European Academy of Neurology/Movement Disorder Society - European Section guideline on the treatment of Parkinson's disease: I. Invasive therapies
This article is co-published by the European Journal of Neurology and Journal of the International Parkinson and Movement Disorder Society
Abstract
Background and Purpose
This update of the treatment guidelines was commissioned by the European Academy of Neurology and the European section of the Movement Disorder Society. Although these treatments are initiated usually in specialized centers, the general neurologist and general practitioners taking care of PD patients should know the therapies and their place in the treatment pathway.
Methods
Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology was used to assess the spectrum of approved interventions including deep brain stimulation (DBS) or brain lesioning with different techniques (radiofrequency thermocoagulation, radiosurgery, magnetic resonance imaging–guided focused ultrasound surgery [MRgFUS] of the following targets: subthalamic nucleus [STN], ventrolateral thalamus, and pallidum internum [GPi]). Continuous delivery of medication subcutaneously (apomorphine pump) or through percutaneous ileostomy (intrajejunal levodopa/carbidopa pump [LCIG]) was also included. Changes in motor features, health-related quality of life (QoL), adverse effects, and further outcome parameters were evaluated. Recommendations were based on high-class evidence and graded in three gradations. If only lower class evidence was available but the topic was felt to be of high importance, clinical consensus of the guideline task force was gathered.
Results
Two research questions have been answered with eight recommendations and five clinical consensus statements. Invasive therapies are reserved for specific patient groups and clinical situations mostly in the advanced stage of Parkinson's disease (PD). Interventions may be considered only for special patient profiles, which are mentioned in the text. Therapy effects are reported as change compared with current medical treatment. STN-DBS is the best-studied intervention for advanced PD with fluctuations not satisfactorily controlled with oral medications; it improves motor symptoms and QoL, and treatment should be offered to eligible patients. GPi-DBS can also be offered. For early PD with early fluctuations, STN-DBS is likely to improve motor symptoms, and QoL and can be offered. DBS should not be offered to people with early PD without fluctuations. LCIG and an apomorphine pump can be considered for advanced PD with fluctuations not sufficiently managed with oral treatments. Unilateral MRgFUS of the STN can be considered for distinctly unilateral PD within registries. Clinical consensus was reached for the following statements: Radiosurgery with gamma radiation cannot be recommended, unilateral radiofrequency thermocoagulation of the pallidum for advanced PD with treatment-resistant fluctuations and unilateral radiofrequency thermocoagulation of the thalamus for resistant tremor can be recommended if other options are not available, unilateral MRgFUS of the thalamus for medication-resistant tremor of PD can be considered only within registries, and unilateral MRgFUS of the pallidum is not recommended.
Conclusions
Evidence for invasive therapies in PD is heterogeneous. Only some of these therapies have a strong scientific basis. They differ in their profile of effects and have been tested only for specific patient groups.
INTRODUCTION
The first European guideline on the treatment of Parkinson's disease (PD) was published in 2006 consisting of two parts, the early uncomplicated disease [1] and late complicated disease [2], and were renewed in 2013 as recommendations of European Federation of Neurological Societies/Movement Disorder Society (MDS) for the diagnosis [3] and management [4] of PD. The European Academy of Neurology (EAN) in collaboration with the European section of the MDS (MDS-ES) has now begun to produce regular updates of the guidelines (GLs [5]) according to GRADE methodology. The GL task force has been set up from the two societies to realize this task. The new PD GLs will be separated into several chapters of which this review on invasive therapies is the first. We have included interventions requiring surgery or invasive medication delivery. Invasive treatments are usually considered for advanced PD and cover deep brain stimulation (DBS), pump therapies, and lesional therapies for the treatment of PD.
Radiofrequency thermocoagulation of the thalamus and pallidus internus has a long history of treatment for PD. Initiated in the 1950s [6], it was practiced around the world in the following decades [7-10]. These treatments were initially used because effective drugs to treat bradykinesia and tremor were not available yet. However, when levodopa (l-dopa) became available in the 1970s, lesional surgeries were largely abandoned with the exception of pallidotomy in some countries and thalamotomies for tremors unresponsive to oral treatments, both on a small scale. However, the occurrence of l-dopa–induced fluctuations and dyskinesia triggered the search for better interventions. As a result, lesional pallidotomies were rediscovered, and subsequently DBS started its steep rise in the 1990s. Because of the general impression that DBS results in fewer complications [11], which was confirmed by a randomized controlled trial in the year 2000 [12], DBS has become the most frequently used intervention, and lesional procedures have usually been reserved for specific situations.
DBS developed out of the aforementioned radiofrequency thermocoagulation of the thalamus [13, 14]. Benabid and colleagues discovered that lesioning of the thalamic ventralis intermedius (Vim) nucleus for tremor can be replaced by electrical stimulation [15]. After the discovery of the pathophysiologic role of the subthalamic nucleus (STN) by Bergman and colleagues [16], DBS electrodes were implanted in this nucleus [17] to improve the broader symptom spectrum of PD. Radiofrequency thermocoagulation pallidotomy [18] was subsequently also largely replaced by electrical stimulation of this nucleus. A large number of case series [19] and finally randomized controlled studies have established the concept of DBS of the STN or the globus pallidus internus (GPi) for the treatment of PD. [20]
Radiosurgery of the Vim with proton beams has also been used since the late 1960s in few centers worldwide [21, 22] initially only for this nucleus. Although these lesional procedures showed some benefits, few recent publications are available and are mostly case series, and detailed reports of adverse effects or long-term consequences are lacking. A very recent development is the introduction of magnetic resonance imaging–guided focused ultrasound surgery (MRgFUS). This is an incisionless therapy that has mainly been assessed for essential tremor, and trials for PD are only beginning. This technique is currently used within the thalamus but lesioning of the STN was also recently reported [23].
Among pharmacological interventions, the concept of reducing dopaminergic hypersensitivity and maintaining constant plasma levels by continuous stimulation of dopaminergic terminals has emerged [24], resulting in the treatments of continuous subcutaneous infusion of apomorphine [25] or intrajejunal application of l-dopa–carbidopa intestinal gel (LCIG) preparations [26]. Apomorphine hydrochloride (apomorphine) has been proposed for the treatment of PD since the 1950s [27], but it had been used only in a few movement disorder centers since then [25, 28]. During the past decade, it has been used more widely, in parallel with the technological advances of infusion pump devices. Apomorphine can also be administered using intermittent subcutaneous pen injection for individual off periods, which is not discussed here.
Human fetal [29, 30] or stem cell transplantation or gene therapy [31-33] are experimental treatments of PD and have currently no relevance for clinical care.
SCOPE
Although there are individual GLs and evidence-based medicine reviews on DBS and radiofrequency thermocoagulation lesions, the whole spectrum of invasive interventions for PD has not been addressed in previous GLs. Furthermore, only the NICE-GL 2017 [34], which had a more limited focus, used GRADE-methodology. The invasive interventions for PD share several features and merit a combined review: All are currently mostly used for advanced stage PD except STN-DBS and MRgFUS. They are all considered invasive procedures as apomorphine infusion requires continuous subcutaneous infusion, LCIG needs a percutaneous jejunostomy, MRgFUS is an incisionless intracerebral lesioning, and DBS and radiofrequency thermocoagulation require brain surgery through a burr hole. Furthermore, all are more expensive than standard drug treatments for PD. Health economics and cost-effectiveness of those interventions are beyond the scope of these GLs, but existing systematic reviews are available [35].
METHODS
General
The methodology for the development of these GLs has followed the framework provided by GRADE [36] and the recommendations of the EAN on the development of a neurological management GL [5]. Population/intervention/comparison/outcome (PICO) questions were constructed according to GRADE standards and are shown in the Appendix S1 Methods section (see Table App 1.1a and b). References used in the current GLs were identified by performing a systematic search in the PubMed, Embase, Web of Science, and Cochrane Library databases as well as clinical trials registration via clinicaltrials.gov in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [37]. The literature search for each PICO was conducted from the earliest possible date to December 31, 2020. Two people (from either EAN [K.S., G.D., E.M., A.A.] or Cochrane Response [G.V., N.H.]) independently screened all citations and abstracts identified by the search. Articles were selected based on the following eligibility criteria: (1) original research and/or (2) randomized clinical trial (RCT) and/or (3) metanalysis/systematic review and (4) performed in people with PD. Data were extracted in DistillerSR and summarized in GRADE evidence profiles. The writing group (G.D., A.A., J.C., K.S., E.M.) of the GLs shared the tasks of the systematic review and developed the first draft of the GLs. Every member of the GL group voted on each of the recommendations. Members with a possible conflict of interest for specific questions abstained from voting. Cochrane Response, a fee-for-service organization of Cochrane, was assisting the GL task force to produce these GLs.
For further details regarding search strategy, study selection, data extraction, assessment of risk of bias, data synthesis, summarizing and interpreting results, see the Appendix S1 Methods section.
Outcomes
- Objective outcomes are usually graded by the clinician with clinical scales and include measures of mobility as well as nonmotor outcomes if they are not patient reported (eg, instrumented measures of mobility, sleep). A typical example is the Unified Parkinson's Disease Rating Scale Part III (UPDRS-III).
- Measures of function and/or well-being of the patient. The majority of reported outcomes are based on patient-reported outcome measures such as health-related quality of life (QoL; eg, 39-Item Parkinson's Disease Questionnaire [PDQ-39]) or are completed by the clinician based on an interview with the patient (eg, Schwab and England scale, Unified Parkinson's Disease Rating Scale Part II [UPDRS-II]).
- Adverse events (AEs) are to be balanced against the benefit. AEs are defined as any undesirable experience associated with the use of a medical (or a device) product in a patient and can cover all aspects of health. AEs are standardized into serious AEs (SAEs) and AEs. Death, life-treating conditions, hospitalization and prolonged hospitalization, disability, and permanent damage are considered SAEs. For surgical studies, surgical SAEs are considered separately.
| High certainty of evidence | |
| Large effect | Intervention results in a large reduction/increase in outcome |
| Moderate | Intervention results in reduction/increase in outcome |
| Small, important | Intervention results in a slight reduction/increase in outcome |
| Small, unimportant | Intervention results in no reduction/increase in outcome |
| Moderate certainty of evidence | |
| Large effect | Intervention likely results in a large reduction/increase in outcome |
| Moderate | Intervention probably results in a reduction/increase in outcome |
| Small, important | Intervention probably results in a slight reduction/increase in outcome |
| Small, unimportant | Intervention likely results in little to no difference in outcome |
| Low certainty of evidence | |
| Large effect | Intervention may result in a large reduction/increase in outcome |
| Moderate | Intervention may reduce/increase outcome |
| Small, important | Intervention may reduce/increase outcome slightly |
| Small, unimportant | Intervention may result in little to no difference in outcome |
| Very low certainty of the evidence | |
| Any effect | The evidence is very uncertain about the effect of intervention on outcome |
Wording of recommendations
- Trivial, small unimportant effect or no effect, if the point estimate is not significant.
- Small effect, if the point estimate is significant and if the GL group considered the effect relevant despite being below the MCIC threshold.
- Small effect if the difference is significant and Cohen's d is 0.2 to 0.35.
- Moderate effect if Cohen's d is >0.35 and <0.65.
- Large effect if the effect is significant and Cohen's d is >0.65.
- Trivial, small unimportant effect or no effect if the difference is not significant.
- Small effect if the difference is significant and Cohen's d is <0.35.
- Moderate effect if the difference is significant and Cohens d is >0.35 and <0.65.
- Large effect if the difference is significant and Cohen's d is >0.65.
The wording of the summary of findings is based on the most recent GRADE recommendation [38] and shown in Table 1.
All recommendations were graded by the GL group in light of clinical circumstances. For some of the PICO questions/interventions, no RCTs could be found, and grading of the evidence was therefore impossible. For each of these interventions, the GL group has worded a clinical consensus statement according to EAN standards [39].
A detailed protocol of the GL development can be found in the full GLs (see the online attachment).
RESULTS AND RECOMMENDATIONS
Search results
In total, the database search yielded 2600 articles on nonlesional therapies (see Appendix S2, Fig. App2.1). Based on the aforementioned criteria, 13 studies were included. Specifically, eight studies on DBS versus best medical treatment (BMT), two studies on STN-DBS versus GPi-DBS, one study on apomorphine, and two studies on LCIG.
For lesional therapies, 1297 articles were screened (see Appendix S2, Fig. App2.2), and three studies were included (two on radiofrequency thermocoagulation of the pallidum and one on MRgFUS therapy). For more details, see the Search and Search Results section in Appendix S2.
Interventions, targeted brain structures, and patient profiles discussed in these GLs
The studies were to be separated by the following intervention types: DBS with implantation of an electrode that reversibly modulates brain circuits, radiofrequency thermocoagulation producing thermal localized lesions. Both procedures require brain surgery through a burr hole for each brain side. Radiosurgery is producing brain lesions with stereotactic radiation through the intact scalp. MRgFUS is producing lesions with high-energetic focused ultrasound. Both procedures are incisionless. Apomorphine pump treatment is applied through subcutaneous continuous infusion, and LCIG requiring a permanent percutaneous jejunostomy for infusion of l-dopa into the duodenum. The studies were also separated for the targeted nucleus in the brain (STN, GPi, and Vim). For early PD with early fluctuations and for early PD without fluctuations, studies have considered only the STN as the DBS target. For advanced PD, four of the six studies included only STN-DBS patients, whereas two studies had patients with mixed targets: one with 51/121 GPi, 60/121 STN, and 134 BMT [40] and the other with 4/178 GPI, 174/178 STN, and 183 BMT [41]. The benefits and risks of STN-DBS versus GPi-DBS are presented in a separate paragraph as there are two large RCTs comparing the effects between the two targets [42, 43]. Mostly uncontrolled trials with radiofrequency thermocoagulation have been published for Vim, GPi, and STN. MRgFUS and radiosurgery trials are available for Vim and STN. Finally, the different RCTs have recruited different patient groups. Most invasive interventions were tested for patients with advanced PD with fluctuations and dyskinesia that can no longer be satisfactorily treated with oral medication. This also includes treatment-resistant tremor [44]. The group is labeled here as advanced PD. Patients with motor fluctuations since <3 years after onset can usually still be treated with medication but were specifically tested in RCTs and are labeled as early PD with early fluctuations. An additional patient group are those who are not yet fluctuating and still have a favorable response to drugs even if extended medication regimens are needed. They are called early PD without fluctuations. Two additional specific patient groups are those with treatment-resistant tremor and those with predominant unilateral symptoms.
Figure 1 gives an overview of the different aspects of these GL recommendations.
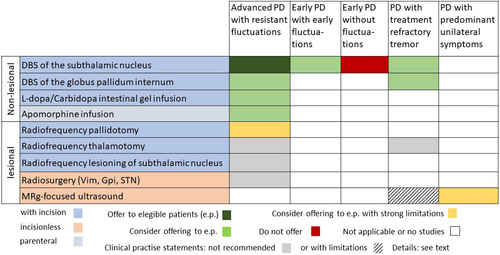
Evidence, summary of findings, and recommendations
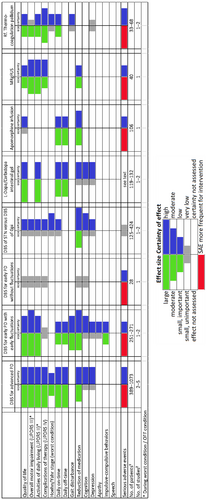
Presentation of the data is separated for nonlesional and lesional therapies. The effects of the interventions are discussed in the next sections and shown in an abbreviated form in Figure 2 and detailed in Appendix S3 (Effects of Intervention section), Appendix S4 (Forest Plots section), and Appendix S5 (Summary of Findings section). They are also partly repeated in the next paragraphs. The wording of the results follows the suggestions of GRADE (see Table 1) [38]. All comparisons of effects and adverse effects are against standard noninvasive treatments (medication).
NONLESIONAL THERAPIES
STN-DBS for advanced PD with medically unresponsive fluctuations or medically unresponsive tremor
The most extensively studied intervention is STN-DBS for advanced PD, with six RCTs against best medical treatment BMT [40, 41, 45-47] of which one had a double-blind design [47]. A study on DBS for the GPi against medical treatment was not identified, but two studies compared stimulation of the STN and GPi [42, 43].
Summary of findings
These RCTs show that STN-DBS probably results in a large improvement of QoL, a large improvement of activities of daily living (ADLs), and a large improvement of motor impairment in these patients. There is likely to be a large effect on motor fluctuations and dyskinesia, the typical complications of long-term medical therapy, particularly with a moderate increase in daily ON time and a moderate reduction in daily OFF time. Hoehn and Yahr stage may be slightly improved. Cognition and depression as important outcomes are likely neither improved nor worsened.
SAEs are more common for the surgically treated patient group than for those on BMT. A total of 152 SAEs are described in 604 patients (25%) in the DBS group versus 52 SAEs/469 patients (11%) in the BMT group. Most surgery-related AEs were reversible. Patients seeking DBS have more suicidal ideation and suicides than the general population [48, 49] but rates in the treated group do not differ from the comparator group on BMT [49] suggesting that this is not an effect of the treatment.
Considerations of the GL task force
Eligibility criteria are important for the selection of patients for this treatment. The most important preoperative predictor of outcome is response to l-dopa during a formal l-dopa test [50]. Most studies reported a minimum of 33% improvement as an inclusion criterion, and lower values have shown poorer treatment results [51]. Tremor as a symptom is responding particularly well to STN-DBS, even if not well responding during the l-dopa test [52]. Age may also be a predictor of response, but this has not yet been convincingly demonstrated because some studies have found no effect of age [53] but others have, and most studies had an age limit at 70 years. There are also neurosurgical contraindications (eg, severe brain atrophy), uncontrolled depression, psychosis, or dementia. The expert group suggests consideration of overall health and biological age rather than the numerical age.
Several uncontrolled retrospective and prospective studies report on the value of STN-DBS for the treatment of several nonmotor symptoms of the disease. This includes a reduction of mood fluctuations, hallucinations, and psychosis and improvements in urinary incontinence and sleep [52, 54-56]. An open 3-year study in 67 STN-DBS patients matched with 84 medically treated patients showed significant differences on the nonmotor symptom scale, particularly for sleep, fatigue, and urinary symptoms [55].
Long-term outcome of STN-DBS cannot be assessed in randomized controlled studies. However, a number of uncontrolled long-term studies of patients with advanced PD and STN stimulation are available and underwent multiple meta-analyses [52, 57, 58]. These reveal that STN-DBS is still effective beyond 15 years after the intervention, with significant improvement in motor complications and a stable reduction of dopaminergic drugs [59]. Certainly, these cohorts are highly selected and most do not report the disease course of patients lost at follow-up. Maintenance of QoL above the preoperative level has been found for 5 years after surgery despite the natural progression of the disease [58, 59].
The GL group concludes that STN-DBS should be offered to eligible patients with PD with medically resistant fluctuations. Stringent inclusion and exclusion criteria need to be applied at specialized centers for each patient.
Recommendation 1: Offer STN-DBS to people with advanced PD if fluctuations are not satisfactorily controlled with medication or if tremor cannot be controlled with medication (15 voters, 100%).
STN-DBS for Early PD with early fluctuations
Two studies were included in the review [60, 61]. Overall, 271 patients with PD aged younger than 60 years and with fluctuations or dyskinesia for less than 3 years were randomly assigned to STN-DBS or BMT.
Summary of findings
STN-DBS in early PD with early fluctuation compared with BMT results in a large improvement of QoL, a large improvement of ADLs, and a large improvement of motor symptoms. The evidence is very uncertain about the effect on complications of therapy. Daily ON time without dyskinesia and OFF time may be improved. STN-DBS may result in no difference regarding cognition and apathy. STN-DBS may improve depression. The effect sizes are similar to advanced PD. DBS may improve impulsive–compulsive behaviors in the long term. STN-DBS probably improves gait [62] and reduces the daily dosage of medication [61]. STN-DBS may increase the likelihood of experiencing SAEs. They occurred in 54.8% of the patients in the neurostimulation group and in 44.1% of those in the best medical treatment group. In the DBS group, 13.7% experienced gait impairment as an AE in contrast to 11.8% in BMT group. Long-term data are not yet published for this cohort.
Considerations of the GL task force
Inclusion criteria are important: Patients in this group were aged younger than 61 years at surgery, the improvement at the preoperative l-dopa test was 50% or higher, and there were no cognitive changes or uncontrolled psychiatric conditions. A secondary analysis showed that the effect on QoL depends on the baseline PDQ-39 score, with those having a worse PDQ-39 baseline score having better postoperative improvement [63]. Thus, a subjectively relevant affection of QoL might be a further inclusion criterion.
The GL group concludes that STN-DBS can be offered to people with early PD and early fluctuations who fulfil the inclusion and exclusion criteria for DBS. Less is known about the long-term course of these patients than for patients with advanced disease.
Recommendation 2: Consider offering STN-DBS to people with early PD and early fluctuations (15 voters, 100%).
STN-DBS for people with early PD without fluctuations
The study published by Charles and colleagues [64, 65] is the only study on implantation of STN-DBS at a stage where fluctuations have not yet occurred. Inclusion criteria were the following: Hoehn and Yahr stage II in the off state, antiparkinsonian medications for more than 6 months but less than 4 years, and no current or prior history of motor fluctuations. A total of 30 patients were randomly assigned, and outcomes were reported for 12 and 24 months of follow-up.
Summary of findings
The critical outcomes PDQ-39, ADLs (UPDRS-II), motor score while OFF (UPDRS-III), and disease complications (Unified Parkinson's Disease Rating Scale Part IV [UPDRS-IV]) were not different between the DBS and BMT groups. Similarly, medication change from baseline to 24 months was not significantly different between the DBS and BMT groups despite substantially increased dosages. Two serious AEs occurred in the DBS group.
Considerations of the GL task force
The study has limitations as the sample size was small (15 people with PD per arm). According to the inclusion criteria, patients eligible for the study did not experience motor fluctuations; however, the difference between the UPDRS-III in the OFF and the ON states was 43% at baseline, suggesting that the patients were already fluctuating. At 24 months of follow-up, the UPDRS-III score in the ON state did not differ from the baseline score, and there was worsening of the UPDRS-III score off medication compared with baseline. The authors stated that a neuroprotective effect can be seen in DBS group; however, no current evidence supports this view. Further studies are underway.
The GL group concludes that STN-DBS should not be offered to people with PD without fluctuations.
Recommendation 3: Do not offer DBS to people with early PD without fluctuations (16 voters, 100%).
STN-DBS versus GPi-DBS in PD
The majority of RCTs for DBS in PD have investigated STN-DBS. Nevertheless, in some countries, GPi-DBS is commonly used. There are only two RCT studies comparing GPi-DBS versus STN-DBS. The American Veterans Administration (VA) study [43] randomly assigned 152 patients to GPi-DBS and 147 to STN-DBS. The Netherlands study randomly assigned 62 patients to GPi-DBS and 63 patients to STN-DBS [42]. Duration of the studies was 24 months [43] and 12 months [42], respectively.
Summary of findings
The analysis of both studies shows that most critical and important outcomes were not different. This applies for QoL (PDQ-39), ADLs (UPDRS-II) at 1 and 2 years, motor score (UPDRS-III in the off medication state) at 1 and 2 years, complications of therapy, ON time without troublesome dyskinesia, SAEs, Hoehn and Yahr staging, and cognition [53, 66]. A minor improvement of depression in the GPi group was found in the US study [43]. There was a greater reduction in l-dopa dosage in the STN groups of both studies. GPi-DBS was associated with a greater increase of daily dose of dopaminergic medication compared with STN-DBS both in the first year of follow-up and during a longer term follow-up.
Considerations of the GL task force
The 3-year, open-label, follow-up data of the VA study [53] showed a stable result of the critical outcome parameters, whereas the 3-year follow-up of the Netherlands study [67] concluded that motor symptoms and functioning during the off-drug phase were more improved after STN-DBS than after GPi-DBS, with no differences for cognition, mood, or behavior.
The studies show only minor differences between STN-DBS and GPi-DBS during the 3-year observation period. Although these differences may be important for particular patients, they do not allow prioritizing one treatment over the other. Therefore, both targets are similarly effective to treat symptoms of advanced PD and can both be recommended. The greater reduction of l-dopa for those treated with STN-DBS was considered an important clinical difference.
Recommendation 4: Both STN-DBS and GPi-DBS are effective to treat symptoms of advanced PD with fluctuations, but dopaminergic medication can be more reduced with STN-DBS (16 voters, 100%).
LCIG FOR ADVANCED PD
Two studies are identified on the LCIG treatment of advanced PD: one 3-month, double-blind, double-dummy study [68] of LCIG versus oral immediate release l-dopa plus in patients with otherwise stable antiparkinsonian treatment and a second study against optimized oral treatment (Results of one of the studies (DYSCOVER) published only on www.clinicaltrials.gov).
Summary of findings
Quality of life assessed by the PDQ-39 and PDQ-8 probably results in a large improvement compared with oral treatment. Similarly, LCIG probably resulted in large improvement of ADLs. ON time with troublesome dyskinesia was probably moderately improved in the overall group. There was moderate decrease in daily OFF time in the LCIG group compared with oral treatment. LCIG application may result in little or no difference in daily dosage of anti-PD medication compared with best medical treatment.
AEs were very common (>95% of the patients). Three patients discontinued treatment: one because of psychosis, one because of peritonitis/pneumonia, and one had postprocedural discharge. No patients died. Most of the SAEs/AEs were related to the gastrojejunostomy, and as both treatment groups had this intervention, there is no difference between the two groups. A total of 63 patients (89%) had device-related complications, including tube dislocations, percutaneous gastrojejunostomy, insertion complications, stoma insertion complications, pump malfunctions, and pneumoperitoneum. The AEs occurred mostly within the first week and resolved in all cases, but the observation period was only 3 months.
Considerations of the GL task force
Given the fact that the controlled study duration was only 3 months, limited evidence is available on long-term benefits and complications. Open-label, longer term (2 years) changes in UPDRS-IV items for OFF time and ON time have been assessed with a multinational national prospective registry. This registry also included assessments of nonmotor symptoms and QoL, showing continued improvement compared with baseline [69]. SAEs have not only been reported in the controlled study but also in open-label studies, including even life-threatening complications [69, 70].
The GL group concluded that the treatment can be considered for people with advanced PD and disabling fluctuations but knowledge about the treatment results beyond 3 months is limited. Its value for dyskinesia has not yet been established.
Recommendation 5: Consider offering LCIG for people with advanced PD if fluctuations are not satisfactorily controlled with medication (15 voters, 100%).
APOMORPHINE INFUSION FOR ADVANCED PD
One recent RCT with a 12-week double-blind phase and a 52-week open-label phase [71] was identified. Patients received either continuously infused subcutaneous apomorphine or placebo. Concomitant medication was reduced when dopaminergic adverse effects (eg, dyskinesia) occurred during the hospital stay of <10 days at the beginning of the treatment phase. Rescue doses up to 300 mg oral l-dopa were allowed.
Summary of findings
Apomorphine showed no relevant effect on QoL or the motor score in the ON condition. There was a moderate improvement in daily on time without troublesome dyskinesia in the apomorphine group compared with BMT. Similarly, there was a moderate improvement in daily off time in the apomorphine group compared with BMT. AEs and SAEs were more common in the apomorphine group. Apomorphine infusion probably results in a decrease in the daily dosage of anti-PD medication in the short term.
Considerations of the GL task force
Multiple open-label studies confirmed the efficacy of apomorphine in the reduction of daily off time. In addition, some the studies reported a reduction of dyskinesia severity; however, these data from open-label trials have to be interpreted accordingly. Some GL members consider apomorphine infusion the least invasive of the treatments discussed here [72].
Recommendation 6: Consider offering apomorphine pump infusion for people with advanced PD if fluctuations are not satisfactorily controlled with medication (15 voters, 100%).
LESIONAL NEUROSURGICAL THERAPIES
Historically, the first invasive treatment for the treatment of advanced PD was radiofrequency thermocoagulation brain surgery, dating back to the 1950s [13]. In the 1990s, localized lesions with radiosurgery (RS) were proposed [22, 73]. However, radiofrequency thermocoagulation lesions have been progressively abandoned because of better results with DBS or the unavailability of these procedures in many countries [74, 75]. The latest and most advanced lesional but incisionless intervention is MRgFUS with few RCTs [74, 75] and several uncontrolled studies published in the past 10 years. As outlined previously, the brain targets for lesional procedures are similar for all available interventions. Clinical trials for different interventions and targets are evaluated separately in this GL.
RADIOFREQUENCY THERMOCOAGULATION
Pallidotomy with radiofrequency thermocoagulation
The success of Leksells' pallidotomy [18] was one of the reasons for the revival of unilateral pallidotomy, particularly in North America at the turn of the century [76]. However, the evidence for this treatment is weak.
Two unblinded RCTs with 36 and 37 patients were included [77, 78] and compared outcomes for the pallidotomy group with a medical therapy group. QoL was reported in one study, and pallidotomy may improve QoL (PDQL, Parkinson's disease quality of life questionaire). The intervention may slightly improve ADLs (UPDRS-II) and the motor score (UPDRS-III). Pallidotomy probably reduces complications of therapy (UPDRS-IV). SAEs were more common in the pallidotomy group. Pallidotomy may make little or no difference to the Hoehn and Yahr score. The evidence is uncertain if pallidotomy improves depression and gait. Long-term data of these trials have not been published, but case series on pallidotomies have reported positive long-term data for up to 5 years [76, 79-81]. The GL committee concluded that unilateral pallidotomy can be considered as a treatment option for advanced PD with medically intractable treatment complications in the absence of other more efficacious and better established treatment options for the particular patient, but the recommendation is considered very weak.
Recommendation 7: Consider offering unilateral pallidotomy with radiofrequency thermocoagulation to people with advanced PD who experience troublesome fluctuations and for which DBS or pump therapies is not a treatment option (16 voters, 100%).
Thalamotomy with radiofrequency thermocoagulation
This procedure has been used to treat thousands of people with tremor-dominant PD and has mostly been applied unilaterally. Bilateral application had the disadvantage of frequent dysarthria in up to 40% of the patients [82] and it has, therefore, only been applied since the second half of the last century. The higher number of AEs with radiofrequency compared with thalamic DBS for unilateral and particularly for bilateral procedures was shown in a controlled trial [12, 83] and was the main reason that this treatment has mostly been abandoned. This GL group identified no RCTs that fulfill the inclusion criteria, and no recommendations according to the GRADE-methodology are possible. Therefore, the procedure cannot be recommended. However, the GL committee is aware that unilateral procedures are still used for selected indications (eg, patients with repeated infected DBS electrodes) when no other treatment options are available or in countries that have no other interventions available.
Clinical Consensus Statement 1: RCTs for unilateral radiofrequency thermocoagulation of the thalamus for parkinsonian tremor or advanced PD are not available, and formal recommendations are not possible. As DBS has a better safety profile, this GL task force does not recommend this treatment if safer treatments are available (16 voters, 100%).
Lesioning of the STN with radiofrequency thermocoagulation
There is only one larger case series on radiofrequency lesioning of the STN, which concluded acceptable feasibility [84, 85] with an open follow-up [86]. It reported some efficacy but also some cases with ongoing surgically induced dyskinesia. The procedure has never been tested in an RCT [87]. It cannot be recommended by this GL group as a treatment for advanced PD.
Clinical Consensus Statement 2: RCTs for unilateral radiofrequency thermocoagulation of the STN for people with PD are not available. Due to potential high risks for AEs, this GL task force does not recommend this treatment (16 voters, 100%).
RADIOSURGERY WITH GAMMA RADIATION
Treatment with radiosurgery is available in only few centers worldwide, and no RCT has been published with this method for the treatment of advanced PD or tremor of PD. Open-label and prospective collections of case series are available. One study used blinded evaluation outcomes [88]. The occurrence of potentially dangerous adverse effects such as continuing evolving lesions long after the application of the radiation were reported [89]. In contrast to all other interventions, there is no possibility to control the effect of the lesion before it is complete because the destruction of the tissue and subsequent clinical effects due to radiation take days to weeks. Therefore, a reversible test is not possible. We are aware that it is an incisionless procedure that may be needed for rare clinical situations, but other interventions are available and should be preferred.
These facts have led this GL group not to consider it as a treatment option. It is not a recommended option for the treatment of advanced PD, neither as thalamotomy, pallidotomy, nor as a lesioning technique for the STN.
Clinical Consensus Statement 3: RCTs for unilateral gamma radiation radiosurgery of any of the three target nuclei are not available for people with PD. Due to potential high risks for AEs, this GL task force does not recommend this treatment (16 voters, 100%).
MAGNETIC RESONANCE IMAGING–GUIDED FOCUSED ULTRASOUND LESIONING
Unilateral thalamotomy with MRgFUS
One MRgFUS system is approved in Europe (CE mark for essential tremor, PD tremor, and neuropathic pain since 2012) [90] and in the United States for treating essential tremor (since 2016) [91]. Its use in parkinsonian tremor is based on a study with 20 patients receiving active treatment and seven receiving sham treatment [75]. As the latter number is below the threshold required for this GL, the study was excluded. There are several additional case series in people with PD with tremor [92, 93].
Although promising, this GL does currently not recommend the treatment because of the lack of appropriate data. Further studies are needed.
Clinical consensus statement 4: No sufficient RCTs available for uni- or bilateral MRgFUS of the thalamus for medically resistant tremor in PD. Despite promising preliminary data, this treatment should only be applied within clinical studies or registries (16 voters, 100%).
Unilateral pallidotomy with MRgFUS
Only one small case series has been published so far [94]. These GLs do not recommend the treatment because appropriate data are lacking.
Clinical consensus statement 5: Do not use MRgFUS of the pallidum for advanced PD with fluctuations outside clinical studies (16 voters, 100%).
Unilateral lesioning of the STN with MRgFUS (MRgFUS subthalamotomy)
In a controlled trial [95], 27 patients were randomly assigned to unilateral subthalamotomy and 13 to sham treatment. The patients were relatively young (57.1 ± 9.1 years) and had a relatively short disease duration (6.2 ± 3.0 years) and pronounced unilateral disease.
Summary of findings
Focused ultrasound subthalamotomy may improve QoL (PDQ-39), probably improves ADLs (UPDRS-II), and probably improves the motor score (UPDRS-III). It may also reduce complications of therapy (UPDRS-IV). No information was available for the following outcomes: the total UPDRS, cognition, depression, apathy, impulsive–compulsive behaviors, gait, and speech. Focused ultrasound may result in a decrease in daily dosage of anti-PD medication in the short term.
Short-term AEs were more common in the subthalamotomy group, including dyskinesia in the off medication state, weakness of the treated body side, facial asymmetry, speech disturbance, gait disequilibrium, somnolence, and binge eating. At 12 months, one patient still had speech disturbance and one patient had unsteadiness. Intraprocedural AEs were headache and dizziness that resolved after 1 day. There were neither intracerebral hemorrhages nor infections. So far only 1-year data are available.
Considerations of the GL task force
This treatment is new, and only one RCT is available. The results are promising regarding the standard outcomes for advanced PD. The AEs are frequent, but longer term sequela are mild and rare. Many key questions, however, remain open regarding this treatment: Long-term data beyond 1 year are lacking. The treatment was applied unilaterally in a highly selected group of people with unilaterally dominant PD. Therefore, preliminary data suggesting that MRgFUS may be cost-effective compared with DBS should be interpreted with caution [96]. The majority of people with advanced PD have bilateral disease, but it is unknown whether MRgFUS subthalamotomy can be safely and efficiently performed bilaterally.
Despite initial promising results, currently the treatment cannot be recommended outside clinical studies.
Recommendation 8: Consider using unilateral MRgFUS of the STN in people with distinctly unilateral PD only within clinical studies or registries due to the limited data on this new treatment (16 voters, 100%).
FUTURE DEVELOPMENTS
These GLs is the first to evaluate all currently available invasive treatments for PD and is part of a series that will cover all the other treatment options and essential diagnostic procedures for PD. There is only limited evidence for several of the invasive treatments for PD. Using the rigorous GRADE methodology, the GLs only consider RCTs for evaluation but describe the spectrum of approved interventions. Economic evaluations of the treatment options are not yet included and will likely differ between regions with different health care systems and the large variation in the availability and costs of these treatments. However, the EAN is working on including this in the future. A number of careful studies are available that compare the costs for the different treatments for specific countries [35, 96-101].
Several invasive interventions for PD have undergone a rapid development, with DBS of the STN and GPi being established treatments for the improvement of motor symptoms and health-related QoL [20]. Further questions on the use of DBS for psychosocial impact and nonmotor symptoms in PD as well as the possible usefulness for axial abnormalities still need to be answered. Limited research activity is available for radiosurgery for PD [102]. No randomized controlled studies on lesional procedures with radiofrequency thermocoagulation have been published, and this treatment may remain a last resort treatment for special cases in the hands of experienced specialized functional neurosurgeons. Research and use of MRgFUS is currently rapidly developing, but important questions are still open [23]. One conceivable indication for this intervention is treatment-resistant parkinsonian tremor, but the first focused ultrasound thalamotomy trial failed the threshold of this GL because the study was underpowered. Infusion therapies are another active field of research, and other new forms of less-invasive interventions are currently being developed. New trials may also explore the treatment of other, particularly nonmotor, symptoms of PD.
The invasive treatments discussed should only be used for appropriately selected patients, but in those can profoundly change the lives of people with PD.
AUTHOR CONTRIBUTIONS
Guenther Deuschl: Conceptualization (lead); investigation (lead); methodology (lead); writing – original draft (lead). Angelo Antonini: Formal analysis (equal); investigation (lead); methodology (equal); validation (lead); writing – review and editing (equal). João Costa: Conceptualization (equal); data curation (equal); investigation (equal); methodology (lead); writing – review and editing (equal). Katarzyna Smilowska: Data curation (equal); formal analysis (lead); methodology (lead); project administration (equal); validation (lead); writing – review and editing (equal). Daniela Berg: Formal analysis (equal); writing – review and editing (equal). Jean-Christophe Corvol: Formal analysis (equal); methodology (equal); writing – review and editing (equal). Giovanni Fabbrini: Formal analysis (equal); methodology (equal); writing – review and editing (equal). Joaquim J Ferreira: Formal analysis (equal); methodology (equal); writing – review and editing (equal). Thomas Foltynie: Formal analysis (equal); methodology (equal); writing – review and editing (equal). Pablo Mir: Formal analysis (equal); methodology (equal); writing – review and editing (equal). Anette Schrag: Formal analysis (equal); methodology (equal); writing – review and editing (equal). Klaus Seppi: Formal analysis (equal); methodology (equal); writing – review and editing (equal). Pille Taba: Formal analysis (equal); methodology (equal); writing – review and editing (equal). Evzen Ruzicka: Formal analysis (equal); methodology (equal); writing – review and editing (equal). M. Selikhova: Formal analysis (equal); methodology (equal); writing – review and editing (equal). Nicolas Henschke: Formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); writing – review and editing (equal). Gemma Villanueva: Data curation (equal); formal analysis (equal); methodology (equal); project administration (equal); validation (equal); writing – review and editing (equal). Elena Moro: Data curation (lead); investigation (lead); methodology (equal); writing – review and editing (equal).
ACKNOWLEDGMENTS
Cochrane Response was contracted by European Academy of Neurology/Movement Disorder Society, European Section (EAN/MDS-ES) to conduct the systematic review. The GL development was further supported by travel grants for meetings and support for online meetings by EAN/MDS-ES. GL team members were not paid. Katarzyna Śmiłowska was supported with a stipend of the Medical Faculty of the Christian-Albrechts-University, Kiel. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
G. Deuschl reports a grant from Medtronic for the Earlystim study; minor fees from Boston Scientific, Aleva, Functional Neuromodulation, and Roche for review and DSMB activities; and minor royalties from Thieme Publishers. A. Antonini reports a major grant from Abbvie. He has received honoraria for talks sponsored by Abbvie and has served on advisory boards for Abbvie. D. Berg reports grants from Abbvie, Alexion Pharma, Alnylam, Apopharma Inc., Biogen, BMWi, BMBF, Christa und Peter Thomsen Foundation, Coppenrath Foundation, Dapm Foundation, Desitin, EU, Else-Kröner-Fresenius Foundation, Gossweiler Foundation, Hoffmann La Roche AG, Icon Ltd + Biohaven Inc., Janssen Pharmaceutica N.V., Jan von Appen Foundation, Lundbeck, Merck Serono, Parkinson Fonds Deutschland GmbH, Pfizer, Roche AG, Sanofi, Sivantos GmbH, Stichting Parkinson Fonds, UCB, and Zambon. She received payments for lectures from Abbvie, Bial, Biogen, Desitin, Movement Disorder Society (MDS), GE Healthcare, Feo GmbH, Nansen Neuroscience Network, Parkinson bewegt e.V., UCB Pharma GmbH, and Zambon. J.-C. Corvol received honoraria for talks sponsored by Ever Pharma. J. Ferreira reports grants from Abbvie, Novartis, Medtronic, and Fundação MSD. He received payment for lectures from Bial, Abbvie, and Ipsen and has served on an advisory board for Bial. He reports consultancies for Bial, Abbvie, Lundbeck, Biogen, Sunovion Pharmaceuticals, and Affiris. T. Foltynie has received grants from the National Institute of Health Research, Edmond J Safra Foundation, The Michael J. Fox Foundation (MJFF), John Black Charitable Foundation, Cure Parkinson's Trust, Innovate UK, Janet Owens Research Fellowship, Rosetrees Trust, Van Andel Research Institute, and Defeat MSA. He has served on advisory boards for Peptron, Voyager Therapeutics, Handl therapeutics, Living Cell Technologies, Bial, and Profile Pharma and has received honoraria for talks sponsored by Bial, Profile Pharma, and Boston Scientific. P. Mir has received grants from the Spanish Ministry of Science and Innovation, the Instituto de Salud Carlos III-Fondo Europeo de Desarrollo Regional, the Consejería de Economía, Innovación, Ciencia y Empleo de la Junta de Andalucía, and the Consejería de Salud y Bienestar Social de la Junta de Andalucía and he has received honoraria for lecturing from Abbott, Allergan, Abbvie, Bial, Britannia, Italfarmaco, Merz, UCB, Roche, Teva, and Zambon. A. Schrag has received grants from GE Healthcare; consulted for Abbvie, Biogen, Roche, Neurotechnology, Biogen, and Bial; and has received honoraria and royalties from Oxford University Press. K. Seppi reports consultancies for Abbott, Stada, Grunenthal, AOP Orphan, Bial, Lundbeck, Roche, and Biogen and grants from AOP Orphan, MJFF, and FWF Austrian Science Fund. He received payment for lectures from AOP Orphan, Abbvie, Stada, Grunenthal, Ever Pharma, Licher Pharma, MDS, UCB, and Teva. N. Henschke and G. Villanueva work for Cochrane Response, an evidence services unit operated by Cochrane, and have no known conflicts of interest. E. Moro reports consultancies for Abbott, Medtronic, and Kyowa and major grants from Medtronic and Ipsen. She received payment for lectures from Abbott and Medtronic. J. Costa, K. Smilowska, P. Taba, E. Ruzicka, M. Selikhova, and G. Fabbrini declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.





