Remote seizures and drug-resistant epilepsy after a first status epilepticus in adults
Abstract
Background and purpose
Long-term consequences after status epilepticus (SE) represent an unsettled issue. We investigated the incidence of remote unprovoked seizures (RS) and drug-resistant epilepsy (DRE) in a cohort of first-ever SE survivors.
Methods
A retrospective, observational, and monocentric study was conducted on adult patients (age ≥ 14 years) with first SE who were consecutively admitted to the Modena Academic Hospital, Italy (September 2013–March 2022). Kaplan–Meier survival analyses were used to calculate the probability of seizure freedom following the index event, whereas Cox proportional hazard regression models were used to identify outcome predictors.
Results
A total of 279 patients were included, 57 of whom (20.4%) developed RS (mean follow-up = 32.4 months). Cumulative probability of seizure freedom was 85%, 78%, and 68% respectively at 12 months, 2 years, and 5 years. In 45 of 57 patients (81%), the first relapse occurred within 2 years after SE. The risk of RS was higher in the case of structural brain damage (hazard ratio [HR] = 2.1, 95% confidence interval [CI] = 1.06–4.01), progressive symptomatic etiology (HR = 2.7, 95% CI = 1.44–5.16), and occurrence of nonconvulsive evolution in the semiological sequence of SE (HR = 2.9, 95% CI = 1.37–6.37). Eighteen of 57 patients (32%) developed DRE; the risk was higher in the case of super-refractory (p = 0.006) and non-convulsive SE evolution (p = 0.008).
Conclusions
The overall risk of RS was moderate, temporally confined within 2 years after the index event, and driven by specific etiologies and SE semiology. Treatment super-refractoriness and non-convulsive SE evolution were associated with DRE development.
INTRODUCTION
Status epilepticus (SE) is a condition characterized by high short-term mortality and morbidity [1-3]. In recent years, most studies have been focused on the optimization of therapeutical management, the marketing of new potentially effective antiseizure medications (ASMs) [4-6], and the identification of short-term prognostic predictors. Different prognostic scores have been proposed to predict short-term SE outcomes [7-9]. Recently, a retrospective, multicenter study developed the so-called ACD score for predicting 2-year mortality after hospital discharge [10]. Nevertheless, long-term outcomes after SE still represent an unsettled issue [11], especially as concerns the risk of remote unprovoked seizures (RS) and SE recurrence [12-14]. From this point of view, data from animal models of SE showed that excessively prolonged critical activity over time may be associated with the formation of epileptogenic networks [15, 16]. However, to date, evidence from clinical practice is limited.
In 1998, a population-based study from Richmond (Virginia, USA) suggested that patients with SE had a significantly greater risk for subsequent unprovoked seizures compared to acute symptomatic seizures, especially in the case of structural etiologies [17]. Thereafter, only a few studies have investigated the risk of subsequent unprovoked seizures and SE recurrence after an incident event in adults [11, 13, 18, 19]. Particularly, progressive symptomatic etiologies [11, 19], female gender [11, 13], and delayed treatment have been proposed as potential risk factors for seizure recurrence [19].
In this study, we evaluated the risk of subsequent RS and of drug-resistant epilepsy (DRE) development after a first incident SE in a cohort of adult patients admitted to a third-level Academic Hospital.
METHODS
Study design and participants
This retrospective, observational, monocentric study enrolled all consecutive adult patients (age ≥ 14 years) prospectively registered at the Ospedale Civile Baggiovara, the hub for neurological emergencies of the Modena district (Italy), for an incident SE from 1 September 2013 to 1 March 2022.
Patients with a SE or seizure cluster [20] prior to the study period as well as patients with a previous history of seizures were excluded. We also excluded patients with a SE due to anoxic brain injury (because they represent a specific etiology with poor outcome), as well as those patients who presented a SE as the onset manifestation of genetic generalized epilepsy (GGE; e.g, absence SE).
Finally, since the aim of the study focused on long-term outcomes after SE, we limited our analysis to 30-day survivors and to the patients residing in the Modena city district. Figure 1 outlines the study flowchart and the final study population.
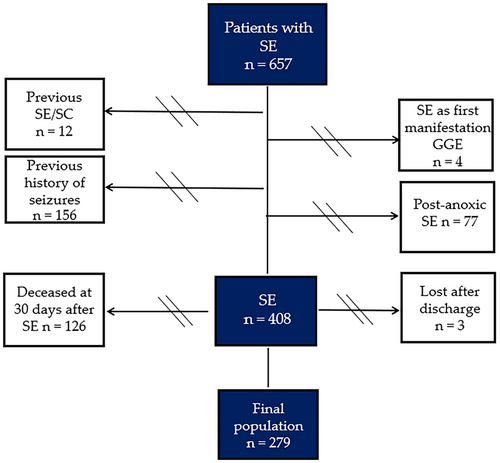
Before 2015, we adopted an operational definition of SE that was defined as a continuous seizure that lasts ≥5 min or two or more discrete seizures between which there was not a complete recovery of consciousness [21]. After 2015, the last International League Against Epilepsy (ILAE) definition of SE was adopted and prospectively applied [22]. In the case of SE without prominent motor semiology, the diagnosis of non-convulsive status epilepticus (NCSE) was reviewed according to Salzburg electroencephalographic (EEG) criteria [23].
According to response to treatment, SE was classified as responsive, refractory, or super-refractory. Treatment responsiveness was defined as SE cessation after first-line therapy with benzodiazepines alone or followed by second-line treatment with ASMs. Refractory SE was considered as a failure of first-line therapy with benzodiazepines and one second-line treatment with ASMs. Super-refractory SE (SRSE) was a status that continued or recurred despite the use of anesthetics for longer than 24 h.
Procedures and data collection
As reported in previous studies by our group [14, 24-26], a specific Status Epilepticus Form was used to collect, for each case, the following information: age, sex, place of residence, site and date of SE observation, date of SE onset, comorbidities, level of disability before SE (using the modified Rankin Scale), level of consciousness at first medical evaluation (using the Glasgow Coma Scale), etiology, semiology of SE before treatment, and type and results of neuroradiologic studies. Type, duration, and dosage of ASM, anesthetic drugs, and other therapies used were recorded as well.
The main outcome measure of the study was the development of RS according to the ILAE definition [27]. A secondary outcome measure was the development of DRE [28]. To identify DRE, the following factors were taken into account: (i) ASM regimen at hospital discharge; (ii) the occurrence of RS as well as breakthrough seizures that occurred in temporal proximity to potentially seizure-provoking external factors (e.g., sleep deprivation, intercurrent febrile illness); these were considered as evidence of inadequate seizure control (treatment failure); and (iii) the occurrence of seizures due to poor treatment compliance or medical-driven treatment reduction; these were not considered as treatment failures. For patients discharged home with one ASM and who experienced RS during the follow-up, the occurrence of further seizures within 12 months after the add-on of a second ASM was considered as evidence of treatment failure and DRE development (according to the ILAE definition) [28]. Conversely, for patients discharged home with two or more ASMs, the ILAE definition of DRE cannot be applied. For these patients, we considered the occurrence of RS during the follow-up as evidence of treatment(s) failure and, consequently, of DRE development, regardless of the time elapsed from the index event. Follow-up data were acquired by the computerized hospital chart review, outpatient visits, and telephones calls. Follow-up data were updated to 1 March 2023.
Predictors of seizure recurrence
We examined age, gender, seizure type, SE cause and etiology, SE duration, Epidemiology-Based Mortality Score in Status Epilepticus (EMSE) and Status Epilepticus Severity Score (STESS) values, treatment response, and the development of neurological deficits after the index event as risk factors for seizure recurrence. See the Supplementary Material for a detailed description of the considered variables.
Statistical analysis
Descriptive statistics was used for the evaluation of demographic and clinical data. Comparisons of clinical variables at the index event between patients who experienced RS and the ones who achieved seizure freedom during the follow-up were performed using Fisher and chi-squared tests with Yates correction for categorical variables, whereas continuous variables were analyzed using the independent samples t-test or the Mann–Whitney U-test, as appropriate. Kaplan–Meier survival analyses were used to calculate the probability of seizure freedom for all patients included in the study. This analysis was repeated by SE cause and etiological classification, semiology, duration, treatment response, and STESS and EMSE scores. The log-rank statistic was used to compare the risk of subsequent seizures for patients with and without the predictor. To assess independent predictors of seizure recurrence, we implemented baseline characteristics associated with p < 0.10 in the univariate analysis in a multivariate Cox regression model. Finally, a competing-risk regression model was performed as sensitivity analysis to assess the impact of mortality as a competing event with the occurrence of RS during the follow-up. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS for Windows, version 21 (SPSS, Chicago, IL, USA) and Stata/IC 13.1 (StataCorp, College Station, TX, USA).
Standard protocol approvals and data availability
The scientific advisory boards of our institution approved the research protocol according to local regulations, and the local ethics committee approved the retrospective analysis of patients' data. This study followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statements [29].
The authors state that the anonymized data on which the article is based will be shared on the request of any qualified investigator.
RESULTS
According to inclusion and exclusion criteria, 279 patients (mean age = 69.9 years, 63% female) with a first incident SE were included in the study. Clinical and demographic features of the cohort are reported in Table 1, as well as the comparison between patients with and without RS during the study period. As concerns specific SE etiologies, the three leading causes of SE were cerebrovascular diseases (102/279, 37%), central nervous system (CNS) tumors (44/279, 16%), and septic–metabolic disorders (24/279, 9%).
| Variable | Total, N = 279 | RS−, n = 222, 79.6% | RS+, n = 57, 20.4% | % RS in each category | p |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 69.9 ± 14.9 | 70.9 ± 13.9 | 65.5 ± 17.6 | N/A | 0.013 |
| Gender, female, n (%) | 176 (63) | 137 (62) | 39 (71) | 22.2% | 0.434 |
| SE etiological classification, n (%) | |||||
| Acute symptomatic | 135 (48) | 118 (53) | 17 (30) | 12.5% | 0.005 |
| Remote symptomatic | 67 (24) | 51 (23) | 16 (28) | 23.8% | |
| Progressive symptomatic | 63 (23) | 45 (20) | 18 (32) | 28.6% | |
| Cryptogenic/unknown etiology | 14 (5) | 8 (4) | 6 (10) | 42.8% | |
| SE causes, n (%) | |||||
| Cerebrovascular diseases | 102 (37) | 82 (37) | 20 (35) | 19.6% | 0.341 |
| CNS tumors | 44 (16) | 33 (14) | 11 (19) | 25% | |
| Sepsis | 11 (4) | 11 (5) | 0 (0) | 0% | |
| Traumatic brain injury | 11 (4) | 8 (4) | 3 (5) | 27.2% | |
| Metabolic disorders | 13 (5) | 12 (6) | 1 (2) | 7.6% | |
| Toxic | 17 (6) | 15 (7) | 2 (4) | 11.7% | |
| Inflammatory disorders | 10 (4) | 7 (3) | 3 (5) | 30.0% | |
| CNS infections | 19 (7) | 15 (7) | 4 (7) | 21.1% | |
| Multifactorial | 29 (10) | 23 (10) | 6 (10) | 20.6% | |
| Unknown | 14 (5) | 8 (4) | 6 (10) | 42.8% | |
| Others | 9 (2) | 8 (4) | 1 (2) | 11.1% | |
| SE semiology, n (%) | |||||
| Prominent motor phenomena | 83 (30) | 70 (32) | 13 (23) | 15.6% | 0.283 |
| Convulsive SE | 27 (10) | 19 (9) | 8 (14) | 29.6% | |
| Focal motor SE | 52 (19) | 48 (22) | 4 (7) | 7.7% | |
| Myoclonic SE | 4 (1) | 3 (1) | 1 (2) | 25.0% | |
| Prominent motor phenomena with evolution into NCSE | 52 (19) | 38 (17) | 14 (24) | 26.9% | |
| NCSE | 144 (51) | 114 (51) | 30 (53) | 20.8% | |
| Treatment response, n (%) | |||||
| Responsive SE | 150 (54) | 118 (53) | 32 (56) | 21.3% | 0.804 |
| Refractory SE | 103 (37) | 84 (38) | 19 (33) | 18.4% | |
| Superrefractory SE | 26 (9) | 20 (9) | 6 (11) | 23.1% | |
| SE duration, days, median [IQR] | 1 [2.75] | 1 [2] | 1.5 [5.75] | N/A | 0.162 |
| Prognostic scores, median [IQR] | |||||
| STESS | 3 [1] | 3 [1] | 3 [2] | N/A | 0.193 |
| EMSE | 43 [44] | 44 [44] | 35 [31] | N/A | 0.040 |
| ASM at hospital discharge, n (%) | 270 (96) | 213 (96) | 57 (100) | 21.1% | 0.211 |
| Functional outcome, median [IQR] | |||||
| mRS at hospital admission | 1 [3] | 1 [3] | 1 [3] | N/A | 0.783 |
| mRS at hospital discharge | 3 [4] | 4 [4] | 3 [3] | N/A | 0.141 |
| mRS at 30 days after SE | 3 [4] | 3 [4] | 3 [3] | N/A | 0.173 |
- Abbreviations: ASM, antiseizure medication; CNS, central nervous system; EMSE, Epidemiology-Based Mortality Score in Status Epilepticus; IQR, interquartile rang; mRS, modified Rankin Scale; N/A, not applicable; NCSE, non-convulsive SE; RS, remote unprovoked seizures; SE, status epilepticus; STESS, Status Epilepticus Severity Score.
We assessed seizure recurrence through a survival analysis where patients lost to follow-up were censored at their last medical contact and deceased subjects at their date of death (mean follow-up time = 32.4 months).
Remote seizure occurrence
Overall, 57 patients (20.4%) experienced RS during the study period. Of note, 24 of 57 (42%) patients presented an episode of SE or a seizure cluster as first relapse after the index event.
The overall cumulative probability of remote seizure development was 15%, 22%, 28%, and 32% at 12 months, 24 months, 36 months, and 5 years after SE, respectively. It is noteworthy that no patient experienced a first RS >5 years after the index event. In most cases (45/57 patients, 81%), RS occurred within the first 2 years of follow-up. The cumulative probability of seizure freedom and the likelihood of presenting a first post-SE unprovoked seizure among patients who experienced RS through follow-up are showed in Figure 2.
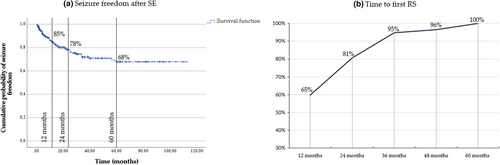
Regarding ASMs at discharge (Table 1), 270 of 279 patients (97%) were discharged home with ASMs: 204 (75%) with one ASM, 53 (20%) with two, and 13 (5%) with three or more. Nine patients were discharged home without any ASMs. All of them had an acute symptomatic SE, and none developed RS during the follow-up.
No patients with remote (n = 67) or progressive symptomatic SE (n = 64) suspended the ASMs by medical indication (only two patients withdrew ASMs on their own and experienced RS). In our cohort, 91 of 135 patients with acute symptomatic SE had a follow-up of 12 months or longer. Excluding those patients who experienced RS in the first year of follow-up (n = 9), 82 patients were seizure-free at 12 months from the index event. Complete discontinuation of ASMs was achieved in 30 of 82 patients, and all but one remained seizure-free at the last medical contact (mean follow-up = 47.2 months). On the other hand, 52 patients were still taking ASMs at the time of the last medical contact; RS occurred in four patients (mean follow-up = 48.3 months).
Factors associated with remote seizures
No statistically significant differences were found in demographic variables, with the exception of a lower mean age at SE onset in the RS group (Table 1).
As concerns SE etiological classification, the risk of RS development was significantly lower in the case of acute symptomatic etiologies when compared to remote and, particularly, progressive disorders (log-rank p = 0.006; Figure 3a).
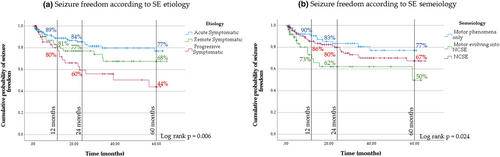
As far as seizure type is concerned, a significantly higher probability of seizure freedom was observed in SE with prominent motor manifestations compared to SE episodes with evolution into NCSE (log-rank p = 0.024; Figure 3b).
Finally, considering SE etiology according to a binary model, structural causes/lesions (187 cases, 64%) versus nonlesional causes (toxic–metabolic, withdrawal of benzodiazepines, or inflammatory etiologies; 92 cases, 36%), the cumulative probability of remote seizures was found to be higher in the case of SE due to structural brain damage (log-rank p = 0.044; Figure 4).
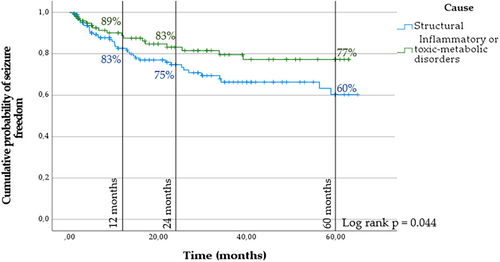
The occurrence of RS was not influenced by age (log-rank p = 0.601), gender (log-rank p = 0.288), treatment response (log-rank p = 0.689), SE duration (log-rank p = 0.279), STESS (log-rank p = 0.618), or EMSE (log-rank p = 0.913).
Table 2 reports the results of univariate and multivariate Cox regression analysis of the factors associated with the risk of seizure recurrence after a first incident SE. After examining the univariate effect of predictors in separate Cox models adjusted for demographic variables (age and gender), we created a final model based upon the variables with p-value ≤ 0.10 in the univariate analysis. SE structural etiology (p = 0.026), etiological classification (p = 0.011), and seizure semiology (p = 0.030) were included in the final model. Patients who experienced an SE incident due to structural brain damage had a twofold increased long-term risk of seizure recurrence (95% confidence interval [CI] = 1.06–4.01, p = 0.032), which increased up to 2.7-fold in the case of progressive disorders (95% CI = 1.44–5.16, p = 0.009) and to 2.9-fold for motor cases with evolution into NCSE (95% CI = 1.37–6.37, p = 0.021).
| Factor | Crude | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| SE cause | ||||||
| Inflammatory or toxic–metabolic | 1.0 | Referent | 0.026 | 1.0 | Referent | 0.032 |
| Structural | 1.9 | 1.08–3.64 | 2.1 | 1.06–4.01 | ||
| SE etiology | ||||||
| Acute symptomatic | 1.0 | Referent | 0.011 | 1.0 | Referent | 0.009 |
| Remote symptomatic | 1.8 | 0.92–3.52 | 1.5 | 0.71–2.99 | ||
| Progressive symptomatic | 2.6 | 1.38–4.82 | 2.7 | 1.44–5.16 | ||
| SE semiology | ||||||
| Prominent motor phenomena | 1.0 | Referent | 0.030 | 1.0 | Referent | 0.021 |
| NCSE | 1.4 | 0.73–2.78 | 1.6 | 0.81–3.12 | ||
| Prominent motor phenomena ➔ NCSE | 2.8 | 1.27–5.82 | 2.9 | 1.37–6.37 | ||
| SE duration | ||||||
| <24 h | 1.0 | Referent | 0.283 | Not included | ||
| 24–72 h | 0.63 | 0.36–1.11 | ||||
| >72 h | 0.78 | 0–34 to 1.83 | ||||
| Treatment response | ||||||
| Responsive | 1.0 | Referent | 0.59 | Not included | ||
| RSE | 0.98 | 0.56–1.72 | ||||
| SRSE | 1.6 | 0.63–3.78 | ||||
| STESS score | ||||||
| <3 | 1.0 | Referent | 0.63 | Not included | ||
| ≥3 | 1.2 | 0.54–2.78 | ||||
| EMSE score | ||||||
| <3 | 1.0 | Referent | 0.74 | Not included | ||
| ≥3 | 1.1 | 0.58–2.1 | ||||
| New neurological deficits (at hospital discharge) | ||||||
| No | 1.0 | Referent | 0.91 | Not included | ||
| Yes | 1.02 | 0.61–1.74 | ||||
- Abbreviations: CI, confidence interval; EMSE, Epidemiology-Based Mortality Score in Status Epilepticus; HR, hazard ratio; NCSE, non-convulsive SE; RSE, refractory SE; SE, status epilepticus; SRSE, super-refractory SE; STESS, Status Epilepticus Severity Score.
- a Adjusted for age, gender, and variables with p ≤ 0.10 at univariate Cox regression analysis.
Finally, a competing-risk regression model was performed as sensitivity analysis to assess the impact of mortality as a competing event with the occurrence of RS during the follow-up. Cumulative incidence of RS ranged from 10% to 20% at 12 months and at 5 years from the index event, respectively. Furthermore, patients who experienced an SE episode with prominent motor manifestations and evolution into NCSE had a 2.1-fold increased long-term risk of RS (95% CI = 0.98–4.54, p = 0.058), which rose to 2.13 in the case of progressive symptomatic CNS disorders (95% CI = 1.15–3.95, p = 0.016). Details are reported in the Supplementary Material (Table S1, Figures S1 and S2).
Development of DRE
According to the proposed criteria, 18 of 57 patients with RS developed DRE (32%).
Specifically, 10 of 18 patients in the DRE subgroup were discharged home with one ASM and experienced further seizures despite the add-on of one or multiple ASMs. On the other hand, eight patients were taking two or more ASMs in combination at the time of first relapse (in all patients, RS occurred within 12 months from the index event).
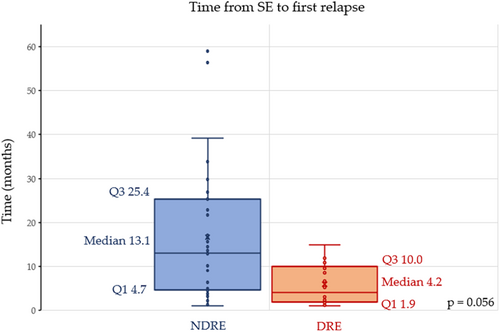
No significant differences were observed between responders and DRE patients according to SE etiology and SE duration. Conversely, patients with DRE more frequently experienced SE with prominent motor phenomena with evolution into NCSE (44% vs. 25%, p = 0.008) and SRSE (27% vs. 3%; p = 0.006). The time between the index SE event and first seizure relapse tended to be shorter in DRE compared to responders (4.2 vs. 13.1 months, p = 0.056). Details of the comparison between DRE and responders are reported in Table 3 and Figure 5.
| Variable | DRE, n = 18 | Responders, n = 39 | p |
|---|---|---|---|
| Age, years, mean ± SD | 60.5 ± 19.2 | 67.7 ± 16.6 | 0.185 |
| Gender, female n (%) | 11 (61) | 27 (69) | 0.762 |
| SE cause, n (%) | |||
| Structural | 13 (72) | 29 (74) | 0.878 |
| Inflammatory or toxic–metabolic | 5 (28) | 10 (26) | |
| SE etiology, n (%) | |||
| Acute symptomatic | 5 (28) | 17 (44) | 0.522 |
| Remote symptomatic | 6 (33) | 10 (25) | |
| Progressive symptomatic | 7 (39) | 12 (31) | |
| SE semiology, n (%) | |||
| Prominent motor phenomena | 6 (33) | 7 (18) | 0.006 |
| NCSE | 4 (23) | 26 (67) | |
| Prominent motor phenomena ➔ NCSE | 8 (44) | 6 (25) | |
| Treatment response, n (%) | |||
| Responsive SE | 10 (56) | 22 (56) | 0.008 |
| Refractory SE | 3 (17) | 16 (41) | |
| Superrefractory SE | 5 (27) | 1 (3) | |
| SE duration, days, median [IQR] | 1.5 [5.75] | 1 [2] | 0.162 |
| SE as first relapse, n (%) | 9 (50) | 13 (33) | 0.363 |
| Recurrent SE, n (%) | 11 (61) | 15 (38) | 0.190 |
| Time from SE to first relapse, months, median [IQR] | 4.2 [8.1] | 13.1 [20.7] | 0.056 |
- Abbreviations: DRE, drug-resistant epilepsy; IQR, interquartile range; NCSE, non-convulsive SE; SE, status epilepticus.
DISCUSSION
In this study, we evaluated the risk of remote unprovoked seizures and DRE development in a prospectively collected cohort of adult first-ever SE survivors. Overall, 57 of 279 patients (20.4%) developed RS during the study period. The cumulative probability of seizure freedom decreased respectively from 85% to 68% at 1 year and 5 years after SE. In the majority of cases (81%), relapses occurred within 2 years after the index event, and no patients experienced a first remote unprovoked seizure >5 years after SE. Our results suggest that the cumulative risk of seizure recurrence after SE is moderate and specifically enclosed within a limited period from the index event. Of note, a similar temporal trend has already been observed in previous studies [11, 17].
In a recent monocentric, retrospective, and observational study on nonpediatric SE, Rodrigo-Gisbert et al. [19] reported that up to 30% of patients can develop RS, with an estimated seizure recurrence rate of 43.7% in 5 years that is higher than the estimated 5-year recurrence rate of 32% observed in our cohort. Discrepancies may be related to differences in the study cohorts, especially regarding etiologies, and to the policy regarding ASMs at discharge and during follow-up. Moreover, in contrast to the Rodrigo-Gisbert population, in our study we included patients at advanced age who may be less likely to develop RS compared to younger patients, for example, due to a reduced life expectancy.
Considering factors associated with seizure recurrence, we observed that the risk of RS development varied according to SE etiology. In particular, we found that remote and progressive symptomatic etiologies were associated with a 1.6-fold and a 2.7-fold increased risk of RS compared to acute symptomatic causes, respectively. These results, which were confirmed by the competing-risk regression model, can be superimposed on those previously reported and corroborate the pivotal role of etiology in predicting the risk of RS after SE [11, 13, 14, 17, 19]. As concerns acute symptomatic etiologies, predicting the risk of post-SE epilepsy development would be of fundamental value to guide clinical practice and eventually withdrawal of ASMs. From this point of view, several factors should be taken into account, such as SE cause, EEG findings, treatment tolerability, patients' comorbidities and compliance. In our cohort, 82 of 135 patients with acute symptomatic SE had a follow-up of 12 months or longer and were seizure-free at 1 year after the index event. Among these patients, complete discontinuation of ASMs was achieved in 30 cases and all but one remained seizure-free at last medical contact (mean follow-up = 47.2 months).
Our results show that the overall risk of seizure recurrence is relatively low, especially in nonlesional etiologies (see Table 1). Future prospective studies are needed to define the risk of post-SE epilepsy development in patients with acute etiologies, eventually considering a more granular classification of SE etiologies as recently proposed by our group [30], as well as other potentially useful tools, such as fluid biomarkers of neurodegeneration/neuroinflammation [31, 32] and structural neuroimaging [33-35].
With reference to SE semiology, cumulative probability of seizure freedom at 5-year follow-up was found to be higher (77%) in the case of SE with prominent motor phenomena compared to NCSE (67%) and, especially, to those cases with initial motor manifestations and subsequent evolution into NCSE (50%). In a previous retrospective, population-based study in Salzburg, Leitinger et al. [2] found that the occurrence of nonconvulsive phases in the semiological sequence of SE was associated with a higher case fatality rate than pure motor episodes (27.6% vs. 3.5%). SE is a dynamic condition characterized by biomolecular processes occurring in neurons [36] and systemic homeostatic mechanisms to compensate for the extreme metabolic demands of the seizing brain [37]. As ictal activity persists over time, convulsive SE episodes may evolve into non-convulsive ones (NCSE), and compensatory mechanisms fail accordingly. Thus, the occurrence of non-convulsive phases in the semiology sequence of SE might be considered as a marker of disease severity and brain damage [2, 26]. The result of the present study highlights the value of SE semiology, expanding its role as a risk factor for RS in the long term after SE.
In our population, we did not find a significant association between SE treatment refractoriness (or SE duration) and the development of remote unprovoked seizures. Data from animal models of SE documented the impact of SE severity and duration on epileptogenesis and epilepsy in the long term [38]. Consequently, it is reasonable to assume that the more severe (and prolonged) the seizure activity, the higher the probability of developing neural epileptogenic networks. However, evidence from clinical practice is controversial, as previous studies reached opposite or inconclusive results [11, 13, 19, 39]. It is still unclear whether treatment refractoriness may influence the risk of RS development or whether this depends on additional factors, mainly the underlying etiology. Of note, under the umbrella of cases that fulfill the actual definition of “refractory status epilepticus” there are conditions with different degrees of refractoriness, and this issue must be investigated in future studies [40]. A similar consideration can be drawn with regard to the relationship between SE duration and post-SE epilepsy development.
Post-SE DRE
As many as 36% of patients in clinic-based cohorts are estimated to developed DRE [41]. DRE can expose patients to increased risks of premature death, injuries, psychosocial dysfunction, and a reduced quality of life [42]. Prevention of (drug-resistant) epilepsy is an unmet medical need, and recent research activity has been focused on the progression of epilepsy after it is established [43]. Thus, identifying patients at higher risk of developing DRE would be of high value for the study of antiepileptogenic treatment and eventually for the design of randomized clinical trials.
In our population, 18 of 57 patients (32%) fulfilled the adopted definition of DRE [28], whereas 39 patients managed to maintain sustained seizure freedom during follow-up.
Comparing DRE and responders, we did not find significant differences in terms of age, gender, and SE etiology between the two groups. Conversely, patients with DRE more frequently experienced SRSE as well as prominent motor episodes with evolution into NCSE at the index event. SRSE being the most advanced stage of SE, it is reasonable to assume that the processes leading to extreme refractoriness to treatment could promote subsequent network reorganization and epileptogenicity. Of note, in our study, SRSE was not significantly associated with an overall increased risk of RS, but when RS occurred, the risk of develop DRE was higher. From this point of view, it is worth noting that mortality is high in the case of SRSE and survivors are left with severe disabilities [44]. Therefore, patients with SRSE may have died prior to experience unprovoked seizures, but once it occurred, post-SE epilepsy was drug-resistance in the majority of cases (5/6 patients).
Finally, median time from SE to first relapse was reduced in the case of DRE compared to responders (4.2 vs. 13.1 months). A similar time trend has been observed in the case of post-stroke epilepsy, where a shorter latency from stroke to first unprovoked seizure was associated with an increased risk of DRE development [45].
Study limitations
This is an observational monocentric study, in which clinical prospectively collected data were reviewed retrospectively; therefore, the results cannot allow definite conclusions regarding risk factors and long-term outcome following a first-ever SE. Second, in our study we were not able to assess the role of other clinical variables, such as time to treatment initiation, in the development of RS. From this point of view, however, it has to be noted that more than half of our patients presented a NCSE, which presents a diagnostic challenge, because SE onset is not always clearly datable in these patients [46, 47]. Unfortunately, not of all patients in our population underwent magnetic resonance imaging (MRI) studies for the detection of peri-ictal MRI abnormalities [48], which could represent a potentially useful tool for the prediction of RS and DRE development [33, 34] and should be considered in future prospective studies in this field.
Conclusions
In this retrospective cohort of first-ever SE survivors, the overall risk of seizure recurrence was moderate and enclosed within the first 2 years after SE in 81% of relapses. Progressive symptomatic etiologies, structural brain damage, and the occurrence of nonconvulsive phases in the semiology sequence of SE were factors associated with an increased risk of seizure recurrence. Notably, late epilepsy may be more refractory to treatment in patients with SRSE. In addition, a shorter latent period characterized DRE development. Our results highlight the importance of etiology and seizure semiology to drive the risk of remote seizures after SE and contribute to expanding the knowledge on the development of DRE after a first SE episode.
AUTHOR CONTRIBUTIONS
Niccolò Orlandi: Conceptualization (lead); data curation (lead); formal analysis (lead); writing–original draft (equal); writing–review and editing (equal); visualization (equal). Giada Giovannini: Data curation (equal); writing–review and editing (equal); conceptualization (equal). Maria Cristina Cioclu: Writing–review and editing (equal). Niccolò Biagioli: Writing–review and editing (equal). Laura Madrassi: Writing–review and editing (equal). Anna Elisabetta Vaudano: Writing–review and editing (equal). Matteo Pugnaghi: Writing–review and editing (equal). Simona Lattanzi: Formal analysis (equal); writing–review and editing (equal). Stefano Meletti: Supervision (lead); conceptualization (equal); visualization (equal); writing–original draft (equal); writing–review and editing (equal).
CONFLICT OF INTEREST STATEMENT
S.M. has received research grant support from the Ministry of Health, and has received personal compensation as a scientific advisory board member for UCB, Jazz Pharmaceuticals, and Eisai. N.O. has received speaker's or consultancy fees from Eisai. S.L. has received speaker's or consultancy fees from Angelini Pharma, Eisai, GW Pharmaceuticals, Medscape, and UCB Pharma, and has served on advisory boards for Angelini Pharma, Arvelle Therapeutics, BIAL, Eisai, GW Pharmaceuticals, and Rapport Therapeutics outside the submitted work. None of the other authors has any conflict of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The authors state that the anonymized data on which the article is based will be shared by request of any qualified investigator.




