ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE Task Force on Nosology and Definitions
Abstract
The International League Against Epilepsy (ILAE) Task Force on Nosology and Definitions proposes a classification and definition of epilepsy syndromes in the neonate and infant with seizure onset up to 2 years of age. The incidence of epilepsy is high in this age group and epilepsy is frequently associated with significant comorbidities and mortality. The licensing of syndrome specific antiseizure medications following randomized controlled trials and the development of precision, gene-related therapies are two of the drivers defining the electroclinical phenotypes of syndromes with onset in infancy. The principal aim of this proposal, consistent with the 2017 ILAE Classification of the Epilepsies, is to support epilepsy diagnosis and emphasize the importance of classifying epilepsy in an individual both by syndrome and etiology. For each syndrome, we report epidemiology, clinical course, seizure types, electroencephalography (EEG), neuroimaging, genetics, and differential diagnosis. Syndromes are separated into self-limited syndromes, where there is likely to be spontaneous remission and developmental and epileptic encephalopathies, diseases where there is developmental impairment related to both the underlying etiology independent of epileptiform activity and the epileptic encephalopathy. The emerging class of etiology-specific epilepsy syndromes, where there is a specific etiology for the epilepsy that is associated with a clearly defined, relatively uniform, and distinct clinical phenotype in most affected individuals as well as consistent EEG, neuroimaging, and/or genetic correlates, is presented. The number of etiology-defined syndromes will continue to increase, and these newly described syndromes will in time be incorporated into this classification. The tables summarize mandatory features, cautionary alerts, and exclusionary features for the common syndromes. Guidance is given on the criteria for syndrome diagnosis in resource-limited regions where laboratory confirmation, including EEG, MRI, and genetic testing, might not be available.
PODCAST
Key Points
- This paper presents International League Against Epilepsy (ILAE) definitions of electroclinically defined epilepsy syndromes with onset in neonates and infants.
- We divided syndromes in two groups: self-limited epilepsy syndromes and developmental and epileptic encephalopathies.
- We introduce the concept of epilepsy syndromes determined primarily by etiology.
- We summarize for each syndrome mandatory, alerts and exclusionary criteria to support an easier use for clinicians.
1 INTRODUCTION
The International League Against Epilepsy (ILAE) Task Force on Nosology and Definitions proposes a framework for classification and definitions of epilepsy syndromes with onset in the neonatal period and infancy. This group includes infants from birth, whether premature or term, up to 2 years of age. The Task Force proposes definitions for well-established electroclinically defined epilepsy syndromes. Furthermore, we introduce the concept of epilepsy syndromes determined primarily by etiology. This group includes syndromes for which there is a specific etiology for the epilepsy that is associated with a clearly defined, relatively uniform, and distinct clinical phenotype in most affected individuals as well as consistent electroencephalography (EEG), neuroimaging, and/or genetic correlates.1 With all novel associations, the phenotypic spectrum will become better defined with time. In common with all ILAE classifications, the focus of our Task Force was to develop a document reflecting the latest scientific knowledge that prepares the epilepsy community for emerging developments in epilepsy diagnosis and management.
A pure biological classification of the epilepsies is not possible given current levels of scientific knowledge; however, broadening the definition of epilepsy syndromes to include etiology reflects the current reality of clinical epilepsy diagnosis and management. Precision therapies for genetically determined epilepsies, which may not only attenuate or stop seizures but also address many of the associated comorbidities, are in development. The concepts presented in this proposal build on the work of many ILAE Commissions and Task Forces over several decades and further develop the 2017 ILAE Framework for Classification of the Epilepsies and the 2021 modification for seizures in the neonate, where etiology is considered at all levels of classification from seizure type, to epilepsy type, and epilepsy syndrome.2, 3 The Task Force proposes the new classification and definitions of epilepsy syndromes as a hybrid combining electroclinical features with etiology. There is a complex relationship between etiology and clinical features in individuals with epilepsy, where one etiology may relate to several different epilepsy syndromes and where one syndrome may be associated with different etiologies. More rarely, specific etiologies are associated with a unique electroclinical syndrome in most affected individuals. This requires that, in any individual with epilepsy, both the electroclinical syndrome and the etiology are considered together when developing a management plan. In resource-limited regions where such an approach is challenging due to limited access to specialized investigations, carefully defining the epilepsy syndrome can often suggest the etiology and guide optimal treatment. International collaborations through global networks and the ILAE may enhance equity of care.
1.1 Definition of an epilepsy syndrome
The Proposal for Classification of Epilepsies and Epileptic Syndromes, published by the ILAE in 1985, defined an epilepsy syndrome as “an epileptic disorder characterized by a cluster of signs and symptoms, customarily occurring together”.4 The most recent Classification of the Epilepsies retained this definition, describing an epilepsy syndrome as a cluster of features incorporating typical seizure types, EEG, and imaging features that tend to occur together, often with age-dependent features such as age at onset and remission (where applicable), seizure triggers, diurnal variation, sometimes prognosis, and distinctive comorbidities such as intellectual and psychiatric dysfunction.2 It was noted that syndromes may have etiological, prognostic, and treatment implications.
“a characteristic cluster of clinical and EEG features, often supported by specific etiological findings (structural, genetic, metabolic, immune, and infectious).” The diagnosis of a syndrome in an individual with epilepsy frequently carries prognostic and treatment implications. Syndromes often have age-dependent presentations and a range of specific comorbidities.
1.2 Epilepsy with onset in the neonatal period and infancy
Epilepsy incidence is age dependent, with the highest incidences (>60 per 100 000) found in individuals younger than the age of 5 years and individual age 65 years or older.5 Several population-based studies have noted a much higher incidence of epilepsy in the first year of life than in older children (82.1–118 vs. 46 per 100 000 person-years).6-8 A recent prospective, population-based study showed an incidence of 75 per 100 000 live births prior to 6 months and 62 per 100 000 between 6 and 12 months, considerably higher than previous estimates from retrospective studies.9 These population-based studies are from high-resource countries, and it is noteworthy that acquired epilepsies have a higher incidence in resource-limited populations.10-12
Children presenting with epilepsy very early in life experience a high burden of cognitive and behavioral comorbidity,13 and higher rates of drug resistance14 and mortality,15 with up to 50% showing global developmental delay 2 years after presentation.9 Comorbidities are more frequent among children who develop drug-resistant seizures14 and those with a high seizure burden.16, 17
Traditionally, syndromes have been defined primarily by electroclinical features; however, in the last two decades, gene discovery in the epilepsies has allowed cohorts of cases with a shared genetic etiology to be studied. Consistent electroclinical phenotypes have emerged, with examples including CDKL5,18 MeCP2,19, 20 PCDH19,21-23 STXBP1,24 and inv dup 15.25 Furthermore, some structural, metabolic, immune, and infectious etiologies also have characteristic electroclinical phenotypes.1 Therefore, epilepsies due to specific genetic, structural, metabolic, immune, or infectious etiologies may also meet criteria for a syndrome, when they are associated with consistent electroclinical features and have management and prognostic implications. Epilepsies in children younger than 3-years-old can be classified by syndrome in 54% of patients and by etiology in 54%, when the latest neuroimaging, metabolic, and gene testing techniques are used.7, 9 In the group younger than 12 months, etiology could be determined in 64%. By comparison, infants with severe epilepsies beginning before 18 months can be classified with an epilepsy syndrome at presentation in 64%, with the etiology being determined in 67%.9, 26
The etiology-defined epilepsy syndromes are restricted in this document to those with homogeneous electroclinical features and which, although they are individually rare diseases, are common enough to be seen in the practice of pediatric epilepsy specialists. The number of recognizable etiology-defined syndromes will increase, and further development of associated precision therapies is anticipated. We have not included response to therapy as part of the epilepsy syndrome definition, although when there is evidence for specificity of response to medication, either reduction or exacerbation of seizure frequency, we have discussed this in the text.
2 METHODS
The methodology of syndrome classification and definition by our Task Force is described in a separate paper “Methodology for classification and definition of epilepsy syndromes with list of syndromes: report of the ILAE Task Force on Nosology and Definitions.”1 The Task Force met face-to-face at ILAE meetings and had online discussions between 2018 and 2021. A working group consisting of Task Force members with expertise in pediatrics was convened. One member of the group was assigned to draft a template for each proposed syndrome, using data from a literature review through to July 2019, with the most recent edition of “Epileptic Syndromes of Infancy, Childhood and Adolescence”27 and current criteria listed on www.epilepsydiagnosis.org. The definitions presented here were based on an iterative process within the Task Force based on further input and clinical experience of Task Force members, together with additional literature searches.1 A Delphi process incorporating two rounds of comments and involving additional expert clinicians outside the authorship group helped build consensus for any areas of disagreement. This revised version addresses the reviewers’ comments and the comments posted on the ILAE site on the first submission, and, where needed, were based on a third Delphi round.
2.1 Framework for classification
- - Mandatory: Criteria that must be present in order to diagnose the syndrome. If a mandatory criterion is absent, the syndrome cannot be diagnosed.
- - Alerts: Criteria that are absent in the vast majority of cases within a syndrome, but rarely can be seen. Alerts alone would not exclude the syndrome but should cause the clinician to rethink the diagnosis and undertake further investigations to rule out other conditions. The more alerts that are present, the less confident one can be about diagnosis of a specific syndrome.
- - Exclusionary: Criteria that must be absent in order to diagnose the syndrome. If an exclusionary criterion is present, the syndrome cannot be diagnosed.
2.2 Syndromes
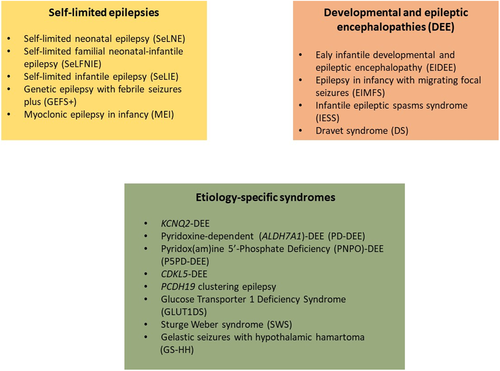
We have divided epilepsy syndromes with onset in neonates and infants into two major groups: self-limited epilepsy syndromes, where there is likely to be spontaneous remission; and the developmental and epileptic encephalopathies (DEEs), diseases where there is developmental impairment related to both the underlying etiology independent of epileptiform activity and the epileptic encephalopathy (Figure 1). Most etiology-specific syndromes that begin in the neonatal or infantile period are DEEs.
Within the group of self-limited epilepsies, there are syndromes in which both de novo and inherited pathogenic variants produce broadly similar electroclinical features in familial and nonfamilial cases. We have, therefore, assigned a name for the syndrome and the inheritance as a secondary descriptor. The reasons for replacing the term “benign” in the epilepsy lexicon with “self-limited” have been described previously.2, 28 In the self-limited epilepsy syndromes beginning under 2 years of age, the seizures are typically drug responsive and the syndromes are associated with normal cognition or minor cognitive impairment.
The concept of the “developmental and epileptic encephalopathy” (or DEE) recognizes that in infants presenting with severe early-onset epilepsy, neurodevelopmental comorbidity may be attributable to both the underlying cause and to the adverse effects of uncontrolled epileptic activity.2
We have divided the DEEs into Early Infantile DEE (EIDEE), with exclusive onset under 3 months of age, and other syndromes that present usually after 3 months of age or have a spectrum of age of onset that includes early and late infantile periods. We discuss the typical age of presentation for each syndrome. We have not sub-divided EIDEE into neonatal onset and later onset conditions, as presentation can occur at any time from birth to a few months of age.
2.2.1 Self-limited epilepsy syndromes
Self-limited (familial) neonatal epilepsy (SeLNE)
Self-limited neonatal epilepsy and self-limited familial neonatal epilepsy have similar clinical and electrical features but can be distinguished on the basis of family history (Table 1).29-31 These entities have similar genetic etiologies, with de novo pathogenic gene variants responsible for nonfamilial cases. A family history should be carefully sought as it can support diagnosis and guide decisions on investigation, treatment, and prognosis. The familial syndrome was known previously as benign familial neonatal seizures or convulsions.
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Seizures are characterized by focal tonic features at onset, affecting the head, face, and limbs. Focal clonic or tonic seizures may alternate sides from seizure to seizure, and may evolve to bilateral tonic or clonic seizures | Clinical history suggestive of in utero seizures |
Epileptic spasms Myoclonic seizures Generalized tonic seizures Generalized tonic-clonic seizures |
| EEG | Interictal: Mild background slowing |
Interictal: Persistent focal slowing or moderate or greater background slowing not limited to the postictal period Burst suppression pattern Hypsarrhythmia Ictal: Lack of EEG correlate with clinical symptoms |
|
| Age at onset | Onset after first month of age | ||
| Development at onset | Any degree of encephalopathy | ||
| Neurological exam | Significant neurological examination abnormalities, excluding incidental findings | ||
| Imaging | Neuroimaging documenting a causal lesion for seizures | ||
| Other studies – genetics |
Lack of pathogenic variant in gene associated with this syndrome, most commonly KCNQ2 or KCNQ3 OR Lack of family history suggesting AD inheritance with incomplete penetrance |
Other acute symptomatic cause of seizures including intracranial infection, ischemic or hemorrhagic stroke, hypoxic-ischemic brain injury, significant metabolic disturbances | |
| Course of illness |
Mild neurodevelopmental delay long-term Lack of remission of epilepsy after 6 months of age Drug-resistant epilepsy |
Moderate to severe neurodevelopmental disability | |
|
Are MRI or ictal EEG required for diagnosis? A nonlesional MRI is required to diagnose this syndrome An ictal EEG is not required for diagnosis |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, SeLNE can be diagnosed without EEG and MRI in a neonate with a family history suggestive of familial SeLNE who meets all other mandatory and exclusionary clinical criteria and has no Alerts. However, the clinical history of affected family members should be consistent with the expected course for SeLNE, and careful follow-up of the patient is required to ensure their course is also consistent with this syndrome | |||
- Abbreviations: EEG, electroencephalogram; MRI, magnetic resonance imaging; SelNE, self-limited neonatal epilepsy.
Seizures typically start between days 2 and 7 of life and often have focal tonic or focal clonic features or may progress to have sequential features.3 Focal seizures may alternate sides from seizure to seizure. Seizures can recur over hours to days. Developmental milestones are usually normal.31
Epidemiology:
The estimated incidence of SeLNE is 5.3/100 000 live births.9
Clinical context:
These syndromes present between days 2 and 7 of life.29-31 If children are born prematurely, seizures may occur within days of the corrected gestational age of 40 weeks. Both sexes are affected equally.
Pregnancy and birth history are unremarkable. Infants appear otherwise developmentally appropriate for age. Head size and neurological examination are normal.
Course of illness:
Seizures usually remit by 6 months of age, the majority ceasing by 6 weeks of age. If antiseizure medication has been commenced, it can often be stopped within weeks. Developmental progress is usually normal, although a minority of cases may have learning difficulties or mild motor impairment. Studies report that up to one third of individuals have seizures in later life.30 These include febrile seizures, clusters of focal seizures, isolated generalized tonic-clonic seizures, and in a minority, self-limited epilepsy with centrotemporal spikes.29, 30, 32 Some patients with specific pathogenic gene variants may have myokymia (continuous muscle activity causing stiffness and subtle twitching), which may present later in infancy.33
Seizures:
Seizures are characterized by focal tonic features at onset, affecting the head, face, and limbs.29, 30, 34 These may progress in a sequential pattern with tonic, clonic, myoclonic, and autonomic features following each other without a single predominant feature. There is often changing lateralization within or between seizures. Vocalization and/or automatisms may be seen. Autonomic features such as apnea and cyanosis are present in one third of seizures and may be the predominant manifestation. A recent paper comparing the presenting features of genetic epilepsies and acute provoked seizures in the neonate reports that seizures in genetic epilepsies (primarily KCNQ2-related SeLNE) tend to have later onset and be of shorter duration than acute provoked seizures associated with stroke or hypoxic ischemic encephalopathy.35 Clusters of seizures in self-limited neonatal epilepsy may occur over hours or days, with the neonate behaving normally between events.36 Clinical examination is normal between events except in the immediate post-ictal period or if the infant is sedated by medication.
EEG:
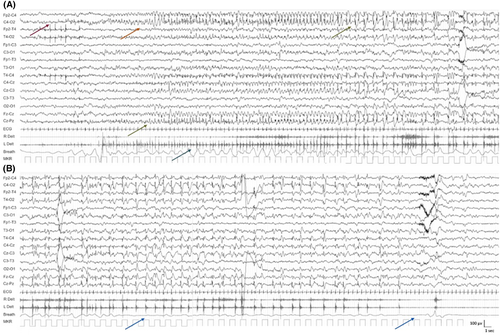
The EEG background may be normal or may show minor nonspecific abnormalities.34 Focal interictal epileptiform abnormalities can be seen in approximately two thirds of cases, most commonly in the central, centrotemporal, or frontotemporal regions with a normal background.34, 35 During periods of more active seizures, focal or widespread slowing may be seen; however, in contrast to KCNQ2-DEE, a burst-suppression pattern, or more marked, persistent slowing is not observed.
A typical ictal pattern has been described with an initial attenuation of the EEG lasting up to 20 s, followed by repetitive spike discharges (mainly centrotemporal, although other regions can be affected; Figure 2), which are often bilateral but asynchronous and with shifting laterality.34, 37 The topography can change from one seizure to the next.
Imaging:
Neuroimaging does not show a causal lesion for the epilepsy.
Genetics:
Autosomal dominant inheritance patterns are seen within families (sometimes with incomplete penetrance). SeLNE may be due to de novo pathogenic variants in the same genes, KCNQ2 and KCNQ3, as self-limited familial neonatal epilepsy. The KCNQ2 and KCNQ3 genes code for potassium channel subunits, which come together to form a heterotetrameric potassium ion channel (the M channel).38-40
A family history of SeLNE is required for self-limited familial neonatal epilepsy. There is often variability in the duration of the epilepsy in affected family members. In more than 90% of families, a pathogenic variant is identified.30 Pathogenic variants in KCNQ2 are the most common cause of the syndrome, being present in over 80%, and include stop codons, deletions, and frameshift mutations resulting in haploinsufficiency, as well as certain missense variants that cause mild to moderate loss of channel function.41, 42 KCNQ3 and SCN2A pathogenic variants are much less frequent.
- Acute provoked seizures due to hypoxic ischemic encephalopathy, metabolic etiologies, electrolyte disturbances, and stroke are more common than self-limited neonatal epilepsy. Provoked seizures tend to have an earlier onset on day 1 of life and be more prolonged. The presence of an encephalopathy excludes self-limited neonatal epilepsy.
- Focal structural causes present with stereotyped focal clonic seizures.
- Benign neonatal sleep myoclonus should be readily distinguished due to the presence of myoclonus from sleep in an otherwise well infant, which can change in frequency, amplitude, and topography.
Self-limited familial neonatal-infantile epilepsy (SeLFNIE)
SeLFNIE is an autosomal dominant syndrome with onset in the neonatal or infantile period in different family members (Table 2).43 This disorder was identified in families and found to be due to dominantly inherited SCN2A pathogenic variants.44 In addition, rare families have KCNQ2 pathogenic variants.45 De novo pathogenic gene variants are likely to cause nonfamilial cases. This syndrome can only be distinguished from the SeLNE or SeLIE if there is a family history documenting onset of self-limited epilepsy in some family members in the neonatal period, and others in the infantile period. Seizures start between day 2 and 7 months of life and have a semiology that is similar to self-limited neonatal epilepsy, with focal clonic or focal tonic features, often occurring in clusters. Seizures can recur over hours to days. Developmental milestones are typically normal.
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Focal tonic seizures with head and eye deviation, followed by other tonic and clonic features and may evolve to bilateral tonic clonic seizures | Sequential seizures |
Epileptic spasms Myoclonic seizures |
| EEG | Interictal: Mild background slowing |
Interictal: Persistent focal slowing or moderate or greater background slowing not limited to the postictal period Burst suppression pattern Hypsarrhythmia Ictal: Lack of EEG correlate with clinical symptoms |
|
| Age at onset | 1 day to 23 months | ||
| Development at onset | A history of prior acute symptomatic seizures including intracranial infection, ischemic or hemorrhagic stroke, hypoxic-ischemic brain injury, significant metabolic disturbances | Encephalopathy | |
| Neurological exam | Significant neurological examination abnormalities, excluding incidental findings | ||
| Imaging | Neuroimaging documenting a causal lesion for seizures | ||
| Other studies – genetics, and so on | Lack of pathogenic variant in genes associated with this syndrome (usually SCN2A) | ||
| Course of illness |
Mild neurodevelopmental delay long-term Lack of remission of epilepsy by age 2 years Drug-resistant epilepsy |
Moderate to severe neurodevelopmental disability | |
|
Are MRI or ictal EEG required for diagnosis? A nonlesional MRI is required to diagnose this syndrome An ictal EEG is not required for diagnosis |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, self-limited neonatal-infantile (SeLFNIE) epilepsy can be diagnosed without EEG and MRI in a neonate with a family history suggestive of familial self-limited neonatal-infantile epilepsy who meets all other mandatory and exclusionary clinical criteria and has no Alerts. However, the clinical history of affected family members should be consistent with the expected course for SeLNIE, and careful follow-up of the patient is required to ensure that their course is also consistent with this syndrome | |||
Epidemiology:
The estimated incidence is unknown.
Clinical context:
SeLFNIE presents from 1 day to 23 months of life (mean 11 weeks, median 13 weeks).46 Both sexes are affected equally. Perinatal history is unremarkable. Infants are developmentally appropriate for age with normal examination and head circumference. No other clinical features are seen (such as movement disorders).
Course of illness:
Seizure frequency varies, with some infants having only a few seizures and not requiring treatment, whereas others have clusters of many seizures per day. Seizures cease by age 12–24 months, with no recurrences later in life. Seizures are readily controlled with antiseizure medications.
Seizures:
Initially focal tonic features are observed with head and eye deviation, followed by other tonic and clonic features. Some have prominent apnea and staring. Seizures vary in duration from 20 s to 4 min. Seizures with fever are rare.
EEG:
The EEG background is typically normal. During periods of more active seizures, focal discharges, which are mainly in posterior regions, or widespread slowing may be seen.47
Imaging:
Neuroimaging does not show a causal lesion for the epilepsy.
Genetics:
Autosomal dominant inheritance with high penetrance is seen with different family members showing a mixture of neonatal and infantile onset. This syndrome is associated primarily with pathogenic variants in the sodium channel subunit gene: SCN2A. Some families with self-limited seizures associated with KCNQ2 may have individuals presenting outside the neonatal period.43, 45
- SeLNE.
- SeLIE.
- Neonatal or infantile acute symptomatic seizures due to hypoxic-ischemic injury, infection, stroke, or metabolic etiologies.
- Other focal structural causes should be considered in infants with persistently focal stereotyped seizures.
Self-limited (familial) infantile epilepsy (SeLIE)
SeLIE, formerly called benign familial (and nonfamilial) infantile seizures, is a syndrome characterized by the onset of seizures in the infantile period (Table 3). Seizures are often frequent and may be difficult to control at onset, but they resolve spontaneously. Children have normal developmental progress. The syndrome was first described in families with a dominant inheritance of infantile seizures.48 Later, it was expanded to include the familial syndrome of Infantile Convulsions Choreo-Athetosis with a movement disorder of paroxysmal kinesigenic dyskinesia/dystonia, with affected family members having either seizures or movement disorder, or both.49
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Focal seizures occur with behavioral arrest, impaired awareness, automatisms, head/eye version, and clonic movements (often alternating from one side to the other and progressing to a hemiclonic or focal to bilateral tonic-clonic seizure). Seizures are usually brief (<3 min) | Prolonged or focal clonic (hemiclonic) seizures (>10 min) |
Epileptic spasms Myoclonic seizures Sequential seizures Tonic seizures |
| EEG | Interictal: Mild background slowing |
Interictal: Persistent focal slowing or moderate or greater background slowing not limited to the postictal period Hypsarrhythmia |
|
| Age at onset | Onset 18–36 months of age | Age at onset <1 month or >36 months | |
| Development at onset | Mild developmental delay |
Moderate to profound delay Neurocognitive regression |
|
| Neurological exam | Significant neurological examination abnormalities, excluding incidental findings | ||
| Imaging | Causal lesion on brain MRI | ||
| Other studies – genetic, etc |
Lack of pathogenic variants found in PRRT2, SCN2A, KCNQ2, or KCNQ3 OR Lack of family history suggesting autosomal dominant inheritance with incomplete penetrance |
||
| Course of illness | Lack of remission by late childhood | Neurocognitive regression with myoclonic seizures, ataxia, spasticity | |
|
Are MRI or ictal EEG required for diagnosis? A nonlesional MRI is required to diagnose this syndrome An ictal EEG is not required for diagnosis |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, SeLIE can be diagnosed without EEG and MRI in an infant with a family history suggestive of familial SeLIE who meets all other mandatory and exclusionary clinical criteria and has no Alerts. However, the clinical history of affected family members should be consistent with the expected course for SeLIE, and careful follow-up of the patient is required to ensure their course is also consistent with this syndrome | |||
De novo and familial SeLIE are clinically identical except for the presence of a family history in the latter. Pathogenic variants in PRRT2 are the most common genetic etiology. Familial cases show autosomal dominant inheritance, with incomplete penetrance.
Epidemiology:
SeLIE is relatively common, accounting for 7%–9% of all epilepsies beginning prior to 2 years of age.50 The incidence is estimated at 14.2/100 000 live births.9
Clinical context:
Age at onset ranges from 3 to 20 months with a peak of 6 months. The antenatal, birth, and neonatal history is typically normal. Head size and neurological examination are normal.
Course of illness:
Seizures may be frequent at onset but usually remit within 1 year from onset. In untreated cases there can be isolated or brief clusters of seizures within the period from onset to remission.51 A minority of individuals may have epilepsy persisting into later life.
Patients with proline rich transmembrane protein 2 (PRRT2) pathogenic variants may develop paroxysmal kinesigenic dyskinesia/dystonia beginning from childhood to adult life.52, 53 Symptoms of the movement disorder should be sought for specifically as the events are very brief, lasting seconds, and the diagnosis is often missed.
Seizures:
Focal seizures are mandatory for diagnosis, and occur with behavioral arrest, cyanosis, staring with impaired awareness, automatisms, head/eye version, and clonic movements. Focal clonic seizures may alternate from one side to the other and progress to a bilateral tonic-clonic seizure but do not migrate from one side to another within the same seizure. Seizures are brief (<3 min) but can be frequent (eg, 5–10 per day over 1–3 days at onset). One third of patients present with a single isolated seizure 10–15 days before frequent seizures commence. Longer seizures can occur but are rare. Seizures remit but recur after 1–3 months in a third of patients.54
Epileptic spasms and/or myoclonic seizures are exclusionary for this diagnosis.
EEG:
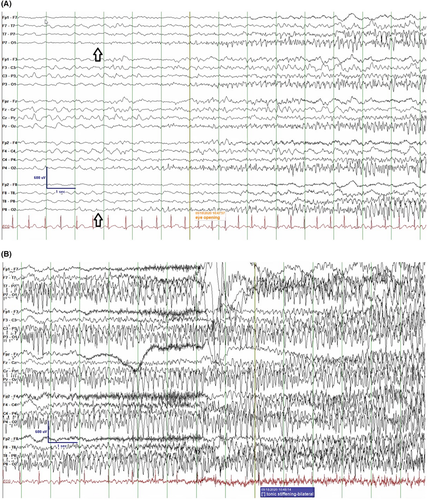
The background EEG is normal, although focal slowing may occur postictally.55 The interictal EEG is typically normal, but a variant with midline spikes during slow sleep has been described.56-58 If there is persistent focal slowing in one area, a structural brain abnormality should be considered. Diffuse, persistent slowing would suggest a different syndrome.
The ictal recording is characterized by focal discharges, which often have onset in the temporal or posterior head regions, and which may spread to both hemispheres (Figure 3).55 The seizure onset may vary from lobe to lobe or from hemisphere to hemisphere in different seizures in the same patient. However, the ictal pattern within the same seizure does not show a migrating pattern.
Imaging:
Neuroimaging does not show a causal lesion for the epilepsy. If the electroclinical diagnosis is clear and there is a family history, and/or a PRRT2 pathogenic variant, neuroimaging is not mandatory.
Genetics:
PRRT2 is the most commonly implicated gene.9, 41, 53 Other genes rarely associated with this syndrome include SCN8A, in which a movement disorder is also observed.59 Infantile onset is also seen in patients with pathogenic variants in SCN2A (see above section on SeLFNIE). In familial cases, inheritance is autosomal dominant with high penetrance. A genetic etiology can be identified in about 80% of cases.9
- SeLFNIE: the distinction is made largely on age at presentation in affected family members (see above section).
- Infantile seizures due to acute causes, for example, bleeding, infection, hypoglycemia.
- Structural etiologies such as malformations of cortical development or brain injury.
- Epilepsy of infancy with migrating focal seizures: neurodevelopmental delay and a migrating pattern on EEG within the same seizure is seen.
- Dravet syndrome (DS): prolonged focal clonic (hemiclonic) seizures, rather than short seizures, should suggest DS.
- Metabolic disorders: progressive encephalopathy and/or other organ dysfunction should prompt consideration of a metabolic disorder.
Genetic epilepsy with febrile seizures plus (GEFS+) spectrum
GEFS+ was described initially as an autosomal dominant familial epilepsy with variable penetrance.60 GEFS+ includes a spectrum of epilepsy phenotypes including epilepsy with myoclonic atonic seizures, DS,61 idiopathic and other genetic generalized epilepsy syndromes,62 and focal epilepsies,63 with heterogeneous phenotypes, usually present in the same family. Although febrile seizures are the hallmark of GEFS+ and occur in many affected family members, not all affected family members have febrile seizures. GEFS+ has heterogeneous genetic etiologies, with pathogenic variants in several genes identified.
Although the most common phenotype in GEFS+ is classical febrile seizures, the next most common phenotype is Febrile Seizures plus (FS+). Children with FS+ may have several different presentations: the most frequent is where typical febrile seizures continue beyond the age of 6 years, the typical age at which most febrile seizures stop. In infancy, a strong family history of GEFS+ phenotypes suggests this diagnosis, but more recently, cases with FS+ phenotypes have been identified without a family history and a de novo pathogenic variant in a GEFS+ gene.64
Epidemiology:
GEFS+ is a common familial syndrome; however, epidemiological data on the incidence are lacking.
Clinical context:
The following describe the specific FS+ phenotype. Specific syndromes are described elsewhere.
Febrile seizures in GEFS+ families may begin prior to 6 months of age unlike typical febrile seizures (which begin after age 6 months and mainly after 12 months) and persist beyond 6 years of age.60, 65 FS+ is the term used to describe febrile seizures persisting after 6 years of age and/or evolving to afebrile seizures. Other afebrile seizure types may develop at various ages. Prolonged focal clonic (hemiclonic) seizures with fever prior to 15 months, particularly if recurrent, should suggest DS. Neurological examination and cognitive abilities are usually normal.
Course of illness:
Seizures in FS+ are typically responsive to antiseizure medications, although not all patients require prophylactic treatment. Patients presenting with only FS+ usually have a self-limited epilepsy with resolution of seizures by puberty.60 The course of illness for individuals presenting other epilepsy types or epilepsy syndromes within the spectrum of GEFS+ depends on the type of epilepsy or syndrome.
Seizures:
Febrile seizures, which may be generalized or focal, are mandatory for diagnosis. In addition, a variety of other generalized or focal afebrile seizures may be seen.60, 62, 63, 65, 66
EEG:
The background EEG is normal. Occasionally focal or generalized spike and wave may be seen. The ictal EEG varies according to the seizure type.
Imaging:
Brain magnetic resonance imaging (MRI), if done, does not show a causal etiology in patients with GEFS+ syndromes.
Genetics:
Inheritance is autosomal dominant, with variable penetrance.60, 62, 65 Members of the same family may present with different types of seizures or epilepsy syndromes that may or may not be associated with fever or febrile seizures.60, 65, 66
Although SCN1B was the first gene identified,67 it is not the most common gene associated with GEFS+, with SCN1A pathogenic variants identified in ~10% of GEFS+ families.62, 68 Other gene variants encoding voltage-gated sodium, calcium, and potassium channels, and ligand-gated ion channels including nicotinic cholinergic receptor subunits, the γ-aminobutyric acid (GABA) A receptor subunits, and syntaxin 1B (STX1B) have also been linked to the syndrome.69, 70
- Familial febrile seizures without a family history suggestive of GEFS+.
- Infantile seizures due to acute causes, for example, ischemia, infection, and hypoglycemia.
- Structural etiologies such as malformations of cortical development or prior brain injury.
Myoclonic epilepsy in infancy (MEI)
This syndrome presents with myoclonic seizures at onset, which may be activated by sudden noise, startle, or touch, and less commonly by photic stimulation (Table 4). Some authors propose that the term “Reflex Myoclonic Epilepsy in Infancy” should be used if myoclonic seizures are activated by triggering factors such as sudden noise or startle, and they propose that children with this syndrome have a slightly earlier age at onset, better response to antiseizure medication, higher remission rate, and more favourable cognitive outcome.71 However, this syndrome could be considered a subgroup of MEI. Seizures are self-limiting in most cases. An EEG, ideally with video and electromyography (EMG), is mandatory to confirm the epileptic nature of the myoclonus and to exclude Infantile Epileptic Spasms Syndrome (IESS), which is much more common and severe than MEI.
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Myoclonic seizures (see text) | Afebrile generalized tonic-clonic seizure or generalized clonic at time of epilepsy onset |
Any of the following seizure types:
|
| EEG | Normal background |
Interictal: Lack of generalized spike-wave discharge on sleep recording PPR at low frequency photic stimulation (suggest CLN2 disease) |
Ictal: Recorded myoclonic event without EEG correlate Interictal: Hypsarrhythmia Generalized slow spike-wave (<2.5 Hz) |
| Age at onset | Age at onset of myoclonic seizures ≤4 months or >3 years | ||
| Development at onset |
Speech delay at time of diagnosis Moderate to profound ID |
||
| Neurological exam | Significant neurological examination abnormalities, excluding incidental findings | Dysmorphism or other congenital anomalies (suggests chromosomal disorder) | |
| Imaging | Significant neuroimaging abnormalities | ||
| Other studies – genetics, and so on | Low CSF glucose or pathogenic SLC2A1 variants (Glut1DS) | ||
| Course of illness | Neurocognitive regression | ||
|
Are MRI or ictal EEG required for diagnosis? A nonlesional MRI is required for diagnosis An ictal EEG is not required for diagnosis but should be strongly considered if the interictal sleep recording does not show generalized spike-wave to confirm that myoclonus is epileptic |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, at a minimum, a sleep EEG showing generalized spike-wave is required to make this diagnosis | |||
Epidemiology:
MEI is a rare disorder, accounting for less than 0.8% of children with epilepsy treated at a specialty center.72 It accounted for 1.1% of all epilepsy with onset prior to 36 months of age in a population-based cohort.9
Clinical context:
The syndrome begins between the ages of 4 months and 3 years, with a peak age of 6–18 months. Males are more commonly affected, with a M:F ratio of ~2:1.72 Development prior to seizure onset is usually normal. However, mild cognitive or behavioral or motor difficulties may coexist at onset and should not exclude the diagnosis, as they might be incidental. Neurological examination is normal.
Course of illness:
Myoclonic seizures remit in nearly all cases, within 6 months to 5 years from onset, and most children can discontinue antiseizure therapy. Rarely, generalized tonic-clonic seizures may be seen in later life. Approximately 10% develop other epilepsies in late childhood or adolescence—mostly juvenile myoclonic epilepsy.72 Patients with photosensitivity may have seizures that are more difficult to control. At long-term follow-up, developmental outcome was normal in 63%–85% of cases.72-77 Occasionally, mild intellectual disability, learning disorders, or attention problems evolve over time. Rarely, moderate to severe intellectual disability can be seen, and it is not necessarily correlated with seizure frequency.
Seizures:
Myoclonic seizures are mandatory for diagnosis and involve the head and the upper arms. They usually occur multiple times per day, both in wakefulness and sleep. They can occur in clusters and can lead to falls. Reflex-induced myoclonic seizures are seen in about one third of cases and are triggered by sudden noise, touch, or startle.72 Febrile seizures are present in up to one third of cases72 and may either precede or follow myoclonic seizures. Epileptic spasms, tonic, absence, and focal seizures are exclusionary. In addition, generalized tonic-clonic or generalized clonic seizures present at epilepsy onset are exclusionary.
EEG:
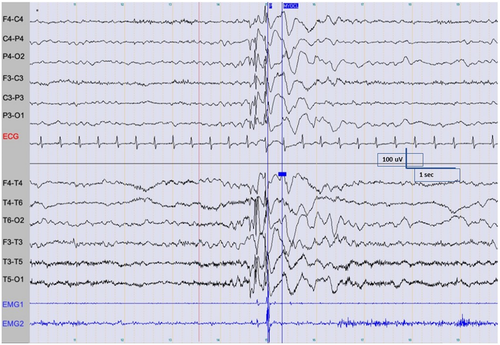
The EEG background in wakefulness is normal. Interictally, generalized discharges in the form of spike-and-wave, or less frequently, polyspike-and-wave, may be seen, and are more common in the early stages of sleep (Figure 4). Photic stimulation does not provoke spike-wave discharge without concomitant myoclonus, but a photoparoxysmal response can be seen after disappearance of myoclonic seizures in a minority of patients. The ictal EEG shows brief bursts of generalized spike-and-wave, polyspike, and polyspike and wave at ~3 Hz during myoclonus. Myoclonic seizures are more commonly recorded from sleep, and may be triggered by sudden noise, touch, or startle, or occasionally by intermittent photic stimulation.73, 78 Concurrent EMG recording facilitates diagnosis.
Imaging:
Brain MRI does not show a causal lesion for the epilepsy.
Genetics:
A family history of epilepsy or febrile seizures is reported in ~10% of cases.72 No causal genes have been found.
Differential diagnosis:
- Infantile epilepstic spasms syndrome (IESS) is distinguished by clusters of epileptic spasms, not myoclonic seizures. Epileptic spasms are most commonly seen shortly after waking, in comparison to myoclonus in MEI, which may be seen both during wakefulness and sleep. Epileptic spasms last longer than 1 s. The interictal EEG in IESS is in most cases very abnormal, with hypsarrhythmia or multifocal discharges. The ictal recording can also differentiate epileptic spasms from myoclonia (Figure 8).
- DS presents with prolonged seizures triggered by fever and status epilepticus. Myoclonus typically presents later.
- Lennox-Gastaut syndrome is distinguished by prominent atonic, tonic, and atypical absence seizures, which are not seen in MEI.
- Epilepsy with myoclonic atonic seizures is distinguished by myoclonic-atonic seizures, atypical absences, generalized tonic-clonic seizures, and episodes of nonconvulsive status epilepticus, which are not seen in MEI, and also present later in the preschool years.
- Early-infantile DEE (EIDEE) is distinguished by multiple seizure types in addition to myoclonus, marked developmental delay, and severely abnormal EEG.
- Various neurometabolic disorders including both small molecule, mitochondrial, and storage disorders, may present with myoclonic seizures in early life. These are often associated with progressive neurological deterioration and other organ dysfunctions.
- Glucose transporter-1 deficiency syndrome (Glut1DS) is distinguished by slight to moderate microcephaly, other seizure types in addition to myoclonus, by low cerebrospinal fluid (CSF) glucose and, a low CSF/plasma glucose ratio in addition to a pathogenic variant in SLC2A1 when genetic testing is available.
- Progressive myoclonus epilepsies are distinguished by the presence of significant language or motor regression, frequent association with other seizure types besides myoclonus, frequent atrophy on MRI, and photoparoxysmal response to low photic frequencies (suggesting CLN2 disease).
- Benign myoclonus of infancy is distinguished by the lack of EEG correlate to the myoclonic jerks.
- Hyperekplexia presents with pathological startle responses, which have no EEG correlate.
- Hypnic jerks are normal episodes of sleep myoclonus seen most frequently in light sleep
- Shuddering attacks present with repetitive, quick shudders, often provoked by excitement; there is no EEG correlate.
2.2.2 Developmental and epileptic encephalopathies (DEEs)
Early-infantile developmental and epileptic encephalopathy (EIDEE)
- Onset of epilepsy in the first 3 months of life with frequent seizures that are typically drug resistant.
- Abnormal neurological examination findings, for example, abnormalities of posture, tone, or movement.
- Moderate to profound developmental impairment evident with time.
- Abnormal inter-ictal EEG, which may include a burst-suppression pattern, diffuse slowing, or multi-focal discharges.
- Neuroimaging, metabolic, and genetic testing allows precise etiological classification in ~80% of cases.9, 26
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Tonic and/or myoclonic seizures | ||
| EEG |
Interictal: Either burst suppression or multifocal discharges Diffuse slowing |
||
| Age at onset | Birth to 3 months (adjusted for prematurity) | ||
| Development at onset | Normal development at onset, although it is acknowledged that this can be challenging to accurately assess historically | ||
| Neurological exam at onset | Normal neurological examination, although it is acknowledged that this can be challenging to assess historically or in an infant who has had very frequent seizures and/or received ASMs that may alter their exam | ||
| Early Comorbidities | Developmental impairment is present prior to or shortly after seizure onset | ||
| Course of illness | Abnormal neurodevelopment including intellectual disability | ||
|
Are MRI or ictal EEG required for diagnosis? An MRI is not required for diagnosis but is strongly recommended to exclude structural causes An ictal EEG is not required in an infant with characteristic clinical features where the interictal EEG shows burst-suppression, multi-focal discharges with diffuse slowing |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, this syndrome cannot be diagnosed without an interictal EEG | |||
Predominant seizure types include focal tonic, generalized tonic, myoclonic, focal clonic, and epileptic spasms. Sequential seizures may occur.3, 79 EIDEE includes neonates and infants previously classified as Ohtahara syndrome and Early Myoclonic Encephalopathy.79, 80 The syndrome may have many and varied underlying etiologies including genetic, metabolic, and structural. The electroclinical descriptions of Ohtahara syndrome (predominantly burst suppression EEG pattern and tonic seizures) and Early Myoclonic Encephalopathy (predominantly myoclonic seizures and either burst-suppression or other significant EEG abnormalities) have been extremely valuable in epilepsy classification.81, 82 This nomenclature allowed clinicians and researchers to study the causes, outcomes, and treatment of neonates and infants with severe early onset epilepsy and provided families with crucial information on prognosis. However, the electroclinical features of these two syndromes have considerable overlap and furthermore share similar underlying etiologies.80, 83-85 The Task Force proposed that separating EIDEE into individuals with Ohtahara vs. Early Myoclonic Encephalopathy no longer provides valuable information for clinical decision-making or determination of prognosis.
Epidemiology:
The incidence of EIDEE is estimated as 10/100 000 live births.9
Clinical context:
This syndrome begins in the early infantile period (range 0–3 months) and affects boys and girls equally. The neurological examination is often severely abnormal, with abnormalities of tone (most frequently central hypotonia), posture, and motor behavior with cortical visual impairment. Abnormal neurological behavior or development often presents prior to onset of seizures but may be challenging to recognize due to extremely early onset (review of early videos can be helpful). Most children have moderate to profound developmental impairment. Family, pregnancy, and birth history are usually normal. Head size varies dependant on etiology but may be normal at birth.
Course of illness:
The seizures are usually drug resistant unless metabolic or genetic targets for precision therapy or structural abnormalities amenable to surgery are identified.86, 87 For instance, patients with pathogenic variants in SCN2A or SCN8A show seizure response to sodium channel agents, often at high dose.88-90 EIDEE, regardless of whether epileptic spasms are a presenting seizure type, may evolve into IESS with the burst-suppression or multi-focal EEG abnormalities evolving in some cases to a hypsarrhythmia pattern. In very young neonates and infants, the extent of any developmental impairment may be difficult to assess; however, almost all infants with EIDEE will have moderate to profound intellectual disability. The exceptions include some individuals with early effective treatment of the underlying etiology, as may be the case in pyridoxine dependant epilepsy or pyridox(am)ine 5'-phosphate deficiency.91
Infants with EIDEE often have comorbid movement disorders including myoclonus, chorea, dystonia, and tremor. These may present prior to seizure onset, early in the evolution of the syndrome, or develop with time. Differentiating paroxysmal movement disorders from seizures can be challenging, particularly in the context of a severely abnormal interictal EEG. In such cases, prolonged video-EEG with EMG leads recordings should be considered to confirm the type of the paroxysmal event.87
Comorbidities associated with global neurological disability including cortical visual impairment, motor impairment, orthopaedic concerns, behavioral problems, feeding difficulties, and early and increased mortality are recognized associations with the syndrome.92
Seizures:
- Tonic seizures.
- Myoclonic seizures.
- Epileptic spasms.
- Sequential seizures, may include tonic, clonic, and/or autonomic components, as well as automatisms without a single predominant seizure type.
Tonic seizures are frequent and can occur in isolation or in clusters with 10–20 clusters a day. If these occur in clusters, distinguishing features from spasms include (1) tonic seizures usually occur independent of the sleep cycle, unlike epileptic spasms that are often appear upon awakening; and (2) tonic seizures last longer than epileptic spasms, which last <3 s. Tonic seizures are focal or asymmetric in the neonatal period.
Focal or multifocal myoclonus may be the predominant seizure type. The frequency of the myoclonus varies from occasional to almost continuous. Myoclonus can be erratic or massive and bilateral. Erratic myoclonus is asynchronous, asymmetric, and random. It can occur in the face or extremities or may be restricted to only an eyebrow, lip, or finger. It occurs during both wakefulness and sleep. Erratic myoclonus is more commonly associated with a metabolic etiology.
Epileptic spasms occur in some patients. They are more frequently seen beyond the first month of life. They usually occur in clusters—often on awakening.
Sequential seizures are characterized by several seizure manifestations occurring in sequence during a seizure.3 For example, an event may begin with focal tonic features followed by focal clonic features and then epileptic spasms without one predominant manifestation. In addition to the above seizure types, focal motor seizures may also occur.
EEG:
Interictal: The background is abnormal and may show burst-suppression, multifocal spikes/spike waves/sharp waves with or without slowing, discontinuity and/or diffuse slowing (Figure 5). The background abnormalities may be scarce very early in the course in rare patients but will deteriorate quickly with increasing seizure frequency. The burst-suppression pattern consists of high-voltage bursts (150-300uV) of mixed spikes, and sharp and slow waves lasting 1–5 s, alternating with periods of marked suppression (<5 μV) lasting 3–10 s; however, the duration might be influenced by concomitant medications. It is usually seen both in wakefulness and sleep and is unresponsive to stimulation. A burst- suppression pattern is usually bilateral but can be asymmetric, asynchronous, or even unilateral. Random focal attenuation can sometimes be seen. In some children, an abnormal EEG background pattern may be seen prior to seizures with the burst-suppression pattern becoming obvious only postictally. The burst-suppression pattern may disappear with age, but the EEG will remain abnormal. For infants who evolve to IESS, hypsarrhythmia may appear with age. If the etiology is treatable (metabolic or structural lesion amenable to surgery), the EEG may improve or even normalize.
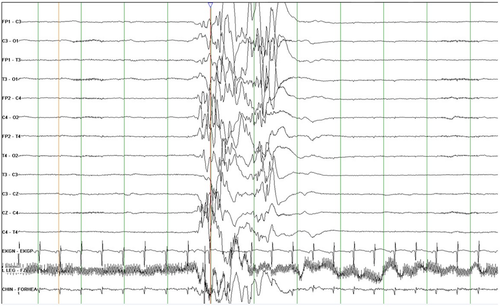
Ictal: The pattern depends on seizure type. In the neonatal period, ictal patterns are focal or asymmetric. With tonic seizures the burst-suppression pattern attenuates with the emergence of low-voltage, high-frequency fast activity. Myoclonus may have a spike/sharp wave correlate. Erratic/fragmented myoclonus may not have an ictal correlate. Focal seizures are associated with a focal ictal recruiting rhythm. The ictal pattern in a sequential seizure will change through the seizure as the clinical manifestations change. Epileptic spasms are accompanied by a high-voltage generalized or focal sharp or slow wave followed by low-amplitude fast activity and attenuation. Furthermore, ictal EEG patterns may be seen with or without clinical seizures.
Imaging:
Structural brain abnormalities are an important and frequent cause of EIDEE and should be sought in all children. Where seizures are drug resistant and focal features are prominent, further imaging modalities should be considered to exclude a surgically remediable lesion. For certain genetic etiologies, imaging is often normal initially or may show reduced brain volume or evidence of white matter hypo-/dysmyelination. Over time cerebral atrophy may develop.
Genetics:
- ○ Chromosomal microarray, karyotype (eg, ring chromosome 14).
- ○ Gene panel, whole exome or genome sequencing—it can be helpful for the quality of the resulting test report to highlight phenotypic features consistent with specific genes, where present (see section below).
Causative pathogenic gene variants can be identified in more than half of patients with EIDEE.9, 84
The seizure type(s) and EEG with other phenotypic features may predict genotype:
- KCNQ2- DEE pathogenic variants are associated with sequential seizures (with a tonic component mostly but also with clonic, tonic, myoclonic, epileptic spasms, or autonomic seizures) (see section below). This variant is also seen with exclusively tonic seizures associated with a burst-suppression or a multifocal EEG. Family history may include individuals with self-limited familial infantile epilepsy.88, 93-98
- SCN2A-DEE pathogenic variants may include sequential seizures with predominantly tonic and autonomic features.90, 99
- SCN8A-DEE pathogenic variants are associated with focal seizures.100
- STXBP1-DEE pathogenic variants are associated with asymmetric tonic or sequential seizures (tonic, autonomic, clonic, and epileptic spasms).101, 102
- CDKL5-DEE is associated with tonic seizures. Sequential seizures typically recur with a “hyperkinetic-tonic-spasms” phenotype.18, 103
- KCNT1-DEE pathogenic variants can present with focal tonic seizures with autonomic symptoms.103
- UBA5-DEE pathogenic variants can present with predominant myoclonic seizures.104
Metabolic studies:
Metabolic studies should be strongly considered, particularly if a clear structural abnormality is not found on imaging.86 Furthermore, imaging or EEG features may suggest a specific metabolic etiology. Other sources should guide detailed neurometabolic testing; however, investigations should include urine organic and amino acids (including s-sulfocysteine), urine alpha aminoadipic semialdehyde, plasma amino acids, lactate, uric acid, copper/ceruloplasmin, ammonia, acylcarnitine profile, transferrin isoelectric focusing, very long-chain fatty acids, and CSF glucose, lactate, pyruvate, amino acids, and neurotransmitters.
- Provoked seizures associated with hypoxic ischemic encephalopathy, infection, acute reversible metabolic disturbance, stroke, or intracranial haemorrhage may be myoclonic, focal clonic, and focal tonic. There may be a severe encephalopathy and a suppression-burst EEG. Provoked seizures are much more common than those associated with EIDEE, and relevant investigations to exclude acute causes should be performed. However, certain genetic causes of EIDEE including molybdenum cofactor deficiency and sulfite oxidase deficiency have imaging features that may mimic hypoxic brain injury.
Epilepsy of infancy with migrating focal seizures (EIMFS)
EIMFS is a rare developmental and epileptic encephalopathy beginning with drug-resistant, focal seizures in the first year of life, with associated severe encephalopathy (Table 6). Focal seizures can arise in both hemispheres and migrate from one cortical region to another within a seizure. Seizures are often prolonged with episodes of status epilepticus.105 The cause is mainly genetic with KCNT1106 as the major gene and more than 25 other genes linked to this syndrome.107 Prognosis is poor, with severe neurological disability and reduced life expectancy, which may be, in part, related to the specific genetic mutation,105, 108 although a milder evolution has been reported in a few children.
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures |
Focal/multifocal tonic or clonic seizures, with or without subtle behavioral arrest and prominent autonomic features Seizures migrate from one hemisphere or lobe to another clinically Seizure frequency rapidly increases in the first weeks and months, often progressing to status epilepticus |
Myoclonic seizures | |
| EEG |
Ictal recording shows a migrating pattern (this might be missed if a prolonged video EEG is not performed) Interictal: Multifocal discharges |
Interictal: Suppression burst pattern prior to medication Single persistent epileptic focus on EEG Hypsarrhythmia |
|
| Age at onset | <12 months | Onset 6–12 months | |
| Development at onset | Severe delay prior to seizure onset | ||
| Neurological exam | Significant abnormalities on neurological examination prior to seizure onset | ||
| Comorbidities | Developmental plateauing or regression with frequent seizures | ||
| Imaging | Abnormal neuroimaging with structural causal lesion | ||
| Course of illness | Neurodevelopmental delay |
Seizure freedom Lack of brain atrophy on MRI |
|
|
Is MRI or ictal EEG required for diagnosis? An MRI is required for diagnosis to exclude a causal structural etiology An ictal EEG may not be required if clinical migration is observed. However, an ictal EEG is strongly recommended to document a migrating pattern |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, EIMFS can be diagnosed on clinical observation of seizure migration without EEG or MRI, provided all other clinical mandatory and exclusionary criteria are met | |||
Epidemiology:
EIMFS has an estimated prevalence of ~0.11/100 000 children.109
Clinical context:
This syndrome usually begins in the first 6 months (mean 3 months), with rare cases beginning in the latter half of the first year of life.105, 108, 110 Males and females are equally affected. Head size and neurological examination are usually normal at onset. Most patients develop microcephaly by 1 year of age.110, 111 Development may be normal at onset; however, regression and subsequent severe delay is typical.105
Course of illness:
Prognosis is poor, with ongoing drug-resistant seizures, severe neurological developmental and motor disability, and reduced life expectancy,105, 110 although a milder evolution has been reported in a few children. Some patients are also affected by severe gut dysmotility and may have a movement disorder,109 as is common to many genetic developmental epileptic encephalopathies.
Seizures:
Focal motor clonic or tonic seizures are mandatory for diagnosis. These are initially sporadic, but the frequency rapidly increases in the weeks and months after seizure onset. Seizures may also be more subtle, with behavioral arrest with or without head and eye version, and prominent autonomic features.105, 112
Focal seizures show a migration pattern on EEG, which might be missed if a prolonged video-EEG is not performed.108, 112, 113 Clinically, migration is characterized by unilateral focal tonic or clonic activity at seizure onset, which then evolves to contralateral focal tonic or clonic activity over the course of the seizure. Status epilepticus is common.112 Rare cases with a history of epileptic spasms have been reported.109, 114-116 Myoclonic seizures are exclusionary.
EEG:
The EEG background can be normal at onset; however, diffuse slowing of the background occurs with time.105, 109, 112 Multifocal discharges appear with time in all cases. The EEG abnormality is enhanced by sleep deprivation and by sleep. Rarely hypsarrhythmia is reported.109, 115
The ictal EEG correlates with clinical semiology, and there is involvement of multiple independent cortical regions consecutively in the same single seizure event (Figure 6).112, 113 The ictal EEG is characterized by monotonous activity in the 4–10 Hz band, beginning in the temporo-occipital regions with a specific and pathognomonic pattern of propagation migration.112, 113 Recently, two EEG markers have been developed to differentiate EIMFS seizures due to KCNT1 from other focal seizures seen in neonates and infants, with variance in time and coherence of ictal rhythms of seizures.113
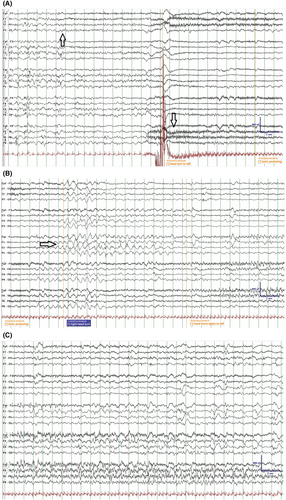
Imaging:
Neuroimaging is normal at the outset, with reports of mild to moderate enlargement of subarachnoid and ventricular spaces. Brain atrophy, predominantly in the cerebellar region, has been reported on follow-up of some cases. Delayed myelination with white matter hyperintensity on MRI and decreased N-acetyl aspartate on MR spectroscopy are often reported.106, 109, 110
Genetics:
Familial inheritance is rare showing interfamilial variability (mildly affected parents with infants with EIMFS).117, 118 De novo gene abnormalities are most commonly implicated. KCNT1 is the major gene and is reported in almost half of cases.106, 107, 119 Other genes associated with this syndrome include mainly SCN1A, SCN2A, SLC12A5, BRAT1, and TBC1D24.107
Metabolic testing:
Some children presenting with EIMFS have been found to have underlying congenital disorders of glycosylation.120
- SeLNE, SeLFNIE, and SeLIE are distinguished by normal development and lack of a migrating pattern within the same seizure on ictal EEG.
- Other focal, early-onset epilepsies due to a structural etiology are distinguished by the presence of stereotyped seizures, often with a single constant focus without a migrating pattern on EEG.
- Other EIDEE. These children may have multifocal and/or generalized seizures, with severe neurodevelopmental delay but do not show the characteristic migrating pattern within the same seizure on EEG. Many of these children may also develop movement disorders.
- Other inborn errors of metabolism.
- DS is distinguished by prolonged focal clonic (hemiclonic) seizures that alternate from side to side with different seizures. However, these patients do not show a migratory pattern within the same seizure.
Infantile epileptic spasms syndrome (IESS)
IESS is a term proposed to encompass both West syndrome as well as infants presenting with epileptic spasms who do not fulfil all the criteria for West syndrome (Table 7). West syndrome classically referred to the triad of epileptic spasms, hypsarrhythmia, and developmental stagnation or regression.121 However, infants with IESS often lack one of these three criteria. For example, the developmental impact may not be apparent or typical hypsarrhythmia may not be present. This concern was previously identified by the West Delphi group, who proposed the term Infantile Spasms syndrome for all cases of infantile spasms, regardless of EEG findings, and retained the term West syndrome for cases in which hypsarrhythmia was associated, regardless of developmental regression.122 This change emphasizes the importance of early diagnosis and therapy because shorter lag time to treatment is associated with a better outcome.123 The addition of the term “epileptic” to the name of the syndrome was done upon the request of many pediatric neurology/epilepsy experts in order to avoid any confusion with nonepileptic spasms and to emphasize the epileptic nature of this syndrome.
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Flexor, extensor or mixed epileptic spasms which often occur in clusters | ||
| EEG |
Interictal: Hypsarrhythmia, multifocal or focal epileptiform discharges (that might be seen quickly after the spasms onset) |
Interictal: Normal EEG Suppression-burst pattern |
Ictal: Normal EEG during recorded clinical events of suspected spasms |
| Age at onset | 1–24 months (while epileptic spasms may begin later, this would not be ISS) | Age at onset 1–2 months | |
| Comorbidities | Developmental slowing after spasms onset but may be absent early in the course (difficult to determine in a child with existing significant developmental disorders) | ||
|
Is MRI or ictal EEG required for diagnosis? An MRI is not required for diagnosis but is highly recommended to evaluate for underlying cause. An ictal EEG is not required for diagnosis provided the interictal study shows hypsarrhythmia or epileptiform abnormalities or developmental delay. In the absence of hypsarrhythmia or epileptiform anomalies, an ictal recording is required |
|||
| Possible evolving syndrome: Infants with preceding brain injury, developmental brain malformations, or specific genetic conditions, including early-infantile DEE, who show significant interictal EEG abnormalities (high amplitude, background slowing, and/or multifocal discharges) should be watched carefully for the development of clinical epileptic spasms. However, the syndrome of ISS cannot be diagnosed prior to onset of the mandatory seizure type | |||
| Syndrome without laboratory confirmation: In resource-limited regions, an interictal EEG is highly recommended. However, if EEG is unavailable, if clear clusters of typical epileptic spasms are witnessed by an experienced clinician (in person or on video recording), with the other clinical mandatory and exclusionary criteria, ISS can be diagnosed | |||
IESS is characterized by the onset of epileptic spasms between 1 and 24 (peak 3 and 12) months of age, although later onset may occur. Infants may have no antecedent history, or the antecedent history may reflect the underlying cause, for example, acquired structural brain or genetic abnormality. In some cases, infants with EIDEE or other early onset epilepsies (usually with focal seizures) may evolve to have clinical and EEG features of IESS after 3–4 months of age.124
Epidemiology:
The estimated incidence of IESS is 30/100 000 liveborn infants, with some studies suggesting higher incidence rates with higher geographic latitudes (Sweden, Finland, Denmark).9, 125-127 A population-based cohort showed that IESS accounted for 10% of epilepsies that begin prior to 36 months.9, 41 Both sexes are affected, with a higher incidence in males.8, 38
Clinical context:
IESS has onset between 3 and 12 months, with a range of 1–24 months. If onset occurs prior to 3 months, other early-onset developmental and epileptic encephalopathies should be considered. Prior to the onset of IESS the development can be normal, but there is often a history of preceding clear or suspected abnormal development. Developmental slowing, arrest, or regression is seen with the onset of spasms, although it may not be apparent very early in the course. Parents may report isolated regression in visual attention or altered social responsiveness in the days or weeks preceding the onset of spasms. Developmental plateauing and regression usually worsen without urgent and effective treatment. Although head size and examination may be normal, careful neurological examination may provide clues to the etiology, including abnormal head size or neurological exam findings. In addition, dermatological exam (for stigmata suggestive of a neurocutaneous disorder such as tuberous sclerosis complex), ophthalmologic assessment, and examination for dysmorphic features are important as they can suggest an underlying cause.
Course of illness:
IESS frequently evolves to other epilepsy types or syndromes, especially Lennox-Gastaut syndrome, or drug-resistant focal epilepsies. Although there are no precise data, it has been suggested that about 30% of patients with IESS may evolve to Lennox-Gastaut syndrome.128-136 Some infants may begin with focal epilepsy that evolves to IESS, and then, as the child ages or in response to therapy, revert back to focal epilepsy. In such cases, focal features are often seen on EEG and typical hypsarrhythmia may be absent. Coexisting focal seizures, asymmetric epileptic spasms, and consistent focal features on EEG should also raise the possibility of a structural brain abnormality.
Epileptic spasms may persist in some cases, particularly with some of the genetic or structural encephalopathies. In some individuals, they resolve with effective therapy and subsequent epilepsy is not seen.
Developmentally, many infants are left with poor developmental outcome, regardless of seizure outcome. The severity of developmental delay relates predominantly to etiology and promptness of treatment. Prognosis is more favorable for infants with preceding normal development, no known cause, and prompt initiation of syndrome-specific treatment.123, 125
Seizures:
Epileptic spasms are mandatory for the diagnosis of IESS, and consist of brief tonic contractions of axial muscles, each typically lasting <3 s, which may be flexor, extensor, or mixed. These usually occur in series or clusters, with increasing prominence of the motor features through the cluster, often over a period of minutes (although clusters may last 30 min or longer) and are often seen on awakening. These may be symmetric or asymmetric and may be subtle, with minor head nods, or eye or chin movements.
Focal seizures may also be seen and may co-occur in an infant with spasms, particularly in the setting of a structural etiology, for example, tuberous sclerosis or focal cortical dysplasia. Focal seizures may occur either independently of spasms or may precede, occur during, or follow a cluster of epileptic spasms, or even occur throughout the series of epileptic spasms. Tonic seizures at onset are atypical and should raise concern for another early onset developmental and epileptic encephalopathy.
EEG:
Interictally, hypsarrhythmia (chaotic, high amplitude, excessive slowing, multifocal epileptiform discharges) is often seen and the yield of detection is greatest if non-REM (rapid eye movement) sleep is recorded (Figure 7A). Some infants may have a very active multifocal epileptiform EEG without the chaotic background that typifies hypsarrhythmia. A consistent focal epileptiform discharge or focal fast activity should suggest an underlying structural abnormality. Very early in the course, or in older children, hypsarrhythmia may also be absent. Clinicians should not withhold standard therapy for children with IESS who do not have hypsarrhythmia.
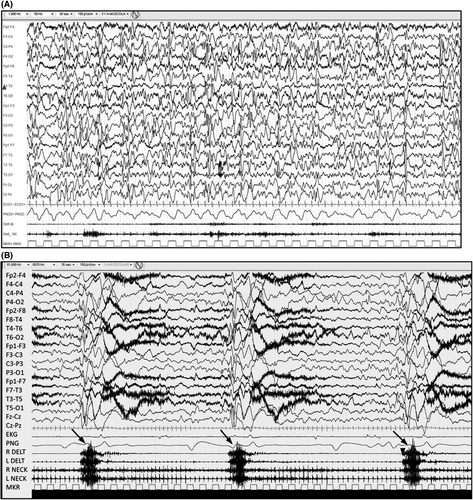
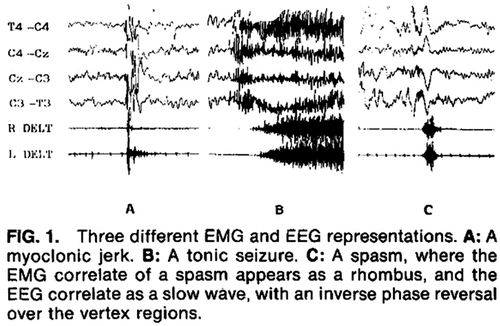
The ictal recording of an epileptic spasm is characterized by a high amplitude, generalized, sharp or slow wave followed by low amplitude, fast activity, which may appear as a brief electrodecrement (Figure 7B). Hypsarrhythmia typically attenuates or stops during a series of epileptic spasms. EMG helps to distinguish epileptic spasms from myoclonic seizures and tonic seizures (see Figure 8).137 A burst-suppression pattern on EEG is suggestive of EIDEE.
Neuroimaging:
Neuroimaging is strongly recommended to clarify the etiology, which may impact treatment decision-making. Brain MRI is abnormal in one half to two thirds of children with IESS,9, 138-142 and can show either acquired or congenital lesions that are focal, multifocal, or diffuse. Early imaging should be repeated after 2 years of age when myelination is likely to be complete, if there is a suspicion of a focal structural lesion, or in infants with refractory infantile spasms of unknown etiology. Optimized imaging and analysis for the detection of subtle focal cortical dysplasia may be necessary, and modalities such as fluorodeoxyglucose positron emission tomography or arterial spin labeling can be useful to detect focal structural anomalies in the presence of an apparently normal MRI.143, 144 Such children should be referred early for epilepsy surgical assessment. In addition, MRI abnormalities may point to specific metabolic disorders.
Genetics:
Genetic studies should be considered if no etiology is found after clinical examination and MRI.145-147 In addition, genetic testing should be considered for patients with structural brain disorders known to be associated with a genetic basis.
Pathogenic variants in many genes have been associated with IESS and often are de novo in the child. A genetic etiology can be defined in up to 41% of cases.9, 41 Etiologies include Trisomy 21, ARX, CDKL5, STXBP1, IQSEC2, TSC1, TSC2, and many others. A genetic mutation can be inherited from a parent with mild symptoms or an unaffected parent. In addition, a range of chromosomal abnormalities and copy number variants have been associated with IESS, so chromosomal microarray and routine karyotype should be considered.
Metabolic and other lab studies:
Metabolic etiologies are a rare but important cause of IESS. Metabolic testing should be considered if an etiology is not found on clinical examination and no structural abnormalities are seen on MRI. In the absence of a known etiology, pyridoxine dependency should be considered. If laboratory studies are unavailable to rapidly exclude this diagnosis, infants should be considered for a trial of pyridoxine.148 However, given the rarity of this disorder, such a trial should be given at the same time as the first-line therapy.
Differential diagnosis:
- EIDEE begins before 3 months of age. Although spasms may be present, other seizure types including tonic, myoclonic, and sequential seizures coexist.
- MEI presents with myoclonic seizures, not epileptic spasms. The EEG and EMG can distinguish myoclonus from epileptic spasms. EEG shows a normal background with generalized spike wave discharges.
- Benign sleep myoclonus: jerks in sleep are a normal phenomenon.
- Benign myoclonus of infancy presents with myoclonus and a normal interictal and interictal EEG.
- Infantile colic presents with intermittent prolonged bouts of crying and stiffening. The EEG is normal.
- Gastroesophageal reflux or Sandifer syndrome.
- Benign shuddering attacks of infancy.
- Benign infantile head drops: frequent head drops with onset at 3–6 months of age. This entity is self-limited and the EEG is normal.
- Hyperekplexia
Dravet syndrome (DS)
DS (previously known as Severe Myoclonic Epilepsy of Infancy), presents in the first year of life in a normal child with prolonged, febrile and afebrile, focal clonic (usually hemiclonic), or generalized clonic seizures (Table 8).149 Other seizure types including myoclonic and atypical absence seizures appear between the age of 1 and 4 years. Seizures are usually intractable, and from the second year of life children demonstrate cognitive and behavioral impairments.149 Gait abnormalities including a characteristic crouch gait are usually seen by late childhood.150 The clinical diagnosis is supported by the identification of pathogenic variants in the sodium channel gene SCN1A (found in over 80% of cases).151
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Recurrent focal clonic (hemiclonic) febrile and afebrile seizures (which often alternate sides from seizure to seizure), focal to bilateral tonic-clonic, and/or generalized clonic seizures |
No history of prolonged seizures (>10 min) Lack of fever sensitivity as a seizure trigger |
Epileptic spasms Early infantile SCN1A DEE |
| EEG | Normal EEG background without interictal discharges after age 2 years | ||
| Age at onset | 1–20 months | 1–2 months or 15–20 months | |
| Development at onset | Developmental delay at seizure onset | ||
| Neurological exam | Focal neurological findings (other than Todd’s paresis) | ||
| Imaging | MRI showing a causal focal lesion | ||
| Other testing: ie, genetics, and so on | Lack of pathogenic SCN1A or other causal variant | ||
| Course of illness |
Drug-resistant epilepsy Intellectual disability |
Good efficacy with prophylactic sodium-channel agents including carbamazepine, oxcarbazepine, and phenytoin | |
|
Is MRI or ictal EEG required for diagnosis? An MRI is not required for diagnosis but is highly recommended to exclude other causes. An ictal EEG is not required for diagnosis |
|||
| Possible evolving syndrome: In a child <12 months who presents with a prolonged hemiclonic or bilateral tonic-clonic seizure with fever, and no other underlying cause, the possibility of Dravet syndrome should be considered. Further convulsive seizures (often with fever, and if prolonged or hemiclonic) would allow more definitive diagnosis of Dravet syndrome. A diagnosis would be further supported by the finding of a pathogenic SCN1A variant | |||
| Syndrome without laboratory confirmation: In resource-limited regions, Dravet syndrome can be diagnosed in children without Alerts who meet all other clinical mandatory and exclusionary criteria, without EEG, MRI, and genetic testing | |||
Epidemiology:
DS affects ~6.5/100 000 live births.9, 41, 152
Clinical context:
Onset of seizures is typically between 3 and 9 months, with a mean and median age of 6 months.149, 153, 154 Rare cases can present as early as 1 month of age, or as late as 20 months of age in a few reported cases; however, onset before 2 months or after 15 months should alert the clinician to review the diagnosis and consider further investigations to exclude other conditions. Development appears normal at seizure onset.153-155 The neurological examination is normal at seizure onset. Walking may be slightly delayed (mean 16–18 months) and gait instability may be present. Head size is normal during the first years. Significant developmental delay, neurological examination abnormalities, movement disorders, or microcephaly at the time of seizure onset suggests an alternative diagnosis.
Course of illness:
Seizures are drug resistant and present through life. Episodes of status epilepticus are more frequent before 5 years of age. They can, however, occur later, even into adult life, especially with an illness or fever.153 By adolescence/early adulthood, status epilepticus and atypical absences are rare—seizures are predominantly brief, with various types (focal with loss of awareness, clonic, generalized tonic-clonic, myoclonic, and atypical absences). Nocturnal seizures tonic and tonic-clonic may appear at this age and become the predominant seizure type.156, 157 Over time, developmental progress slows and delay may be evident from 12 to 60 months following onset of seizures.158-160 Speech delay is predominant. Most patients manifest a degree of intellectual disability ranging from mild to severe (50%).155, 158 Many patients develop behavior disorders and some have inattention and hyperactivity.153, 160, 161 Developmental regression can be seen following episodes of status epilepticus. In most patients, however, the pattern is more of developmental slowing and consequent intellectual impairment.158 Over time, most patients develop subtle pyramidal signs and gait disorder evolving to crouch gait, typically by late childhood to adolescence.150
Seizures:
- Myoclonic seizures.
- Focal impaired awareness seizures.
- Focal to bilateral tonic-clonic seizures.
- Atypical absence seizures.
- Atonic seizures.
- Nonconvulsive status epilepticus (originally termed obtundation status),
- tonic and tonic-clonic seizures mainly in sleep and in clusters.
By this age, in addition to illness, seizures can also be triggered by physical activity, change in environmental temperature, visual patterns (rarely), photic stimulation (15% of patients), and excitement.139, 153, 159 Tonic and tonic-clonic seizures mainly in sleep and in clusters, may appear later in the course of the disease, from around age 4–5 years, and become more evident in adult life.156, 157, 162 Epileptic spasms are exclusionary. Seizures are exacerbated with the use of sodium channel–blocking drugs (this can be a clue to the diagnosis) such as carbamazepine, lamotrigine, oxcarbazepine, and phenytoin.145 However, lamotrigine may rarely have a role in older patients as suggested in one small case series.163
EEG:
Background may be normal or slow prior to age 2 years. Slowing is typical after 2 years of age.154, 159, 164 Interictal discharges are often focal, multifocal, and generalized, and appear after 2 years of age.164 In patients with sleep clusters of seizures, interictal frontal discharges are often seen.162, 164 A photoparoxysmal response occurs in 15% of patients and is more frequent in younger children.164 Ictal recordings depend on seizure type.
Neuroimaging:
MRI is normal at seizure onset.165 Over time, mild cerebral and cerebellar atrophy may evolve. A minority of patients have hippocampal sclerosis165, 166; however, epilepsy surgery is not indicated.
Genetics:
Genetic testing is recommended at all ages, including in adults in whom the diagnosis is suspected but details of history in infancy may be difficult to access. A pathogenic variant in SCN1A is present in more than 80%–85% of cases.151 Most are de novo; however, up to 10% of patients who are thought to have a de novo mutation will have one parent who is mosaic for the variant.167 This carries implications for reproductive counseling. DS may occur in one member of a family with GEFS+. SCN1A pathogenic variants may be found in other epilepsy syndromes such as GEFS+ and early infantile SCN1A encephalopathy with profound impairment. The diagnosis of DS requires the typical clinical features and cannot be made on the basis of the genetic variant alone, and the absence of a gene variant should not preclude a clinical diagnosis of the syndrome.161 Treatment should not be delayed in the setting of a clinical diagnosis.
Other genes have rarely been associated with DS including dominant, pathogenic variants in GABRG2, GABRA1, STXBP1, and rare recessive cases with SCN1B variants.168
A family history of febrile seizures or other epilepsies may be seen in 30%–50% of cases, and the semiology may be suggestive of GEFS+.
Metabolic and other lab studies:
No consistent abnormalities found.
Differential diagnosis:
- FS+: Although this condition also may present with febrile seizures in early life, the presence of recurrent, prolonged, focal clonic seizures (hemiclonic) in infancy should suggest DS.
- Lennox-Gastaut syndrome: Lennox-Gastaut syndrome can readily be distinguished from DS, as tonic seizures are prominent early on, and prolonged focal clonic (hemiclonic) seizures do not occur. Furthermore, the EEG in Lennox-Gastaut shows a slow background, with prominent, frontally predominant slow spike-wave (<2.5 Hz) and paroxysmal fast activity in sleep.
- Epilepsy with myoclonic-atonic seizures: Epilepsy with myoclonic-atonic seizures begins later than DS. Although some cases may have a history of febrile seizures, prolonged, focal clonic (hemiclonic) seizures and other focal seizures are not seen. Myoclonic atonic seizures are typical. Children may develop myoclonic nonconvulsive status epilepticus but recurrent convulsive status epilepticus is also rare.
- Protocadherin 19 Clustering Epilepsy typically presents with clusters of seizures, as opposed to prolonged focal clonic (hemiclonic) seizures. However, similar to DS, seizures occur mainly in infancy and are triggered by fever. PCDH19 Clustering Epilepsy predominantly affects females, and there is an X-linked mode of inheritance that spares males.
- SCN1A-DEE is distinguished from DS by very early onset (<3 months), preceding developmental delay and prominent movement disorder. Some cases of early onset SCN1A-EIDEE such as Thr226Met169 are linked to gain-of-function variants, and thus responsive to sodium channel–blocking agents.170
- Structural focal epilepsy may begin with prolonged focal seizures triggered by fever; however, recurrent seizures affect the same side or limb, as opposed to DS, which results in focal clonic (hemiclonic) seizures that often alternate sides. Myoclonic and atypical absence seizures are unusual. MRI often shows a causal lesion.
- Mitochondrial disorders: Children with mitochondrial disorders may also present with multiple seizure types early in life. However, there are other signs of mitochondrial disease, such as other organ dysfunction, elevated lactate, and characteristic abnormalities on MRI.
- Intracranial infection such as meningitis or encephalitis must be excluded in the presence of a prolonged febrile seizure.
2.2.3 Etiology-specific syndromes
Increasingly, consistent electroclinical phenotypes are being identified with strong associations to specific etiologies. Some known syndromes have specific etiologies (ie, SCN1A pathogenic variants in DS); however, for other etiologies, novel characteristic phenotypes are associated. In some cases, the etiology has just a single phenotype, whereas in others, particularly certain genetic disorders, the phenotype may vary depending on age and nature of the variant. Etiology-specific syndromes can be identified, where there is a specific etiology for the epilepsy that is associated with a clearly defined, relatively uniform, and distinct clinical phenotype in most affected individuals (clinical presentation, seizure types, comorbidities, course of illness, and/or response to specific therapies), as well as consistent EEG, neuroimaging, and/or genetic correlates.1 Our Task Force did not aim to identify and describe all Etiology-Specific Syndromes, but provided definitions on a limited number, including the DEEs associated with KCNQ2, CDKL5, PCDH19, SCL2A1, pyridoxine and pyridox(am)ine 5'-Phosphate-dependent epilepsy, Sturge-Weber syndrome, and Gelastic Seizures with Hypothalamic Hamartoma.
KCNQ2-DEE
KCNQ2-DEE causes a neonatal onset encephalopathy and is due to de novo missense variants that produce a disorder distinct from self-limited neonatal epilepsy. Seizures may respond to sodium channel blockers (Table 9).
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Tonic, myoclonic, and/or focal seizures | ||
| EEG | Either burst suppression or multifocal discharges; diffuse slowing | ||
| Age at onset | < 3 months | Onset beyond the first week of life (corrected gestational age) | |
| Neurological exam | Normal neurological examination | ||
| Comorbidities | Neurodevelopmental slowing/encephalopathy is apparent at seizure onset | ||
| Other testing: ie genetics etc | Pathogenic variant in KCNQ2 | ||
| Course of illness | Abnormal neurodevelopment, with profound to moderate impairment | ||
|
Is MRI or ictal EEG required for diagnosis? An MRI is not required for diagnosis but is strongly recommended to exclude other causes An ictal EEG is not required for diagnosis |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, KCNQ2-DEE cannot be diagnosed without genetic testing | |||
Epidemiology:
The incidence of KCNQ2-DEE is unknown.
Clinical context:
Seizure onset is within the first few days of life in the context of a severe neonatal encephalopathy with abnormal neurological examination and behavior.93, 97, 171-176 Seizures are typically not responsive to first-line medications such as phenobarbitone. Sodium channel–blocking agents such as carbamazepine and phenytoin should be considered early in this clinical context.88
Course of illness:
Seizures may respond partially or completely to sodium channel blockers. Epilepsy frequently remits; however, developmental outcome is typically moderately to severely impaired.174 Over half of patients will become seizure-free, varying from a few months of age to several years.174 As genetic testing becomes more readily available it is likely that more cases with intermediate outcome between self-limited neonatal epilepsy and KCNQ2-DEE will be identified. Milder phenotypes may be seen in cases with mosaicism.
Seizures:
Focal tonic seizures are seen most frequently, although other seizure types including focal clonic and myoclonic may also be seen.97, 172, 174 Autonomic features, apnea, and ictal crying may be prominent during seizures. Epileptic spasms have been recorded in some individuals; however, the evolution to IESS is seen less frequently in KCNQ2-DEE than in other severe EIDEEs. The seizure semiology in neonates is similar to that seen in SeLNE; however, seizure frequency, EEG background abnormalities, and abnormal neurological examination in KCNQ2-DEE allow the syndromes to be distinguished.174
EEG:
In more than 60% of cases the EEG shows a burst suppression pattern, which may be asymmetric at times (Figure 5).96, 173 In other cases, multifocal abnormalities including spikes, sharp waves, and hemispheric suppression may be seen.
Neuroimaging:
MRI signal abnormalities may be seen in the basal ganglia or thalamus during the neonatal period. In some cases, hyperintensities seen on T1 sequences in the globus pallidus may disappear with time.90, 92 Mild atrophy of the frontal lobe and thin corpus callosum have been reported.94, 96
Genetics:
De novo missense variants in particular regions (hot spots) of the KCNQ2 gene produce a dominant negative, more severe loss of channel function than is seen in SeLNE.173, 175, 176
Pyridoxine-dependent (ALDH7A1)-DEE (PD-DEE) and pyridox(AM)INE 5'-phosphate deficiency (PNPO)-DEE (P5PD-DEE)
PDE-DEE and P5P-DEE are caused by genetic-metabolic defects within the same lysine degradation pathway (Table 10).177 Seizure control can be achieved in almost all cases with pharmacological doses of pyridoxine and pyridoxal-5'-phosphate, respectively, emphasizing the importance of early recognition. Some infants with P5PD-DEE respond partially or completely to pyridoxine therapy.177
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures |
Variable seizure types, which may include:
Seizures are drug resistant and frequent (often evolving to status epilepticus) but rapidly respond to pyridoxine (pyridoxine-dependent-DEE) or pyridoxal−5-phosphate (pyridox(am)ine 5'-phosphate-DEE) supplementation |
||
| EEG | Interictal: Abnormal with slowing and focal/multifocal discharges or burst suppression pattern | ||
| Age at onset | Age >3 years at onset (there are rare, later-onset forms of pyridoxine-dependent epilepsy) | ||
| Neurological exam | Lack of encephalopathy and irritability | ||
| Other testing: ie genetics etc |
Laboratory testing providing confirmatory evidence, which may include: 1. Metabolic features: Increased α-aminoadipic semialdehyde and/or pipecolic acid in urine, plasma, and/or CSF (pyridoxine-dependent-DEE) or low pyridoxal−5-phosphate in CSF (pyridox(am)ine 5'-phosphate-DEE) OR 2. Genetic features: pathogenic variants in ALDH7A1 or PLBP (pyridoxine dependent-DEE) or PNPO gene (pyridox(am)ine 5'-phosphate-DEE) |
||
| Course of illness | Seizures that show sustained marked reduction or cessation with lifelong pyridoxine or pyridoxal−5-phosphate. | Normal neurodevelopmental outcome | |
|
Is MRI or ictal EEG required for diagnosis? An MRI is not required for diagnosis but is strongly recommended to exclude other causes An ictal EEG is not required for diagnosis |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, pyridoxine or pyridox(am)ine 5'-phosphate-DEE can be diagnosed in children without Alerts who meet all other mandatory and exclusionary clinical criteria and whose seizures cease with pyridoxine or P5P supplementation, recur when supplementation is stopped, and cease again with re-introduction of supplementation | |||
Epidemiology:
Estimates of incidence are available only for PD-DEE due to pathogenic variants in ALDH7A1 and vary from 1 in 65 000 births, 1 in 273 000 births, to 1 in 783 000 births.178-180 The incidence of P5P-DEE is unknown.
Clinical context:
Patients with PD-DEE and P5P-DEE present shortly after birth with encephalopathy and seizures or with intrauterine convulsions. However, up to 25% of patients with pyridoxine-dependent epilepsy may present outside the newborn period, mainly in the first 3 years of life, although new onset of seizures has been reported at 17 years of age.181, 182 Patients with P5PD-DEE are often born prematurely, and those with either PD-DEE and P5PD-DEE may show signs of neonatal distress, irritability, and vomiting at times with acidosis and low Apgar scores, leading to a misdiagnosis of neonatal hypoxic-ischemic encephalopathy.182, 183 There may be a family history of EIDEE, infertility, and death in siblings.184 Seizures are resistant to standard antiseizure medications.
Course of illness:
Evidence from small case series and observational studies suggests that lysine reduction therapies including a lysine-restricted diet and L-arginine therapy may provide additional benefit in terms of seizure control and cognitive outcome.148 Despite adequate seizure control, the majority of people have varying degrees of intellectual disability from mild to severe.185, 186 Later-seizure onset is associated with better cognitive outcome; however, this can be normal for patients with onset at any age with both PD-DEE and P5PD-DEE, emphasizing the importance of early and adequate treatment.187 Seizure relapse may occur during febrile illnesses, and treatment doses of pyridoxine may be doubled at these times.148 Withdrawal of pyridoxine leads to a recurrence of seizures; therefore, treatment should be lifelong with dose adjustments as needed. Chronic use of pyridoxine may result in peripheral neuropathy, but this is rare if doses do not exceed 200 mg/day and can be monitored through testing of deep tendon reflexes and nerve conduction studies.188 People with P5PD-DEE may be exquisitely sensitive to dosing and timing of pyridoxal-5-phosphate, with some benefiting from multiple doses per day.
Cirrhosis of the liver has been reported in P5PD-DEE and surveillance for this association is appropriate.189
Seizures:
Seizures may manifest antenatally as excessive fetal movements and typically present in the first hours to days of life. Infants may be acidotic and hypotonic; however, seizures may manifest as frequent, at times continuous, multifocal myoclonus affecting limbs, trunk, eyes, and facial muscles. A variety of seizure types may occur including focal seizures, spasms, and generalized tonic-clonic seizures.148 The semiology of a hyperkinetic, seemingly distressed and agitated infant with multifocal myoclonus and spasms should alert the clinician to the possibility of PD-DEE or P5PD-DEE. In older infants, presentation may be with febrile or febrile generalized tonic-clonic seizures, status epilepticus, or clusters of focal seizures. If doses of pyridoxal-5-phosphate are missed or not tolerated during vomiting illnesses, patients with P5PD-DEE may present with semiology, suggesting occipital network involvement, including colored lights, ictal blindness, and darting eye movements. Presentation with infantile spasms later in infancy is rare but has been reported in PD-DEE.190 The wide variety of seizure types at presentation necessitates that PDE-DEE and P5PD-DEE be considered in all infants with drug-resistant seizures in infancy. Some children with PDE-DEE may be partially responsive to antiseizure medications.
EEG:
EEG in PD-DEE and P5PD-DEE in neonates with severe encephalopathy prior to treatment can show a burst-suppression pattern. In other cases, focal or multifocal discharges may be seen against a background of slow rhythms. If pyridoxine is given intravenously to an encephalopathic patient (ideally this should be done under EEG control), it must be done in a setting where the child can be intubated for respiratory support should treatment cause apnea. A burst-suppression EEG or EEG with multifocal sharp or spike complexes can become diffusely suppressed following pyridoxine administration and may take many hours or days to return to show normal background rhythms. Hypsarrhythmia has been reported in 1 of 30 patients in one series.181
Neuroimaging:
Neuroimaging may be normal, but in both PD-DEE and P5PD-DEE, over half of patients have MRI abnormalities. These including white matter edema in severely encephalopathic cases.181, 183 Intraventricular hemorrhage, ventricular dilatation, and corpus callosum hypoplasia can lead to misdiagnosis of a structural etiology for the epilepsy.181
Genetics:
Most cases of PD-DEE are associated with biallelic variants in ALDH7A1, also known as antiquitin, with a minority associated with biallelic variants in PLBP (previously known as PROSC).184, 186, 191 Pyridox(am)ine 5' phosphate deficiency is associated with biallelic variants in the PNPO gene.191 The disorder, previously termed folinic acid responsive epilepsy, is a form of PD-DEE associated with variants in ALDH7A1 and has a better response to pyridoxine than folinic acid alone.91 If a single pathogenic variant is identified, in the appropriate clinical context, then multiplex ligation probe amplification and chromosomal microarray should be undertaken to identify intragenic or whole gene deletions, or duplications involving the relevant gene on the other allele. If variants of uncertain significance are identified, metabolic investigations will help in assessment of pathogenicity. Antenatal genetic testing and maternal treatment with pyridoxine should be considered in subsequent pregnancies.
Metabolic testing
The biomarkers ⍺-aminoadipic semialdehyde (⍺-AASA) and pipecolic acid are elevated in urine, plasma, and CSF.148 Ideally urine and plasma samples should be taken prior to treatment with pyridoxine; however, this should not delay therapy in suspected cases.188 Following treatment, these biomarkers may be reduced but typically remain elevated. ⍺-AASA is considered the more reliable test. With the use of biomarkers and gene testing, withdrawal of therapy as a diagnostic test is now obsolete.
CDKL5-DEE
CDKL5-DEE, also known as CDKL5 deficiency disorder, is a DEE that is the result of pathogenic variants in the cyclin-dependent kinase like 5 (CDKL5) gene. It is an important cause of very early-onset epilepsy (median age 6 weeks) with pronounced hypotonia (Table 11). The combination of clusters of infantile spasms and tonic seizures in the first few months of life is characteristic, but multiple seizure types can occur. Seizures often have multiple phases, with a classic sequential hypermotor (hyperkinetic)-tonic-spasms seizure. Severe to profound global delay is present in essentially all cases.
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures |
Seizures, which may include tonic seizures, epileptic spasms, generalized tonic-clonic seizures, and/or focal seizures Hyperkinetic-tonic-spasms sequence seizures are characteristic but not seen in all cases |
Absence of epileptic spasms in the first year of life | |
| EEG | Normal EEG background without interictal discharges after 4 months of age | ||
| Age at onset | Onset of epilepsy >3 months | ||
| Development at onset | Normal development prior to seizure onset | ||
| Neurological exam |
Normal tone Lack of encephalopathy |
||
| Other testing: ie genetics etc | Pathogenic variant in the CDKL5 gene (X-linked but females outnumber males by 4:1) | ||
| Course of illness |
Profound to severe intellectual disability Drug-resistant epilepsy |
||
|
Is MRI or ictal EEG required for diagnosis? An MRI is not required for diagnosis but is strongly recommended to exclude other causes An ictal EEG is not required for diagnosis |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, CDKL5-DEE cannot be diagnosed without confirmatory genetic testing | |||
Epidemiology:
CDKL5-DEE is rare, with estimated incidence of between 1/40 000 and 1/60 000 live births.41, 192, 193 It is X-linked and females outnumber males by a ratio of 4:1.194, 195
Clinical context:
The median age of seizure onset is 6 weeks, and 90% of cases have onset before 3 months.196, 197 Developmental concerns are present at the time of seizure onset but become more pronounced with time. True regression is rare.194 Neurological examination shows diffuse hypotonia but normal head circumference at onset.18, 194 Cortical visual impairment, with poor eye contact and lack of visual tracking is common.194 Subtle dysmorphic features with deep set eyes, broad forehead, prominent lips, deep philtrum, and puffy phalanges with tapered fingers have also been described.194
Course of illness:
Epilepsy typically remains drug resistant and most patients are left with severe intellectual disability. Most patients continue to have daily seizures, although occasional periods of seizure freedom up to 2 months or longer are seen in less than half of cases.194 Independent walking and ability to speak single words is achieved in less than one quarter of cases.194 Movement disorders including choreoathetosis, akathisia, dystonia, and parkinsonism can affect a minority of patients.194 Males are more severely affected.
Seizures:
The initial seizure type can vary, but most commonly tonic seizures, spasms, generalized tonic-clonic seizures, or focal seizures are seen.195 Over time, other seizure types can occur. The majority will have epileptic spasms and/or tonic seizures. One characteristic seizure type, seen in many but not all cases, is hypermotor(hyperkinetic)-tonic-spasms sequence seizures.198 The first part of this seizure begins with a hypermotor phase with rocking, kicking, and vocalization that lasts 10–60 s. This is followed by a tonic phase, either with extension of all limbs or extension of the upper limbs and flexion of the lower limbs lasting 20–45 s. The seizure evolves to a series of extensor spasms, which lasts 1–15 minutes. Similar seizures that involve multiple phases with clustering of tonic seizures and spasms, but with variable order of seizures' types, are common.195 Autonomic features are commonly seen with the above seizures, with facial flushing, pupillary dilatation, and irregular respirations. Myoclonic, clonic, absence, and atonic seizures may be seen with time.
Stage 1: Early epilepsy onset with brief tonic seizures, often with facial flushing.
Stage 3: Late multifocal and myoclonic epilepsy with tonic seizures, myoclonia, absences, or multifocal seizures.
EEG:
In Stage 1, the interictal EEG is normal but ictal recordings show generalized attenuation followed by fast activity in frontal or central head regions during the tonic seizure.199 A burst-suppression pattern is not seen in this stage. In Stage 2, the interictal EEG is severely abnormal, showing bilateral or generalized slowing with spikes or polyspikes.199 A burst-suppression pattern has rarely been reported in this stage.200 In Stage 3, the interictal recording shows diffuse, high-amplitude delta slowing with pseudo-periodic bursts of spikes, polyspikes, and spike-wave complexes that are maximal in the central, temporal, or temporal-occipital regions.199
Genetics:
A pathogenic or likely pathogenic variant in the CDKL5 gene is required to confirm the diagnosis of CDKL5-DEE, and multiple variants have been reported in affected individuals. There are limited data on genotype-phenotype correlation; however, missense variants may correlate with a slightly less-severe disorder than truncating variants.194
PCDH19 clustering epilepsy
PCDH19 Clustering Epilepsy is an X-linked disease, seen predominantly in females, caused by pathogenic variants in the PCDH19 gene (Table 12). Few males are reported. Epilepsy onset is often during the first year of life (mostly during the first 3 years), and the most characteristic feature is clusters of seizures often induced by fever. Intellectual disability and psychiatric symptoms are reported in about two thirds of cases. The severity of the phenotype seems to be correlated with the age of epilepsy onset.22, 201
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Focal seizures (fearful screaming typical) and tonic-clonic seizures, in clusters; may be triggered by fever |
Prolonged focal clonic (hemiclonic) seizures in infancy (consider Dravet) No clustering |
|
| EEG | Absence of epileptiform discharges (which is usually focal, but rarely may be generalized) | ||
| Age at seizure onset | 1.5–60 months in females; 5–96 months in males | ||
| Other testing: ie, genetics, and so on | PCDH19 pathogenic variant: (see following text for further detail on inheritance pattern) | ||
|
Is MRI or ictal EEG required for diagnosis? An MRI is not required for diagnosis but is strongly recommended to exclude other causes An ictal EEG is not required for diagnosis |
|||
| Possible evolving syndrome: This syndrome should be considered in an infant girl who presents with a first cluster of febrile seizures | |||
| Syndrome without laboratory confirmation: In resource-limited regions, PCDH19 Clustering Epilepsy could be a provisionally diagnosed without confirmatory genetic testing, specifically in the setting of a family history suggestive of X-linked dominant inheritance with male sparing | |||
Epidemiology:
Data on incidence are limited but one study reports an estimated incidence of 1/42 000 live births.41 Large cohorts of females with seizure clusters triggered by fever show rates of PCDH19 pathogenic variants ranging from 2% to 20%.22
Clinical context:
Seizure onset is typically before 1 year of age, with a mean age of 10 months (1.5–60 months in females).21, 202 Development and neurological examination are normal at seizure onset. Head circumference is normal.
Course of illness:
Seizures occur in clusters, which are triggered by fever and often drug resistant. After the first decade, a decrease in the frequency of the seizure clusters generally occurs regardless of the treatment, and remission of seizures may occur in at least one quarter, usually in adolescence to mid-adulthood.21, 22, 202-204
Signs of intellectual disability and autism spectrum disorder, affecting up to 70%, emerge during the second year of life and often become the most relevant symptoms after the first decade. Behavioral disorders, with prominent hyperactivity and possible psychosis in up to 25% of women, are often problematic in adolescence and adults.205
Seizures:
At onset, seizures are focal impaired aware with tonic extension of the upper arms, deviation of head and eyes, pallor of the face, expression of fear, and screaming reported in half of the patients.21, 202
Atypical absences may also be seen.202 Seizures occur in clusters, often related to fever, and status epilepticus has been reported.206
EEG:
Interictal EEG showed slow background activity with rare focal spikes and slow waves that increase in frequency during clusters. With age, background activity may normalize. One third of patients show a photoparoxysmal response and few patients had generalized bursts of spike and waves.21, 202
Seizures recorded on ictal EEG often arise from temporal regions, but parieto-occipital, frontal, or central onset may also be seen. In half of cases, seizures appear focal but are not well lateralized or localized on EEG.21
Neuroimaging:
MRI is typically normal at seizure onset.
Genetics:
PCDH19 pathogenic variants were initially recognized in large pedigrees in which only females were affected by epilepsy and intellectual disability (Epilepsy in Females with Mental Retardation). Currently, approximately half of reported cases are de novo.22
Although the PCDH19 gene is located on Xq22, this condition has an unusual X-linked mode of inheritance sparing transmitting males. Only heterozygous female and mosaic males are affected due to presumed cellular interference. Few affected males with a similar phenotype are reported to date (nine cases reported in the literature).201, 207
SMC1A DEE can mimic PCDH19 Clustering Epilepsy and can present with prolonged clusters of multiple focal and generalized seizures resistant to antiseizure medication, sometimes lasting days. Infants with this disorder have a severe developmental encephalopathy and mild dysmorphic features.208
Metabolic and other lab studies:
No consistent metabolic abnormalities are found.
Glucose transporter 1 deficiency syndrome (Glut1DS)
Glut1DS is a complex neurological disorder associated with a range of neurological symptoms including infantile onset epilepsy, movement disorders, and intellectual disability (Table 13).209, 210 Epilepsy is the most common presenting feature of Glut1DS and is a drug-resistant unless treated with the ketogenic diet.211-213 The syndrome is associated with pathogenic variants in the SLC2A1 gene encoding the glucose transporter type 1, impairing glucose transport across the blood-brain barrier.209
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Seizures, which may be focal or generalized, including absence seizures (often beginning before 3 years of age) | ||
| Neurological exam | Focal neurological findings (other than Todd’s paresis) | ||
| Other testing: ie genetics etc |
Pathogenic SLC2A1 variant OR Low fasting CSF glucose and CSF/plasma glucose ratioa |
Other documented etiology for hypoglycorrhachia | |
| Course of illness | Intellectual disability |
Seizures that are controlled with medication Lack of improvement in seizures with ketogenic diet Lack of movement disorders such as ataxia, paroxysmal exercise-induced dyskinesia, dystonia |
|
|
Is MRI or ictal EEG required for diagnosis? An MRI is not required for diagnosis but is strongly recommended to exclude other causes An ictal EEG is not required for diagnosis |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, GLUT1DS can be diagnosed without EEG, MRI, or genetic studies in children without Alerts who meet all other mandatory and exclusionary clinical criteria. CSF studies are required for diagnosis | |||
- a CSF glucose may not be as low in later-onset epilepsies associated with GLUT1 deficiency syndrome.
Epidemiology:
The estimated incidence of Glut1DS presenting as epilepsy in infancy is 1/24 000 live births; however, the syndrome as a whole may be more common, as individuals may present later in childhood and with symptoms other than seizures.41
Clinical context:
Infants may present with many different seizure types but generalized-onset seizures are more common than focal.211, 214 In any child presenting with epilepsy and a movement disorder Glut1DS should be considered.215 A history of seizures associated with fasting or in the early morning may be present. Other clues to diagnosis include eye-head gaze saccades (consisting of rapid, multidirectional eye movements, accompanied by head movements in the same direction) in early infancy and microcephaly (present in 50% of cases) or deceleration of head growth.209, 213, 216 Diagnosis is confirmed by lumbar puncture identifying low CSF glucose with normal or low CSF lactate after a 4–6 hour fast in the context of a normal blood glucose.217 In Glut1DS, CSF glucose fifth percentile values range from 1.8–2.9 mmol/L, and CSF/plasma glucose ratio fifth percentile values range from 0.41–0.510. In the presence of a highly typical phenotype with a pathogenic SLC2A1 variant, a lumbar puncture may not be necessary.213 In later-onset epilepsy associated with GLUT1DS, CSF glucose levels may not be as low.218
Course of illness:
Seizures vary in frequency from multiple per day to only a few per year and are resistant to antiseizure medications. Seizure frequency tends to decline later in childhood and adult life, where intellectual disability, movement disorders, and migraine may be the predominant features.213, 219 Ketogenic diet with adequate ketosis may completely control seizures. Although this therapy may ameliorate further cognitive decline, many patients are still left with variable degrees of intellectual disability.
Seizures:
Generalized seizures are typically myoclonic, myoclonic-atonic, generalized tonic-clonic, or atypical or early onset absences. Early onset absences (less than age 4 years), often seen with a myoclonic component, should be investigated by lumbar puncture and genetic testing.220 In addition, this disorder should be considered in persons with epilepsy with myoclonic-atonic seizures or drug-resistant absence epilepsy, particularly if cognitive concerns are present. Epileptic spasms and generalized tonic-clonic status epilepticus have also been reported.41
EEG:
Interictal EEG is often normal. There is some evidence for age-specific changes, with focal or generalized slowing of background rhythms in infancy with or without intermittent focal or generalized spike and wave. In children older than 2 years, generalized 2.5–4 Hz spike-wave is seen.221 In some cases, pre-prandial EEG abnormalities may be improved during the recording by feeding as glucose crosses the blood-brain barrier and EEG background rhythms may be less abnormal on the ketogenic diet.222
Neuroimaging:
Approximately 25% of patients have neuroimaging abnormalities including hyperintensity of subcortical U fibers, prominence of perivascular Virchow spaces, prominent ventricles, and delayed myelination for age.209, 223, 224 18F-Deoxyglucose positron emission tomography may show a specific imaging signature including reduced signal from cerebral cortex, cerebellum, and thalamus with apparent increased glucose in the striatum.225
Genetics and other investigations:
Gene sequence analysis identifies heterozygous and less commonly recessive pathogenic variants in SLC2A1 in 81%–89% of cases.209 Another 11%–14% of cases with deletions or duplications in the gene may be identified by multiplex-ligation probe amplification and chromosomal microarray.209 With a highly suspicious clinical phenotype, but nondiagnostic lumbar puncture and genetic testing, other investigations including erythrocyte uptake tests and measurement of glucose transporter type 1 on the surface of red blood cells should be considered.225, 226
Sturge-Weber syndrome (SWS)
SWS is a congenital neurocutaneous syndrome defined by the association of a facial capillary malformation referred to as a port-wine stain birthmark with ipsilateral leptomeningeal angioma and frequent ipsilateral glaucoma. It is caused by somatic activating mutations in the guanine nucleotide–binding protein alpha-q (GNAQ) gene (Table 14).227 The prognosis of SWS is highly variable and related to the potential complications that develop often in early childhood, including epilepsy, focal neurological deficits, and glaucoma.228 The diagnosis is confirmed by brain imaging showing direct or indirect evidence of the leptomeningeal angioma.
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Focal motor or autonomic seizures with or without impaired awareness, which may evolve to bilateral tonic-clonic seizures | ||
| EEG |
Interictal: Lack of asymmetrical background with reduction in voltage and slowing over the affected hemisphere |
||
| Neurological exam | Lack of facial capillary hemangioma affecting the V1 dermatome | ||
| Imaging | MRI showing leptomeningeal enhancement suggestive of leptomeningeal angioma, with cortical calcification and focal cerebral atrophy developing with time | ||
| Course of illness |
Lack of abnormal neurological examination—may be limited to visual field deficit Lack of intellectual disability ranging from mild to profound |
||
|
Is MRI or ictal EEG required for diagnosis? An MRI is required for diagnosis. Changes may be very subtle or absent on MRI done prior to 2 months of age An ictal EEG is not required for diagnosis |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, Sturge-Weber syndrome can be presumptively diagnosed without EEG or MRI in persons without Alerts who meet all other mandatory clinical criteria | |||
Epidemiology:
The estimated incidence of SWS is 1/20 000 to 1/50 000 live births. Patients with a facial port-wine stain on the forehead and/or the upper eyelid have an estimated risk of 20%–70% of developing SWS.229, 230
Clinical context:
The diagnosis of SWS is suspected at birth in newborns presenting a facial port-wine stain covering the forehead and/or the upper eyelid. Careful examination under the hairline is important to detect more subtle lesions. Contrast-enhanced MRI can detect the leptomeningeal angioma before 3 months of age.231 Rarely, the facial angioma may be absent.232
Seizures are usually the first manifestation, affecting 75% to 85% of patients at a median age of 6 months.233 Rare cases with onset of seizures in adulthood have also been reported.233 In addition to epilepsy, 40% to 60% of SWS patients will develop glaucoma with a risk of early visual impairment.233
Course of illness:
Natural history is highly variable but is usually marked by a progressive course with age-dependent neurological manifestations. Early manifestations during infancy include epilepsy, hemiparesis, psychomotor delay, and stroke-like events. Later signs and symptoms at school age include headaches, academic difficulties, and behavioral problems. In adulthood, psychiatric disorders including depression can be significant, and epilepsy and stroke-like events can continue throughout life.
Early seizure onset (before age 12 months), high seizure frequency, and drug resistance are the most reliable predictors of poor outcome.233, 234 Extensive unilateral or bilateral intracranial involvement is associated with earlier onset of seizures and worse cognitive development compared to unilateral leptomeningeal angioma.235 Presurgical evaluation should be considered in patients with unilateral disease who are drug resistant.236
Seizures:
The first seizures are usually focal motor.237 Focal autonomic seizures with variable degrees of impaired awareness are also frequent.238 Seizures can be subtle, and their prompt recognition is important because prolonged seizures and status epilepticus can occur frequently.237 About 30% of cases may have onset of seizures during febrile episodes and there is an increased susceptibility of fever-induced seizures at any age.237 Infantile spasms, myoclonic atonic seizures, and gelastic seizures have also been reported.239 Seizure clustering following a prolonged period of seizure freedom is common (40% of cases).238, 239
Due to the high incidence of early-onset seizures and their potential deleterious effects on the developing brain, parental education in early seizure recognition and individualized emergency plans including the use of rescue benzodiazepine therapy is recommended.240
EEG:
The EEG characteristically shows asymmetric reduction in voltage and slowing of the background over the affected hemisphere (Figure 9).241 The background might be normal, however, during the first year of life. Interictal epileptiform abnormalities may appear later and consist of focal sharp waves or frequent spike-wave bursts.241 Such interictal epileptiform abnormalities before seizure onset might be a useful marker to identify patients with SWS who are at risk of developing epilepsy.242 Ictal activity varies depending on seizure focus.
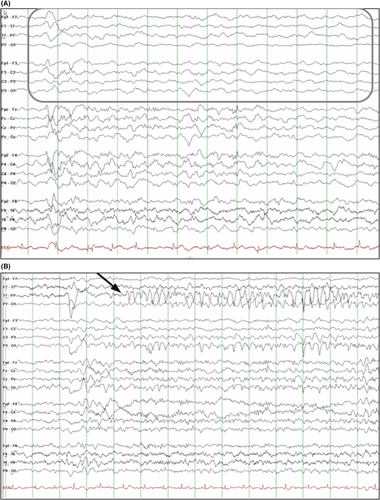
Neuroimaging:
Contrast-enhanced, cerebral MRI confirms the diagnosis of SWS by the direct visualization of leptomeningeal enhancement (Figure 10). Detection can be challenging in very young infants. Other imaging features such as ipsilateral choroid plexus enlargement, enlarged transmedullary veins, and T2 shortening of the white matter can help establish the diagnosis.231 Cortical calcifications and cerebral atrophy appear over time.
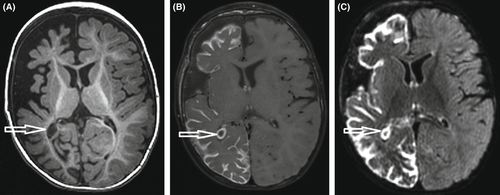
Genetics:
Isolated port-wine stain and SWS have a common genetic etiology, with a somatic mosaic pathogenic variant recently identified in the GNAQ gene.227
Gelastic seizures with hypothalamic hamartoma
Hypothalamic hamartomas are very rare, congenital, non-neoplastic lesions, which are characteristically associated with gelastic (laughing episodes without mirth) or, less commonly, dacrystic (crying) seizures that typically begin in infancy or early childhood (Table 15). Other seizure types including focal impaired awareness or various generalized seizures may evolve, and with time there is progressive cognitive plateauing or regression and progressive behavioral abnormalities including impulsiveness and aggression. Precocious puberty is present in some cases. Seizures remain drug resistant but may improve significantly with surgical intervention. Early surgical therapy should be considered for seizure control and to prevent progressive cognitive and behavioral decline.
| Mandatory | Alerts | Exclusionary | |
|---|---|---|---|
| Seizures | Gelastic seizures with mechanical, mirthless laughter, inappropriate to context | Seizure frequency less than daily | |
| EEG |
Interictal: Generalized or focal background slowing (excluding immediate postictal period) Ictal: Gelastic seizures may lack ictal EEG correlate |
||
| Age at onset | Onset >5 years of age | ||
| Development at onset | Clear developmental delay at seizure onset | ||
| Neurological exam | Focal neurological findings (other than Todd’s paresis) or generalized hypotonia | ||
| Imaging | Hypothalamic hamartoma (may require thin slices through the hypothalamic region to confirm) | ||
| Course of illness | Drug-resistant epilepsy | Lack of behavioral problems including aggression, impulsivity, and hyperactivity | |
|
Is MRI or ictal EEG required for diagnosis? An MRI is required for diagnosis An ictal EEG is not required for diagnosis. Furthermore, gelastic seizures may lack ictal correlate on EEG |
|||
| Syndrome without laboratory confirmation: In resource-limited regions, HH-GS cannot be diagnosed in the absence of an MRI, as gelastic seizures may arise from other brain regions | |||
Epidemiology:
A single study documented a prevalence of hypothalamic hamartoma with gelastic seizures of 0.5/100,000 in children <20 years of age.243
Clinical context:
Onset is in the first year of life in ~85% of cases.244 A minority of cases can begin in early to mid-childhood.243, 245 There is no sex predisposition. Neurological examination is normal; however, general physical examination may reveal features of precocious puberty.
Course of Illness:
Epilepsy due to hypothalamic hamartoma is drug resistant. There is progression over time in most cases, with development of focal impaired awareness and generalized seizures.246, 247 Some patients may develop tonic, atonic, or atypical absences suggestive of Lennox-Gastaut syndrome. Surgical therapy targeting the hypothalamic hamartoma can mitigate this unfavorable evolution. Cognition is typically normal at seizure onset, but over time, developmental plateauing or regression is usually seen. Children can also develop progressive behavioral problems including aggression, impulsivity, hyperactivity, and autism spectrum disorder.
Seizures:
Gelastic seizures are the distinctive seizure type and mandatory for diagnosis. They are seen at epilepsy onset, and are brief, typically lasting less than 1 minute. They consist of mechanical and mirthless laughter, inappropriate to context. Awareness is often not impaired and postictal confusion is absent. Seizure frequency is high, typically multiple per day, and seizures may cluster. Seizures with smiling alone, but without distinctive mirthless laughter, are not gelastic seizures. Dacrystic seizures, characterized by stereotypic lacrimation, and sobbing, grimacing, or yelling, inappropriate to context may also be present. The combination of gelastic and dacrystic seizures in the same patient is particularly suggestive of a hypothalamic hamartoma. Other seizure types that can occur include focal seizures with frontal or temporal lobe semiology and rarely, epileptic spasms. Later in childhood, tonic and drop attacks, as well as atypical absences, may develop.
EEG:
The background is usually normal. Interictal discharges typically appear after infancy and initially are most commonly seen in the temporal regions, although focal spikes from any region may be present. Children with infantile spasms may show a hypsarrhythmia pattern.248
By later childhood, generalized slow spike-wave, or generalized spike or spike-wave can occur, in addition to focal or multifocal discharges.
Ictal recordings of gelastic seizures may show no change, or alternatively may show subtle and nonspecific changes, such as a decrease in amplitude, or reduction in frequency of interictal spikes. On scalp recording, seizures may appear to localize to the temporal or frontal region. However, depth electrodes in the hamartoma will confirm it as the focus of ictal onset,244, 249 and thus surgery should target the hamartoma, as opposed to focal temporal or frontal resection. By later childhood, patients with generalized seizure types will show generalized ictal onset.
Neuroimaging:
MRI shows a pedunculated or sessile lesion (Figure 11) that lies between the infundibular stalk anteriorly and the mammillary bodies posteriorly.250 The lesions are typically isointense to slightly hypointense to gray matter on T1-weighted studies, and hyperintense on T2-weighted studies. They usually do not enhance with contrast. In cases of suspected gelastic seizures, thin slices through the hypothalamic region should be obtained.
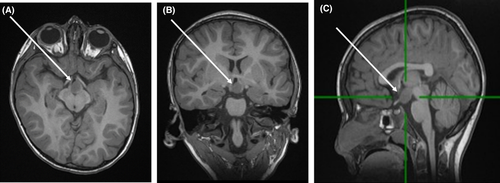
Genetics:
Most cases are sporadic. Approximately 5% of cases have Pallister Hall syndrome with a GL13 pathogenic variant.251
- Gelastic seizures are not always associated with hypothalamic hamartomas but may arise from other foci (most commonly temporal and frontal). In patients without hypothalamic hamartomas, an epilepsy protocol MRI should be obtained to evaluate for other structural lesions.
- Complex stereotypies.
- Infantile self-gratification.
3 DISCUSSION
In defining epilepsy syndromes in neonates and infants, we focus on the electroclinical picture, with careful descriptions of seizure type(s), significant antecedent factors, neurological examination, associated comorbidities, and the interictal and ictal EEG patterns. We hope that this classification will be relevant to all clinicians, regardless of health care resources. Although the proportion of infants with known etiologies is expanding, many are still left with unknown cause, but still fulfill criteria for an epilepsy syndrome, which provides physicians and families guidance regarding optimal therapies, comorbidities, and prognosis.
The Nosology Task Force wished to move away from eponymous names, with some exceptions. We elected to maintain a few syndromes, including Dravet syndrome (or DS), due to the ubiquitous use of this term in research, ongoing precision clinical trials, and orphan drug designation and registration.
We propose using transparent terms that describe the clinical condition, such as IESS. By defining the syndrome by the characteristic seizure type, our aim is to enable early diagnosis and appropriate treatment. Many infants do not fulfill the full triad of West syndrome, as they may lack hypsarrhythmia or regression—thus we propose the term IESS. There is electroclinical overlap between Ohtahara syndrome and Early Myoclonic Encephalopathy, with both syndromes sharing genetic and structural etiologies. In addition, many infants do not meet criteria for either syndrome, highlighting the broad spectrum of presentations within EIDEE. Thus, our Task Force merged both entities into one syndrome called EIDEE.
We aligned our nomenclature with previous classification efforts.2 Syndrome names that contained terminology such as severe (severe myoclonic epilepsy in infancy), malignant (malignant migrating partial seizures in infancy), and benign (benign neonatal seizures) were changed to align with the most recent Classification.2 Similarly, the term “partial seizures” was replaced by “focal seizures.” To avoid any confusion between seizure types and epilepsy syndrome, we replaced the term “convulsions” with “epilepsies” in some syndromes such as Self-Limited Neonatal Epilepsy. Furthermore, because only family history differentiates between Familial and Non-familial SeLNE and SeLIE, we merged these together using the term “Self-limited (Familial) Neonatal Epilepsy” and “Self-limited (Familial) Infantile Epilepsy,” which allows the term “familial” to be used where appropriate.
Finally, we introduce the concept of Etiology-Specific Syndromes for certain genetic and structural etiologies. Gene discoveries have allowed delineation of new electroclinical syndromes, such as PCDH19 Clustering Epilepsy and CDKL5-DEE. Etiology-specific syndromes inform rapid diagnosis and optimization of medical care, and they ensure readiness for precision medicine trials. Given the devastating consequences of many infantile epilepsies, prompt etiological diagnosis offers the hope that novel precision therapies will improve long-term prognosis. Progress in this area relies not only on advances in genetics, imaging, and immunology, but also requires clinicians to carefully phenotype electroclinical and developmental features and long-term outcome in children with early-life epilepsies.
ACKNOWLEDGMENTS
We gratefully acknowledge the input from the following persons outside of our Nosology Task Force who assisted with the Delphi Panels: Drs Birinus Adikaibe, Raidah Al Baradi, Danielle Andrade, Thomas Bast, Ahmed Beydoun, Christian Bien, Roberto Caraballo, Ana Carolina Coan, Mary Connolly, John Dunne, Sheryl Haut, Floor Jansen, Barbara Jobst, Reetta Kalviainen, Angela Kakooza, Mitsuhiro Kato, Kelly Knupp, Silvia Kochen, Lieven Lagae, Luis Carlos Mayor, Natela Okujava, Kurupath Radakishnan, Eliane Roulet-Perez, Loreto Rios, Lynette Sadleir, Daniel San Juan-Orta, Jose Serratosa, Renee Shellhaas, Meng-Han Tsai, Vrajesh Udani, Helen Yue-Hua Zhang, and Dong Zhou.
DISCLOSURES
SM Zuberi has received research support from Epilepsy Research UK, Tenovus Foundation, Glasgow Children's Hospital Charity, and Scottish Government Digital Health & Care. His institution has undertaken commercial trials for GW Pharma, Zogenix, Stoke Therapeutics, Encoded Therapeutics, and Marinus Pharmaceuticals. He has received honoraria for educational symposia, advisory boards, and consultancy work from GW Pharma, UCB Pharma, Eisai, Zogenix, Arvelle Therapeutics, GRIN Therapeutics, Jaguar Gene Therapy, and Encoded Therapeutics. E Wirrell has served as a paid consultant for Encoded Therapeutics and Biomarin. She is the Editor-in-Chief of Epilepsy.com. JM Wilmshurst has received paid honorarium for activities as Associate Editor of Epilepsia. N Specchio has served on scientific advisory boards for GW Pharma, BioMarin, Arvelle, Marinus, and Takeda; has received speaker honoraria from Eisai, Biomarin, LivaNova, and Sanofi; has served as an investigator for Zogenix, Marinus, Biomarin, UCB, and Roche. R Pressler has acted as an investigator for studies with UCB and Johnson & Johnson. She received consulting fees and/or honoraria from UCB, Eisai, Natus, and GW. Her research is supported by the National Institute of Health Research (NIHR) Biomedical Research Centre at Great Ormond Street Hospital, Cambridge Biomedical Research Centre, NIHR and GOSH Charity. S Auvin has served as consultant or received honoraria for lectures from Biocodex, Biomarin, Eisai, GW Pharma, Neuraxpharma, Nutricia, UCB Pharma, Xenon, and Zogenix. He has been investigator for clinical trials for Eisai, UCB Pharma, and Zogenix. He is Associate Editor of Epilepsia. E Hirsch has received honoraria from UCB, Eisai, Livanova, Novartis, and GW Pharmaceuticals. S Wiebe has received research support- from the Canadian Institutes of Health Research and Alberta Innovates Health Solutions. He chairs the Clinical Research Unit at the University of Calgary, which receives support from Cumming School of Medicine. His institution has received unrestricted educational grants from UCB Pharma, Eisai, and Sunovion. JH Cross has acted as an investigator for studies with GW Pharma, Zogenix, Vitaflo, Ovid, and Marinus. She has been a speaker and on advisory boards for GW Pharma, Zogenix, Stoke Therapeutics, and Nutricia; all remuneration has been paid to her department. Her research is supported by the National Institute of Health Research (NIHR) Biomedical Research Centre at Great Ormond Street Hospital. She holds an endowed chair at UCL Great Ormond Street Institute of Child Health; she holds grants from NIHR, EPSRC, GOSH Charity, ERUK, and the Waterloo Foundation. P Tinuper received speaker's or consultancy fees from Arvelle, Eisai, GW Pharma, LivaNova, UCB Pharma, Xenon Pharma, and Zogenix. IE Scheffer has served on scientific advisory boards for UCB, Eisai, GlaxoSmithKline, BioMarin, Nutricia, Rogcon, Chiesi, Encoded Therapeutics, and Xenon Pharmaceuticals; has received speaker honoraria from GlaxoSmithKline, UCB, BioMarin, Biocodex, and Eisai; has received funding for travel from UCB, Biocodex, GlaxoSmithKline, Biomarin, and Eisai; has served as an investigator for Zogenix, Zynerba, Ultragenyx, GW Pharma, UCB, Eisai, Anavex Life Sciences, Ovid Therapeutics, Epigenyx, Encoded Therapeutics, and Marinus; and has consulted for Zynerba Pharmaceuticals, Atheneum Partners, Ovid Therapeutics, Care Beyond Diagnosis, Epilepsy Consortium, and UCB. E Perucca received speaker and/or consultancy fees from Angelini, Arvelle, Biogen, Biopas, Eisai, GW Pharma, Sanofi group of companies, SK Life Science, Takeda, UCB Pharma, Xenon Pharma, and Zogenix; and royalties from Wiley, Elsevier, and Wolters Kluwer. SL Moshé is the Charles Frost Chair in Neurosurgery and Neurology and acknowledges grant support by U.S. National Institutes of Health (NIH) U54 NS100064 and NS43209, U.S. Department of Defense (W81XWH-18-1-0612), the Heffer Family and the Segal Family Foundations, and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. SL Moshé is serving as Associate Editor of Neurobiology of Disease. He is on the editorial board of Brain and Development, Pediatric Neurology, Annals of Neurology, MedLink Neurology, and Physiological Research. He receives compensation from Elsevier for his work as Associate Editor in Neurobiology of Disease and from MedLink for his work as Associate Editor; and royalties from two books he co-edited. R Nabbout has served as principal investigator in clinical trials for Novartis, Nutricia, Eisai, UCB, GW Pharma, and LivaNova. She received consulting and lecturer honoraria from Biogene, BioMarin, Praxis, GW Pharma, Zogenix, Novartis, Nutricia, Stoke, Ionis, Targeon, Neuraxpharma, Takeda, Nutricia, Biocodex, Advicenne, and Eisai. She received unrestricted research grants from Eisai, UCB, LivaNova, and GW Pharma, and academic research grants from EJP-RD (horizons 2020). E Yozawitz, K Riney, P Samia, S Galicchio, C Triki, and OC Snead report no conflicts of interest.
ETHICAL STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.




