Who seizes longest? Impact of clinical and demographic factors
Abstract
Objective
To investigate the impact of clinical and demographic parameters on the duration of focal onset seizures with and without secondary generalization using precise duration measurements from intracranial electroencephalographic (iEEG) recordings.
Methods
Patients with unifocal epilepsy syndromes and iEEG recording were retrospectively identified from the database of the local epilepsy center (2006-2016). Seizure duration was defined as time difference of iEEG seizure pattern onset and cessation. The seizure semiology was classified based on video recordings. Clinical and demographic data were extracted from patient reports.
Results
In total, 69 adults were included, and 654 focal onset seizures were analyzed. Focal to bilateral tonic-clonic seizures (FBTCSs; 98/654) were significantly longer than focal seizures (FSs) without generalization (FS-BTCs; 556/654, P < .001), and most FSs (545/654, 83.3%) terminated within 2 minutes. The duration of FSs was prolonged with increasing age of the patients (P = .003) and was significantly shortened (P < .001) by evolution into an FBTCS. FBTCSs with lateralizing semiologies like version (P = .015) and sign of four (P = .043) were associated with longer bilateral tonic-clonic manifestations. Furthermore, FBTCSs with preceding aura, frontal origin, or onset during sleep were by trend shorter. Age (P < .001) and disease duration (P = .028) were essential for prediction of FS-BTC duration, whereas the vigilance state (P = .085) was the main prediction factor for the duration of FBTCSs.
Significance
The identified modifiers of seizure duration are of great relevance for clinical risk evaluation, especially in the aging epilepsy patient suffering from temporal lobe epilepsy with secondary generalized seizures.
Key Points
- This is the first study to systematically analyze the association of the duration of focal seizures and clinical and demographic factors.
- A positive association was revealed for the duration of focal seizures and age of the patients.
- Male gender, preceding aura, frontal seizure onset, and seizure onset during sleep showed a large although nonsignificant effect and might also influence the duration.
- Besides age, predicted seizure duration was prolonged with shortened disease duration and occurrence of version/sign of four.
- We suspect degenerating inhibition mechanisms in elderly patients as well as an essential role of the frontal lobe in seizure termination.
1 INTRODUCTION
Epileptic seizures are associated not only with social stigmatization, but also with a high injury risk. The risk for seizure complications is mainly influenced by the seizure severity, that is, clinical seizure manifestation and seizure duration.1-4 Thus, prolonged, bilateral tonic-clonic seizures are associated with higher complication rates than shorter, focal seizures (FSs) without generalization. It is for example known that the risk for musculoskeletal injuries, lung edema, ictal asystole, and sudden unexpected death in epilepsy rises with increasing seizure duration.2, 5, 6
Although the seizure duration seems to be a crucial risk factor, little is known about its drivers. It is common experience that most seizures stop without intervention mainly due to metabolic mechanisms like changes in ionic concentrations, increasing acidity, or neuromodulator release.7, 8 Nevertheless, the seizure duration varies between epilepsy syndromes with, for example, longer focal impaired awareness seizures (FIASs) in mesial temporal than neocortical extratemporal epilepsy syndromes.9 Differences also exist between certain seizure semiologies like FIASs versus focal to bilateral tonic-clonic seizures (FBTCSs) or generalized tonic-clonic seizures.9-11 Furthermore, seizures are longer in patients with history of status epilepticus and magnetic resonance imaging (MRI) lesion, and shorter in those with ictal dystonia, ictal hypoxia, and anticonvulsive treatment.11, 12 This suggests that, in addition to common metabolic processes, the seizure duration is modulated by patient-specific parameters. However, systematic investigations of the interaction of clinical/demographic parameters and seizure duration are scarce.
All but three13-15 hitherto available studies on seizure duration used surface electroencephalographic (EEG) recordings or video-based analyses,5, 10, 11, 16 although intracranial EEG (iEEG) recording is superior in precise determination of seizure duration. The three studies addressing seizure duration using iEEG electrodes examined either the duration of FIASs in temporal and extratemporal epilepsy syndromes13 or characteristics of sleep-related hypermotor seizures,15 or focused on the impact of epilepsy syndromes and semiology only.14
To better understand the drivers of seizure duration, we aimed to systematically evaluate the impact of clinical and demographic factors like epilepsy syndrome, disease duration, vigilance state at seizure onset, age, and sex on the duration of focal onset seizures with and without secondary generalization in patients with iEEG recordings.
2 MATERIALS AND METHODS
This study was approved by the institutional review board of Human Studies Research at the University of Munich. All participants gave written informed consent to the scientific use of their clinically acquired data.
2.1 Participants
A retrospective database search was performed to identify all adult drug-resistant epilepsy patients who underwent an iEEG evaluation between January 2006 and June 2016 (n = 128). The study was restricted to iEEG recordings as this allowed a more precise determination of the seizure duration compared to analyses of surface EEG recordings. All patients had surface EEG recordings prior and in parallel to the iEEG recording.
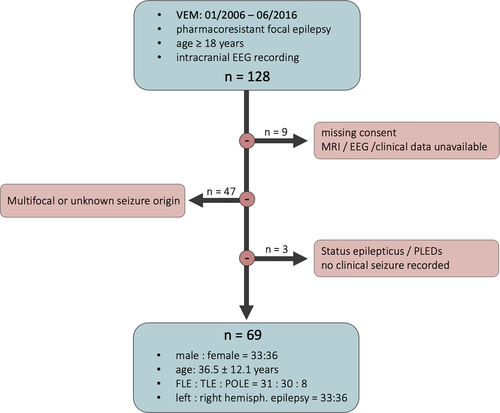
To analyze the association of clinical and demographical parameters and seizure duration, study inclusion required at least one recorded clinical seizure with determinable seizure duration. Thus, patients with no recorded seizures, status epilepticus with undeterminable seizure pattern duration, or seizures with electrographic transition to periodic lateralized discharges or periodic bursting were excluded from statistical analyses (n = 3). Furthermore, only the most clear-cut epilepsy cases, that is, patients with unifocal epilepsy syndromes, were included in the study, to also assess the association of seizure onset zone and seizure duration. The epilepsy syndrome classification resulted from an interdisciplinary patient management conference and was based on the available EEG data, seizure semiology, electrical cortical stimulation, neuropsychological test results, and functional and structural imaging data. The latter were acquired on a 3-T MRI scanner using a specific epilepsy protocol (high-resolution three-dimensional [3D] T1 and 3D fluid-attenuated inversion recovery [FLAIR] sequence, coronal FLAIR, T2, and inversion recovery T1 sequence). Patients with multifocality or unknown epileptic focus were excluded (n = 47), as were patients with unavailable MRI, relevant clinical, or EEG data (n = 5), or missing consent for scientific use of their data (n = 4). The patient selection process is summarized in Figure 1.
2.2 Clinical and demographic parameters
Clinical and demographic data with relevance for the study were extracted from the patient reports, including sex, age at disease onset, age at monitoring, epilepsy syndrome, disease etiology, antiepileptic drugs (AEDs), and type of EEG recording (stereo-EEG [sEEG] vs subdural electrodes).
As generally accepted standard procedure, the AEDs were withdrawn during the presurgical evaluation in the epilepsy monitoring unit (EMU) to increase the likelihood of epileptic seizures. AED withdrawal was started upon admission to the EMU, that is, after implantation of the iEEG electrodes and postoperative exclusion of an intracranial hemorrhage. In patients with one or two AEDs, the medication was completely withdrawn within 1 day. In patients with at least three AEDs or history of status epilepticus, all but one AED were stopped on the first day and the remaining AED was tapered during the following 1-3 days. If a patient experienced a generalized convulsive seizure or a series of three or more focal onset seizures, lorazepam 2.5 mg was administered sublingually. As reported separately, AED withdrawal did not influence the seizure duration in our study cohort.17
2.3 Seizure duration determination
The seizure duration was determined based on the iEEG recordings, that is, subdural and sEEG. Subdural electrodes were typically used in patients with a neocortical epilepsy focus, whereas sEEG electrodes were preferred for the EEG evaluation of more extensive or mesial or subcortical brain areas. The iEEG electrodes were individually placed according to the suspected location of the seizure onset zone and associated evolution pathways. The EEG signal from the intracranial electrodes (AD-Tech Medical Instrument Corporation) was recorded using XLTEK Neuroworks software and an XLTEK EMU128FS amplifier (Natus Medical Incorporated) with a sampling rate of 1024 Hz and 12- to 16-bit A-D conversion. The seizure duration (in seconds) was determined as the time difference between EEG seizure pattern onset and cessation and validated by at least two experienced epileptologists who were blinded to the clinical and demographic parameters. Thereby, the seizure pattern end was defined as the cessation of ictal rhythmic activity in all EEG channels. In case of an asynchronous seizure pattern cessation,18 the seizure end was always set at the time point of cessation of the latest detectable seizure pattern. For FBTCSs, the durations of the focal and the generalized phase were noted separately.
2.4 Semiological seizure evaluation
The video recordings of all seizures were evaluated with regard to seizure semiology,19 including lateralizing signs like dystonia, sign of four, or a forced head and trunk rotation (ie, version). Especially ictal dystonia was reported to inhibit secondary generalization, presumably caused by an activation of the basal ganglia.12 Furthermore, the occurrence of aura symptoms and the time of day and vigilance state at seizure onset (sleep vs awake) were noted.
2.5 Statistical analysis
Mean and standard deviation were calculated for quantitative parameters. F tests were used to compare variances, and group differences were calculated using t tests. A negative binomial mixed model was fitted to analyze the association between the clinical and demographic parameters and the length of epileptic seizures, in a univariate as well as in a multivariate setting. Separate models were calculated for FSs without generalization (FS-BTCs), FBTCSs, and the focal and the generalized phase of FBTCSs. Sex, age, lobe, disease duration or age at disease onset, aura, and vigilance at seizure onset (sleep vs wakefulness) were thereby chosen as fixed effects. For FS-BTCs, dystonia was added as a fixed effect, whereas version and sign of four were considered as fixed effects for FBTCSs. In addition, a random intercept per patient was included in the model to account for the repeated measurements (multiple seizures per patient). The model selection was done via Akaike information criterion (AIC). This criterion is based on the goodness of fit (not P values) but penalizes model complexity. A P value < .05 was considered statistically significant.
3 RESULTS
3.1 Demographics and clinical characteristics
This study included 69 adults (33 males, 36 females) with unifocal epilepsy. Most patients (47/69, 68.1%) had a structural etiology, and a similar number of patients were diagnosed with frontal (31/69, 44.9%) or temporal (30/69, 43.5%) lobe epilepsy (Table 1). The minority had a parieto-occipital epilepsy onset (8/69, 11.6%). The cohort was further characterized by an equal distribution of left (33/69, 47.8%) and right (36/69, 52.2%) hemispheric epilepsy syndromes. Upon admission, mean disease duration was 20.4 ± 13.2 years, with a minimum of 2 and a maximum of 56 years. Age at monitoring was 36.51 ± 12.08 years. Most of the patients (40/69, 58.0%) were evaluated with sEEG, whereas subdural electrodes were used in 29 patients (29/69, 42.0%).
| FLE | TLE | POLE | Total | |
|---|---|---|---|---|
| Total number of patients | 31 | 30 | 8 | 69 |
| Sex, male/female | 18/13 | 11/19 | 4/4 | 33/36 |
| Epilepsy syndrome lateralization, left/right | 12/19 | 19/11 | 2/6 | 33/36 |
| MRI findings | Nonlesional (n = 7); focal cortical dysplasia (n = 9); gyral malformation (n = 1); tumor (n = 7); porencephalic cyst (n = 1); lesion/gliosis after TBI, bleeding, or abscess (n = 6) | Nonlesional (n = 13); focal cortical dysplasia (n = 1); tumor (n = 5); vascular malformation (n = 1); focal atrophy (n = 1); sclerosis (n = 5); lesion/gliosis after TBI, resection, or encephalitis (n = 4) | Nonlesional (n = 2); focal cortical dysplasia (n = 2); diffuse atrophy (n = 1); gliosis after bleeding, bullet wound, or perinatal hypoxia (n = 3) |
- Abbreviations: FLE, frontal lobe epilepsy; MRI, magnetic resonance imaging; POLE, parieto-occipital lobe epilepsy; TBI, traumatic brain injury; TLE, temporal lobe epilepsy.
3.2 Seizure characteristics
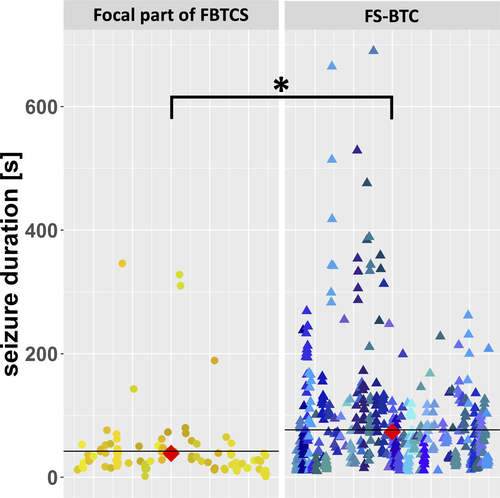
In total, 654 FSs were recorded, of which 15.0% (98/654) showed an evolution to FBTCS. Their characteristics are summarized in Table 2. On average, the patients experienced 9.5 seizures, with a maximum of 24 seizures/patient. The 556 FS-BTCs had a mean duration of 76.7 seconds and an expected duration of 73.5 seconds (95% confidence interval [CI] = 60.5-89.1), with a wide variability ranging from 10 seconds to a maximum of 690 seconds. In comparison, the 98 FBTCSs lasted significantly longer (mean = 102.2 seconds, range = 15-429 seconds, expected value = 104.3 seconds, 95% CI = 89.8-121.1, P < .001). Most FSs (545/654, 83.3%) self-terminated within 2 minutes, that is, 84.0% (467/556) of the FS-BTCs and 79.6% (78/98) of the FBTCSs. A prolonged duration of >5 minutes was detected in 21 of 654 (3.2%) seizures of nine of 69 (13.0%) patients.
| FLE | TLE | POLE | Total | |
|---|---|---|---|---|
| Total number of seizures | 343 | 225 | 86 | 654 |
| Seizure onset, left/right | 148/195 | 142/83 | 7/79 | 297/357 |
| Clinical/subclinical seizures | 303/40 | 183/42 | 70/16 | 556/98 |
| Preceding aura | 68 (19.8%) | 54 (24.0%) | 38 (44.2%) | 160 (24.5%) |
| Seizure onset during sleep | 255 (74.3%) | 132 (58.7%) | 38 (44.2%) | 425 (65.0%) |
| Secondary generalization/FBTCSs | 51 (14.9%) | 31 (7.3%) | 16 (18.6%) | 98 (15.0%) |
| Version | 31 (60.8%) | 19 (61.3%) | 8 (50.0%) | 58 (59.2%) |
| Sign of four | 13 (25.5%) | 4 (12.9%) | 2 (12.5%) | 19 (19.4%) |
| Dystonia | 0 (0%) | 7 (3.1%) | 1 (1.2%) | 8 (1.2%) |
- Abbreviations: FBTCS, focal to bilateral tonic-clonic seizure; FLE, frontal lobe epilepsy; POLE, parieto-occipital lobe epilepsy; TLE, temporal lobe epilepsy.
In addition, FSs were shortened by evolution into a FBTCS, meaning that the focal phases of FBTCSs (38.7 seconds, 95% CI = 29.9-50.1) lasted significantly shorter than FS-BTCs (73.5 seconds, 95% CI = 60.5-89.1, P < .001; Figure 2).
3.3 Association of seizure duration and demographic parameters
The duration of the FS-BTC was significantly associated with the age of the patient. Per year of life, the duration of FS-BTCs was prolonged by a factor of 1.022 (P = .003) in a univariate model. No significant association was found for the age of the patients and the duration of FBTCSs and their focal and generalized phases. Disease duration did not influence the seizure duration and thus did not account for the observed age dependency. Male gender did not alter the overall duration of FSs with or without generalization significantly, but, compared to women, male patients showed a tendency of shortened focal phases in FBTCSs (factor = 0.783, P = .350).
3.4 Association of seizure duration and epilepsy syndrome
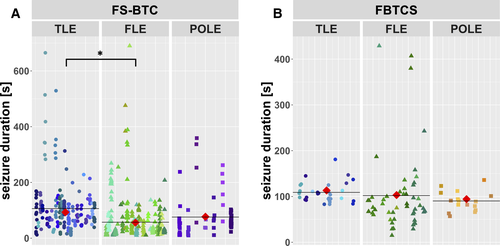
In a univariate model, FS-BTC durations were shortest in patients with frontal lobe epilepsy (FLE; FS-BTCs: 55.9 seconds, 95% CI = 33.5-93.3) and longest in patients with temporal lobe epilepsy (TLE; FS-BTCs: 93.1 seconds, 95% CI = 71.1-122.0, P = .010; Figure 3). No significant differences were found for the duration of FBTCSs between FLE (94.2 seconds, 95% CI = 57.9-153.4), TLE (113.3 seconds, 95% CI = 86.5-148.5, P = .590), and parieto-occipital lobe epilepsy (103.2 seconds, 95% CI = 66.4-160.4, P = .398). The multivariate regression model, however, revealed no significant association between the epilepsy syndrome and the seizure duration.
3.5 Association of seizure duration and seizure semiology
A preceding aura had no significant impact on the seizure duration in the univariate model, but it tended to shorten the focal phase of FBTCSs (factor = 1.621, P = .068). Strong lateralizing semiologies like version and sign of four, however, were significantly associated with prolonged generalized phases in FBTCSs in the univariate (version factor = 1.349, P = .004; sign of four factor = 1.329, P = .012) as well as the selected multivariate (version factor = 1.290, P = .015; sign of four factor = 1.254, P = .043) models. Dystonia was observed in only eight seizures and did not reveal an association with seizure duration.
The vigilance state at seizure onset, that is, sleep or wakefulness, had no significant impact on the duration of FSs in the univariate and multivariate models. However, the average duration of FBTCSs with seizure onset during sleep tended to be shorter than of those starting during wakefulness (factor = 0.825, P = .085). This nonsignificant effect was also observed for the generalized phase of FBTCSs (factor = 0.828, P = .077).
3.6 Seizure duration prediction
Model selection was done according to AIC, with a better model having a lower AIC. For FS-BTCs, age (factor = 1.034/y, P < .001) and disease duration (factor = 0.982, P = .028) remained in the selected model. The age dependency of FS-BTCs is visualized in Figures 4 and 5 contrasts the predicted and observed FS-BTC durations.
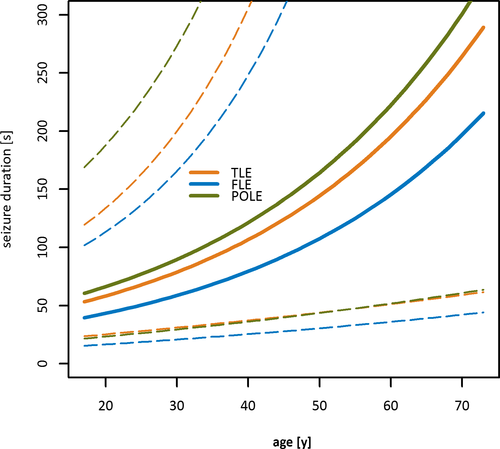
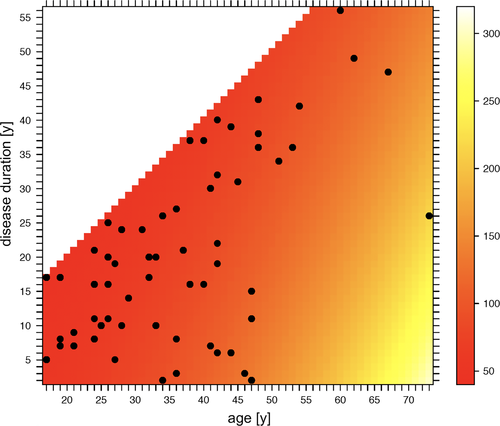
In contrast, the vigilance state (sleep vs wakefulness; factor = 0.825, P = .085) was the core influencing factor in the FBTCS prediction model. Furthermore, the focal phase of FBTCSs was mainly influenced by the occurrence of an aura (factor = 1.663, P = .068), whereas the duration of the generalized phase was significantly associated with the appearance of lateralizing signs, that is, version (factor = 1.290, P = .015) or sign of four (factor = 1.254, P = .043).
The detailed results of the univariate, multivariate, and selected models are available online as a supplement.
4 DISCUSSION
This study is the first to systematically analyze the association of the duration of focal onset seizures and demographic and clinical parameters, using iEEG to ensure precise seizure duration determination.
In accordance with previous publications,9, 10, 14, 20 the seizure duration was significantly prolonged in the case of an evolution to FBTCS and the epileptic activity typically self-terminated within 2 minutes. Similar to previous reports,14, 21 only 3.2% of seizures lasted >5 minutes. Thereby, the duration of FBTCSs appeared more stereotypic, that is, revealed a smaller variance, than the duration of FS-BTCs. Furthermore, the FS phase was significantly shortened in the case of an evolution to a bilateral tonic-clonic seizure. Both suggest that early seizure evolution already differs between FBTCSs and FS-BTCs. This is supported by a recent description of unequal activation patterns in the regions surrounding the seizure onset zone during the preictal and early ictal phase of FBTCSs and FS-BTCs.22
4.1 Clinical and demographic modifiers of seizure duration
Of note, the seizure duration was significantly prolonged by increasing age (FS-BTCs) and occurrence of version/sign of four (generalized phase of FBTCSs). Furthermore, the predicted FS-BTC duration was significantly impacted by the disease duration.
The longer seizure durations in aging epilepsy patients might indicate an age-dependent impairment of seizure termination. This could be explained by physiological, age-related neuronal degeneration, as it involves a gradual decrease of inhibitory networks with a reduced release of inhibitory neurotransmitters like γ-aminobutyric acid.23, 24 Thus, seizure termination might be less efficient in elderly patients, leading to the observed seizure prolongation. Accordingly, among adults, elderly patients have the highest incidence of epilepsy as well as the highest rate of status epilepticus.25-27 Further support of our hypothesis comes from a recent report that also described longer durations of interictal epileptiform discharges in aging epilepsy patients.28 No significant association, however, was found between the age of the patients and the duration of FBTCSs, which is most likely explained by the difference in group sizes, with a presumably underpowered analysis for FBTCSs (556 FS-BTCs vs 96 FBTCSs).
Longer FS-BTC durations were further observed in patients with shorter disease durations (selected/prediction model). The constellation of older age and short disease duration is mainly given in older epilepsy patients with adult onset epilepsy syndromes and thus short disease histories. In this subgroup, the inhibitory networks are thought to be less definite than in patients with inherent epilepsy syndromes,29, 30 which might contribute to the differences in seizure duration.
The lateralizing signs "version" and "sign of four" typically occur at the beginning of secondary generalizations,31, 32 and went along with significantly longer durations of FBTCSs. Both semiologies are associated with an epileptic activation of frontal brain regions, including the frontal eye field and the supplementary motor area.32-35 Based on our results, we suggest that the frontal lobe might play an essential role in seizure inhibition: (1) as in earlier studies,11, 20 FSs were shortest in FLE, indicating that they were more efficiently terminated by the surrounding frontal brain regions than focal onset seizures with temporal or parieto-occipital origin; and (2) the extensive epileptic activation of the frontal lobe during version/sign of four might cause a functional inhibition of the frontal inhibitory mechanisms, leading to the prolonged seizure duration in FBTCSs.
In line with the first reports,11 seizures also tended to be shorter in male patients compared to female patients—although we did not observe a significant association, most likely due to the insufficient cohort size. On a pathophysiological level, this observation might correlate with gender differences in cerebral perfusion and a higher glucose uptake in females.36, 37 Furthermore, female patients are characterized by a higher cerebral density of excitatory receptors and transmitters like serotonin and dopamine, which might facilitate seizure evolution and maintenance in women.38-40 Prospective gender studies including functional measurements are needed to substantialize this hypothesis.
4.2 Limitations
Although the 69 patients represent the typical drug-resistant patient population that can be found in adult EMUs, it is a highly selected cohort. Thus, our results cannot be transferred to a broader epilepsy population without limitations. Especially the elderly patients with late onset epilepsy syndromes due to, for example, vascular lesions were underrepresented, as they usually become seizure-free under medication and thus do not undergo presurgical evaluations. Furthermore, our analyses were restricted to iEEG recordings only. However, presurgical iEEG recording is typically required in less clear-cut epilepsy cases with unclear seizure focus localization. Consequently, lateralizing semiologies like dystonia are less frequent in this patient cohort and were relatively underrepresented in our study compared to literature reports.32, 35, 41 Significant impact, however, was revealed for version and sign of four, which should be confirmed in a less selected epilepsy cohort. Moreover, iEEG electrodes cover only a limited brain region. Therefore, we cannot totally rule out that an earlier EEG seizure onset or termination in more distant regions was missed. However, this is very unlikely, as we also paid attention to remote seizure patterns, correlated the EEG with the respective video recordings, and additionally used surface electrodes covering the rest of the brain. The analysis is further biased by an unequal number of seizures per patient, which was accounted for by including a random estimate in the statistical model. To record enough seizures during the video-EEG evaluation, it is common practice to withdraw the antiepileptic drugs after EEG electrode implantation. Although AED withdrawal increases the likelihood of seizures, it was shown not to alter seizure duration.17 Individual patients, however, received lorazepam as rescue medication after a series of focal onset seizures or bilateral tonic-clonic seizures. The respective seizures could not all be identified retrospectively and were thus not excluded from the analysis. Consequently, we cannot rule out a certain bias of the rescue medication on the seizure durations and thus our statistical analysis. However, the potential impact on our results is thought to be low, as the rescue medication was typically administered after the respective seizure end. Last but not least, we might have missed further essential modifiers of the seizure duration that we were not aware of and that were thus not included in the statistical model.
4.3 Significance
Age, epilepsy syndrome, and lateralizing semiologies were identified as relevant modifiers of seizure duration in focal epilepsy patients. Although these parameters are noninfluenceable, they provide valuable insight into developmental network changes, seizure evolution, and termination mechanisms, and are of great relevance for risk evaluation in adult epilepsy patients.
ACKNOWLEDGMENTS
The authors are indebted to the patients for their participation in this study.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
All data used for analysis are presented in the article. The discussion and conclusions only rely on the data presented.