Insights into phenotypic variability caused by GARS1 pathogenic variants
Abstract
Background and Purpose
Pathogenic variants of the glycyl-tRNA synthetase 1 (GARS1) gene have been described as a cause of Charcot–Marie–Tooth disease type 2D, motor axonal neuropathy with upper limb predominance (distal hereditary motor neuropathy [dHMN] type V), and infantile spinal muscular atrophy.
Methods
This cross-sectional, retrospective, observational study was carried out on 12 patients harboring the c.794C>T (p.Ser265Phe) missense pathogenic variant in GARS1. The patients' clinical data, nerve conduction studies, magnetic resonance imaging (MRI), and intraepidermal nerve fiber density in skin biopsies were reviewed.
Results
The mean age at onset was 9.5 years; the intrinsic hand muscles were affected before or at the same time as the distal leg musculature. The clinical examination revealed greater weakness of the distal muscles, with a more pronounced involvement of the thenar complex and the first dorsal interosseous in upper limbs. Electrophysiological studies were concordant with an exclusively motor axonal neuropathy. A pathologic split hand index was found in six patients. Muscle MRI showed predominant fatty infiltration and atrophy of the anterolateral and superficial posterior compartment of the legs. Most patients reported distal pinprick sensory loss. A reduced intraepidermal nerve fiber density was evident in skin biopsies from proximal and distal sites in nine patients.
Conclusions
GARS1 variants may produce a dHMN phenotype with “split hand” and sensory disturbances, even when sensory nerve conduction studies are normal. This could be explained by a dysfunction of sensory neurons in the dorsal ganglion that is reflected as a reduction of dermal nerve endings in skin biopsies without a distal gradient.
INTRODUCTION
Charcot–Marie–Tooth disease (CMT) encompasses a group of genetically diverse inherited motor and sensory neuropathies that share the main pathological feature of progressive motor and sensory degeneration [1]. There is some genetic overlap between the axonal forms of CMT (CMT2) and the inherited neuropathies classified as distal hereditary motor neuropathy (dHMN) or distal spinal muscular atrophy (dSMA), all of which produce length-dependent lower motor neuron degeneration with no detectable sensory loss [2]. In this way, pathogenic variants in the glycyl-tRNA synthetase 1 (GARS1) gene have been identified in patients with a CMT2 (CMT2D) or dHMN/dSMA phenotype, frequently displaying characteristic upper limb predominance (dHMN type V) [3].
GARS1 encodes mitochondrial and cytoplasmic isoforms of the glycyl-tRNA synthetase (GlyRS), a member of the ubiquitously expressed aminoacyl-tRNA synthetase (ARS) family, which is responsible for charging tRNA with glycine [4]. This ubiquitous “housekeeping” enzyme is necessary for adequate protein translation in all cells, yet pathogenic variants of this gene specifically lead to degeneration of peripheral motor and sensory nerves, both in humans and in mouse models [5]. Furthermore, autosomal dominant variants of the genes encoding other members of the ARS family (YARS, AARS, HARS, MARS, and WARS) have also been associated with the development of intermediate or axonal forms of CMT, whereas autosomal recessive changes may produce more complex phenotypes including epileptic encephalopathy, sensorineural hearing loss, developmental delay, and liver dysfunction [6-11].
More than 15 missense GARS1 variants have been described in patients with inherited neuropathy, and although most of them affect the catalytic domain of the protein, others reside throughout the primary GlyRS sequence. Studies in yeast, fly, and mouse models of CMT2D revealed that dominant disease-causing variants do not always affect canonical GlyRS activity. Sometimes the prevailing mechanism is a toxic gain of function, with or without alteration of GlyRS activity [12]. The mechanisms underlying the specific vulnerability of peripheral nerve axons to ARS dysfunction remain largely unresolved, although they may be related to an impairment of axonal transport and protein translation, involving both loss- and gain-of-function processes [13, 14].
The phenotypic spectrum of GARS1-associated neuropathy ranges from sensory–motor to purely motor forms of the disease, named CMT2D and dMHN/dSMA, respectively. In both phenotypes, the onset of symptoms may appear in adolescence or early adulthood, manifested with cramps in distal limb muscles followed by symmetrical weakness and atrophy in the lateral aspects of the hands, which subsequently progress to the lower limbs [15, 16]. There is significant overlap between the dHMN and CMT phenotypes, and some patients previously diagnosed with dHMN subsequently develop clinical or electrical sensory deficits [15, 17, 18]. The reasons underlying this variable sensory involvement remain largely unknown, but according to mouse models, the sensory damage appears to be present at birth, is nonprogressive, and is probably caused by aberrant interactions of the mutant protein with Trk receptors [19]. Less frequently, pathogenic variants in GARS1 located near the anticodon-binding and catalytic domains of the gene have been associated with a rare and severe infantile spinal muscular atrophy (iSMA) phenotype that appears within the first 12 months of life [20].
Here, we present in-depth phenotyping of 12 patients harboring a known pathogenic variant in GARS1 that causes a dHMN phenotype with mild sensory symptoms.
METHODS
This cross-sectional, retrospective, and observational study enrolled patients with GARS1 germline variants identified at a tertiary neuromuscular center in Valencia, Spain. Written informed consent was obtained from all the participants, and the protocols were approved by the Drug Research Ethics Committee of Hospital Universitari i Politècnic La Fe, with the approval number 2019/0123. Photographs and videos of the patients were taken and reproduced only after specific informed consent was given.
A standardized questionnaire was employed to collect the basal information on the participant's medical history and symptoms. The severity of the neuropathy was evaluated using the Charcot–Marie–Tooth Neuropathy Score version 1 (CMTNS), as a significant proportion of the patients had not undergone the mandatory neurophysiological radial nerve testing required for CMTNS version 2 [21]. For patients on whom no nerve conduction studies (NCS) had been performed, the Charcot–Marie–Tooth Examination Score version 2 (CMTES) was employed.
Motor and sensory NCS were performed using standard techniques at a controlled temperature of ≥32°C. The split hand index (SHI) was determined by multiplying the compound muscle action potential (CMAP) amplitude of the first dorsal interosseous and the abductor pollicis brevis (APB) muscles and dividing the product by the CMAP amplitude of the abductor digiti minimi (ADM) muscle with a cutoff value of 5.2 [22]. Sensory evoked potentials (SEPs) were obtained following standard procedures.
Genetic studies were carried out by amplification of all exons and their intronic flanking sequences in the proband of Family 1 (F1) and using a customized gene panel in the proband of Family 2 (F2). Segregation analysis was performed by Sanger sequencing on the available family members, and the GARS1 variant identified was classified following the American College of Medical Genetics and Genomics guidelines [23]. Muscle magnetic resonance imaging (MRI) of the feet and lower limbs was performed following a previously described protocol [24]. Fatty infiltration of the muscles of the feet and lower limbs was analyzed in T1 sequences and graded from 0 to 4 according to the modified Mercuri score [25]. T2-weighted short-tau inversion recovery (STIR-T2w) sequences were used to evaluate the existence of muscle edema.
Skin biopsies from the thigh and the lateral distal aspect of the leg (above the external malleolus) were obtained with a 3-mm punch and immediately fixed overnight at 4°C in 4% paraformaldehyde prepared in phosphate-buffered saline (PBS). After washing in PBS (4 × 15 min), the tissue was cryoprotected by immersion in sucrose and stored in liquid nitrogen at −80°C prior to obtaining 50-μm sections on a cryomicrotome at −25°C. The sections were then washed twice in PBS and transferred for long-term storage. The biopsies were then probed with a primary antibody (rabbit anti-PGP 9.5 polyclonal antibody, ab-5925; Millipore, Burlington, MA, USA), washed in supplemented PBS, and then incubated with the secondary antibody at room temperature (biotinylated antirabbit IgG [H + L] BA-1000; Vector, Newark, CA, USA). Finally, the intraepidermal nerve fiber (IENF) density was determined under an optical microscope (Olympus BX41; Hachioji, Tokyo, Japan and Nikon Digital, Tokyo, Japan).
RESULTS
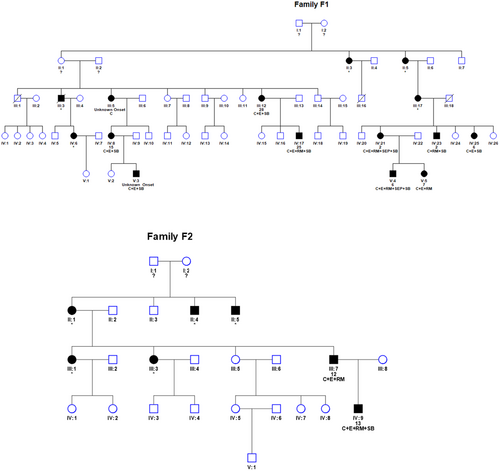
Twelve patients were identified who harbored the same missense pathogenic variant in the well-conserved catalytic domain of GARS1, c.794C>T (p.Ser265Phe). The patients belonged to two apparently nonrelated families: F1 with 10 affected individuals and F2 with two affected individuals studied (Figure 1). Both families had ancestors from the same municipality, although no common progenitor could be identified.
Clinical assessments
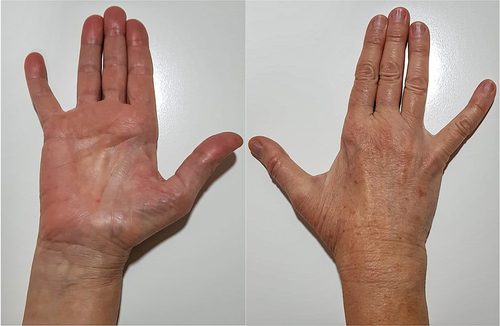
Demographic and clinical characteristics of all the individuals are detailed in Table 1 and Figure 2. The mean age at which patients were evaluated was 45.8 years (range = 27–72 years), and the median age of symptom onset was 9.5 years (range = 2–28 years), with 50% of patients being symptomatic in the first decade of life, 30% in the second decade, and 20% in the third. In both families the age of onset was similar. Most patients (90.9%) manifested symptoms in the upper extremities before or at the same time as those in the lower extremities. The most frequent clinical presentation was clumsiness in handling and walking. Only one individual had motor symptoms restricted to the lower extremities at onset, and only in two patients the initial presentation included sensory symptoms. Clinical examination revealed weakness in the intrinsic muscles of the hands in all patients. In eight patients (66.7%), there was a clear muscular atrophy affecting the first dorsal interosseous and the thenar eminence, with a relatively well-preserved muscle bulk of the hypothenar eminence that gave a split hand appearance (Figure 2). Distal weakness and atrophy of the lower legs and/or deformities of the feet (pes cavus, hammer toes) and Achilles tendon retractions were observed in 10 patients.
| Case (sex) | Onset (years) | Age, years | Motor | Sensory LL | Reflexes | Foot deformities | MRI | Split hand (SHI) | Skin biopsy, fiber count | CMTES | Functional situation (years) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UL | LL | Pinprick | Vibratory | UL/LL | |||||||||
| F1/V:5 (F) | Walking and handling clumsiness, pes cavus (7) | 27 |
Proximal: − Distal: ++ |
Proximal: − Distal: ++ |
+ | nl | −/+ | PC, ATR, HT | Legs: distal posterior and anterolateral compartments fatty infiltration and atrophy | No (10.47) | − | 6 | Self-sustained gait |
| F1/V:4 (M) | Walking and handling clumsiness (6) | 32 |
Proximal: − Distal: + |
Proximal: − Distal: ++ |
++ | nl | ++/++ (patellar +++) | PC, ATR |
Feet: atrophy and fatty infiltration Legs: anterolateral compartment atrophy and proximal posterior compartment fatty infiltration Thighs: mild distal fatty infiltration in biceps femoris (long head) |
No (10.9) |
Proximal: 6 Distal: 3.7 |
6 | Self-sustained gait |
| F1/V:3 (M) | Unknown onset | 27 |
Proximal: − Distal: ++ |
Proximal: − Distal: ++ |
nl | nl |
++/+ (Achilles −) |
PC, ATR | − |
Yes (4.63) |
Proximal: 6.6 Distal: 3.8 |
5 | Self-sustained gait |
| F1/III:12 (F) | Handling clumsiness (27) | 60 |
Proximal: − Distal: ++ |
Proximal: − Distal: ++ |
nl | nl |
−/− (patellar +) |
No | − | − |
Proximal: 5.5 Distal: 5 |
− | − |
| F1/III:5 (F) | Handling clumsiness (unknown) | 72 |
Proximal: − Distal: ++ |
Proximal: − Distal: − |
nl | nl |
+++/+++ (Achilles −) |
No | − | Yes | − | − | − |
| F1/IV:17 (M) | Handling clumsiness, sensory alteration in hands (24) | 45 |
Proximal: − Distal: ++ |
Proximal: − Distal: ++ |
+ | nl |
+/+ (Achilles −) |
PC |
Feet: severe atrophy Legs: distal posterior and anterolateral compartment fatty infiltration; mild edema in posterior compartment |
Yes (NR to APB) |
Proximal: 5 Distal: 2.9 |
10 | DAFOs |
| F1/IV:25 (F) | Walking and handling clumsiness, sensory alteration in hands, pes cavus (6) | 42 |
Proximal: − Distal: + |
Proximal: − Distal: − |
+ | nl | ++/++ | PC | − |
No (10.65) |
Proximal: 8 Distal: 5 |
2 | Self-sustained gait |
| F1/IV:23 (M) | Walking and handling clumsiness, pes cavus (2) | 47 |
Proximal: − Distal: ++ |
Proximal: + Distal: ++ |
++ | + |
−/− (patellar +) |
PC |
Feet: severe atrophy Legs: distal posterior and anterolateral compartments fatty infiltration and atrophy Thighs: mild proximal and diffuse fatty infiltration |
Yes |
Proximal: 4.4 Distal: 4 |
18 | DAFOs (−−) |
| F1/IV:21 (F) | Walking clumsiness (2) | 53 |
Proximal: − Distal: ++ |
Proximal: + Distal: ++ |
++ | + | ++/− | PC, ATR | Legs: distal atrophy in anterolateral compartment |
Yes (0.05) |
Proximal: 7.6 Distal: 4.3 |
20 | Cane and DAFOs (48) |
| F1/IV:8 (F) | Handling clumsiness (15) | 59 |
Proximal: − Distal: ++ |
Proximal: − Distal: ++ |
nl | + |
+/+ (Achilles −) |
No | − |
Yes (0.41) |
Proximal: 4.2 Distal: 1.5 |
8 | Self-sustained gait |
| F2/IV:9 (M) | Handling clumsiness (13) | 27 |
Proximal: − Distal: ++ |
Proximal: − Distal: ++ |
+ | nl | +/++ | PC, ATR, HT |
Feet: mild intrinsic muscle atrophy Legs: moderate anterolateral compartment atrophy and distal posterior fatty infiltration; mild posterior edema |
Yes (0.32) |
Proximal: 5.5 Distal: 1.5 |
6 | Self-sustained gait |
| F2/III:7 (M) | Walking and handling clumsiness (12) | 59 |
Proximal: − Distal: ++ |
Proximal: − Distal: ++ |
++ | + | −/− | PC |
Feet: severe atrophy Legs: severe atrophy; mild edema |
Yes (NR to APB) |
− | 14 | Self-sustained gait, foot surgery (58) |
- Note: Motor examination: +, mild weakness; ++, moderate weakness. Sensory examination: nl, normal; +, reduced at toe; ++, reduced at ankle. Reflexes: −, absent; +, reduced; ++, normal; +++, increased.
- Abbreviations: APB, abductor pollicis brevis; ATR, Achilles tendon retractions; CMTES, Charcot–Marie–Tooth Examination Score; DAFO, dynamic ankle foot orthosis; F, female; HT, hammer toes; LL, lower limbs; M, male; MRI, magnetic resonance imaging; NR, no response; PC, pes cavus; SHI, split hand index; UL, upper limbs.
Of the 12 individuals, 10 (83.3%) reported sensory symptoms or reduced sensation at examination. Paresthesia, numbness, and pain were the most frequently reported symptoms. Sensory disturbances included pain hypoesthesia in a stocking pattern in eight patients (66.7%), and in some individuals, there was also vibratory (four cases, 33.3%), tactile (two patients, 16.7%), and thermal (two patients, 16.7%) impairment that followed a distal gradient. In one patient (F1/IV:8), the sensory and motor symptoms were accompanied by axial and appendicular ataxia, as well as postural and intention tremor with dystonic characteristics. In this patient, we identified a likely pathogenic c.844C>T/p.Arg282Trp variant in the gene TUBB4A (tubulin beta 4A class IVa) that was deemed responsible for the dystonic features [26, 27].
At the moment of evaluation, three patients used dynamic ankle/foot orthoses, one required crutches, and the rest maintained independent ambulation. The CMTES score at the last assessment was 9.5 points (ranging between 2 and 20 points), concordant with mild-to-moderate severity. No individual experienced respiratory failure, dysphagia, or other associated symptoms.
Nerve conduction studies
NCS were performed in 10 individuals, all of whom displayed abnormalities exclusively affecting the motor component (Table 2). Nerve conduction velocities were within the normal range in all nerves, as were sensory nerve action potentials. The latter were normal even in patients with long disease duration, and in those patients who had had more than one study, we were not able to detect any clear amplitude decrease with age. By contrast, CMAP amplitude decreased throughout the series. In most cases, CMAP amplitude was reduced more prominently in the lower limb nerves following a length-dependent distribution, but in three patients, CMAP amplitude reduction was proportionally greater in the median nerve than in the peroneal nerve. The drop in CMAP amplitude was consistently more prominent in the median nerve to the APB (mean = 4.92 mV) than in the ulnar nerve to the ADM (mean = 9.9 mV). The SHI was calculated in nine patients, and it was decreased in six of them. SEPs were obtained from four patients, and no abnormalities were detected in the tibial or median nerves studied.
| Case (sex) | Current age/onset (years) | Motor median | Motor ulnar to FDI | Motor ulnar to ADM | Motor peroneal | SHI | Sensory ulnar | Sensory median | Sural | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CMAP, mV | CV, m/s | DL, ms | F-wave, ms | CMAP, mV | CV, m/s | DL, ms | F-wave, ms | CMAP, mV | CV, m/s | DL, ms | F-wave, ms | CMAP, mV | CV, m/s | DL, ms | F-wave, ms | SNAP, μV | CV, m/s | SNAP, μV | CV, m/s | SNAP, μV | CV, m/s | |||
| F1/V:5 (F) | 21/7 | 8.9 | 51.2 | 5 | 30.45 | – | – | – | – | 13.8 | 50.7 | 3.7 | 30.75 | 1.5 | 46.2 | 6.1 | 43.95 | – | 14.3 | 59 | 34.7 | 51.4 | 21.2 | 47.4 |
| 27 | 13.4 | 50.6 | 4.9 | – | 8.12 | – | 3.9 | – | 13.9 | 45 | 3.2 | – | 2.2 | 30.8 | 5.7 | – | 7.83 | 12.7 | 61.5 | 24.1 | 52.8 | 25.3 | 48.8 | |
| 30 | 10.9 | 45.9 | 4.20 | – | 7.3 | – | 4.75 | – | 7.6 | 53.7 | 3.8 | – | 2.5 | 30.9 | 6.35 | – | 10.47 | 11.6 | 62.2 | 21.0 | 55.3 | 13.5 | 44.4 | |
| F1/V:4 (M) | 30/6 | 13.6 | 47.4 | 5.35 | – | – | – | – | – | 9.3 | 46.9 | 3.45 | 35.3 | 0.8 | – | 9.40 | – | – | – | – | 13.1 | 46.3 | 25.8 | 44.4 |
| 35 | 13.2 | 47.2 | 4.85 | – | 9.5 | – | 4.08 | – | 11.5 | 46.6 | 3.38 | 32.3 | 6.1 | – | 2.81 | – | 10.9 | – | – | – | – | 22.6 | 45.7 | |
| F1/V:3 (M) | 27/−− | 15.5 | 53.1 | 4.1 | – | – | – | – | – | 14.1 | – | 3.05 | – | 4 | 45.5 | 5.3 | – | – | – | – | – | – | 25.8 | 44.4 |
| 29 | 10 | 56.6 | 3.52 | – | 3.7 | – | 4.08 | – | 8 | 65.3 | 2.96 | – | – | – | – | – | 4.63 | 13.7 | 62 | – | – | – | – | |
| F1/III:12 (F) | 60/28 | 0.3 | 41.2 | 4.2 | – | – | – | – | – | 9.8 | 41.0 | 3.3 | – | 5.6 | 39.7 | 4.7 | – | – | 5.2 | 61.1 | 18.0 | 50.0 | 12 | 60.0 |
| F1/III:5 (F) | −−/−− | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| F1/IV:17 (M) | 28/25 | 12.5 | 49.2 | 3.5 | – | – | – | – | – | 2.9 | 49.1 | 2.7 | – | – | – | – | – | – | – | – | 29 | 50.7 | – | – |
| 56 | NR | NR | NR | – | 0.9 | – | 4.85 | – | 14.3 | 51.5 | 3.5 | – | – | – | – | – | NR | – | – | – | – | – | – | |
| F1/IV:25 (F) | 44/6 | 11.7 | 51.1 | 4.0 | – | 13.3 | – | 4.55 | – | 14.6 | 50.7 | 3.2 | – | 7 | 32.4 | 5.55 | 62.35 | 10.65 | 10.8 | 56.4 | 14.5 | 55.3 | 19.8 | 41.4 |
| F1/IV:23 (M) | 36/2 | – | – | – | – | – | – | – | – | 7.8 | 52 | 3.3 | – | – | – | – | – | – | 16.3 | 61 | 20.4 | 46 | 11.2 | 50 |
| F1/IV:21 (F) | 43/2 | 2.8 | 40.3 | 4.8 | 37.25 | – | – | – | – | 12.7 | 48.8 | 2.9 | 30.09 | 0.9 | 43.7 | 7.1 | – | – | 6.3 | 56.4 | 26 | 49.8 | 34.5 | 54.6 |
| 47 | 1.1 | 28.2 | 5.2 | – | – | – | – | – | 14.5 | 50 | 3.1 | 30.4 | 0.7 | 36.1 | 7.5 | – | – | 10.4 | 59.5 | 48.6 | 50 | 33.5 | 44 | |
| 52 | 1 | 38.9 | 5.7 | – | – | – | – | – | 12.2 | 45.7 | 2.85 | 28.35 | 0.08 | 29.2 | 6.8 | – | – | 9.0 | 60.0 | 20.3 | 57.1 | 20.4 | 46.9 | |
| 58 | 0.4 | – | 6.44 | – | 1.2 | 44.4 | 3.12 | – | 10.7 | 51.9 | 3.08 | – | – | – | – | – | 0.05 | – | – | – | – | – | – | |
| F1/IV:8 (F) | 59/15 | 2.3 | 37.1 | 5.55 | – | 2.4 | – | 3.95 | – | 13.4 | 60.0 | 2.55 | 26.10 | 0.1 | – | 10.5 | – | 0.41 | 10.2 | 59.1 | 18.2 | 56.4 | 8.9 | 45.3 |
| F2/IV:9 (M) | 9/13 | 7.8 | 59.2 | 3.5 | – | – | – | – | – | 12.2 | 56.8 | 3.1 | – | 7.8 | 48.4 | 4.2 | – | – | 7.2 | 42.4 | 15.7 | 37.8 | 13 | 35.3 |
| 19 | 0.9 | 67.6 | 5.3 | – | – | – | – | – | 9.1 | 58.6 | 3.3 | – | 8.5 | 47.6 | 4.7 | – | – | 18 | 57.1 | 34 | 58.3 | 27 | 48.6 | |
| 28 | 0.7 | – | 4.7 | – | – | – | – | – | 11.3 | 58.0 | 2.9 | 25.3 | 5.6 | 49.2 | 4.25 | – | – | 16.3 | 51.2 | 20.4 | 54.9 | 9.7 | 46.2 | |
| 29 | 0.3 | – | 4.95 | – | 11.9 | – | 2.8 | – | 11.1 | 57.9 | 3.15 | – | – | – | – | – | 0.32 | – | – | – | – | – | – | |
| F2/III:7 (M) | 60/12 | 0.1 | – | 5.52 | – | NR | NR | NR | NR | 0.1 | – | 4.35 | – | NR | NR | NR | – | NR | – | – | – | – | 10.0 | 54.3 |
- ADM, abductor digiti minimi; CMAP, compound muscle action potential (peak-to-peak amplitudes); CV, conduction velocity; DL, distal latency; F, female; FDI, first dorsal interosseus; M, male; NR, no response; SHI, split hand index; SNAP, sensory nerve action potential.
Muscle-imaging features
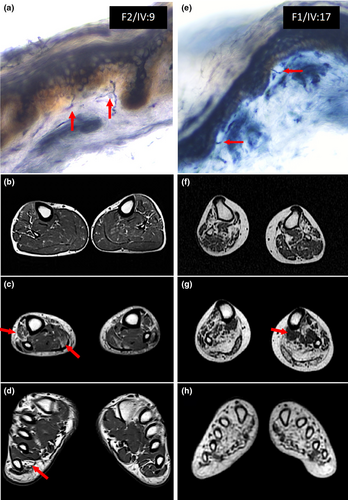
MRI scans of the lower limbs were reviewed in seven patients. Five scans included the feet; four of them showed severe atrophy and fatty infiltration of the intrinsic feet muscles, whereas in one patient the involvement was mild. Regarding the leg muscles, atrophy and fatty infiltration was observed in all the patients following a distoproximal gradient in the involved muscles (Figure 3). Atrophy was more prominent in the muscles of the anterolateral and superficial posterior compartment, and fatty infiltration was greater in the superficial posterior compartment (which showed a median Mercuri score of 3) than in the anterior or lateral compartments (median Mercuri score = 2). Small areas of muscle tissue with normal signal intensity surrounded by areas of fat intensity, named “muscle islands,” were observed in muscles with a Mercuri score of 3 or 4 (Figure 3) [28]. There was a relative sparing of the deep posterior compartment of the legs (flexor digitorum longus, tibialis posterior, and flexor hallucis longus) in most of the patients. Muscles of the thighs were only affected in three individuals, with mild distal fatty infiltration without a specific pattern. Mild hyperintensities in STIR-T2w sequences were evident in four patients, predominantly in the muscles of the superficial posterior compartment of the leg and anterolateral compartment in one of them.
Skin biopsies
Skin biopsies were obtained from nine patients from the proximal and distal lower limbs. A reduction in IENF density was observed in all of them (Figure 3 and Table 1). The average density in proximal regions (thighs) was 5.65 fibers/mm2 (cutoff at 95% lower value of a control group of 30 subjects with similar age range and equal sex representation = 8.5 fibers/mm2), and in distal regions it was 3.8 fibers/mm2 (cutoff value for age and sex = 4.5 fibers/mm2). In seven patients, IENF density was reduced both proximally and distally, whereas in two of them it was decreased only proximally.
DISCUSSION
GARS1-associated neuropathy encompasses different phenotypes including purely motor distal forms (dHMN or dSMA type V), sensory and motor distal forms (CMT2D), and severe iSMA-like phenotypes with respiratory failure. A continuum exists between dHMN and CMT2D phenotypes, even in individuals from the same family, with some of the members displaying sensory alterations whereas others do not [15, 18]. Weakness and atrophy usually appear and predominate in the hands, progressing to the distal muscles of lower limbs in approximately half of the individuals, with variable clinical severity that ranges from weakness and atrophy of the extensor digitorum brevis and dorsiflexors of the fingers, to weakness and peroneal muscle atrophy with foot drop and pes cavus [29]. Patients classified as CMT2 also present a distal sensory axonopathy that is associated with an impairment of all sensory modalities with vibratory and tactile predominance, related to disease severity but not duration [17].
The c.794C>T (p.Ser265Phe) variant in GARS1 was previously described in a Korean family in which three patients were affected by a dHMN type V phenotype [30]. The clinical phenotype of this Korean family is quite similar to those described in our families, with an age at onset around the 2nd decade of life, a purely or predominantly motor presentation, and relevant upper limb involvement. As patent in our families, predominant upper limb weakness and atrophy have been described as an initial or early clinical feature related to GARS1 neuropathy. In most of the reported cases, symptoms first appear in the intrinsic hand muscles, predominantly in the thenar musculature and first interosseus [15, 17, 31]. The preferential involvement of the first dorsal interosseous and the abductor pollicis brevis muscles, with relative sparing of the abductor digiti minimi muscle, is known as split hand [32]. This clinical sign was described in amyotrophic lateral sclerosis (ALS), where >70% present a split hand at some stage of the disease [22, 33]. In any case, split hand is not pathognomonic for ALS, although it has only rarely been described in other diseases like spinal and bulbar muscular atrophy (Kennedy disease), spinocerebellar ataxia type 3, and postpolio syndrome, and in a single dHMN family with a pathogenic variant in GARS1 [34-36]. In our patients, upper limb involvement predominated in 10 of 12 affected members, and clinical examination revealed a split hand in nine of them (75%), confirming that GARS1-associated neuropathy should be considered a potential cause for split hand.
There are several ways electrophysiological testing can help to confirm and quantify a split hand, most of which include ratios of the CMAP amplitudes from the relevant hand muscles, whereas others include distal latencies, F-wave persistence, quantitative motor unit number index, or muscle echography [36-39]. The SHI was used in our cohort to attempt to confirm and quantify split hand, which is a validated diagnostic and prognostic marker in ALS [40]. The SHI was decreased in six of nine (66.6%) individuals. Those six patients had clinically evident split hand on examination, whereas the other three patients did not present atrophy in the hands. Therefore, in our experience, SHI did not outperform clinical examination and did not supersede the clinical findings.
MRI showed atrophy and fatty muscle replacement in all of the patients. We also identified the presence of small foci of normal muscle within fatty muscle tissue, which has been coined as “islands,” reported as a hallmark of neurogenic conditions [28]. Most patients followed a similar pattern of muscle involvement. In early stages, the involvement of the anterolateral muscle compartment of the legs predominated; subsequently, in most patients, fatty infiltration of the posterior compartment was observed. Finally, in advanced stages, MRI showed a severe atrophy and fatty replacement of all leg muscles, with a relative sparing of deep posterior compartment (predominantly tibialis posterior and flexor digitorum longus). These findings showed a length-dependent pattern of involvement in all patients except one (F2/IV:9) who presented a minimal atrophy of foot muscles with a greater involvement of distal portions of the calves, although this was also mild (Figure 3). Nevertheless, in all patients, fatty infiltration followed a distoproximal pattern in every muscle compartment.
All patients in this series had a dHMN phenotype, as sensory nerve conduction studies and SEP were preserved consistently, even in patients with decades of progression (Table 2). Interestingly, all individuals except two did report sensory symptoms throughout the disease course (distal paresthesia, numbness, or pain), yet only on rare occasions did these symptoms appear at the initial stages of the disease. On examination, 66.7% of patients reported pain sensory loss in lower limbs, which was more frequent than vibratory deficit (present in 33.3% of patients) in contrast to previous reports [17]. Only two patients (16.7%) described altered pain sensation in the hands. In any case, sensory impairment was mild, distal, nonprogressive, and limited to the lower limbs in most patients.
The discordance between clinical examination and electrophysiology has been described in a few other families with GARS1 neuropathy, yet the reason behind it has not been fully elucidated [15, 18, 41]. In keeping with the sural nerve biopsy findings described in patients with CMT2D and dHMN phenotypes, one possibility is that GARS1 pathogenic variants predominantly affect medium and small myelinated axons, whereas there is relative sparing of the thickest myelinated sensory fibers [15, 18, 42]. The involvement of smaller fibers could explain the presence of mild sensory symptoms/signs, despite the inability to demonstrate a reduction of sensory potentials. For this reason, we further investigated the epidermal small sensory fibers in our dHMN type V patients through determination of fiber density in skin biopsies.
The IENF density in skin biopsies has been established as one of the main tools to study sensory and autonomic neuropathies. This technique enables the study of terminals of the C- and A-delta fibers involved in the recognition of painful and thermal stimuli [43]. Although reduced IENF density is characteristic of small fiber neuropathies, it can also be seen following large sensory fiber damage [43]. This technique has made it possible to recognize the involvement of small fibers in a wide variety of peripheral neuropathies, both hereditary and acquired, even in entities considered to be purely motor neuropathies. This is the case with ALS, in which a significant length-dependent reduction of IENF was demonstrated, predominantly in spinal onset forms [44]. It is not clear whether these findings correlate with the presence of sensory alterations or with disease evolution; nevertheless, they support the existence of a distal sensory axonopathy [45, 46]. Significant reductions of IENF have also been reported in genetic forms of ALS (e.g., SOD1, SETX, VAPB), facial onset sensory and motor neuronopathy, and exceptionally, in certain forms of dHMN like those associated with BICD2 variants [46-48].
We detected a reduced IENF density in all of our cohort of dHMN patients who underwent skin biopsy. In most patients, the reduction of IENF was greater at the proximal than at the distal biopsy sites with respect to cutoff values. Moreover, in two patients, the loss of fibers was only evident at the proximal sites. There were sensory alterations on examination in all of these patients except two, Patients FI/V:3 and F1/III:12, who had reduced proximal and distal and proximal fiber density, respectively, but did not develop sensory symptoms during the course of the disease. There was no clear relation between age or disease duration and reduction of IENF, nor with the altered sensory modality or its severity.
In any case, IENF density reduction was clearly not length dependent and would not be concordant with a distal sensory axonopathy, but rather with more proximal sensory neuron damage. This is consistent with certain mouse models, in which there was a sensory and nonprogressive dorsal ganglion dysfunction present at birth, consisting of an alteration of the proportion of neurons dedicated to each sensory modality [19]. Therefore, it is possible that human variants in GARS1 cause a developmental dorsal ganglionopathy, which can be detected as a loss of IENF without a clear length-dependent pattern and manifesting clinically as a mild, nonprogressive multimodal sensory impairment. This sensory neuron reordering could explain the findings in examination without manifesting at NCS, because the total number of fibers evaluable by this technique would be maintained or minimally reduced. Along with earlier data, these findings indicate that the pattern of sensory neuropathy associated with GARS1 variants may range from a clear large and small fiber axonopathy to the loss of unmyelinated or thinly myelinated sensory fibers secondary to a possible ganglionopathy [18, 42].
There are some limitations in our study, such as the low number of patients or the lack of studies in some of them. In future studies, it would be interesting to incorporate a larger number of participants, including patients with different pathogenic variants and younger ages, which would allow a more accurate phenotyping and characterization of disease progression.
In summary, in-depth phenotypic characterization of our families allowed us to identify several significant phenotypic aspects of GARS1 neuropathy. On the one hand, as previously known, there is a distinctive motor phenotype affecting predominantly upper limbs, but with an important proportion of patients showing a split hand conformation and an involvement of the muscles of the anterolateral and posterior compartment of the legs, as was confirmed by MRI. On the other hand, we found a greater decrease in proximal intraepidermal fibers compared to distal fibers in skin biopsies, which could reflect an impairment of sensory neurons in the dorsal ganglia and be the origin of mild sensory symptoms without neurophysiological alterations. Consequently, although the clinical spectrum in these patients covers both purely motor forms and CMT-like forms, there is significant overlap that often makes them indistinguishable, requiring a deeper study beyond the NCS.
AUTHOR CONTRIBUTIONS
Jesús Jiménez-Jiménez: Investigation; writing – original draft; methodology; validation; writing – review and editing; formal analysis; data curation; visualization. Irene Navarrete: Investigation; methodology; visualization; writing – review and editing; formal analysis. Inmaculada Azorín: Investigation; methodology; visualization; formal analysis; data curation. Pilar Martí: Investigation; methodology; visualization; formal analysis; software; data curation. Roger Vílchez: Investigation; methodology; visualization; formal analysis; data curation. Nuria Muelas: Writing – review and editing; validation; visualization; formal analysis; data curation; supervision. Javier Cabello-Murgui: Writing – review and editing; visualization; investigation; validation. Elvira Millet: Investigation; writing – review and editing; visualization; validation; methodology; data curation; formal analysis. Juan Francisco Vázquez-Costa: Writing – review and editing; validation; visualization. Juan J. Vílchez: Writing – review and editing; visualization; validation; formal analysis; investigation; data curation. Teresa Sevilla: Conceptualization; investigation; writing – original draft; writing – review and editing; visualization; validation; methodology; formal analysis; project administration; supervision; data curation; resources; funding acquisition. Rafael Sivera: Conceptualization; investigation; writing – original draft; methodology; validation; visualization; writing – review and editing; formal analysis; project administration; data curation; supervision; resources; funding acquisition.
ACKNOWLEDGMENTS
We deeply appreciate the commitment of the families studied. R.S., J.J.V., N.M., J.F.V.-C., and T.S. are all members of the European Reference Network for rare neuromuscular diseases (EURO-NMD).
FUNDING INFORMATION
This work was supported by Instituto de Salud Carlos III through the project PI22/01830 to R.S., PI19/01178 to T.S., and PI21/00737 to J.F.V.-C. (cofunded by the European Regional Development Fund “A Way of Making Europe”).
CONFLICT OF INTEREST STATEMENT
The authors report no disclosures relevant to the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




