The ILAE classification of seizures and the epilepsies: Modification for seizures in the neonate. Position paper by the ILAE Task Force on Neonatal Seizures
Abstract
Seizures are the most common neurological emergency in the neonatal period and in contrast to those in infancy and childhood, are often provoked seizures with an acute cause and may be electrographic-only. Hence, neonatal seizures may not fit easily into classification schemes for seizures and epilepsies primarily developed for older children and adults. A Neonatal Seizures Task Force was established by the International League Against Epilepsy (ILAE) to develop a modification of the 2017 ILAE Classification of Seizures and Epilepsies, relevant to neonates. The neonatal classification framework emphasizes the role of electroencephalography (EEG) in the diagnosis of seizures in the neonate and includes a classification of seizure types relevant to this age group. The seizure type is determined by the predominant clinical feature. Many neonatal seizures are electrographic-only with no evident clinical features; therefore, these are included in the proposed classification. Clinical events without an EEG correlate are not included. Because seizures in the neonatal period have been shown to have a focal onset, a division into focal and generalized is unnecessary. Seizures can have a motor (automatisms, clonic, epileptic spasms, myoclonic, tonic), non-motor (autonomic, behavior arrest), or sequential presentation. The classification allows the user to choose the level of detail when classifying seizures in this age group.
Key points
- The International League Against Epilepsy (ILAE) presents a new classification and framework for seizures in the neonatal period in line with 2017 ILAE classifications.
- It emphasizes the key role of electroencephalography (EEG) for the diagnosis of seizures in this age group.
- Seizures are considered focal at onset, and thus a division into focal and generalized is unnecessary.
- Seizures can occur with clinical manifestations or without clinical manifestations (electrographic-only).
- Descriptors are determined by the predominant clinical feature and divided into motor, non-motor, and sequential.
1 DEFINITIONS
For the purpose of this report, the following definitions are used1, 2:
- Gestational age (GA): time elapsed between the first day of the last menstrual period and the day of delivery (completed weeks).
- Postmenstrual age (PMA): gestational age plus chronological age (in weeks).
- Preterm infant: born before GA of 37 weeks.
- Neonatal period: period from birth up to 44 weeks PMA.
2 INTRODUCTION
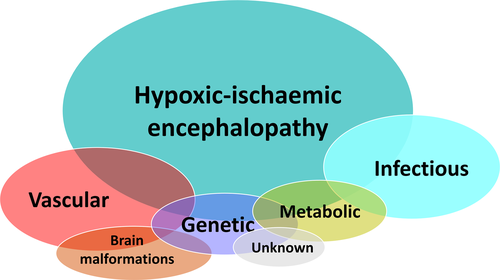
Seizures are the most common neurological emergency in the neonatal period, occurring in 1–5 per 1000 live births.3-5 The majority of neonatal seizures are provoked by an acute illness or brain insult with an underlying etiology either documented or suspected, that is, these are acute provoked seizures (previously also called acute symptomatic, although acute provoked is now the preferred term). They do not meet the criteria for the diagnosis of epilepsy, which is defined as meeting any of the following conditions: (a) at least two unprovoked seizures occurring >24 hours apart; (b) one unprovoked seizure and a probability of further seizures similar to the general recurrence risk after two unprovoked seizures; and (c) diagnosis of an epilepsy syndrome.6, 7 Epilepsy syndromes may present in the neonatal period and, with the increasing availability of genetic testing, expanding numbers of neonatal epilepsies with genetic and metabolic etiologies are recognized.5, 8 Although many causes can give rise to neonatal seizures, a relatively small number account for most seizures (Figure 1) including hypoxic-ischemic encephalopathy, stroke or hemorrhage, infections, cortical malformations, errors of metabolism (acute and inborn), and genetic etiologies. Less common but important causes are neonatal drug withdrawal and birth-related head trauma.
Neonatal seizures have been categorized previously as clinical only, electroclinical, or electrographic-only.9, 10 A clinical-only seizure has been defined as a sudden paroxysm of abnormal clinical changes without a definite EEG association. Currently there is no evidence that these clinical-only events are epileptic in nature (see historical review below). An electro-clinical seizure features definite clinical signs simultaneously coupled with an electrographic seizure. An electrographic-only seizure refers to the presence of an electrographic seizure seen on EEG that is not associated with any evident clinical signs (synonyms: clinically silent or subclinical seizures). The term electrographic-only is preferred, as this depends on observational methods used and the seizure may not be truly subclinical.
The clinical diagnosis of neonatal seizures is difficult, particularly in critically ill infants, due to the multitude of epileptic and nonepileptic clinical manifestations within the intensive care setting.9, 11 In the study by Malone, 20 video clips of paroxysmal events in neonates were presented to 137 health professionals (mostly neonatologists and intensivists) with the aim of classifying movements as seizure or nonseizure.12 The average of correctly identified events was 10 of 20. There was poor inter-observer agreement independent of observers’ specialty. The immature state of the motor pathways13, 14 in term and preterm neonates may account for some of the difficulties in differentiating seizures from nonepileptic movements.15 In selected populations, particularly in infants with hypoxic-ischemic encephalopathy (HIE), 50%–80% of seizures are electrographic-only and, as a result, the extent of the seizure burden may be greatly underestimated.8-11, 16, 17 Seizure burden can be defined as ictal (or seizure) electrographic activity in a given period of EEG recording and expressed as summed electrographic seizure seconds.18 Seizure burden should be differentiated from seizure frequency, which does not take duration of seizures into account. Treatment of seizures, particularly with phenobarbital, can result in the so-called “uncoupling” or ”decoupling,” meaning electroclinical seizures become electrographic-only.9, 10, 17, 19-21 Although therapeutic hypothermia for HIE reduces the overall seizure burden, it can also increase electroclinical uncoupling of seizures.11 There is evidence that electrographic-only seizure burden has an effect on neurological outcome comparable to that of electroclinical seizures.16, 22-26
The American Clinical Neurophysiology Society has recently defined an electrographic neonatal seizure as “a sudden, abnormal EEG event, defined by a repetitive and evolving pattern with a minimum 2 μV peak-to-peak voltage and duration of at least 10 seconds.” “Evolving” is defined as an unequivocal evolution in frequency, voltage, morphology, or location,27 for example, increasing amplitude and decreasing frequency of discharges over time. This definition does not require any evident clinical change.
3 HISTORICAL REVIEW
Historical efforts to characterize and classify neonatal seizures have been directed toward emphasizing how they differ from those of older children and adults. In this report, our aim is to use terminology consistent with the 2017 ILAE Classification of Seizures and the Epilepsies.7, 28
Studies in the 1950s and early 1960s focused on motor and behavioral changes, were based on direct observation with or without EEG recordings, and included focal clonic and generalized tonic seizures,29-31 and later also myoclonus.32
Early investigators recognized autonomic nervous system changes including variation in respiratory rate, vasomotor changes, salivation, heart rate, and blood pressure as seizure manifestations.33 Polymorphic and atypical clinical events were described, the latter including staring, sudden awakening and alerting, eye deviation, eye blinking, nystagmus, chewing, and limb movements such as swimming, rowing, and pedaling,34 classified as “anarchic,”30 “minimal”35 or “subtle.”36 These findings resulted in the classification proposed by Volpe, which included: multifocal clonic, focal clonic, tonic, myoclonic, and subtle seizures.36, 37
Correlating contemporaneous visual analysis of clinical seizures as well as electroencephalographic and polygraphic measures, Watanabe and colleagues recognized a wide range of motor, behavioral, and autonomic signs and provided detailed electroclinical correlations. Using video-EEG recordings, Mizrahi and Kellaway also documented electroclinical correlations and noted that many clinical events previously reported as seizures presumed to be of epileptic origin were in fact nonepileptic.9 Events such as generalized tonic episodes and so-called subtle seizures, both of which occur without EEG correlate, could be provoked by stimulation and suppressed by restraint. This led to a reconsideration of the classification of neonatal seizures based on pathophysiology (epileptic vs nonepileptic); electroclinical relationships (electroclinical, clinical only, electrical only); or behavioral (focal clonic, focal tonic, myoclonic, spasms, generalized tonic, motor automatisms—each with additional modifiers to suggest whether they were considered to be of epileptic or nonepileptic origin). The term motor automatisms included ocular movements, oral-buccal-lingual movements, and “progression movements of the limbs” (pedaling, swimming, rowing).9
With the advent of prolonged bedside electrographic monitoring in the neonatal intensive care unit (NICU), it has been increasingly recognized that electrographic-only seizures without clinical correlates are frequent, particularly in critically ill neonates. As a result, the definition of neonatal seizures has been reconsidered, now with a focus on the electrographic basis of the events, either with or without clinical manifestations.38
The 2017 ILAE Position Papers on Classification of Seizure Types and the Epilepsies presented a framework for classification including seizure types, epilepsy types, and syndromes.7, 28 A seizure is currently defined as a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain.6 However, a seizure does not necessarily mean that a person has epilepsy. It is of note that electrographic-only seizures are not included in this definition. Seizure semiology is the description of signs and symptoms associated with an ictal event and is valuable in localizing the epileptogenic zone. In the neonate, the development within the limbic system with its connections to midbrain and brainstem is more advanced than the cerebral cortical organization, which may, in part, account for some differences in neonatal seizure semiology compared to that in older children.39
The ILAE Commission on Classification & Terminology recognized that seizures in the neonate require special considerations and therefore a Neonatal Task Force was established with the aim of integrating seizures and epilepsies in this age group into the 2017 ILAE Classification.
4 METHODS
The goal of the task force was to develop a classification of seizures in neonates that can fulfil the following criteria:
- Integrate into the 2017 ILAE Classifications.
- Be based on electroclinical phenotype.
- Emphasize the key role of EEG in the diagnosis of neonatal seizures.
- Have implications for management and treatment of events.
- Be acceptable to neonatologists, pediatricians, epileptologists, neurophysiologists, and neurologists.
- Be applicable in all healthcare settings.
The task force followed the process for a Position Paper outlined by the ILAE (https://www.ilae.org/files/dmfile/Process-of-Publishing-ILAE-Commision-and-Task-Force-Reports-25-Jan-2020.pdf). This process includes the appointment of a task force (group of experts selected by the League), which produces an initial proposal, posting of this proposal on the ILAE website, soliciting comments, and criticism by all stakeholders (public consultation), and finally appointing a second expert panel to review and incorporate the public comments as well as the peer review by Epilepsia.
During the 5-month public consultation, we received comments from individuals as well as learned bodies and interested groups, all of which were reviewed by the second Task Force (see Report of the second neonatal seizure Task Force, Appendix S1). Most of the comments and criticisms were constructive and provided invaluable feedback, which informed the content of the Position Paper.
5 CLASSIFICATION
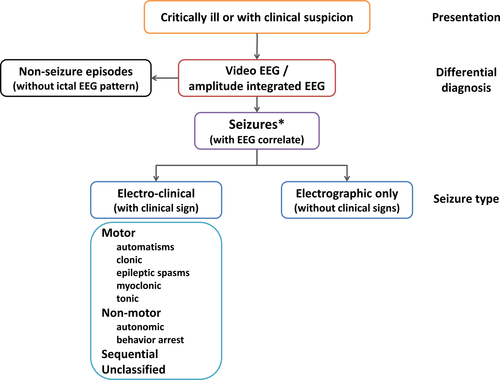
Figure 2 depicts the diagnostic framework for seizures in the neonatal period, which includes the classification of seizures.
5.1 Presentation
Newborns may present with paroxysmal clinical events suspected to be seizures of epileptic origin; these include motor and non-motor phenomena. However, as mentioned earlier, many neonates will have mostly or exclusively electrographic-only seizures, which will only become apparent on EEG or amplitude-integrated EEG (aEEG, see below).
5.2 Diagnosis/Differential diagnosis
In neonates, video-EEG recording is the gold standard for diagnosis.4, 9, 18, 40-42 However, it is recognized that many neonatal units have only limited or no access to EEG. Instead, many neonatologists use aEEG, which is a simplified bedside neurophysiology tool displaying one or more commonly two channels of EEG in a filtered and compressed manner.43, 44 In situations when and where full EEG is not readily available, aEEG may be used with co-registration of raw channels, although its limitations are well recognized.4, 45
A proportion of seizures are electrographic-only, particularly in encephalopathic and critically ill patients.10, 11, 46 In the neonate, the immaturity of the central nervous system may also contribute. Hence, electrographic-only seizures should be part of the classification. The initial stage of description of a neonatal seizure should specify whether a seizure is with (electroclinical) or without clinical signs (electrographic-only). Instances have been described where clinical seizures occur both with and without an associated rhythmic EEG discharge in a given patient; however, this is considered to be a rare occurrence and by definition implies that electrographic seizures (with or without clinical correlate) also occur in that given patient.19, 21 Therefore, only events with EEG correlate are included in this classification. Theoretically, focal seizures originating from subcortical cerebral areas such as the limbic and peri-limbic systems may be missed. However, this notion is not, at present, provable or disprovable. Studies have shown that the most of clinical-only events are not of epileptic origin9, 15 and that in epileptic seizures an electrographic ictal pattern will become apparent during more prolonged EEG monitoring.16, 47 Polygraphic video-EEG can help to evaluate any manifestations in question such as autonomic features or automatisms and decrease the risk of over-diagnosing of common nonseizure events as epileptic.9, 15, 48, 49
5.3 Seizure types
We used the definition of seizure type as suggested by Fisher and colleagues: a useful grouping of seizure characteristics for purposes of communication in clinical care, teaching, and research.7
The basic principles of the 2017 ILAE classification of seizure types7 (see online Appendix S2) are based on the 1981 classification with the initial division of seizures into those of focal and generalized onset.50, 51 Newborns have been shown to have seizures with exclusively focal onset,38, 52 thus the initial division into focal and generalized is unnecessary. Nevertheless, in some rare conditions, seizures may rapidly engage bilaterally distributed networks such as spasms or myoclonic seizures, for example, in inborn errors of metabolism. Even in genetic early infantile developmental and epileptic encephalopathies, tonic seizures are initially focal or asymmetric in the neonatal period9, 53 and subsequently may become generalized in infancy. The second level in the 2017 ILAE classification is the division into aware and impaired awareness seizures; however, this is not applicable to neonates, as it is not possible to confidently and reproducibly assess awareness and responsiveness in this age group.
This is followed by the division into motor and non-motor seizures, and finally by the seizure type (Table 1). Although seizures in neonates can present with a variety of clinical signs, in the majority of cases a single predominant feature can be determined. Pragmatically, it appears best to classify seizures according to the predominant clinical manifestation, as this is more likely to have clinical implications for etiology than determination of the seizure-onset zone. This may or may not be the first clinical manifestation. For example, a neonate may present with focal tonic posturing, and in addition have some ocular myoclonus—this can still be classified as a tonic seizure. Regardless, as in adults, localization within the brain should be specified when known and appropriate.
| Type | Description6, 7 | Special considerations | Clinical context of seizure type | Source |
|---|---|---|---|---|
| Automatisms | A more or less coordinated motor activity usually occurring when cognition is impaired. This often resembles a voluntary movement and may consist of an inappropriate continuation of preictal motor activity. | Typically oral in neonates. Behavior in term and preterm infants may mimic ictal automatisms, thus EEG / aEEG mandatory. | Seen in HIE and preterm infants. Often part of sequential seizures. | 9, 83, 84 |
| Clonic | Jerking, either symmetric or asymmetric, that is regularly repetitive and involves the same muscle groups. | Seizure type, which is more reliably diagnosed clinically. | Typical seizure type in neonatal stroke or cerebral hemorrhage. May be seen in HIE. | 9, 12, 85-87 |
| Epileptic spasms | A sudden flexion, extension, or mixed extension–flexion of predominantly proximal and truncal muscles that is usually more sustained than a myoclonic movement but not as sustained as a tonic seizure. Limited forms may occur: Grimacing, head nodding, or subtle eye movements. |
Brief in neonates, thus may be difficult to differentiate from myoclonic seizures without EMG channel. May occur in clusters. |
Rare. May be seen in inborn errors of metabolism or early-infantile DEE. | 53, 96 |
| Myoclonic | A sudden, brief (<100 msec) involuntary single or multiple contraction(s) of muscles(s) or muscle groups of variable topography (axial, proximal limb, distal). | Clinically difficult to differentiate from non-epileptic myoclonus, requires EEG, ideally with EMG channels. | Typical seizure type in inborn errors of metabolism and preterm infants. May also be seen in early-infantile DEE. | 88, 90, 91, 93, 94, 97 |
| Tonic | A sustained increase in muscle contraction lasting a few seconds to minutes. | Focal, unilateral or bilateral asymmetric. Generalized tonic posturing not of epileptic origin. | Typical seizure type early-infantile DEE and genetic neonatal epilepsies. | 57, 62, 88, 91, 96, 98, 99, 101 |
| Autonomic | A distinct alteration of autonomic nervous system function involving cardiovascular, pupillary, gastrointestinal, sudomotor, vasomotor, and thermoregulatory functions. | May involve respiration (apnea). EEG / aEEG mandatory. | Rare in isolation. Seen in intraventricular hemorrhage as well as temporal or occipital lobe lesions. Also described in early-infantile DEE. | 9, 53, 99, 102-104 |
| Behavioral arrest | Arrest (pause) of activities, freezing, immobilization, as in behavior arrest seizure. | EEG / aEEG mandatory. | Rare as an isolated seizure type. More commonly seen as part of sequential seizure. | 53, 105 |
| Sequential seizure | This term is used in the instruction manual for the ILAE 2017 operational classification of seizure types for events with a sequence of signs, symptoms, and EEG changes at different times.6 | No predominant feature can be determined, instead the seizure presents with a variety of clinical signs. Several features typically occur in a sequence, often with changing lateralization within or between seizures. | Often seen in genetic epilepsies such as self-limited neonatal epilepsy or KCNQ2 encephalopathy. | 54, 58, 62, 83, 98-100 |
| Electrographic-only seizure | Subclinical, without clinical manifestation. | EEG / aEEG mandatory. | Often seen in preterm infants, HIE (particularly in those with basal ganglia/thalamus injury), critically ill and neonates undergoing cardiac surgery. | 9, 11, 15, 81, 106-109 |
| Unclassified seizure type | Due to inadequate information or unusual clinical features with inability to place in other categories. | EEG / aEEG mandatory. |
- Abbreviations: aEEG, amplitude-integrated EEG; early infantile DEE, early infantile developmental and epileptic encephalopathy; EEG, electroencephalography; EMG, electromyography; HIE, hypoxic-ischemic encephalopathy; ILAE International League Against Epilepsy; msec, milliseconds.
In some situations, it may be difficult to identify the dominant feature, typically in longer seizures where a sequence of clinical features can be seen, often with changing lateralization. Events with a sequence of signs, symptoms, and EEG changes at different times have been described as a sequential seizure in the 2017 ILAE classification manual.6 Because this is often seen in neonates, this term was added to the seizure types. Sequential refers to several seizure manifestations occurring in sequence (not necessarily simultaneously) in a given seizure, and not manifestations in different seizure types (eg, a neonate may present with epileptic spasms and other focal seizures). Typical examples for sequential seizure are seen in neonates with self-limited neonatal epilepsy, which have been described as stereotyped with a variety of manifestations including tonic, clonic, automatisms, and autonomic features (including apnea), which show varying lateralization during a single seizure.54, 55 Similar seizures have been reported in neonates with KCNQ2 or SCN2A encephalopathy.56-58 Sequential seizures need to be differentiated from migrating focal seizures, which is an electroclinical phenomenon described in some genetic syndromes.59
Several seizure types described in the 2017 ILAE classification cannot be diagnosed in newborns due to lack of verbal and limited nonverbal communications. These include sensory, cognitive, and emotional seizures. Sensory seizures are defined as a perceptual experience not caused by appropriate stimuli in the external world. Such seizures may in rare cases produce semiology such as grimacing or crying, but it is assumed that in the vast majority of cases they would appear as electrographic-only events. Awareness and responsiveness cannot be accurately assessed in neonates and hence is not readily classified; however, this may change with more advanced technology or detailed observation. Similarly, somatosensory or visual auras cannot be determined in neonates. Due to the relatively low muscle tone and supine position of newborns, the occurrence of atonic seizures cannot be evaluated clinically without invasive methods.53 These seizure types are therefore not included in the new classification. Motor seizures can be further described using descriptors as listed in Table 2. The framework allows the user to classify the seizure in as much detail as required in a certain situation. The full description would include manifestation, a descriptor, and etiological diagnosis.
| Seizure type | Descriptors |
|---|---|
| Automatisms |
Unilateral Bilateral asymmetric Bilateral symmetric |
| Clonic seizures |
Focal Multifocal Bilateral |
| Epileptic spasms |
Unilateral Bilateral asymmetric Bilateral symmetric |
| Myoclonic seizures |
Focal Multifocal Bilateral asymmetric Bilateral symmetric |
| Tonic seizures |
Focal Bilateral asymmetric Bilateral symmetric |
5.4 Epilepsy syndromes
Although the majority of seizures in the neonatal period occur in the context of an acute illness, in some cases the seizures may be the first manifestation of early infantile epilepsy. Early differentiation of provoked seizures from neonatal-onset epilepsies has important diagnostic, therapeutic, and prognostic implications because the evaluation and long-term management of neonatal epilepsies are distinct from those of provoked seizures.60 Syndromes presenting in the neonatal period include the following:61 self-limited neonatal epilepsy (previously benign familial neonatal seizures) and early infantile developmental and epileptic encephalopathy (previously early myoclonic epilepsy and early infantile epileptic encephalopathy) (see also proposal by ILAE Task Force on Nosology and Definitions, in preparation).
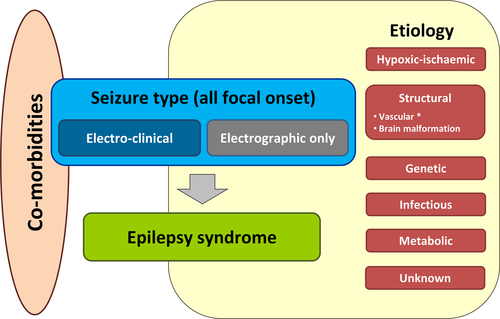
Recent advances in neuroimaging and genomic technology as well as the implementation of video-EEG in the NICU, allow for the identification of more discrete, etiology-specific neonatal epilepsy syndromes than previously recognized.61-63 It is likely that the combination of more sophisticated genetic testing and video-EEG monitoring will allow the identification and stratification of distinct etiology-specific electroclinical phenotypes,58 as suggested in the new ILAE classification of the epilepsies.28 This framework has been adapted for neonates (Figure 3).
6 Discussion
In line with the new ILAE seizure classification7 and ILAE framework for the epilepsies,28 a new ILAE classification for seizures in the neonatal period has been developed by the ILAE neonatal task force. This classification emphasizes the role of EEG in the diagnosis of seizures and includes a classification of seizure types relevant to this age group. The seizure type is typically determined by the predominant clinical feature. In most electroclinical seizures in neonates, the first feature is also the predominant feature. Review of the literature suggests that seizure semiology in neonates may have diagnostic value with respect to etiology and/or outcome and thus implications for management (Table 1). For example, focal clonic movements can frequently be observed as the first and also predominant feature of seizures in perinatal stroke.
However, many of these clinical associations are based on small case studies or with very limited description of semiology and will need to be tested on a larger data set.
Clancy and colleagues described electrographic-only seizures in newborns as sudden, repetitive, evolving stereotyped waveforms with a definite beginning, middle, and end and a minimum duration of 10 s.46 However, the choice of 10 s duration was explicitly arbitrary. Similarly, an arbitrary minimum duration of 10 s is also applied to the definition of a seizure in critically ill adults.64 This is in contrast to some electroclinical seizures such as myoclonic seizures or spasms, which are by definition shorter than 10 s.6, 7, 65 Both in neonates and critically ill adults it has been suggested that brief rhythmic discharges (so-called BRDs [brief rhythmic discharges] or BIRDs [brief interictal/ictal rhythmic discharges]) are associated with more sustained electrographic seizures with the same morphology in the same or subsequent EEG recording66-69 and an increased risk of abnormal neurodevelopmental outcome.67 BRDs are defined as very brief (<10 s) runs of focal or generalized sharply contoured rhythmic activity, with or without evolution, that are not consistent with any known normal or benign pattern, which in adults have a frequency greater than 4 Hz.70
BRDs may be considered part of the ictal-interictal continuum. It is of interest that the presence or absence of evolution is not part of the definition. It has been suggested that definite BRDs with an evolution represent “very brief” electrographic seizures (Figure 4).69, 70
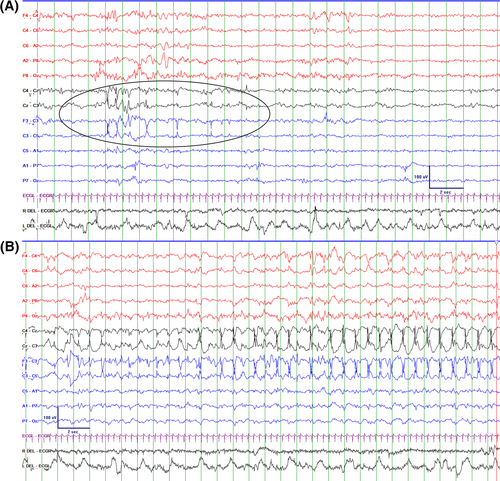
We define seizures in the neonatal period as:
An electrographic event with a pattern characterized by sudden, repetitive, evolving stereotyped waveforms with a beginning and end. The duration is not defined but has to be sufficient to demonstrate evolution in frequency and morphology of the discharges and needs to be long enough to allow recognition of onset, evolution, and resolution of an abnormal discharge.
This is a conceptual definition and how this relates to decisions on therapy is discussed below. Although it has been suggested that 10 s may allow better interrater reliability, in some cases shorter ictal patterns may be identified as seizures because of their evolution and morphology similar to other events in the same recording that are longer and thus meet duration criterion. BRDs without evolution are not considered seizures but may serve as an early predictor of seizures during subsequent EEG monitoring and as a prognostic indicator. Notable exceptions are certain clinical seizures such as myoclonic seizures and spasms.
In defining electroclinical and electrographic-only seizures, we acknowledge that the decision of when to treat neonatal seizures depends not only on the correct diagnosis but just as much on the seizure burden. The seizure burden (electrographic seizure seconds in a given period), but not seizure frequency (number of seizures in a given period regardless of duration) or clinical manifestation, is associated with poor outcome.71 It is generally agreed that rare brief seizures may not require treatment but should initiate EEG monitoring so that seizure burden can be evaluated.72 It has been suggested that a seizure burden of >30–60 s per hour should be considered as an indication to start treatment.72 Electrographic seizure burden and seizure frequency may impact the treatment approach, but the presence or absence of clinical signs should not.25, 26 The ILAE Neonatal Seizure Guideline Task Force is currently updating the 2011 World Health Organization (WHO) guidelines for neonatal seizures,73 which will be addressing these specific aspects of treatment related decision-making.
The task force accepts that the current reality in many regions of the world is that access to even the most basic EEG studies is not possible.4, 74 Acknowledging this, the role of the Task Force was to define the gold standard approach to diagnosis and recognition of neonatal seizures. This can be used to lobby for better facilities even if the process is challenging and takes many years to achieve.
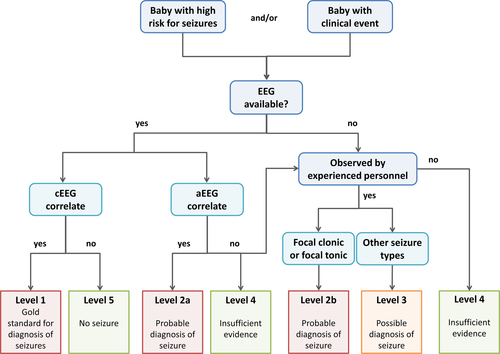
If EEG is not available, we would like to refer to an algorithm developed by the Brighton collaboration defining different degrees of diagnostic certainties4 depending on diagnostic tests available (Figure 5). EEG is regarded as the gold standard (definite diagnosis), whereas events seen on aEEG can be considered to be seizures with “probable certainty.” If only clinical evaluation is available, focal clonic seizures and focal tonic seizures can also be considered “probable seizures,” whereas other clinical events such as automatisms, autonomic seizures, and seizures with behavioral arrest would always require EEG confirmation and thus can be deemed “possible seizure,” only if no EEG is available. Electrographic-only seizures will, by definition, be missed without EEG. Generalized tonic extensor posturing events, without clear asymmetry, are not considered seizures and bedside maneuvers can help in identifying clinical events as exaggerated reflex behaviors and nonepileptic in origin.9 If stimulation of the infant provokes behaviors similar to a spontaneously observed clinical event suspected of being seizures and restraint of infant limbs during spontaneous events prompts an arrest of the events, they may be considered to be nonepileptic events. Although these infants may not have clinical seizures, the onset of these paroxysmal movements warrants further assessment, since they too can be associated with significant central nervous system disorders and subsequent neurological impairment.
This position paper does not address the definition or classification of status epilepticus in neonates. Neonatal status epilepticus is relatively common and is associated with poor outcome but no widely accepted definition exists.75 The recent report of the ILAE task force of status epilepticus76 is only partially applicable to neonates, as it does not address seizure burden and electrographic-only seizures and does not take into account that status epilepticus-induced hippocampal injury is age dependent and less likely to occur in the young.77
Although this framework was developed for seizures in the neonatal period, we believe that some aspects can be readily applied to acute seizures in critically ill patients of any age, particularly within the intensive care setting. Nonconvulsive seizures are common in critically ill patients78 and electrographic-only presentation due to electroclinical uncoupling has been described in two thirds of critically ill children with seizures.79, 80 However, the etiologies may vary with age. Further prospective evaluations of this classification are recommended in neonates.
ACKNOWLEDGMENTS
Special thanks are given to all members of the ILAE and other stakeholders who have contributed to the public comments; their contribution to finalizing this classification was invaluable. Additional helpful key comments were received from the International Federation of Clinical Neurophysiology (an ad hoc group led by Dr Monika Eisermann, Paris).
CONFLICT OF INTEREST
Ronit M. Pressler has no conflicts of interest in regards to this article. She is an investigator for studies with UCB and Johnson & Johnson. She served as a Consultant and on Advisory Boards for Esai and UCB. Her research is supported by the National Institute of Health Research (NIHR) Biomedical Research Centre at Great Ormond Street Hospital, Cambridge Biomedical Research Centre, NIHR and GOSH Charity. Solomon L. Moshé has no conflicts of interest in regards to this article. He is the Charles Frost Chair in Neurosurgery and Neurology and partially funded by grants from National Institutes of Health (NIH) U54 NS100064 and NS43209, US Department of Defense (W81XWH-13-1-0180 and EP170020), CURE Infantile Spasms Initiative, and the Heffer Family and the Segal Family Foundations and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. He is serving as Associate Editor of Neurobiology of Disease and is on the editorial board of Brain and Development, Pediatric Neurology and Physiological Research. He receives from Elsevier an annual compensation for his work as Associate Editor of Neurobiology of Disease and royalties from two books he co-edited. He received a consultant fee from Eisai, Mallinckrodt, Pfizer, and UCB. Eli M. Mizrahi has no conflicts of interest with regard to this article. He has received consultant fees from Eisai and royalties from Elsevier, McGraw-Hill and Springer publishers. Sameer M. Zuberi has no conflicts of interest in relation to this article. He has received research funding from Epilepsy Research UK, UCB Pharma, Dravet Syndrome UK, and Glasgow Childrens Hospital Charity. He has served as a Consultant and on Advisory Boards for Encoded Genomics, Zogenix, UCB Pharma, Biocodex. He receives an honorarium from Elsevier for his role as Editor-in-Chief of the European Journal of Paediatric Neurology. Jo M. Wilmshurst has no conflicts of interest in regard to this article. She has received a stipend from Wiley for her role as an Associate Editor for Epilepsia. Magda L. Nunes has no conflicts of interest in regards to this article. She is a researcher 1D supported by CNPq–Brazil, PQ grant number 306338/2017-3. Sampsa Vanhatalo has no conflicts of interest in regards to this article. He is supported by the Finnish Academy (SV: 313242, 288220, 3104450), Pediatric foundation, and HUS Children’s Hospital. Maria Roberta Cilio has no conflict of interest in regards to this article. She served as Consultant and on Advisory Boards for GW Pharmaceuticals, UCB, Sanofi Pharma, and Biocodex. She receives royalties from Elsevier as co-editor of a book. The other authors have no conflict of interest to disclose in relation to this publication. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.