Effect of diet on body size and survival of omnivorous crickets
Abstract
Omnivory is a feeding habit in which animals prey on organisms from two or more trophic levels. Omnivorous species play important roles in predator–prey interactions, food web dynamics, and ecosystem functions. Although cricket species have been considered omnivores, quantified investigations of their feeding habits have not been carried out. Therefore, we compared the developmental time, body size and mortality rate of the band-legged ground cricket, Dianemobius nigrofasciatus (Matsumura) (Orthoptera: Trigonidiidae) reared on diets meant for carnivores and herbivores. Furthermore, to examine whether the crickets can balance their nutritional conditions through their food choices, we set up two additional diet treatments, which comprised diets for carnivores and herbivores provided simultaneously or by rotation. The mortality rate of the carnivore diet treatment was higher than that of the other diet treatments. This result suggests that D. nigrofasciatus is a “herbivore” that also obtains nutrients from resources other than plant materials. Although developmental time did not significantly differ among the dietary treatments, the body size of the crickets that were provided both diets simultaneously was significantly larger than that of the crickets provided with only the diets for either carnivores or herbivores. These results suggest that an appropriate amount of carnivorous feeding is advantageous for the development of the cricket, and indicates that crickets can balance nutrition on their own.
INTRODUCTION
Omnivory is a feeding habit in which animals feed on organisms from two or more trophic levels (Polis & Strong 1996). Coll and Guershon (2002) summarized that omnivory includes “predators” that feed on herbivores as well as other predators (i.e. intraguild or interguild predators), and true omnivores that feed on animals and plants. Omnivorous animals play important roles in predator–prey interactions, food web dynamics, and ecosystem functions (Coll & Guershon 2002). Therefore, it is important to study the evolutionary and ecological significance of omnivory.
The benefits of omnivory that have been proposed include being less susceptible to a lack of food resources (Pearson et al. 2011), a decrease in interspecific competition (Coll & Guershon 2002; Singer & Bernays 2003), and a decrease in the uptake of toxins (Bernays et al. 1994). In particular, the practice of mixing complementary foods can be used to achieve balanced diets by including a variety of macronutrients, vitamins and minerals (Denno & Fagan 2003; Behmer 2009). Conversely, omnivorous species have to consume multiple prey and plant items to maintain optimal nutritional balance.
Crickets (Orthoptera: Grylloidea) are omnivorous insects that feed on weed seeds, seedlings and other dead or living arthropods (Bechinski et al. 1983; Burgess & Hinks 1987; Lundgren & Rosentrater 2007; White et al. 2007; Bidau 2014; Ichihara et al. 2014a, 2014b). Recently, the nutrient ecology of cricket species has been studied in terms of its ecological stoichiometry and nutritional geometry frameworks. These studies showed the effect of macronutrient balance, including carbohydrates, nitrogen and phosphate, on fitness components (Bertram et al. 2006; Harrison et al. 2017; Reifer et al. 2018; Gutiérrez et al. 2020). For example, protein-rich diets were found to shorten developmental time and increase body size, whereas calling activity was promoted by a carbohydrate-rich diet in Gryllus assimilis (Reifer et al. 2018). A high-protein diet was also found to increase fecundity in Acheta domesticus (Gutiérrez et al. 2020) and G. veletis (Harrison et al. 2017). Hunt et al. (2004) showed that male Teleogryllus commodus reared on a diet of high protein exhibited a shorter life span because they had invested more resource in calling while young (but see Judge et al. 2008). These studies showed that crickets reared on high-protein diets generally exhibit higher growth rates than those reared on lower protein diets. Under laboratory conditions, crickets can be reared by being fed with either artificial diets meant for carnivorous animals (i.e. domestic cats) or those meant for herbivorous animals (i.e. guinea pigs or rabbits) (Judge et al. 2010). However, although crickets have been considered to be more like herbivores, it is not known whether crickets are more phytozoophagous (i.e. prey-taking herbivores) or zoophytophagous (plant-feeding carnivores) species (Bidau 2014).
The cricket species occur in various habitats such as forests, grasslands, farmlands, riversides and seashores, and they have a wide range of feeding habits (Bidau 2014). Crickets also have a variety of predators and parasitoids (Zuk & Kolluru 1998; Lagos 2017); therefore, crickets have an important ecological role in ecosystems. Although feeding habits are important for understanding the ecological function of crickets, there is no quantitative research on feeding habits. Whether the cricket is phytozoophagous or zoophytophagous will significantly change its ecological role in the ecosystem. For example, if the cricket is phytozoophagous, it will mainly play a role as a herbivore; if the cricket zoophytophagous, it will mainly play a role as a predator and/or decomposer. Furthermore, if crickets change their feeding habit to compensate for nutrient deficiencies in the environment, they will be both predator and herbivore depending on the environmental conditions. The effect of the changes would propagate to the population dynamics and antipredatory traits of plants and prey being foraged. In this study, we examined the effect of artificial diets, meant for carnivorous or herbivorous animals, on the growth of the ground cricket, Dianemobius nigrofasciatus (Matsumura) (Orthoptera: Trigonidiidae). Although artificial diets cannot examine the actual feeding habits in the field, we can determine whether they are closer to herbivores or carnivores by approximation.
Dianemobius nigrofasciatus is commonly found around crop fields, weedy river margins or lawns between 30° and 44°N on the islands of Japan (Masaki 1972; Matsuda et al. 2019). In the southern part of Japan, adults emerge in the summer and produce nondiapause eggs, whereas those of the next generation emerge in autumn and produce diapause eggs (Masaki 1972; Matsuda et al. 2019). Because of its interesting diapause traits, the life history of D. nigrofasciatus has been investigated and they have mostly been reared while feeding on plant materials, such as carrots, in addition to protein-rich artificial diets (Masaki 1972; Shiga & Numata 1996; Matsuoka & Ishihara 2010). To determine whether the crickets are phytozoophagous or zoophytophagous, we investigated the developmental time, body size and mortality rate of crickets reared on either carnivore or herbivore diets. Furthermore, we compared the growth of crickets when both feeds were given simultaneously and in rotation. If the cricket feeds only on the diet it prefers, then giving it even two types of diet at the same time should most likely result in poorer growth than those given the feed in rotation and also result in growth that is similar to those fed a single diet. However, if the cricket can balance its own nutrition, those reared on both diets would exhibit higher rates of growth than those observed in the other treatments.
MATERIALS AND METHODS
Study species
Approximately 70 adult D. nigrofasciatus individuals were collected from an agricultural field at Kagoshima University (31°34′05.3″N 130°32′46.6″E). The crickets were reared in a container 30 × 20 × 20 cm), between 26°C and 28°C, under a photoperiod of 15 h light : 9 h dark. The crickets were supplied with egg cartons for shelter, soil in a 200 mL plastic cup, along with excess guinea pig food (Marmot selection; Yeaster Co., Ltd., Hyogo, Japan) and cat chow (Purina One Metabolic Energy Control; Purina, Kobe, Japan). Furthermore, the soil was sprinkled with water every 2–3 days to provide the crickets with a source of water and a site suitable for oviposition. Progenies of the second generation were used in the experiment.
Experimental design
The nymphs, aged 13–16 days, were randomly assigned to the following four treatments: (i) the herbivorous diet treatment (HD), where crickets were provided with pellets originally meant for guinea pigs; (ii) the carnivorous diet treatment (CD), where crickets were provided with pellets originally meant for domestic cats; (iii) the treatment comprising both diets (BD), where crickets were simultaneously provided with both pellets meant for cats and those for guinea pigs; and (iv) the rotation treatment (RD), where crickets were provided pellets meant for cats and guinea pigs in alternate shifts every 4–6 days. The actual assignment of the crickets to each treatment is as follows. First, we prepared 160 containers (4 treatment × 40 replications) with one cricket. Then we allocated a treatment (one of the four diet treatments) to each container by random numbers. The final sample size is less than 40 due to accidental deaths and escapes. The dietary constituents of the feeds for guinea pigs and domestic cats that were used in this study are summarized in Tables 1 and 2. The guinea pig food did not contain any animal materials, but the cat food did contain some plant materials such as soybean and corn. Before they were given to the crickets, these pellets were milled using an electric coffee mill (Melitta Ltd., Tokyo, Japan). The crickets were housed individually in a container (6 cm diameter, 3 cm high) with food, a folded filter paper for shelter, and water in a cotton-plugged plastic vial. The experimental conditions were between 26°C and 28°C, and under a photoperiod of 15 h light : 9 h dark. We recorded the developmental times from the experimental setting to adulthood. We also measured mortality rates at emergence and at 10 days after emergence (i.e. when almost all individuals were sexually mature). The body size of the adults aged 10–20 days after the final molt was also measured. The head width, including the compound eyes, was measured as an index of the body size in accordance with the method used in previous studies (Masaki 1972; Matsuda et al. 2019). The head width was measured using a digital microscope (3R-MSUSB401; 3R Solution Corp., Tokyo, Japan).
| Guinea pig food | Cat food | |
| Protein (%) | >18 | >42 |
| Fat (%) | >2 | >8 |
| Carbohydrate (%) | ca. 60 | <29 |
| Crude fiber (%) | <22 | <5 |
| Guinea pig food | Cat food |
|---|---|
| Timothy meal | Turkey meal |
| Wheat flour | Corn gluten |
| Alfalfa meal | Chicken meal |
| Defatted soybean | Defatted soybean |
| Brewerʼs yeast | Rice |
| Plant-derived fermenting substance | Corn |
| Dandelion meal | Soybean protein |
| Plantain meal | Yeast |
| Mushroom meal | Integument of soybean |
| Oligosaccharide | Beef fat |
Statistical analyses
To examine the effect of diet on the mortality rates, generalized linear model (GLM) with binomial error and logit link was used. The explanatory variable in this model was the dietary treatment. To test the effect of diet on developmental period, we used GLM with gamma error and log link was used because the response variable could not assume normality (Shapiro–Wilk normality tests, W = 0.97, P < 0.01). Because the gamma distribution is appropriate for analysis of latency such as death and failure (Crawley 2005), we used GLM with gamma error and log link. Indeed, the Akaike information criterion value of the GLM with gamma error was lower than that of the linear model (gamma, 811.1; linear, 817.5). The explanatory variables used in this case were dietary treatment and sex. To analyze the effect on body size, we used linear model because normality and equal variance of response variable could be assumed (Shapiro–Wilk normality tests: W = 0.99, P = 0.55; Bartlett test of homogeneity of variances: χ2 = 4.79, d.f. = 3, P = 0.19). We used a likelihood ratio test to evaluate the statistical significance of each coefficient in the models. We used an extension of Tukeyʼs multiple comparison that can be applied to GLMs with the R add-on package “multcomp1.4.16” (Hothorn et al. 2016) to compare the treatment groups. All statistical calculations were undertaken using R 4.0.3 software (R Core Team 2020).
RESULTS
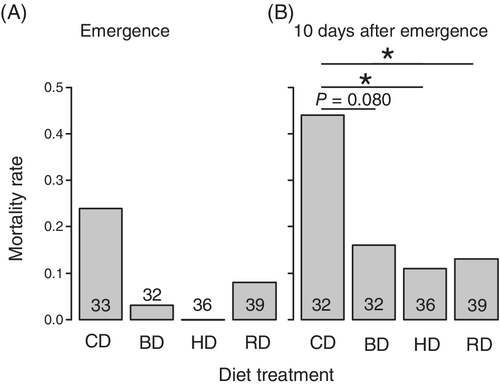
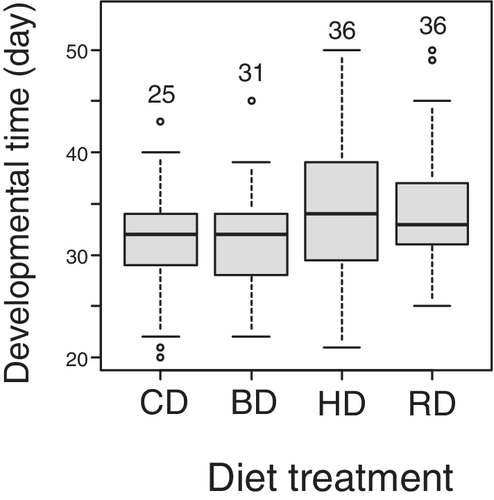
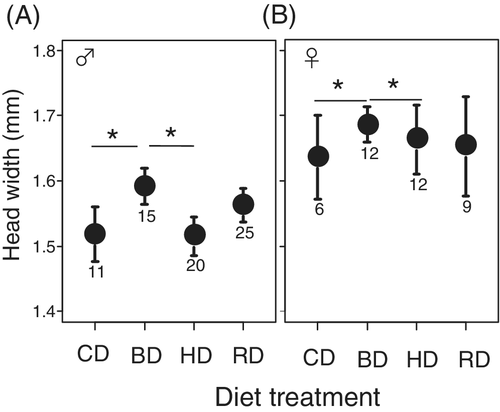
We did not analyze the mortality rate at emergence because the response variable had little variance and could not be used in GLM analysis (Fig. 1a). The reason for the little variance would be that there were almost no mortalities in the three treatments due to the benign rearing environments. There was a significant effect of diet on mortality rate from the period of setting to 10 days after the final eclosion (Fig. 1b; χ2 = 13.68, d.f. = 3, P = 0.0045). When residual deviance in the model is larger or smaller than degree of freedom, the model has over- or under-dispersion (Crawley 2005). To check the possibility, we divided residual deviance (126.58) by degree of freedom (135), and the result was 0.938. We concluded the model did not exhibit over- or under-dispersion because the result was close to 1. In the results obtained from extended Tukeyʼs multiple comparison, the mortality rate of CD was significantly higher than that of HD and RD (Fig. 1b, Table 3). Meanwhile, there was a marginal difference in mortality rate between CD and BD treatments (Fig. 1b, Table 3). Sex was also included as an explanatory variable in the analysis, except for those individuals that died before the stage when the sex could be distinguished (11 crickets), but there was no significant difference in mortality rate between the sexes (χ2 = 0.0062, d.f. = 1, P = 0.94). Developmental time was not significantly affected by diet (Fig. 2; χ2 = 7.39, d.f. = 3, P = 0.061) or sex (χ2 = 0.014, d.f. = 1, P = 0.91). However, body size was significantly affected by diet (Fig. 3; χ2 = 14.03, d.f. = 3, P = 0.0029) and males were significantly smaller than females (Fig. 3; χ2 = 57.88, d.f. = 1, P < 0.001). In Tukeyʼs multiple comparison results, the crickets that were provided the BD diet were significantly larger than those provided with either diet only (CD or HD) (Fig. 3, Table 4).
| Diet treatment | Z-value | P-value |
|---|---|---|
| BD vs. CD | 2.38 | 0.080 |
| HD vs. CD | 2.86 | 0.022 |
| RD vs. CD | 2.79 | 0.027 |
| HD vs. BD | 0.55 | 0.950 |
| RD vs. BD | 0.34 | 0.990 |
| RD vs. HD | 0.23 | 1.000 |
- Tukeyʼs multiple comparisons test was used to achieve the multiple comparisons. BD, crickets simultaneously provided with both diets for herbivores and for carnivores; CD, crickets provided with a diet meant for carnivores; HD, crickets provided with a diet meant for herbivores; RD, crickets provided with diets for carnivores and herbivores in alternate shifts.
| Diet treatment | Z-value | P-value |
|---|---|---|
| BD vs. CD | 3.12 | 0.0098 |
| HD vs. CD | 0.43 | 0.9700 |
| RD vs. CD | 1.88 | 0.2400 |
| HD vs. BD | 3.20 | 0.0074 |
| RD vs. BD | 1.57 | 0.4000 |
| RD vs. HD | 1.73 | 0.3100 |
- Tukeyʼs multiple comparisons test was used to achieve the multiple comparisons. BD, crickets simultaneously provided with both diets for herbivores and for carnivores; CD, crickets provided with a diet meant for carnivores; HD, crickets provided with a diet meant for herbivores; RD, crickets provided with diets for carnivores and herbivores in alternate shifts.
DISCUSSION
The mortality rate of the crickets reared on cat food (i.e. diet for carnivores) was higher than that of the crickets reared on the other diet treatments. This result suggest that D. nigrofasciatus is “herbivore” that also obtains nutrients from resources other than plant materials (phytozoophagous). However, there are previous studies showing that other cricket species exhibit better growth in high-protein diets (e.g. Hunt et al. 2004; Reifer et al. 2018; Gutiérrez et al. 2020). This difference could be due to the fact that D. nigrofasciatus is smaller and might be less predatory than these field crickets, which may result in slightly different nutrient requirement. For example, ascorbic acid is sometimes an essential nutrient in herbivorous insects (Berenbaum 2001). Cat food used in the present study did not contain such plant-specific nutrition. The micronutrients could have elicited the high mortality rate.
Although developmental time from experimental setting to emergence did not significantly differ among the dietary treatments, the body size of the crickets reared on only cat food or only guinea pig food was smaller than that of the crickets fed with both types of foods simultaneously. These results indicate that the cricket are basically herbivorous, but they will enhance their fitness when they occasionally consume prey. This will be because plant foliage is low in nutritional value compared with prey tissue, such as protein content (Coll & Guershon 2002). In that case, the crickets are basically herbivorous, but also consume supplementary carnivorous diets. For example, the feeding habits of crickets may include them feeding on plant tissues and seeds most of the time, and occasionally foraging dead or living insects when they are available. In this case, the crickets also play the roles of primary consumers, predators and decomposers (or scavengers) in their habitats. In our personal observation, D. nigrofasciatus preyed on living aphids and dead dragonflies and stick insects in laboratory conditions.
Furthermore, our results suggest that crickets can forage to achieve their own nutritional balance. Although the diet choice has been shown in generalist herbivores such as grasshoppers and caterpillars (Waldbauer & Friedman 1991; Behmer 2009), only a few studies have investigated the choice behavior in omnivore species (Xu et al. 2013). Although the mechanism for maintaining nutritional balance is still unclear, experiencing “boredom” with a certain diet could be a contributing mechanism. Indeed, the grasshopper Taeniopoda eques become more receptive to novel plants when fed continually on the same plant (Bernays et al. 1992). Because body size of this cricket is small, we could not measure its consumption by feeding weight. In the future, we may be able to measure it by feeding time to examine the differences in food intake due to boredom.
The quantity and quality of plants and prey will vary temporally and spatially (Coll & Guershon 2002). The cricket D. nigrofasciatus is bivoltine in the southern part of Japan: adults of the first generation emerge early summer and the second generation emerge autumn (Masaki 1972). The difference in seasons could lead to different feeding habits depending on internal and external factors. For example, the amount and composition of plants and prey would be different in summer and autumn. The differences will be reflected in different nutrient intakes, and diet requirements will vary accordingly. Nutrient requirements might be also different for internal factors such as diapause, cold tolerance and heat tolerance, depending on the seasons. Similarly, differences in nutritional conditions among habitats can also affect food preferences of the cricket. In protein-rich vegetation, such as that dominated by Leguminosae, crickets may be almost herbivores, while in low-protein vegetation, they may be more like carnivores. The effect of such temporal and spatial variations on feeding habits will be an interesting subject.
Cricket species are used as important model organisms in behavioral ecology, especially in the field of sexual selection. In laboratory settings, the crickets have often been reared on cat food only. Our study highlights the important fact that the nutritional conditions of laboratories do not always reflect the feeding habits of the organisms in the wild. For example, Judge et al. 2010 reported that rabbit (i.e. herbivore) food was of higher quality than cat food for the field cricket G. pennsylvanicus.
A high-protein diet increases female fecundity in A. domesticus (Gutiérrez et al. 2020) and G. veletis (Harrison et al. 2017). In addition, higher protein diets reduce male life span, although the diets enhance survival rates of nymphs and females in the field cricket T. commodus (Hunt et al. 2004). Thus, there may be cases where the effect of the type of diet is different between the adult and nymph stages. We did not examine the effect of diet content on reproductive traits. The examination of the possibility is a subject for future study.
Although the present study suggests that D. nigrofasciatus is phytozoophagous, its feeding habits and trophic positions in the wild are still unknown. In light of this, DNA metabarcoding (de Sousa et al. 2019) and stable isotope analysis (Hyodo 2015) will be useful tools for quantifying the feeding habits and trophic positions of cricket species. The cricket population is broadly distributed and abundant in rural and urban environments. Therefore, the cricket is an important subject for the investigation of the ecological function of omnivorous species.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Naoki Matsuda and Dr. Hideharu Numata for providing valuable information on Dianemobius nigrofasciatus. We would also like to thank Editage for providing the English language editing service that was used to facilitate the completion of this manuscript. This study was supported in part by the Special Budget of the Ministry of Education, Culture, Sports, Science and Technology (MEXT; Establishment of Research and Education Network on Biodiversity and Its Conservation in the Satsunan Islands) and the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Number 19K21244).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVAL
All applicable institutional guidelines for the care and use of animals were adhered to.




