European Academy of Neurology/Peripheral Nerve Society Guideline on diagnosis and treatment of Guillain–Barré syndrome
Abstract
Guillain–Barré syndrome (GBS) is an acute polyradiculoneuropathy. Symptoms may vary greatly in presentation and severity. Besides weakness and sensory disturbances, patients may have cranial nerve involvement, respiratory insufficiency, autonomic dysfunction and pain. To develop an evidence-based guideline for the diagnosis and treatment of GBS, using Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology a Task Force (TF) of the European Academy of Neurology (EAN) and the Peripheral Nerve Society (PNS) constructed 14 Population/Intervention/Comparison/Outcome questions (PICOs) covering diagnosis, treatment and prognosis of GBS, which guided the literature search. Data were extracted and summarised in GRADE Summaries of Findings (for treatment PICOs) or Evidence Tables (for diagnostic and prognostic PICOs). Statements were prepared according to GRADE Evidence-to-Decision (EtD) frameworks. For the six intervention PICOs, evidence-based recommendations are made. For other PICOs, good practice points (GPPs) are formulated. For diagnosis, the principal GPPs are: GBS is more likely if there is a history of recent diarrhoea or respiratory infection; CSF examination is valuable, particularly when the diagnosis is less certain; electrodiagnostic testing is advised to support the diagnosis; testing for anti-ganglioside antibodies is of limited clinical value in most patients with typical motor-sensory GBS, but anti-GQ1b antibody testing should be considered when Miller Fisher syndrome (MFS) is suspected; nodal–paranodal antibodies should be tested when autoimmune nodopathy is suspected; MRI or ultrasound imaging should be considered in atypical cases; and changing the diagnosis to acute-onset chronic inflammatory demyelinating polyradiculoneuropathy (A-CIDP) should be considered if progression continues after 8 weeks from onset, which occurs in around 5% of patients initially diagnosed with GBS. For treatment, the TF recommends intravenous immunoglobulin (IVIg) 0.4 g/kg for 5 days, in patients within 2 weeks (GPP also within 2–4 weeks) after onset of weakness if unable to walk unaided, or a course of plasma exchange (PE) 12–15 L in four to five exchanges over 1–2 weeks, in patients within 4 weeks after onset of weakness if unable to walk unaided. The TF recommends against a second IVIg course in GBS patients with a poor prognosis; recommends against using oral corticosteroids, and weakly recommends against using IV corticosteroids; does not recommend PE followed immediately by IVIg; weakly recommends gabapentinoids, tricyclic antidepressants or carbamazepine for treatment of pain; does not recommend a specific treatment for fatigue. To estimate the prognosis of individual patients, the TF advises using the modified Erasmus GBS outcome score (mEGOS) to assess outcome, and the modified Erasmus GBS Respiratory Insufficiency Score (mEGRIS) to assess the risk of requiring artificial ventilation. Based on the PICOs, available literature and additional discussions, we provide flow charts to assist making clinical decisions on diagnosis, treatment and the need for intensive care unit admission.
OBJECTIVES AND SCOPE
The aim was to develop an evidence-based international guideline on the diagnosis and treatment of Guillain–Barré syndrome (GBS) according to the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology1 and to formulate evidence-based recommendations and consensus-based good practice points (GPPs) for clinical practice. The target population for the diagnostic part of the Guideline are patients of any age (including children), presenting with clinical features suggestive of GBS. The treatment recommendations apply to patients diagnosed with GBS. The Guideline is intended for neurologists, paediatric neurologists and other physicians in secondary and tertiary care settings. The aim is to optimise diagnostic accuracy and to improve patient outcomes.
BACKGROUND
Epidemiology of GBS
GBS is the most common cause of acute flaccid paralysis with an annual global incidence of approximately 1–2 per 100 000 person-years.2, 3 Worldwide, GBS affects about 100 000 people per year.2 GBS can affect people at any age, but the incidence increases with age and reaches its peak between 50 and 70 years.4 Males are about 1.5 times more likely to be affected than females.2-4 Only a few outbreaks and reports on seasonal variations have been reported on associations of GBS with infections.5 The diagnosis of GBS relies upon a combination of clinical features, often with support of electrodiagnostic and laboratory features. Most diagnostic criteria for GBS require a combination of history, neurological examination, cerebrospinal fluid (CSF) and electrodiagnostic results. Commonly used diagnostic criteria for GBS are the revised NINDS (1990)6 and the Brighton criteria (2011).7 The current Guideline on GBS is based upon the results of extensive literature searches on the diagnosis, treatment and prognosis of GBS. The evidence from randomised clinical therapeutic trials allows evidence-based recommendations about treatments according to GRADE.
METHODS
The methodology for the development of this Guideline followed the frameworks provided by AGREE II8 and GRADE,1 and the recommendations of the EAN on the development of a neurological management guideline.9 During the initial meeting in March 2018, the Task Force (TF) formulated a list of relevant questions potentially to be addressed in this GBS Guideline. Based on priorities and practical limitations (mainly relevance and likelihood to find relevant literature), we finally selected 14 questions that were constructed in the Population/Intervention/ Comparison/Outcome question (PICO) format (Box 1). The following databases were searched for identification of eligible studies for each PICO question, according to predefined selection criteria: Medline, via the PubMed interface; Embase, via the embase.com interface; the Cochrane Library, consisting of the Cochrane Database of Systematic Reviews; the Database of Abstracts of Reviews (DARE); and the Cochrane Central Register of Controlled Clinical Trials (CENTRAL). The literature search for each PICO was conducted between April 2018 and November 2019 without restrictions regarding publication date. The Task Force (TF) additionally included relevant papers published during the preparation of this Guideline. Unpublished data were not used.
BOX 1. POPULATION/INTERVENTION/COMPARISON/OUTCOME QUESTIONS (PICOs)
Diagnostic PICOs (systematic literature search and consensus)
PICO 1. Antecedent events: In patients with clinically suspected GBS, does enquiring about antecedent events* compared with not enquiring about antecedent events influence the diagnostic test accuracy, treatment response and patient outcome? *Includes: infection, vaccination, surgery, immune drug exposure within 6 weeks before onset of GBS, pregnancy or transplant within 6 months before onset of GBS or use of immunomodulatory drugs (especially monoclonal antibodies) within 1 year before onset of GBS.
PICO 2. Cerebrospinal fluid (CSF) examination: In patients with clinically suspected GBS, does examination of the CSF* compared with no CSF examination influence the diagnostic test accuracy, treatment response and patient outcome? *Includes: leucocyte count, increased total protein level, presence of unmatched oligoclonal bands and presence of other biomarkers such as neurofilament.
PICO 3. Antibody testing: In patients with clinically suspected GBS, does testing for serum antibodies* compared with not testing for these antibodies influence the diagnostic test accuracy, particularly for GBS subtypes such as AMAN and Miller Fisher syndrome, and patient outcome? *Includes, but not limited to anti-ganglioside antibodies (GM1, GQ1b), antibodies against Campylobacter jejuni, glycolipids, nodal–paranodal structures.
PICO 4. Electrodiagnosis: In patients with clinically suspected GBS, is a combination of clinical examination and electrophysiology/electrodiagnosis* compared with clinical examination only helpful in the early diagnosis (up to 4 weeks from onset of neurological symptoms) and outcome of GBS? *Includes motor and sensory nerve conduction studies, somatosensory evoked potentials, root stimulation, triple stimulation technique, nerve excitability studies, sural sparing and needle electromyography.
PICO 5. Nerve MRI or ultrasound (US) imaging: In patients with clinically suspected GBS, does using (contrast)-MRI (thickening, enhancement of cervical/lumbar nerve roots or brachial/lumbar plexus) or US (increased cross-sectional area of peripheral nerves or roots) compared with not using MRI or US influence the diagnostic accuracy in the early diagnosis (≤4 weeks from onset of weakness or ≤1 week from hospital admission of GBS?
PICO 6. Prediction of acute-onset CIDP (A-CIDP): In patients initially diagnosed with GBS, does the presence of certain clinical symptoms* or laboratory features** compared with their absence predict the subsequent diagnosis of A-CIDP as confirmed by neurological worsening at >8 weeks from onset? *Includes: progression of weakness, number of treatment-related fluctuations, antecedent events, oculomotor, facial, bulbar or respiratory weakness, autonomic dysfunction. **Includes: EDX abnormalities, CSF pleocytosis, MRI or nerve US abnormalities, anti-ganglioside or nodal–paranodal antibodies.
Treatment PICOs (systematic literature search and GRADE—except consensus for PICO 7)
PICO 7. Monitoring for admission to intensive care unit (ICU): In patients diagnosed with GBS, does the use of formal protocols or criteria* to decide when admission to ICU is necessary for ventilation and other mechanical methods of life support, compared with no pre-planned management strategy to guide ICU admission, influence mortality, respiratory arrest, length of ICU stay and mechanical ventilation, disability or grip strength? * Includes: criteria sets, automatic protocols, mEGRIS score, respiratory monitoring by FVC, assessment of bulbar weakness, cardiac monitoring and monitoring of dysautonomia.
PICO 8. Plasma exchange (PE): In patients diagnosed with GBS, does treatment with PE, influence disability, grip strength, mortality, ICU admission, quality of life, time to ambulation or length of mechanical ventilation at 4 and 26 weeks after onset, compared with not using PE or using a different number/volume of PE sessions, a different replacement volume (albumin vs. plasma) or immunoadsorption.
PICO 9. Intravenous immunoglobulins (IVIg): In patients diagnosed with GBS, does treatment with IVIg, with or without corticosteroids or PE influence disability, grip strength, mortality, ICU admission, quality of life, time to ambulation or length of mechanical ventilation at 4 and 26 weeks after onset compared with not using IVIg?
PICO 10. Corticosteroids: In patients diagnosed with GBS, does treatment with corticosteroids (IV or oral), alone or in combination with IVIg or PE, influence disability, grip strength, mortality, ICU admission, quality of life, time to ambulation or length of mechanical ventilation at 4 and 26 weeks after onset compared with not using corticosteroids?
PICO 11. Other disease-modifying treatments than PE, IVIg or corticosteroids: In patients diagnosed with GBS, does treatment with pharmacological therapies other than PE, IVIg, corticosteroids (or eculizumab, mycophenolate, interferons or other) influence disability, grip strength, mortality, ICU admission, quality of life, time to ambulation or length of mechanical ventilation at 4 and 26 weeks after onset compared with not using these therapies?
PICO 12. Treatment of pain: In patients diagnosed with GBS, does pharmacological treatment (anti-epileptics, antidepressants, opioids or opioid analogues, cannabinoids, acetaminophen, NSAIDs or other analgesia), yoga or meditation relieve pain prevalence or intensity, fatigue or quality of life, compared with no pharmacological intervention, yoga or meditation?
PICO 13. Treatment to reduce fatigue: In patients diagnosed with GBS do pharmacological interventions to treat fatigue (amantadine, modafinil, SSRI, dexamphetamine, other psychoactive amines or drugs), or behavioural and physical management strategies (graded exercise programmes, physiotherapy, other therapies) compared with not using these treatments influence the presence and severity of fatigue, quality of life, return to work, disability or grip strength?
Prognostic PICO (systematic literature search and consensus)
PICO 14. Prognosis: In patients diagnosed with GBS, does the presence of certain clinical (e.g., age, severity of weakness) or non-clinical risk factors (e.g., electrodiagnostics, anti-ganglioside antibodies), GBS disability scores/I-RODS scores, grip strength, ability to walk/run at 6 or 12 months, or fatigue compared with the absence of these factors are related to the prognosis? Factors related to the requirement of mechanical ventilation are included in PICO 7.
The six PICOs on intervention were subjected to GRADE assessment. Data were extracted and summarised in GRADE Summary of Findings Tables (treatment PICOs) or Evidence Tables (diagnostic, intensive care unit [ICU] admission and prognostic PICOs). To reach consensus, TF members prepared draft statements about definition, diagnosis and treatment, according to the elements of the GRADE Evidence-to-Decision (EtD) frameworks.10 The TF made a strong recommendation for or against an intervention when it judged that almost all informed people would make the recommended choice.11 A weak recommendation was made when it judged that most informed people would choose the recommended course of action, but a substantial number would not, either because it was applicable or available only to a subgroup, the evidence had low certainty, or the risk/benefit ratio might not be favourable for all patients. For the six diagnostic PICOs, and those on assessing the need for ICU admission and for prognosis, a formal GRADE approach was not considered useful, because of limited evidence. When appropriate, the TF offered GPPs,12 phrased as ‘advises’ or ‘suggests’ depending on the strength of the GPP. The recommendations and GPPs were collated into a single document, which was then revised iteratively by the TF until consensus was reached. The patient representative from the GBS/CIDP Foundation International reviewed all recommendations and GPPs and participated in consensus votes in the capacity of TF member. A detailed protocol of the guideline development process can be found in the Supplementary Material online. It is planned to update the Guideline every 5 years.
The TF decided to focus on a number of relevant diagnostic and treatment PICO questions, and acknowledges that some practical issues related to treatment and care of patients with GBS are not covered in this Guideline. Almost all studies referred to in this Guideline are based on GBS diagnostic criteria and usually the motor-sensory or motor form. If studies specifically focus on Miller Fisher syndrome (MFS) or other variants of GBS, this is mentioned.
RESULTS
This GBS Guideline is divided into four parts. The first part describes the clinical features and diagnostic criteria. The second part includes additional information and GPPs based upon the six diagnostic PICOs. The third part focuses on treatment (PICOs 7–13). The fourth part focuses on the prognosis and outcome (PICO 14). For all recommendations and GPPs, consensus was reached by all members of the TF.
Classification and clinical diagnostic criteria
Clinical features and diagnostic criteria for GBS
The symptoms and severity of GBS vary greatly. There are several published sets of criteria for the diagnosis of GBS, of which the most frequently used are the NINDS criteria revised by Asbury and Cornblath (1990),6 The Brighton Collaboration Consensus Criteria (2011),7 the Wakerley and Yuki (2015) criteria,13 and the consensus guideline by Leonhard et al. (2019).14 For making a clinical diagnosis at the onset of disease, the TF predominantly used the Leonhard et al. consensus guideline14 and further updated these recommendations after extensive discussions (Table 1).
| Features required |
|
|
|
| Features that support diagnosis |
|
|
|
|
|
|
|
| Laboratory findings that support diagnosis |
|
|
|
| Findings which make GBS less likely |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- Abbreviations: A-CIDP, acute-onset chronic inflammatory demyelinating polyradiculoneuropathy (see: PICO 6); BBE, Bickerstaff brainstem encephalitis; CMAP, compound muscle action potential; CSF, cerebrospinal fluid.
- a Weakness may start in the legs (or other location in regional variants of GBS).
- b Only applies if duration of progression is known (e.g., to separate from CIDP).
- c Hyponatraemia may occur in some patients with GBS.
- Progressive weakness of arms and legs
- Absent or decreased deep tendon reflexes in affected limbs
- Progressive worsening for no more than 4 weeks
The maximal duration of progression originates from large studies showing that progression does not exceed 2 weeks in most patients, and almost never exceeds 4 weeks.4, 14, 15 The maximal duration of progression is often not known when a patient is admitted and is included because it helps to separate GBS from CIDP or other forms of chronic neuropathy. In addition to these main criteria, we list features and laboratory findings that support the diagnosis and findings that make GBS less likely (Table 1). GBS is a spectrum, and most cases have limb weakness and fulfil the requirements for the diagnosis. Some GBS variants and MFS do not fulfil all requirements for GBS since there is not always progressive weakness of arms and legs. In some patients, the deep tendon reflexes initially can be normal or hyperactive.
- Motor-sensory and motor GBS
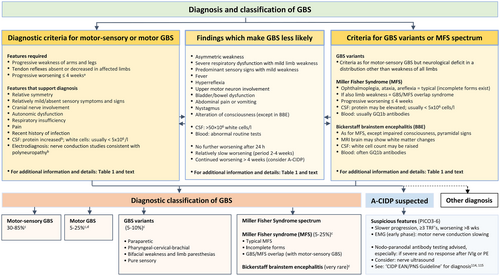
- GBS variants
- MFS spectrum
Patients with typical MFS have ophthalmoplegia, ataxia and areflexia.3, 7, 13 However, a fairly large proportion of MFS patients also have features of motor-sensory GBS (defined as GBS/MFS overlap syndrome) or an incomplete form of MFS (without all three typical clinical features). Bickerstaff brainstem encephalitis (BBE) is considered a rare variant of MFS that clinically has a combination of ophthalmoplegia, ataxia, pyramidal tract signs and impaired consciousness. These patients may have white matter changes on a brain MRI scan.2, 3, 21, 22 Patients with MFS and BBE usually have antibodies against ganglioside GQ1b (PICO 3).
Acute-onset chronic inflammatory demyelinating polyradiculoneuropathy
About 5% of patients initially diagnosed with GBS later turn out to have acute-onset chronic inflammatory demyelinating polyradiculoneuropathy (A-CIDP) and should be treated as for CIDP.23 Clinical and laboratory features possibly related to A-CIDP are discussed (PICO 6). A few of these patients, especially those not responding well to IVIg or PE, may have antibodies against nodal–paranodal antigens (like NF155, Caspr1 or CNTN1), which define a different disease (autoimmune nodopathy) (PICO 3).
Pathogenetic subtypes
Largely based on a combination of clinical, electrodiagnostic and morphological features, GBS has been classified into different pathogenetic subtypes: acute inflammatory demyelinating polyradiculoneuropathy (AIDP), acute motor axonal neuropathy (AMAN) and acute motor and sensory axonal neuropathy (AMSAN).14 Multiple papers on this subject in humans and animal models have been published.24-27
In AMAN cases, the pathophysiology is either (a) reversible conduction failure, due to reversible axonal conduction block at the nodes of Ranvier or the motor nerve terminal without axonal degeneration, followed by rapid recovery,28, 29 or (b) extensive axonal degeneration associated with inexcitable nerves and a worse outcome.30 In AIDP cases, there is conduction slowing and/or conduction block, associated with segmental demyelination with or without secondary axonal degeneration, usually followed by recovery through regeneration of myelinating Schwann cells.27
Preceding infections are relevant, especially Campylobacter jejuni, which can induce cross-reactive antibodies that bind to human gangliosides GM1 or GD1a at the nodes of Ranvier or motor nerve terminal, activate complement and disrupt sodium-channel clusters and axoglial junctions, leading to nerve conduction failure.3, 31 C. jejuni infections are particularly associated with AMAN and/or motor GBS, and worse outcome32 (see: PICO 14). The demyelinating subtype is predominant in all global regions tested, but AMAN and motor GBS are more frequent in Asia (especially Bangladesh) than Europe and America.4, 31
Some important issues remain only partially resolved. (a) The best electrophysiological criteria to distinguish AIDP from AMAN are currently unknown, and all electrophysiological criteria have limitations. This led to the introduction of the concept of ‘nodo-paranodopathy’ to describe situations in which nodal membrane injury of the axon or through detachment of terminal myelin loops (or both) accounts for acute conduction failure, in the absence of segmental demyelination.29 (b) Anti-GM1 antibodies can also be found in some cases classified as AIDP,33 although much less commonly, and both subtypes of GBS can be mediated by complement-fixing anti-GM1 antibodies.34 (c) There is some overlap, as both motor GBS and AMAN may have electrophysiology mimicking demyelination, AIDP often also has axonal degeneration, and there appears to be a spectrum of sensory involvement in AMAN and AMSAN.34
The TF acknowledges the relevance of studying the pathophysiological mechanisms leading to different subtypes of GBS. However, the TF deliberately avoided giving or endorsing specific diagnostic criteria for these entities, because there is currently no gold standard to choose between the various published criteria, and the distinction between these subtypes of GBS currently does not affect clinical management.
Pain and fatigue
Pain often occurs in GBS, at any time in the disease course, even before the onset of weakness, and may be severe.35 Fatigue is an important and frequently occurring residual complaint that may persist when weakness has recovered.36, 37
Outcome measures
For assessment of outcome in GBS, various instruments are available such as the Medical Research Council (MRC) sum score, GBS disability scale (GBS-DS) formerly also known as the Hughes Disability Scale,38 Inflammatory neuropathy cause and treatment (INCAT) disability scale and sensory sum score,39 and the inflammatory Rasch-built overall disability scale (I-RODS).39-41 For assessing the severity of disability in GBS, most randomised controlled trials (RCTs) have used the GBS-DS.38 This seven-point scale ranges from 0 (no symptoms), 1 (minor symptoms and capable to run), 2 (able to walk 10 m without assistance but unable to run), 3 (able to walk 10 m across an open space with help; ‘unable to walk unaided’), 4 unable to walk 10 m even with help (wheelchair bound or bedridden), 5 (requiring assisted ventilation for at least part of the day) to 6 (dead). Most RCTs have included GBS patients with a GBS-DS ranging from 3 to 5, a level of severity that is often considered to be ‘severe GBS’. The GBS-DS primarily focuses on important clinical aspects in ambulation and respiration, but not on arm function, disability in many other daily activities, cranial nerve function, pain, fatigue or quality of life.39
Differential diagnosis
Many disorders may mimic GBS.14 Additional diagnostic tests should be considered according to the differential diagnosis (Table 2).
| Adapted from: Leonhard et al.14 |
| CNS |
|
|
|
|
|
| Anterior horn cells |
|
| Nerve roots |
|
|
| Peripheral nerves |
|
|
|
|
|
|
|
|
| Neuromuscular junction |
|
|
|
|
| Muscles |
|
|
|
|
|
| Conversion or functional disorder |
- This table is not intended to be comprehensive.
- Abbreviations: CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; ICU, intensive care unit; MOG, myelin oligodendrocyte glycoprotein.
Diagnostic PICOs
The diagnosis of GBS can be difficult, especially in the first days, which may delay treatment (Tables 1 and 2). A diagnostic delay in pre-school children seems partly due to non-specific presentations, pain and the rarity of GBS especially in this age group.42
Antecedent events (PICO 1)
Many GBS patients report an infectious illness in the 6 weeks prior to onset of GBS.
Good practice points
- The TF advises enquiry about antecedent events (especially diarrhoea, respiratory infection or fever), which if present may support the diagnosis of GBS, especially if there is clinical uncertainty about the diagnosis.
- Although not assessed in controlled studies, it seems that the risk of developing GBS may be increased shortly after receiving a few specific biological drugs affecting the immune system.
- Following a few specific vaccinations (such as influenza, herpes zoster, SARS-CoV2 adenovirus-vector vaccines), a very small increased risk of GBS has been reported, but the benefits of vaccination far outweigh this risk.3, 43-48
Considerations supporting the GPP (supporting information)
Evidence summary: In patients with suspected GBS, the diagnosis of GBS is more likely if there is a history of recent (within the previous 6 weeks) diarrhoea (sensitivity 13%–18%, specificity 89%–100%),49-53 Campylobacter infection,54 respiratory infection (sensitivity 21%–68%, specificity 59%–98%),49-53 fever (specificity up to 100%)49, 52 or influenza-like illness (specificity up to 100%).48, 53 However, around one third of GBS patients report none of these. Case–control studies showed that GBS is associated with infections (C. jejuni, cytomegalovirus (CMV), Epstein–Barr virus, Hepatitis E virus, Zika virus), and with Mycoplasma pneumoniae in children.14, 54-57 Although large case–control studies are lacking, from the epidemiological and cohort studies that exist, GBS is not or marginally measurable increased in incidence after SARS-CoV-2 infections.48, 58 As this PICO assesses the clinical history prior to the onset of GBS, infections with non-specific symptoms that typically require serological diagnosis fall outside the scope of this Guideline. Specific testing for these is generally not clinically useful, as laboratory results likely come after the diagnosis has been made, but may be warranted in selected cases.59 There is some evidence about surgery related to GBS as retrospective and epidemiological studies reported an association between GBS and recent surgery (sensitivity 39%, specificity 98%), with a stronger association for surgery on bones or digestive organs.60-62
Although not assessed in controlled studies, the risk of GBS may be increased shortly after receiving select biological drugs affecting the immune system,52, 63 especially immune checkpoint inhibitors or anti-tumour necrosis factor alpha agents.5, 64-66
Rationale: Asking about these antecedent events may increase diagnostic confidence and is generally more useful when present than absent.
Vaccinations: Prior vaccinations were not originally part of this PICO. However, the TF considered it helpful to comment, although did not systematically review the evidence. Most vaccinations have no proven association with any increased risk of GBS.3, 45 The risk of GBS after standard seasonal influenza vaccine (trivalent or quadrivalent) is about one excess GBS case per million vaccines, though historically higher after the 1976 monovalent H1N1 ‘swine’ influenza vaccine.46 In a retrospective questionnaire study in 245 patients with previous GBS, subsequent influenza vaccination gave no significant increased risk of recurrence of GBS.67 After a recombinant herpes zoster vaccine, there was possibly a similar very small increased risk of GBS.47 Large studies of SARS-CoV2 vaccines found a small excess risk of 4–7 excess cases of GBS per million after adenovirus-vector vaccines,43, 44, 48 without association with any specific clinical features,44 but no increased risk after pegylated mRNA vaccines.44 When assessing a patient with GBS, a history of recent vaccination is likely of negligible importance towards making the diagnosis of GBS. For all these diseases, the benefits of vaccination (reducing morbidity and mortality from infection and infection-associated GBS) exceed any extremely small increased risk of developing vaccination-induced GBS.
CSF (PICO 2)
CSF examination is often considered helpful to support the diagnosis of GBS, especially in cases of uncertainty.
Good practice points
- The TF advises CSF examination, particularly when the diagnosis is uncertain or when an alternative diagnosis needs to be excluded.
- Results supportive of GBS are an increased CSF protein concentration, and a normal or only slightly increased CSF white blood cell count (usually <5 cells/μL, rarely 5–50 cells/μL and very rarely >50 cells/μL).
- Normal CSF protein is common during the first week of the disease and does not exclude GBS.
- A CSF white blood cell count of >50 cells/μL should raise suspicion for alternative diagnoses (Table 2).
Considerations supporting the GPP (supporting information)
Evidence summary: CSF examination in patients suspected to have GBS classically shows an increased CSF protein and normal CSF white cell (leucocyte) count.4 Diagnostic sensitivity of an increased CSF protein depends on the time CSF is examined after onset of weakness.15 In a study with over 1000 GBS patients, 50% had an elevated CSF protein at fewer than 3 days from onset (median protein level 0.45 g/L), and 84% at more than 7 days from onset (median protein level 0.98 g/L).4 A CSF white cell count <5 cells/μL was found in 80%, a mildly elevated white cell count (5–50/μL) in 19% and more than 50 white cells/μL in 2% of patients.4 The specificity of a raised CSF protein is unknown, and it does not rule out some mimics of GBS (Table 2). Normal values of CSF protein are higher in older people.68, 69 Both CSF protein and cell count may be artefactually raised after IVIg.70
Rationale: Although CSF examination is often performed, because increased protein may support the diagnosis and to exclude mimics with increased cell count, the diagnostic accuracy is unknown.
Antibody testing (PICO 3)
Serum antibodies against gangliosides and other antigens have been found in GBS, particularly in motor GBS and MFS.
Good practice points
- In most patients with motor-sensory GBS, the TF does not advise routine testing for serum antibodies against gangliosides, because of low–moderate diagnostic sensitivity and test assay variability.
- In some suspected motor GBS, GBS variants or some other cases with diagnostic uncertainty, the TF suggests that testing for anti-ganglioside antibodies could be helpful.
- The TF does not advise testing for serum antibodies against gangliosides for the purpose of subtyping into AIDP, AMAN or AMSAN, as this currently has no specific treatment implications.
- In patients suspected to have MFS (or MFS spectrum), the TF advises testing for serum antibodies against GQ1b.
- In patients with poor response to treatment, continuous worsening or relapse after treatment, the TF suggests considering an autoimmune nodopathy (PICO 6). When this is suspected, antibodies against nodal–paranodal antigens should be tested. When testing for nodal–paranodal antibodies, the TF advises using cell-based assays using plasmids encoding human recombinant proteins and validation with a second technique (ELISA or immunohistochemistry).
Considerations supporting the GPPs (supporting information)
Evidence summary: Serum antibodies that have been tested in GBS cases include GM1,71-75 GM1b,76 GD1a,77 GQ1b,78-83 GalNAc-GD1a,84-86 GT1a,87 GD1b, GD3, O-GD3, GT3, O-GT3,88 sulfatide,89, 90 galactocerebroside,91, 92 CNTN1,93 NF155 and NF 186,94, 95 cardiolipin,96 LM1,97-99 sulphated glycolipids,89 P0 and PMP22.100 The test accuracy varies depending on GBS subtype, tested antigen and control group. Anti-GM1 IgG antibody sensitivity for the whole group of GBS patients ranges from 20% to 69% and varies between geographical regions.71-75, 101 Anti-GM1 specificity is reported to be high (94%–98%) in GBS compared with healthy controls and other neurological diseases.25, 54, 73, 102 In GBS, the sensitivity of anti-ganglioside antibody panels is reported to vary between 32% and 64%, depending on the presence of a recent infection or GBS subtype (AIDP or AMAN).103-105 For MFS, sensitivity for anti-GQ1b antibodies is high (88%–100%),79-81 with a very high specificity (100%).83 Especially, when there is some clinical doubt and test results can be obtained within reasonable time, testing for anti-GQ1b antibodies is considered helpful. The INCAT group recommended using standardised methods for ELISA to test for anti-ganglioside antibodies.106 Several studies looked for antibodies against microorganisms (Zika virus,107, 108 CMV,109, 110 C. jejuni,111, 112 Haemophilis influenzae113) with varying sensitivity and specificity (PICO 1). For patients initially diagnosed with GBS but who are subsequently considered to have an autoimmune nodopathy, see also the EAN/PNS CIDP Guideline for further details.114, 115
Rationale: To diagnose motor-sensory GBS, there is currently no indication to test for anti-ganglioside antibodies because of their low–moderate sensitivity and frequent delay in reporting results of antibody assays beyond the therapeutic window. GQ1b antibody testing is useful when MFS is suspected.
Electrodiagnosis (PICO 4)
When GBS is suspected clinically, the diagnostic certainty can be increased using confirmatory tests, especially electrodiagnostic studies.
Good practice points
- The TF advises using electrodiagnosis to support the early diagnosis of GBS.
- Electrodiagnostic studies are helpful in the differential diagnosis of disorders, which may mimic GBS.
- Electrodiagnostic subtype classification into AIDP, AMAN or AMSAN is not helpful in the early diagnosis of GBS and currently has no bearing on management and treatment.
- In patients with suspected GBS examined within the first week after disease onset, the following electrodiagnostic features are supportive of the diagnosis, but do not exclude GBS mimics (high sensitivity but low specificity):
- Sensory and/or motor conduction abnormalities consistent with a polyneuropathy.
- Absent H-reflexes.
- Facial nerve direct responses showing either increased distal motor latency or decreased compound muscle action potential (CMAP) amplitude.
- Blink responses either absent or showing prolonged ipsilateral R1 and R2 responses and contralateral R2 response.
- The diagnosis of GBS is supported by the following electrodiagnostic abnormalities with low to moderate sensitivity but high specificity:
- Sural sparing pattern (abnormal median or ulnar sensory nerve action potential (SNAP) with normal sural nerve SNAP, after excluding carpal tunnel syndrome).
- Indirect discharges (often multiple and resembling A-waves and distinct from F waves).
- Distal CMAP duration prolongation >8.5 ms (time from onset of first negative deflection to return to baseline of last negative deflection, using a filter bandpass of 2 Hz–10 kHz).
- The presence of H-reflexes makes the diagnosis less likely.
- Normal electrodiagnostic examination in the first week does not exclude the diagnosis. Performing a second electrodiagnostic study later during the disease course can be helpful since abnormalities may take several weeks to develop.
- In patients with suspected MFS, the diagnosis may be supported by the following electrodiagnostic abnormalities:
- Sural sparing pattern.
- Any sensory and motor conduction abnormalities consistent with polyneuropathy.
Considerations supporting the GPPs (supplementary material online)
Evidence summary: Prospective and retrospective studies that evaluated up to 84 patients with clinically suspected GBS or up to 66 AIDP cases with a variable number of controls concluded that numerous nerve conduction parameters are abnormal early after disease onset.116-131 However, some studies included small numbers of patients and/or did not include controls. Sensitivity and specificity vary according to electrodiagnostic criteria and the control groups used. Absence of H-reflexes has a high sensitivity (95%–100%) for AIDP105, 121 with 33% specificity.119 It was concluded that the presence of H-reflexes renders the diagnosis of GBS unlikely.118, 122 A sural sparing pattern is present early in the disease course with 16%–100% sensitivity116, 118, 119, 122, 124 and 91%–98% specificity.116, 118 Sural sparing pattern may occur in all subtype classifications (AIDP, AMAN, unclassified) and in MFS.125 Distal CMAP duration prolongation can especially be found in AIDP117 and was reported to have 32%–88% sensitivity with high specificity (91%–100%).118, 117, 128 Decreased motor nerve conduction velocities and increased distal motor latencies were found in 78% of AIDP cases.122 If a patient meets published criteria for AIDP,24, 132 this may support the diagnosis of GBS, but the TF considered the evidence of insufficient certainty, and does not advise electrodiagnostic subtyping in clinical practice. Indirect discharges (often multiple and resembling A-waves) are common in AIDP (sensitivity 59%–100%),121, 126, 133, 134 with a reported specificity of 73%, as these have also been reported in AMAN.118 Facial nerve direct responses and blink responses are often abnormal early in the disease, but specificity is low.121, 123, 124, 135 Nerve root stimulation can detect very proximal conduction block by electrical stimulation of lumbar roots136 or magnetic stimulation of the ulnar nerve,127 but there is only low certainty evidence for this. In MFS, sensory and motor conduction abnormalities are present in >50% of cases (high sensitivity but low specificity), and sural sparing pattern has low sensitivity but high specificity.129-131
Rationale: Electrodiagnosis is an important test that is relatively easy to conduct and that may help to diagnose GBS. Since there is no consensus on definitions for electrophysiological subtyping between AIDP, AMAN and AMSAN,24, 132, 137-139 and currently it has no impact on management and treatment, the TF decided that electrodiagnostic subtyping was not further considered in this Guideline.
Nerve MRI or nerve ultrasound (PICO 5)
The TF explored whether nerve MRI or ultrasound (US) could be useful in selected cases with suspected GBS.
Good practice points
- The TF advises against using nerve MRI or US as routine add-on tests for the diagnosis of GBS with typical presentation.
- The TF suggests that nerve MRI and US should be considered in selected cases with atypical presentation.
- The presence of MRI nerve root enhancement is supportive of GBS, but does not rule out other causes of polyradiculopathy (Table 2). When the disease course is considered compatible with A-CIDP, the presence of widespread nerve enlargement on nerve US or MRI may favour the diagnosis of A-CIDP, but is not specific for the diagnosis. Whole spine MRI with contrast may aid in ruling out spinal cord compression, transverse myelitis, spinal cord tumours or other mimics.
Considerations supporting the GPPs (supporting information)
Evidence summary: Studies identified on nerve MRI in GBS were small (range 11–40 patients) and uncontrolled; in these circumstances showing high sensitivities (82%–95%), but with unknown specificity.140-144 Studies identified on nerve US in GBS were small (range 17–50 patients) and were mostly controlled but used highly selected patient populations and often compared with healthy controls only. Furthermore, most studies lack objective cut-off values for abnormalities. Within these significant limitations, they report sensitivities ranging from 47% to 95% and specificities ranging from 36% to 91%.145-153
Rationale: Nerve MRI or US should only be considered if the diagnosis of GBS is uncertain, possibly to rule out other causes. Abnormal nerve MRI and US may help to localise the pathology to the nerve roots, but the tests lack specificity and do not rule out GBS when normal. Further research to define cut-off values and to evaluate the specificity of nerve MRI and US in patients suspected to have GBS and its mimics are indicated.
A-CIDP (PICO 6)
Some patients with clinically suspected GBS do not clearly improve, but continue to progress or relapse after 8 weeks. After excluding alternative diagnoses, some patients may be diagnosed with A-CIDP, requiring another or an alternative treatment consistent with CIDP management (EAN/PNS CIDP Guideline for further details114, 115).
Good practice points
- The TF suggests that the possibility of changing the diagnosis from GBS to A-CIDP may be considered a few weeks after onset in some patients initially diagnosed with GBS, especially if the patient worsens again after initial improvement or stabilisation (known as a treatment-related fluctuation, TRF), or presents as mild or slowly progressive GBS and continues to worsen.
- A-CIDP is more likely if there are three (or more) TRFs.
- A-CIDP is possibly more likely if there are any of the following: (a) marked sensory abnormality (including sensory ataxia); (b) absence of facial, bulbar or respiratory weakness; (c) slower disease onset (threshold not defined but possibly >2 weeks from onset to nadir); (d) US evidence of widespread peripheral nerve enlargement; or (e) early significant reduction in motor nerve conduction velocity (MNCV).
- A-CIDP cannot be confirmed unless there is further worsening at least 8 weeks after onset.
- If A-CIDP is considered (especially if there appears a poor response to treatment), the TF advises to test for antibodies against nodal–paranodal antigens (see also PICO 3).
- In case of a TRF, the TF suggests to consider re-treatment with IVIg or PE.
Considerations supporting the GPPs (supporting information)
Evidence summary: In a prospective study of 170 patients initially diagnosed as GBS, 16 (9%) had a TRF and another 8 (5%) were subsequently diagnosed with A-CIDP.23 A-CIDP was reported more likely (>5%) if there are marked sensory disturbances on examination (sensory ataxia is the most specific), but facial or bulbar weakness and preceding infections were less frequently observed in A-CIDP compared with GBS.149, 154, 155 Confirmation of these results however warrant additional studies in larger numbers of patients. A-CIDP is likely in patients initially diagnosed with GBS, if there is further worsening after 8 weeks from onset (sensitivity 100%, specificity 92%), or when there are three (or more) TRFs (episodes of worsening following treatment-induced improvement/stabilisation) (sensitivity 52%, specificity 96%).23 Reduced MNCV < 90% of lower limit of normal (or <85% if small distal CMAP) was more frequently found in A-CIDP than in GBS.23, 149, 155 Patients with A-CIDP tested with nerve US within 4 weeks of onset had greater enlargement of peripheral nerves compared with those with GBS (sensitivity 88%, specificity 84%).147, 149 The absence of IgG anti-ganglioside antibodies has a sensitivity of 96% and specificity of 35% for A-CIDP compared with GBS.23, 24, 147, 155 Some patients suspected to have A-CIDP may have an autoimmune nodopathy.156 These patients have a poorer response to conventional therapies for CIDP, and there is anecdotal evidence that these patients may response to rituximab (see EAN/PNS Guideline CIDP).114, 115
There is no evidence from an RCT, but observational data indicate that a repeated course of IVIg or PE can be effective in case of a TRF.157, 158
Rationale: It is important to diagnose A-CIDP because treatment differs from GBS.114, 115 A-CIDP however should not be over-diagnosed in severely weak patients with slow or no improvement. If in doubt, the presence of muscle wasting and denervation on electromyography indicates that GBS-related axonal degeneration is more likely than A-CIDP.
Part 3: Treatment
Prediction of the need for ICU admission (PICO 7, Figure 2)
GBS can present with rapidly progressive weakness leading to respiratory insufficiency in hours to days. Failure to recognise impending respiratory failure can result in death or hypoxia-induced disability. Elective transfer to an ICU may result in earlier recognition of the need for ventilatory support and intubation. This should result in fewer unanticipated emergencies and better outcomes.
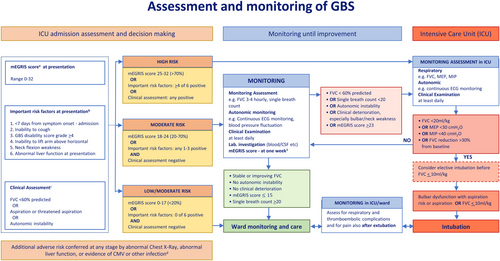
Good practice points
- The TF advises assessing the risk of respiratory failure early in the course of GBS.
- The TF advises using a prognostic model at hospital admission to quantify the risk of requiring mechanical ventilation. This can be quantified using the modified Erasmus GBS Respiratory Insufficiency Score (mEGRIS).159 mEGRIS requires four clinical factors to estimate the risk of requiring mechanical ventilation at any time during the first 2 months from disease onset. The risk of requiring mechanical ventilation is greater in patients with rapid disease progression, bulbar palsy and weaker (lower MRC scores of) neck flexion and bilateral hip flexion.
-
The TF advises regularly assessing risk factors for requiring mechanical ventilation during hospital admission.
These include: rapid progression of limb weakness during hospital admission; GBS-DS grade 4 (unable to walk 10 m even with aid); neck flexion, facial or bulbar weakness, and especially the inability to cough; autonomic instability such as fluctuations in blood pressure or heart rate.
- The TF advises regularly assessing any decline in respiratory function by measuring forced vital capacity (FVC), and single breath count (SBC). Optionally (when available) measurement of maximum inspiratory pressure (MIP) or maximum expiratory pressure (MEP) can be helpful to indicate respiratory insufficiency.
- The TF suggests that some laboratory tests may indicate a greater risk of requiring mechanical ventilation (e.g., elevated liver function tests, infection with HSV or CMV, or electrodiagnostic features of demyelination).
- The TF advises frequent monitoring of the following quantitative measures of ventilatory function:
- FVC should be checked between three and six times a day, depending on severity, and until significant worsening seems unlikely. While the patient is still declining and there is a reduction in FVC, 4-hourly monitoring is likely appropriate.
- A fall of the FVC > 30% below the predicted baseline should alert concern, a fall of >30% in 24 h likely indicates immediate transfer to ICU, or a 50% decline in under 24 h likely indicates the need for ventilation. Elective ventilation should be considered when FVC ≤ 20 mL/kg, and when the FVC is ≤10 mL/kg ventilation is almost inevitable.
- SBC < 20 (inability to count in a single breath out loud from 1 to 20) is a useful bedside tool to assess the need for transfer to ICU.
- If measured, an MEP of <30 cmH2O or MIP < 40 cmH2O indicates the need for elective mechanical ventilation to be considered imminently.
- After discharge from ICU, the TF advises that frequent careful monitoring for potential late respiratory or cardiovascular complications should continue.
Considerations supporting the GPPs (supporting information)
Evidence summary:
mEGRIS prognostic model: An individual patient's risk of requiring mechanical ventilation can be estimated by using EGRIS160, 161 the updated version of EGRIS,162 and now with the modified (and simplified) version mEGRIS.159 An advantage of mEGRIS is that it can be used across the full spectrum of GBS, including mild cases and variants, and in patients from different regions. The original EGRIS is based upon 397 GBS patients in a prospective cohort, and 191 patients in a validation cohort, and has good discriminative ability (area under the curve [AUC] 0.84) (moderate certainty evidence). EGRIS was externally validated in a retrospective cohort of 177 Japanese patients.163 The updated version of EGRIS is based upon 1023 patients from the International GBS Outcome Study IGOS (Europe/North America n = 842, Asia n = 104, other n = 77), aged ≥6 years, and includes GBS variants and those with mild symptoms.162 In this cohort, 104 (10%) required mechanical ventilation within the first week from study entry.
Individual factors were assessed for the certainty of evidence to predict respiratory failure (requiring mechanical ventilation) based on both controlled and observational studies. Predictors of respiratory failure (high certainty evidence) are: (1) Shorter time from onset to admission,160, 164, 165 (2) Bulbar involvement,102, 160, 164, 166 and (3) MRC sum score <20/60.161, 167 Probable predictors of respiratory failure (moderate certainty evidence) are: (1) neck muscle weakness,167-169 (2) greater GBS disability score,160, 164, 170 (3) lower vital capacity and164, 166, 171 (4) hypoalbuminemia.172 Factors that may predict respiratory failure (low certainty evidence) are: (1) inability to lift elbows,165 (2) inability to stand,165 (3) inability to cough,165 (4) dysautonomia,102 (5) lower single breath count (may be more an indicator than a predictor),166 (6) increased liver enzymes,165 (7) lower proximal/distal CMAP ratio,171 AIDP versus AMAN or intermediate GBS subtype163, 173 and (8) longer phrenic nerve latency.166 The modified version (mEGRIS) is based upon the first 1500 patients included in the IGOS.159 Of these, 1133 (76%) patients met the study criteria. Independent predictors of MV were a shorter time from onset of weakness until admission, the presence of bulbar palsy and weakness of neck flexion and hip flexion. mEGRIS was based on these factors and accurately predicts the risk of MV with an AUC of 0.84 (0.80–0.88). The model was internally validated within the full IGOS cohort and within separate regional subgroups, which showed AUC values of 0.83 (0.81–0.88) and 0.85 (0.72–0.98), respectively.
Assessment of important risk factors: The TF found 10 studies assessing predictive factors of the need for mechanical (endotracheal) ventilation. In a large retrospective series of 722 adults, 313 were ventilated and six important predictors (see Figure 2) of subsequent ventilation were identified165: (1) <7 days from symptoms to admission (OR 2.51); (2) inability to cough (OR 9.09); (3) GBS disability grade ≥4 (wheelchair bound or bedridden) (OR 2.53); (4) inability to abduct shoulder to horizontal (OR 2.99); (5) neck flexion weakness (OR 4.34); and (6) abnormal liver function tests at presentation (OR 2.09). If 4 out of 6 predictors were present, mechanical ventilation was required in 85% of patients.
In a subpopulation, it was found that three individual features were strongly associated with the need for subsequent ventilation. If there was neck flexion weakness (OR 5.00), a duration between onset and nadir of <7 days (OR 5.00) and an FVC of <60% predicted (OR 2.86) were all present, then mechanical ventilation was required in >85% of patients. Ventilation was also associated with the presence of facial, bulbar or clinical markers of autonomic failure.174 Co-existing CMV (OR 8.81, CI 2.34–33.1) or herpes simplex virus (HSV) infections (OR 4.83, CI 1.16–20.1) were associated,175 and pulmonary infection (abnormal chest x-ray) is possibly associated (49 vs. 29%, p = .06) with a subsequent need for ventilation.174
Clinical assessment of respiratory function: Quantitative indicators of the likelihood of a need for ventilation are FVC of <20 mL/kg, MIP < 40 cm H2O, MEP < 30 cm H2O or a recorded reduction of >30% in FVC from baseline.174 A decline of FVC of >50% was associated with mechanical ventilation within 36 h, and a drop in the FVC < 1 L was associated with artificial ventilation within 18 h.176 SBC < 20 predicted the need for subsequent ventilation.166
Risk of prolonged mechanical ventilation: Risk factors for prolonged mechanical ventilation (14 days or more) in individuals with GBS have been studied in a cohort of 552 participants (recruited from RCTs and observational studies).177 Prolonged mechanical ventilation in GBS may be predicted by (1) inability to lift arms177 or (2) axonal GBS or inexcitable nerves (low certainty evidence).
Complications after ICU discharge: It was reported that two thirds of deaths of GBS patients occur in the period following ICU discharge and during the recovery phase, most frequently from respiratory or cardiovascular complications.178
Rationale: It is very important to regularly assess indicators of progressive respiratory impairment (ventilatory insufficiency due to muscle weakness and also infections) and autonomic dysfunction in the early and progressive phases of GBS to avoid emergency intubations or otherwise life-threatening situations. The TF has constructed a flow chart that may aid (Figure 2).
Immune treatment of GBS
There are Cochrane Systematic Reviews of the effects of plasma exchange (PE),179 Intravenous Immunoglobulin (IVIg),180 corticosteroids181 and other treatments for GBS.182 Most RCTs have been conducted in GBS patients unable to walk unaided (GBS-DS grade 3 or more). Recommendations are given for each treatment separately.
PE (PICO 8)
The first large PE trials evaluated the effect of PE in GBS patients unable to walk unaided within the first 4 weeks from onset of weakness. Later PE trials also investigated the number of PE sessions (volume exchanged) according to disease severity (Figure 3).

Recommendations
- The TF strongly recommends starting PE as soon as possible in GBS patients unable to walk unaided (GBS-DS grade 3 or more) and within 4 weeks from onset.
- The TF strongly recommends four to five exchanges over 1–2 weeks with a total exchanged volume of 12–15 L in patients who are severely disabled (unable to walk unaided, bedridden or ventilated).
- The TF weakly recommends two exchanges in GBS patients still able to walk unaided but who cannot run (GBS-DS grade 2) within the first 2 weeks from onset of weakness.
Good practice points
- The TF advises PE (four to five exchanges over 1–2 weeks) also in patients who are still ambulatory, but who have a fast rate of deterioration, a risk of requiring ventilatory support, swallowing difficulties or other poor prognostic factors. These patients are considered at high risk of further deterioration, which may potentially be prevented by starting treatment early (PICO 7 and 14).
- The TF does not advise to start PE (a) in very mildly affected patients (GBS-DS grade 1) with stable disease within the first 2 weeks from onset of weakness; (b) in patients still mildly affected (GBS-DS grade 1 or 2) at weeks 2–4 from onset of weakness. It is considered unlikely that these patients will further deteriorate to a higher GBS-DS grade within the time frame of progression of GBS (max 4 weeks).
- The TF does not advise on the specific type of PE (most often continuous flow machines are used) and on the use of specific replacement fluids.
Considerations supporting the recommendations (supporting information)
Evidence summary: The TF considered the effectiveness of PE compared with supportive treatment only (evidence from 6 RCTs including 649 patients), its rather limited adverse effects and the high disease burden, crucial in decision making.179, 183-188 PE reduced the time to regain ability to walk with aid, reduced the time to onset of motor recovery and increased the proportion of patients who recovered the ability to walk unaided (low to moderate certainty evidence). After 1 year, full recovery of muscle strength was somewhat more likely and severe residual weakness somewhat less likely in patients treated with PE (moderate certainty evidence). Moderate certainty evidence showed that the effect of PE was only demonstrable when started within the first 4 weeks of onset of weakness, and most effective when started within the first week of onset of weakness.185, 186
The recommended total number and exchanged volume of PE sessions vary between studies.179 The advice of the TF mainly originates from three large studies.185-187 Two trials compared different numbers of PEs related to the level of GBS disease severity.186, 187 The largest study in 161 ventilated GBS patients showed that six PE sessions (each of 40 mL/kg or 1.5 plasma volumes) may be as effective as four PE sessions (low certainty evidence).187 In 304 moderately severe GBS patients (not ventilated), four PE sessions (in total 4 × 3.75 L plasma removed) may be superior to two PE sessions (low to moderate certainty evidence).187 In the 91 GBS patients still able to walk or stand up unaided, two PE sessions appeared to be better than no PE,187 but this is considered to be a low certainty evidence. In the North American PE trial (including 245 GBS patients), in total, 200–250 mL plasma/kg was removed in five exchanges over 7–14 days.185 According to the most frequently used PE schedules in the largest trials, the TF recommends four to five exchanges of about 3 L plasma each, with a total removal of 12–15 L plasma (dependent on body weight and possible side effects).158, 179 There is no RCT on the effect of PE (or IVIg) in very mildly affected patients (GBS-DS 1) within the first 2 weeks from onset of weakness, nor in (very) mildly affected patients (GBS-DS grade 1–2) 2–4 weeks after onset of weakness. In one trial, 57 participants were randomly allocated to receive PE with albumin and crystalloids, and 52 participants received fresh frozen plasma as the replacement fluid. No significant differences were found between the two arms in any of the outcomes. Although fibrinogen and prothrombin decreases were greater in participants receiving albumin, adverse events were more frequently found when fresh frozen plasma was used as replacement fluid.186, 189, 190
A single report of a small number of patients suggested that small volume plasma exchange (SVPE)—a technique in which multiple small blood volumes are sequentially exchanged—can be used relatively safely without requiring specialised equipment (very low certainty evidence).191 Efficacy data on SVPE are not available.
Rationale and implementation considerations: RCTs showed that PE is an effective treatment for patients with GBS. PE is a less favourable option than IVIg in young children due to limited vascular access and the child's possible fear of the procedure. Patients with severe cardiovascular autonomic instability have a relative contra-indication to PE with discontinuous filtration machines because of the large volume shifts and possible blood pressure changes, but these are a lesser problem with continuous flow machines.
In very mildly affected patients (GBS-DS grade 1) within the first 2 weeks from onset of weakness, or in mildly affected patients (GBS-DS grade 1–2) without additional difficulties (like swallowing or autonomic disturbances) within 2–4 weeks from onset of weakness, there is no indication that PE (or IVIg) is beneficial. Because treatment may induce side effects and is expensive, it is not advised (GPP) to start treatment in these patients. PE requires local availability of specialised equipment and skills, not readily available in all hospitals. In under-resourced parts of the world where standard care for GBS (including ICU facilities, PE or IVIg treatment) is unaffordable or unavailable for many patients, efforts to find innovative treatment alternatives should be encouraged. Whether SVPE is as effective as standard PE and partially overcomes this problem requires additional studies.
IVIg (PICO 9)
There are no RCTs investigating the effect of IVIg in comparison with placebo. As PE was considered a proven effective treatment for GBS at the time of the IVIg studies, studies investigated whether IVIg was as effective as PE (Figure 3).
Recommendations
- The TF strongly recommends starting IVIg as soon as possible in patients unable to walk unaided (GBS-DS grade 3 or more) if still within the first 2 weeks from onset of weakness.
- The TF weakly recommends the most frequently used and proven effective standard course of IVIg (0.4 g/kg/day for 5 days) rather than a low-dose (0.4 g/kg/day for 3 days) or a high-dose (0.4 g/kg/day for 6 days) regimen or a 2-day regimen (1 g/kg/day).
- The TF strongly recommends that patients with a poor prognosis should be treated with only one standard course of IVIg (0.4 g/kg/day for 5 days), rather than giving also a second 5-day IVIg course (PICO 14).
- The TF has no preference for treatment with either IVIg or PE.
Considerations supporting the recommendations (supplementary material online)
- IVIg versus PE: Both IVIg and PE are effective treatments for GBS. The effect of IVIg versus PE has been studied in seven RCTs with a total of 613 participants, of which on RCT had insufficient data to analyse.180 Six of these trials (567 participants) investigated improvement of at least one grade on the GBS-DS 4 weeks after randomisation in patients unable to walk unaided, and started within the first 2 weeks of the onset of weakness.38, 192-197 Five of these trials (536 participants) investigated the change in disability grade 4 weeks after randomisation.192-196 No significant difference in treatment efficacy could be demonstrated between IVIg and PE (moderate certainty evidence), although IVIg treatment was less frequently discontinued than PE (high certainty evidence).180
- IVIg dose and treatment schedule: In an open study of 51 children, participants were randomised to a 2-day 1 g/kg/day IVIg or a 5-day 0.4 g/kg/day IVIg schedule.198 This small study did not observe significant differences in time to regain unaided walking nor in the secondary outcome measures (low certainty evidence). TRFs were more frequently found in the 2-day regimen (5/23) as compared with the 5-day (0/23) IVIg regimen.180, 198 The TF considered there is too little evidence to support the use of a 2-day IVIg regimen.
- High versus low IVIg dose: One study investigated lower dose IVIg (3 days 0.4 g/kg) versus higher dose IVIg (6 days 0.4 g/kg) in 39 GBS patients with contra-indications for PE.180, 199 Patients treated with the high-dose regimen had a non-significant faster improvement in time to walking with assistance (low certainty evidence). There was a significant difference in the group of ventilated patients in favour of the high-dose IVIg treatment. The TF considers there is too little evidence to support the use of lower or a higher dose IVIg compared with the standard IVIg regimen.
- Second course of IVIg: Almost all studies with IVIg in GBS were conducted with a total dose of 2 g/kg administered over 5 days. The second IVIg dose RCT (SID-GBS trial) investigated, in 93 GBS patients with a poor prognosis (mEGOS ≥6), whether an early second course of IVIg (5 days of 0.4 g/kg/day started 7–9 days after starting the first IVIg course) was more effective than placebo.200 There was no significant benefit on any outcome measure (moderate certainty evidence). Patients receiving a second IVIg course more frequently had severe side effects, particularly thromboembolic events temporally associated with IVIg administration (moderate certainty evidence).200
Rationale: IVIg is generally associated with few adverse events and is readily available in most hospitals. PE requires special facilities, good intravenous access and has a slightly higher adverse event (AE) rate (at least when non-continuous flow apheresis was used). In children, the relative burden of PE may be a reason to prefer IVIg. Due to the high costs, patients in some countries may be unable to afford either treatment.
Good practice points
- The TF advises to start IVIg (or PE) also in patients who are still able to walk unaided (GBS-DS grade 2) within 4 weeks from onset of weakness, but who have a fast rate of deterioration, a risk of requiring ventilatory support, swallowing difficulties, autonomic disturbances or poor prognostic factors (PICO 7 and 14, Figure 3).
- The TF suggests treating GBS patients unable to walk unaided 2–4 weeks from onset, with either PE or IVIg.
- The TF suggests considering IVIg (or PE) in GBS patients still able to walk unaided (GBS-DS grade 2) within 2 weeks from onset, with stable or slowly deteriorating disease, in particular when there are also other features of GBS (like weakness of the arms or cranial nerve involvement).
- The TF does not advise to start IVIg (a) in very mildly affected patients (GBS-DS grade 1) with stable disease within the first 2 weeks from onset of weakness; (b) in mildly affected patients (GBS-DS grade 1 or 2) with stable disease presenting 2–4 weeks from onset of weakness because there is no indication that IVIg (or PE) is beneficial in this clinical condition.
Rationale: The effect of IVIg in GBS has only been studied in RCTs in patients unable to walk unaided, when started within 2 weeks from onset of weakness. As PE and IVIg are considered equally effective in this group of patients, it seems likely (GPP) that IVIg (like PE) is also indicated to treat severely affected patients 2–4 weeks from onset of weakness. Therefore, it seems likely (GPP) that IVIg can also be considered if PE is indicated in mildly affected GBS patients (GBS-DS grade 2) early in the course of disease (Figure 3). Starting IVIg (or PE) in mildly affected patients possibly could prevent additional neural damage and further complications. There are no RCTs conducted in very mildly affected patients (GBS-DS grade 1). The chance that this group of patients, especially if stable, benefit from IVIg (or PE) seems low, treatment may induce side effects and is expensive. Therefore, it is not advised (GPP) to treat this group of patients with IVIg (or PE).
IVIg immediately after PE, or PE after IVIg
Recommendation
- The TF strongly recommends against treatment with PE followed immediately by IVIg, compared with PE or IVIg alone.
Good practice point
- In patients who have not clearly improved or who further deteriorate after IVIg or PE, the TF does not suggest subsequently treating with the alternative treatment (PE or IVIg), as there is no trial evidence of efficacy to support this regimen.
Considerations supporting the recommendations (supporting information)
Evidence summary: This recommendation is based upon one RCT comparing IVIg or PE, with PE immediately followed (irrespective of the prognosis) by IVIg in 249 participants.193 Treatment with IVIg or PE (standard treatment schedules) was started within 14 days of GBS onset. In the combined treatment group, the patients first received PE, followed by IVIg (0.4 g/kg/day for 5 days), which was started on the day after the last PE (irrespective on the effect PE). No significant differences were found in the three arms of this RCT (moderate certainty evidence). There is no indication that a subgroup of patients would benefit more from the combined treatment with PE followed by IVIg.
A small open-label study in nine selected paediatric patients with severe GBS describes the effect of alternating PE/IVIg (one PE session, immediately followed by IVIg (0.4 g/kg), both repeated alternatively five times, ‘Zipper method’).201 This unconventional treatment schedule washes out IVIg very rapidly by repeating the PE sessions. To assess whether it may have an objective positive clinical effect requires an appropriate-scale RCT.
There are no appropriate data on the possible effect of giving the alternative treatment (PE or IVIg) in patients who further deteriorate despite standard treatment with IVIg or PE.
Rationale: As the combined treatment of PE followed immediately by IVIg (in an RCT irrespective of the clinical course after PE) has probably no increased effectiveness, potential additional (limited) adverse events and an increase in costs, this treatment schedule is not recommended.180, 193 PE and IVIg seem equally effective, and there are currently no subgroups identified which do better after either PE, IVIg or PE followed by IVIg. Starting PE immediately after IVIg washes out the treatment that has just been infused, which theoretically seems unattractive and expensive. It is not excluded that some patients who do not do well after their first treatment might possibly do better after a different treatment. However, based on a lack of trial evidence, and the arguments mentioned above, the TF does not advise giving the alternative treatment as a standard procedure in patients who further deteriorate despite a course of IVIg or PE.
Immunoadsorption (IA) and IA followed by IVIg
Immunoadsorption (IA) is a technique to remove possible pathogenic antibodies.
Recommendations
- The TF does not recommend IA for treatment of GBS.
Considerations supporting the recommendation (supporting information)
Evidence summary: One small open pilot study, a prematurely stopped RCT and a very small retrospective cohort study were identified.195, 202, 203 The open randomised pilot study included 45 GBS patients, of which 11 received PE, 13 IA and 21 IA followed by IVIg, which may suggest some advantages for the sequential regimen.202 The RCT aimed to recruit 279 patients but was prematurely stopped after including 23 patients treated with IVIg, 26 with PE and 18 with IA.195 No significant benefit was found for IA alone, nor for the combination of IA followed by IVIg (very low certainty evidence).180, 195, 202
Rationale: Because IVIg is a proven effective treatment for GBS and there is insufficient data on the effectiveness of IA, the TF concluded that there currently is insufficient information to support the use of IA for treatment of GBS. IA is expensive and in most countries has only limited availability. Practical experience suggests that IA may be safe and effective; however, this requires proper validation.
Corticosteroids (PICO 10)
GBS is considered an immune-mediated disease, therefore corticosteroids have been studied. Trials have separately investigated the effect of oral and of IV corticosteroids.
Recommendations
- The TF strongly recommends against oral corticosteroids for the treatment of GBS.
- The TF weakly recommends against IV methylprednisolone (IVMP) alone or combined with IVIg for treatment of GBS.
Considerations supporting the recommendations (supporting information)
Evidence summary: The TF considered the probable lack of efficacy of IVMP (moderate certainty evidence from a trial with 242 patients),181, 204 the probable lack of efficacy of the combination of IVIg and IVMP compared with IVIg and placebo (moderate certainty evidence from a trial with 225 patients),205 the probable harm (delayed recovery) of oral corticosteroids (low certainty evidence from 4 trials with a total of 120 patients) using various oral regimens of the equivalent of 40 mg prednisolone daily for at least 2 weeks,38, 181, 205-208 and the high certainty evidence of adverse effects (diabetes more common), despite that hypertension was less common in the corticosteroid-treated patients, which is crucial in decision making.204, 205
Rationale: Corticosteroids are inexpensive, but there is no trial evidence to support the use of corticosteroids in patients with GBS. Intermediate high dosages of oral corticosteroids may even harm GBS patients.
Other treatments (PICO 11)
A variety of other agents has been tested in small studies and case series. Non-pharmacological treatments like physiotherapy, speech/swallowing therapy, occupational therapy and other forms of rehabilitation treatment are often used in patients with GBS.
Pharmacological treatments
Eculizumab
There is evidence for complement activation from pathological studies and from animal models of GBS.209-211 Eculizumab, a complement blocking agent, was beneficial in an animal model.212
Recommendations
- The TF weakly recommends against eculizumab for treatment of GBS.
Considerations supporting the recommendation (supporting information)
Evidence summary: In two small phase 2 trials (including a total of 41 patients), no beneficial effects of eculizumab could be demonstrated (very low to low certainty evidence).182, 213, 214 Both trials used the same protocol. One trial in seven patients was prematurely ended because recruitment was very slow.214 The Japanese eculizumab trial (JET-GBS) in 33 patients (randomised 2:1 for eculizumab compared to placebo) did not show significant effects on pre-specified outcome measures; however, more patients were able to run after 6 months compared with placebo.213
Rationale: The lack of demonstrated efficacy, the known adverse effects (all patients had some adverse effects) and the high cost currently result in a weak recommendation against eculizumab. Further trials are awaited.
Other pharmacological treatments
Recommendations
- The TF strongly recommends against using alemtuzumab, brain-derived neurotrophic factor, CSF filtration, cyclophosphamide, interferon beta 1a, muronomab-CD3, mycophenolate mofetil or tripterygium polyglycoside for the treatment of GBS.
- The TF does not make a recommendation either for or against using neuromuscular electrical stimulation within 2 weeks after onset of GBS.
- The TF weakly recommends against using 3,4-diaminopyridine to improve muscle strength in patients with chronic disability after GBS.
Considerations supporting the recommendation (supporting information)
Evidence summary: Only small, very low certainty RCTs and case series evaluating these drugs (see: recommendation 1) were identified, for which no clinically important differences in any of the outcome measures could be demonstrated for any of the interventions described.182, 215-224 Minor clinical benefit or harm cannot be excluded. As these treatments potentially can have side effects and important clinical benefit is unlikely, the TF recommended strongly against using these treatments.
The TF considered that the effectiveness of neuromuscular electrical stimulation during early rehabilitation phase of disease is uncertain, based on one small RCT (12 participants).225, 226 Both the effectiveness and safety of 3,4-diaminopyridine (seizures have been observed in other studies) are considered uncertain (one observational study, four GBS patients).227, 228 In a cross-over study (seven participants) with chronic disability after GBS (over 12 months after onset), some improvement of motor function after 3,4-diaminopyridine was found.229 Because of the small trial size, it is not possible to draw a conclusion about the effectiveness in patients with long-term stable weakness after GBS.
Rationale: Given the costs and safety profiles, and the lack of proven clinical benefit, the TF currently recommended against alemtuzumab, brain-derived neurotrophic factor, CSF filtration, cyclophosphamide, interferon beta 1a, muronomab-CD3, mycophenolate mofetil and tripterygium polyglycoside. Because of the uncertainty about an effect on motor function and the potential side effects, the TF weakly recommended against using 3,4-diaminopyridine for the treatment of GBS. Further research needs to be conducted before a recommendation about the possible effectiveness of neuromuscular electrical stimulation can be given.
Physiotherapy, speech, swallowing, occupational therapy or other forms of rehabilitation treatment
Most patients with GBS are treated with physiotherapy, and many with speech, swallowing, occupational therapy or other forms of rehabilitation treatment after GBS. Psycho-emotional aspects and careful daily care are very important as well.
Recommendations
- The TF does not make a recommendation on the use of high-intensity over a low-intensity rehabilitation programme in the chronic phase of GBS.
Considerations supporting the recommendation (supporting information)
Evidence summary: No RCTs on physiotherapy, occupational therapy or rehabilitation treatment in the acute phase of GBS were identified. Only a small randomised study (79 patients) in the chronic phase of GBS was found. This study indicated that a high-intensity intervention may reduce disability more than a low-intensity intervention, but this requires additional studies.230 Despite the absence of RCTs, TF is unanimous in their support of the use of physiotherapy, occupational therapy, speech or respiratory therapy (when indicated) and rehabilitation treatment in the various phases of GBS.
Good practice points
- The TF advises to start physiotherapy, occupational therapy, speech therapy and rehabilitation treatment for patients with GBS during the acute phase (already during hospital admission).
- The TF advises transitioning to rehabilitation centres when available, to assist both patients and their family/partners. The TF advises to continue home and/or outpatient physiotherapy, occupational therapy, speech therapy and respiratory therapy (or other forms of therapy when indicated) for more than 6 months (when limitations persist) since function can continue to improve for many months after acute disease.
- The TF acknowledges that psycho-emotional effects of having GBS can be very important both for patients and their caregivers, and therefore careful attention and professional help are often indicated.
Rationale: Despite the lack of appropriate studies, the TF considers physiotherapy, occupational therapy and other forms of rehabilitation treatment important for GBS patients both in the acute and more chronic phase of disease.
Treatment of pain (PICO 12)
Pain often occurs in the acute and recovery phase of GBS, may be severe, and often precedes the onset of weakness.35 Pain is heterogeneous in location and type (nociceptive or neuropathic). Pain may occur in the back, interscapular region, muscles, in a radicular pain distribution, or as painful par- or dysesthesias in the extremities.35 Recommendations are made based upon trial evidence. Because pain is frequent and its treatment may be complex, the TF additionally provides GPPs to guide practical care. Both pharmacological and a variety of activities to improve emotional well-being are considered.
Pharmacological treatment
Recommendations
- The TF weakly recommends using gabapentin or carbamazepine for the treatment of pain in GBS.
- The TF weakly recommends against using high-dose corticosteroids for the treatment of pain in GBS.
Good practice points
- The TF advises asking about the presence of pain during the acute and recovery phases of GBS.
- The TF advises assessing the cause(s) of pain and whether it is neuropathic or nociceptive (especially musculoskeletal pain) because treatment may differ.
- The TF advises first using gabapentinoids (gabapentin and pregabalin) or tricyclic antidepressants prior to using carbamazepine. Care should be taken when using certain (like tricyclic anti-depressive) drugs in patients with autonomic failure.
- The TF suggests that co-administration of medications may be feasible and useful. Opioids might be used but care should be given to adverse reactions (constipation, ileus, confusion, suppression of respiratory drive and addiction).
- The TF advises using published guidelines for treatment of chronic neuropathic pain for treatment of pain or dysesthesias in GBS. These guidelines recommend tricyclic antidepressants, pregabalin, gabapentin or serotonin-noradrenaline reuptake inhibiters (duloxetine or venlafaxine) as first-line treatments.
Considerations supporting the recommendations (supporting information)
Evidence summary: There are only small short-term studies in the acute phase of GBS available that provide low certainty evidence. Carbamazepine (100–200 mg three times daily) was beneficial in two small trials versus placebo (low certainty evidence).231, 232 Two small RCTs support the use of gabapentin (5 mg/kg or 300 mg, three times a day) for the treatment of neuropathic pain (low certainty evidence).232, 233 It has been argued that pain in the acute phase in some patients may be due to proximal localised endoneurial inflammatory oedema that may be responsive to corticosteroids.234 Methylprednisolone (500 mg for 5 days) probably offers no clear benefit to the treatment of either pain or disability (moderate certainty evidence).35, 235 Guidelines for treatment of chronic neuropathic pain are available.236, 237
Rationale: There currently is no clear indication that neuropathic pain in GBS should be treated differently from neuropathic pain in other peripheral nerve diseases.
Yoga, meditation and other activities to improve emotional well-being
Complementary techniques such as yoga, meditation and mindfulness, and some other forms of activities have been described in the management of GBS, but very few have any evidence of efficacy. The only therapies with RCTs in the literature are pranayama yoga and meditation, and therefore these were considered specifically in this GBS Guideline.
Recommendations
- The TF does not make a recommendation for or against using pranayama yoga and meditation techniques for the treatment of GBS.
- The TF does not make a recommendation for or against other activities that improve emotional well-being for treatment of GBS.
Considerations supporting the recommendations (supporting information)
Evidence summary: Only one small trial (22 patients) was identified that compared pranayama yoga (breathing practices) and meditation as add-on therapy to usual care.238 The TF is uncertain whether pranayama yoga and meditation as add-on therapy influences sleep quality and pain (very low certainty evidence), or changes in daily activities (low certainty evidence). There is no evidence that patients with GBS may benefit from activities that improve emotional well-being more or less than the general population.
Rationale: Although there is no evidence to show that patients with GBS may benefit from yoga (in all its forms) and other complementary therapies, patients who have had GBS may find that careful introduction of one of these activities may be worthwhile to try.
Treatment of fatigue (PICO 13)
Fatigue is an important and frequently (38%–86%) occurring residual complaint in patients with GBS, especially during the recovery phase.36, 37 The causes and treatment of fatigue are complex and poorly understood. Pharmacological and non-pharmacological strategies (graded exercise programmes, cognitive behavioural therapy and physical fatigue management strategies) are used.
Pharmacological interventions
Recommendations
- The TF weakly recommends against using amantadine to reduce fatigue in patients with GBS.
- The TF does not make a recommendation for or against other pharmacological agents for the treatment of fatigue in patients recovering from GBS.
Considerations supporting the recommendation (supporting information)
Evidence summary: There is low certainty evidence from a cross-over trial in 80 GBS patients that there is no effect of amantadine to reduce fatigue, improve quality of life or increase participation when used at standard doses for 6 weeks.36 No other studies appropriately assessing other drugs to treat fatigue in GBS have been found.239
Rationale: Although amantadine might reduce fatigue in other disorders, trial evidence in GBS did not show a positive effect. Other long-term conditions with associated fatigue are sometimes treated with anti-fatigue medication, including stimulants (modafinil, methylphenidate), androgenic steroids (DHEA) and antidepressants (serotonin reuptake inhibitors), but this has not systematically been studied in GBS.240
Non-pharmacological interventions
Good practice point
- The TF does not advise for or against exercise training for fatigue.
Considerations supporting the GPP (supporting information)
Evidence summary: An open 12-week study of bicycle exercise training in 16 GBS patients and 4 CIDP patients with severe fatigue, who were otherwise relatively well recovered from their disease, indicated that training was well tolerated and fatigue scores improved.241
Rationale: It is very unlikely that exercise would be harmful in physically relatively well-recovered GBS patients. Exercise is often feasible and anecdotal reports from patients, clinicians and unpublished studies suggest that it has at least subjective and maintained benefits.
Part 4: Prognosis
Prognosis (PICO 14)
The outcome of GBS is highly variable, and predicting the prognosis of GBS is relevant for treatment purposes and for counselling. Both the short-term prognosis (e.g., likelihood of needing mechanical ventilation, see Part 3 Prediction of ICU admission, PICO 7) and the long-term prognosis (e.g., likelihood of being able to walk unaided after 6 months) are relevant. Although outcome is more than the ability to walk, this is a main outcome parameter in the RCTs.
Good practice points
- The TF advises assessing the risk of poor outcome in GBS at an early stage of the disease.
- For clinical decision making and counselling, the TF advises to estimate the risk of being unable to walk unaided after 4 and 26 weeks. This risk is increased in older patients, those with preceding diarrhoea/gastroenteritis and in patients with higher GBS disability score or severe limb weakness at hospital admission, and can be calculated using the mEGOS score.
- Because the risk of being unable to walk unaided after 3–6 months is increased in patients with low mean distal compound muscle action potential (CMAP) amplitude (<20% of lower limit of normal, averaged over at least 3 motor nerves), the TF advises to take this finding also into consideration when discussing outcome.
Considerations supporting the recommendations (supporting information)
Evidence summary:
Risk of poor outcome (inability to walk unaided after 3–6 months): Both controlled and observational studies have been found and were used to identify factors related to poor outcome in GBS.
Predictors for poor outcome (high certainty evidence) are (1) older age,164, 168, 171, 242-248 (2) preceding diarrhoea/gastroenteritis,102, 167, 245-249 (3) higher GBS disability scores at admission,102, 164, 167, 247, 249 (4) lower MRC sum scores at admission247, 248, 250 and (5) decreased CMAP amplitude.243, 244, 251, 252
Probable predictors of poor outcome (moderate certainty evidence) are (1) preceding C. Jejuni infection,102, 247 (2) severe arm weakness,246 (3) higher EGOS scores,161, 249, 253 (4) higher mEGOS scores,161, 247 (5) higher GBS disability scores at nadir,164, 242, 243 (6) absence of CMAPs167/Inexcitable motor nerves,242, 246 (7) decreased proximal/distal CMAP amplitude171 and (8) hypoalbuminemia.172 Preceding CMV infection probably does not independently predict poor outcome in GBS at 6 months, but may predict poor outcome at 8 weeks.248 The presence of any preceding infection may not independently predict poor outcome in GBS.243, 254
Factors that may predict poor outcome (low certainty evidence) are: (1) short time from onset to admission,242, 244, 247, 248 (2) short duration of active disease,245 (3) upper or lower limb power < MRC grade 3,242 (4) respiratory failure /history of mechanical ventilation,242, 244, 250, 254, 255, 164 (5) short time to nadir disability,245, 243 (6) lower MRC sum scores at nadir,254, 256 (7) being bedbound/having respiratory insufficiency at nadir,245 (8) increased CSF protein,257 (9) denervation with needle EMG,258 (10) recruitment pattern of hypothenar muscles with needle EMG,258 (11) anti-GM1 or GD1a antibodies,102 (12) absence of IgM anti GM1 antibodies,246 (13) diabetes,255 and (14) BCII glucocorticoid receptor gene polymorphisms.259 Neck muscle weakness may not independently predict poor outcome in GBS.250, 254 Increased serum neurofilament light chain (NFL) levels are associated with axonal damage and poor outcome after GBS, but it is not yet known if this is independent of other prognostic factors.260, 261
Prognostic model: Risks of poor outcome for individual patients can be calculated by the mEGOS247 (moderate certainty evidence). An IGOS validation study showed that mEGOS also accurately predicts the inability to walk after 4–26 weeks in patients in countries outside The Netherlands. The region-specific version of mEGOS for patients from Europe/North America enables an even more accurate prediction of the inability to walk unaided for patients in those continents.32
Rationale: The prognosis of GBS varies greatly. Prognostic models help to estimate the prognosis in an early stage of disease.
AUTHOR CONTRIBUTIONS
Pieter A. van Doorn: Conceptualisation—lead, funding acquisition—lead, methodology—equal, project administration—equal, writing—original draft—lead, writing—review and editing—lead. Peter Y. K. Van den Bergh: Conceptualisation—equal, funding acquisition—equal, methodology—equal, project administration—equal, writing—original draft—supporting, writing—review and editing—supporting. Robert D. M. Hadden: Conceptualisation—equal, funding acquisition—supporting, methodology—equal, project administration—supporting, writing—original draft—supporting, writing—review and editing—supporting. Bert Avau: Methodology—lead, software—supporting, supervision—supporting, writing—original draft—supporting, writing—review and editing—supporting. Patrick Vankrunkelsven: Methodology—supporting, project administration—supporting, supervision—supporting. Shahram Attarian: Investigation—supporting, writing—original draft—supporting, writing—review and editing—supporting. Patricia H. Blomkwist: Funding acquisition—supporting, investigation supporting. David R. Cornblath: Investigation—supporting, writing—original draft—supporting, writing—review and editing—supporting. H. Stephan Goedee: Investigation—supporting, writing—original draft—supporting, writing—review and editing—supporting. Thomas Harbo: Investigation—supporting, writing—original draft—supporting, writing—review and editing—supporting. Bart C. Jacobs: Investigation—supporting, writing—original draft—supporting, writing—review and editing—supporting. Susumu Kusunoki: Investigation supporting, writing—original draft—supporting, writing—review and editing—supporting. Helmar C Lehmann: Investigation—supporting, writing—original draft—supporting, writing—review and editing—supporting. Richard A. Lewis: Investigation—supporting, writing—original, draft—supporting, writing—review and editing—supporting. Michael P. Lunn: Investigation—supporting, writing—original draft—supporting, writing—review and editing—supporting. Eduardo Nobile-Orazio: Investigation supporting, writing-original draft-supporting, writing-review and editing—supporting. Luis Querol: Investigation—supporting, writing—original draft—supporting, writing—review and editing—supporting. Yusuf A. Rajabally: Investigation—supporting, writing—original draft—supporting, writing—review and editing—supporting. Tirugnanam Umapathi: Investigation—supporting, writing—original draft—supporting, writing—review and editing—supporting. Haluk A. Topaloglu: Investigation—supporting, writing—original draft—supporting, writing—review and editing—supporting. Hugh J. Willison: Investigation—supporting, writing—original draft—supporting, writing—review and editing—supporting.
ACKNOWLEDGEMENTS
The GBS Guideline Task Force is grateful to the European Academy of Neurology (EAN), the Peripheral Nerve Society (PNS), the GBS/CIDP Foundation International and GAIN Charity UK for granting unrestricted financial support to develop this Guideline. The TF especially thanks Prof RAC Hughes for his continuous support and guidance during the development of this first international evidence-based GBS Guideline, and Prof KC Gorson for critically reviewing and his helpful comments on the manuscript of this Guideline.
CONFLICT OF INTEREST STATEMENT
Pieter A. van Doorn received funding for research from Zon-MW, Prinses Beatrix Spierfonds, Grifols and Takeda; and payments to his department for serving on advisory boards for Annexon Biosciences, Argenx, Hansa Medical AB, Octapharma AG, Hoffmann-la Roche and Sanofi-Genzyme. Peter Y. K. Van den Bergh received fees for review activities or boards from Alnylam, CSL, Pfizer, Janssen, Novartis and Genzyme. Support for travel from CSL Behring, LFB, Pfizer, Natus, Alnylum, UCB, Genzyme and Novartis. Robert D. M. Hadden received conference expenses from CSL Behring; speaker fees from CSL Behring and Alnylam; served on advisory boards for Takeda, Janssen and Argenx; and received payments to his department from CSL Behring and Grifols. Bert Avau reports no conflicts of interest. Patrik Vankrunkelsven reports no conflicts of interest. Shahram Attarian reports speaker honorary and travel support: LFB, Alnylam, Akcea, Pfizer, Sanofi, Pharnext, Inflectis, Biogen, Novartis Hansa, UCB, Argenx, Alexion and Roche. Board members: Sanofi, Pfizer, Pharnext, InFlectis, Hansa, LFB, Biogen, Alnylam, Argenx, Alexion and Roche. Research funding: LFB, Pfizer and Biogen. Patricia H. Blomkwist-Markens is Liaison (volunteer) of the GBS|CIDP Foundation International and Volunteer of the GBS|CIDP|MMN group of Spierziekten Nederland (Dutch patient organisation for neuromuscular diseases). David R Cornblath declares interests as consultant: Annexon Biosciences, Boehringer Ingelheim, Cigna Health Management, Inc., Grifols S.A., Johnson & Johnson, Neura Bio, Octapharma AG, Pfizer Inc., Roche, Seattle Genetics Inc., Valenzabio; Data Safety Monitoring Board: PledPharma AB, Hansa Medical AB, Mitsubishi Tanabe Pharma Corporation; Technology Licensing: AstraZeneca Pharmaceuticals, LP, Genentech Inc., Levicept Inc., Seattle Genetics, Inc., Merrimack Pharmaceuticals, Disarm Therapeutics; SAB: Sinomab, Algotherapeutics, Nervosave. H. Stephan Goedee has received research grants from Prinses Beatrix Spierfonds, travel support from Shire/Takeda and speaker fees from Takeda paid to the institution. Thomas Harbo has received speaker/consultancy honoraria from CSL Behring, Annexon, Argenx, Beigene and Roche. Bart C. Jacobs declares honoraria as speaker for Grifols, as consultant for Annexon Biosciences and Hoffmann-la Roche, technology licensing for Hoffmann-la Roche and received funding for research from Zon-MW, EU Horizon 2020, Prinses Beatrix Spierfonds, GBS-CIDP Foundation International, CSL-Behring, Grifols, Hansa Medical AB, Annexon Biosciences and Hoffmann-la Roche. Susumu Kusunoki: received grants and honoraria for lecturing from Teijin, Nihon Pharm, CSL Behring and from the Japan Blood Products Organization, and Data Safety Monitoring Board for Argenx. Helmar C. Lehmann: received honoraria for speaking and advisory board engagements by Alnylam, Biogen, CSL Behring, Grifols, Gruenenthal, LFB Pharma, Takeda and UCB. Richard A. Lewis provided advisory and consultancy services to Akcea, Alexion, Alnylam, Annexon, Argenx, Boehringer Ingelheim, CSL Behring, Grifols, Johnson &Johnson, Pfizer, Roche, Sanofi and Takeda. Medical Advisory Board: GBS-CIDP Foundation International and Myasthenia Gravis Foundation of America; Board of Directors: Peripheral Nerve Society—President. Speaker: Medscape; Royalties: Up to Date. Michael P. Lunn has advised NHS England for their IVIG Working group and IVIG Commissioning Panels as well as advising the MHRA on vaccine safety in COVID-19 on an invitational basis. He is Joint Coordinating Editor Cochrane Neuromuscular; has given advice on ad hoc advisory boards, particularly on trial design to Roche, AstraZeneca, Sanofi, UCB, Sanofi, Takeda, Polyneuron and BeiGene. Unrestricted conference expenses have been received from BeiGene and CSL Behring. Eduardo Nobile-Orazio reports personal fees for Advisory or Steering Board from Argenx, Takeda, CSL Behring, Janssen, Kedrion, LFB, Roche and Honorarium for Lectures from Takeda, CSL Behring and Kedrion. Luis Querol received speaker honoraria from Merck, Sanofi-Genzyme, Roche, Biogen, Grifols and CSL Behring; provided expert testimony for Grifols, Johnson & Johnson, Annexon Pharmaceuticals, Sanofi-Genzyme, Novartis, Takeda and CSL-Behring; and received research funds from Roche, UCB and Grifols. Yusuf A. Rajabally has received speaker/consultancy honoraria from LFB, Polyneuron and Argenx and has received educational sponsorships from LFB and CSL Behring and has obtained research grants from LFB and CSL Behring. Thirugnanam Umapathi reports no conflicts of interest. Haluk A. Topaloglu reports no conflicts of interest. Hugh J. Willison provided advisory and consultancy services to Alexion, Argenx SE, Annexon, Polyneuron AG, GSK (US), Janssen (NL), Buhlmann Labs AG, F.Hoffman-La Roche, GeneTx Biotherapeutics, Grifols, and received research funding from Annexon, Argenx, and Polyneuron AG.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.