Association Between Stress Hyperglycemia Ratio and Parkinson's Disease Across Different Glucose Metabolism Statuses—A Prospective Study From UK Biobank
Funding: The present study was supported by the Natural Science Research Project of Anhui Education Committee China (grant no. KJ2019A0339) and the Natural Science Research Project of Bengbu Medical College (grant no. BYKY1467).
Haiyan Zhou and Yuzhi Mi contributed equally.
ABSTRACT
Background
The stress hyperglycemia ratio (SHR) has been linked to adverse outcomes in various conditions, yet its association with Parkinson's disease (PD) remains unclear. This study investigates the relationship between SHR and PD risk across sex and glucose metabolism statuses using data from the UK Biobank.
Methods
In this prospective cohort study, 406,271 participants without baseline PD from the UK Biobank were included. SHR was calculated as [FPG (mmol/L)]/[1.59 × HbA1c (%)−2.59] and divided into tertiles. Incident PD cases were identified via linked medical records. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs), with analyses stratified by sex and diabetes status (nondiabetic, prediabetic, diabetic).
Results
Over a median follow-up of 9 years, 2837 PD cases were identified. In men, elevated SHR was associated with increased PD risk, with the highest tertile (T3) showing a significantly higher risk compared with the lowest (T1) (HR: 1.20, 95% CI: 1.06–1.37). This association was strongest in nondiabetic men (T3 vs. T1: HR: 1.25, 95% CI: 1.08–1.45). No significant associations were observed in women or in prediabetic or diabetic men, either across tertiles or as a continuous variable.
Conclusion
Elevated SHR is independently linked to an increased PD risk in men, particularly those without diabetes, but not in women or other glucose metabolism groups. These findings suggest a sex-specific role of acute metabolic stress in PD pathogenesis and emphasize the need to consider glucose metabolism status in PD risk assessment.
1 Introduction
Parkinson's disease (PD) is the second most prevalent and fastest-growing neurological disorder globally, characterized by progressive motor symptoms such as bradykinesia, muscle rigidity, and tremor, which severely impair quality of life and impose significant societal and economic burdens [1]. In 2019, PD affected over 10 million individuals worldwide [2]. The disease's primary pathology involves the irreversible loss of dopaminergic neurons in the substantia nigra pars compacta, and to date, no curative treatment exists, underscoring the critical need for effective primary prevention strategies.
Current risk factors for PD, such as advanced age, male gender, and genetic predisposition, are largely nonmodifiable [3]. Although modifiable environmental factors, such as exposure to air pollutants, have been implicated in PD risk, intervention strategies remain complex and challenging to implement [3]. Thus, identifying new modifiable risk factors that can be targeted for early intervention is of paramount importance. Notably, the higher incidence of PD in males compared to females suggests potential sex-specific mechanisms warranting further investigation.
Emerging evidence indicates that hyperglycemia, a marker of metabolic stress, plays a role in various adverse outcomes, including cardiovascular diseases and neurodegenerative disorders [4-6]. However, traditional glycemic metrics, such as fasting plasma glucose (FPG) and glycated hemoglobin (HbA1c), provide limited insights into acute and chronic glycemic interplay. To address this gap, the stress hyperglycemia ratio (SHR) has been introduced as a novel marker that integrates acute glycemic changes (admission glucose) and chronic glycemic status (HbA1c) [7]. Studies have linked elevated SHR to increased risks of cardiovascular events and mortality, even in nondiabetic individuals [8-10]. Despite these findings, the potential relationship between SHR and PD risk remains unexplored, especially in the context of sex-specific and diabetes-specific differences.
This study aimed to investigate the association between SHR and the risk of developing PD, leveraging the UK Biobank's large prospective cohort. By stratifying analyses by sex and diabetes status, we seek to elucidate whether SHR serves as a significant predictor of PD and explore potential mechanisms underlying sex-specific vulnerability to metabolic stress.
2 Method
2.1 Study Design and Participants
This study utilized data from the UK Biobank, a large, ongoing prospective cohort that recruited over 500,000 middle-aged and older participants aged 37–73 years from across England, Scotland, and Wales between 2006 and 2010. Participants completed touchscreen questionnaires, underwent physical examinations, and provided biological samples. Detailed descriptions of the cohort have been published previously [11]. Briefly, the UK Biobank aims to explore the sociodemographic, lifestyle, environmental, and genetic factors contributing to various complex diseases. The project received ethical approval from the Northwest Multi-Center Research Ethics Committee (reference: 21/NW/0157), and all participants provided informed consent prior to enrollment.
In the present study, individuals with a baseline diagnosis of Parkinson's disease (PD) (n = 930) and those with missing glucose or HbA1c data (n = 95,068) were excluded, resulting in a final cohort of 406,271 participants.
2.2 Assessment of Covariates
Participants' demographic and clinical information, including age, sex, smoking status, drinking status, and comorbidities (hypertension, diabetes, hyperlipidemia, and stroke), was collected via a self-completed touchscreen questionnaire. Smoking and drinking statuses were classified into three categories: never, former, and current. Socioeconomic deprivation was assessed using the Townsend Deprivation Index, calculated based on participants' postal codes and corresponding national census data. Higher scores indicate greater socioeconomic deprivation [12]. Ethnicity was self-reported and categorized into four groups: White, Black, Asian, and other ethnic backgrounds. Participants also provided information on their sleep duration, which was classified as normal (7–9 h), long (> 9 h), or short (< 7 h). Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Physical activity levels were quantified using metabolic equivalent of task (MET)-hours/week, reflecting the energy expenditure associated with various activities.
2.3 Calculation of SHR
The stress hyperglycemia ratio (SHR) was calculated using the formula:
(Fasting plasma glucose [FPG] [mmol/L])/ (1.59 × HbA1c [%]−2.59) as described previously [7]. Participants were stratified into three groups based on SHR tertiles: Tertile 1: SHR ≤ 0.78. Tertile 2: 0.78 < SHR < 0.87. Tertile 3: SHR ≥ 0.87. The distribution of SHR values is presented in Figure S1.
2.4 Definition of Incident PD
Incident PD cases were identified using the International Classification of Diseases, Tenth Revision (ICD-10) code G20, recorded in the “First occurrence fields” of the UK Biobank. These cases were determined by integrating data from self-reported medical conditions, primary care records, hospital admissions, and death registrations. The earliest recorded date across these sources was used as the date of PD diagnosis. Follow-up time was calculated from the recruitment date to the earliest of the following events: PD diagnosis, death, or the censoring date.
2.5 Definitions of Glucose Metabolism Status
According to the standards of the American Diabetes Association (ADA), participants were categorized into three groups based on their diabetes status: nondiabetes (non-DM) (FPG < 5.6 mmol/L and HbA1c < 5.7%, with no prescriptions for glucose-lowering drugs), prediabetes (Pre-DM) (FPG 5.6–6.9 mmol/L or HbA1c 5.7%–6.4%, with no prescriptions), and diabetes (DM) (FPG ≥ 7.0 mmol/L or HbA1c ≥ 6.5%, either condition, or with prescriptions) [13].
2.6 Statistical Analyses
The baseline characteristics of the participants were summarized using medians (interquartile ranges [IQR]) for continuous variables and frequencies (n) with percentages (%) for categorical variables. Differences between participants stratified by PD status during follow-up were assessed using the Mann–Whitney U-test for continuous variables and chi-squared (χ2) tests for categorical variables. Given the sex-specific differences in PD incidence, all analyses were stratified by sex.
To estimate the hazard ratios (HR) and 95% confidence intervals (CI), Cox proportional hazards models were employed to examine the associations between SHR and the risk of incident PD using the coxph function from the survival package (version 3.7.0) in R (version 4.3.0). The proportional hazards assumption was tested using Schoenfeld residuals, and no significant violations were detected. Two models with increasing levels of covariate adjustment were used in the primary analyses: Model 1: adjusted for age, ethnicity, and Townsend Deprivation Index. Model 2: additionally adjusted for BMI, smoking status, alcohol consumption, physical activity (MET hours/week), hypertension, stroke, high cholesterol, sleep duration, and C-reactive protein levels.
Kaplan–Meier survival analyses were performed to compare event-free survival across the three SHR tertile groups using the log-rank test. Restricted cubic spline analyses were conducted to explore potential nonlinear associations between SHR and the risk of incident PD. This model used four knots and was adjusted for the same covariates as Model 2. The analyses were conducted using the plotRCS package (version 0.1.4). Subgroup analysis stratifies the association between SHR and PD based on glucose metabolic status.
To minimize the potential influence of reverse causality, sensitivity analyses were conducted by excluding participants diagnosed with PD within the first 2 years of follow-up. In the subgroup analysis, we excluded patients who progressed from non-DM and pre-DM to DM. The sensitivity analysis included the same covariates as Model 2.
Statistical significance was defined as a two-sided p value < 0.05. All statistical analyses and figure preparations were performed using R software (version 4.3.0, R Core Team 2023) [14].
3 Results
3.1 Baseline Characteristics of the Study Population
A total of 406,271 participants (53.8% female, median age 58 [50, 63] years) were included in this study, with 2837 incident PD cases identified over an average follow-up period of 9 (standard deviation 2.2) years. The study flowchart is provided in Figure 1. Table S1 presents the baseline characteristics stratified by sex. Among male participants, 1801 developed PD, while 1036 cases were observed in females. Across both sexes, participants who developed PD were older and had higher rates of comorbidities, including hypertension, diabetes, stroke, and hyperlipidemia, compared to those who did not. Additionally, a higher proportion of current alcohol consumers was observed in the PD group, alongside elevated FPG and HbA1c levels.
3.2 Relationship Between SHR and PD
Table 1 details the associations between SHR and PD stratified by sex.
| Quantiles of the SHR | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| T1 | Ref | Ref | Ref | Ref | ||||
| T2 | 0.87 (0.75–1.01) | 0.061 | 0.86 (0.72–1.03) | 0.093 | 1 (0.89–1.12) | 0.945 | 0.98 (0.85–1.12) | 0.751 |
| T3 | 0.96 (0.83–1.12) | 0.627 | 0.92 (0.76–1.1) | 0.341 | 1.17 (1.04–1.3) | 0.006 | 1.2 (1.06–1.37) | 0.004 |
| Continuous | ||||||||
| SHR | 0.99 (0.63–1.58) | 0.982 | 0.81 (0.45–1.43) | 0.458 | 1.57 (1.16–2.12) | 0.003 | 1.65 (1.17–2.32) | 0.004 |
- Note: Model 1 age, Ethnic background, Townsend Deprivation Index. Model 2: age, Ethnic background, Townsend Deprivation Index, BMI, smoking status, alcohol consumption, physical activity (MET hours/week), hypertension, stroke, high cholesterol, sleep duration, and C-reactive protein levels.
- Abbreviations: CI, confidence interval; HR, hazard ratio.
In females, after adjustment for model 1 covariates, neither the second tertile (T2: HR 0.87 [95% CI: 0.75–1.01]) nor the third tertile (T3: HR 0.96 [95% CI: 0.83–1.12]) of SHR was associated with PD risk compared to the first tertile (T1). Adjusting for Model 2 covariates yielded similar results, with T2 (HR 0.86 [95% CI: 0.72–1.03]) and T3 (HR 0.92 [95% CI: 0.76–1.1]) remaining nonsignificant. When SHR was analyzed as a continuous variable, no significant association was observed (Model 1: HR 0.99 [95% CI: 0.63–1.58]; Model 2: HR 0.81 [95% CI: 0.45–1.43]).
In males, after Model 1 adjustment, T2 was not significantly associated with PD risk (HR 1.00 [95% CI: 0.89–1.12]), but T3 was linked to an increased risk of PD (HR 1.17 [95% CI: 1.04–1.30]). In Model 2, T2 remained nonsignificant (HR 0.98 [95% CI: 0.85–1.12]), while T3 was still associated with an elevated PD risk (HR 1.20 [95% CI: 1.06–1.37]). When analyzed as a continuous variable, SHR was positively associated with PD risk (Model 1: HR 1.57 [95% CI: 1.16–2.12]; Model 2: HR 1.65 [95% CI: 1.17–2.32]).
Sensitivity analyses, excluding participants diagnosed with PD within the first 2 years of follow-up (n = 1414, 63.4% men), confirmed the findings in males, with T3 remaining significantly associated with PD risk (HR 1.21 [95% CI: 1.06–1.38]) and SHR as a continuous variable showing a consistent association (HR 1.66 [95% CI: 1.17–2.35]) (Table S2).
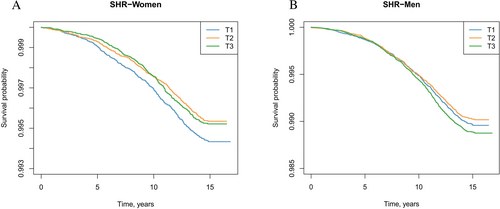
Kaplan–Meier survival analyses illustrating event-free survival across SHR tertiles are presented in Figure 2.
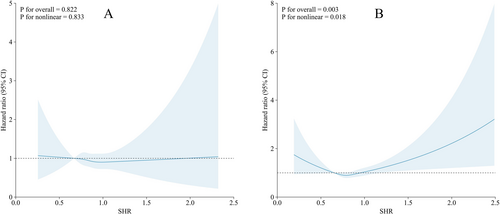
Restricted cubic spline Cox regression analyses revealed that the association between SHR and PD risk was nonlinear in men (p for nonlinearity = 0.018), with a U-shaped curve suggesting increased risk at both lower and higher ends of SHR distribution (Figure 3B). In contrast, no significant association or nonlinearity was observed in women (p for nonlinearity = 0.833, Figure 3A). These findings suggest that the relationship between SHR and PD risk in men may not follow a simple linear trend, highlighting the complexity of metabolic stress responses in neurodegeneration.
3.3 Subgroup Analysis
Subgroup analyses were conducted to assess the relationship between SHR and PD in participants with different glucose metabolic status.
In participants with DM, no significant association was observed between SHR tertiles and outcomes in either men (T3: HR 1.10 [95% CI: 0.47–2.53]) or women (T3: HR 1.49 [95% CI: 0.19–11.36]). SHR as a continuous variable also showed no significant association in men (HR 1.49 [95% CI: 0.71–3.13]) or women (HR 1.07 [95% CI: 0.20–5.64]) (Table S3).
In participants with pre-DM, SHR tertiles were not significantly associated with outcomes in either men (T3: HR 0.81 [95% CI: 0.53–1.26]) or women (T3: HR 0.53 [95% CI: 0.26–1.07]). Similarly, SHR as a continuous variable showed no significant association in men (HR 0.97 [95% CI: 0.32–2.94]) or women (HR 0.65 [95% CI: 0.10–4.20]) (Table S3).
In participants with non-DM, among women, SHR tertile T3 was not associated with outcomes (HR 0.97 [95% CI: 0.78–1.20]). However, in men, SHR tertile T3 was significantly associated with increased risk (HR 1.25 [95% CI: 1.08–1.45]) (Table S3).
Subgroup analysis excluding patients who progressed02013;3.13]) or women (HR 1.07 [95% CI: 0.20–5.64]) (Table S3).
In participants with pre-DM, SHR tertiles were not significantly associated with outcomes in either men (T3: HR 0.81 [95% CI: 0.53–1.26]) or women (T3: HR 0.53 [95% CI: 0.26–1.07]). Similarly, SHR as a continuous variable showed no significant association in men (HR 0.97 [95% CI: 0.32–2.94]) or women (HR 0.65 [95% CI: 0.10–4.20]) (Table S3).
In participants with non-DM, among women, SHR tertile T3 was not associated with outcomes (HR 0.97 [95% CI: 0.78–1.20]). However, in men, SHR tertile T3 was significantly associated with increased risk (HR 1.25 [95% CI: 1.08–1.45]) (Table S3).
Subgroup analysis excluding patients who progressed from non-DM and pre-DM to DM (non-DM to DM: n = 23,244, 45.8% men; pre-DM to DM: n = 3099, 49.4% men) is presented in Table S4, showing consistent results with the main findings.
4 Discussion
This study aimed to explore the association between the SHR and the risk of PD, with a particular focus on sex- and diabetes-specific factors. Our findings revealed a significant association between elevated SHR and an increased risk of PD in men, particularly those without diabetes, but not in women, men with prediabetes, or men with diabetes. These results highlight the complex interplay of metabolic stress in PD pathogenesis and underscore the need to investigate sex-specific mechanisms and the modifying effects of glucose metabolism status.
SHR is a novel marker that integrates both FPG and HbA1c to provide a more comprehensive measure of an individual's glycemic status, reflecting both acute and long-term glycemic control [15]. Elevated SHR signifies an acute state of metabolic stress, which is thought to be linked to various adverse outcomes, including cardiovascular disease, stroke [16, 17], and now, as demonstrated in this study, neurodegenerative diseases such as PD. While much of the literature has focused on chronic metabolic conditions like diabetes and their links to PD [18, 19], our findings suggest that acute metabolic stress, as indicated by a high SHR, may also play a significant role in the development of PD. This is an important step in understanding how fluctuations in blood glucose may affect the brain, potentially through inflammatory pathways, oxidative stress, or other mechanisms that influence neuronal health.
A striking finding in our study was the marked difference in the association between SHR and PD risk between men and women. While elevated SHR was associated with an increased risk of PD in men, this association was not observed in women. Several factors may contribute to these sex-specific differences.
First, there are well-documented differences in the neurobiological effects of sex hormones on the brain. Estrogen, a hormone that is more prominent in women, has been shown to have neuroprotective effects [20]. It is thought to reduce oxidative stress, modulate mitochondrial function, and enhance neuronal survival [21, 22], all of which could counteract the potentially harmful effects of elevated SHR on the brain. In contrast, men have lower levels of estrogen and may therefore be more susceptible to the harmful effects of metabolic stress on the brain. This might explain why we observed an association between SHR and PD risk in men but not in women.
Second, men and women may experience different patterns of metabolic disease and glycemic dysregulation. Men are more likely to develop metabolic syndrome, including central obesity and higher levels of visceral fat, which have been linked to systemic inflammation and oxidative stress [23, 24]. These metabolic disturbances could exacerbate the neurotoxic effects of elevated blood glucose levels, making men more vulnerable to the development of PD. In contrast, women tend to have a more protective fat distribution pattern and may be less prone to the systemic inflammation that exacerbates PD risk [25]. These biological differences may explain why SHR is a significant predictor of PD in men but not in women.
Third, lifestyle factors may also differ between the sexes and contribute to the observed differences. Men may have higher exposure to environmental toxins, engage in more physically demanding occupations, all of which may amplify the effects of metabolic stress on the brain [26-28]. Women, on the other hand, may benefit from a combination of lifestyle factors that mitigate these risks, such as lower alcohol consumption, greater health awareness, and better management of chronic conditions [29]. However, this hypothesis requires further investigation through more detailed lifestyle and environmental exposure data.
Furthermore, our restricted cubic spline analyses revealed a U-shaped association between SHR and PD risk in men, indicating that both relatively low and high SHR values may confer increased risk. This nonlinear pattern suggests that not only hyperglycemia but potentially relative hypoglycemia or other stress-induced glycemic instability may adversely affect neurological outcomes. This complexity underscores the need to consider SHR as a dynamic marker rather than a strictly linear predictor, and further mechanistic studies are warranted to elucidate how both extremes of glycemic stress influence PD pathogenesis.
Our study also investigated the relationship between SHR and PD risk in individuals with and without diabetes, revealing another important aspect of this relationship. In nondiabetic men, elevated SHR was associated with an increased risk of PD, while no significant association was observed in men with prediabetes or diabetes. Similarly, in women, SHR was not associated with PD risk regardless of their diabetes status, including prediabetes.
In nondiabetic men, elevated SHR may represent a transient but significant metabolic disruption, indicating a heightened acute response to stressors such as inflammation, hyperglycemia, or other metabolic disturbances. Nondiabetic individuals, particularly those without a history of chronic glycemic dysregulation, may experience a stronger acute inflammatory response to elevated glucose levels, which could contribute to the acceleration of neurodegenerative processes [30]. This suggests that the effects of SHR might be most pronounced in individuals without underlying chronic conditions like prediabetes or diabetes, where acute metabolic stress may have a more significant impact on brain function.
On the other hand, diabetic men, who are chronically exposed to higher levels of glucose and insulin resistance, may experience a desensitization of their neural tissues to the acute effects of elevated glucose levels [31]. Chronic hyperglycemia in diabetes could lead to a prolonged inflammatory state, which may not have the same acute impact on neuronal health as a transient spike in glucose [32, 33]. Similarly, in men with prediabetes, the intermediate state of glycemic impairment might not trigger the same acute metabolic stress response as seen in nondiabetic individuals, nor the chronic adaptation seen in diabetes, potentially explaining the lack of association with PD risk [34].
Recent studies have proposed that glucose metabolism abnormalities observed in patients with PD may not simply reflect systemic insulin resistance or chronic hyperglycemia, but rather arise from distinct pathophysiological processes within the central nervous system [35, 36]. For example, impairments in mitochondrial function, neuroinflammation, and altered dopamine–glucose coupling in the brain may independently drive central insulin resistance in PD, even in the absence of systemic metabolic disorders. This central dysregulation may partly explain why SHR, which primarily reflects acute peripheral glycemic stress, shows limited association with PD risk in individuals with established diabetes. In these individuals, chronic hyperglycemia may have already triggered long-term neuroadaptations or neuronal injury, potentially obscuring the added effect of transient glycemic excursions captured by SHR. Therefore, the lack of statistical significance in diabetic subgroups does not necessarily negate a pathophysiological link between glucose dysregulation and PD, but may instead reflect distinct temporal and mechanistic patterns of metabolic involvement in neurodegeneration.
These findings underscore the complex relationship between chronic metabolic conditions and acute metabolic stress. In individuals with diabetes, the body's adaptive mechanisms to long-term glucose fluctuations may reduce the impact of acute metabolic disturbances [37]. In individuals with prediabetes, the transitional glycemic state may mute the effects of SHR, while in those without diabetes, the absence of such adaptation may heighten susceptibility to the neurodegenerative effects of stress hyperglycemia, making SHR a more potent risk marker for PD in these populations.
The relationship between SHR and PD that we observed in men, particularly nondiabetic men, underscores the potential role of metabolic stress in PD pathogenesis. Chronic and acute disturbances in glucose metabolism may contribute to neurodegeneration through several potential mechanisms. Elevated glucose levels are known to induce inflammation, oxidative stress, and mitochondrial dysfunction—all of which are implicated in the development and progression of neurodegenerative diseases like PD [38, 39].
This study has several notable strengths. First, the large sample size and the use of a well-characterized cohort from the UK Biobank enabled robust analyses with comprehensive adjustment for potential confounders. Second, the sex-stratified and subgroup analyses added depth to the findings, uncovering nuanced associations that might be overlooked in a generalized analysis. Finally, the use of SHR, a novel and clinically relevant marker that integrates acute and chronic glycemic measures, adds a unique perspective to the study of PD risk factors.
However, several limitations should be acknowledged. First, the observational nature of this study precludes causal inference, and residual confounding cannot be ruled out despite comprehensive adjustments. Second, SHR was calculated using a single measurement of fasting glucose and HbA1c at baseline, which may not reflect glycemic variability over time. Third, while we stratified participants by glycemic status using ADA-defined cutoffs, the prediabetes definition did not include impaired glucose tolerance (IGT) due to the lack of oral glucose tolerance test (OGTT) data in the UK Biobank. This may have led to misclassification of individuals with isolated IGT, potentially underestimating associations within the prediabetes subgroup. Fourth, the study population consisted predominantly of individuals of European descent, which may limit the generalizability of our findings to other ethnic groups. Future studies incorporating OGTT measurements and more diverse populations are warranted.
5 Conclusion
In conclusion, this study provides evidence that elevated SHR is associated with an increased risk of PD in men, particularly in nondiabetic men, but not in women, prediabetic individuals, or diabetic individuals. These findings highlight the importance of considering sex- and diabetes-specific metabolic factors in PD research. While the study adds a novel perspective on PD risk, further studies are needed to validate these findings in diverse populations and to investigate the underlying mechanisms. Understanding the role of metabolic stress in PD development may pave the way for targeted prevention strategies and early interventions.
Author Contributions
H.Z., Y.M., X.Z., and W.X. designed the study and initial analysis plan. H.Z. and Y.M. contributed to the statistical analysis and wrote the draft of the manuscript. H.Z., Y.M., X.Z., W.X., and W.S. contributed to revision of the manuscript. All authors mentioned above made substantial contributions to the content of the paper.
Acknowledgements
The present study utilized the UK Biobank Resource (https://www.ukbiobank.ac.uk). The authors extend their appreciation to the investigators, staff, and participants of the UK Biobank for their contributions.
Disclosure
The authors have nothing to report.
Ethics Statement
The UK Biobank was established with ethical clearance from the North West Multi-Centre Research Ethics Committee (reference: 21/NW/0157).
Consent
Written informed consent have been provided by all participants.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




