Time-dependent risk factors for epileptic seizures in glioblastoma patients: A retrospective analysis of 520 cases
Abstract
Objective
Epilepsy is a common comorbidity of glioblastoma. Seizures may occur in various phases of the disease. We aimed to assess potential risk factors for seizures in accordance with the point in time at which they occurred.
Methods
We retrospectively analyzed medical files of adult patients with de novo glioblastoma treated at our institution between January 2006 and January 2020. We categorized seizures as preoperative seizures (POS), early postoperative seizures (EPS; before initiation of radio[chemo]therapy [RCT]), seizures during radiotherapy (SDR; during or <30 days after RCT), and posttherapeutic seizures (PTS; ≥30 days after completion of RCT). We addressed associations between patients' characteristics and their seizures.
Results
In the final cohort (N = 520), 292 patients experienced seizures. POS, EPS, SDR, and/or PTS occurred in 29.6% (154/520), 6.0% (31/520), 13.8% (70/509), and 36.1% (152/421) of patients, respectively. POS occurred more frequently in patients with higher Karnofsky Performance Scale scores (odds ratio [OR] = 3.27, p = .001) and tumor location in the temporal lobe (OR = 1.51, p = .034). None of the parameters we analyzed was related to the occurrence of EPS. SDR were independently associated with tumor location (parietal lobe, OR = 1.86, p = .027) and POS, but not EPS, and were independent of RCT. PTS were independently associated with tumor progression (OR = 2.32, p < .001) and with occurrence of SDR (OR = 3.36, p < .001), and negatively correlated with temporal lobe location (OR = .58, p < .014). In patients with tumors exclusively located in the temporal lobe, complete tumor resection was associated with a decreased risk of postoperative seizures.
Significance
Seizures in glioblastoma patients have various, time-dependent risk factors. Temporal lobe localization was a risk factor for preoperative seizures; surgery may have had a protective effect in these patients. RCT did not have dose-dependent pro- or anticonvulsive effects. PTS were associated with tumor progression.
Key points
- We conducted a retrospective analysis with the purpose of addressing risk factors for seizures in glioblastoma patients, categorized as preoperative seizures, early postoperative seizures, seizures during radiotherapy, and posttherapeutic seizures
- Seizures were associated with various time-dependent risk factors
- Temporal lobe localization was an independent risk factor for preoperative seizures; surgery may have had a protective effect in these patients
- We did not detect dose-dependent pro- or anticonvulsive effects of RCT
- The occurrence of posttherapeutic seizures was associated with tumor progression
1 INTRODUCTION
Epileptic seizures are a common symptom in patients with glioblastoma.1-3 Approximately 25% of all patients with the disease present with preoperative seizures (POS), and 50%–70% of all patients with glioblastoma will develop seizures at some point during the disease's course.4-7
The association between seizure occurrence and glioblastoma survival has been extensively discussed. The point in time at which the seizures occur is likely to be a decisive factor in whether this association is positive or negative. Whereas some work has noted an association of POS with improved survival,8, 9 seizures occurring later in the disease's course may be a marker for tumor progression and are associated with worse outcomes.10, 11 These findings may prompt the assumption that risk factors for epileptic seizures may also change during the course of glioblastoma. Identifying patients at risk of seizures during certain periods of the disease may help guide treatment decisions and improve quality of life for these patients.
Research has yet to definitively identify the mechanisms underlying epileptogenesis in brain tumors. A multifactorial genesis, dependent on the type of tumor, is likely.4 Previous studies examining risk factors for seizures in glioblastoma patients reported results that were in part contradictory, but drew on small, heterogenous glioma cohorts (high- and low-grade tumors) or did not take the point in time of the seizures' occurrence into account.12-17 Whereas a few studies have examined risk factors for POS,9, 18 we as yet know little about the frequency of, and risk factors for, early postoperative seizures (EPS), seizures during radiotherapy (SDR), and seizures occurring in the posttherapeutic setting (PTS). The highly malignant, dynamic character of glioblastomas and the apparent differences in implications for patient survival when seizures occur earlier or later in the disease's treatment suggest the importance of examining underlying risk factors for these seizures relating to when they occur, as we may safely assume that risk factors might likewise change over time. The knowledge potentially arising from this analysis may be of clinical utility in counseling patients at various points in time during the course of their disease. The probability of a patient experiencing a seizure in the future in light of the patient's history is important information in this regard.
We conducted a retrospective analysis of glioblastoma patients treated at our institution to assess risk factors for epileptic seizures, taking into account the point in time at which the seizures occurred alongside various clinical, radiographic, and molecular parameters.
2 MATERIALS AND METHODS
We performed a retrospective analysis of electronic medical records of patients treated at our institution that covered inpatient stays and follow-up appointments. All consecutive cases with newly diagnosed, de novo glioblastoma seen between January 2006 and January 2020 were eligible for this study. The exclusion criteria were (1) pediatric cases (<18 years old at diagnosis), (2) previous history of epilepsy, and (3) infratentorial or extracranial location. We conducted this retrospective study in accordance with the STROBE guidelines; the study received approval from the institutional ethics committee of Friedrich-Alexander-Universität Erlangen-Nürnberg (No. 390_20Bc). We reviewed records relating to demographic information; Karnofsky Performance Scale (KPS) at admission; radiographic, histological, and molecular characteristics of the tumor; oncological treatment received; seizure occurrence; seizure semiology, wherever possible; and treatment with antiseizure medication (ASM). We determined tumor volume on presurgical magnetic resonance imaging (MRI) scans in the contrast-enhancing tumor part.
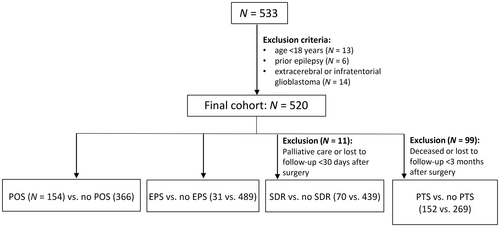
Epilepsy was consequently diagnosed according to the International League Against Epilepsy criteria,19 counting both focal seizures and focal to bilateral tonic–clonic seizures. For our analysis of risk factors for seizure occurrence, we defined four time periods in the course of treatment: preoperative, early postoperative, during radiochemotherapy (SDR), and after primary oncological treatment (PTS). Seizures that occurred prior to the first surgery (i.e., the surgery that led to the tumor diagnosis) and that had no identifiable cause other than the underlying oncological disease were counted as POS. We regarded EPS occurring after surgery and prior to the initiation of radiotherapy as EPS. Seizures occurring during adjuvant radiotherapy or <30 days after radiotherapy were classified as SDR. Finally, we categorized seizures occurring ≥30 days after primary oncological treatment (i.e., first surgery and adjuvant radiotherapy) as PTS. As the records did not contain sufficient information on seizure frequency, we evaluated dichotomously for each patient whether they had experienced seizures during each time period. To evaluate the risk factors for the occurrence of the event of interest (POS, EPS, SDR, PTS), we compared the following patient groups: patients with POS versus those without POS, patients with EPS versus those without EPS, patients with SDR versus those without SDR, and patients with PTS versus those without PTS. We considered any seizures occurring in time periods prior to the given time period as covariates. Figure 1 provides an illustrative overview of the analysis design.
Statistical analyses were performed with SPSS (version 26, IBM). Patients' baseline characteristics were expressed, as appropriate, as percentages of patients, mean ± SD, and median values with interquartile ranges for data on overall survival, progression-free survival, and median follow-up duration. Associations between potential risk factors and the outcome parameters were first tested using univariate analysis. The significant correlations (p < .05) from univariate analyses as well as age, preceding seizures (as they may influence the risk for further seizures), and follow-up in months as potential confounders were then evaluated in a multivariate analysis using binary logistic regression analysis for the identification of independent risk factors for POS, EPS, SDR, and PTS. As this was an exploratory study, significance level was set at p < .05, without correction for multiple testing.
Any data not published within the article will be shared in anonymized form upon reasonable request.
3 RESULTS
3.1 Patient population
After the exclusion of ineligible cases (age < 18 years, n = 13; prior epilepsy, n = 6; extracerebral or infratentorial glioblastoma, n = 14), 520 individuals were included in the study. Of these, 10 were admitted to palliative care shortly after primary surgery and did not receive radiotherapy, and one patient was lost to follow-up within <30 days after surgery. We consequently excluded these patients from the analysis of SDR. After 3 months of follow-up, 99 patients had died or were lost to follow-up, and were therefore excluded from the analysis of PTS. The total cohort, therefore, included 520 patients for the analysis of POS and EPS, 509 patients for the analysis of SDR, and 421 patients for the analysis of PTS. Table 1 shows the baseline characteristics of the final cohort. Tables S1a and S1b display the characteristics of each patient group.
| Parameter | n (%) or mean ± SD |
|---|---|
| Age, years | 61.7 ± 12.2 |
| Sex, % female | 44.2% |
| KPS at admission | |
| ≥70% | 361 (70.8%) |
| <70% | 148 (29%) |
| Missing | 10 (1.9%) |
| Tumor location | |
| Frontal | 185 (35.6%) |
| Parietal | 127 (24.4%) |
| Temporal | 213 (41%) |
| Occipital | 79 (15.2%) |
| Insular | 8 (1.5%) |
| Right | 243 (46.7%) |
| Left | 228 (43.8%) |
| Bilateral | 49 (9.4%) |
| Multifocal | 125 (24%) |
| EOR | |
| Biopsy | 152 (29.3%) |
| Partial | 140 (27%) |
| Gross | 226 (43.6%) |
| Missing | 2 (.4%) |
| Tumor volume, cm3 | 29.8 (32.3) |
| Missing | 49 (9.4%) |
| Postsurgical treatment | |
| None | 10 (1.9%) |
| RT only | 41 (7.9%) |
| RT + TMZ | 468 (90%) |
| TTF | 32 (6.2%) |
| Immunotherapy: Avastin | 69 (13.3%) |
| Cortisone during RT | 365 (74.9%) |
| Seizure occurrence | 292/520 (56.2%) |
| POS | 154/520 (29.6%) |
| EPS | 31/520 (6.2%) |
| SDR | 70/509 (13.8%)a |
| PTS | 152/421 (36.1%)a |
| Treatment with ASM | 330 (63.5%) |
| Initial drug | |
| Levetiracetam | 278 (53.5%) |
| Valproic acid | 11 (2.1%) |
| Lacosamide | 6 (1.7%) |
| Oxcarbazepine | 14 (4.2%) |
| Benzodiazepine | 15 (2.9%) |
| Lamotrigine | 3 (.9%) |
| Phenytoin | 5 (1.5%) |
| Topiramate | 2 (.6%) |
| Pregabalin | 3 (.9%) |
| None | 13 (3.9%) |
| Molecular status | |
| MGMT methylated | 92 (41.6%) |
| Missing | 299 (57.5%) |
| IDH1 mutation | 26 (5%) |
| Missing | 150 (28.8%) |
| ATRX lost | 18 (8.1%) |
| Missing | 298 (57.3%) |
| MIB1 mutation, % | 21.8 [range = 2–80] |
| Missing | 190 (36.5%) |
| Progression occurred | 279 (53.7%) |
| Death observed | 415 (78.8%) |
| Progression-free survival, median, months (IQR) | 9 (6–15) |
| Overall survival, median, months (IQR) | 11 (4–18) |
| Follow-up, median, months (IQR) | 11 (4–19) |
- Abbreviations: ASM, antiseizure medication; ATRX, alpha thalassemia/mental retardation syndrome X-linked; EOR, extent of resection; EPS, early postoperative seizures; IDH1, isocitrate dehydrogenase 1; IQR, interquartile range; KPS, Karnofsky Performance Scale; MGMT, O(6)-methylguanine-DNA methyltransferase; MIB1, mitotic index marker; POS, preoperative seizures; PTS, posttherapeutic seizures; RT, radiotherapy; SDR, seizures during radiotherapy; TMZ, temozolomide; TTF tumour-treating fields.
- a Of the 520 individuals included in the study, 11 were admitted to palliative care shortly after primary surgery or were lost to follow-up within <30 days after surgery, and were consequently excluded from the analysis of SDR. After 3 months of follow-up, 99 patients were either lost to follow-up or had died and were thus excluded from the analysis of PTS.
3.2 Seizures: Occurrence and management
A total of 292 of 520 patients (56.2% of the final cohort) had at least one epileptic seizure at some point during the course of their disease. The median time interval between primary surgery and first seizure was −2.5 days (range = −250 to 1473 days). The cumulative numbers of patients who experienced POS, EPS, SDR, and PTS were 154 of 520 (29.6%), 31 of 520 (6.0%), 70 of 509 (13.8%) and 152 of 421 (36.1%), respectively. The onset of epilepsy was preoperative in 154 (52.8%) patients with epilepsy (PWE) and postoperative in 138 (47.3%) PWE.
At the time of their first seizure, all patients had visible lesions on MRI scan and the seizure was attributed to the tumor.
Of the initial seizures, 150 of 292 (51.3%) were focal to bilateral tonic–clonic seizures. During the entire course of their disease, a total number of 177 of 292 (60.6%) PWE developed focal to bilateral tonic–clonic seizures.
ASM treatment was initiated after the occurrence of first seizure(s) in all cases except for 13 PWE (of whom 10 experienced their initial seizures as POS and three as PTS). Fifty-nine of 520 patients (11.3%) received "prophylactic" ASM treatment without preceding seizures. Levetiracetam was the most frequently prescribed ASM (Table 1).
3.3 Risk factors for seizures at different points in time during treatment
On univariate analysis, POS were more frequent in PWE with a higher preoperative KPS score (p < .001) and with temporal lobe tumor localization (odds ratio [OR] = 1.511, p = .033), and in females (p = .02). A greater tumor volume was negatively associated with POS occurrence (OR = .982, p < .001; Table S2). In the multivariate analysis, smaller tumor volume, temporal lobe localization, and higher KPS score were each independently associated with POS (Table 2, Model 2). As data on presurgical tumor volume were missing in 9.4% of all cases, we calculated a second model that excluded tumor volume and similarly revealed the association between temporal lobe localization and higher KPS.
| Parameter | Model 1 | Model 2 | ||
|---|---|---|---|---|
| aOR (95% CI) | p | aOR (95% CI) | p | |
| Age | .994 (.978–1.010) | .429 | .994 (.977–1.012) | .530 |
| Sex = female | .720 (.480–1.080) | .113 | .700 (.454–1.081) | .108 |
| Localization in temporal lobe | 1.569 (1.054–2.335) | .026a | 1.761 (1.148–2.700) | .010a |
| Tumor volume, cm3 | – | – | .991 (.987–.996) | <.001a |
| KPS at admission < 70% | .321 (.191–.540) | <.001a | .394 (.228–.681) | <.001a |
- Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; KPS, Karnofsky Performance Scale.
- a Statistically significant.
We did not detect any significant risk factors for EPS (on univariate analysis; see Table S2). Of the 21 EPS patients who experienced their first seizure during the perioperative course, five (23.8%) developed further seizures.
None of the radio(chemo)therapy (RCT) parameters (dose regimen, duration of chemotherapy, and radiotherapy) was associated with an increased or decreased risk of SDR on univariate analysis. SDR were independently related to tumor location in the parietal lobe (adjusted OR [aOR] = 1.831, p = .034) and occurrence of preceding POS (aOR = 1.729, p = .046), but not EPS (OR = 2.060, p = .145; Table 3).
| Parameter | aOR (95% CI) | p |
|---|---|---|
| Age | 1.002 (.979–1.025) | .892 |
| Localization in parietal lobe | 1.863 (1.075–3.230) | .027a |
| POS | 1.729 (1.011–2.959) | .046a |
| EPS | 2.060 (.780–5.439) | .145 |
| Follow-up, months | .984 (.964–1.004) | .114 |
- Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; EPS, early postoperative seizures; POS, preoperative seizures.
- a Statistically significant.
We noted an independent association of both occurrence of tumor progression (aOR = 2.323, p < .001) and occurrence of SDR (aOR = 3.358, p < .001) with an increased risk of PTS. The tendency we observed toward increased occurrence of PTS with rising duration of follow-up did not attain statistical significance (aOR = 1.010, p = .093). Neither parameters of the primary adjuvant RCT nor the use of secondary therapy modalities (surgery or radiotherapy) affected the occurrence of late seizures. Tumors located in the temporal lobe were independently associated with a decreased risk for PTS (aOR = .576, p = .014) in contrast to the increased risk of POS observed with this location. Younger age was significant on univariate but not on multivariate analysis (Table 4).
| Parameter | aOR, 95% CI | p |
|---|---|---|
| Age | .993 (.975–1.012) | .481 |
| Localization in temporal lobe | .576 (.371–.894) | .014a |
| POS | 1.285 (.823–2.006) | .270 |
| EPS | 1.004 (.402–2.505) | .994 |
| SDR | 3.358 (1.824–6.183) | <.001a |
| Tumor progression | 2.323 (1.435–3.759) | <.001a |
| Follow-up, months | 1.010 (.998–1.021) | .093 |
- Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; EPS, early postoperative seizures; POS, preoperative seizures; SDR, seizures during radiochemotherapy.
- a Statistically significant.
We addressed the number of missing data on IDH mutation by performing additional multivariate analyses that included only patients with known, negative IDH status (n = 344). Due to a loss of power occasioned by reduced sample size, some results did not retain significance, particularly tumor progression as predictive of late seizures and location in the parietal lobe as predictive of SDR. However, no new or unexpected findings emerged. We did not detect significant differences in seizure occurrence between patients with missing and complete data on IDH1 mutation and MGMT promotor methylation status (data not shown).
3.4 Temporal lobe location
As temporal lobe resection was associated with an increased risk of POS but a decreased risk of PTS, we further analyzed the subgroup of POS patients whose tumor was located exclusively in the temporal lobe (n = 49). In this group, an extensive (“gross”) tumor resection was significantly associated with a decreased risk of seizures after tumor resection; none of the other parameters survived on multivariate analysis (Table S3). This was not the case in the other subgroups (patients with exclusively frontal/parietal/occipital location, data not shown).
4 DISCUSSION
This study sought to retrospectively identify risk factors for epileptic seizures in glioblastoma patients in four different periods during the course of the disease and its treatment, namely preoperatively, early postoperatively, during radiotherapy, and posttherapeutically. The total incidence of epilepsy was 56.2%; seizures were the presenting symptom of the tumor in 29.6% of all cases. These figures are comparable to findings of previous studies.4-7 More than 10% of all patients in our cohort received “prophylactic” ASM without preceding seizures, a course of action current guidelines do not recommend.20 Our findings are in line with previous studies.21, 22 We speculate that this may reflect general insecurities and risk aversion in a setting where higher risks for seizures cannot be excluded while tapering ASM. At the last follow-up, only three of these patients were no longer on ASM. Levetiracetam was the most frequently used drug.
POS were associated with higher KPS and temporal lobe involvement. We observed a negative association between higher tumor volume and POS occurrence. In 9.4% of all cases, data on presurgical tumor volume were missing, mainly because patients did not receive in-house presurgical MRI. Although these missing cases may have caused bias, we note that both models (including and excluding tumor volume) produced significant findings on higher KPS and temporal lobe location. As a potential explanation for the associations linking POS to smaller tumor volume and higher KPS, we hypothesize that tumors presenting with an epileptic seizure may be diagnosed earlier than those presenting with more subtle signs such as headache, cognitive changes, or slowly evolving neurological deficits.8, 23, 24 Previous work has identified temporal lobe involvement as a risk factor for POS in both low- and high- grade astrocytomas, suggesting a lower seizure threshold of the temporal lobe compared to other brain regions.10, 23 Although some studies found an association between younger age and POS, this was not the case in our study.9, 17, 25
In our cohort, 6.0% of patients experienced EPS, a figure similar to previous studies' reports of EPS occurring in 3%–8% of all patients after supratentorial tumor resection.9, 26-28 We did not detect any significant association between potential risk factors and EPS occurrence; that the group of patients with EPS was considerably smaller than that of patients experiencing POS, SDR, and PTS may explain the lack of significant results for the risk factors explored in this analysis. The current study observed no significant association of EPS with an increased risk of further seizures (SDR or PTS). This contrasts with the findings for POS and reinforces the view that EPS may not carry an increased risk of epilepsy.29 However, the small number of patients experiencing EPS in our study urges cautious interpretation of our findings.
Unlike EPS, SDR were associated with an increased risk of further seizures (PTS). As irradiation causes inflammation, seizures may occur de novo, or pre-existing epilepsy may worsen during radiotherapy. A prospective trial with the purpose of examining seizure activity during RCT in glioblastoma patients is currently in progress, addressing the absence thus far of data on seizure activity during RCT.30 Our study does not show dose-dependent associations between RCT and SDR. Discontinuation of treatment was not linked to SDR occurrence. We therefore hypothesize that seizures during RCT may promote susceptibility to seizures, independently of the RCT dosage within the treatment range, and, unlike EPS, should probably not be considered acute symptomatic seizures. A limitation of our study is, however, that the prevalence of SDR may have been underestimated given the possibility that the effects of radiation therapy can last longer than 30 days.
Tumor location in the parietal lobe was associated with an increased risk of SDR. The parietal lobe is particularly rich in eloquent areas. We speculate that these patients are most likely to receive smaller resections, potentially leaving the epileptogenic zone around the tumor in place. Confirmation of this hypothesis requires further research on how the involvement of specific eloquent brain areas influences seizure risk. The association found in previous work between a frontoparietal (i.e., central) localization and an increased risk of seizures of other etiologies, such as cerebral abscesses, may suggest the central lesion itself as an alternative explanation for our findings.31 Given the small number of patients in our study with parietal lobe localization and SDR, interpretation of the results requires caution.
In this context, we note the finding, which may initially appear contradictory, that tumors primarily localized in the temporal lobe were more strongly associated with POS, but less strongly associated with PTS. As the temporal lobe is amenable to more extensive removal than is, for example, the parietal lobe, we hypothesize that the complete removal of the temporal epileptogenic lesion led to better seizure control after surgery. Our study found that patients with tumors located in the temporal lobe were most likely to receive complete tumor resection. This finding is in line with outcomes after surgery for refractory focal epilepsy, where temporal lobe lesions tend to result in better postoperative seizure control than do extratemporal lobe foci.32 However, our temporal lobe subgroup was small, and the picture is less clear for multifocal tumors. Our findings were independent of right or left hemispheric location, contrasting with reports from some previous studies that identified dominant hemispheric location as a risk factor for seizures in high-grade glioma.16, 25
It is well known that seizures in brain tumor patients are a warning sign for tumor progression.10, 11 In our study, 76 of all PWE (26.0%) developed new onset epilepsy after completion of the oncological first-line therapy. PTS in general, and especially new onset PTS (data not shown), were strongly associated with tumor progression on multivariate analysis. These findings reinforce those of previous work, confirming that new onset epilepsy can occur at any stage during the disease.
4.1 Limitations
Our analysis has a number of limitations. We lacked information on seizure frequency, particularly for seizures scored on the basis of reports made during patients' admissions for surgery or RCT. Therefore, rather than including seizure frequency in the analyses, we scored whether a patient had or had not had a seizure during each of the four time periods under study (0 = no seizure, 1 = seizure[s]). This limitation suggests a need for prospective trials evaluating seizure frequency in more detail to improve monitoring and ASM therapy in glioblastoma patients. A major limitation of this study is the number of missing neuropathological data. Until recently, our hospital did not routinely perform analysis of MGMT promotor methylation, a known predictor of glioblastoma survival; this explains the >60% of data missing in relation to this characteristic. This said, the study's intent was not to assess associations between epilepsy and tumor survival, but rather to explore risk factors for seizures. Although a novel study found an association between MGMT gene promotor methylation and postoperative seizure control, it remains unclear whether this is a causality or a mere association.18 Data regarding IDH1 status were missing in 30% of the cases. The new 2021 World Health Organization (WHO) classification for central nervous system tumors no longer classifies astrocytomas with an IDH1 mutation as glioblastoma,33 and includes other molecular markers for the definition of glioblastoma that were not present in most of our patients. Our results therefore apply only to tumors diagnosed according to the old classification; we believe that the new classification will be especially of value for studies diving deeper into the associations between novel molecular markers and seizures, a concern that goes beyond the bounds of this study. As this analysis was conducted before 2021 when the old WHO classification was in place, we controlled for this factor by collecting information on IDH1 status in our cases. Although previous studies on low-grade gliomas had found seizures to be associated with IDH mutations,14, 34 the picture is less clear for glioblastoma patients, and previous studies found contradictory results.14, 34-39 In our study, IDH1 mutation was not a crucial factor in seizures. However, the patient groups were small (see also Table S1); although we did not detect significant differences in seizure occurrence between patients where data on IDH1 mutation and MGMT promotor methylation status were absent versus present (data not shown), we cannot exclude the possibility of bias caused by the missing data.
5 CONCLUSIONS
In this retrospective, monocentric study, seizures in glioblastoma patients had various, time-dependent risk factors. Temporal lobe localization was a risk factor for POS, and surgery may have had protective effects in these patients. RCT did not have dose-dependent pro- or anticonvulsive effects. The occurrence of PTS was associated with tumor progression. Further studies may make use of these findings to identify patients at risk of seizures during certain periods of disease and their treatment.
AUTHOR CONTRIBUTIONS
Study concept and design: Jenny Stritzelberger, Hajo Hamer. Acquisition, analysis, or interpretation of data: Anna Gesmann, Imke Fuhrmann, Stefanie Balk, Sebastian Brandner, Felix Eisenhut, Arnd Dörfler, Roland Coras, Werner Adler, Stefan Schwab, Florian Putz, Rainer Fietkau, Luitpold Distel, Tamara M. Welte, Jenny Stritzelberger. Critical revision of the manuscript for important intellectual content: Anna Gesmann, Imke Fuhrmann, Stefanie Balk, Sebastian Brandner, Felix Eisenhut, Arnd Dörfler, Roland Coras, Werner Adler, Tamara M. Welte, Florian Putz, Rainer Fietkau, Luitpold Distel, Stefan Schwab, Hajo Hamer. Statistical analysis: Anna Gesmann, Jenny Stritzelberger, Werner Adler. Drafting of the manuscript: Jenny Stritzelberger. Supervision: Hajo Hamer.
ACKNOWLEDGMENTS
The following work was performed in (partial) fulfillment of the requirements for obtaining the degree “Dr. med.” We thank the language service of Friedrich-Alexander-Universität Erlangen-Nürnberg for their assistance in proofreading the manuscript for style and grammar. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
This research did not receive any grant from public, commercial, or not-for-profit sector funding agencies.
CONFLICT OF INTEREST STATEMENT
H.H. has served on the scientific advisory boards of Arvelle, Bial, Corlieve, Eisai, GW, Novartis, Sandoz, UCB Pharma, and Zogenix. He has been part of the speakers' bureaus of or received unrestricted grants from Amgen, Ad-Tech, Alnylam, Bracco, Desitin, Eisai, GW, Nihon Kohden, Novartis, Pfizer, and UCB Pharma. The remaining authors have no conflicts of interest.




