Treatment of seizures in the neonate: Guidelines and consensus-based recommendations—Special report from the ILAE Task Force on Neonatal Seizures
Abstract
Seizures are common in neonates, but there is substantial management variability. The Neonatal Task Force of the International League Against Epilepsy (ILAE) developed evidence-based recommendations about antiseizure medication (ASM) management in neonates in accordance with ILAE standards. Six priority questions were formulated, a systematic literature review and meta-analysis were performed, and results were reported following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 standards. Bias was evaluated using the Cochrane tool and risk of Bias in non-randomised studies - of interventions (ROBINS-I), and quality of evidence was evaluated using grading of recommendations, assessment, development and evaluation (GRADE). If insufficient evidence was available, then expert opinion was sought using Delphi consensus methodology. The strength of recommendations was defined according to the ILAE Clinical Practice Guidelines development tool. There were six main recommendations. First, phenobarbital should be the first-line ASM (evidence-based recommendation) regardless of etiology (expert agreement), unless channelopathy is likely the cause for seizures (e.g., due to family history), in which case phenytoin or carbamazepine should be used. Second, among neonates with seizures not responding to first-line ASM, phenytoin, levetiracetam, midazolam, or lidocaine may be used as a second-line ASM (expert agreement). In neonates with cardiac disorders, levetiracetam may be the preferred second-line ASM (expert agreement). Third, following cessation of acute provoked seizures without evidence for neonatal-onset epilepsy, ASMs should be discontinued before discharge home, regardless of magnetic resonance imaging or electroencephalographic findings (expert agreement). Fourth, therapeutic hypothermia may reduce seizure burden in neonates with hypoxic–ischemic encephalopathy (evidence-based recommendation). Fifth, treating neonatal seizures (including electrographic-only seizures) to achieve a lower seizure burden may be associated with improved outcome (expert agreement). Sixth, a trial of pyridoxine may be attempted in neonates presenting with clinical features of vitamin B6-dependent epilepsy and seizures unresponsive to second-line ASM (expert agreement). Additional considerations include a standardized pathway for the management of neonatal seizures in each neonatal unit and informing parents/guardians about the diagnosis of seizures and initial treatment options.
Key Points
- This paper presents guidelines and recommendations from the International League Against Epilepsy regarding the treatment of neonatal seizures
- The Clinical Practice Guideline group consisted of an international team of experts including neurologists, neonatologists, pediatricians, epileptologists, and a parent representative
- Recommendations are based on a systematic review and expert-based consensus via Delphi methodology if insufficient evidence was available
- Recommendations include choice of first- and second-line medication, treatment duration, effect of therapeutic hypothermia on seizures, and use of pyridoxine
1 INTRODUCTION
Seizures are the most common neurological emergency in the neonatal period. Most seizures in newborns are acute provoked (or symptomatic), typically related to hypoxic–ischemic brain injury, intracranial hemorrhage, arterial ischemic stroke, or intracranial infection.1-3 In 10%–15% of infants, seizures are the manifestation of neonatal epilepsy, usually due to cortical malformations, genetic defects, or inborn errors of metabolism.4-6 The 2022 International League Against Epilepsy (ILAE) classification of epilepsy syndromes with onset in neonates and infants addresses etiology-specific epilepsy syndromes.7
Electroencephalography (EEG) is required for seizure diagnosis, because most seizures in neonates have no clinical manifestations (electrographic-only),8, 9 and differentiating between seizures and other abnormal movements is difficult.10 In addition, treatment with antiseizure medication (ASM) may cause electroclinical uncoupling in which the clinical correlate ceases but electrographic seizures persist.8, 11, 12 EEG monitoring using conventional video-EEG (cEEG) is recommended to identify seizures in neonates by multiple clinical practice guidelines and consensus statements,2, 13-15 as well as in clinical trials of neonatal seizure management.16 In the clinical setting, amplitude-integrated EEG (aEEG) can be used in addition to or in the absence of access to cEEG, although it is recognized that sensitivity and specificity of aEEG is rather variable and as such it cannot be recommended as the mainstay for seizure detection.17
There is considerable variation in clinical practice regarding neonatal seizure management.18-21 The most recent international guideline regarding neonatal seizure management was published in 2011 by the World Health Organization (WHO), ILAE, and International Bureau of Epilepsy, which included articles until 2008.13 It was intended for clinicians practicing in a wide range of health care facilities, and it was developed based on all published studies (including randomized controlled trials [RCTs], quasi-RCTs, and observational studies) in full-term neonates with clinical and/or electrographic seizures in the initial 28 days of life. New evidence regarding neonatal seizure management has emerged in the past decade necessitating an update of the evidence-based recommendations on seizure management in term and preterm neonates.
This article provides guidelines and consensus-based recommendations for six priority questions related to neonatal seizure management: (1) first-line ASM, (2) second-line ASM, (3) duration of ASM treatment, (4) impact of therapeutic hypothermia on seizure burden in neonates with hypoxic–ischemic encephalopathy (HIE), (5) impact of electrographic seizure treatment on outcome, and (6) administration of pyridoxine. The target users are clinicians who care for neonates with seizures, including neonatologists, pediatric neurologists, pediatricians, and pharmacologists. Recommendations on the acute management of neonates with seizures, including identification and treatment of other correctable etiologies (e.g., hypoglycemia, hyponatremia), other than ASM are outside the scope of these guidelines. These are covered in the previous WHO/ILAE guidelines of neonatal seizures and elsewhere.13, 22
2 MATERIALS AND METHODS
2.1 Clinical Practice Guideline working group
The ILAE Commission for Pediatrics identified the need to update the original Neonatal Seizure Guideline published in 2011.13 In response, ILAE's Executive Committee established a Clinical Practice Guidelines (CPG) working group in 2017, comprised of 27 members of the Neonatal Task Force, including 19 child neurologists and clinical neurophysiologists and three neonatologists representing all ILAE regions, two methodologists, one parent representative, and two senior advisors. Fifteen members declared no conflicts of interest, six members declared nonrelated conflicts of interest, and six members declared related conflicts of interest. Overall, 78% of the CPG working group were void of conflicts of interest. There was no representation from the pharmaceutical or medical device industry. Guideline development adhered to the ILAE handbook and toolkit.23
2.2 Priority questions
Six priority questions were formulated following the PICO (population, intervention[s], comparator[s], and outcome[s]) format. The questions addressed first-line ASM, second-line ASM, duration of ASM treatment, impact of therapeutic hypothermia on seizure burden in neonates with HIE, impact of electrographic seizure treatment on outcome (neurodevelopment and epilepsy), and administration of pyridoxine (Table 1). For questions on efficacy, only studies with EEG-confirmed seizures were included, to reduce the risk of including events other than true epileptic seizures (i.e., inclusion of non-seizure events).2, 16
| Priority question | Population | Indication | Comparator | Outcome |
|---|---|---|---|---|
| 1. Which is the most efficacious first-line ASM in neonates with seizures requiring pharmacological treatment (specifically regarding cessation of seizures and adverse effects)? | Neonates with EEG-confirmed seizures | Pharmacologic treatment: phenobarbital, phenytoin, levetiracetam, midazolam, lorazepam | No or other pharmacological treatment | Cessation of seizure |
| 2. Which is the most efficacious second-line ASM in neonates (specifically regarding cessation of seizures and adverse effects)? | Neonate with seizures not responding to first-line ASM treatment | Pharmacologic treatment: phenobarbital, phenytoin, levetiracetam, midazolam, lidocaine, lorazepam, topiramate, bumetanide, carbamazepine | No or other pharmacological treatment | Cessation of seizure |
| 3. Will continuation of ASM improve neurodevelopmental outcome and reduce the risk of developing subsequent epilepsy? | Neonates after cessation of seizures | Medication withdrawal | Not discontinuing medication | Neurodevelopmental outcome and development of epilepsy |
| 4. In neonates with HIE, does therapeutic hypothermia reduce seizure burden? | Neonates with HIE | Therapeutic hypothermia | Neonate with HIE not undergoing therapeutic hypothermia | EEG seizure burden (min/h) |
| 5. Is a reduction of electroclinical and/or electrographic seizure burden in neonates associated with improved outcome (neurodevelopment, reduction of subsequent epilepsy)? | Neonates with seizures | Effective electrographic seizure treatment | No or ineffective electrographic seizure treatment | Neurodevelopmental outcome including epilepsy |
| 6. In neonates with seizures, is the use of pyridoxine effective and safe? | Neonates with seizures not responding to ASM or clinical and EEG findings suggestive of vitamin B6-dependent epilepsy | Treatment with add-on pyridoxine or pyridoxal 5′-phosphate | No treatment | Cessation of seizures, safety, neurodevelopment |
- Abbreviations: ASM, antiseizure medication; EEG, electroencephalography; HIE, hypoxic–ischemic encephalopathy; PICO, population, intervention(s), comparator(s), and outcome(s).
2.3 Systematic review
The systematic review protocol was registered with PROSPERO (CRD42017071825), and the results were reported following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 standards.24 MEDLINE, Embase, and Cochrane Central Register of Controlled Trials were searched. Both keywords and MeSH terms were included. Appendix S1 provides the search strategies for each database. The search was limited to seven languages (English, French, Italian, German, Spanish, Dutch, Portuguese). The searches covered the years 2008–2020, and 131 earlier references from the previous systematic review in the 2011 guideline13 were included. Because therapeutic hypothermia was not included in the 2011 guideline, the searches for studies about the effect of therapeutic hypothermia on seizure burden in neonates with HIE covered 2004–2020. The search was limited to humans. Case reports of fewer than five neonates and conference abstracts were excluded. Review articles were collated only to ensure that no key references were missed. The first search was performed on August 14, 2017, and the search was repeated on June 28, 2020; thus, all relevant articles until June 2020 were included in this review. All abstracts and full text articles were reviewed independently by two members of the working group, with involvement of a third reviewer to help resolve disagreements. Data extraction forms for all priority questions were drafted and pilot-tested by members of the working group.
2.4 Evaluation of evidence (GRADE)
Studies meeting inclusion criteria and considered relevant to a priority question were included for further evaluation. The risk of bias was assessed using the Cochrane Risk of Bias tool (for RCTs) and risk of Bias in non-randomised studies - of interventions (ROBINS-I) (for non-RCT studies).25, 26 Grading of recommendations, assessment, development and evaluation (GRADE) was applied to questions on first-line ASM, second-line ASM, and the impact of therapeutic hypothermia on seizure burden to rate the quality of evidence as high, moderate, low, or very low. The quality rating was upgraded or downgraded based on factors that could influence the quality of the evidence, in line with the GRADE method.26, 27 For the remaining questions (duration of ASM administration, impact of electrographic seizure treatment on outcome, and administration of pyridoxine), only uncontrolled studies were identified, so the quality of evidence was judged to be very low.
2.5 Delphi consensus process
If no evidence or insufficient evidence was obtained from RCTs, then expert opinion was sought using the Delphi methodology.28 In addition, questions addressing specific scenarios and further important points on care pathways and parent support were included in the Delphi consensus process. As these were included without systematic review, these were added as considerations rather than recommendations. Statements regarding the priority questions were drafted by a subgroup consisting of seven child neurologists, one neonatologist, and one methodologist. All members of the working group, except methodologists and the parent representative, were invited to respond to an online questionnaire (Survey Monkey) anonymously, yielding involvement of medical professionals from the relevant specialties (child neurology, epileptology, clinical neurophysiology, and neonatology) and from all ILAE regions. Each statement was evaluated using a 5-point Likert scale (completely agree, mostly agree, partially agree, mostly disagree, completely disagree). Consensus was achieved when at least 66% agreement (completely agree or mostly agree) or disagreement (mostly disagree or completely disagree) was reached. The Delphi consensus process consisted of five rounds of questionnaires.
2.6 Strength of recommendations and level of agreement
The strength of recommendations was defined according to GRADE and the ILAE CPG development tool.23 Besides the quality of the evidence, clinical benefits and harms of the intervention were considered. If the quality of evidence according to GRADE was at least moderate, then the strength of the recommendation was considered "strong." If GRADE could not be applied but the Delphi process yielded an agreement of >66%, then a recommendation was made based on the Delphi process. Agreement was labeled “high” (>75% agreement) or “moderate” (66%–75% agreement).
3 RESULTS
A total of 556 studies were identified as relevant to the priority questions and underwent full text review (Figure 1). Studies were excluded because they were conference abstracts (n = 35), because diagnosis of neonatal seizures was not confirmed by EEG (n = 212), or because full text review showed that the information given was not relevant for the priority questions (n = 136). The remaining 218 studies were allocated to one or more priority questions.
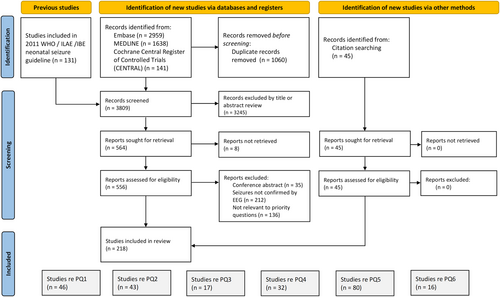
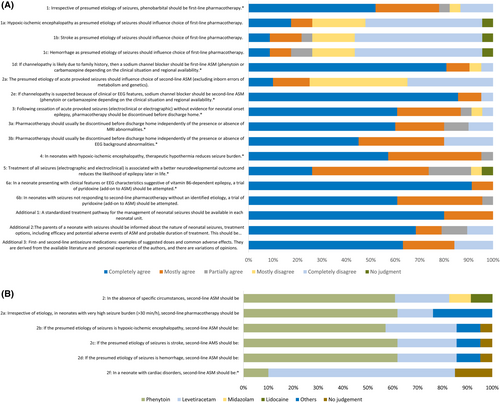
Regarding priority questions 1, 2, and 5, one or more randomized controlled trials were identified, and GRADE could be used to evaluate the evidence regarding these priority questions (Table S1). Figure S1 provide the risk of bias for priority questions 1 and 2. GRADE could not be applied to priority questions 3, 4, and 6. The Delphi process included 21 statements, and consensus was reached for 10 statements (Figure 2A,B). Recommendations were evidence-based for priority questions 1 and 4, and recommendations were consensus-based for the other priority questions.
In line with the ILAE handbook and toolkit, the manuscript was disseminated via the ILAE website for public comments for a 4-week period.23 All comments were addressed and, if considered relevant by the guidelines development group, incorporated into the final guidelines.
4 RECOMMENDATIONS
4.1 Recommendation 1: First-line ASM
Evidence-based recommendation
In neonates with seizures requiring ASM, phenobarbital should be the first-line ASM.
Strength of recommendation: Moderate.
Consensus-based recommendations:
Phenobarbital should be the first-line ASM regardless of etiology (including HIE, stroke, and hemorrhage).
Level of agreement: High.
If channelopathy is the likely cause for seizures due to family history, then phenytoin or carbamazepine (sodium channel blocker) may be the first-line ASM.
Level of agreement: High.
Question 1: Which is the preferred first-line ASM in neonates with seizures requiring pharmacological treatment (specifically regarding cessation of seizures and adverse effects)?
PICO: Table 1
- Studies allocated for full text review: 46
- Studies included after full text review: 11 (two RCTs, three prospective observational, six retrospective)
- Studies analyzed by GRADE: 2
- Evidence level from GRADE: Moderate to low quality (Table S1a)
Delphi: Figure 2A
Forty-six studies evaluated first-line treatment of neonatal seizures and were selected for full text review. The most common reason for study exclusion was a focus on clinical seizures without EEG to diagnose seizures or assess response to therapy (see Figure 1). There were no placebo-controlled studies. Eleven studies were included (Table S2a) assessing phenobarbital,12, 29-36 phenytoin,33 and levetiracetam30, 34, 37, 38 as first-line treatment for neonatal seizures. Overall, phenobarbital was the most widely used first-line ASM in term and preterm infants with seizures, with a variable response rate.29, 34 As nearly all studies in the neonatal period used phenytoin rather than fosphenytoin and the latter is not widely available, we refer to phenytoin throughout most of these guidelines.
Two RCT studies were included in the GRADE analysis (Table S2a).33, 34 The first study assessed the efficacy of phenobarbital and phenytoin for the treatment of seizures in term and preterm neonates with heterogeneous etiologies.33 Study inclusion required EEG-confirmed seizure(s), and efficacy was evaluated by EEG monitoring. Dosing of both ASMs was adjusted based on plasma levels, but the actual dosing was not stated. The primary outcome was complete seizure control within 24 h. Seizures were controlled with phenobarbital in 13 of 30 (43%) and phenytoin in 13 of 29 (45%) neonates. There was no difference in efficacy between phenobarbital and phenytoin as first-line treatment (relative risk [RR] = .97, 95% confidence interval [CI] = .54–1.72). The level of evidence was downgraded to low quality due to confounding factors (Table S1). The second study assessed the efficacy of phenobarbital and levetiracetam for the treatment of seizures in term neonates with heterogeneous etiologies.34 Seizures were assessed by EEG monitoring for eligibility and efficacy. The efficacy analysis included 83 neonates, and the safety data analyzed 106 treated neonates. Initial dosing was 20 mg/kg for phenobarbital and 40 mg/kg for levetiracetam. Neonates who continued to have seizures (assessed every 15 min) received an additional 20 mg/kg of phenobarbital or an additional 20 mg/kg of levetiracetam. The primary outcome was seizure cessation on EEG within 15 min and sustained seizure freedom on EEG for 24 h after the infusion. Seizures were controlled with phenobarbital in 24 of 30 (80%) and levetiracetam in 15 of 53 (28%) neonates. Phenobarbital was more effective than levetiracetam as first-line treatment (RR = .35, 95% CI = .22–.56). The level of evidence was moderate quality (Table S1). No studies evaluated efficacy of ASM according to etiology of acute provoked seizures.
Nine studies (controlled and observational) did not report adverse events, and nine studies indicated that no adverse events were observed for phenobarbital, phenytoin, or levetiracetam (Table S2a). One RCT with phenobarbital and phenytoin reported that no adverse events were observed.33 Only one study with phenobarbital and levetiracetam used standardized adverse events tables, and it reported that there was a trend toward hypotension being more common with phenobarbital (17%) than levetiracetam (5%).34
To determine whether the etiology of seizures should influence the choice of first-line ASM, five additional questions were assessed with the Delphi process (Figure 2). Results indicated that 78% of respondents completely or mostly agreed that regardless of presumed etiology of seizures (HIE, stroke, hemorrhage), phenobarbital should be first-line ASM. Furthermore, 91% of respondents completely or mostly agreed that if self-limited familial neonatal epilepsy (channelopathy caused by pathogenic variants of the KCNQ2 or KCNQ3 genes)7, 39 was considered as etiology due to positive family history, then phenytoin or carbamazepine (sodium channel blockers) should be the first-line ASM.
4.2 Recommendation 2: Second-line ASM
Consensus-based recommendations
In neonates with seizures not responding to first-line ASM, phenytoin or levetiracetam may be used as a second-line ASM for most etiologies (HIE, stroke, or hemorrhage). Other possible options include midazolam or lidocaine.
Level of agreement: Moderate.
If channelopathy as an etiology for the seizures is suspected because of clinical or EEG features, then a sodium channel blocker may be used as a second-line ASM. This can be phenytoin or carbamazepine, depending on the clinical state of the neonate (critically ill or otherwise well baby) and the regional availability of ASM and monitoring of drug levels.
Level of agreement: High.
In neonates with cardiac disorder(s), levetiracetam may be preferred as a second-line ASM.
Level of agreement: Moderate.
Question 2: Which is the preferred second-line ASM in neonates (specifically regarding cessation of seizures and adverse effects)?
PICO: Table 1
- Studies allocated for full text review: 43
- Studies included after full text review: 22 (three RCTs, five prospective observational, 14 retrospective)
- Studies analyzed by GRADE: 3
- Evidence level from GRADE: Very low quality (Table S1)
Delphi: Figure 2A,B
Seizures are often refractory to the first-line ASM, prompting use of a second-line ASM. Forty-three studies referred to the topic of second-line treatment of neonatal seizures and were selected for full text analysis (Table S2b). There were no placebo-controlled studies. Additionally, all studies of second-line ASM were of add-on design, because there was no washout phase after the first-line ASM and often both ASMs were administered concurrently. Twenty-two studies were included, and they assessed levetiracetam,34, 40-44 phenobarbital,30, 33, 34 phenytoin,29, 30, 33, 45 midazolam,46-51 lidocaine,46, 48, 51-54 clonazepam,46 bumetanide,55 topiramate,56 paraldehyde,29 diazepam,29 and carbamazepine.45, 57 There was substantial variability in study methods, including outcome measures, with substantial variability in efficacy across studies of the same ASM.
Three RCTs assessed phenytoin, midazolam, levetiracetam, and/or lidocaine as second-line ASM (Tables S1a and S2b).33, 34, 46 One study assessed second-line therapy in neonates with seizures persisting after either phenobarbital or phenytoin.33 In this study, phenobarbital and phenytoin met criteria for success as first-line ASM in 13 of 30 (43%) and 13 of 29 (45%), respectively, as the first-line ASM. Addition of the other ASM due to persistent seizures was followed by seizure control in five of 13 (39%) neonates with phenobarbital and four of 15 (27%) neonates with phenytoin. There was no difference in efficacy between phenobarbital and phenytoin as second-line ASM (RR = 1.44, 95% CI = .49–4.27), but the sample size was small. A second study assessed second-line therapy in neonates with seizures persisting after either phenobarbital or levetiracetam.34 Phenobarbital and levetiracetam met criteria for success (partly with dose escalation) in 24 of 30 (80%) neonates and 15 of 53 (28%) neonates as the first-line ASM. Addition of the other ASM due to persistent seizures was followed by seizure control in 20 of 37 (54%) neonates with phenobarbital and one of six (17%) neonates with levetiracetam. There was no difference in efficacy between phenobarbital and levetiracetam as second-line ASM (RR = .31, 95% CI = .05–1.89). A third study assessed second-line ASM in neonates with seizures persisting after phenobarbital.46 Seizures were controlled with lidocaine in three of five neonates and midazolam in zero of three neonates. There was no significant difference in efficacy between lidocaine and midazolam as second-line ASM (RR = 4.67, 95% CI = .32–68.03), but the sample size was small. Many studies did not address adverse events,30, 44, 46-49, 51 whereas some studies indicated that no adverse events were observed.33, 40-43, 45, 57 Only two RCTs used a systematic approach to adverse event assessment when assessing phenobarbital versus levetiracetam34 and bumetanide55 (Table S2b).
The level of quality of the evidence was very low regarding second-line ASM because of imprecision of estimates due to the very small number of patients included; hence, the two RCTs included were not sufficiently informative. Consequently, expert opinion was sought via the Delphi process. Specifically, we evaluated whether phenobarbital, phenytoin, levetiracetam, midazolam, or lidocaine should be used after no response or insufficient response to first-line ASM, and whether the choice of second-line ASM should be influenced by seizure etiology (HIE, stroke, hemorrhage, channelopathy) or comorbidity (cardiac disorders; Figure 2A,B). Although experts agreed on which ASM could be used as second-line (phenytoin, levetiracetam, midazolam, or lidocaine), there was no agreement as to which was the best. Three questionnaire rounds yielded no agreement for choice of second-line ASM, although more favored phenytoin (61%) and levetiracetam (22%) compared to midazolam or lidocaine (each 8.5%). Consequently, we concluded that all four ASMs may all be considered as second-line ASM for most etiologies (HIE, stroke, or hemorrhage). However, there was consensus that phenytoin or carbamazepine (sodium channel blockers) may be preferred for neonates with presumed channelopathy. Clinical features suggesting a channelopathy include no other etiology for seizures, tonic or sequential seizure type, with or without encephalopathy, normal ultrasound and/or magnetic resonance imaging (MRI), and certain EEG features.7, 15 In the absence of a positive family history of a channelopathy, experts agreed that phenobarbital should be administered as the first-line ASM to avoid unnecessary delay of treatment (>95% completely or mostly agreed). Notably, an increase of seizure frequency may occur as a response to sodium channel blockers in rare channelopathies with onset in the neonatal period (i.e., loss-of-function SCN1A variants), in which case these should be stopped or avoided if the mutation is known. Additionally, there was consensus that levetiracetam should be preferred for neonates with cardiac disorder(s) due to potential cardiac toxicity of phenytoin and lidocaine (75% completely or mostly agreed; Figure 2A).
4.3 Recommendation 3: Duration of treatment with ASM
Consensus-based recommendations
Following cessation of acute provoked seizures (electroclinical or electrographic) without evidence for neonatal-onset epilepsy, ASMs should be discontinued before discharge home, regardless of MRI or EEG findings.
Level of agreement: High.
Question 3: Will continuation of ASM improve neurodevelopmental outcome and reduce the risk of developing subsequent epilepsy?
PICO: Table 1
- Studies allocated for full text review: 17
- Studies included after full text review: 3 (zero RCTs, one observational prospective study, two retrospective trials)
- Studies analyzed by GRADE: 0
- Evidence level from GRADE: Not applicable
Delphi: Figure 2A
Clinicians must determine how long to continue ASM administration after the acute management phase. There were 17 studies addressing this topic, and three studies were included for full text analysis, including two retrospective studies58, 59 and one prospective observational study (Table S2c).6 These studies reported that the risk of subsequent developmental delay,6 seizure recurrence,58, 59 epilepsy,6 or neurologic impairment59 was not different in patients with ASM (mostly phenobarbital) discontinued prior to discharge or continued after discharge.
As only insufficient evidence on duration of ASM from RCTs or other controlled studies was found, expert opinion was evaluated by the Delphi process. In the Delphi process, 87% of respondents completely or mostly agreed that following cessation of acute provoked seizures (electroclinical or electrographic) without evidence for neonatal-onset epilepsy, ASM should be discontinued before discharge home (Figure 2).
Furthermore, 80% of respondents completely or mostly agreed that ASM should usually be discontinued before discharge home regardless of the presence or absence of MRI abnormalities, and 80% of respondents completely or mostly agreed that ASM should be discontinued before discharge home regardless of the presence or absence of EEG background abnormalities. Some participants noted their responses were influenced by a prospective, observational, multicenter comparative effectiveness study published after completion of the systematic literature review that indicated neurodevelopment and risk for epilepsy at age 24 months were not different among children with acute symptomatic neonatal seizures whose ASM was discontinued or maintained at hospital discharge.60
4.4 Recommendation 4: Impact of therapeutic hypothermia on seizure burden
Evidence-based recommendation
Therapeutic hypothermia may reduce seizure burden in term neonates with HIE. However, the impact of therapeutic hypothermia as a specific seizure therapy was not assessed.
Strength of evidence: Weak.
Consensus-based recommendations:
Therapeutic hypothermia may reduce seizure burden in neonates with HIE.
Level of agreement: High.
Question 4: In neonates with HIE, does therapeutic hypothermia reduce seizure burden?
PICO: Table 1
- Studies allocated for full text review: 32
- Studies included after full text review: 9 (zero RCTs, six observational prospective studies, three retrospective trials)
- Studies analyzed by GRADE: 3
- Evidence level from GRADE: Low quality (Table S1b)
Delphi: Figure 2A
Therapeutic hypothermia (brain/body cooling) is a neuroprotective technique for term neonates with HIE. Neonates with HIE have a high risk for seizures, and EEG monitoring is often performed to identify electroencephalographic seizures. There were 32 articles addressing this topic, and although all had access to comparison groups, none was an RCT. Nine studies fulfilled the recommended requirements for therapeutic hypothermia in the setting of term infants with HIE (Table S2d).61-69 Six studies were excluded because they focused on head cooling, during which EEG cannot be performed,61, 69 did not access continuous EEG,61 or did not have comparators.64-66, 68, 69 Among the remaining three studies, two had historical control group comparators62, 63 and one had both historical and real-time comparators.67
GRADE assessment concluded with low quality that seizure burden was higher in the normothermia groups for all three studies and that the mean seizure frequency was lower in the therapeutic hypothermia group of two studies (Tables S1b and S2d).62, 67 There was very low certainty regarding reduced progression to status epilepticus (as defined by each study) in the therapeutic hypothermia group. Two studies did not find a difference in the occurrence of status epilepticus between non-hypothermia and hypothermia groups,63, 67 whereas one study found a higher occurrence of status epilepticus in the non-hypothermia group than in the hypothermia group (Table S1b).62
Due to the lack of RCTs, we aimed to confirm the weak evidence from observational studies via the Delphi process (Figure 2A). Nearly all (95%) respondents completely or mostly agreed that therapeutic hypothermia may reduce seizure burden in neonates with HIE.
4.5 Recommendation 5: Associations between seizure burden and outcome
Consensus-based recommendations
Treating neonatal seizures (including electrographic-only seizures) to achieve a lower seizure burden may be associated with improved outcomes (neurodevelopment, reduction of subsequent epilepsy).
Level of agreement: Moderate.
Question 5: Is a reduction of electroclinical and/or electrographic-only seizure burden in neonates associated with improved outcomes (neurodevelopment, reduction of subsequent epilepsy)?
PICO: Table 1
- Studies allocated for full text review: 80
- Studies included after full text review: 10 (two RCTs, four observational prospective studies, four retrospective trials)
- Studies analyzed by GRADE: 0
- Evidence level from GRADE: Not applicable
Delphi: Figure 2A
Seizure identification and effective management are intended to reduce secondary brain injury and improve outcomes. Ten studies were included after full text review (Table S2e). Two studies randomized neonates to different approaches for seizure detection and management. One study assessed outcome for the full cohort (not separating the different treatments),70 and the other study assessed MRI before discharge (but not long-term outcome).71 Both studies were underpowered to assess outcomes, so there was no GRADE assessment. The first RCT performed cEEG monitoring in term neonates with moderate or severe HIE and randomized them to treatment of both electrographic and clinical seizures (n = 15) or treatment of only clinical seizures (n = 20), and the study demonstrated that seizure burden was lower with treatment of electrographic seizures.70 Twenty-four surviving subjects from both groups were combined for outcome analysis, and higher seizure burden was associated with significantly worse neurodevelopmental outcomes at 18–24 months. The second RCT randomized term neonates with moderate to severe HIE and subclinical seizures on aEEG to treatment of both clinical and subclinical seizures (n = 19) or treatment of only clinical seizures (n = 14).71 Treatment addressing subclinical seizures was associated with a trend toward lower seizure burden. Twenty subjects from both groups were combined for outcome analysis, and lower seizure burden was associated with less severe injury on MRI. One study, published after the literature search, randomized neonates to treatment of aEEG-identified seizures versus clinical seizures.72 Death or severe disability assessed at 2 years were not significantly different between the two groups. However, the overall seizure burden between both groups was not different, consistent with no difference in outcome between the two groups.72 Numerous studies have indicated that a high seizure burden is associated with unfavorable outcomes.1, 58, 62, 73-77 However, these studies focused on associations between seizure burden in neonates and outcome(s), as opposed to the impact of seizure reduction on outcome. Thus, based on the available data, we could not establish whether clinical efforts to reduce seizure burden are associated with improved outcomes.
As there was no evidence from RCT or other controlled studies to inform our recommendations, expert opinion was evaluated using the Delphi process. Results indicated that 74% of respondents completely or mostly agreed that treatment of seizures (electroclinical and electrographic-only) may be associated with a better neurodevelopmental outcome and reduced the likelihood of epilepsy later in life.
4.6 Recommendation 6: Treatment with pyridoxine and pyridoxal 5´-phosphate
Consensus-based recommendations
A trial of pyridoxine (add-on to ASM) may be attempted in neonates presenting with clinical features or EEG characteristics suggestive of vitamin B6-dependent epilepsy and neonates with seizures unresponsive to second-line ASM without an identified etiology.
Level of agreement: High.
Question 6: In neonates with seizures with unknown etiology, is the use of pyridoxine or pyridoxal 5′-phosphate (PLP) effective and safe?
PICO: Table 1
- Studies allocated for full text review: 16
- Studies included after full text review: 8 (zero RCTs, zero not randomized controlled studies, eight retrospective studies)
- Studies analyzed by GRADE: 0
- Evidence level from GRADE: Not applicable
Delphi: Figure 2A
Several independent genetic disorders have been found to interfere with the bioavailability of pyridoxine and PLP, resulting in vitamin B6-dependent epilepsy.78, 79 These include antiquitin deficiency (due to pathogenic ALDH7A1 variants), hypophosphatasia, hyperphosphatasia, pyridox(am)ine 5′-phosphate oxidase (PNPO) deficiency, and PLP binding protein deficiency (formerly called PROSC deficiency).80 Neonates with vitamin B6-dependent epilepsy may initially present with features suggesting HIE or systemic manifestations including lactic acidosis and acute abdomen.7, 81 The systematic review of the literature failed to identify any randomized or controlled studies investigating the effect of pyridoxine or PLP. Eight retrospective case series addressing safety of pyridoxine and PLP in neonates responding to pyridoxine or PLP were included after full text review (Table S2f). Typical features of seizure semiology (myoclonic jerks, spasms), abnormal movements (eye movements, grimacing), and EEG (burst suppression, discontinuity) were described in antiquitin and PNPO-deficient patients.81-83 Although some neonates with vitamin B6-dependent epilepsy respond immediately to pharmacological doses of pyridoxine or PLP, delayed responses are described; therefore, treatment with pyridoxine or PLP should be continued for at least 3–5 days before concluding that it is not effective.84 Other authors have suggested a trial with repeated doses of pyridoxine up to a total dose of 500 mg.78 One retrospective study of 10 neonates with treatment-resistant seizures reported that pyridoxine treatment led to immediate flattening of the EEG in two of six patients with ALDH7A1 variants versus one of four patients with undetermined seizure etiology.85 Adverse effects of pyridoxine and PLP included acute respiratory depression,81 depression of EEG amplitude,85 peripheral neuropathy on long-term high-dose pyridoxine >500 mg/day,86 and liver toxicity on high-dose PLP 50 mg/kg/day.86 These disorders are rare; pathogenic variants of the ALDH7A1 gene is the most common and has an estimated incidence of 1:65 000–1:396 000.87, 88 Given the low incidence of the independent genetic disorders presenting with vitamin B6-dependent epilepsy, controlled studies of pyridoxine or PLP as first-line or second-line therapy for neonatal seizures may not be feasible.
In the Delphi process, 100% of respondents completely or mostly agreed that a trial of pyridoxine (add-on to ASM) should be performed in a neonate or infant presenting with clinical features or EEG characteristics suggestive of vitamin B6-dependent epilepsy, and 96% of respondents completely or mostly agreed a trial of pyridoxine (add-on to ASM) should be attempted in all neonates with seizures without an identified etiology not responding to second-line ASM. The risk of apnea should be considered when a trial of pyridoxine is attempted. Neonates with PNPO developmental and epileptic encephalopathies may only respond to PLP. Therefore, if vitamin B6-dependent epilepsy is suspected, following an unsuccessful trial of pyridoxine, PLP treatment may be tried even though this product is not licensed as medication. If vitamin B6-dependent epilepsy is suspected, then treatment should not be delayed, as a therapeutic trial with either pyridoxine or PLP can be started before diagnostic samples are collected without affecting the results. Furthermore, partial or transient response to ASM does not rule out vitamin B6-dependent epilepsy.84
4.7 Additional consideration: Need for standardized pathways in each neonatal unit
Consensus-based consideration
A standardized pathway for the management of neonatal seizures should be available in each neonatal unit.
Level of agreement: High.
Delphi: Figure 2A
The treatment of neonatal seizures is time-sensitive; studies have shown that neonates who are diagnosed and treated earlier respond better to treatment.31, 89, 90 Using standardized pathways may improve the time to effective treatment. As assessed by the Delphi process, 100% of respondents completely or mostly agreed that neonatal units should have a standardized pathway for the management of neonatal seizures.
4.8 Additional consideration: Need for communication with parents/guardians
Consensus-based consideration
The parents/guardians of a neonate with seizures should be informed of the neonate having seizures, possible etiologies, and initial treatment options with subsequent discussions based on the neonate's condition.
Level of agreement: High.
Delphi: Figure 2A
Neonatal seizures, particularly in the context of acute brain injury, cause parental anxiety and concern about management and long-term prognosis.91 Parents/guardians need to be informed that the neonate has seizures, possible etiologies, and treatment options, including potential adverse effects of ASM and the probable duration of treatment. However, this has to be within the scope of feasibility in an acutely ill child and should not delay treatment. As assessed by the Delphi process, 79% of respondents completely or mostly agreed with the above statement.
5 DISCUSSION
Seizures are common in neonates, yet there is substantial variability in management.18-21 These guidelines address the management of seizures in neonates based on the best available evidence and consensus-based expert opinion.
Recent guidelines have emphasized the need for EEG monitoring for the reliable diagnosis of neonatal seizures, as well as the importance of timely seizure identification through EEG-based approaches2, 14, 15 In this systematic review, only studies with EEG-confirmed seizures were included, consistent with recommendations from the ILAE,15 International Neonatal Consortium,16 European Medicines Agency,16 US Food and Drug Administration,16 Brighton Collaboration,2 and American Clinical Neurophysiology Society.14 For clinical trials to be meaningful and transferable, it is essential that outcome measures are well-defined and can be measured accurately and precisely.92, 93 It is well established that the accuracy and validity of seizure outcome measures in drug trials are questionable if EEG is not used, including treating nonseizure events, underestimating total electrographic seizure burden, and lack of ability to assess whether electrographic-only seizures cease. Thus, trials using clinical diagnosis should not be used for licensing ASM or to inform clinical guidelines or recommendations. Our conclusions can be considered applicable to neonatal seizures in general, provided that diagnostic certainty for neonatal seizures as defined by the Brighton Collaboration2 and ILAE15 is taken into account. Conventional EEG (gold standard) and aEEG are considered reliable methods for clinical management. Only focal clonic and focal tonic seizures can be diagnosed by clinical observation alone, and all seizure types require confirmation with EEG or aEEG.2, 15
No guidelines have addressed seizure management since the WHO/ILAE/International Bureau for Epilepsy (IBE) guideline was published in 2011.13 A systematic review in 2012 summarized pharmacokinetic data for second-line ASM, and a systematic review in 201394 came to similar conclusions as the WHO/ILAE/IBE guideline. Four additional systematic reviews reviewed first-line treatment with phenobarbital and/or levetiracetam, but without consensus-based guidelines.95-98 In line with previous guidelines, phenobarbital is recommended as first-line ASM but now with better evidence. Phenobarbital should be used for the shortest duration possible with early discontinuation for neonates with acute provoked seizures responding to treatment. As phenobarbital has been associated with some potential adverse effects,34, 99 these considerations should ensure phenobarbital is only administered when necessary and that exposure is as brief as possible. In contrast to previous guidelines, we added special considerations for neonates where a channelopathy is likely due to a family history (use of sodium channel blocker). However, in the absence of a positive family history, phenobarbital should be the first-line ASM to avoid treatment initiation delays.
As in previous guidelines, the choice of second-line ASM therapy remains unclear. We concluded that in neonates with seizures not responding to first-line ASM, phenytoin or levetiracetam may be used as a second-line ASM for most etiologies (HIE, stroke, or hemorrhage), with midazolam and lidocaine being other less favorable options. However, there are some important caveats regarding second-line ASM selection that have not been discussed in the previous guideline. First, if a channelopathy (such as self-limited neonatal epilepsy or KCNQ2 encephalopathy) is suspected because of clinical and/or typical EEG7, 100 features, then a sodium channel blocker may be the second-line ASM. Second, in a neonate with cardiac disorders, levetiracetam may be preferred as the second-line ASM. Third, a trial of pyridoxine (add-on to ASM) may be attempted in neonates presenting with clinical features or EEG characteristics suggestive of vitamin B6-dependent epilepsy and neonates with seizures unresponsive to a second-line ASM without an identified etiology. Furthermore, if vitamin B6-dependent epilepsy is suspected, following an unsuccessful trial of pyridoxine, a trial of PLP may be considered. Although some other ASMs are emerging for use in neonates (such as oxcarbazepine, brivaracetam, and lacosamide), no controlled studies on their efficacy or safety have been published and thus their use can currently not be recommended.
We recommend that following cessation of acute provoked seizures (electroclinical or electrographic-only seizures) without evidence for neonatal-onset epilepsy, ASM should be discontinued before discharge, regardless of MRI or EEG findings. This differs from the prior WHO/ILAE/IBE guidelines.13 This conclusion is further supported by a study published after completion of the systematic review that indicated neither neurodevelopment nor epilepsy at age 24 months was different among children with acute provoked neonatal seizures whose ASMs were discontinued or maintained at hospital discharge.60
We reviewed evidence regarding whether treating neonatal seizures (including electrographic-only seizures) to lower seizure burden is associated with improved outcomes. However, because studies focused on associations between seizure burden and outcome, as opposed to the impact of seizure reduction on outcome, the available data could not establish whether clinical efforts to reduce seizure burden are associated with improved outcomes. Given indirect evidence,31, 70, 71, 73, 75, 90, 101 experts agreed that treatment of neonatal seizures (including electrographic-only seizures) to achieve a lower seizure burden may be associated with improved outcomes. In many low- and middle-income settings, lack of access to EEG or aEEG precludes recognition of electrographic seizures or leads to misinterpretation of clinical events, potentially adding to seizure burden or overuse of ASM, with effects on outcomes for neonates in these regions. Thus, clinicians should be aware that diagnostic certainties of neonatal seizures depend on the available diagnostic method (EEG, aEEG, or observation by experienced personnel).2, 15 We concluded that therapeutic hypothermia may reduce seizure burden in neonates with HIE. However, the impact of therapeutic hypothermia as a nonpharmacological treatment of seizure could not be assessed.
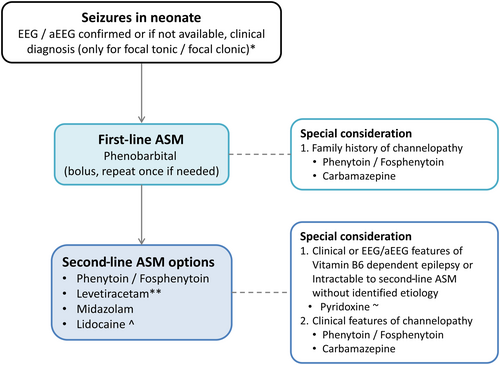
Based on the conclusions of each priority question, Figure 3 provides a sample neonatal seizure management pathway and Table 2 provides suggested ASM doses.19, 102 As with all pathways, adaptation is needed based on individual patient characteristics and practice settings. All experts agreed that neonatal units should have a standardized pathway for the management of neonatal seizures.
| Medication | Dosage | Common adverse effects | Remarks |
|---|---|---|---|
| Phenobarbital |
Loading dose: 20 mg/kg iv Second loading dose: 10–20 mg/kg iv if required Maintenance: 5 mg/kg/day iv or orally in one dose |
Respiratory depression Somnolence, depressed consciousness, and poor feeding Hypotension |
If a second loading dose of 20 mg/kg is given, respiratory support should be available Prolonged half-life first week of life and preterm Renal and hepatic excretion can be affected in HIE Consider plasma levels if on maintenance |
| Phenytoin/fosphenytoin |
Loading dose: 20 mg/kg PE iv over 30 min Maintenance: 5 mg/kg/day iv or orally in two divided doses, adjusted according to response and plasma concentration to max. per dose 7.5 mg/kg Target level: 10–20 μg/mL |
Infusion site irritation/necrosis Hypotonia Arrhythmia, bradycardia Respiratory depression/arrest |
Phenytoin has poor oral bioavailability Levels likely higher in infants receiving therapeutic hypothermia, thus adjust dosage according to local target levels Cardiac monitoring required If used for channelopathies, switch to carbamazepine for maintenance once oral administration is possible |
| Levetiracetam |
Loading dose: 40 mg/kg iv Second loading dose: 20 mg/kg iv if required Maintenance: 40–60 mg/kg/day iv or orally in three divided doses |
Mild sedation Irritability |
Usually well tolerated but limited information regarding dosing and adverse effect for the neonatal population |
| Lidocaine |
Loading dose: 2 mg/kg iv over 10 min Maintenance: 7 mg/kg/h iv for 4 h, reduce to 3.5 mg/kg/h for 12 h, reduce to 1.75 mg/kg/h for 12 h, then stop Adapt dose for birth weight, PMA, and therapeutic hypothermia103 |
Cardiac (arrhythmias, atrioventricular block, cardiac arrest) Hypotension Methemoglobinemia |
Not to be given to a patient with congenital heart disease and/or who was or is on proarrhythmic drugs like (fos)phenytoin Cardiac monitoring required |
| Midazolam |
Loading dose: .05–.15 mg/kg, followed by: Maintenance: 1 μg/kg/min (=60 μg/kg/h) continuous infusion, titrate up in steps of 1 μg/kg/min (=60 μg/kg/h) to max. of 5 μg/kg/min (=300 μg/kg/h)a |
Respiratory depression Somnolence, depressed consciousness, and poor feeding Hypotension |
Needs to be tapered when maintenance treatment has been used |
| Carbamazepine | 10 mg/kg/day orally in two divided dosesb |
Transient somnolence Gastrointestinal symptoms Hyponatremia and skin reactions reported in safety studies in children 1 month—17 years |
Usually well tolerated but limited information regarding dosing and adverse effect for the neonatal population |
| Pyridoxine HCl | Loading dose: 100 mg iv or orally, followed by 30 mg/kg/day iv or orally in two divided doses for 3–5 days |
Respiratory depression Hypotension Prolonged treatment with high dosages may cause peripheral neuropathy |
Ventilatory support should be available when loading dose is administered If effective, continue until genetic results are available |
| Pyridoxal 5′-phosphate | 30 mg/kg/day orally in three divided doses for 3–5 days |
Respiratory depression Hepatotoxic; cirrhosis described in prolonged use |
Not licensed as medical product, but most promising approach in PNPO-deficient patients If effective, continue until genetic results are available |
- Note: The suggested doses have been derived from the available literature19, 34, 36, 45, 47, 57, 78, 79, 100, 102-104 and personal experience of the authors, and there are variations of opinions. Local/regional availability has to be taken into account. Recommended dosages were approved by the CPD working group via Delphi (84% of experts mostly or completely agree).
- Abbreviations: HIE, hypoxic–ischemic encephalopathy; iv, intravenous; max., maximum; PE, phenytoin equivalent; PMA, postmenstrual age; PNPO, pyridox(am)ine 5′-phosphate oxidase.
- a Higher doses (up to 18 μg/kg/min = 1080 μg/kg/h) have been used by some47 without serious adverse effects.
- b Higher doses (up to 20 mg/kg/day) have been used for KCNQ2 developmental and epileptic encephalopathies in some case series, but no safety studies have been performed in neonates.7, 45, 57, 100
Despite a growing number of publications over recent years, the evidence and consensus-based recommendations identified several key limitations in the existing literature. First, many studies are small, lack EEG-based seizure diagnosis, lack EEG-based assessment of ASM efficacy, assess cohorts that are heterogeneous in terms of etiology and postmenstrual age, and only partially address confounding factors. Varied data approaches across studies made formal analyses combining the data across studies difficult. Development and implementation of more standard common data elements may improve these issues.15 Second, there is an alarming lack of safety reporting in studies evaluating ASM in neonates. All observational studies and RCTs should include standardized safety reporting for both old and new drugs. Third, studies are needed to determine when to start an ASM. According to recommendations by the International Neonatal Consortium, treatment of seizures should be started when the seizure burden reaches 30 s/h. However, this number is arbitrary and based on clinical trial design rather than meaningful clinical endpoints.16 Fourth, studies are needed to determine which ASM is optimal in neonates with seizures refractory to an initial ASM. Finally, studies have mostly assessed seizure cessation in response to ASM, but not whether overall strategies to reduce seizure exposure (incorporating EEG-based diagnosis and optimized multifaceted management approaches) improve long-term patient-centered neurobehavioral outcomes. General research priorities for the treatment of neonatal seizures include (1) pharmacokinetic and pharmacodynamic studies for term and preterm neonates; (2) appropriate dose-finding studies for new ASMs as well as safety studies for new ASMs and existing ASMs if higher doses are used; and (3) RCTs aiming to license further ASMs in neonates.
The ILAE recommends that guidelines be updated every 5 years, and the ILAE Task Force on Neonatal Seizures is developing an approach to periodically update these recommendations.
ACKNOWLEDGMENTS
We would like to thank librarian Dilshaad Brey, Department of Pediatrics and Child Health, University of Cape Town for her help with the literature searches.
CONFLICT OF INTEREST STATEMENT
S.A. has received honoraria or consultancy fees from Arvelle, Angelini, Biomarin, Eisai, GW Pharma, Jazz Pharma, Neuraxpharm, Nutricia, UCB Pharma, Vitaflo, Xenon, Zogenix. S.A. is a deputy editor for Epilepsia. G.B. has a consultancy with UCB and Nihon Kohden to provide advice on neonatal EEG monitoring. She is a cofounder of startup company Kephala, which provides EEG reviewing services for industry and academia. M.R.C. is an associate editor of Pediatric Research. She receives consultant fees from UCB, Eisai, Biocodex, Sanofi, and Jazz. Her research is funded by the European Joint Programme on Rare Diseases and the Fonds Recherche Clinique, Cliniques Universitaires Saint-Luc. M.E. has served as a consultant, as a speaker, and on advisory boards for Eisai, UCB Pharma, Neuraxpharm, Lusofarmaco, and Proveca. His research is supported by the Italian Ministry of Health, Ricerca Corrente 2022. C.D.H. receives an honorarium for his work as an associate editor of the Journal of Clinical Neurophysiology. He has served as a consultant on clinical trial design for UCB Pharma and Takeda, and receives grant support from the Canadian Institutes of Health Research (PJT-166076) and the US National Institutes of Health (NIH; R01 HD101419-01). H.H. serves as an associate editor of Neuropediatrics and section editor of the Journal of the International Child Neurology Association, both with no financial compensation. N.J. was the Bludhorn Professor of International Medicine during the preparation of this article. She has received grant funding paid to her institution for grants unrelated to this work from the National Institute of Neurological Disorders and Stroke (NIH U24NS107201, NIH IU54NS100064, NIH U24NS113849). She receives an honorarium for her work as an associate editor of Epilepsia. L.N. has received honoraria for talks from UCB, Novartis, Biogen. E.M.M. has served as a consultant for Eisai Pharmaceutical and UCB Biosciences, and has received royalties from McGraw-Hill Medical Publishers, Springer Publishing Company, and Demos Medical Publishers. S.L.M. is the Charles Frost Chair in Neurosurgery and Neurology and partially funded by grants from NIH U54 NS100064 (EpiBioS4Rx), RO1-NS4320, and RO1-NS127524, the US Department of Defense (EP210041, W81XWH-21-ERP-IDA, and EP210022, W81XWH-21-ERP-RPA), the Heffer Family and the Segal Family Foundations, and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. He was an associate editor of Neurobiology of Disease until December 2021. He is on the editorial board of Brain and Development, Pediatric Neurology, Annals of Neurology, MedLink, and Physiological Research. He received compensation from Elsevier for his work as an associate editor of Neurobiology of Disease and from MedLink for his work as an associate editor, and royalties from two books he coedited. A.K.-M. has received research support from Training Health Researchers Into Vocational Excellence in East Africa (THRIVE-2), Swedish Research Council-VR, and Fogarty National Institutes of Health. She also has grants and nonfinancial support from the Duke Global Health Institute (USA) and Epilepsy Foundation (USA). M.L.N. is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)-Brazil (PQ 306338/2017–3). R.M.P. is an investigator for studies with UCB and Johnson & Johnson. She has served as a consultant, as a speaker, and/or on advisory boards for Natus, Persyst, Aeglae, GW, and UCB. Her research is supported by the National Institute of Health Research (NIHR) Biomedical Research Centre at Great Ormond Street Hospital and Cambridge University Hospital, NIHR and James Bradfield Memorial Grant (via the Evelyn trust). She is on the editorial boards of Journal of Clinical Neurophysiology, Neurophysiologie Clinique, and European Journal of Pediatric Neurology and is an associate editor for Epilepsia Open. R.A.S. is a consultant for the Epilepsy Study Consortium, and receives royalties from UpToDate for authorship of topics related to neonatal seizures. She is president-elect of the Pediatric Epilepsy Research Foundation. Her research has been funded by the NIH, Patient-Centered Outcomes Research Institute, and Pediatric Epilepsy Research Foundation. K.P.V. has received honoraria from MedLink Neurology and the Dravet Syndrome Foundation. J.M.W. has received an honorarium for her role as an associate editor for Epilepsia. LSDV is an associate editor for Neuropediatrics and is on the editorial board of European Journal of Pediatric Neurology and Neonatology. She has received royalties from three books she coedited and a honorarium for two chapters in UpToDate. The remaining authors have no conflicts of interest.




