International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions
Abstract
The 2017 International League Against Epilepsy classification has defined a three-tier system with epilepsy syndrome identification at the third level. Although a syndrome cannot be determined in all children with epilepsy, identification of a specific syndrome provides guidance on management and prognosis. In this paper, we describe the childhood onset epilepsy syndromes, most of which have both mandatory seizure type(s) and interictal electroencephalographic (EEG) features. Based on the 2017 Classification of Seizures and Epilepsies, some syndrome names have been updated using terms directly describing the seizure semiology. Epilepsy syndromes beginning in childhood have been divided into three categories: (1) self-limited focal epilepsies, comprising four syndromes: self-limited epilepsy with centrotemporal spikes, self-limited epilepsy with autonomic seizures, childhood occipital visual epilepsy, and photosensitive occipital lobe epilepsy; (2) generalized epilepsies, comprising three syndromes: childhood absence epilepsy, epilepsy with myoclonic absence, and epilepsy with eyelid myoclonia; and (3) developmental and/or epileptic encephalopathies, comprising five syndromes: epilepsy with myoclonic–atonic seizures, Lennox–Gastaut syndrome, developmental and/or epileptic encephalopathy with spike-and-wave activation in sleep, hemiconvulsion–hemiplegia–epilepsy syndrome, and febrile infection-related epilepsy syndrome. We define each, highlighting the mandatory seizure(s), EEG features, phenotypic variations, and findings from key investigations.
PODCAST
Key Points
- The ILAE produced a classification of epileptic syndromes presenting in childhood
- Syndromes with onset in childhood are divided into three categories: self-limited focal epilepsies, generalized epilepsies, and developmental and/or epileptic encephalopathies
- Each syndrome has mandatory seizure types, EEG features, age at onset, and findings from key investigations
- Precise identification of an epileptic syndrome can provide useful information on prognosis and management
1 INTRODUCTION
The goal of this paper is to describe epilepsy syndromes that begin in childhood (age 2–12 years). Additional syndromes that have a variable age at onset, including in childhood, are described in the paper on epilepsy syndromes with onset at a variable age.1 The childhood onset syndromes can be broadly divided into three main groups: (1) self-limited focal epilepsies (SeLFEs); (2) generalized epilepsy syndromes, which are thought to have a genetic basis; and (3) developmental and/or epileptic encephalopathies (DEEs), which often have both focal and generalized seizures, including Lennox–Gastaut syndrome (LGS), developmental epileptic encephalopathy with spike-and-wave activation in sleep (DEE-SWAS), and epileptic encephalopathy with spike-and-wave activation in sleep (EE-SWAS), or may have generalized seizures alone, such as epilepsy with myoclonic atonic seizures (EMAtS), or focal/multifocal seizures alone, such as hemiconvulsion–hemiplegia–epilepsy syndrome (HHE) and febrile infection-related epilepsy syndrome (FIRES).
Childhood is also the typical age of onset of childhood absence epilepsy (CAE); this syndrome is covered in a separate paper on the idiopathic generalized epilepsy (IGE) syndromes.2
Recognition of these childhood syndromes requires careful analysis of seizure semiology, evolution over time, and the developmental course of the child, as well as electroencephalographic (EEG) features (background, interictal, and ictal patterns) and, in some cases, brain magnetic resonance imaging (MRI) and genetic studies. At times, childhood syndromes may have evolved from other epilepsy syndromes or types, such as infantile epileptic spasms syndrome, which may evolve to LGS, or self-limited epilepsy with centrotemporal spikes (SeLECTS; formerly known as benign rolandic epilepsy or benign epilepsy with centrotemporal spikes) or structural focal epilepsy evolving to EE-SWAS. In other syndromes, children with prior normal development present with a severe, acute encephalopathy followed by drug-resistant epilepsy, as typically seen in FIRES, or HHE. Moreover, for some SeLFEs, there may be overlap with the IGEs or even evolution to them, reflecting the patient's underlying susceptibility to epileptic seizures.3, 4
The exact proportion of children with epilepsy who meet criteria for a specific syndrome has not been well studied prospectively; however, retrospective data suggest that an epilepsy syndrome is identified in at least one third of cases.5, 6
This paper will address the specific clinical and laboratory features of epilepsy syndromes that begin in childhood and provide rationale for any significant nomenclature or definitional changes. Table 1 summarizes the epilepsy syndromes, with updated nomenclature and acronyms used in this paper.
| Self-limited focal epilepsies | Genetic generalized epilepsies | DEEs | |||||
|---|---|---|---|---|---|---|---|
| Epilepsy syndromes with focal seizures | Formerly known as | Epilepsy syndromes with generalized seizures | Formerly known as | DEEs | Formerly known as | ||
| SeLECTS | Childhood epilepsy with centrotemporal spikes, (benign) Rolandic epilepsy, (benign) epilepsy with centrotemporal spikes | CAEa | Pyknolepsy, petit mal | EMAtS | Doose syndrome | ||
| SeLEAS | Panayiotopoulos syndrome, early onset (benign) occipital epilepsy | EEM | Jeavons syndrome | LGS | No changes | ||
| COVE | Late onset (benign) occipital epilepsy or idiopathic childhood occipital epilepsy–Gastaut type | EMA | Bureau and Tassinari syndrome |
DEE-SWAS EE-SWAS Landau–Kleffner syndrome (subtype of EE-SWAS) |
Epileptic encephalopathy with continuous spike-and-wave in sleep, atypical (benign) partial epilepsy (pseudo-Lennox syndrome) | ||
| POLE | Idiopathic photosensitive occipital lobe epilepsy | FIRES | AERRPS, DESC | ||||
| HHE | No changes | ||||||
Note
- This table includes identified syndromes of this age group and not all epilepsy types.
- Abbreviations: AERRPS, acute encephalitis with refractory, repetitive partial seizures; CAE, childhood absence epilepsy; COVE, childhood occipital visual epilepsy; DEE, developmental and/or epileptic encephalopathy; DEE-SWAS, developmental epileptic encephalopathy with spike-and-wave activation in sleep; DESC, devastating epileptic encephalopathy in school-aged children; EEM, epilepsy with eyelid myoclonia; EE-SWAS, epileptic encephalopathy with spike-and-wave activation in sleep; EMA, epilepsy with myoclonic absence; FIRES, febrile infection-related epilepsy syndrome; HHE, hemiconvulsion–hemiplegia–epilepsy syndrome; LGS, Lennox–Gastaut syndrome; POLE, photosensitive occipital lobe epilepsy; SeLEAS, self-limited epilepsy with autonomic seizures; SeLECTS, self-limited epilepsy with centrotemporal spikes.
- a CAE is addressed in the paper on idiopathic generalized epilepsies.2
2 METHODOLOGY
The methodology for syndrome definitions is described in “Methodology for Classification and Definition of Epilepsy Syndromes with List of Syndromes: Report of the ILAE Task Force on Nosology and Definitions.”7 A working group consisting of Task Force members with expertise in pediatrics was convened. One member of the group was assigned to draft a template for each proposed syndrome, using data from a literature review through July 2019, the most recent edition of “Epileptic Syndromes of Infancy, Childhood and Adolescence,”8 and current criteria listed on www.epilepsydiagnosis.org, which was circulated to all members. Every draft was discussed at either an on-line or an in-person meeting of Task Force members and modified based on further input and clinical experience of Task Force members, together with additional literature searches.
For each syndrome, mandatory features (must be present for diagnosis) and exclusionary features (must be absent for diagnosis) were proposed, along with alerts (features that are absent in the vast majority of cases, but rarely can be seen). Alerts should lead to caution in diagnosing the syndrome and consideration of other conditions. A Delphi process was then undertaken, surveying all Task Force members, in addition to recognized external experts in pediatric epilepsy, from all International League Against Epilepsy (ILAE) regions (Europe, Oceania/Asia, North America, Latin America, Africa, and the Eastern Mediterranean region), to reach consensus.
For each syndrome, the core diagnostic criteria, along with a summary of other features, are provided. Based on the Delphi process, tables with the mandatory and exclusionary criteria and alerts for each syndrome are provided at the end of the article.
Proposed syndromes are subdivided into (1) SeLFEs of childhood, (2) genetic generalized epilepsies, and (3) developmental and/or epileptic encephalopathies of childhood.
3 SELF-LIMITED FOCAL EPILEPSIES OF CHILDHOOD
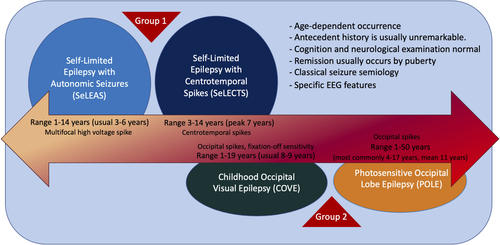
Focal epilepsies with onset during childhood are often self-limited and usually of unknown cause.9, 10 Many self-limited childhood focal epilepsies have a characteristic electroclinical presentation and fall within one of the SeLFE syndromes (Figure 1). These conditions have been referred to in the past as “benign” or “idiopathic.” The term “benign” is no longer recommended, as it fails to acknowledge the comorbidities present in some individuals. The term “idiopathic epilepsy” is now restricted to describing the four syndromes termed the idiopathic generalized epilepsies. Given the typical evolution of these conditions, with age-dependent onset and remission, it has been proposed to use the term “self-limited” when referring to such epilepsies.11 The Nosology and Definitions Task Force of the ILAE proposes the term “self-limited focal epilepsies of childhood” to encompass this group of epilepsy syndromes.
Presumed genetic factors play an important etiological role, as supported by the higher incidence of a positive family history of epilepsy and age-dependent, focal EEG abnormalities. However, no specific genetic variants have been identified so far. Rarely, genetic variants may be associated with more severe phenotypes of these syndromes (i.e., GRIN2A in SeLECTS evolving to EE-SWAS).12-15
- Age-dependent occurrence, specific for each syndrome.
- No significant structural lesion of the brain.
- Birth, neonatal, and antecedent history is usually unremarkable.
- Cognition and neurological examination are typically normal.
- Remission usually occurs by puberty.
- Pharmacoresponsiveness if treated.
- Genetic predisposition for the EEG trait.
- Classic seizure semiology for each syndrome. Seizures are focal motor or sensory with or without impaired awareness and may evolve to bilateral tonic–clonic seizures.
- Specific EEG features: epileptiform abnormalities with distinctive morphology and location (depending on the epilepsy syndrome), often activated with sleep. The EEG has a normal background.
In most cases, children with SeLFEs have features characteristic of one specific syndrome. However, some have a mixed picture, or may evolve from one syndrome to another over time.18 Furthermore, rare cases also show overlap with the IGEs.3, 4
Within the SeLFEs, we recognize two levels of syndromes, based on the long-term prognosis.
- Self-limited epilepsy with centrotemporal spikes (SeLECTS; formerly called childhood epilepsy with centrotemporal spikes, benign epilepsy of childhood with centrotemporal spikes, or benign Rolandic epilepsy).
- Self-limited epilepsy with autonomic seizures (SeLEAS; formerly called Panayiotopoulos syndrome or early onset benign occipital epilepsy).
- Childhood occipital visual epilepsy (COVE; formerly called late onset benign occipital epilepsy, Gastaut syndrome, or idiopathic childhood occipital epilepsy–Gastaut type; rare cases may begin around puberty/adolescence).
- Photosensitive occipital lobe epilepsy (POLE; formerly called idiopathic photosensitive occipital lobe epilepsy).
In the first group, remission in both SeLECTS and SeLEAS is expected in all cases by adolescence, and if treatment is started it should not be continued beyond that age.
In COVE and POLE, the remission is highly likely; however, a few patients may experience a persistence of seizures after adolescence. Chronic treatment with antiseizure medications (ASMs) is often prescribed. In most cases, ASMs can be successfully discontinued without seizure recurrence; however, rare cases may require a longer duration of ASM treatment.
All the above nomenclature changes were carefully evaluated by our working group. The main goal was to have a uniform classification and terminology for the self-limited childhood focal epilepsy syndromes. Our aim was to improve diagnosis and management of these epilepsy syndromes, for both counseling and treatment purposes.
3.1 Self-limited epilepsy with centrotemporal spikes
SeLECTS is a self-limited epilepsy syndrome, formerly known as benign Rolandic epilepsy or benign epilepsy with centrotemporal spikes, which begins in children in their early school years (Table 2).19 Seizures are often brief, and typically involve focal clonic or tonic activity of the throat/tongue and one side of the lower face, which may then evolve to a focal to bilateral tonic–clonic seizure. This epilepsy syndrome occurs in children who are otherwise neurologically and cognitively normal, and imaging studies, if done, show no causal lesion. The EEG shows a normal background with high-amplitude centrotemporal sharp-and-slow-wave complexes, which are activated in drowsiness and sleep.20 Seizures cease by puberty. The finding of a positive family history and focal EEG abnormalities in family members supports underlying genetic factors contributing to the etiology of SeLECTS.21, 22
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures |
Focal seizures with dysarthria, sialorrhea, dysphasia, and unilateral clonic or tonic–clonic movement of mouth in wakefulness or sleep and/or nocturnal focal to bilateral tonic–clonic seizures in sleep only If seizures occur during sleep, they are seen within 1 h of falling asleep or 1–2 h prior to awakening |
Focal motor or generalized convulsive status epilepticus >30 min Usual seizure frequency more than daily Daytime seizures only |
Generalized tonic–clonic seizures during wakefulness Atypical absences Seizures with gustatory hallucinations, fear, and autonomic features |
| EEG | High-amplitude, centrotemporal biphasic epileptiform abnormalities |
Sustained focal slowing not limited to the postictal phase Persistently unilateral centrotemporal abnormalities on serial EEGs Lack of sleep activation of centrotemporal abnormalities |
|
| Age at onset | >12 years | <3 years or >14 years | |
| Development at onset | Moderate to profound intellectual disability | Neurocognitive regression with a continuous spike-and-wave pattern in sleep (suggests EE-SWAS) | |
| Neurological exam | Hemiparesis or focal neurological findings, other than Todd paresis | ||
| Imaging | Causal lesion on brain MRI | ||
| Course of illness |
Remission by mid to late adolescence No developmental regression |
Neurocognitive regression with a continuous spike-and-wave pattern in sleep suggests evolution to EE-SWAS | |
|
An MRI is not required for diagnosis but should be strongly considered in cases with alerts. An ictal EEG is not required for diagnosis. |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, SeLECTs can be diagnosed without EEG and MRI in children without alerts who meet all other mandatory and exclusionary criteria. | |||
Note
- Alert criteria are absent in the vast majority of cases, but rarely can be seen. Their presence should result in caution in diagnosing the syndrome and consideration of other conditions.
- Abbreviations: EEG, electroencephalogram; EE-SWAS, epileptic encephalopathy with spike-and-wave activation in sleep; MRI, magnetic resonance imaging; SeLECTs, self-limited epilepsy with centrotemporal spikes.
3.1.1 Epidemiology
SeLECTS is the most frequent SeLFE and accounts for approximately 6%–7% of all childhood epilepsies.5, 23 Its incidence is approximately 6.1 per 100 000 children aged <16 years per year.24, 25
3.1.2 Clinical context
The age at onset is usually between 4 and 10 years (range = 3–14 years) in 90% of patients, with a peak at approximately 7 years.26 Both sexes are affected, with a slight male predominance (60%).25, 27, 28
Antecedent, birth, and neonatal history is typically normal. A history of febrile seizures is seen in 5%–15% of cases. Rarely, a history of SeLEAS may be present.29 Development, cognition, neurological examination, and head size prior to seizure onset are typically normal. SeLECTS may be seen in children with a history of prior neurological injury or intellectual disability, but these features are considered coincidental and not causal. Prior to epilepsy onset, attention-deficit/hyperactivity disorder and specific cognitive function deficits, mainly related to language and executive function, may be seen.30
3.1.3 Course of illness
Seizures usually resolve by puberty but can occasionally continue until 18 years of age.31 While the epilepsy is active, behavioral and neuropsychological deficits may rarely emerge or worsen, particularly in language and executive functioning.32, 33 These deficits often improve or resolve with age.34 The social outcome in adults is very good.35 Seizures typically respond well to ASM. The prognosis for seizure remission is excellent even for those whose seizures are initially difficult to control.36
3.1.4 Seizures
Focal seizures with characteristic frontoparietal opercular features and/or nocturnal bilateral tonic–clonic seizures are mandatory for diagnosis. Seizures are brief, typically <2–3 min, usually few in number (most children have fewer than 10 lifetime seizures), and may occur sporadically, with frequent seizures seen over a few days or weeks and then several months passing before the next seizure.
Characteristic semiology of the focal seizures includes (1) somatosensory symptoms, with unilateral numbness or paresthesia of the tongue, lips, gums, and inner cheek27; (ii) orofacial motor signs, specifically tonic or clonic contraction of one side of the face, mouth, and tongue, then involving one side of the face; (iii) speech arrest—children have difficulty or are unable to speak (dysarthria or anarthria) but can understand language; and (iv) sialorrhea, a characteristic ictal symptom—it is unclear whether this is due to increased salivation, swallowing disturbance, or both. In some cases, focal seizures in sleep evolve rapidly to tonic–clonic activity of the ipsilateral upper limb, to an ipsilateral hemiclonic seizure, or to a focal to bilateral tonic–clonic seizure. Todd paresis may occur postictally. In nocturnal seizures, the initial focal component may often not be witnessed.
Seizures occur during sleep in 80%–90% of patients and only while awake in <20% of children.37 In seizures associated with SeLECTS, cognitive (e.g., gustatory hallucinations), emotional (e.g., fear), and autonomic features are not seen. Moreover, focal motor or focal to bilateral tonic–clonic status epilepticus, defined as seizure persisting for >30 min, is rare37 and, if present, should lead to review of the diagnosis. The occurrence of atypical absence seizures, focal atonic seizures, and focal motor seizures with negative myoclonus with loss of balance and falls should suggest evolution to EE-SWAS, and evidence of cognitive impairment or regression should be sought.
Generalized tonic–clonic seizures, as distinct from focal to bilateral tonic–clonic seizures, during wakefulness are exclusionary, but may be difficult to differentiate clinically.
3.1.5 Electroencephalogram
Background activity is typically normal, with the presence of normal sleep architecture. If sustained focal slowing without centrotemporal spikes or diffuse slowing is recorded, another epilepsy syndrome or a structural lesion should be considered, and brain imaging is recommended.
High-amplitude (>200 μV, peak to trough),38 centrotemporal sharp-and-slow-wave complexes that activate in drowsiness and sleep are mandatory for diagnosis. They are triphasic, high-voltage (100–300 µV) sharp waves (initial low-amplitude positivity, then high-amplitude negativity followed again by low-amplitude positivity), with a transverse dipole (frontal positivity, temporoparietal negativity), often followed by a high-voltage slow wave.37, 39 The abnormalities may be isolated or occur in trains of doublets and triplets, and focal, rhythmic, slow activity is occasionally observed in the same region as the spikes. The abnormalities may be unilateral or bilateral and independent (Figure 2A). There may be abnormalities seen outside the centrotemporal region (midline, parietal, frontal, occipital). If a continuous spike-and-wave pattern is present in sleep, the child should be evaluated for progressive language or cognitive impairment or regression. This EEG pattern should only lead to a diagnosis of EE-SWAS if developmental plateauing or regression is present.21, 40
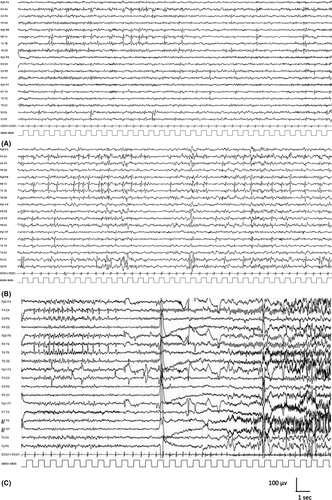
A marked increase in the frequency of epileptiform activity in drowsiness and sleep is typical. The EEG pattern may also change such that sharp waves or spike-and-wave complexes have a broader field and become bilaterally synchronous (Figure 2B). In 10%–20% of children, centrotemporal sharp waves or spike-and-wave complexes may be activated by sensory stimulation of the fingers or toes.41
Seizures are typically infrequent; it is rare to obtain an ictal recording, and there are few published reports in the literature.42 Seizures may be accompanied by a brief decrease in amplitude of the background EEG, followed by diffuse sharp wave abnormalities of increasing amplitude, predominantly in one centrotemporal region,42 followed by high-amplitude slowing and then a return to the usual interictal EEG (Figure 2C). With focal to bilateral tonic–clonic seizures, ictal rhythms may become bilaterally synchronous (as opposed to generalized) sharp wave or spike-and-wave activity.43-45
3.1.6 Imaging
Neuroimaging is normal or may show nonspecific findings. If the electroclinical diagnosis of SeLECTS is made and there are no atypical features, neuroimaging is not required. If there are clinical, developmental, or EEG features or evolution inconsistent with this diagnosis, neuroimaging should be considered. Nonspecific MRI findings, such as hippocampal asymmetry, white matter abnormalities, and enlargement of the lateral ventricles, should not exclude a diagnosis of SeLECTS.46 Patients with focal epilepsy due to structural abnormalities such as focal cortical dysplasia, heterotopia, or low-grade brain tumors may mimic SeLECTS but usually show atypical features such as unilateral EEG abnormality or drug resistance.
3.1.7 Genetics
Genetic factors play an important etiological role, as supported by the higher incidence of a positive family history for epilepsy or febrile seizures, and age-dependent, focal EEG abnormalities in the relatives of SeLECTS patients. Siblings may show the EEG trait of centrotemporal abnormalities in an age-dependent, autosomal dominant fashion without clinical seizures.22 However, the clinical epilepsy syndrome is likely complex in inheritance, as pedigrees with multiple individuals with SeLECTS are very rare.42 At this time, there are no identified pathogenic gene variants found in most children with SeLECTS. Heterozygous pathogenic variants in GRIN2A can be found in individuals with SeLECTS, who may show evolution to EE-SWAS, with associated language and cognitive impairment.13-15 Also, copy number variants have been detected in rare cases.47 Other genetic etiologies such as fragile X syndrome (FraX) should be considered.
3.1.8 Differential diagnosis
- DEE-SWAS or EE-SWAS: Patients with DEE-SWAS may present with similar seizures but can be distinguished by cognitive and language regression. Children with SeLECTS may rarely evolve to this syndrome.
- Focal seizures due to structural brain abnormality.
- Other SeLFEs: The morphology of the EEG abnormalities in the various SeLFEs may overlap, and their seizure localization may change with age. If patients present with prolonged focal nonmotor seizures with prominent autonomic features, especially ictal vomiting, SeLEAS should be considered.
- FraX should be excluded in males with intellectual impairment, as EEG changes in FraX may mimic those seen in SeLECTS.48, 49 In FraX, seizures are most commonly focal impaired awareness seizures, and less likely focal motor without impaired awareness or focal to bilateral tonic–clonic seizures.
3.2 Self-limited epilepsy with autonomic seizures
SeLEAS, formerly known as Panayiotopoulos syndrome or early onset benign occipital epilepsy, is characterized by the onset in early childhood of focal autonomic seizures that are often prolonged. EEG shows high-amplitude (>200 μV, peak to trough)38 focal spikes with variable localization that are typically activated by sleep. Seizures are infrequent in most patients, with 25% only having a single seizure. The epilepsy is self-limited, with remission typically within a few years from onset.50 The mean duration of the disease is approximately 3 years (Table 3).51
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures |
Focal autonomic seizures, with or without impaired awareness Autonomic symptoms often involve prominent retching and vomiting, but may also include malaise, pallor, flushing, abdominal pain, and pupillary or cardiorespiratory changes |
Seizure frequency greater than monthly | |
| EEG | High-amplitude, focal or multifocal epileptiform abnormalities that increase in drowsiness and sleep |
Sustained focal slowing not limited to the postictal phase Unilateral focal abnormalities in a consistent focal area across serial EEGs |
|
| Age at onset | <3 years or >8 years | <1 year or >14 years | |
| Development at onset | Moderate to profound intellectual disability | Neurocognitive regression with a continuous spike-and-wave pattern in sleep (suggests EE-SWAS) | |
| Neurological exam | Hemiparesis or focal neurological findings, other than Todd paresis | ||
| Imaging | Causal lesion on brain MRI | ||
| Course of illness |
Remission by early to mid adolescence No developmental regression |
Neurocognitive regression with a continuous spike-and-wave pattern in sleep suggests evolution to EE-SWAS | |
|
An MRI is not mandatory for diagnosis but should be done in the presence of any alerts. An ictal EEG is not required for diagnosis. |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, at a minimum, an interictal EEG is required to confidently diagnose this syndrome. | |||
Note
- Alert criteria are absent in the vast majority of cases, but rarely can be seen. Their presence should result in caution in diagnosing the syndrome and consideration of other conditions.
- Abbreviations: EEG, electroencephalogram; EE-SWAS, epileptic encephalopathy with spike-and-wave activation in sleep; MRI, magnetic resonance imaging.
3.2.1 Epidemiology
The prevalence of SeLEAS depends on the age range studied. It accounts for 5% of childhood epilepsies between 1 and 14 years51 and 13% of childhood epilepsies between 3 and 6 years.52 SeLEAS is the most common cause of afebrile nonconvulsive status epilepticus in childhood.53
3.2.2 Clinical context
The usual age at onset is between 3 and 6 years (70% of cases), and ranges from 1 to 14 years.54 Both sexes are affected equally. Antecedent and birth history is normal. A history of febrile seizures is seen in 5%–17% of patients. Head size and neurological examination are normal. Development and cognition are normal.51, 55, 56
3.2.3 Course of illness
Seizure frequency is typically low, with approximately 25% of children having a single seizure only, and the majority having fewer than five seizures in total.57 Seizures typically remit within 1–2 years, with normal neurodevelopment, although approximately 20% of patients may evolve to other SeLFEs, most commonly SeLECTS.57 Rarely, SeLEAS may evolve to EE-SWAS.
3.2.4 Seizures
Focal autonomic seizures, with or without impaired awareness, are mandatory for diagnosis. Autonomic features at onset may vary, but most frequently include retching, pallor, flushing, nausea, malaise, or abdominal pain. Vomiting, the most common autonomic manifestation, occurs in approximately 75% of children and leads to misdiagnosis of acute gastroenteritis or migraine. Additional autonomic features include pupillary (e.g., mydriasis), temperature, and cardiorespiratory (breathing, pallor, cyanosis, and heart rate) changes. Syncope may rarely occur. Seizures frequently evolve with eye and/or head deviation, generalized hypotonia, and focal clonic (hemiclonic) or focal to bilateral tonic–clonic seizure activity. Awareness is usually preserved at seizure onset and may fluctuate in degree of impairment as the seizure progresses. More than 70% of seizures occur from sleep. Seizures are often prolonged and can last longer than 30 min.17
3.2.5 Electroencephalogram
The background activity is normal. If persistent focal slowing is present, a structural brain abnormality should be sought as an alternative etiology. Diffuse slowing is not seen except in the postictal period.
Multifocal, high-voltage sharp waves or spike-and-wave complexes are typically seen, often over the posterior regions at disease onset. Abnormalities may show marked variability in terms of localization in sequential EEGs, and the predominance of abnormalities might move to either centrotemporal region or frontopolar region. Generalized abnormalities may also be seen.29 EEG abnormalities are activated both by sleep deprivation and by sleep, when abnormalities often have a wider field and may be bilaterally synchronous (Figure S1A,B). Eye closure (elimination of central vision and fixation-off sensitivity) typically activates posterior abnormalities, but this finding is not pathognomonic of this syndrome.
If seizures are recorded, ictal onset varies, but most have posterior onset. The ictal pattern shows rhythmic slow activity intermixed with small spikes and/or fast activity (Figure S1C).58
3.2.6 Imaging
Neuroimaging, if performed, shows no causal lesion. MRI should be considered in cases with recurrent seizures or atypical presentations. Nonspecific MRI findings should not exclude a diagnosis of SeLEAS.
3.2.7 Genetics
SeLEAS is probably genetically determined; however, no causative gene variants have been detected so far. There is a higher prevalence of febrile seizures in first-degree relatives and case reports of siblings with other SeLFEs.18, 51 There is no clear indication to perform genetic testing in most patients; however, rare cases with SCN1A pathogenic variants have been reported.59-61
3.2.8 Differential diagnosis
- Focal seizures due to structural brain abnormalities. Temporal lobe epilepsy in early childhood and structural occipital epilepsies may present with ictal vomiting.
- SeLECTS should be diagnosed if seizures have prominent frontoparietal–opercular features.
- COVE is distinguished by prominent visual symptoms, as opposed to autonomic features.
- Familial focal epilepsy with variable foci. Different focal epilepsies occur in other family members, but SeLEAS is not usually seen.
- Migraine-associated disorders such as benign paroxysmal vertigo.
- Syncope.
- Other medical disorders associated with intermittent vomiting.
3.3 Childhood occipital visual epilepsy
COVE syndrome, formerly known as late onset benign occipital epilepsy, Gastaut syndrome, or idiopathic childhood occipital epilepsy–Gastaut type, begins in later childhood and is self-limited in the majority of patients. This syndrome occurs in developmentally normal children, with frequent, brief seizures during wakefulness, with visual phenomena without altered awareness, which are often followed by headaches with migrainous features. Seizures may be controlled, and remission of seizures often, but not invariably, occurs within 2–7 years from onset (Table 4).62
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures |
Focal sensory visual seizures with elementary visual phenomena (multicolored circles), with or without impaired awareness, and with or without motor signs (deviation of the eyes or turning of the head) Seizures arise predominantly or exclusively from wakefulness |
Prolonged seizure lasting >15 min GTCS during wakefulness |
Drop (tonic or atonic) seizures Atypical absences Progressive myoclonus |
| EEG | Occipital spikes or spikes-and-wave abnormalities (awake or sleep) | Sustained focal slowing not limited to the postictal phase | |
| Age at onset | <6 years >14 years | <1 year or >19 years | |
| Development at onset | Intellectual disability | Neurocognitive regression | |
| Neurological exam | Any significant neurological examination abnormality | Persistent visual field deficit | |
| Imaging |
Causal lesion on brain MRI Cerebral occipital lobe calcifications |
||
| Course of illness |
Neurocognitive regression Development of myoclonic seizures, ataxia, spasticity |
||
|
An MRI is required for diagnosis to exclude a causal lesion. An ictal EEG is not required for diagnosis. |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, at a minimum, an interictal EEG and MRI are required to confidently diagnose this syndrome. | |||
Note
- Alert criteria are absent in the vast majority of cases, but rarely can be seen. Their presence should result in caution in diagnosing the syndrome and consideration of other conditions.
- Abbreviations: EEG, electroencephalogram; GTCS, generalized tonic–clonic seizures; MRI, magnetic resonance imaging.
3.3.1 Epidemiology
COVE has a prevalence of 0.3% of children with newly diagnosed, afebrile seizures.26
3.3.2 Clinical context
Age at onset is typically at 8–9 years, with a range from 1 year to 19 years.63 Both sexes are equally affected. Antecedent and birth history is normal. Patients have normal development and cognition, although mild cognitive impairment has been described. Head size and neurological examination are normal.64
3.3.3 Course of illness
Remission occurs in 50%–80% of patients by puberty with or without administration of ASM.65, 66 Seizures are often responsive to ASM. Remission is more likely in the 90% of patients who only have focal seizures.64 Occurrence of bilateral tonic–clonic seizures is associated with a lower rate of remission. Development usually remains normal.
3.3.4 Seizures
Focal sensory visual seizures during wakefulness are mandatory for diagnosis. They have abrupt onset, are brief (typically seconds, most lasting <3 min, rarely up to 20 min), and frequent without treatment. Typically, elementary visual phenomena occur, described as small multicolored circles seen in the peripheral vision, increasingly involving more of the visual field and moving horizontally across to the other side. This may be followed by deviation of the eyes or turning of the head (to the side ipsilateral to the hemisphere of seizure onset).67
Other features consistent with occipital lobe onset may occur, including ictal blindness, complex visual hallucinations or illusions (such as palinopsia, micropsia, metamorphopsia), orbital pain, eyelid fluttering, or repeated eye closure.68, 69 The seizure may spread outside the occipital lobe, resulting in hemiparesthesia, impaired awareness (14%), and hemiclonic (43%) or focal to bilateral tonic–clonic (13%) seizure.63 Typical absence seizures may rarely occur in some patients after onset of the focal sensory seizures.70
There may be ictal or postictal headache, nausea, or vomiting. Postictal headache with migrainelike features is common (in 50% of patients) and may be associated with nausea and vomiting.
3.3.5 Electroencephalogram
The background activity is normal. Interictal occipital sharp waves or spike-and-wave complexes are typically seen but may only occur in sleep. Centrotemporal, frontal, or generalized abnormalities are also present in 20% of cases.71 Fixation-off sensitivity (facilitation of epileptiform abnormalities with elimination of central vision) is seen in 20%–90% of patients but is not pathognomonic of this syndrome.63, 66, 72 EEG abnormalities are enhanced by sleep deprivation and sleep (Figure S2A,B). COVE may rarely evolve to EE-SWAS; therefore, if cognitive regression occurs, a sleep EEG should be performed.
At ictal onset, there is a decrease in the usual background occipital spikes or spike-and-wave complexes with the sudden appearance of unilateral occipital fast rhythms with spikes of low amplitude. There may be slower spike-and-wave abnormalities during oculoclonic seizures or ictal blindness (Figure S2C).68, 69
3.3.6 Imaging
Neuroimaging is normal. Brain MRI is required to exclude a structural brain abnormality.73
3.3.7 Genetics
Genetic testing is not required, as there are no genes identified for this epilepsy syndrome. It is presumed that the etiology is genetic, and likely complex/polygenic in inheritance.18 A family history of febrile seizures or epilepsy occurs in up to one third of cases, and a family history of migraine is reported in 9%–16% of cases.63, 66
3.3.8 Differential diagnosis
- Focal seizures due to a structural brain abnormality.
- Celiac disease, epilepsy, and cerebral calcification syndrome is distinguished by occipital lobe calcification, best seen on brain computerized tomography scan.
- Mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes (MELAS).
- Lafora disease is distinguished by the presence of regression, prominent myoclonus, progressive ataxia, and spasticity.
- Migraine with visual aura can be distinguished by the more gradual development and longer duration of the aura, and the character of the visual phenomena (linear, zig-zag, or fortification spectral phenomena as opposed to colored circles or light flashes that change in size or move horizontally).
- Posterior reversible encephalopathy syndrome presents with acute symptomatic seizures, which resolve with control of hypertension.
3.4 Photosensitive occipital lobe epilepsy
POLE is a rare epilepsy syndrome that has onset in childhood and adolescence and is characterized by the presence of photic-induced, focal seizures involving the occipital lobe in individuals with normal development, neurological examination, and intellect (Table 5). At seizure onset, the patient experiences a visual aura with involuntary head version with intact awareness. Prognosis is variable.
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures |
Focal sensory visual seizures (see text), which may evolve to bilateral tonic–clonic seizures Seizures are triggered by photic stimuli, such as flickering sunlight |
Prolonged seizures lasting >15 min |
Eyelid myoclonia Progressive myoclonus |
| EEG | Occipital epileptiform abnormalities facilitated by eye closure and IPS |
Sustained focal slowing not limited to the postictal phase Photoparoxysmal response at slow photic frequency (1–2 Hz; suggest CLN2 disease) |
|
| Age at onset | <4 years or >17 years | <1 year or >50 years | |
| Development at onset | Moderate to profound intellectual disability | Neurocognitive regression | |
| Neurological exam | Any significant neurological examination abnormality | Permanent visual field deficit | |
| Imaging | Causal lesion on brain MRI | ||
|
An MRI is required for diagnosis to exclude a causal lesion. An ictal EEG is not required for diagnosis. |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, at a minimum, an EEG and MRI are required to confidently diagnose this syndrome. | |||
Note
- Alert criteria are absent in the vast majority of cases, but rarely can be seen. Their presence should result in caution in diagnosing the syndrome and consideration of other conditions.
- Abbreviations: CLN2, ceroid lipofuscinosis type 2; EEG, electroencephalogram; IPS, intermittent photic stimulation; MRI, magnetic resonance imaging.
3.4.1 Epidemiology
The prevalence of POLE is low. Epidemiological data are limited, but estimates suggest that POLE accounts for 0.7% of childhood epilepsies.74
3.4.2 Clinical context
Age at onset is between 1 and 50 years, but is most commonly between 4 and 17 years (mean = 11 years), although rare cases with adult onset are also reported.75 There is a strong female predominance.74 Antecedent and birth history is unremarkable, and development is normal. Head size and neurological examination are normal.
3.4.3 Course of illness
Prognosis varies; some patients will have only a few seizures, others have seizure remission over time, and others continue to have photic-induced seizures.76
3.4.4 Seizures
Photic-induced, focal sensory visual seizures (induced for example by flickering sunlight) are mandatory for diagnosis and the main seizure type. Young children may find the aura hard to describe but they can sometimes draw a picture of what they see. Visual sensory symptoms include lights, colored spots, formed visual hallucinations, or visual blurring/loss that moves across the visual field. There is associated head and eye version in which the patient feels they are following the visual phenomenon. Seizures can be induced by video games or other photic stimuli, and in the past were often induced by older analog televisions with slower frequency outputs.77
Seizures are typically brief (<3 min), although prolonged seizures may occur. Seizures may progress to a cephalic sensation (including headache), autonomic epigastric sensation or vomiting, and impaired awareness or to a focal to bilateral tonic–clonic seizure.74, 78 Infrequently, seizures can arise from sleep without photic induction. Some patients also have focal sensory visual occipital seizures without visual induction.74 An overlap between this syndrome and the IGEs is well described,79-81 and thus myoclonic, absence, and generalized tonic–clonic seizures may also be seen. The frequency of seizures is variable.
3.4.5 Electroencephalogram
The background EEG is normal. Interictal occipital spikes or spike-and-wave abnormalities may be seen. Generalized spike-and-wave complexes or centrotemporal spikes may coexist. Occipital spike-and-wave or polyspike-and-wave complexes are facilitated by eye closure and intermittent photic stimulation (Figure S3). Generalized spike-and-wave or polyspike-and-wave (with posterior predominance) complexes may also occur with photic stimulation.74 Epileptiform activity is elicited by sleep deprivation and by sleep.
Ictal onset is in the contralateral occipital lobe to the visual field containing the visual sensory phenomena, and to the direction of head and eye deviation.74, 76 Occipital ictal patterns may spread to the ipsilateral temporal lobe or the contralateral occipital lobe.
3.4.6 Imaging
Neuroimaging is normal.
3.4.7 Genetics
A family history is reported in one third of patients.74 A few families with affected members in multiple generations have been reported.79, 82, 83 There is considerable overlap with the IGEs and with SeLECTS.80, 84 No known gene exists.
3.4.8 Differential diagnosis
- Epilepsy with eyelid myoclonia (EEM) is differentiated by the prominent eyelid myoclonia and by the absence of visual hallucinations and head and eye version.
- SeLEAS is differentiated by prominent dry retching/vomiting and other autonomic features that are seen at seizure onset.
- COVE is distinguished by frequent focal sensory seizures with visual symptoms that are not triggered by photic stimuli.
- Focal seizures due to a structural brain abnormality: If present, focal sensory seizures with visual symptoms are not triggered by photic stimuli.
- Ceroid lipofuscinosis type 2 (CLN2) disease presents in younger children (<5 years of age), and the EEG characteristically shows a photoparoxysmal response at low frequencies (1–3 Hz). Children have progressive cognitive regression, ataxia, and visual loss.
- Lafora disease presents with focal sensory visual seizures but is associated with a progressive myoclonic epilepsy with disabling myoclonus, cognitive impairment, and ataxia.
- Migraine with visual aura has visual phenomena that are longer in duration and qualitatively different (linear, zig-zag, or fortification spectral phenomena as opposed to colored circles or light flashes that change in size or move horizontally).
4 GENETIC GENERALIZED EPILEPSY SYNDROMES OF CHILDHOOD
Essentially all generalized epilepsy syndromes that have onset in childhood have a genetic etiology. They are regarded as following complex inheritance, which means they have a polygenic basis, with or without a contribution from environmental factors. Among the genetic generalized epilepsies with onset in childhood, the most common and best delineated is the IGE syndrome of CAE, which is discussed in the IGE paper.2 Recent studies have highlighted that some IGE syndromes may also be due to monogenic disorders such as glucose transporter 1 (GLUT1) deficiency syndrome.85 Genetic etiologies such as Angelman syndrome or 15q inversion-deletion are important causes of the more severe DEEs, and typically arise de novo in the patient.
Other childhood genetic generalized epilepsy syndromes include two distinct syndromes, epilepsy with myoclonic absence (EMA) and epilepsy with eyelid myoclonia (EEM). These syndromes have a more variable prognosis than CAE, with a higher proportion of cases having drug-resistant seizures and more frequent cognitive comorbidities. Although there is often a positive family history of epilepsy, the type of epilepsy in family members may include IGE syndromes as well as genetic epilepsy with febrile seizures plus. Epilepsy with myoclonic-atonic seizures (EMAtS) is also a generalized epilepsy syndrome that is classified under the DEEs, as children typically show developmental stagnation or regression during the period of frequent seizures. See Figure 3.
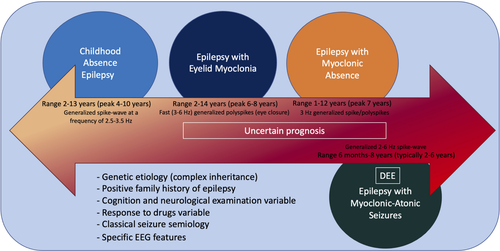
4.1 Epilepsy with eyelid myoclonia
4.1.1 Overview
This syndrome (previously known as Jeavons syndrome) is characterized by the triad of frequent eyelid myoclonia, with or without absences, induced by eye closure and photic stimulation. Eyelid myoclonia is often most prominent on awakening (Table 6).
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Eyelid myoclonia (see text) |
Inability to induce eyelid myoclonia in the office by slow eye closure during exposure to bright light in an untreated patient Myoclonic jerks affecting limbs—strongly consider JME |
Any of the following seizure types:
|
| EEG | Eye closure and intermittent photic stimulation elicits fast (3–6 Hz) generalized polyspikes or polyspike-and-wave complexes |
Focal slowing Consistently unilateral focal spikes Generalized slow spike-and-wave pattern at frequency < 2.5 Hz (unless it is at the end of a higher frequency burst) Diffuse background slowing that is not limited to the postictal period Lack of EEG correlate with typical clinical event |
|
| Age at onset | <2 years or >14 years | ||
| Neurological exam | Focal neurological findings | ||
| Imaging | Potentially relevant abnormal neuroimaging, excluding incidental findings (see text) | Abnormal neuroimaging with causative lesion | |
| Course of illness | Progressive cognitive decline over the course of the epilepsy | ||
|
An MRI is not required for diagnosis. An ictal EEG is not required for diagnosis, provided that eyelid myoclonia has been observed clinically by the diagnosing provider and the interictal study shows fast (3–6 Hz) generalized polyspikes or polyspike-and-wave complexes induced by eye closure or intermittent photic stimulation. However, most untreated patients will have recorded photoparoxysmal response with eyelid myoclonia on a routine EEG performed during intermittent light stimulation. |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, epilepsy with eyelid myoclonia can be diagnosed in persons who meet all other mandatory and exclusionary clinical criteria if they have eyelid myoclonia witnessed by the examiner or captured on home video. | |||
Note
- Alert criteria are absent in the vast majority of cases, but rarely can be seen. Their presence should result in caution in diagnosing the syndrome and consideration of other conditions.
- Abbreviations: EEG, electroencephalogram; JME, juvenile myoclonic epilepsy; MRI, magnetic resonance imaging.
A subgroup of patients with EEM have prominent photic induction of eyelid myoclonia (with or without absence), and absence or myoclonic seizures.86 This subgroup has previously been referred to as “sunflower syndrome,” due to sun-seeking behavior, as they may turn their faces to the sun as a light source at seizure onset.87 This subgroup can be termed “EEM with prominent photic induction.”
4.1.2 Epidemiology
This syndrome is rare, and there are no population-based studies on incidence. Several studies from epilepsy centers have shown that it accounts for 1.2%–2.7% of all epilepsy cases seen.88, 89
4.1.3 Clinical context
The peak age at onset is 6–8 years, with a range of 2–14 years.89-91 There is a 2:1 female:male predominance.89-91 Antecedent and birth history is normal. Development and cognition are often normal, although individuals with borderline intellectual functioning and intellectual disability are seen. In the subgroup with prominent photic induction, approximately half have intellectual disability or attentional problems, which may become more apparent with age.87 Neurological examination is normal.
4.1.4 Course of illness
EEM is often, but not invariably, drug-resistant.92-94 Generalized tonic–clonic seizures are often controlled with ASMs, whereas eyelid myoclonia are not fully controlled. In adult life, eyelid myoclonia alone may not be associated with EEG change, and thus represents a movement disorder.95 EEM is often a life-long condition.93, 94
In the subgroup with prominent photic induction, behavioral management may be important to avoid excessive medication, but is very challenging, particularly in those with intellectual disability. Environmental measures to reduce light exposure are important in these patients, which include wearing wide-brimmed hats and wrap-around sunglasses. Specific blue lenses (Z1) may attenuate the photosensitive response in some patients.96
4.1.5 Seizures
Eyelid myoclonia, consisting of brief, repetitive, and often rhythmic 3–6-Hz myoclonic jerks of the eyelids, with simultaneous upward deviation of the eyeballs and extension of the head, is mandatory for diagnosis. These seizures are very brief (typically <1 to 3 s, always <6 s) and occur multiple times each day, even many times per hour. They are typically induced by involuntary or voluntary slow eye closure or exposure to bright light or sunlight.97 During eyelid myoclonia, awareness may be intact or mildly impaired; impaired awareness may be subtle and not recognized by the patient.
Up to 20% of patients develop eyelid myoclonic status epilepticus, with repetitive, recurrent eyelid myoclonia associated with mildly impaired awareness and responsiveness. Eyelid myoclonia may also be associated with absence seizures, with mildly impaired awareness. In addition, some patients have typical absence seizures without eyelid myoclonia.
Generalized tonic–clonic seizures occur in the majority of cases but are usually infrequent. They may be provoked by sleep deprivation, alcohol, or photic stimulation.
In the patients with prominent photic induction, eyelid myoclonia (with or without absence), absence, or myoclonic seizures are typically associated with behaviors such as facing a light source and hand-waving in front of the eyes, rubbing the forehead, going up close to an analog television, or using other means to create a flickering effect of light.86, 87, 98 Sustained triggering can result in a generalized tonic–clonic seizure.
Febrile seizures occur in 3%–13% patients.92, 99 Patients may also have myoclonic and typical absence seizures even if at relatively lesser frequency than eyelid myoclonias. The presence of frequent limb myoclonus should suggest an alternative syndrome diagnosis. Focal seizures are exclusionary.
4.1.6 Electroencephalogram
The background activity is normal; significant EEG background slowing should suggest an alternative diagnosis. Interictally, brief bursts of fast (3–6 Hz) irregular generalized polyspike-and-wave complexes are frequent. Fixation-off sensitivity, which can be induced by eye closure, and intermittent photic stimulation activate the epileptiform abnormality and often elicit eyelid myoclonia with/without absence seizures (Figure 4).100, 101 Young patients typically show photic sensitivity, which becomes less apparent with age and ASM. Similarly, sensitivity to eye closure may reduce with age. The epileptiform activity is also elicited by hyperventilation.
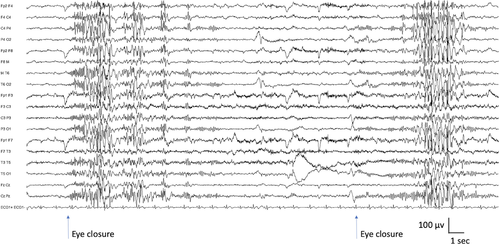
In the subgroup with photic induction, generalized spike-and-wave abnormalities are also provoked by photic induction. In those patients, intermittent photic stimulation may trigger brief eyelid myoclonia, typical absence, or myoclonic seizures.
Bursts of generalized spike-and-wave activity often become briefer and fragmented in sleep. Fragmented generalized spike-and-wave complexes can appear as focal or multifocal spike-and-wave complexes but are not consistently localized to one area. The morphology of the focal spike-and-wave complexes resembles that of the generalized spike-and-wave pattern.
Ictal recordings of eyelid myoclonia show high-amplitude, irregular generalized polyspike or polyspike-and-wave complexes, which may be followed by rhythmic spike or polyspike-and-wave complexes at a frequency of 3–6 Hz. Eyelid myoclonia with/without absence is terminated with complete elimination of light. Eyelid myoclonia may or may not be associated with loss of awareness.
4.1.7 Imaging
An MRI is not required with a typical clinical presentation, but if done, shows no causal abnormality.
4.1.8 Genetics
This syndrome likely has shared genetic etiologies with other idiopathic generalized epilepsies. A family history of seizures or epilepsy is present in 25%–83% of cases, with nearly all affected relatives having generalized seizures.92, 99 In approximately 20% of cases, there is a family history of an IGE—CAE, juvenile absence epilepsy, juvenile myoclonic epilepsy, or generalized tonic–clonic seizure alone—and nearly half the patients have a family history consistent with genetic epilepsy with febrile seizures plus.99
No single pathogenic gene variant is identified in the majority of patients. In patients with this syndrome in the setting of a DEE, several monogenic disease genes have been implicated, including CHD2,102 SYNGAP1103 and NEXMIF104 (formerly known as KIAA2022); some patients with pathogenic variants in these genes have this syndrome without a DEE.
4.1.9 Differential diagnosis
- IGE syndromes with absence seizures (CAE, juvenile absence epilepsy, and juvenile myoclonic epilepsy) may have photosensitivity on EEG; however, prominent eyelid myoclonia is not seen.
- POLE presents with visually induced seizures but without eyelid myoclonia.
- Other early onset epilepsies with myoclonus and photosensitivity,97 including rare monogenic epilepsies such as neuronal ceroid lipofuscinosis.
- Facial tics.
- Compulsive blinking.
4.2 Epilepsy with myoclonic absence
EMA is a very rare childhood epilepsy syndrome that presents with daily myoclonic absence seizures (Table 7).
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Myoclonic absence seizures as predominant type (see text) |
Focal seizures Atonic, myoclonic–atonic, or tonic seizures |
|
| EEG | Regular 3-Hz generalized spike-and-wave pattern time-locked with myoclonic jerks |
Focal slowing Consistently unilateral focal spikes Generalized slow spike-and-wave pattern at frequency < 2 Hz (unless it is at the end of a higher frequency burst) Diffuse background slowing that is not limited to the postictal period |
|
| Age at onset | <1 year or >12 years | ||
| Neurological exam |
Moderate or greater intellectual disability Focal neurological findings |
||
| Imaging | Abnormal neuroimaging with causative lesion | ||
| Course of illness | Progressive cognitive decline over the course of epilepsy | ||
|
An MRI should be considered to exclude other causes. An ictal EEG is not required for diagnosis, provided that myoclonic absences have been observed clinically by the diagnosing provider and the interictal study shows regular 3-Hz generalized spike-and-wave complexes. However, most untreated patients will have recorded myoclonic absence seizure on routine EEG. |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, epilepsy with myoclonic absences can be diagnosed in persons who meet all other mandatory and exclusionary clinical criteria if they have myoclonic absence seizures witnessed by the examiner or captured on home video. | |||
Note
- Alert criteria are absent in the vast majority of cases, but rarely can be seen. Their presence should result in caution in diagnosing the syndrome and consideration of other conditions.
- Abbreviations: EEG, electroencephalogram; MRI, magnetic resonance imaging.
4.2.1 Epidemiology
The exact incidence is unknown. This syndrome accounted for 0.5%–1% of all epilepsies observed in a specialty epilepsy clinic, Saint Paul Center in Marseille.105
4.2.2 Clinical context
Peak age at onset is approximately 7 years, with a range of 1–12 years, and males are more commonly affected (70%).105, 106 The antecedent and birth history is unremarkable; however, at presentation approximately half of patients have developmental impairment. Intellectual disability may become evident with age, and is ultimately seen in 70% of cases.105-107 Neurological examination is typically normal.
4.2.3 Course of illness
The evolution of EMA is variable.105, 106 Remission occurs in approximately 40% of patients. In the remainder, myoclonic absences persist, or the epilepsy may evolve with the development of other generalized seizure types. Prognosis is more favorable if myoclonic absence seizures are the only seizure type and are controlled with medication.105
4.2.4 Seizures
Myoclonic absence seizures are mandatory for diagnosis.105 Absence seizures are associated with rhythmic 3-Hz jerks of the upper limbs, superimposed on tonic abduction of the arms during the seizure (giving a ratcheting appearance). The seizures have an abrupt onset and offset. The patient, if standing, typically bends forward during the seizure, but falling is uncommon. The myoclonic jerks are typically bilateral and symmetric but can be unilateral or asymmetric. Perioral myoclonia and rhythmic jerks of the head and legs may also occur. Seizures last 10–60 s and occur multiple times per day.107 Alertness varies from complete loss of awareness to retained awareness. Occasionally, autonomic manifestations, such as a change in breathing or urinary incontinence105 or complex gestural automatisms, may be seen.108 Myoclonic absences are the only seizure type seen in one third of patients. Myoclonic absence status epilepticus is rare. Generalized tonic–clonic (seen in 45%), clonic, atonic, or typical absence seizures may also occur; multiple seizure types may indicate a more unfavorable prognosis. Only 4% of patients also have typical absence seizures without myoclonic jerks. Focal seizures are exclusionary.
4.2.5 Electroencephalogram
The background activity is normal. Occipital intermittent rhythmic delta activity is typically not seen.105 Interictal 3-Hz generalized spike-and-wave and polyspike-and-wave abnormalities may occur (approximately one third of cases). Focal abnormalities that arise consistently from one region should raise consideration of an alternative diagnosis of a structural etiology.
Generalized spike-and-wave discharges may be provoked by hyperventilation, which may also trigger myoclonic absence seizures. Intermittent photic stimulation triggers generalized spike-and-wave abnormalities in a minority of patients (14%). Generalized spike-and-wave complexes are also activated by sleep deprivation, drowsiness, and sleep. Similar to other generalized epilepsies, generalized spike-and-wave complexes often become fragmented with sleep deprivation or sleep. The pattern may appear as focal or multifocal spike-and-wave complexes but is not consistently seen in a single area. The morphology of the focal abnormalities appears similar to the generalized spike-and-wave activity.
Regular 3-Hz generalized spike-and-wave complexes accompany myoclonic absence seizures. The 3-Hz discharge is time-locked with the myoclonic jerks (Figure 5). The electromyographic (EMG) recording shows that the myoclonic jerks precede the marked tonic contraction of both deltoids.105
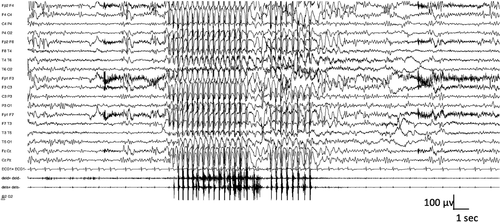
4.2.6 Imaging
An MRI should be considered to exclude other causes, but if done, it should be normal or may show mild diffuse atrophy.
4.2.7 Genetics
A family history (usually of generalized seizures) is present in 20% of cases. Rarely, there is a family history of febrile seizures. Although EMA is considered to be genetic, there are only isolated case reports of specific pathogenic genetic variants,109, 110 with most cases likely to be polygenic. This syndrome is presumed to have shared genetic etiologies with the IGEs.111
4.2.8 Differential diagnosis
- CAE: Although subtle myoclonic jerks may be seen with absences in CAE, they are low amplitude, do not have the sustained rhythmicity, and are not associated with the stepwise (ratcheting) tonic abduction of the arms.
- LGS often has atypical absences with rhythmic jerking or loss of tone; however, the presence of slow spike-and-wave (≤2.5 Hz), generalized paroxysmal fast activity and tonic seizures should suggest the diagnosis.
- Myoclonic absence seizures may rarely be seen in other DEEs but are not the predominant seizure type.110
5 DEES OR EPILEPTIC ENCEPHALOPATHIES WITH ONSET IN CHILDHOOD
“Epileptic encephalopathies” are defined as diseases in which the epileptic activity itself contributes to severe cognitive and behavioral impairments above and beyond that expected from the underlying etiology alone. These disorders are characterized by frequent epileptiform activity associated with developmental slowing and often regression. They may occur on a background of normal or abnormal development.
In the 2017 Classification of the Epilepsies, additional terminology was introduced with the word “developmental” added to denote those children who had abnormal development secondary to the underlying cause in addition to an epileptic encephalopathy.112 This term was introduced because many pathogenic gene variants cause developmental impairment in their own right, with the epileptic encephalopathy superimposed on the pre-existing impairment, further impacting developmental outcome.113
Conversely, a “developmental encephalopathy” refers to developmental impairment without frequent epileptiform activity, such as in a child or adult with intellectual disability.112
Moreover, the task force reviewed the use of the term “developmental encephalopathy” in persons who had fully completed development and agreed to establish a wider term “epilepsy syndromes with progressive neurological deterioration” in addition to developmental encephalopathy. This can be applicable in older individuals with FIRES or Rasmussen syndrome.
In this section, we describe EMAtS, LGS, and DEE-SWAS. We also include two syndromes characterized by acute encephalopathy, followed by a developmental and epileptic encephalopathy, namely, FIRES and HHE.
5.1 Epilepsy with myoclonic atonic seizures
EMAtS, formerly known as Doose syndrome, begins in early childhood, in the setting of normal development in two thirds of cases.114 The full complement of clinical and EEG features may be absent early in the course and take time to appear. These children typically show developmental stagnation or even regression during the active seizures (stormy) phase, which improves once seizures are controlled. See Table 8.
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Myoclonic–atonic seizures | Tonic seizures within 12 months of epilepsy onset |
Epileptic spasms or IESS prior to diagnosis Focal seizures |
| EEG | Generalized 2–6-Hz spike-wave or polyspike-and-wave abnormalities |
Generalized paroxysmal fast activity in sleep Generalized slow spike-and-wave complexes of <2 Hz Photoparoxysmal response at low frequencies (suggests CLN2 disease) |
Persistent focal abnormalities Hypsarrhythmia |
| Age at onset | <6 months or >8 years | ||
| Development at onset | Moderate to severe developmental delay preceding seizure onset | ||
| Neurological exam | Focal neurological findings | ||
| Imaging | Causal lesion on MRI | ||
|
An MRI is not required for diagnosis but is typically done to exclude other causes. An ictal EEG is not required for diagnosis. However, in a child with alerts or with clinical features that may suggest Lennox–Gastaut syndrome or infantile epileptic spasms, a video at least is essential and ideally an ictal EEG should be recorded. Syndrome-in-evolution: Epilepsy with myoclonic atonic seizures should be suspected in the case of explosive onset of multiple generalized seizure types in an appropriately aged child without other alerts or exclusionary features. |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, epilepsy with myoclonic atonic seizures can be presumptively diagnosed without EEG if the clinician has personally witnessed myoclonic atonic seizures, either directly by observing the patient, or on video provided by the family. However, an EEG is strongly recommended. | |||
Note
- Alert criteria are absent in the vast majority of cases, but rarely can be seen. Their presence should result in caution in diagnosing the syndrome and consideration of other conditions.
- Abbreviations: CLN2, ceroid lipofuscinosis type 2; EEG, electroencephalogram; IESS, infantile epileptic spasms syndrome; MRI, magnetic resonance imaging.
5.1.1 Epidemiology
EMAtS has an incidence of approximately 1 in 10 000 children and accounts for approximately 2% of childhood epilepsies.115
5.1.2 Clinical context
EMAtS typically begins at between 2 and 6 years (range = 6 months to 8 years). Boys are more commonly affected.116 Approximately one quarter of children have a history of a febrile seizure,117-120 and such a history is associated with a more favorable long-term outcome.120 Development prior to seizure onset is normal in two thirds of patients, and neurological examination is typically unremarkable at onset. Moderate to severe developmental delay preceding seizure onset should be considered as an alert for diagnosis.121
5.1.3 Course of illness
The onset of EMAtS is often abrupt, with explosive “stormy” onset of many seizures and seizure types often generalized tonic–clonic and myoclonic. In other cases, it evolves more slowly, requiring careful follow-up over the first year to distinguish it from LGS. Seizures often are drug-resistant, particularly during the high seizure frequency (explosive or stormy) phase, and recurrent bouts of nonconvulsive status epilepticus with increased frequency of other generalized seizure types are seen. During this phase, developmental plateauing or even regression, predominantly on behavior and executive functions, and ataxia are often evident. Behavior disorders such as hyperactivity and aggression, and sleep disturbances are also common during the active phase, and typically improve or remit after seizure control is achieved.
Despite seizures being drug-resistant initially, two thirds of children achieve remission, usually within 3 years of onset, and can be weaned off antiseizure therapies.120, 122 In the remaining third, persisting seizures, cognitive impairment, aggression, and hyperactivity are often seen. Once seizures are controlled and the EEG improves, developmental progress is seen. Development may return to premorbid levels of function, or the child may be left with a variable degree of intellectual disability. Factors predictive of poorer outcome include tonic seizures, recurrent nonconvulsive status epilepticus, and an EEG showing very frequent or nearly continuous irregular generalized spike-and-wave, slow spike-and-wave, or generalized paroxysmal fast activity.120, 122-125
5.1.4 Seizures
Myoclonic–atonic seizures are mandatory for diagnosis and are characterized by a brief myoclonic jerk affecting the proximal muscles, often associated with a slight vocalization, followed by a very brief atonic component, which may be subtle, with a head nod, or more prominent, with an abrupt fall (Video S1). Conversely, pure atonic seizures, which are also commonly seen, lack the myoclonic component at onset, and lead to an abrupt but brief loss of axial tone, with head nods or a sudden fall.
Other seizures that are frequently seen include myoclonic (which are brief [<100 ms] and can also lead to falls),126 absence, and generalized tonic–clonic seizures. The latter may occur with or without fever and are the presenting seizure type in approximately two thirds of cases.117, 119, 122
Tonic seizures appear in some patients later in the course and are associated with a poorer long-term outcome.120
Nonconvulsive status epilepticus is also common and may be inaugural. It manifests as impaired awareness, lasting hours to days, with atypical absence, myoclonic, and atonic features, associated with somnolence, unsteadiness, drooling, speech disorders, and erratic myoclonus predominating in the face and upper limbs. Recurrent nonconvulsive status epilepticus is associated with a less favorable outcome.120, 124 Epileptic spasms and focal seizures are exclusionary.
5.1.5 Electroencephalogram
The background activity shows a normal, age-appropriate posterior dominant rhythm at onset. Monomorphic, biparietal theta rhythms are characteristic of EMAtS but are not seen in all patients. With increased seizure frequency, generalized, higher amplitude background slowing may be seen.
Interictal abnormalities comprised of generalized 2–6-Hz spike-and-wave or polyspike-and-wave complexes often occurring in bursts lasting 2–6 s are seen (Figure 6A). Long sequences of generalized irregular spike-and-wave discharges should raise the question of nonconvulsive status epilepticus. The generalized discharges can become fragmented, and a consistent spike focus is not seen. Generalized spike-and-wave abnormalities are activated with sleep. Generalized paroxysmal fast activity, consisting of bursts of diffuse or bilateral fast (10 Hz or more) polyspikes during sleep, is rarely seen and should suggest LGS. Hyperventilation may elicit generalized spike-and-wave discharges and absence seizures. Photosensitivity is rare.
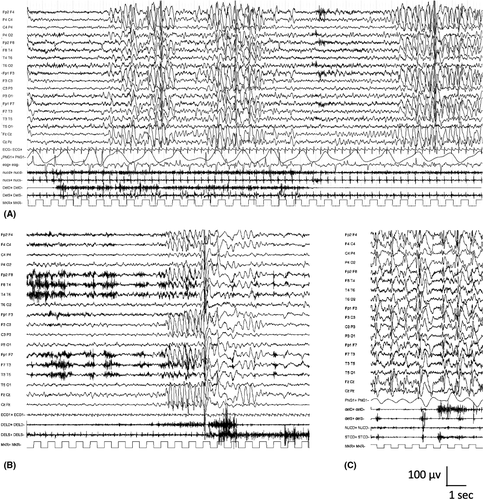
Ictal recordings of myoclonic–atonic seizures show generalized polyspike or spike discharges with the myoclonus, followed by a high-voltage slow wave accompanying the atonic component (Figure 6B,C). Simultaneous recording of EMG with EEG is recommended for ictal recordings; polyspikes correlate with brief myoclonus in the neck muscles, whereas the slow wave correlates with loss of muscle activity in the proximal limb muscles. Absence seizures are associated with 2–6-Hz generalized spike-and-wave complexes.
During nonconvulsive status epilepticus, the EEG shows long runs of high-amplitude, 2–3-Hz irregular, generalized spike-and-wave activity, with background slowing.
5.1.6 Imaging
The MRI is normal.
5.1.7 Genetics
A family history of epilepsy or febrile seizures is found in approximately one third of cases117, 119, 122, 123, 127 and is associated with a more favorable long-term outcome.120 The familial epilepsy syndrome of genetic epilepsy with febrile seizures plus is seen in families of probands with EMAtS.128, 129
In the majority of children, EMAtS has complex inheritance with a polygenic pattern. In some cases, pathogenic variants have been seen in genes including SCN1A,130 SCN1B,131 SCN2A,132 STX1B,133 SLC6A1,134 CHD2,102 SYNGAP1,103 NEXMIF104 KIAA2022.135 Approximately 5% of patients with EMAtS have GLUT1 deficiency associated with pathogenic variants in SLC2A1.85
5.1.8 Differential diagnosis
- LGS can be distinguished by the presence of tonic seizures early in the disease and the EEG, which shows slow spike-and-wave <2.5-Hz and generalized paroxysmal fast activity in sleep. Additionally, children with LGS more commonly have delayed development prior to seizure onset and may have a history of infantile spasms syndrome.
- Myoclonic epilepsy of infancy is distinguished by the lack of myoclonic–atonic and atypical absence seizures, and typically presents earlier than EMAtS.
- Dravet syndrome is distinguished by prolonged, hemiclonic seizures triggered by fever/illness in the first year of life and absence of myoclonic–atonic seizures.
- DEE-SWAS or EE-SWAS is associated with regression and marked activation of epileptiform abnormalities in sleep, with nearly continuous diffuse spike-and-wave complexes; myoclonic–atonic seizures are not seen.
- Subacute sclerosing panencephalitis is a rare condition associated with fulminant/rapid progression of myoclonic seizures, and episodes of falls. The EEG pattern is diagnostic.
- CLN2 disease typically begins in children with normal development or isolated speech delay. Children may present with a phenotype of EMAtS; however, there is progressive motor and cognitive decline and ataxia. The EEG shows a photoparoxysmal response at 1–3 Hz, so low-frequency testing is important.
5.2 Lennox–Gastaut syndrome
LGS is a DEE associated with a wide range of etiologies. It results from high-frequency, synchronized activity in bilaterally distributed brain networks that develops in a susceptible age period in childhood.136 This syndrome is characterized by the presence of (1) multiple types of drug-resistant seizures with onset prior to 18 years (one of which must include tonic); (2) cognitive and often behavioral impairments, which may not be present at seizure onset; and (3) diffuse slow spike-and-wave and generalized paroxysmal fast activity on EEG (Table 9). Many clinicians use the term “LGS” to describe any severe, early onset epilepsy with intractable seizures leading to falls. This approach is incorrect, as it fails to recognize the specific features of LGS, and to distinguish it from EMAtS, which often has a markedly better outcome, and many other severe epilepsies starting in childhood. The full complement of clinical and EEG features is often absent early in the course and takes time to appear. Young children presenting with characteristic seizure types but lacking all the features need close follow-up for evolution to LGS. In particular, a number of severe infantile epilepsy syndromes, such as infantile epileptic spasms syndrome, early infantile DEE, and epilepsy of infancy with migrating focal seizures, often evolve to LGS. Repetitive assessment for LGS criteria may be helpful to access to ASMs licensed for LGS.
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Tonic seizures (see text) In addition to tonic seizures, at least one additional seizure type must be present, which may include any of the following:
|
||
| EEG |
Generalized slow spike-and-wave complexes of <2.5 Hz (or history of this finding on prior EEG) Generalized paroxysmal fast activity in sleep (or history of this finding on prior EEG) |
Photoparoxysmal response at low frequencies (consider CLN2 disease) | Persistent focal abnormalities without generalized spike-and-wave pattern |
| Age at onset | <18 years | >8 years | |
| Long-term outcome |
Drug-resistant epilepsy Mild to profound intellectual disability |
||
|
An MRI is not required for diagnosis but is usually performed to evaluate for underlying etiology. An ictal EEG is not required for diagnosis. However, it should be strongly considered in a child with alerts or with clinical features that may suggest epilepsy with myoclonic atonic seizures syndrome. Syndrome-in-evolution: Approximately 50% of infants with a severe DEE, e.g., IESS or early infantile DEE, evolve over time to Lennox–Gastaut syndrome. |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, at a minimum, an interictal EEG showing characteristic generalized slow spike-and-wave pattern during wakefulness is required for diagnosis. | |||
Note
- Alert criteria are absent in the vast majority of cases, but rarely can be seen. Their presence should result in caution in diagnosing the syndrome and consideration of other conditions.
- Abbreviations: CLN2, ceroid lipofuscinosis type 2; IESS, infantile epileptic spasms syndrome; DEE, developmental and/or epileptic encephalopathy; EEG, electroencephalogram; MRI, magnetic resonance imaging.
5.2.1 Epidemiology
LGS accounts for approximately 1%–2% of all persons with epilepsy. In children, LGS is rarely diagnosed at initial seizure onset (0.6%). LGS often evolves from another severe infantile epilepsy syndrome or etiology, with approximately 20% of cases evolving from infantile epileptic spasms syndrome.137 Ultimately, 3.6% of all children with epilepsy, and 19% of children with seizures starting in infancy, evolve to LGS.138
5.2.2 Clinical context
LGS usually begins between 18 months and 8 years of age, with a peak age at onset of 3–5 years. Onset in the second decade is rare.139 It is slightly more common in males. Abnormalities on neurological examination (for example pyramidal signs) are often found and are related to the underlying etiology. Most children have developmental impairment that predates seizure onset in LGS, but developmental stagnation or decline can occur with onset of frequent seizures. Less commonly, development and behavior may be normal at seizure onset.
5.2.3 Course of illness
LGS persists into adulthood in nearly all cases, and seizures remain drug-resistant.139 Atypical absence and tonic seizures remain frequent in adults, whereas atonic seizures often settle.140
Over time, there is developmental slowing, plateauing, or regression, culminating in moderate to severe intellectual disability in >90% of patients.140-142 Behavior disorders such as hyperactivity, aggression, autism spectrum disorder, and sleep disturbances are common in childhood and adolescence.140, 141
5.2.4 Seizures
Tonic seizures, consisting of a sustained increase in axial and limb muscle contraction lasting from 3 s to 2 min, are mandatory for diagnosis and are most prominent in sleep. They may be subtle, with slow upward eye rolling or deviation, at times with facial grimace or flexor movements of the head and/or trunk, or more clinically obvious, with a brief cry, apnea, abduction, and elevation of the limbs with a vibratory component and bilateral fist clenching. If occurring while the patient is standing, they may forcefully throw the patient off balance, leading to a fall (drop attack), with the patient often sustaining an injury. Tonic seizures may be exacerbated by medications that lead to increased sleepiness, such as acute use of high-dose benzodiazepines.
- Atypical absence seizures: These are often frequent and consist of periods of impaired awareness. They may be challenging to identify with confidence due to their gradual onset and offset in a patient with underlying cognitive impairment.
- Atonic seizures: These lead to an abrupt loss of axial tone, with head nods or a sudden fall (drop attacks), often causing injury. They are frequent, particularly in younger children with LGS. They are typically brief, lasting only one to a few seconds.
- Myoclonic seizures: Myoclonic seizures are also very brief (<100 ms) and may lead to falls (drop attacks). If myoclonic–atonic seizures are present, the diagnosis of EMAtS should be strongly considered.
- Focal impaired awareness seizures: These may remain focal or evolve to bilateral tonic–clonic seizures.
- Generalized tonic–clonic seizures.
- Nonconvulsive status epilepticus: Approximately half to three quarters of patients with LGS have one or more episodes of nonconvulsive status epilepticus, which consist of ongoing atypical absence seizures with altered awareness, with erratic, generalized, or multifocal myoclonic and atonic components, and interspersed clusters of brief tonic seizures.
- Epileptic spasms.
5.2.5 Electroencephalogram
- Generalized slow spike-and-wave: This interictal slow spike-and-wave pattern is characterized by spikes (<70 ms) or sharp waves (70–200 ms), followed by negative high-voltage slow waves (350–400 ms), which are bilaterally synchronous, often anterior predominant, and occur at a frequency of ≤2.5 Hz (Figure 7A). The slow spike-and-wave pattern is abundant and often occurs in runs. It can be associated with atypical absence seizures, but often waxes and wanes without any clinical correlate both in wakefulness and particularly in sleep. Generalized slow spike-and-wave (≤2.5 Hz) complexes are more frequently present in young children, whereas in adolescence and adulthood there is a decrease in the frequency of the spike-and-wave pattern. After the age of 16 years, the majority of patients no longer exhibit the typical slow spike-and-wave pattern.143-145
- Generalized paroxysmal fast activity: This pattern consists of bursts of diffuse or bilateral fast (10 Hz or more) activity often seen during sleep. These typically are brief, lasting a few seconds or less (Figure 7B).
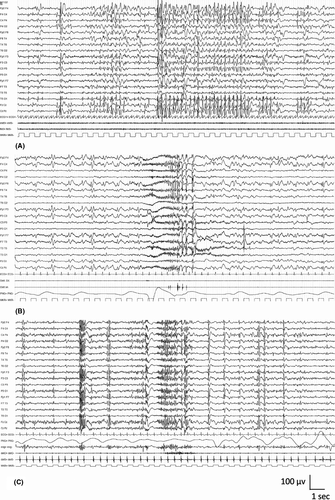
Focal or multifocal slow spike-and-wave pattern may also be seen. Abnormalities are not typically activated by photic stimulation.
Tonic seizures, which are often subtle, and may not be recognized by families, are typically recorded on sleep EEG. The EEG pattern of tonic seizures consists of a burst of bilateral 10-Hz or higher frequency fast activity with a recruiting rhythm—an initial diffuse decrement followed by gradual increase in amplitude (Figure 7C). Polygraphic recordings during tonic seizures often show a brief apnea with electromyographic axial muscle contraction. Because of these characteristic findings, a sleep recording can be beneficial to distinguish LGS from other epilepsy syndromes.
Atypical absence seizures are associated with slow spike-and-wave (<3 Hz) complexes, although it can be challenging to clearly distinguish between ictal and interictal slow spike-and-wave patterns.
5.2.6 Imaging
As structural causes are the most common etiology, MRI at onset is strongly recommended, as this may impact on treatment decision-making.146 A variety of structural etiologies may be found, including focal or diffuse cortical malformations, tuberous sclerosis complex, tumors, or acquired brain injury such as hypoxic–ischemic encephalopathy. Reinvestigation of older patients with LGS can result in identification of structural etiologies missed on previous imaging.147 MRI may also be normal.
5.2.7 Genetics
Pathogenic variants in many genes have been associated with the etiologies that causes LGS and are usually de novo in the child.148, 149 A range of chromosomal abnormalities and copy number variants have been associated with LGS, so chromosomal microarray is essential. A range of next generation sequencing approaches can be taken, ideally with whole exome sequencing, or an epilepsy gene panel, particularly if no etiology is found after clinical examination and MRI. Furthermore, genetic testing should also be considered for patients with structural brain disorders suggestive of an underlying genetic cause.
5.2.8 Metabolic testing
Rarely, LGS can be due to a neurometabolic disorder. Metabolic testing should be considered if an underlying etiology is not found with imaging or genetic studies.
5.2.9 Differential diagnosis
- Infantile Epileptic Spasms syndrome may progress to LGS, and distinction between these syndromes during the transition can be challenging. In distinction to spasms, tonic seizures are typically longer than 3 s and do not occur in clusters on waking.
- EMAtS is distinguished by normal development prior to seizure onset in many cases, myoclonic–atonic seizures, and faster generalized spike-and-wave pattern, which is typically >3 Hz.
- Dravet syndrome is distinguished by prolonged, hemiclonic seizures triggered by seizures in the first year of life; tonic seizures (if present) do not occur until later.
- Other early onset DEEs with multiple seizures types.
- DEE-SWAS or EE-SWAS is associated with regression and marked activation of epileptiform abnormalities in sleep, with nearly continuous diffuse spike-and-wave complexes.
- Ring (20) syndrome is associated with refractory epilepsy, intellectual disability, and behavioral abnormalities; tonic seizures usually appear during sleep, whereas awake patients frequently experience nonconvulsive status epilepticus.
- Frontal lobe epilepsy may present with bilateral tonic seizures, often with asymmetrical features. Slow spike-and-wave and generalized paroxysmal fast activity are not seen.
- Rare metabolic disorders may lead to an LGS phenotype. CLN2 disease typically begins in children with normal development or isolated speech delay. Following onset of seizures, there is progressive motor and cognitive decline and ataxia. The EEG characteristically shows a photoparoxysmal response at 1–3 Hz.
5.3 DEE-SWAS and EE-SWAS
DEE-SWAS and EE-SWAS refer to a spectrum of conditions that are characterized by various combinations of cognitive, language, behavioral, and motor regression associated with marked spike-and-wave activation in sleep. Regression is seen within weeks from the EEG pattern. DEE-SWAS and EE-SWAS share similar clinical features (Table 10) and management implications. They are grouped together because they carry similar implications, and the syndrome highlights the need to inquire about specific clinical features when seeing a child, such as auditory agnosia, global regression of behavior and motor skills, and negative myoclonus. This syndrome is intended to replace syndromes previously named epileptic encephalopathy with continuous spike-and-wave in sleep and atypical benign partial epilepsy (pseudo-Lennox syndrome). Landau–Kleffner syndrome (LKS) is a specific subtype of EE-SWAS, where regression affects mainly language, with an acquired auditory agnosia, and the eponym used to describe this syndrome should be retained (Figure 8).
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Tonic seizures during sleep | Epileptic spasms | |
| EEG |
Slow (1.5–2 Hz) spike-and-wave abnormalities in N-REM sleep Abnormalities are markedly activated in sleep |
Generalized paroxysmal fast activity in sleep (consider Lennox–Gastaut syndrome) Generalized slow spike-and-wave complexes of <2.5 Hz in both awake and asleep states (consider Lennox–Gastaut syndrome) |
|
| Age at onset | >1 and <2 years | <1 year or >12 years | |
| Development at onset | Cognitive, behavioral, or motor regression or plateauing temporally related to SWAS on EEG | ||
| Long-term outcome | Remission of SWAS pattern on EEG by mid adolescence, although EEG often remains abnormal | ||
|
An MRI is not required for diagnosis but is often performed to evaluate for underlying etiology. A sleep EEG is mandatory for diagnosis. |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, this syndrome cannot be presumptively diagnosed without a sleep EEG. | |||
Note
- Alert criteria are absent in the vast majority of cases, but rarely can be seen. Their presence should result in caution in diagnosing the syndrome and consideration of other conditions.
- Abbreviations: EEG, electroencephalogram; MRI, magnetic resonance imaging; N-REM, non-rapid eye movement; SWAS, spike-and-wave activation in sleep.
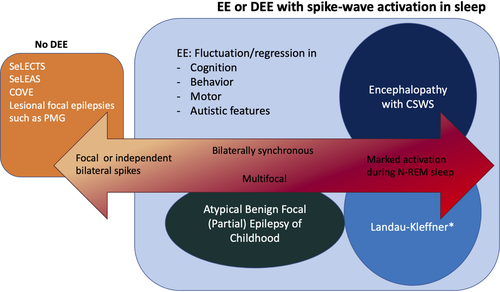
Sleep EEG must be performed to confirm the diagnosis. The EEG pattern associated with EE-SWAS and DEE-SWAS was known as electrical status epilepticus in sleep (ESES).11, 150 Historically, ESES was defined as nearly constant epileptiform activity that occupies >85% of slow wave sleep; however, lower percentages of sleep may also be associated with significant regression in cognitive or behavioral function.
EE-SWAS is now recognized in patients with pre-existing normal development with an activation of slow (1.5–2 Hz) spike-and-wave complexes in non-rapid eye movement (N-REM) sleep.
DEE-SWAS occurs in patients with pre-existing neurodevelopmental disorders and is defined on the basis of a documented persisting worsening of various combinations of cognitive, language, behavioral, and motor functions concomitant with significant activation of spike-and-wave complexes during sleep. Caution is needed not to overdiagnose the syndrome in the absence of clear and persisting regression.
Specific focal epilepsy syndromes, such as SeLECTs and SeLEAS, or other structural focal epilepsies, may evolve to EE-SWAS, either transiently or for a prolonged period.
5.3.1 Epidemiology
DEE-SWAS and EE-SWAS are rare, accounting for 0.5%–0.6% of all epilepsy presentations seen at pediatric tertiary epilepsy centers.151-153
5.3.2 Clinical context
DEE-SWAS and EE-SWAS are characterized by onset of seizures at between 2 and 12 years of age (peak = 4–5 years), with the EEG developing spike-and-wave activation in sleep 1–2 years after seizure onset in association with cognitive/behavioral regression or plateauing. Both sexes are affected equally. Antecedent and birth history are often normal; however, structural brain lesions are a risk factor for DEE-SWAS and EE-SWAS. Specifically, thalamic injury in early life154 and malformations such as bilateral perisylvian polymicrogyria are associated with this syndrome; therefore, neuroimaging, particularly MRI, is recommended whenever possible. Neurological examination and developmental level may be normal or reflect an underlying structural brain abnormality. Regression in cognitive, behavioral, or psychiatric functioning is the cardinal symptom of this syndrome. All cognitive domains can be affected, including language and communication, temporospatial orientation, attention, and social interaction. Motor regression with dyspraxia or dystonic features may also occur.155 Follow-up visits with clinical assessment, EEGs, and neuropsychological testing should be scheduled at baseline and during the follow-up to assess the evolution.156
5.3.3 Course of illness
Clinical seizures typically remit around puberty, even in patients with a structural lesion.157 Resolution of clinical seizures may precede, coincide with, or follow the resolution of the EEG pattern.157 The SWAS pattern on EEG also resolves, typically by adolescence.158, 159 Focal abnormalities may persist during both wakefulness and sleep. Sleep architecture normalizes with resolution of SWAS.158
Neurocognitive and behavioral improvement is typically seen, with resolution of the SWAS on EEG.160 However, many patients have residual impairment, which is severe enough to limit independent functioning in approximately half of the patients.161, 162 The duration and etiology of DEE-SWAS and EE-SWAS are the most important predictors of cognitive outcome; the risk of poor outcome is higher if it is present for >2 years.163 Poorer outcomes are also seen with younger onset of DEE-SWAS.163 Thus, early diagnosis is of paramount importance to enable initiation of treatment to improve long-term outcome even if some causes are not treatable (the etiology prevails), and there may be no clinical improvement for some when the EEG pattern is abolished. Nevertheless, residual deficits may remain following remission of seizures and SWAS, which may occur from months to 7 years after onset.
5.3.4 Seizures
There is no mandatory seizure type. Seizure type is dependent on the underlying etiology. Furthermore, EE-SWAS and DEE-SWAS may occur in patients who do not have clinical seizures.
In most patients, infrequent and drug-responsive seizures are observed during the initial phase at between 2 and 5 years of age. These early seizures are typically focal motor, with or without impaired awareness, and focal to bilateral tonic–clonic seizures. Seizures typically worsen with the evolution of multiple seizure types. These include focal seizures with or without impaired awareness, typical and atypical absence seizures, atonic seizures, and focal motor seizures with negative myoclonus.
5.3.5 Electroencephalogram
The EEG pattern depends on the underlying etiology. The background activity during wakefulness may show focal or diffuse slowing and often contains focal or multifocal abnormalities, but it may be normal (Figure 9A). Epileptiform abnormalities during wakefulness are not continuous. In drowsiness and sleep, there is marked activation of epileptiform activity, with slow (1.5–2 Hz) spike-and-wave complexes in N-REM sleep. Typically, this activity is also seen in Stage II sleep (Figure 9B). SWAS is usually diffuse but may occur more focally (typically frontally) or multifocally. In rapid eye movement sleep, the abnormalities become less frequent or may even be absent. Normal sleep architecture (vertex sharp waves, sleep spindles, and K complexes) is absent or difficult to distinguish. An overnight sleep EEG may be required, as slow wave sleep may not be achieved on an outpatient sleep EEG. The ictal EEG correlates with the seizure type.
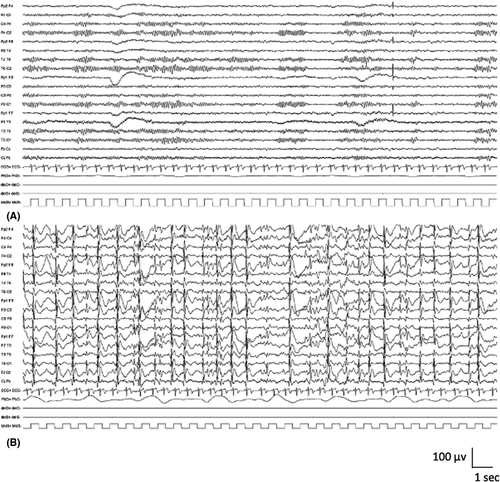
5.3.6 Imaging
Neuroimaging may be normal or demonstrate underlying structural brain abnormalities that may be developmental (e.g., perisylvian polymicrogyria) or acquired (thalamic abnormalities can be observed).
5.3.7 Genetics
Some cases have a genetic basis and may follow monogenic or complex inheritance. A family history of seizures is seen in up to 50% patients with DEE-SWAS or EE-SWAS.164 The major monogenic cause is GRIN2A, which encodes the N-methyl-D-aspartate (NMDA) receptor glutamate receptor alpha 2 subunit.12 Pathogenic variants are associated with a range of severity of DEE-SWAS phenotypes.13-15 These individuals have a characteristic speech pattern, which may persist into adult life.165
5.3.8 Differential diagnosis
- SeLFEs can have marked activation of epileptiform abnormalities in sleep, but there is not temporally related cognitive or behavioral regression with the EEG finding of SWAS.
- Structural focal epilepsies may have abundant focal abnormalities that may activate in sleep, but there is not temporally related cognitive or behavioral regression with the EEG finding of SWAS.
- LGS is distinguished by the EEG, which shows prominent slow spike-and-wave complexes during both wakefulness and sleep, and by the sleep EEG, which shows generalized paroxysmal fast activity, and often tonic seizures are captured.
- Children with autism spectrum disorders with or without intellectual disability but without regression may show activation of epileptiform abnormalities in sleep.
- Cognitive regression due to other etiologies.
5.4 Febrile infection-related epilepsy syndrome
FIRES (previously also known as acute encephalitis with refractory, repetitive partial seizures, or devastating epileptic encephalopathy in school-aged children) is one cause of new onset refractory status epilepticus, which occurs predominantly in children and adolescents (Table 11). A prior febrile infection occurs, starting between 24 h and 2 weeks, prior to an explosive onset of superrefractory status epilepticus; there may or may not be fever at onset of status epilepticus.166 The acute phase, during which the seizure burden is very high, lasts 1–12 weeks,167 and during this phase, mortality and morbidity are significant. This is followed by a chronic phase, where most survivors are left with drug-resistant multifocal epilepsy and a variable degree of intellectual disability or learning difficulties. The cause is not known, but growing evidence suggests a heterogeneous etiology resulting in fulminant non-antibody-mediated neuroinflammation.168, 169
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures |
History of nonspecific febrile illness in the 2 weeks preceding seizure onset Focal and multifocal seizures that often evolve to bilateral tonic–clonic seizures Seizures progress in frequency and severity to culminate in superrefractory status epilepticus typically within 2 weeks of onset |
History of epilepsy prior to onset of symptoms | |
| EEG | Slowing of the background activity with multifocal epileptiform abnormalities and frequent, focal electrographic and electroclinical seizures | Unifocal seizures | |
| Age at onset | <2 years | <1 year or >30 years | |
| Development at onset | Acute encephalopathy with onset of frequent seizures | Intellectual disability prior to seizure onset | |
| Neurological exam | Neurological exam abnormalities prior to onset of seizures | ||
| Imaging | At presentation, MRI shows an epileptogenic lesion concordant with seizure onset (see text) | ||
| Other testing |
Lumbar puncture showing evidence of central nervous system infection Causal antibody on CSF or plasma autoimmune testing Documented metabolic or genetic etiology Documented toxic encephalopathy |
||
| Long-term outcome |
Lack of drug-resistant focal or multifocal epilepsy Lack of learning difficulties or intellectual disability Lack of variable degrees of cerebral atrophy on MRI |
||
|
An MRI is required for diagnosis to exclude a causal lesion. An ictal EEG is required for diagnosis to confirm frequency and multifocality of seizures. |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, this syndrome cannot be presumptively diagnosed without EEG and MRI studies. | |||
Note
- Alert criteria are absent in the vast majority of cases, but rarely can be seen. Their presence should result in caution in diagnosing the syndrome and consideration of other conditions.
- Abbreviations: CSF, cerebrospinal fluid; EEG, electroencephalogram; MRI, magnetic resonance imaging.
5.4.1 Epidemiology
This is a rare syndrome, which is likely underrecognized, with an estimated incidence of 1 per million.170
5.4.2 Clinical context
FIRES occurs most commonly in school-aged children (mean = 8 years) with a typical range of 2–17 years.166, 169, 171 It is exceedingly infrequent under the age of 2 years and can begin at a variable age, but may rarely occur in young adulthood. Both sexes are affected, with a slight male predominance.171 Perinatal history is typically normal. At presentation, children are developmentally normal, without a history of prior neurological disease including epilepsy, and have normal head size.
All children have a history of a prior febrile infection, most commonly upper respiratory or gastrointestinal, between 24 h and 2 weeks before onset of refractory status epilepticus. At the time of seizure onset, patients may still be febrile, or may have had recent resolution of the fever.
At presentation, patients are typically encephalopathic and have frequent seizures despite ASMs. Head size is normal. Persistent focal abnormalities on examination are unusual, but transient Todd paresis may be seen.
5.4.3 Course of illness
The prognosis is variable but often poor.171 Mortality is approximately 10% in the acute phase, due to intensive care complications such as sepsis, or uncontrolled status epilepticus. Following the acute phase, most children are left with drug-resistant, multifocal epilepsy.
Developmentally, in the acute stage, most children regress, and at follow-up, in the chronic phase, the majority are left with varying degrees of intellectual disability.171 Approximately one third of survivors have normal or borderline cognition (often learning disorders), one third have mild to moderate intellectual disability, and one third have severe to profound disability or are vegetative. Poorer outcome was associated with longer duration of medically induced burst-suppression coma and younger age at onset.171 Attention and behavior problems, including aggression, are also common in survivors.
In the chronic phase, many patients will have evidence of motor dysfunction.
5.4.4 Seizures
Focal or multifocal seizures are mandatory for diagnosis and may evolve to bilateral tonic–clonic seizures. Common ictal symptoms are eye deviation and hemifacial twitching. Seizures progress in frequency and severity rapidly to culminate in superrefractory status epilepticus (defined as >24 h) in the acute phase.
5.4.5 Electroencephalogram
The EEG background activity is typically abnormal, with slowing and multifocal abnormalities. Recurrent extreme delta brush, consisting of a paroxysmal beta–delta complex of 15–18-Hz beta superimposed on 1–3-Hz delta in the frontal and central head regions, is often seen (Figure S4A,B).172 This pattern may be modified by anesthetic agents used for the treatment for status epilepticus.
Prolonged video-EEG monitoring at diagnosis shows a gradual increase in seizure burden over the first days to week of illness. Initially, seizure burden may be low, but over time, frequent, multifocal subclinical and clinical seizures are recorded, usually with a frequency of several per hour.172 A typical seizure pattern, consisting of focal activity of >10 Hz of low to moderate amplitude, evolving to well-formed, rhythmic spikes and spike-and-wave complexes, is seen, and ictal activity often shifts from one hemisphere to the other (Figure S4C).172
5.4.6 Neuroimaging
During the acute stage, the MRI is normal in approximately two thirds of cases. Approximately one third may show T2 hyperintense changes in bilateral temporal regions, insula, basal ganglia, and/or thalami, which may be subtle. Leptomeningeal enhancement may also be seen but is not specific to this syndrome.173
During the chronic stage, the MRI usually shows variable degrees of diffuse cerebral atrophy, and/or signal changes over the temporal lobes, cerebral cortex, periventricular white matter, hippocampi, and basal ganglia.173
5.4.7 Genetics
This disorder is not suspected to be genetic, and no causal genes have been identified. There is usually no family history of seizures.
5.4.8 Other laboratory studies
Examination of cerebrospinal fluid (CSF) is required to exclude infection. The CSF is typically normal but may show a mild pleocytosis. CSF protein and lactate are normal. Oligoclonal bands are negative. An immune etiology should be excluded, but in FIRES, no causal antibodies have yet been found.169 Serum and CSF autoimmune panels are negative. It has been reported that Th1 chemokines (CXCL9, CXCL10, etc.) are predominantly upregulated in CSF, independently of interleukin-1 receptor (IL-1R) signaling.174 Metabolic studies are unremarkable. In some cases, excessive neuroinflammation, which may be secondary to functional deficiency in IL-1R antagonist, has been reported.175, 176
5.4.9 Differential diagnosis
- Dravet syndrome is distinguished by its presentation predominantly in the first year of life, and history of intermittent prolonged seizures with interval recovery, as opposed to a singular superrefractory status epilepticus with the development of persistent morbidity.
- PCDH19 clustering epilepsy is distinguished by its presentation in the first 3 years of life, and history of a cluster of seizures usually induced by fever. Superrefractory status epilepticus is unusual.
- Meningitis or encephalitis.
- Specific autoimmune-mediated encephalopathies such as anti-NMDA receptor encephalitis.
- Toxic encephalopathies.
- Metabolic disorders such as mitochondrial disease.
5.5 Hemiconvulsion–hemiplegia–epilepsy syndrome
HHE is a rare consequence of focal motor status epilepticus in infancy and early childhood (Table 12). The initiating event for this syndrome is a focal clonic status epilepticus typically occurring in the context of a febrile illness in children <4 years of age.177 Neuroradiological studies at the time of the status epilepticus show unilateral edematous swelling of the affected hemisphere. The acute phase is followed by hemispheric atrophy with subsequent appearance of focal seizures, which are drug-resistant. The majority of patients have a resultant permanent motor deficit. The etiology and the underlying mechanisms are not understood.
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures |
Diagnosis requires both a history of acute stage and chronic stage disease Acute stage: Episode of febrile, hemiclonic status epilepticus, which is immediately followed by permanent hemiparesis Chronic stage: After a variable time (usually <3 years after initial status epilepticus), unilateral focal motor or focal to bilateral tonic–clonic seizures appear |
Transient hemiparesis (Todd paresis) Unilateral focal motor seizures that progress in a crescendo pattern over months to years, with late development of progressive hemiparesis (consider Rasmussen encephalitis) |
|
| EEG |
Slowing of background activity over the affected hemisphere Focal or multifocal epileptiform abnormalities over the affected hemisphere in the chronic phase |
||
| Age at onset | >4 years | >6 years | |
| Development at onset | Intellectual disability prior to seizure onset | ||
| Neurological exam |
Focal neurological abnormalities prior to initial episode of febrile status epilepticus Facial angioma suggestive of Sturge–Weber syndrome |
||
| Imaging |
MRI immediately following febrile status epilepticus (acute stage) shows diffuse signal change with T2 hyperintensity and restricted diffusion of the subcortical region of the affected hemisphere, often with severe edema Over time (chronic stage), there is atrophy of the affected hemisphere |
Other structural causes predisposing to focal status epilepticus | |
| Other testing | Alternative cause of hemiparesis found such as acute ischemic stroke, intracranial infection, etc. | ||
| Long-term outcome |
Drug-resistant epilepsy Permanent focal motor deficit |
||
|
An MRI is required to diagnosis. An ictal EEG is not required for diagnosis. Syndrome-in-evolution: Children with acute permanent hemiparesis following an episode of focal convulsive febrile status epilepticus, with mandatory MRI findings, but who have not yet progressed to the chronic phase of the disease with recurrent, drug-resistant focal motor or focal to bilateral tonic–clonic seizures should be suspected of having emerging hemiconvulsion–hemiplegia–epilepsy syndrome. |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, hemiconvulsion–hemiplegia–epilepsy syndrome can be presumptively diagnosed without EEG in cases that meet all mandatory and exclusionary clinical criteria without alerts. However, an imaging study (CT or MRI) is required to exclude other causes. | |||
Note
- Alert criteria are absent in the vast majority of cases, but rarely can be seen. Their presence should result in caution in diagnosing the syndrome and consideration of other conditions.
- Abbreviations: CT, computed tomography; EEG, electroencephalogram; MRI, magnetic resonance imaging.
5.5.1 Epidemiology
HHE is a rare syndrome and its incidence has declined markedly in resource-equipped countries over the past 30 years since the instigation of aggressive treatment for prolonged seizures or status epilepticus.178
5.5.2 Clinical context
Age at onset is typically <4 years, and there is no sex predilection.178, 179 Birth and antecedent history are noncontributory, and previous development and neurological examination are normal. Children present with prolonged focal status epilepticus and then develop immediate hemiparesis. The diagnosis of HHE should be considered when a persistent hemiplegia is observed after febrile status epilepticus in a child younger than 4 years. Aphasia may also be present acutely in up to one quarter of cases, if the dominant hemisphere is involved.180
5.5.3 Course of illness
The majority of children are left with a permanent motor deficit. However, this deficit may be minimal or resolve within 12 months in 20%.177 If present, aphasia most commonly resolves within 2 months180 but may persist.181 Subsequent focal seizures appear after a variable duration, with 85% having seizure onset within 3 years of the initial status epilepticus.178 Focal seizures during the chronic phase are typically drug-resistant,179 but may be amenable to surgical treatment, such as hemispherotomy.178, 182 Many children are also left with variable degrees of intellectual disability.178
5.5.4 Seizures
The first seizure is typically focal clonic febrile status epilepticus. The clonic component may be subtle. There is typically a seizure-free period after the focal status epilepticus that can last months to years. After a variable period, focal motor seizures and/or focal to bilateral tonic–clonic seizures appear, and usually become drug-resistant. Seizures may localize solely to the temporal lobe or may arise from extratemporal regions or be multifocal.182
5.5.5 Electroencephalogram
If an EEG is obtained during the acute focal status epilepticus, the ictal discharge is characterized by rhythmic (2–3 Hz) slow waves that are usually bilateral with higher amplitude over the affected hemisphere (Figure S5A,B).178 In addition, over the affected hemisphere, recruiting rhythms (10 Hz) are frequently seen.178 The background activity may be normal at onset, but during the chronic phase, there is excess slowing (often asymmetric) and epileptiform abnormalities, which are most prominent over the affected hemisphere, but may be bilateral.
5.5.6 Imaging
MRI immediately following the status epilepticus demonstrates diffuse hemispheric signal abnormalities with T2 hyperintensity and restricted diffusion, predominantly of the subcortical white matter of the affected hemisphere.183 Edema of the affected hemisphere can be severe, leading to mass effect and possible herniation (Figure S5C–F).178 If magnetic resonance spectroscopy is done, it shows decreased N-acetyl aspartate and mildly increased lactate in the affected hemisphere. On Day 8–15 after status epilepticus, cytotoxic edema decreases, with normalization of apparent diffusion coefficient images and ongoing T2 hyperintensity, with evolving volume loss. Within 1 month, atrophy of the affected cerebral hemisphere is clearly evident. Hippocampal sclerosis is also commonly seen (Figure S5G–J).182
5.5.7 Genetics and other testing
Genetic testing and evaluation for coagulation, metabolic, infective, and immune disorders are typically normal.178, 184
5.5.8 Differential diagnosis
- Dravet syndrome presents in infancy with prolonged hemiclonic seizures in the context of a febrile illness, which may result in transient Todd paresis. However, this deficit resolves, and the typical MRI abnormalities of HHE are not present.
- Sturge–Weber syndrome may present with focal motor status epilepticus, but the skin lesions and MRI showing the typical features of this syndrome should suggest the diagnosis.
- Rasmussen syndrome presents with unilateral focal motor seizures, but the progression is much slower, and focal status epilepticus is seen later in the evolution and is a more persistent feature when it occurs. MRI may be normal at seizure onset or show mild insular atrophy but evolves to focal white matter changes and hemispheric atrophy over months to years.
- Focal febrile status epilepticus or focal status epilepticus due to other etiologies can be followed by Todd paresis, which typically resolves within 24 h.
- Meningitis and encephalitis.
- Hemorrhagic or ischemic stroke.
- Polymerase gamma- or MELAS-related mitochondrial disease.
6 DISCUSSION
Although not every child with epilepsy can be classified as having a specific epilepsy syndrome, identification of a syndrome can provide guidance on management and prognosis. An electroclinical approach, combining a detailed clinical history and an EEG recording, is needed to reach a syndrome diagnosis. Most of the syndromes described above have a mandatory seizure type or types and often mandatory interictal EEG features. A diagnostic hypothesis is crucial to ensure adequate EEG studies, including both wakefulness and sleep, are recorded to inform syndrome diagnosis. A sleep EEG is required to identify mandatory EEG patterns in some syndromes, such as LGS, DEE-SWAS, and EE-SWAS. Moreover, detailed seizure semiology based on history is adequate to diagnose many seizure types without obtaining an ictal recording. However, for seizure types in certain syndromes, an ictal EEG recording is required for diagnosis. For example, it is not easy to determine a specific seizure type for a “drop attack” based on history alone. Even the use of home video recordings, which are undoubtedly helpful in many cases, cannot always confirm a definitive seizure type. Based on the diagnostic hypothesis, a specific EEG type (sleep-deprived, prolonged video-EEG) may be required to confirm a syndrome diagnosis.
In many but not all cases, syndrome identification informs likely etiology. This allows clinicians to initiate the highest yield investigations to minimize discomfort and invasive investigations for the patient, and to reach a specific diagnosis, in the most cost-effective manner. Specific comorbidities also correlate strongly with specific syndrome, and thus identification of an epilepsy syndrome may assist in their earlier recognition and management. In the context of a specific epilepsy syndrome, the occurrence of comorbidities is of paramount importance, as they may be responsible for a greater burden for the patient than the seizures. Increasingly, precision therapies are being identified that target specific etiologies, and recent clinical trials in epilepsy are targeting specific syndromes.
Whereas some syndromes are highly correlated with specific etiologies, others are associated with a diverse group of etiologies. Despite well-recognized electroclinical epilepsy syndromes, evolution and outcome are often still challenging to predict accurately and often depend upon the underlying etiology. With rapid advances in genetics, immunology, and imaging, it is likely that further etiology-specific syndromes will be identified, and it might be possible to predict which patients will respond best to a specific treatment, or to identify a specific or novel therapy based on the causative genes or pathway responsible for the disorder. Use of therapies that target the underlying neurobiological process that leads to epileptogenesis may significantly ameliorate comorbidities as well as seizures.
Furthermore, it is well recognized that specific ASMs may exacerbate certain conditions, such as sodium channel agents for many of the IGEs. Moreover, some ASMs are more likely to be effective for several seizure types, such as absence seizures and generalized tonic–clonic seizures. Thus, early syndrome identification will allow for selection of the optimal therapy, which is most likely to lead to early seizure control and to prevention of other seizure types that may evolve into a specific syndrome.
Accurate syndrome definition will often inform natural history and likelihood of remission. Some syndromes are self-limited over time. For these, we can provide reassurance to families of the favorable long-term outcome and can also avoid excessively prolonged use of chronic ASMs, and unnecessary diagnostic tests or treatments. Conversely, other syndromes have a much poorer outcome, such as LGS, HHE, or FIRES. In those, we understand from their onset that evolution will be unfavorable, typically with drug-resistant and life-long seizures and adverse neurodevelopmental sequelae. In such cases, a more aggressive treatment approach may be undertaken, with regular review, to attempt to ameliorate overall function and quality of life outcomes. However, it should be acknowledged that treatment options for these syndromes are often limited, the choice of the most appropriate medication is not always clear from studies to date, and polytherapy might increase the risk for adverse events or in some cases, cause seizure aggravation. Many patients with these syndromes might benefit from participation in future clinical trials of novel medications. Some syndromes do not fall clearly into a self-limited epilepsy or DEE, but rather they might have an uncertain evolution: EMA, EEM, EMAtS, and EE-SWAS. Outcome is variable, in terms of both seizure remission and cognitive and psychiatric comorbidities. In these latter conditions, there is a spectrum of severity; patients might present with or evolve to intellectual disability ranging from mild to severe, with variable degrees of neurological impairment. Sometimes, even if seizures remit, neurological sequalae persist.
As described above, some syndromes may evolve to another syndrome over time, such as SeLEAS to SeLECTS, and SeLECTS to EE-SWAS. This raises the question of the possible neurobiological links between these syndromes. To date, it is unclear why the majority of children have just one syndrome, whereas others evolve. Such evolution is likely to be due to underlying neurobiological factors. As future research provides insights into the underlying etiology, we may be able to more accurately distinguish the patients who will not show progression from one syndrome to another. Such insights will modify therapeutic approaches from the onset of their epilepsy. Identification of biomarkers may allow intervention to prevent such evolution.
The most significant nosological changes in the childhood syndromes are in the SeLFEs, which were formerly known as “benign” or “idiopathic” focal epilepsies, and DEE-SWAS or EE-SWAS, which was formerly known by the terms LKS, ESES, and epileptic encephalopathy–continuous spike-and-waves during sleep.
The nomenclature “SeLFE” was chosen to reflect the key features of the natural history, and the clinical phenotype. The term “benign” is inappropriate, as many children have associated cognitive and psychiatric comorbidities. For each syndrome, the nomenclature used reflects the major phenotypic features, such as centrotemporal spikes in SeLECTs, autonomic seizures in SeLEAS, occipital semiology and EEG findings in COVE, and photic-induced focal sensory visual seizures and genetic predisposition in POLE. Similarly, the terms DEE-SWAS and EE-SWAS comprise the two essential components, cognitive regression and the characteristic EEG pattern.
We elected to keep the term LGS for several reasons. Most importantly, the term LGS is crucial in allowing patients to acquire the multiple supports including medical and disability support therapies that they require on a daily basis. Replacing this term would lead to a lapse in services that these patients critically require. Additionally, the syndrome comprises multiple seizure type and etiologies, which would be challenging to capture in a succinct name.
Our hope is that using clearer language, with terms directly expressing the seizure semiology that are consistent with the 2017 Epilepsy and Seizure classification, will facilitate both recognition and accurate diagnoses, for health care professionals and families caring for children with epilepsy. The definitions for epilepsy syndromes provided in this paper will require validation in longitudinal studies and may be further refined as new data are published over time. Future work may expand on the definitions of more etiology-specific epilepsy syndromes. This may aid earlier clinical recognition of some etiologies that benefit from prompt targeted treatment.
ACKNOWLEDGMENTS
We gratefully acknowledge the input from the following persons outside of our Nosology and Definitions Task Force who assisted with the Delphi Panels: Drs Birinus Adikaibe, Raidah Al Baradi, Danielle Andrade, Thomas Bast, Ahmed Beydoun, Christian Bien, Roberto Caraballo, Ana Carolina Coan, Mary Connolly, John Dunne, Sheryl Haut, Floor Jansen, Barbara Jobst, Reetta Kalviainen, Angela Kakooza, Mitsuhiro Kato, Kelly Knupp, Silvia Kochen, Lieven Lagae, Luis Carlos Mayor, Natela Okujava, Kurupath Radakishnan, Eliane Roulet-Perez, Loreto Rios, Lynette Sadleir, Daniel San Juan-Orta, Jose Serratosa, Renee Shellhaas, Meng-Han Tsai, Vrajesh Udani, Helen Yue-Hua Zhang, and Dong Zhou.
S.L.M. is the Charles Frost Chair in Neurosurgery and Neurology and acknowledges grant support from the National Institutes of Health (U54 NS100064 and NS43209), US Department of Defense (W81XWH-18-1-0612), the Heffer Family and the Segal Family Foundations, and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. R.P.'s research is supported by the National Institute of Health Research (NIHR) Biomedical Research Centre at Great Ormond Street Hospital, Cambridge Biomedical Research Centre, the NIHR, and the Evelyn Trust. The views expressed are those of the authors and not necessarily those of funders.
CONFLICT OF INTEREST
N.S. has served on scientific advisory boards for GW Pharma, BioMarin, Arvelle, Marinus, and Takeda; has received speaker honoraria from Eisai, BioMarin, LivaNova, and Sanofi; and has served as an investigator for Zogenix, Marinus, BioMarin, UCB, and Roche. E.C.W. has served as a paid consultant for Encoded Therapeutics and BioMarin. She is the Editor-in-Chief of Epilepsy.com. I.E.S. has served on scientific advisory boards for UCB, Eisai, GlaxoSmithKline, BioMarin, Nutricia, Rogcon, Chiesi, Encoded Therapeutics, and Xenon Pharmaceuticals; has received speaker honoraria from GlaxoSmithKline, UCB, BioMarin, Biocodex, and Eisai; has received funding for travel from UCB, Biocodex, GlaxoSmithKline, BioMarin, and Eisai; has served as an investigator for Zogenix, Zynerba, Ultragenyx, GW Pharma, UCB, Eisai, Anavex Life Sciences, Ovid Therapeutics, Epigenyx, Encoded Therapeutics, and Marinus; and has consulted for Zynerba Pharmaceuticals, Atheneum Partners, Ovid Therapeutics, Care Beyond Diagnosis, Epilepsy Consortium, and UCB. R.N. has served as principal investigator in clinical trials for Novartis, Nutricia, Eisai, UCB, GW Pharma, and LivaNova. She received consulting fees from Biogene, BioMarin, GW Pharma, Zogenix, Novartis, Nutricia, Stoke, Ionis, Targeon, and Takeda and honoraria from Nutricia, Biocodex, Zogenix, GW Pharma, Advicennes, and Eisai. She has received unrestricted research grants from Eisai, UCB, LivaNova, and GW Pharma and academic research grants from EJP-RD (horizons 2020) and IDEAL-EPISTOP. S.M.Z. has received research support from Epilepsy Research UK, Tenovus Foundation, Glasgow Children's Hospital Charity, and Scottish Government Technology Enabled Care. He has received honoraria for educational symposia, advisory boards, and consultancy work from GW Pharma, Zogenix, Arvelle Therapeutics, and Encoded Therapeutics. J.M.W. has received paid honorarium for activities as Associate Editor of Epilepsia. R.P. is an investigator for studies with UCB and does consultancy work for Kephala, Ireland. She has served as a speaker and/or on advisory boards for Natus, GW, Eisai, and UCB. E.H. has received honoraria from UCB, Eisai, LivaNova, Novartis, and GW Pharmaceuticals. S.W. has received unrestricted educational grants from UCB Pharma, Eisai, and Sunovion. H.J.C. has acted as an investigator for studies with GW Pharma, Zogenix, Vitaflo, and Marinius. She has been a speaker and on advisory boards for GW Pharma, Zogenix, and Nutricia; all remuneration has been paid to her department. Her research is supported by the NIHR Biomedical Research Centre at Great Ormond Street Hospital. She holds an endowed chair at UCL Great Ormond Street Institute of Child Health; she holds grants from NIHR, EPSRC, GOSH Charity, ERUK, and the Waterloo Foundation. P.T. received speaker's or consultancy fees from Arvelle, Eisai, GW Pharma, LivaNova, UCB Pharma, Xenon Pharma, and Zogenix. S.A. has served as consultant or received honoraria for lectures from Biocodex, Biomarin, Eisai, GW Pharma, Neuraxpharm, Nutricia, UCB Pharma, Xenon, and Zogenix. He has been an investigator for clinical trials for Eisai, UCB Pharma, and Zogenix. He is an Associate Editor for Epilepsia. K.R. has received speaker honoraria, advisory board payments, and/or research funding from UCB, Eisai, Novartis, Zogenix, SK Lifesciences, AFT Pharmaceuticals, LivaNova, Queensland Genomic Health Alliance, Department of Health (Australia), Medicure International, Novartis, and Janssen-Cilag. S.L.M. serves as an Associate Editor of Neurobiology of Disease. He is on the editorial board of Brain and Development, Pediatric Neurology, Annals of Neurology, MedLink, and Physiological Research. He receives compensation from Elsevier for his work as Associate Editor of Neurobiology of Disease and from MedLink for his work as Associate Editor; and royalties from two books he coedited. E.P. has received speaker and/or consultancy fees from Angelini, Arvelle, Biogen, Biopas, Eisai, GW Pharma, Sanofi, SK Life Science, Takeda, UCB Pharma, Xenon Pharma, and Zogenix, and royalties from Wiley, Elsevier, and Wolters Kluwer. None of the other authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.




