown, MA, USA) at every visit before the 3DGA. In the 6MD test, participants were instructed to walk at their maximum speed for 6 min on a flat surface. In the balance test, participants were instructed to stand relaxed and motionless on the force plates for 15 s. The anteroposterior center of pressure sway was used in the final analysis. All evaluations were conducted by an experienced pediatric physiotherapist.
2.3 3DGA data analysis
The gait variable data were recorded, preprocessed, and analyzed using Vicon Nexus 2.8 software (Vicon Motion Systems Ltd.). The data were normalized as a percentage of one gait cycle using Vicon Polygon 4.2 software (Vicon Motion Systems Ltd.) and then exported into Excel software (ver. 16.0; Microsoft Corp., Redmond, WA, USA). We assessed walking speed, step length (/length of lower extremities), gait variability, and the GDI.
Gait variability was assessed based on the coefficient of variation (CV; standard deviation [SD]/mean value × 100) of the step length.20, 21 A higher CV indicates greater variability in gait. The GDI was calculated from the kinematics of gait data for the pelvis and hip in the sagittal, coronal, and horizontal planes, and the knee and ankle in the sagittal plane, as well as the foot progression angle.12
2.4 Outcome measures
In addition to the results of the 3DGA, we collected demographic and clinical data from participants with DS, including age, sex, SCN1A gene mutation status, age at which they started to walk without support, degree of intellectual disability, age at seizure onset, history of status epilepticus seizures, frequency of epileptic seizures, antiseizure medication, score on the Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS Part 2), score on the Gillette Functional Assessment Questionnaire (FAQ), and results of the physical examination (muscle tone, ataxia, pes planovalgus, and scoliosis).
The intelligence quotient (IQ) and developmental quotient (DQ) were evaluated using the Tanaka–Binet Intelligence Scale and Enjoji Infantile Developmental Scale, respectively. Intellectual disability was classified as mild (51 ≤ IQ or DQ ≤ 75), moderate (36 ≤ IQ or DQ ≤ 50), or severe (IQ or DQ ≤ 35). The MDS-UPDRS Part 2 is used to determine the extent of the movement disorder associated with parkinsonism, which was evaluated by the parents of the individual with DS.22 It consists of 13 questions about motor disability associated with parkinsonism, and every item is scored on a scale ranging from 0 to 4. Therefore, the total score ranges from 0 to 52, and higher scores represent more severe disability. The FAQ is used to assess walking ability in daily life, and it was completed by the parents.23 Scores range from 1 to 10, and higher scores indicate better gait performance. We evaluated muscle tone abnormalities and ataxia. For the latter, we checked for intention tremor during reaching movements and unsteadiness during walking, standing, and sitting. Scoliosis was classified by severity as mild (10° ≤ Cobb angle < 25°), moderate (25° ≤ Cobb angle < 40°), or severe (40° ≤ Cobb angle).24
In the present study, to validate the effectiveness of levodopa, the primary outcome was the difference in the GDI between the period with and without levodopa/carbidopa hydrate medication. An increase in the GDI ≥5 points was considered to indicate a clinically meaningful improvement, as reported previously.25 Secondary outcomes included adverse events associated with levodopa/carbidopa hydrate and changes in walking speed, step length/length of lower extremities, CV of step length, 6MD, and anterior posterior center of pressure sway. In addition, we obtained MDS-UPDRS Part 2 and FAQ scores at all three visits for the 3DGA.
Finally, we performed subgroup analyses to identify factors associated with improvement in gait analysis parameters after the administration of levodopa. Participants were divided into two groups based on median age and the GDI, 6MD, and FAQ score at the first visit.
2.5 Statistical analysis
The effectiveness of levodopa for gait pathology was analyzed using a mixed-effects model. Primary and secondary outcome measures were compared between the periods with and without levodopa. The model included treatment (levodopa or no levodopa) and crossover period as fixed covariates, and participant as a random effect. In addition, subgroup analysis stratified using age as a factor for each outcome measure was performed considering the higher variance of the gait parameters in younger participants. Furthermore, we performed a subgroup analysis of levodopa effectiveness, as indicated by the GDI, again using a mixed-effects model.
Because no previous studies have assessed improvements in the GDI after levodopa administration, our crossover study can be considered exploratory. Based on feasibility, we set a recruitment target of 20 cases.
SAS software (version 9.4; SAS Institute, Cary, NC, USA) was used to evaluate the effectiveness of levodopa for gait pathology. A two-sided p-value < .05 was considered statistically significant.
3 RESULTS
During the study period, nine individuals (six male and three female) with DS were recruited. The included participants with DS were 6–20 years of age (median age, 15 years). Various mutations in the SCN1A gene were confirmed in all nine participants. Missense, nonsense, and splice-site mutations were confirmed in three participants, five participants, and one participant, respectively. All participants who started having seizures in infancy were taking at least two antiseizure medications at the first 3DGA. The number of status epilepticus cases before the present study ranged from 0 to 5 times (median, 2 times). Intellectual disability was severe in seven people and moderate in two. Age at beginning to walk without support was from 12 to 24 months (median, 14 months). The participants' total scores on the MDS-UPDRS Part 2 ranged from 15 to 39 points (median, 26 points). The median FAQ score was 8 points (range, 5–8 points). Generalized rigidity, ataxia (appendicular, axial, and gait), pes planovalgus, and scoliosis were seen in one, four, nine, and three participants, respectively.
Five participants were assigned randomly to group A (levodopa precedence group) and four to group B (no levodopa precedence group). One participant in group A developed fever while taking levodopa and stopped the medication. This case was excluded from the crossover study, and the remaining eight participants (four in group A and four in group B) were included in the analysis. There were no statistically significant differences in the demographic data or 3DGA results between the participants in groups A and B, except that the group B participants had a larger CV of step length. Although somnolence, menoxenia, and excitability were experienced in one case each, all three of these participants continued taking levodopa.
Figure 3 shows plots of the 3DGA outcomes. Compared with the off-levodopa period (third 3DGA in group A and second 3DGA in group B), in the on-levodopa period (second 3DGA in group A and third 3DGA in group B), seven of eight participants had a higher GDI, longer 6MD, and smaller anteroposterior center of pressure sway. Moreover, the FAQ score was higher in the on-levodopa period in four participants and was not different between the on- and off-levodopa periods in the remaining four participants. Representative data for the participants who exhibited a good response to levodopa are shown in Figure S1.
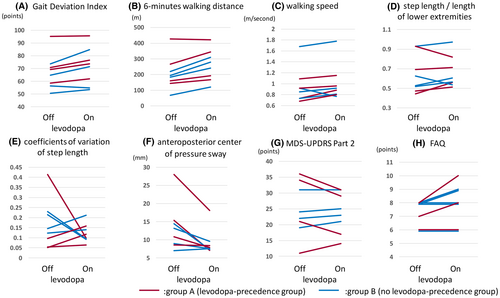
According to the results of the mixed-effects model, levodopa improved the GDI by 4.2 points (p = .029), the 6MD by 52 m (p = .002), and the balance test result by 4.1 mm (p = .011) in participants with DS (Table 1). Results of the subgroup analysis using age as a factor are shown in Table 2. Walking speed and GDI improved significantly in the younger participant group, whereas no statistically significant difference was observed in older participants. The 6MD result improved significantly in younger and older participants.
| Outcome measures | Estimate | 95% confidence interval | p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Walking speed, m/s | 0.059 | −0.02 | 0.137 | .116 |
| Step length/length of lower extremities | 0.018 | −0.055 | 0.092 | .569 |
| Coefficient of variation of step length | −0.044 | −0.146 | 0.058 | .369 |
| Gait Deviation Index | 4.15 | 0.582 | 7.718 | .029 |
| 6-min walking distance, m | 52.079 | 28.328 | 75.829 | .002 |
| Anteroposterior center of pressure sway, mm | −4.129 | −7.552 | −0.706 | .026 |
| MDS-UPDRS Part 2, points | −0.875 | −3.29 | 1.54 | .409 |
| FAQ score | 0.625 | 0.039 | 1.211 | .040 |
- Note: To evaluate the effectiveness of levodopa for gait pathology, a mixed-effects model was constructed to compare outcome measures before and after levodopa administration.
- Abbreviations: FAQ, Gillette Functional Assessment Questionnaire; MDS-UPDRS, Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale.
| Outcome measures | Estimate | 95% confidence interval | p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Walking speed, m/s, all cases | 0.059 | −0.02 | 0.137 | 0.116 |
| Age at first 3DGA | ||||
| <16 years, n = 4 | 0.082 | 0.017 | 0.148 | 0.032 |
| ≥16 years, n = 4 | 0.035 | −0.259 | 0.329 | 0.659 |
| Gait Deviation Index, all cases | 4.15 | 0.582 | 7.718 | 0.029 |
| Age at first 3DGA | ||||
| <16 years, n = 4 | 6.988 | 1.831 | 12.144 | 0.028 |
| ≥16 years, n = 4 | 1.313 | −4.36 | 6.985 | 0.424 |
| 6-min walking distance, m, all cases | 52.079 | 28.328 | 75.829 | 0.002 |
| Age at first 3DGA | ||||
| <16 years, n = 4 | 71.425 | 23.242 | 119.608 | 0.024 |
| ≥16 years, n = 4 | 32.732 | 0.804 | 64.661 | 0.048 |
| FAQ score, all cases | 0.625 | 0.039 | 1.211 | 0.040 |
| Age at first 3DGA | ||||
| <16 years, n = 4 | 0.750 | −0.326 | 1.826 | 0.095 |
| ≥16 years, n = 4 | 0.500 | −1.651 | 2.651 | 0.423 |
- Abbreviations: 3DGA, three-dimensional gait analysis; FAQ, Gillette Functional Assessment Questionnaire.
Table 3 shows the results of the subgroup analysis. Younger participants with a higher GDI and FAQ score in the first 3DGA achieved a clinically meaningful increase in the GDI of >5 points after levodopa administration. The estimated effectiveness was 6.99 points (p = .028) for participants younger than 16 years, 7.4 points (p = .043) for participants with a GDI of 66 points or higher at the first 3DGA, and 5.24 points (p = .021) for participants with a FAQ score of 8 or higher at the first 3DGA.
| Estimate | 95% confidence interval | p-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| All participants (n = 8) | 4.15 | 0.582 | 7.718 | 0.029 |
| Age at first 3DGA | ||||
| <16 years, n = 4 | 6.988 | 1.831 | 12.144 | 0.028 |
| ≥16 years, n = 4 | 1.313 | −4.36 | 6.985 | 0.424 |
| GDI at the first 3DGA | ||||
| <66, n = 4 | 2.975 | −7.106 | 13.056 | 0.332 |
| ≥66, n = 4 | 7.375 | 0.603 | 14.147 | 0.043 |
| 6MD at the first 3DGA | ||||
| <217, n = 4 | 2.625 | −2.912 | 8.162 | 0.178 |
| ≥217, n = 4 | 5.675 | −0.812 | 12.162 | 0.064 |
| FAQ score at the first 3DGA | ||||
| <8, n = 2 | 0.875 | −11.538 | 13.288 | 0.535 |
| ≥8, n = 6 | 5.242 | 1.289 | 9.195 | 0.021 |
- Note: To evaluate the effect of levodopa on the Gait Deviation Index, a mixed-effects model was constructed to compare outcome measures before and after levodopa administration.
- Abbreviations: 3DGA, three-dimensional gait analysis; 6MD, 6-minute walking distance; FAQ, Gillette Functional Assessment Questionnaire; GDI, Gait Deviation Index.
4 DISCUSSION
This is the first study to evaluate quantitatively the effectiveness of levodopa for pathological gait in participants with DS using 3DGA. Levodopa significantly improved the GDI, 6MD, FAQ score, and anteroposterior center of pressure sway in the participants. Our findings indicate that levodopa can improve walking ability in people with DS, and that 3DGA is suitable for objectively and quantitatively analyzing changes in gait skills.
The underlying pathology of movement disorders in DS, including gait disturbance, remains to be elucidated. Gait abnormality in DS may be caused by multiple pathophysiologies that vary among people. Dysfunction of the dopaminergic system, cerebellar ataxia, motor neuropathy, muscle hypotonia, orthopedic misalignment, intellectual disability, and a high body mass index may contribute to gait abnormality.26-28 In the present study, the GDI and 6MD were improved by levodopa, which suggests the involvement of dopaminergic neurotransmission in the gait pathology of DS. The sodium channel encoded by SCN1A is expressed extensively in the frontal lobe and basal ganglia.10, 29 Wyers et al. reviewed pathological gait in people with DS. They suggested that basal ganglia dysfunction explains levodopa-responsive parkinsonism symptoms, and that the vulnerability of the dopaminergic system to aging explains why parkinsonism is seen only in adult individuals with DS.5 In a recent case report of a 19-year-old man with DS, dopamine transporter single-photon emission computed tomography (SPECT) did not reveal presynaptic dopaminergic cell loss.30 However, marked reductions in the cerebral spinal fluid levels of several metabolites, including neopterin, tetrahydrobiopterin, 5-hydroxyindoleacetic acid, and homovanillic acid, were seen.30 These findings are similar to those of dopa-responsive dystonia.31 In fact, the present crossover study showed that a small amount of levodopa/carbidopa hydrate, consistent with the treatment dose in people with dopa-responsive dystonia, improved gait in participants with DS.32 These findings suggest that abnormalities of the dopaminergic system may have an important role in the gait abnormalities seen in DS.
The underlying pathology of ataxic gait in people with DS seems to be more complicated. In their systematic review, Wyers et al. pointed out that the cerebellar signs and ataxia in individuals with DS are controversial, and that the results of DS evaluations depend on the definition of ataxia used.5 A recent study reported functional mobility limitations in participants with DS, mainly during activities requiring stability such as stair climbing, circumventing obstacles, kicking a ball, and running, with limitations increasing in severity from the age of 18 years.28 As well as ataxia, an abnormal posture and poor muscle tone can undermine stability. In the present study, anteroposterior center of pressure sway was improved by administration of levodopa. Our results suggest that a more-complicated mechanism involving dopaminergic neurotransmission rather than cerebellar dysfunction may account for ataxic gait in people with DS.
It remains unclear when levodopa should be started in DS. Studies of individuals with DS of varying age revealed progressive worsening of gait abnormality and orthopedic misalignment with older age, although adolescents exhibited wide variations in their mobility skills.26 In a study of adults with DS, three participants started levodopa, of whom the youngest participant (29 years of age at the final evaluation) showed gait improvement, whereas the two older participants (aged 47 and 51 years) exhibited progressive gait disturbance, even after starting levodopa.6 In our study, the effectiveness of levodopa was greater in younger participants with higher gait performance before treatment started. Our findings suggest that levodopa treatment may be beneficial in younger people with DS and progressive gait abnormality, although long-term efficacy has not been established.
In this study, levodopa was well tolerated in participants with DS. High fever was the only severe adverse event leading to medication cessation. Whether there was a causal relationship between levodopa and fever in this participant was unclear, and the fever resolved after stopping levodopa. Other adverse events during levodopa treatment included somnolence, menoxenia, and excitability. All these adverse events were mild, and no participant needed to stop levodopa without additional treatment. We used a relatively low dose of levodopa for a short period. If higher doses of levodopa are administered for a longer period to adults with Parkinson's disease, adverse events can be more serious. Another issue associated with levodopa is a malignant syndrome following discontinuation of medication. However, in the Earlier versus Later Levodopa Therapy in Parkinson Disease (ELLDOPA) study conducted by the Parkinson Study Group, a 3-day levodopa step-down withdrawal period was implemented, and none of the 361 participants experienced neuroleptic malignant syndrome.33 We set the tapering period to 6 days, twice as long as that of the ELLDOPA study, to ensure safe withdrawal. Although none of our participants experienced malignant syndrome, this result should be interpreted with caution considering the small sample size.
Our study had several limitations. First, the sample size was small, particularly to determine age-related effectiveness. Although we set a sample target of 20 cases, it was difficult to arrange for people with DS to attend the hospital three times, in part due to the coronavirus disease 2019 (COVID-19) pandemic. Because of the small number of participants, some clinical factors potentially influencing the effectiveness of levodopa were not fully considered. Second, the effect of levodopa was considered over only a short period, and its long-term effectiveness remains to be determined. Nevertheless, our study provides evidence of the effectiveness of levodopa, as well as useful information for clinicians and individuals with DS, as well as for caregivers about the treatment of gait disturbance in DS.
5 CONCLUSION
In conclusion, our randomized crossover trial provides evidence that levodopa is a generally well tolerated and potentially beneficial treatment for improving gait disturbance in people with DS.
AUTHOR CONTRIBUTIONS
Conception and organization: T.S., J.N., Y.I., H.W., Y.T., and H.K. Participant recruitment: T.S., J.N., Y.I., T.F., T.T., K.I., H.K., K.K., T.O., S.S., and H.S. Physical examination: T.S. and N.O. Gait analysis: T.I. Statistical analysis: T.S. and F.K. Data interpretation: T.S., J.N., Y.I., T.I., and N.O. Manuscript writing: T.S. Manuscript review: J.N., Y.I., T.I., N.O., F.K., T.F., T.T., K.I., H.K., K.K., T.O., S.S., H.S., H.W., Y.T., and H.K.
ACKNOWLEDGMENTS
This study was supported by The Japan Foundation for Pediatric Research (Grant No: 20-003). The grant was awarded for the project entitled “Randomized clinical trial of L-DOPA for gait pathology in Dravet syndrome” (principal investigator: Takeshi Suzuki). The funders had no role in designing the study; collecting, analyzing or interpreting the data; or preparing the manuscript.
CONFLICT OF INTEREST STATEMENT
The corresponding author, Jun Natsume, is affiliated with the endowed department of Aichi Prefecture. The remaining authors have no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ETHICS STATEMENT
This study was approved by the Clinical Research Review Board of Nagoya University Graduate School of Medicine (approval number: N0028). The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
No materials were reproduced from other sources in this paper.
PATIENT CONSENT STATEMENT
Written informed consent was obtained from the parents of all participants.
CLINICAL TRIAL REGISTRATION
This study was registered to jRCT (registration number: jRCTs041190116). Date of registration was December 25, 2019, and date of first patient enrollment was June 8, 2020.
STATEMENTS RELATING TO OUR ETHICS AND INTEGRITY POLICIES
The study was performed in accordance with ethics and integrity policies of Epilepsia.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




