Higher susceptibility to 6 Hz corneal kindling and lower responsiveness to antiseizure drugs in mouse models of Alzheimer's disease
Abstract
Objective
Epileptic spikes and seizures seem present early in the disease process of Alzheimer's disease (AD). However, it is unclear how soluble and insoluble amyloid beta (Aβ) and tau proteins affect seizure development in vivo. We aim to contribute to this field by assessing the vulnerability to 6 Hz corneal kindling of young female mice from two well-characterized transgenic AD models and by testing their responsiveness to selected antiseizure drugs (ASDs).
Methods
We used 7-week-old triple transgenic (3xTg) mice that have both amyloid and tau mutations, and amyloid precursor protein Swedish/presenillin 1 dE9 (APP/PS1) mice, bearing only amyloid-related mutations. We assessed the absence of plaques via immunohistochemistry and analyzed the concentrations of both soluble and insoluble forms of Aβ1–42 and total tau (t-tau) in brain hippocampal and prefrontal cortical tissue. Seven-week-old mice of the different genotypes were subjected to the 6 Hz corneal kindling model. After kindling acquisition, we tested the anticonvulsant effects of three marketed ASDs (levetiracetam, brivaracetam, and lamotrigine) in fully kindled mice.
Results
No Aβ plaques were present in either genotype. Soluble Aβ1–42 levels were increased in both AD genotypes, whereas insoluble Aβ1–42 concentrations were only elevated in APP/PS1 mice compared with their respective controls. Soluble and insoluble forms of t-tau were increased in 3xTg mice only. 3xTg and APP/PS1 mice displayed more severe seizures induced by 6 Hz corneal kindling from the first stimulation onward and were more rapidly kindled compared with control mice. In fully kindled AD mice, ASDs had less-pronounced anticonvulsive effects compared with controls.
Significance
Mutations increasing Aβ only or both Aβ and tau in the brain enhance susceptibility for seizures and kindling in mice. The effect of ASDs on seizures measured by the Racine scale is less pronounced in both investigated AD models and suggests that seizures of young AD mice are more difficult to treat.
Key Points
- Soluble amyloid beta 1–42 (Aβ1–42) is already increased in the brains of 7-week-old triple transgenic (3xTg) and amyloid precursor protein Swedish/presenillin 1 dE9 (APP/PS1) mice.
- t-tau levels are increased in the brains of 7-week-old 3xTg mice.
- Seven-week-old 3xTg and APP/PS1 mice are more susceptible to 6 Hz corneal kindling.
- The anticonvulsant effects of several antiseizure drugs (ASDs) are less pronounced in 6 Hz corneally kindled Alzheimer's disease mice than in control mice.
1 INTRODUCTION
Alzheimer's disease (AD) is the leading cause of dementia and brain accumulation of amyloid beta (Aβ) plaques, and hyperphosphorylated tau tangles are the main pathologic hallmarks.1 Epilepsy is more prevalent in patients with AD, and the connection between both diseases is further supported by increased Aβ plaque and hyperphosphorylated tau load in patients with epilepsy and by the finding that seizures worsen AD neuropathology in mice.2-4 Seizures seem already present in an early stage of AD and are more frequent in patients with familial AD.5 Before AD symptoms occur, Aβ and tau aggregation have had the chance to affect molecular brain mechanisms for years. Much attention has recently been paid to early changes in the soluble, most toxic forms of Aβ1–42 and tau biomarkers and how these may alter brain excitability.
Soluble Aβ1–42 is found consistently to increase neuronal activity, but the effect of increased soluble total tau (t-tau) is subject to debate, with both neuronal hypoactivity and hyperactivity described.6, 7 A calcium imaging study in mice demonstrated that increasing tau reduced neuronal activity and that by increasing both Aβ1–42 and tau, the effect of the second dominated the first, resulting in reduced neuronal firing rates.8 Seizures are more complex than “just” neuronal hyperexcitability: they are a symptom of increased neuronal synchronization, and the contribution of glial cells is crucial.9, 10 It thus remains to be unveiled how seizure susceptibility and epilepsy are affected in vivo in mouse models exhibiting elevations in both Aβ1–42 and t-tau.
A handful of studies assessed the effect of only amyloid or only tau mutations on seizure susceptibility in AD mice, and just one study was conducted in a model combining both mutations.11-16 The latter found increased susceptibility to an acutely evoked audiogenic seizure in 3-week-old triple transgenic (3xTg) mice.13 However, whether tau biomarkers are increased at this young age and how the combination of soluble Aβ and tau affect seizure susceptibility remains unknown.
Our first aim of the present study was therefore to use a genetic mouse model with aberrant changes in both soluble Aβ1–42 and t-tau, and to subject these presymptomatic mice to a chronic kindling paradigm to assess seizure susceptibility and kindling. We chose the triple transgenic (or 3xTg) mouse model that expresses amyloid precursor protein (APP) with the Swedish mutation, presenilin 1 (PS1) with the M146V mutation and microtubule-associated protein tau (MAPT) with the P301L mutation, in which both Aβ1–42 and t-tau biomarker levels are expected to be increased. For comparison we repeated the experiment in amyloid precursor protein Swedish/presenillin 1 dE9 (APP/PS1) mice, a model overexpressing APPSwe, and PS1d9E mutations, that presumably lead to an increase in Aβ1–42 concentrations only.
Finding an adequate treatment for seizures in patients with AD is complicated by the side effects of antiseizure drugs (ASDs) exacerbating certain AD symptoms.17-19 Little evidence exists on the optimal ASD for these specific patients: a small randomized-controlled trial compared phenobarbital, levetiracetam (LEV), and lamotrigine (LTG) in epileptic patients with AD and found no difference in efficacy.19 Recently, brivaracetam (BRV), a more potent analogue of LEV, was approved.20 Because certain ASD targets are less expressed in the brain of AD patients and their neuronal networks have manifest abnormalities, the efficacy of some ASDs might be affected.21, 22 Therefore, our second aim was to assess how seizures in fully kindled AD and control mice will respond to this aforementioned selection of ASDs.
2 MATERIALS AND METHODS
2.1 Mice
Seven-week-old female 3xTg, APP/PS1 mice and their respective age- and sex-matched wild-type controls were acquired from the Jackson Laboratory (Bar Harbor, USA). The 3xTg mice (MMRRC stock #34830) are homozygous for a PS1M146V knock-in and an APPSwe and a MAPTP301L transgene on a mixed C57BL/6J - 129Sv background. Their controls are B6129SF2/J mice (stock #101045). The APP/PS1 mice (MMRRC stock #34832) are heterozygous for APPSwe and PS1dE9 transgenes on a C57BL/6J congenic background, and wild-type mice with the same background (MMRRC stock #34832) were used as controls. Mice were group-housed (4–5 mice per cage), maintained on a 12-12-h light–dark cycle, and had access to water and food ad libitum. The experiments were approved by the ethical committee of the Vrije Universiteit Brussel (project 19-213-4). Animal Research: Reporting of In Vivo Experiments guidelines and the Basel declaration were considered when designing the experiments. The mice's genotype was verified with polymerase chain reaction performed on ear-punched tissue by Transnetyx (Cordiva, USA).
2.2 (Immuno)histochemistry
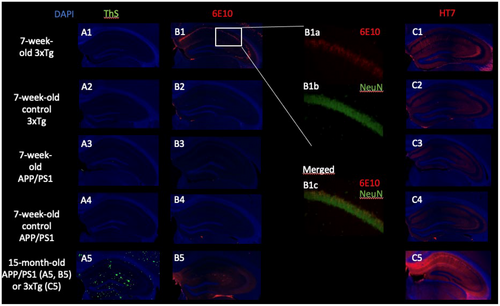
We stained brain sections of three experimentally naïve 7-week-old female mice of all four genotypes with thioflavin S (ThS) to detect cored amyloid plaques; used an anti-amyloid β 1–16 (6E10) antibody to detect amyloid plaques, APP, or its metabolites; anti-human tau (HT7) antibody to detect human t-tau; and anti-neuronal nuclear protein (NeuN) antibody to detect neuronal nuclei. Experimental details can be found in Appendix S1.
2.3 Biomarker analysis in prefrontal cortex and hippocampal homogenates
For protein analysis, 7-week-old experimentally naïve female mice of all four genotypes were killed with cervical dislocation, after which the prefrontal cortex (PFC) and hippocampus (HC) were dissected bilaterally and homogenized. The homogenates were analyzed for human Aβ1–42 and t-tau with enzyme-linked immunosorbent assays (ELISAs). Details can be found in Appendix S1.
2.4 6 Hz corneal kindling
Corneal kindling (6 Hz) was performed as described previously.23, 24 Briefly, 7-week-old mice were stimulated (10 mA, direct current, 3 s duration, 0.2 ms pulse width) with corneal electrodes connected to a stimulator (ECT Unit 57 800, Ugo-Basile, Italy) for 5 weeks twice daily (first stimulation between 8 and 10 am, second stimulation between 2 and 4 pm) 5 days per week (Monday–Friday; details in Appendix S1). Seizure severity was scored by researchers blinded to genotype using a modified Racine scale. Once a mouse exhibited 10 sequential generalized seizures (modified Racine score ≥3), it was considered fully kindled and kept on a maintenance protocol (two stimuli per day, 2 days per week) until the start of the ASD testing.
2.5 Antiseizure drugs
All fully kindled mice were assigned randomly to sequential treatments with different ASDs at different dosages using a Latin square design.25 For BRV these were 0.3, 3, 10, 30, and 100 mg/kg; for LEV they were 2, 6, 20, 60, 200, and 600 mg/kg; and for LTG they were 3, 10, 30, and 60 mg/kg. Dosages were based on the median effective dose (ED50) reported previously in corneal kindling experiments and in-house experience.26 Considering time-to-peak effect, LTG and BRV were administered intraperitoneally (i.p.) 30 min and LEV 60 min before stimulation.20, 27 Drug wash-out of at least 3 days was ensured between different treatments. LTG was purchased from Tocris Bioscience (#1611/10); BRV and LEV were kindly provided by UCB (Belgium).
2.6 Statistical analysis
Statistical analysis was performed using RStudio (version 1.2.1335). Normal distribution of data was verified with a Shapiro test and a qqplot, and variance with a fitted plot. Repeated measures from kindling acquisition and ASD treatment were assessed with a linear mixed-effects model and speed of full kindling with a Cox proportional hazard model. For ELISA, differences between the AD mice and their respective controls were evaluated with a Mann–Whitney U (MWU) test. Linear correlations were evaluated with Pearson's product. ɑ was set at 0.05. Additional details can be found in Appendix S1.
3 RESULTS
3.1 AD biomarkers in the brains of 7-week-old APP/PS1 and 3xTg mice
We first used ThS staining to detect amyloid plaques. As expected, no fluorescent signal was present in any of the slices indicating the absence of cored Aβ plaques at 7 weeks (Figure 1A1–A4). As a positive control, we used a 15-month-old APP/PS1 mouse, the brain slices of which exhibited numerous areas of ThS fluorescence (Figure 1A5). In slices of all three 3xTg mice incubated with the anti-6E10 antibody, we saw an intense fluorescent signal that coincided with NeuN, thus localized in neuronal cell bodies (Figure 1B1a–c). No signal was present in the 7-week-old APP/PS1 or either of the control genotypes (Figure 1B2–B4). As a positive control, widespread extracellular plaque-like deposition was found in 15-month-old APP/PS1 mice (Figure 1B5). In 7-week-old 3xTg mice, weak anti-HT7 signal was found in the hippocampus, with a pattern corresponding to neuronal distribution (Figure 1C1). As expected, no signal was present in the other genotypes (Figure 1C2–C4). As a positive control for this t-tau staining, we used a 15-month-old 3xTg mouse, in which an intense signal spread throughout hippocampal neurons was found (Figure 1C5).
In addition to the morphologic and qualitative information obtained from immunohistochemistry (IHC), we quantified levels of Aβ1–42 and t-tau in PFC and HC homogenates with ELISA. We chose to separate a tris-buffered saline (TBS) soluble fraction, corresponding to soluble forms, and a TBS-insoluble fraction, corresponding to membrane-bound or insoluble plaques or tangles of the proteins.
TBS-soluble Aβ1–42 levels were higher in 3xTg (MWU, W = 81, p < .001) and APP/PS1 (W = 16, p = .03) mice compared with their respective controls (Figure 2A). In the insoluble fraction, APP/PS1 mice show an increased concentration of Aβ1–42 (W = 16, p = .03) compared with their control mice, whereas for 3xTg there was a strong trend toward an increase (W = 63, p = .05) compared with their controls (Figure 2B).
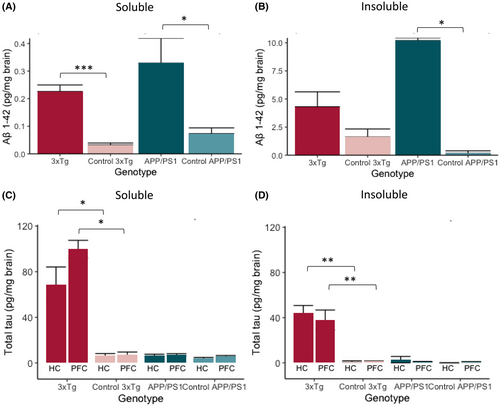
In 3xTg mice, TBS-soluble t-tau levels in PFC and HC are ~10 times higher compared with levels in the respective brain areas of control mice (W = 16, p = .029 and W = 42, p = .026, respectively), and TBS-insoluble t-tau concentrations are 25 times higher than in control mice, both in HC (W = 49, p = .002) and PFC (W = 42, p = .001) (Figure 2C,D). We found no difference between APP/PS1 mice and controls in TBS-soluble or TBS-insoluble fractions of the t-tau protein in HC (W = 25, p = .3 and W = 21, p = .4) or PFC (W = 27, p = .2 and W = 20, p = .8) homogenates (Figure 2C,D).
3.2 Seven-week-old 3xTg and APP/PS1 mice are more prone to 6 Hz corneal kindling and reach the kindled state faster
We assessed whether increased soluble Aβ1–42 or t-tau levels were associated with increased seizure susceptibility and kindling in young mice, before amyloid plaques or neurofibrillary tangles were present. 3xTg, APP/PS1, and their control mice were subjected to identical corneal stimuli during the entire kindling acquisition phase. Seizure severity worsened over time (linear mixed effects model, 3xTg: F(1,1860) = 871, p < .001; APP/PS1: F(1,1369) = 564, p < .001) as expected during kindling acquisition (Figure 3A). 3xTg and APP/PS1 mice exhibited more severe seizures from the first stimulation onward, with an estimated seizure score that is, respectively, 1.89 and 2.71 points higher on day 1 (3xTg: F(1,53) = 89, p < .001; APP/PS1: F(1,54) = 50, p < .001). The difference in evoked seizure severity between AD and control mice decreased over time (3xTg: F(1,1860) = 30, p < .001; APP/PS1: F(1,1369) = 42, p < .001).
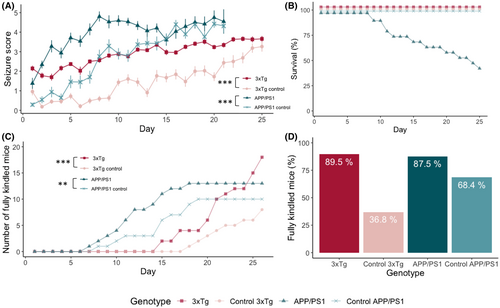
From the eighth day of stimulation onward, APP/PS1 mice were found dead in their home cage. All had previously attained a seizure severity of 6, and by the end of the kindling acquisition, 11 of 19 APP/PS1 mice died (58%; Figure 3B). There was no mortality in the 3xTg or control lines.
The odds of developing a fully kindled state were higher for 3xTg (Cox proportional hazard model, odds ratio 3.7, p < .001) and APP/PS1 mice (odds ratio: 1.4, p = .002) compared with their respective controls (Figure 3C). Seventeen of 19 (89.5%) of the 3xTg mice and seven of 19 (36.8%) of the 3xTg control mice reached a fully kindled state (Figure 3D) after 5 weeks of stimulations. Of the surviving APP/PS1 mice, all but one (7/8) reached a fully kindled state. In the APP/PS1 control group, 13 of 19 (68.4%) was fully kindled by the end of acquisition.
3.3 Tested ASDs are less effective in 6 Hz fully kindled Alzheimer's disease mice
Next, we tested the efficacy of BRV, LEV, and LTG against 6 Hz corneally evoked seizures in fully kindled mice. An identical electrical current as during kindling acquisition phase was administered. Mice were stimulated 30 min after vehicle injection, and the next stimulation was done at the same time the following day after ASD injection. As expected, seizure severity reduced with escalating ASD dose (linear mixed-effects model, 3xTg: F(1,164) = 16, p < .001; APP/PS1: F(1,210) = 43, p < .001), except for plateauing of BRV in the 3xTg control mice (Figure 4A,B). Seizures remained more severe in both AD strains compared to controls after ASD administration (3xTg: F(1,25) = 5, p = .04; APP/PS1: F(1,22) = 23, p < .001) (Figure 4A,B). LTG was less effective than LEV and BRV (3xTg: F(2,163) = 5, p = .01; APP/PS1: F(2,211) = 17, p < .001) (Figure 4A,B). We were not able to further increase the LTG dose because 60 mg/kg exceeds this molecule's median toxic dose in mice.27
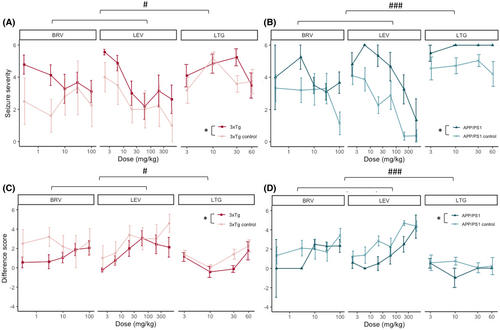
Given that seizures after vehicle injection were on average less severe in wild-type mice compared with AD mice (AD = 5.36, control = 5.11), we introduced the “difference score” to further differentiate the effect of the ASDs. Here we subtract the seizure score after ASD injection from the seizure score after vehicle in the same mouse on the previous day. A positive difference score means the ASD reduced seizure severity, zero indicates a lack of ASD effect, and a negative value represents a more severe seizure after ASD than after vehicle. As expected, the difference score increased with escalating ASD dose (3xTg: F(1,167) = 16, p < .001; APP/PS1: F(1,208) = 38, p = .006) and LTG was less effective than LEV and BRV (3xTg: F(2,166) = 5, p = .01; APP/PS1: F(2,211) = 16, p < .001) (Figure 4C,D). Finally, by using the difference score we can confidently state that the tested ASDs reduce the seizure severity to a smaller extent in 3xTg and APP/PS1 mice compared with their respective controls (Figure 4C,D) (linear mixed-effects model estimate for 3xTg genotype = 1.1, F(1,23) = 7, p = .016; linear mixed-effects model estimate for APP/PS1 genotype = 1.5, F(1,20) = 7, p = .014).
Frequent and severe seizures are one of hypothesized mechanisms of ASD resistance in patients and because evoked seizures were more severe in AD mice vs controls (3.35 vs 2.21) during the acquisition phase, we investigated whether the reduced efficacy of ASDs in AD mice was linked to seizure severity during acquisition.28 We assessed the correlation between mean seizure severity during acquisition in each mouse and their mean difference score after ASD treatment. No correlation was present (Pearson's product, Figure 5A,R = −0.088, p = .07), excluding that the significantly smaller anticonvulsive effect in AD mice is due to a higher seizure severity score during acquisition.
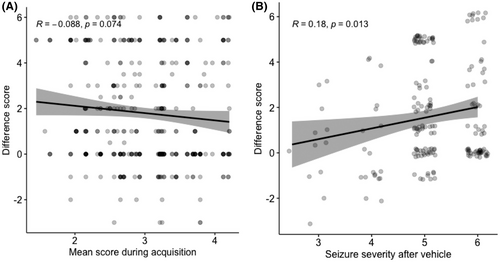
Because the mean seizure severity after vehicle was higher in AD mice, we needed to verify whether ASDs had less effect against more severe seizures. This would be represented by an inverse correlation between seizure severity after vehicle and its associated difference score. We found a very weak, albeit not inversed, correlation (Figure 5B,R = 0.18, p = .01). This indicates that more severe seizures are, on the contrary, more reduced by ASDs, and thus do not explain the reduced effect of ASDs in AD mice.
4 DISCUSSION
We subjected young (7-week-old) 3xTg and APP/PS1 mice to a 6 Hz corneal kindling paradigm.29 We are the first to show enhanced seizure susceptibility and more rapid 6 Hz corneal kindling in AD mice at an age when both soluble Aβ1–42 and t-tau brain levels are increased. Moreover, we showed that selected ASDs have a less pronounced anticonvulsive effect in the fully kindled AD mice of both strains compared to control mice.
4.1 Amyloid beta 1–42 and t-tau biomarkers in homogenates of PFC and HC of AD mice
At 7 weeks of age, both AD models have increased soluble Aβ1–42 concentrations, neither has plaques yet, and only 3xTg mice have increased t-tau brain levels. In APP/PS1 mice, we demonstrated increased levels of TBS-soluble and TBS-insoluble Aβ1–42. Because neither the ThS nor the 6E10 antibody IHC showed any signal, the TBS-insoluble Aβ1–42 seems not to correspond to Aβ fibrils from plaques but rather stems from membrane-bound Aβ oligomers that are characterized as part of this TBS-insoluble fraction.30 Moreover, the earliest report of Aβ plaques in APP/PS1 mice is at only 12 weeks.31 As reported by others, t-tau levels were not different between APP/PS1 mice and controls at this age.32 In 3xTg mice, the increase in TBS-soluble, but not TBS-insoluble, Aβ1–42 aligns with previous findings.33 There is, however, a trend toward increased insoluble Aβ1–42 compared with controls and an increased variability. A possibility is that membrane-bound oligomers start to develop around this age in 3xTg mice and that they were present in only some of our mice. The 6E10 signal has a neuronal distribution and does not correspond to Aβ plaques, as is further supported by the absence of ThS staining and because Aβ plaques are expected at only 6–15 months in this strain.34 The 6E10 antibody staining was vastly more abundant in 3xTg than in APP/PS1 mice, despite 3xTg mice not having higher levels of Aβ1–42. This detected signal might come partly from other forms of Aβ (predominantly Aβ1–40), but a large amount probably arises from full-length or partly processed APP.34 Both soluble and insoluble t-tau levels are markedly elevated in different brain regions of 3xTg compared with control mice and our IHC approach detected t-tau in the HC.
4.2 Susceptibility to seizures and seizure development in the 6 Hz corneal kindling model
There is an increased incidence of seizures in patients with AD, but it is notable that also measures of brain hypoactivity have been described.5, 35 Spontaneous epileptiform activity is detected in several rodent models with established Aβ or tau deposition in the brain.36, 37 Whereas APP and Aβ models almost invariably lead to increased neuronal excitability and seizures in different models, the effects of tau diverge between studies.7, 38, 39 The presence of soluble Aβ1–42 and t-tau, key AD biomarkers, in the mice in this study may allow for the interpretation of potential mechanisms underlying the increased susceptibility to seizures and kindling presently observed. The clinical stage of AD and the aggregation forms of Aβ1–42 and t-tau play a crucial role: there is compelling evidence that, especially in its soluble form, Aβ1–42 leads to neuronal hyperactivation in vivo, whereas t-tau is proposed to have both pro-convulsive and anti-convulsive effects.7 A calcium-imaging study demonstrated that a mutation increasing soluble Aβ leads to hyperactivity of cortical neurons in mice, whereas increasing soluble tau leads to hypoactivity of these neurons.8 When both are increased, the effect of tau dominates Aβ and neurons become hypoactive.
Despite calcium imaging being a magnificent window into a mouse's brain, it is crucial to acknowledge that seizures are more than neuronal hyperexcitability. First, synchronization of neuronal depolarization is at least as important as the activity level of the neurons per se.9 Second, astrocytes play a central role in seizures by regulating neurotransmitter reuptake, cytokine release, neural network reorganization, and blood–brain barrier function.10 All these astrocytic processes are dysfunctional in AD too.40 Activated microglia and microgliosis are also key features of AD and epilepsy.41, 42 Of interest, the connection between AD-related microglial abnormalities and seizures was recently underscored. Hypofunction of triggering receptor on myeloid cells 2 (TREM2), a protein highly expressed in microglia, is a strong genetic risk factor for AD. A haploinsufficient TREM2 mouse model had prolonged and more severe epileptic activity after kainic acid injection compared with wild-type controls.43 With respect to AD and epilepsy, looking at neurons thus shows only part of the story, whereas an in vivo model of seizures and kindling in AD mice allowed us to study the “net effect” on brain excitability.
Different groups looked at the effect of tau reduction on seizure susceptibility, with or without the presence of amyloid alterations and most found that tau reduction led to less-severe seizures.12, 43, 44
One report of a study of seizures in an AD model with both amyloid and tau mutations showed that 3xTg mice were more susceptible to audiogenic seizures at the age of 3 weeks.13 Biomarker analysis was limited to IHC with the 6E10 antibody, which demonstrated the presence of Aβ or APP in HC neurons. It is important to note that the presence of tau was not assessed. Drawing conclusions on the mechanisms underlying this increased seizure susceptibility and whether it is amyloid or tau related is thus impossible. We show here for the first time that young AD mice, in which both Aβ1–42 and t-tau are increased, are more susceptible to kindling. In this regard, it is interesting that neuronal activity and seizures promote APP processing and Aβ generation.45 More severe seizures, as a result of Aβ accumulation, would in turn lead to increased Aβ accumulation, generating a vicious circle.46
With respect to seizure susceptibility or kindling in exclusively “amyloid” mouse models, APP/PS1 mice are more susceptible to pentylenetetrazol-induced acute seizures at 4–9 months and 12-month-old Tg2576 mice are more susceptible to amygdala kindling.47, 48 A correlation between Aβ plaque load and seizure susceptibility was proposed.47 However, our present data and those of others suggest that plaques are not required to increase seizure susceptibility in AD mice.16 Conversely, 2-month-old PS2 knockout mice are less susceptible to 60 Hz corneal kindling compared with controls. This difference is no longer present at 8 months, suggesting potential age-related changes in kindling susceptibility in AD-associated models.11 On the other hand, 4-month-old mice with a MAPT P301L mutation are more susceptible to amygdala kindling, which is fully in line with our data.12
Genetic background differences not only influence susceptibility to the first seizure, but also alter the rate of kindling over time.49 For this reason, it is inappropriate to compare seizure susceptibility or kindling acquisition data between our two transgenic mouse lines in this study.
4.3 Mortality
Even without experimental manipulation, about 15% of APP/PS1 mice die prematurely from the age of 4 months and a sudden unexpected death in epilepsy (SUDEP)–like mechanism is one of the proposed causes.40 Because the corneal kindling procedure induces seizure development, it probably expedited mortality and increased its percentage in this predisposed genotype. Contrary to many 50 or 60 Hz corneal kindling experiments in which even non-transgenic mice occasionally die over the course of the experiment, 3xTg and wild-type mice all survived the 6 Hz corneal kindling acquisition in our study.11, 25, 50 This seems to make the 3xTg genotype very suitable for this type of experiment, especially if a therapeutic intervention is to be assessed afterwards.
4.4 Antiseizure drug response
Once mice were fully kindled, we assessed the effect of ASDs. ASD resistance in our AD mice versus controls is not explained by differences in kindling acquisition or seizure severity. Possible mechanisms underlying the less-pronounced anticonvulsive effect are changes in the drug target itself. Indeed, synaptic vesicle 2A (SV2A), the protein on which LEV and BRV exert their effect, is less expressed in the brains of AD mice and patients, already before reaching the dementia stage.22, 51 Similarly, voltage-gated sodium channels (Nav1.1) are less expressed in mouse models and patients with AD, which could impact the sodium channel blocker LTG.21, 36, 52 Alterations in neuronal networks are another mechanism postulated to underlie ASD resistance, and as Aβ1–42 and tau have a profound impact on neuronal activity, they could be involved.38 In addition, neuroinflammation and blood–brain barrier dysfunction may also play a role in the less-pronounced anticonvulsive effect.28
Several ASDs are successful at reducing (to some extent) spontaneous epileptiform activity in AD mouse models.53 Besides reducing these network abnormalities in mice, LEV, BRV, LTG and valproic acid even rescued cognitive deficits.14, 53, 54
One previous study assessed the effect of ASDs on seizures in an AD mouse model. They found that LEV—but not sodium-channel blocking ASDs—was less effective against seizures in a fully kindled PS2 knockout mouse model.11 In this regard, further studies are needed to better define whether there are seizure protocol–specific differences in the anticonvulsive effect of ASDs in various AD-associated mouse models.
The modest effect of LTG in all genotypes was also surprising, as previous kindling experiments in wild-type mice report an ED50 well below 60 mg/kg.11, 27 Because efficacy of ASDs varies between epilepsy models and rodent strains, a possible explanation is that our corneal kindling was performed with 6 Hz, a model of pharmacoresistant epilepsy, and not 50 or 60 Hz stimulations.25, 50, 55
The dose–response curve is least clear for BRV, as we saw an effect that quickly plateaued in the 3xTg control line. To obtain pharmacologically equivalent dosages, we based the BRV dose on SV2A affinity, ED50 in seizures models, and dosage used in patients with epilepsy compared with LEV (ratio of 1/20).20 A more classic dose–response curve might have been present if even lower doses of BRV had been pursued in our present studies.
The 6 Hz corneal kindling model is a clinically relevant model that reflects pharmacoresistant limbic seizures, as well as a variety of neurobehavioral epilepsy comorbidities in fully kindled mice.24 As these young AD mice express different key AD brain biomarkers, have increased seizure susceptibility, and are more rapidly kindled, 6 Hz corneal kindling seems an interesting way to assess the proconvulsive effects of different aspects of AD pathology and to test multiple ASDs sequentially, without the need to age mice, or use resource-intensive post-status epilepticus models.55
Our study comes with limitations that are worth considering for future studies. We used only female mice, as studies showed a more consistent biochemical and behavioral profile in female 3xTg mice. Because hormonal factors influence both AD and epilepsy, our findings should be confirmed in male mice. In addition, our study does not pinpoint an exact cause of increased susceptibility to seizures in either AD-associated genotype. More direct evidence could arise from inducible transgenes, by treating transgenic mice with Aβ1–42 or tau-neutralizing antibodies, or by injecting specific forms of Aβ1–42 or tau proteins in the naive mouse brain. Although the corneal kindling model and its behavioral score are a well-established and important model in ASD discovery and validation, it comes with limitations. The seizure severity after vehicle differed between AD and control mice, which is a confound to interpret the ASD effect via the behavioral score. We tried to correct for this by using the difference score, but it is important to note that an identical difference score can represent different ASD effects (e.g., a difference score of 3 can be a seizure severity of 6 becoming 3, or a 3 becoming a 0). ASDs reduced seizures a little over 1 point less on the modified Racine scale in AD mice compared to control mice, and it is unclear if this difference is biologically relevant.
Although the behavioral score we use has the advantage of comprehensively staging the clinical severity of a seizure, EEG would provide an additional numerical evaluation of the brain's seizure activity. However, it is not traditionally employed in corneal-kindled mouse studies due to the fit-for-purpose application of this model to moderate-throughput ASD screening. The smaller effect of ASDs on the behavioral score in AD mice thus could benefit confirmation in (EEG-measured) seizure models. In addition, it is unknown whether our findings hold true for ASDs other than those tested in these experiments.
In conclusion, our results show that young female transgenic AD mice with increased soluble Aβ1–42 levels or both increased soluble Aβ1–42 and soluble t-tau levels, are more susceptible to 6 Hz kindled seizures and are more rapidly kindled. The anticonvulsive effects of the tested ASDs were less pronounced in the fully kindled AD mice, pointing to a possible pharmacoresistance of both AD mouse strains.
AUTHOR CONTRIBUTIONS
MV and IS conceptualized and designed the study. Study design was further adapted with valuable suggestions from MBH, NA, GN, MB, SE, and DDB. MVV performed the experiments and analyzed the data. MV and IS drafted the manuscript. All authors reviewed the manuscript.
ACKNOWLEDGMENTS
We thank UCB for providing levetiracetam and brivaracetam. We kindly thank Marie-Laure Custers, Gino De Smet, Anke De Smet, Niel Ravyts, Chloë Van Cauwenberghe, Jana Van der Cruyssen, and Lore Winters for their assistance with the experiments and Wilfried Cools for his help with the statistical analysis. This work was supported by grants of the FWO Research Foundation - Flanders (11E4819N and G040419N), Wetenschappelijk Fonds Willy Gepts of Vrije Universiteit Brussel, and the National Institutes of Health (KL2TR002317 and R01AG067788).
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.