European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force—Second revision
This article is co-published by the European Journal of Neurology and the Journal of the Peripheral Nervous System.
See editorial by J.-M. Vallat et al. on page 3545
Abstract
Objective
To revise the 2010 consensus guideline on chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).
Methods
Seventeen disease experts, a patient representative, and two Cochrane methodologists constructed 12 Population/Intervention/Comparison/Outcome (PICO) questions regarding diagnosis and treatment to guide the literature search. Data were extracted and summarized in GRADE summary of findings (for treatment PICOs) or evidence tables (for diagnostic PICOs).
Results
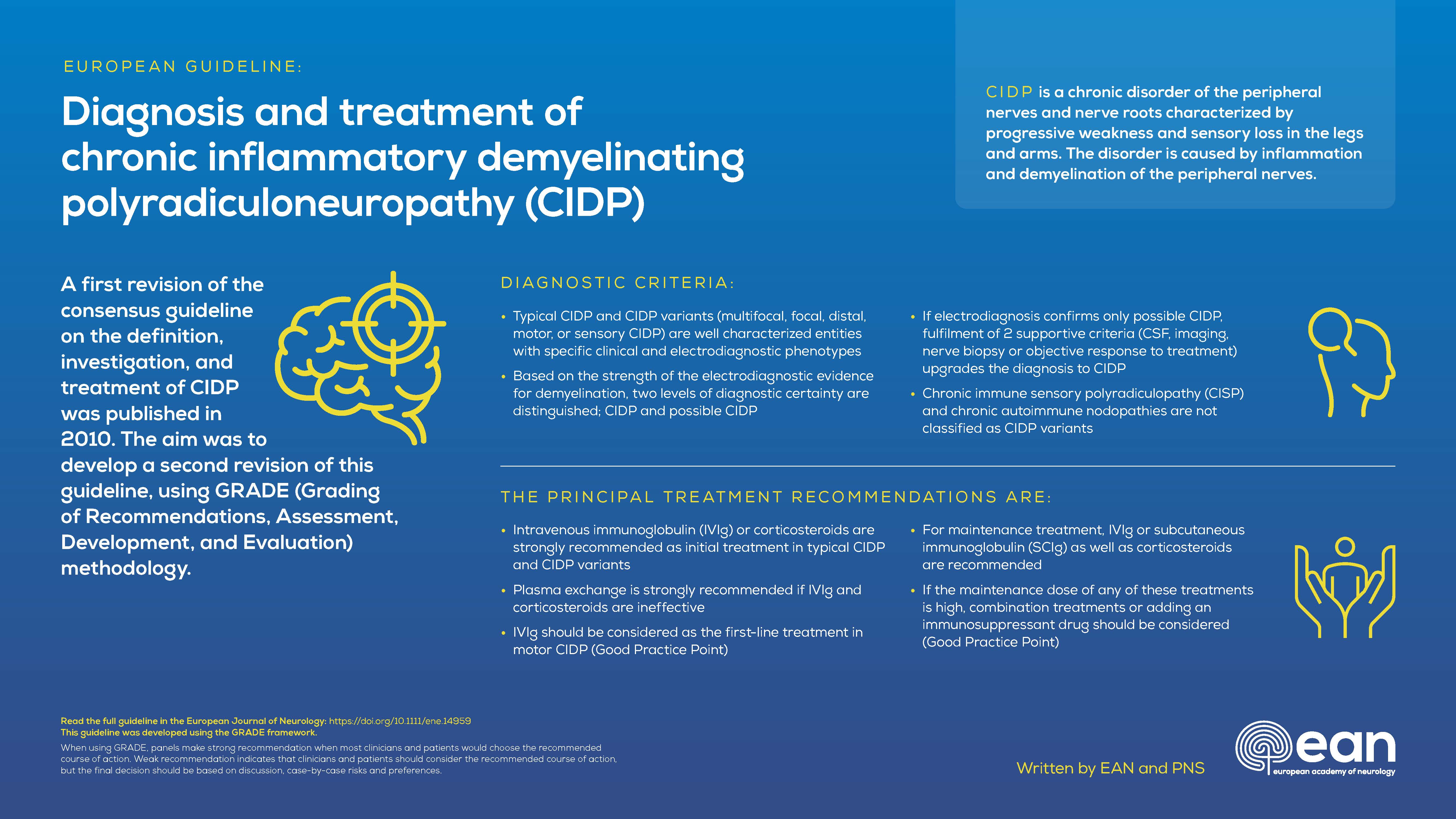
Statements were prepared according to the GRADE Evidence-to-Decision frameworks. Typical CIDP and CIDP variants were distinguished. The previous term “atypical CIDP” was replaced by “CIDP variants” because these are well characterized entities (multifocal, focal, distal, motor, or sensory CIDP). The levels of diagnostic certainty were reduced from three (definite, probable, possible CIDP) to only two (CIDP and possible CIDP), because the diagnostic accuracy of criteria for probable and definite CIDP did not significantly differ. Good Practice Points were formulated for supportive criteria and investigations to be considered to diagnose CIDP. The principal treatment recommendations were: (a) intravenous immunoglobulin (IVIg) or corticosteroids are strongly recommended as initial treatment in typical CIDP and CIDP variants; (b) plasma exchange is strongly recommended if IVIg and corticosteroids are ineffective; (c) IVIg should be considered as first-line treatment in motor CIDP (Good Practice Point); (d) for maintenance treatment, IVIg, subcutaneous immunoglobulin or corticosteroids are recommended; (e) if the maintenance dose of any of these is high, consider either combination treatments or adding an immunosuppressant or immunomodulatory drug (Good Practice Point); and (f) if pain is present, consider drugs against neuropathic pain and multidisciplinary management (Good Practice Point).
OBJECTIVES AND SCOPE
The EFNS/PNS consensus guideline on the diagnosis and management of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) was published first in 2005 [1, 2] and revised in 2010 [3, 4]. The aim of this second revision is to update the 2010 guideline according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology [5] and to formulate evidence-based recommendations and consensus-based Good Practice Points for clinical practice. The target population for the diagnostic part consists of patients of any age, presenting with clinical features suggestive of CIDP. Patients with any comorbidity are considered excluding those with a confirmed alternative cause of their neuropathy. The treatment recommendations apply to patients diagnosed with CIDP. This guideline revision is intended for neurologists and paediatric neurologists in secondary and tertiary care settings. The aim is to optimise diagnostic accuracy and to improve patient outcomes.
BACKGROUND
The diagnosis of CIDP rests upon a combination of clinical, electrodiagnostic, and laboratory features with exclusions to eliminate other disorders that may mimic CIDP. Criteria for CIDP have been most closely linked to electrodiagnostic criteria for detection of peripheral nerve demyelination. Comparison of different published diagnostic criteria sets for CIDP showed that the 2010 EFNS/PNS guideline criteria [3, 4] have very good diagnostic accuracy [6-8]. World-wide acceptance and use of these criteria in CIDP research have been documented [9]. Nevertheless, misdiagnosis commonly occurs, particularly in those classified as CIDP variants [10-12]. Although this may be related to errors in the interpretation of diagnostic test results [11, 13] and to non-compliance or lack of awareness of guidelines [14], some patients fulfilling diagnostic criteria based on correctly interpreted test results do not have CIDP [10, 13]. The current guideline revision attempts to improve specificity of the criteria. The evidence from randomized clinical therapeutic trials has significantly increased since 2010 and allows evidence-based recommendations about treatments according to GRADE.
METHODS
The methodology for the development of this guideline followed the frameworks provided by AGREE II [15] and GRADE [5], and the recommendations of the EAN on the development of a neurological management guideline [16]. Twelve research questions were constructed in the Population/Intervention/Comparison/Outcome question (PICO) format during a kick-off meeting in March 2018 (Box 1). The following databases were searched for identification of eligible studies for each PICO, according to predefined selection criteria: Medline, via the PubMed interface; Embase, via the embase.com interface; the Cochrane Library, consisting of the Cochrane Database of Systematic Reviews; the Database of Abstracts of Reviews (DARE); and the Cochrane Central Register of Controlled Clinical Trials. The literature search for each PICO was conducted between June 2018 and July 2019 without restrictions regarding publication date. The Task Force (TF) additionally included relevant papers published during the preparation of this Guideline. Unpublished data known to the TF was not used. Data were extracted and summarized in GRADE summary of findings tables (treatment PICOs) or evidence tables (diagnostic PICOs). To reach consensus, the TF members prepared draft statements about definition, diagnosis, and treatment, according to the elements of the GRADE Evidence-to-Decision frameworks [17, 18]. The TF made a strong recommendation (for or against an intervention) when it judged that almost all informed people would make the recommended choice [19]. A weak recommendation was made when it judged that most informed people would choose the recommended course of action, but a substantial number would not, either because it was applicable (or available) only to a subgroup, or the evidence had low certainty, or the risk/benefit ratio might not be favourable for all patients. For diagnostic PICOs, a formal GRADE approach to all evidence was not considered useful, because of limited evidence. The TF reached consensus and offered advice as Good Practice Points [20]. Only PICO 1 on electrodiagnosis was subjected to GRADE, which led to the decision to treat the other diagnostic PICOs as consensus-based PICOs, supported by a systematic literature search without formal GRADE assessment. The recommendations and Good Practice Points were revised and collated into a single document, which was then revised iteratively by the TF until consensus was reached. The patient representative from the GBS/CIDP Foundation International reviewed all recommendations and Good Practice Points and participated in consensus votes in her capacity as TF member. A detailed protocol of the guideline development can be found in supporting information. It is planned to update the guideline every 5 years.
BOX 1. Population/Intervention/Comparison/Outcome questions (PICOs)
DIAGNOSTIC PICOS (systematic literature search and consensus—except GRADE for PICO 1)
PICO 1. Electrodiagnosis—In patients with suspected CIDP, does the use of electrophysiology/electrodiagnosis (motor and sensory nerve conduction studies, somatosensory evoked potentials, root stimulation, triple stimulation technique, nerve excitability studies, and electromyography), compared to not using electrodiagnosis, influence diagnostic accuracy and patient outcome?
PICO 2. Response to treatment as diagnostic criterion—In patients with suspected CIDP, does the use of patients' response to treatment (subjective vs objective), compared to not considering response to treatment, influence diagnostic accuracy, and patient outcome?
PICO 3. MRI or ultrasound—In patients with suspected CIDP, does the use of imaging—MRI (thickening or abnormal enhancement of cervical/lumbar nerve roots or brachial/lumbar plexus) or nerve ultrasound (increased cross-sectional area of peripheral nerves or roots compared with normal values), compared to no imaging, influence diagnostic accuracy and patient outcome (treatment response and clinical course)?
PICO 4. CSF—In patients with suspected CIDP, does the use of CSF examination compared to not using CSF examination, influence diagnostic accuracy and patient outcome? Are thresholds for raised protein different in children <16 years old or in any patient, or in subgroups with diabetes, or previous spinal surgery?
PICO 5. Antibodies—In patient with suspected CIDP, does testing for the presence of serum auto-antibodies, including anti-nodal and paranodal antibodies (contactin1, contactin1/contactin-associated protein1 complex, neurofascin155, neurofascin140/neurofascin186, contactin-associated protein1), anti-ganglioside antibodies, and anti-MAG antibodies, compared to not testing for antibodies, influence diagnostic accuracy and patient outcome?
PICO 6. Nerve biopsy—In patients with suspected CIDP, does nerve biopsy (looking for macrophage-associated demyelination, onion bulb formation, demyelinated and to a lesser extent remyelinated nerve fibres, endoneurial oedema, endoneurial mononuclear cell infiltration, loss of transverse bands or paranodal loop detachment, teased fibre analysis), compared to no nerve biopsy, influence diagnostic accuracy and patient outcome?
PICO 7. Monoclonal gammopathies—In patient with suspected CIDP, does testing for the presence of IgG, IgA, IgM, or light chain monoclonal gammopathies, compared with not testing for monoclonal gammopathies and patient outcome?
TREATMENT PICOS (systematic literature search and GRADE - except consensus for PICO 12)
PICO 8. Corticosteroids—In patients with CIDP, does treatment with corticosteroids, compared to no treatment with corticosteroids or corticosteroids in a different dose/timing influence impairment, disability, and quality of life? Are treatment effects different in CIDP variants and in children (<16 years)?
PICO 9. Immunoglobulin—In patients with CIDP, does treatment with IV or SC immunoglobulins, compared to no treatment with immunoglobulins or immunoglobulins in a different dose/timing, influence impairment, disability, and quality of life? Are treatment effects different in CIDP variants and in children (<16 years)?
PICO 10. Plasma exchange—In patients with CIDP, does treatment with plasma exchange, compared to no treatment with plasma exchange or plasma exchange in a different dose/timing, influence impairment, disability, and quality of life? Are treatment effects different in children (<16 years)?
PICO 11. Other immune treatments—In patients with CIDP, does treatment with immunomodulatory drugs other than corticosteroids, immunoglobulins and plasma exchange, compared to no treatment with immunomodulatory drugs or immunomodulatory drugs in a different dose/timing, influence impairment, disability, and quality of life? Are treatment effects different in children (<16 years)?
PICO 12. Pain treatment—In patients with CIDP, do drugs for pain relief (anti-epileptic, antidepressant, opiates or opiate analogues, cannabinoids, acetaminophen, NSAIDs or other typical or atypical analgesia), compared to no pain relief or other analgesia influence pain, fatigue, and quality of life?
RESULTS
Diagnostic criteria for CIDP
Clinical criteria
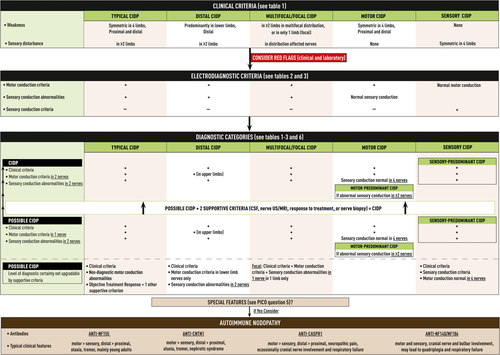
The TF refined the clinical criteria for defining CIDP into “typical CIDP” and “CIDP variants”. Since they are more a matter of definition than research questions, these criteria are formulated as consensus expert opinion. The TF replaced the label “atypical CIDP,” used in the 2010 EFNS/PNS guideline [3, 4], by “CIDP variants” because these are now well characterized entities, each presenting with a specific clinical and electrodiagnostic phenotype (Table 1, Flowchart 1).
| Typical CIDP |
| All the following: |
|
|
|
| CIDP variants |
| One of the following, but otherwise as in typical CIDP (tendon reflexes may be normal in unaffected limbs): |
|
|
|
|
|
- Abbreviation: CIDP, chronic inflammatory demyelinating polyradiculoneuropathy.
Typical CIDP
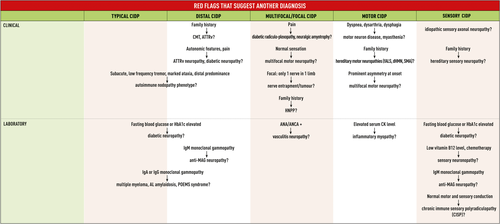
Most commonly, the disease begins with paraesthesia and weakness in the distal limbs as well as difficulty walking. The clinical examination shows progressive symmetric proximal and distal muscle weakness, sensory loss, and decreased or absent deep tendon reflexes. The disease course is steadily progressive for more than 8 weeks, but can be relapsing-remitting. In contrast with Guillain-Barré syndrome (GBS), cranial nerves are less frequently affected and respiratory [21, 22] or autonomic involvement is exceptional [23-26]. Typical CIDP is more common in males and can occur at any age, but most commonly between 40 and 60 years. Onset during infancy and childhood can occur [27-30]. Typical CIDP may present acutely (acute-onset CIDP [A-CIDP]) in up to 13% of patients, who rapidly progress within 4 weeks and initially may be diagnosed with GBS [31, 32]. Therefore, distinguishing A-CIDP from GBS can be challenging as 5% of patients initially diagnosed with GBS are later reclassified as A-CIDP [32]. In contrast with GBS patients, A-CIDP patients continue to deteriorate more than 8 weeks after onset or do relapse at least three times after initial improvement. Often, A-CIDP patients remain able to walk independently, are less likely to have facial weakness, respiratory or autonomic nervous system involvement, and are more likely to have sensory signs [32, 33]. Although these features may favour the diagnosis of A-CIDP, there are no specific clinical features or laboratory tests that can distinguish GBS from A-CIDP in the acute stage of the disease.
CIDP variants
- Distal CIDP, also known as distal acquired demyelinating symmetric neuropathy [37], presents with sensory loss in the distal upper and lower limbs as well as gait instability. Weakness may occur and is usually distally accentuated in lower more than upper limbs. Approximately two thirds of patients with this phenotype have IgM paraproteinaemic neuropathy, often with antibodies against myelin-associated glycoprotein (MAG) [38-40]. Distal neuropathy with an IgM paraprotein and anti-MAG antibodies, anti-MAG neuropathy, is considered outside the scope of CIDP as the majority of patients have specific electrodiagnostic and pathologic findings and do not respond to intravenous immunoglobulin (IVIg) or corticosteroids.
- Multifocal CIDP (synonyms: multifocal demyelinating neuropathy with persistent conduction block, Lewis-Sumner syndrome [LSS] [41]; multifocal acquired demyelinating sensory and motor neuropathy [MADSAM] [42]; multifocal inflammatory demyelinating neuropathy [43]) usually affects the upper limbs first. Lower limbs may become involved later or sometimes are affected from the onset. [42, 43] Cranial nerves, including oculomotor, trigeminal, facial, vagal, and hypoglossal nerves, are probably more frequently involved than in other CIDP forms [38, 44-49].
- Focal CIDP is rare and usually affects the brachial or lumbosacral plexus, but can affect individual peripheral nerves as well [50, 51].
- Motor CIDP presents as relatively symmetric proximal and distal weakness but with normal sensation clinically and electrodiagnostically [52, 53]. This is in contrast to both typical CIDP, where sensation is abnormal, and multifocal motor neuropathy (MMN), where the pattern of weakness is asymmetric and mainly affecting the upper limbs [54]. If sensory nerve conduction is abnormal in clinically motor CIDP [55], the diagnosis is motor-predominant CIDP. Patients with motor CIDP may deteriorate after corticosteroids (PICO 8) [36, 52, 55, 56].
- Sensory CIDP is usually characterized by gait ataxia, impairment of vibration and position sense and changes in cutaneous sensation [35, 57, 58]. By definition, muscle weakness is not present. If motor nerve conduction slowing or motor conduction block are present [57, 59, 60], the diagnosis is sensory-predominant CIDP. Long-term follow-up studies have shown that sensory CIDP is often a transient clinical stage that precedes the appearance of weakness in about 70% of patients [36, 61].
Disorders not classified as CIDP
Chronic immune sensory polyradiculopathy (CISP): Patients suspected to have clinically sensory CIDP, but with normal motor and sensory nerve conduction studies may have CISP [62-64]. Somatosensory evoked potentials may be absent or show very proximal slowing in CISP because sensory axons proximal to the dorsal root ganglia are affected. Because the sensory neurons in the dorsal root ganglia remain intact, standard sensory nerve conduction studies are normal. Although most likely immune-mediated and responding to immune treatment, there is not enough evidence to determine if CISP is demyelinating or related to sensory CIDP, and has therefore not been included in the CIDP variant classification (see Flowchart 2).
Autoimmune nodopathies: Antibodies against nodal-paranodal cell-adhesion molecules (contactin-1 [CNTN1], neurofascin-155 [NF155], contactin-associated protein 1 [Caspr1], and neurofascin isoforms NF140/186) have been discovered in a small subset of patients fulfilling 2010 EFNS/PNS criteria for CIDP [3, 4] (PICO 5, Flowchart 1). Patients with these antibodies often have specific clinical characteristics [65, 66]. Antibodies against CNTN1 were reported in patients diagnosed with CIDP, who presented with acute or subacute disease onset, motor or ataxic features, and had no or poor response to IVIg treatment [67-69]. Antibodies against NF155 were observed in patients diagnosed with CIDP who were younger at onset, and had a subacute or chronic disease course, distal weakness, ataxia, tremor, and no or poor response to IVIg treatment [70-72]. Antibodies against Caspr1 present as an acute/subacute neuropathy frequently associated with ataxia, neuropathic pain, cranial nerve involvement and poor response to IVIg [73-75]. Antibodies to all neurofascin isoforms lead to a severe phenotype, in particular when of the IgG3 isotype [76, 77]. The TF proposed to name these conditions “auto-immune nodopathies” and not to regard them as CIDP variants because they have distinct clinical features, no overt inflammation or macrophage-mediated demyelination [68, 78, 79] and do poorly respond to CIDP treatment, IVIg in particular. Rituximab, however, may be effective [73, 76, 80].
CIDP has been associated with numerous conditions (eg, diabetes mellitus, IgG or IgA monoclonal gammopathy of undetermined significance [MGUS], IgM monoclonal gammopathy without antibodies to MAG, HIV infection, malignancies) [81]. There is insufficient evidence to consider CIDP associated with these diseases different from idiopathic CIDP. In some cases, CIDP may occur as an immune-related adverse event induced by drugs or biologics [82-84]. In those cases, most physicians would stop the drug/biologic but this decision should be based on the individual clinical situation. In most published reports, treatment has not differed from that used in idiopathic CIDP. The differential diagnosis of typical CIDP and CIDP variants is extensive and needs to be carefully addressed by appropriate investigations (Tables 4 and 5, Flowchart 2).
Electrodiagnostic criteria (PICO 1)
The TF strongly recommended electrodiagnosis (nerve conduction studies) to support the clinical diagnosis of typical CIDP and CIDP variants (Tables 2 and 3). The TF decided to reduce the levels of electrodiagnostic certainty, as used in the 2010 EFNS/PNS guideline [3, 4], from three (definite, probable, possible CIDP) to only two (CIDP and possible CIDP), because of empirical evidence showing that the sensitivity and specificity of electrodiagnostic criteria for probable and definite CIDP do not significantly differ [8, 92]. Since there is no gold standard for the diagnosis of CIDP, the TF decided to avoid the label “definite CIDP.” The TF decided to require not only motor but also sensory conduction studies to define the diagnostic categories of typical CIDP and CIDP variants (Table 6, Flowchart 1).
| (1) Strongly supportive of demyelination: |
| At least one of the following: |
| (a) Motor distal latency prolongation ≥50% above ULN in two nerves (excluding median neuropathy at the wrist from carpal tunnel syndrome), or |
| (b) Reduction of motor conduction velocity ≥30% below LLN in two nerves, or |
| (c) Prolongation of F-wave latency ≥20% above ULN in two nerves (≥50% if amplitude of distal negative peak CMAP <80% of LLN), or |
| (d) Absence of F-waves in two nerves (if these nerves have distal negative peak CMAP amplitudes ≥20% of LLN) + ≥1 other demyelinating parametera in ≥1 other nerve, or |
| (e) Motor conduction block: ≥30% reduction of the proximal relative to distal negative peak CMAP amplitude, excluding the tibial nerve, and distal negative peak CMAP amplitude ≥20% of LLN in two nerves; or in one nerve + ≥ 1 other demyelinating parametera except absence of F-waves in ≥1 other nerve, or |
| (f) Abnormal temporal dispersion: >30% duration increase between the proximal and distal negative peak CMAP (at least 100% in the tibial nerve) in ≥2 nerves, or |
| (g) Distal CMAP duration (interval between onset of the first negative peak and return to baseline of the last negative peak) prolongation in ≥1 nerveb + ≥1 other demyelinating parametera in ≥1 other nerve |
| • (LFF 2 Hz) median > 8.4 ms, ulnar > 9.6 ms, peroneal > 8.8 ms, tibial > 9.2 ms |
| • (LFF 5 Hz) median > 8.0 ms, ulnar > 8.6 ms, peroneal > 8.5 ms, tibial > 8.3 ms |
| • (LFF 10 Hz) median > 7.8 ms, ulnar > 8.5 ms, peroneal > 8.3 ms, tibial > 8.2 ms |
| • (LFF 20 Hz) median > 7.4 ms, ulnar > 7.8 ms, peroneal > 8.1 ms, tibial > 8.0 ms |
| (2) Weakly supportive of demyelination |
| As in (1) but in only one nerve. |
- Note 1. These criteria have been established by using a frequency filter bandpass of 2 Hz to 10 kHz for all parameters, except for distal CMAP duration prolongation where separate criteria were defined for four different LFFs of 2, 5, 10, and 20 Hz. Skin temperature should be maintained to at least 33°C at the palm and 30°C at the external malleolus.
- Note 2. Extensiveness of motor nerve conduction studies (number of nerves to be studied and proximal studies): • To apply motor nerve conduction criteria, the median, ulnar (stimulated below the elbow), peroneal (stimulated below the fibular head), and tibial nerves on one side are tested. • If criteria are not fulfilled, the same nerves are tested at the other side, and/or the ulnar and median nerves are stimulated at the axilla and at Erb's point. • Motor conduction block or slowing is not considered in the ulnar nerve across the elbow or the peroneal nerve across the knee. • Between Erb's point and the wrist, at least 50% CMAP amplitude reduction is required for conduction block in the ulnar and median nerves. Proximal studies of the median nerve may require collision techniques to avoid ulnar nerve components in the median nerve CMAP when recorded from the abductor pollicis brevis muscle (but not when recorded from the flexor carpi radialis muscle) [3, 4, 49, 85, 86]. • For ulnar motor conduction block in the forearm, a Martin-Gruber anastomosis should be ruled out with stimulation of the median nerve at the elbow recording over the abductor digiti minimi muscle. • For median motor conduction block in the forearm, co-stimulation of the ulnar nerve at the wrist must be ruled out. Stimulation of the median nerve at the wrist while simultaneously recording over the abductor pollicis brevis muscle and the abductor digiti minimi muscle can detect ulnar nerve co-stimulation; stimulation should be adapted so that no CMAP is recorded from the ulnar nerve-innervated abductor digiti minimi muscle. • If distal CMAP amplitudes are severely reduced (<1 mV), recording from more proximal muscles innervated by the peroneal, median, ulnar or radial nerve may be attempted to demonstrate motor nerve conduction abnormalities meeting electrodiagnostic criteria.
- Abbreviations: CMAP, compound muscle action potential; LFF, low frequency filter; LLN, lower limit of normal values; ULN, upper limit of normal values.
- a Any nerve meeting any of the criteria (a-g).
- b Mitsuma et al. [87]
| (1) CIDP |
|
| (2) Possible CIDP |
|
|
- Note 1. Skin temperature should be maintained to at least 33°C at the palm and 30°C at the external malleolus. 1. Since these criteria do not permit to identify normal reference values compatible with sensory nerve demyelination, sensory CIDP cannot be more than a possible diagnosis as based on clinical and electrophysiological criteria.
- Note 2. Decline in sural nerve action potential amplitude occurs with age and use of age-dependent reference values after age 60 is advised [91].
- Abbreviations: CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; LLN, lower limit of normal; SNAP, sensory nerve action potential. [Correction added on 18 December 2021 after first online publication: The term ‘but in only one nerve’ has been removed under the subheading ‘Possible CIDP’ in Table 3 in this version.]
Recommendation 1—Typical CIDP
- To confirm the clinical diagnosis of typical CIDP, at least two motor nerves must have abnormalities which fulfil the motor conduction criteria. If criteria are fulfilled in only one nerve, the diagnosis is possible typical CIDP.
- Sensory conduction abnormalities must be present in at least two nerves.
- In patients suspected of having typical CIDP because they fulfil clinical criteria but not minimal electrodiagnostic criteria, the diagnosis of possible typical CIDP may be made if there is objective improvement following treatment with IVIg, corticosteroids or plasma exchange and if at least one additional supportive criterion (PICO 2-4, 6) is fulfilled.
Recommendation 2—Distal CIDP
- Motor conduction criteria fulfilment is required in at least two upper limb nerves to confirm the clinical diagnosis of distal CIDP. The distal negative peak CMAP amplitude should be at least 1 mV. When criteria are fulfilled in two lower limb but not upper limb nerves or if criteria are fulfilled in only one upper limb nerve, the maximum diagnostic certainty is possible distal CIDP.
- Sensory conduction abnormalities must be present in at least two nerves.
Recommendation 3—Multifocal and focal CIDP
- Motor conduction criteria fulfilment is required in at least two nerves in total in more than one limb to confirm the clinical diagnosis of multifocal CIDP and in at least two nerves in one limb for the diagnosis of focal CIDP. When criteria are fulfilled in only one nerve, the maximum diagnostic certainty is possible multifocal or possible focal CIDP.
- Sensory conduction abnormalities must be present in at least two nerves of the affected limbs for the diagnosis of multifocal or focal CIDP and in one nerve of the affected limb for the diagnosis of possible focal CIDP.
Recommendation 4—Motor CIDP (and motor-predominant CIDP)
- Motor CIDP must fulfil motor conduction criteria in at least two nerves and sensory conduction must be normal in all of at least four nerves (median, ulnar, radial, and sural) to confirm the clinical diagnosis of motor CIDP. If criteria are fulfilled in only one motor nerve, the diagnosis is possible motor CIDP.
- Motor CIDP with sensory conduction abnormalities in two nerves is diagnosed as motor-predominant CIDP.
Recommendation 5—Sensory CIDP (and sensory-predominant CIDP)
- Sensory CIDP must fulfil sensory conduction criteria and motor conduction must be normal in all of at least four nerves (median, ulnar, peroneal, and tibial) to confirm the clinical diagnosis. The maximum diagnostic certainty is possible sensory CIDP.
- Sensory CIDP with motor conduction criteria fulfilled in one nerve is diagnosed as possible sensory-predominant CIDP. If motor conduction criteria are fulfilled in two nerves, the diagnostic certainty increases to sensory-predominant CIDP.
Considerations supporting the Recommendations (supporting information)
Evidence summary: Data extracted from 38 cohort studies assessing the usefulness of a total of 27 electrodiagnostic parameters or criteria sets were subjected to GRADE analysis. The certainty of the evidence of effect estimates was low to very low for all outcomes.
Rationale: The recommendation of the TF for electrodiagnostic testing in patients with clinically suspected CIDP is based on the very good diagnostic accuracy of 2010 EFNS/PNS electrodiagnostic criteria [3, 4] with high sensitivity/specificity for CIDP of 95%/96% [6], 81%/96% [7], and 73%/91% [8] reported in different patient populations. The advantages of electrodiagnostic testing include the long history of clinical experience, availability, inexpensiveness, and low burden for the patient. The TF expanded the 2010 EFNS/PNS electrodiagnostic criteria [3, 4] by including sensory nerve conduction studies and by defining criteria specific for CIDP variants (Tables 2 and 3). Since up to 20% of patients with clinically typical CIDP do not fulfil minimal electrodiagnostic criteria, the TF considered that such patients may be diagnosed as possible typical CIDP as proposed by Koski et al. [93] if there is an objective response to a trial with any of the three proven CIDP treatments (PICO 2) and if at least one other supportive criterion is fulfilled.
Supportive criteria
Response to treatment (PICO 2), imaging (PICO 3), cerebrospinal fluid (CSF) (PICO 4), or nerve biopsy (PICO 6) may support the diagnosis of CIDP in patients who fulfil clinical criteria for CIDP, but whose electrodiagnostic criteria only allow for possible CIDP. Since sensory nerve conduction studies are now part of the electrodiagnostic criteria set, they have been removed as general supportive criterion, except for diagnosing patients with sensory CIDP without motor nerve conduction abnormalities, in whom fulfilment of the sensory conduction criteria is required.
(a) Response to treatment (PICO 2)
- The TF considered that an objective response to treatment with immunomodulatory agents (IVIg, plasma exchange, corticosteroids) supports the clinical diagnosis of CIDP in patients in whom clinical, electrodiagnostic and other supportive criteria allow only a diagnosis of possible CIDP.
-
Objective response to treatment requires improvement on at least one disability and one impairment scale. Lack of improvement following treatment does not exclude CIDP and a positive response is not specific for CIDP. Many outcome scales are used in CIDP. Some examples of disability and impairment scales are given:
- ○ Disability can be assessed by the Inflammatory Rasch-built Overall Disability Scale (I-RODS) [94-96] and the Inflammatory Neuropathy Cause and Treatment (INCAT) disability scale [97, 98].
- ○ Impairment can be assessed by the MRC sum score [96, 98, 99], the Modified INCAT Sensory Sum scale (mISS) [98, 100], the Neuropathy Impairment Score [101], and by measuring grip strength using handheld dynamometry [98, 102-104].
-
The changes required to define improvement have not been adequately validated. The following which have been used in clinical trials can serve as a guide:
- ○ I-RODS: + ≥4 centile points
- ○ INCAT disability scale: − ≥1 point
- ○ mISS: − ≥2 points
- ○ MRC sum score (0-60): + ≥2 to 4 points*
- ○ Grip strength:
- Martin Vigorimeter: + ≥8 to 14 kPa*
- Jamar hand grip dynamometer: + ≥10%**
*higher values may improve diagnostic specificity.
**values averaged over 3 consecutive days improve diagnostic specificity [104].
Considerations supporting the Good Practice Points (supporting information)
Evidence summary: Data from six cohort studies assessing response to treatment with IVIg, plasma exchange, or corticosteroids were extracted and analysed in evidence tables. There is moderate certainty evidence that corticosteroids and plasma exchange and high certainty evidence that IVIg improves impairment [105] (PICO 8-10). Uncontrolled studies report a positive response to IVIg, plasma exchange, or corticosteroids in variable proportions of patients (68%-99%) [35, 49, 106-108]. Reasons for therapeutic failure likely include inadequate treatment dosing or duration [12]. Misdiagnosis is also an important consideration for patients who do not respond to first line CIDP treatment [10-12].
Rationale: Current immunomodulatory treatments are not specific for CIDP, since other auto-immune conditions may also respond to these. Treatment response therefore needs to be carefully considered in the clinical and electrophysiological context to avoid overdiagnosis. If patients have an objective response to treatment, the probability of the diagnosis of CIDP increases. A minority of non-responders to at least one of the three proven effective treatments (PICO 8-10) still may have CIDP. These patients would require additional testing to rule out other disorders which mimic CIDP before considering other immunosuppressive treatment strategies.
(b) Imaging (PICO 3)
Ultrasound
- The TF suggested to use ultrasound in adult patients to diagnose CIDP in patients fulfilling diagnostic criteria for possible CIDP but not for CIDP. The diagnosis of CIDP may be more likely if there is nerve enlargement* of at least two sites in proximal median nerve segments and/or the brachial plexus (see NOTE below on excluding mimics).
- There is currently no evidence to support ultrasound in paediatric patients.
Considerations supporting the Good Practice Points (supporting information)
Evidence summary: Data extracted from 12 cohort studies assessing the usefulness of ultrasound were analysed. Enlargement mainly of proximal nerve segments in arm nerves and spinal nerve roots are the most characteristic feature in CIDP [109-112]. The yield of stringent cut-off values using a practical sonographic protocol (brachial plexus and proximal median nerve segments bilaterally) has been validated in a prospective cohort of patients with suspected chronic inflammatory neuropathies [113, 114]. In contrast to the adult population, systematic studies on yield of ultrasound in children with suspected CIDP are lacking. Only a few smaller studies reported on reference values for sonographic nerve sizes in different age categories [115-117], but stringent cut-off values based on disease controls are lacking.
Rationale: Since in children inherited demyelinating neuropathies are much more prevalent than CIDP and since rater experience on nerve ultrasound in children is limited, the TF suggested not to use ultrasound to support the diagnosis in children. Ultrasound is a low-cost, widely available, non-invasive procedure with moderate diagnostic accuracy.
MRI
-
The TF suggested not to use MRI in adult patients to diagnose CIDP except in patients fulfilling diagnostic criteria for possible CIDP but not for CIDP. CIDP may be more likely if there is enlargement and/or increased signal intensity of nerve root(s) on T2 weighted MRI sequences (DIXON/STIR, coronal + sagittal planes)* (see NOTE below on excluding mimics).
*preferably quantitative assessment of the spinal nerve root sizes (nerve root diameter right next to the ganglion, measured as height in coronal plane with cut-off value >5 mm), or semi-quantitative scoring of abnormalities of the spinal nerve roots and trunks using the following categories: normal, possibly abnormal, clearly abnormal.
- There is currently no evidence to support MRI in paediatric patients.
Considerations supporting the Good Practice Points (supporting information)
Evidence summary: Data from 18 studies assessing the usefulness of MRI were extracted and analysed. MRI of the brachial and lumbosacral plexus may aid in the diagnosis of CIDP by showing nerve root hypertrophy, increased signal intensity or contrast enhancement [109, 118-121]. Advanced MRI sequences have improved tissue discriminating properties [122]. Most MRI studies only evaluated patients with established CIDP, using different study designs (with/without control group), whereas only a few investigated its added diagnostic value that would approach a more routine clinical setting [123, 124]. An important limitation is the lack of objective cut-off values for abnormality. Two studies found low reproducibility of results in patients with chronic inflammatory neuropathies and disease controls, even among experienced raters [125-127]. Only a few studies used objective cut-offs for abnormal nerve root sizes (>5 mm) to improve performance and consistency of plexus MRI [123, 128].
Rationale: Conditions under which MRI may be considered in patients fulfilling only possible electrodiagnostic criteria include unavailability of ultrasound or when ultrasound results are non-contributory. In children with suspected CIDP, systematic studies on MRI are lacking, inherited demyelinating neuropathies are more prevalent than CIDP and can also show nerve size increase, and rater experience in children is limited. The low inter-rater reliability, lack of objective cut-off values and high cost of MRI contribute to the statement against using MRI.
NOTE: Before concluding that ultrasound or MRI abnormalities are supportive of CIDP, there should be no laboratory/clinical features that suggest other diseases such as MMN, demyelinating Charcot-Marie-Tooth (CMT) disease, IgM paraproteinaemic neuropathy (especially with anti-MAG antibodies), polyneuropathy-organomegaly-endocrinopathy-M-protein-skin changes (POEMS) syndrome, diabetic radiculoplexus neuropathy, amyloid neuropathy, neuralgic amyotrophy, leprosy, neurofibromatosis or neurolymphomatosis.
(c) CSF analysis (PICO 4)
- The TF suggested not to perform CSF analysis if diagnostic criteria are already met.
-
CSF analysis should be considered to exclude other diagnoses or to support the diagnosis of CIDP in the following circumstances:
- ○ Patients fulfilling diagnostic criteria for possible CIDP but not CIDP.
- ○ In cases of acute or subacute onset.
- ○ When an infectious or malignant aetiology is suspected or possible.
- ○ CSF protein elevation should be interpreted cautiously in the presence of diabetes.
- ○ In view of higher normative values for CSF protein in individuals older than 50 years, higher levels are required to support a diagnosis of CIDP; there is insufficient research to date to establish rigorous cut-offs.
Considerations supporting the Good Practice Points (supporting information)
Evidence summary: From 42 clinical cohort studies identified, 9 were included for data extraction and analysis. CSF protein is often increased in CIDP patients (sensitivity of 42%-77%), but with unknown specificity to discern CIDP from CIDP mimics [7, 47]. In suspected CIDP with unusual features or in the presence of systemic symptoms and signs, CSF analysis is suggested to exclude an underlying malignancy or infection [129]. There is a risk of misdiagnosis in cases where electrodiagnosis is non-confirmatory and only CSF protein is increased [11]. Specificity for CIDP is uncertain using newly established higher normative cut-off values for CSF protein elevation in older subjects (>0.6 g/L above age 50) [130]. Liberatore et al. [131] found that, using cut-offs of ≥0.5 g/L under the age of 50 years and >0.6 g/L over the age of 60 years, sensitivity of CSF protein elevation for CIDP was 68%. In children, the interpretation of CSF protein levels is complex and validated reference values for different ages categories are lacking.
Rationale: The independent diagnostic value of CSF testing remains unproven. When CSF protein levels are normal, doubt may unnecessarily be cast upon the diagnosis. In selected cases, where the clinical diagnosis and electrodiagnostic results are not fully confirmatory, CSF analysis could either support the diagnosis or exclude alternative diagnoses. The sensitivity of CSF in CIDP variants is uncertain. It may be advisable to consider more extensive electrodiagnostic testing prior to performing a lumbar puncture.
(d) Nerve biopsy (PICO 6)
Good Practice Points
- In cases where CIDP is suspected but cannot be confirmed with the clinical, laboratory, imaging, and electrodiagnostic studies.
- In cases where CIDP is suspected, but there is little or no response to treatment, such that an alternative diagnosis such as CMT, amyloidosis, sarcoidosis, or nerve sheath tumours/neurofibromatosis might be considered.
-
Nerve biopsies should be considered only when:
- ○ skilled (neuro)surgeons and neuropathologists and specialized and experienced pathology laboratory facilities are available.
- ○ symptoms are severe enough to justify the potential morbidity associated with a nerve biopsy.
- ○ the low accuracy of the test is fully understood by the patient before undergoing the biopsy.
-
When a nerve biopsy is taken:
- ○ current expert consensus on minimal standards for processing and evaluating nerve biopsies should be observed [132].
- ○ most often the sural or the superficial peroneal nerve is biopsied but biopsy of a clinically affected nerve is more likely to provide useful information.
- ○
factors probably supporting the diagnosis of CIDP may be:
Considerations supporting the Good Practice Points (supporting information)
Evidence summary: Data from 26 studies identified for assessing the usefulness of nerve biopsy were extracted and analysed to reach consensus. Several studies tried to estimate nerve biopsy accuracy in diagnosing CIDP, but the variability between them was huge and they could not be combined because of the wide range of outcomes used. Even when using the same parameters, there is an important level of heterogeneity in the sensitivity for findings suggestive of CIDP, which can be due to the subjectivity in studying the biopsies, the timing of the biopsy in the disease course, and comorbidities [137]. Several studies assessed the clinical outcomes when initiating treatment after a nerve biopsy. Clinical outcomes in patients with suspected CIDP, treated with immunomodulating agents after a biopsy-guided diagnosis of CIDP, have been successful [137-139]. However, lacking a control group, these data could not be used for analysis. Since nerve biopsy can reveal findings suggestive of a different or differential diagnosis, a biopsy may save patients from the unnecessary complications of immune treatment and lead to appropriate therapy. Nerve biopsies have poor sensitivity and specificity, and their contribution to the diagnosis is limited by these inaccuracies.
Rationale: The statement on nerve biopsy is intended to reduce the number of unnecessary biopsies for suspected CIDP, given the low diagnostic accuracy and invasive nature. The TF expects that only a small number of carefully selected nerve biopsies will contribute to a more accurate diagnosis of CIDP and to a lower probability of misdiagnosis, especially in unusual cases when all other investigations are non-diagnostic, including some patients considered to have CIDP who have not responded to treatment. Sural nerve biopsy is associated with numbness in the area of innervation [140-142]. Other complications include acute pain [143], chronic pain [142], allodynia [144], dysaesthesia [145], neuroma formation [143], infections, and wound dehiscence [144].
Criteria for immunological testing
Monoclonal gammopathy testing (PICO 7)
- The TF strongly advised testing for serum monoclonal proteins in adult patients with a clinical suspicion of CIDP.
- Testing should include serum protein electrophoresis and immunofixation (to increase sensitivity to detect relevant low level paraproteins and identify paraprotein class and light chain), spot urine immunofixation for light chains (Bence Jones protein). Measurement of serum free light chains (SFLC) may detect an abnormality not otherwise detected. Note that relevant monoclonal proteins may still have normal light chain and ratio measurements in SFLC assays. If a gammopathy is found, further evaluation may be required and haematology-oncology consultation should be strongly considered.
- In patients with distal CIDP, if no IgM paraprotein is found or anti-MAG antibody testing is negative, repeat testing should be considered.
- Testing of vascular endothelial growth factor (VEGF) serum levels is indicated in patients with a distal and painful CIDP phenotype, in whom a lambda light chain associated IgA or IgG paraprotein is found, when POEMS syndrome is suspected.
Considerations supporting the Good Practice Points (supporting information)
Evidence summary: Data from 35 observational studies assessing the presence and significance of monoclonal proteins and anti-MAG antibodies were extracted and summarized in evidence tables. Neuropathies with MGUS may behave like typical CIDP [146-148]. However, monoclonal gammopathies may be associated with neuropathies mimicking CIDP, such as anti-MAG IgM neuropathy [37, 149], POEMS syndrome [150-152], multiple myeloma or AL-amyloidosis [151].
Rationale: In patients with suspected CIDP and a monoclonal gammopathy, correct diagnosis of both the neurological and oncological condition is of paramount importance because of the implications for management and treatment. Patient burden is negligible. These tests are low cost and are available in most hospitals.
Antibody testing (PICO 5)
- The TF suggested to consider testing for nodal and paranodal antibodies in all patients with clinical suspicion of CIDP:
- ○ when nodal and paranodal (anti-NF155, anti-CNTN1, anti-Caspr1) and possibly anti-NF140/186 antibody testing is available and meeting quality standards.
- ○ testing of nodal and paranodal antibodies is advised in CIDP patients with the following features:
- resistance to standard therapy with IVIg and corticosteroids.
- acute or subacute aggressive onset, previous diagnosis of GBS or A-CIDP.
- low-frequency tremor, ataxia disproportionate to the sensory involvement or other cerebellar features or predominantly distal weakness.
- respiratory failure and cranial nerve involvement.
- associated nephrotic syndrome.
- very high CSF protein levels.
- The TF advised using for nodal and paranodal autoantibody testing:
-
a cell-based assay using mammalian expression vectors encoding human NF155, CNTN1, NF186/NF140, and Caspr1. Expression vectors should avoid the use any protein tag at the N-terminal site, any protein tag at the C terminal site for CNTN1 and avoid the use, in general, of GFP-tagged expression vectors.
-
a confirmatory test with ELISA (using human recombinant proteins) or teased-nerve immunohistochemistry. The order of assays can be interchanged. The application of additional confirmatory tests to the protocol is strongly recommended for low titre sera or dubious staining on the cell-based assay to avoid false positives.
-
- The TF advised anti-MAG antibody testing in all patients with an IgM paraprotein fulfilling CIDP diagnostic criteria (especially distal CIDP) because a high titre of anti-MAG antibodies (>7000 Bühlmann Titre Units, BTU) [153] would strongly imply a different diagnosis than CIDP.
- The TF advised for anti-MAG antibody testing:
- ○ Bühlmann test ELISA, or
- ○ Locally validated ELISA, Western blot or immunohistochemistry assays.
Considerations supporting the Good Practice Points (supporting information)
Evidence summary: Data from 16 cohort studies assessing the presence of nodal-paranodal and anti-MAG antibodies were extracted and analysed. Diagnostic utility seems strong for anti-NF155 and anti-CNTN1 IgG [67, 69, 72], and anti-Caspr1 IgG [73-75]. More evidence is needed for anti-NF155 IgM [154], anti-nodal NF140/186 IgG [76, 77], and anti-MAG without an apparent paraprotein [155]. For autoantibodies against CNTN1 and NF155, replication studies and a systematic review [156] are available with clear associations to clinically relevant features and a high diagnostic specificity. For autoantibodies against Caspr1, nodal NF, and MAG, only small case series or anecdotal cases have been reported. Evidence that autoantibody detection may inform treatment remains anecdotal. Several case reports and case series associate the detection of nodal-paranodal antibodies, especially anti-NF155 and anti-CNTN1 with poorer responses to conventional therapies [66, 156]. There is anecdotal evidence that these patients may respond well to rituximab [80, 157]. Although the evidence is weak due to the low numbers of patients, the response to rituximab has been replicated in independent cohorts and the magnitude of the effect is, at least for a subset of patients, very significant.
Rationale: Nodal-paranodal or MAG antibody testing should be considered in patients who fulfil criteria for CIDP, when they present with particular characteristics (Flowchart 1) and when they do not respond well to proven effective treatments for CIDP. Anti-MAG antibodies are relevant, if associated with a distal CIDP phenotype and an IgM paraprotein. The antibody testing has a low cost and positive results have significant implications for diagnosis and treatment. Access to antibody testing requires specialized laboratory procedures that are not available worldwide and standardization of the assays through interlaboratory validation needs to be performed. Patient burden is negligible.
Advised strategy for the diagnosis of CIDP
CIDP should be considered in any patient with a progressive symmetric or multifocal polyradiculoneuropathy in whom the clinical course is relapsing and remitting or progresses for more than 8 weeks, especially if there are sensory symptoms, proximal weakness, areflexia without wasting, or preferential loss of vibration or joint position sense (Flowcharts 1 and 2, Table 6). Electrodiagnostic tests are mandatory and the major features suggesting a diagnosis of CIDP are listed in Tables 1 to 3 and Flowchart 1. The sensitivity of electrodiagnostic criteria for motor nerves may be improved by examining more than four nerves, and by including proximal stimulation in the upper limbs. If electrodiagnostic criteria for CIDP are not met initially, a repeat study at a later date should be considered. Supportive criteria (PICOs 2-4, 6) can be used to confirm the diagnosis of CIDP in patients with a possible diagnosis as based on clinical and electrodiagnostic criteria. CSF examination, ultrasound of proximal median nerve segments, cervical spinal roots, and the brachial plexus or MRI of spinal roots, brachial or lumbar plexus, and a trial of immunotherapy with objective assessment of endpoints may assist the diagnosis. Biopsy of the sural nerve, but occasionally the superficial peroneal nerve, can provide supportive evidence for the diagnosis of CIDP, but positive findings are not specific and negative findings do not exclude the diagnosis. Monoclonal gammopathy testing should be performed in all patients with suspected CIDP (PICO 7). If an IgM paraprotein is present, anti-MAG antibodies should be tested (PICO 5). When specific clinical features are present, testing of nodal-paranodal antibodies may be indicated to diagnose auto-immune neuropathies (PICO 5, Flowchart 1). There is only low certainty evidence concerning all these matters. Since other conditions may mimic CIDP, investigations to discover possible other diseases should be considered (Tables 4 and 5, Flowchart 2). The diagnostic categories for typical CIDP and CIDP variants are defined by mandatory clinical and electrodiagnostic criteria, and if these give a diagnosis of only possible CIDP then two additional supportive criteria are required (Flowchart 1, Table 6).
| Typical CIDP |
|
|
|
|
|
|
|
|
|
|
| Distal CIDP |
|
|
|
|
|
| Multifocal and focal CIDP |
|
|
|
|
|
|
|
| Motor CIDP |
|
|
|
|
| Sensory CIDP |
|
|
|
|
|
|
|
- Abbreviation: CIDP, chronic inflammatory demyelinating polyradiculoneuropathy.
- a The differential diagnosis includes the disorders listed but is not limited to these.
| Investigations strongly advised in typical CIDP and in CIDP variants: |
|
|
|
|
|
|
| Investigations to be performed if indicated, in typical CIDP and in CIDP variants: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Additional investigations if indicated in CIDP variants: |
| Distal CIDP |
|
| Multifocal and focal CIDP |
|
|
|
| Motor CIDP |
|
|
|
| Sensory CIDP |
|
|
|
|
|
- Abbreviation: CIDP, chronic inflammatory demyelinating polyradiculoneuropathy.
| Typical CIDP |
| Typical CIDP |
|
|
| Possible typical CIDP |
|
|
| Distal CIDP |
| Distal CIDP |
|
|
| Possible distal CIDP |
|
|
| Multifocal or focal CIDP |
| Multifocal or focal CIDP |
|
|
| Possible multifocal or focal CIDP |
|
|
| Motor CIDP |
| Motor CIDP |
|
|
| Possible motor CIDP |
|
| Motor-predominant CIDP |
| As in motor CIDP but with sensory conduction abnormalities in two nerves |
| Sensory CIDP |
| Possible sensory CIDP |
|
| Sensory-predominant CIDP |
| Possible sensory-predominant CIDP |
|
| Sensory-predominant CIDP |
|
Treatment of CIDP
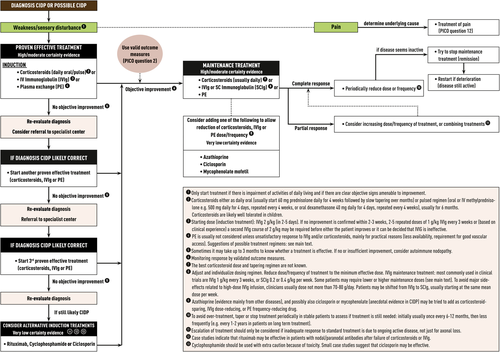
Corticosteroids (PICO 8)
- The TF strongly recommended treatment with corticosteroids.
- The best corticosteroid regimen is not known.
- Pulsed high-dose corticosteroid treatment with oral dexamethasone or IV methylprednisolone may be considered as an alternative to daily oral prednisone/prednisolone or dexamethasone both for induction and maintenance treatment.
- Long-term corticosteroid treatment may induce significant-side effects.
- Since patients with motor CIDP may deteriorate after corticosteroids, IVIg should be considered as the first-line treatment in motor CIDP (Good Practice Point).
Considerations supporting the recommendation (supporting information)
Evidence summary: Although it is uncertain (very low certainty evidence with 1 trial, 28 participants) [158] whether daily oral prednisone (120 mg daily slowly tapered over 4 months) improved impairment compared with no treatment, observational studies and the abundant clinical practice experience strongly suggest that corticosteroids are effective in CIDP. Daily oral corticosteroid doses commonly used are prednisone or prednisolone 60 mg equivalent to methylprednisolone 48 mg, slowly tapered over 6 to 8 months, depending on clinical response and possible side-effects. Although some centres prefer to start with a daily dose of 1 to 2 mg/kg of prednisolone, there is no evidence that this usually higher dose is superior. An alternative to daily corticosteroid regimens could be pulsed treatment with oral or IV corticosteroids. There is moderate certainty evidence (1 trial, 41 participants) [159] that 6 months' treatment with pulsed high-dose oral dexamethasone (4 days 40 mg monthly) did not improve disability more than daily oral prednisolone (60 mg, slowly tapered over 8 months). There is very low certainty evidence from open follow-up studies or randomized controlled trials that pulsed corticosteroid treatment (40 mg/day for 4 days per month) gave similar improvement in disability to daily oral prednisolone (60 mg, slowly tapering over 8 months). There is very low certainty evidence from open follow-up studies or randomized controlled trials that pulsed corticosteroid treatment (40 mg/day oral dexamethasone or 500 mg/day IV methylprednisolone, each daily for 4 days per month for 6 months) may induce more frequent and longer remission than daily oral corticosteroid treatment [10, 160]. Low to moderate certainty evidence suggests that there are fewer side-effects and a faster response with pulsed high-dose corticosteroid compared with daily oral corticosteroid treatment. Some patients with CIDP may deteriorate after corticosteroid treatment, especially those with motor CIDP [36, 52, 55]. Therefore, corticosteroids are not recommended as first-line treatment in these patients [105].
Rationale: Because of abundant clinical practice experience, corticosteroid treatment can be used as first-line treatment. However, in patients with (relative) contraindications for long-term high-dose corticosteroid treatment, IVIg (or subcutaneous immunoglobulin [SCIg]) may be the preferred treatment. Patients should be carefully monitored for treatment response, which usually starts after several weeks or months. Reduction of the corticosteroid dose should be attempted regularly to investigate whether the current high dose is still required or whether the patient is in remission. Addition of calcium and bisphosphonate treatment should be considered. Potential side-effects of corticosteroids (eg, osteoporosis, gastric ulceration, diabetes, cataracts, avascular necrosis of long bones, arterial hypertension) may outweigh the benefit from treatment in low disability disease.
1 Immunoglobulin (PICO 9)
Recommendations and supporting considerations (supporting information)
- The TF strongly recommended treatment with IVIg.
- Induction treatment: The usual total IVIg dose is 2 g/kg, divided over 2 to 5 days. Since not all patients respond to this first course, two to five repeated doses of 1 g/kg IVIg every 3 weeks may be required before either the patient improves or it can be decided that IVIg is ineffective. Alternatively, clinical experience indicates that a second course of 2 g/kg a few weeks after the first course may be sufficient to decide whether IVIg is ineffective.
- Maintenance treatment: Most patients require IVIg maintenance treatment. The best IVIg maintenance dose and schedule are not known. The most commonly used IVIg maintenance regimen in clinical trials is 1 g/kg every 3 weeks, but in clinical practice lower doses and longer treatment intervals maintaining maximal sustained improvement should be considered (eg, 0.4-1 g/kg every 2-6 weeks)
- Objective end-of-dose deterioration before the next IVIg infusion should be minimised. If it occurs, the IVIg dose may be increased or the infusion interval shortened.
- If the patient is clinically stable, it is recommended to check periodically whether the IVIg dose can be reduced (eg, by 25% per infusion), the treatment interval lengthened, or the treatment discontinued. Based on clinical experience, this could be done once every 6 to 12 months for the first 2 to 3 years of treatment, then less frequently (eg, every 1-2 years).
Evidence summary: According to high certainty evidence (5 trials, 269 participants) [104], induction treatment with IVIg produced more short-term improvement than placebo. Adverse events were more common with IVIg than placebo (high certainty evidence), but serious adverse events were not observed (moderate certainty evidence, 3 trials, 315 participants) [105]. The ICE randomized controlled trial showed that 94% of patients responded to 2 g/kg induction treatment and two subsequent treatments of 1 g/kg at 3 weeks intervals [161]. The open PRIMA and PRISM studies indicated that a treatment response sometimes may only be observed after three to five infusions of 1 g/kg every 3 weeks [162, 163]. Alternatively, clinical experience indicates that most patients respond objectively to no more than two initial courses of 2 g/kg [164]. It is not well known whether an objective response following only after several treatments is due to a delayed treatment response or to the requirement of a different treatment regimen. The 1 g/kg every 3 weeks regimen used in the ICE trial for 6 months is often considered as a standard maintenance treatment [161, 165], although the IMC trial comparing IVIg with corticosteroids used an IVIg maintenance dose of 2 g/kg every 4 weeks [166]. Experience from clinical practice indicates that the IVIg maintenance dose can be lower (0.4-1 g/kg every 2-6 weeks), but this should be individually adjusted [164, 167-169]. There is no evidence of a difference in efficacy between different IVIg preparations for treating CIDP. A randomized controlled trial in 27 patients with CIDP comparing 5% freeze-dried and 10% liquid IVIg preparations showed no difference in treatment efficacy [170]. Clinical experience indicates that a switch to another preparation may be helpful to relieve side-effects.
Rationale: The TF considered that the demonstrated efficacy of IVIg in trials, together with extensive practical experience of effectiveness, outweigh the frequent minor and the rare but more serious side-effects. IVIg treatment is acceptable and feasible. The major barriers are the high cost, the inconvenience for the patients, and the need for venous access. The initial IVIg treatment course is usually given in a hospital or day care facility. Maintenance IVIg infusions usually can be administered at a day care facility, infusion centre, or in some countries at home with proper monitoring. Potential burden of repeated infusions and high health care costs of IVIg may outweigh the benefit from treatment in low disability disease.
- Both IVIg and oral or IV corticosteroids are first-line treatments for CIDP. Based on the level of evidence, the TF did not recommend an overall preference for either treatment modality and weakly recommended either IVIg or corticosteroid treatment.
-
Both short- and long-term effectiveness, risks, ease of implementation, and cost should be considered:
- ○ IVIg may be preferable when it comes to short-term treatment effectiveness, or when (relative) contraindications for corticosteroids exist.
- ○ There is some indication that pulsed corticosteroids may be preferable for long-term treatment effectiveness, because of a possible higher rate and longer duration of remission, or when IVIg is unaffordable or unavailable.
Evidence summary: There is little or no difference in short-term improvement of disability with IVIg in comparison with oral prednisolone (moderate certainty evidence; 1 trial, 29 participants) or long-term improvement after IV methylprednisolone (high-certainty evidence; 1 trial, 45 participants) [105]. Clinical improvement after IVIg, however, may be faster and the adherence to the treatment seems to be better after IVIg than after IV methylprednisolone [166]. Side-effects of long-term treatment are probably in favour of IVIg (real-life experience). Pulsed IV corticosteroid treatment, however, may increase the rate and duration of remission after 6 months as compared with IVIg based on one small study (low certainty evidence) [160]. A trial comparing standard oral prednisolone vs pulsed dexamethasone treatment did not show a difference in remission rate [159].
Rationale: The reason for selecting either IVIg or corticosteroid treatment is based on a series of patient-oriented considerations. Chronic high-dose oral corticosteroid treatment probably has a higher chance of side-effects compared with IVIg, but data on long-term (>6 months) corticosteroid treatment in CIDP are not available. IVIg is considerably more costly than corticosteroids. Co-morbidity may be important for the choice of treatment. IVIg is preferable when there is an increased risk of developing osteoporosis or diabetes. In children, tablets are better tolerated than regular IV treatments but an effect on growth should be considered.
- Although the evidence from studies is limited, the TF weakly recommended treatment with IVIg compared with plasma exchange, mainly based on the ease of administration of IVIg.
- In some patients with good vascular access, plasma exchange may be an acceptable option for chronic treatment.
Evidence summary: Both treatments are considered effective, although the research evidence based on comparative studies is sparse (very low certainty evidence). For induction treatment, plasma exchange and IVIg seem equally effective [105, 171, 172]. Doses used in comparative studies are for IVIg: 0.4 g/kg weekly for 3 weeks, then 0.2 g/kg weekly for the next 3 weeks, and for plasma exchange: 2×/week for 3 weeks, then 1×/week for 3 weeks. For maintenance treatment, no proper studies on long-term efficacy and safety of plasma exchange exist. Long-term treatment effects of IVIg are much better known. Especially in small children, IVIg is preferred over plasma exchange, mainly for practical reasons. Non-controlled studies indicated that plasma exchange can still be effective if treatment with IVIg or corticosteroids fails [106].
Rationale: The main advantage of IVIg is the relative ease of administration (although plasma exchange often can be delivered through peripheral vein access if using a centrifugal machine). IVIg infusion does not require special equipment. If plasma exchange can be delivered through a peripheral vein, the side-effect profile is usually good. Both treatments are expensive, but IVIg is usually even more expensive than plasma exchange. The cost of plasma exchange is dependent not only on the costs of the equipment, but also on the costs of replacement fluids such as albumin or fresh frozen plasma. These costs may vary in different countries. In children, IVIg is preferred over plasma exchange, mainly for practical reasons.
- The TF strongly recommended using SCIg for maintenance treatment in CIDP.
- The TF recommended no preference for either IVIg or SCIg for maintenance treatment in CIDP.
- During follow-up, the dose should be tailored according to individual treatment response.
- The TF weakly recommended against using SCIg for induction treatment in CIDP.
Evidence summary: Efficacy of SCIg, compared with placebo, has been demonstrated in two randomized controlled trials with high certainty evidence (PATH trial in 172 patients [173]) and another randomized controlled trial in 30 patients [174] in CIDP patients previously responsive to IVIg. There is insufficient evidence that a higher dose (0.4 g/kg weekly) is superior to a lower dose (0.2 g/kg weekly) for maintenance treatment [95]. However, a 24-week open-label extension study indicated that there were lower relapse rates in the higher dose group [175]. Therefore, long-term dosing should be individualized and tailored to find the most appropriate dose. There are frequent minor side-effects (mainly skin reactions). Limited available information indicates that patients with CIDP might in some cases require higher mean doses of SCIg compared with their previous IVIg dose. There is only very low certainty evidence for using SCIg as induction treatment (one randomized controlled cross-over trial in 20 patients) [175].
Rationale: When CIDP patients switch from IVIg to SCIg, it is reasonable to start using the same mean dose (1:1) per week. If the treatment effect is insufficient, the dose should be adjusted using reliable outcome measures. If the dose is high (>20-30 g/infusion), an option is to split doses, increase frequency or to use multiple injection sites for subcutaneous infusions. Patients' personal preferences should be considered in choosing SCIg or IVIg. Arguments favouring SCIg include the autonomy and convenience of self-treatment at home, avoiding intravenous cannulation, and possibly fewer systemic side-effects. Disadvantages of SCIg include local side-effects (subcutaneous swelling and pain) and more frequent infusions. Maintenance treatment with SCIg is acceptable and usually feasible.
Plasma exchange (PICO 10)
Recommendation
- The TF strongly recommended treatment with plasma exchange.
- The initial treatment may start with 5 exchanges over 2 weeks; thereafter, the plasma exchange interval should be individually adapted. If possible, peripheral veins should be used.
Considerations supporting the recommendation (supporting information)
Evidence summary: According to moderate certainty evidence (2 trials, 59 participants), twice-weekly plasma exchange produced more short-term (at 3 or 4 weeks) improvement in disability than sham exchange [105, 176-178]. In the largest observational study, 3.9% of plasma exchange procedures had complications [179].
Rationale: Plasma exchange requires good vascular access and specialized equipment. In patients with difficult vascular access, who require multiple exchanges in a short period of time, a catheter inserted in a non-peripheral vein can be used. For single exchanges during long-term maintenance treatment, tunnelled catheters may be used. These drawbacks make plasma exchange, despite its effectiveness and relative safety, the third option for chronic treatment after corticosteroids and IVIg.
Other treatments (PICO 11)
Recommendations and supporting considerations (supporting information)
- The TF weakly recommended against using methotrexate.
Evidence summary: According to low certainty evidence (1 randomised parallel-group trial, 60 participants) [180], increasing methotrexate doses to 15 mg weekly for 32 weeks did not allow more participants to reduce corticosteroid or IVIg doses by more than 20% (primary outcome). Serious adverse events were no more common with methotrexate (three cases) than with placebo (one case).
Rationale: In making this recommendation, the lack of efficacy in one trial was crucial [180]. However, it is acknowledged that the patient selection (insufficient assessment of active disease prior to enrolment) and the relatively low 15 mg weekly methotrexate dose used in this trial may have led to an underestimation of the potential efficacy of methotrexate. Observational data that suggest methotrexate might work in some cases [29, 181-183]. Nevertheless, given the current lack of demonstrated efficacy and the potential side-effects such as teratogenicity, abnormal liver function, and pulmonary fibrosis [105], methotrexate is not recommended in patients with CIDP.
- The TF strongly recommended against using interferon beta-1a.
Evidence summary: According to moderate certainty evidence (2 trials, 87 participants), interferon beta-1a (IFN beta-1a), in comparison with placebo, did not allow more patients with CIDP to withdraw from IVIg [184, 185]. A possible increase in serious adverse events could not be confirmed (low certainty evidence). The drug may have serious adverse events (none in the cross-over trial with 20 participants, but 4 in the IFN beta 1a and none in the placebo group in the randomized controlled trial with 67 participants).
Rationale: In making this recommendation, the TF judged the demonstrated lack of efficacy from two randomized controlled trials to be crucial [184, 185]. The drug may have serious side-effects.
- The TF weakly recommended against using fingolimod.
Rationale: The TF did not favour the use of fingolimod to treat CIDP given the current lack of demonstrated efficacy and the associated safety profile of fingolimod.
(d) Other immunosuppressive drugs
• Although there is only very low certainty evidence, the TF advised to use azathioprine, cyclophosphamide, ciclosporin, mycophenolate mofetil, and rituximab (after failure of proven effective treatments or as add-on medication).
• The TF advised not to use alemtuzumab, bortezomib, etanercept, fampridine, fludarabine, immunoadsorption, interferon alpha, abatacept, natalizumab, and tacrolimus.
- Azathioprine, mycophenolate mofetil, or ciclosporin may be considered as immunoglobulin or corticosteroid-sparing agents in CIDP patients treated with either immunoglobulin or corticosteroids as maintenance treatment.
- Cyclophosphamide, ciclosporin, or rituximab may be considered in patients who are refractory to the proven effective treatments (IVIg, corticosteroids, and plasma exchange).
Evidence summary: Azathioprine and mycophenolate mofetil are frequently used in CIDP as immunoglobulin- or corticosteroid-sparing agents, although their effectiveness to lower immunoglobulin or corticosteroid dose is uncertain [187-194]. Although there is only very limited evidence from case series, cyclophosphamide [195-200], ciclosporin [201-204], and rituximab [205-207] may be considered in patients insufficiently responding or refractory to conventional treatment. The TF suggested that rituximab may be tried in children after failure of proven effective treatments, instead of cyclophosphamide because of a better side-effect profile. The TF considered the available evidence on effectiveness too limited, and potential harms too great, to support the use of alemtuzumab [208], bortezomib [209], etanercept [210], fampridine [211], fludarabine [212], immunoadsorption [213, 214], interferon alpha [215], abatacept [216], natalizumab [217], and tacrolimus [218]. The TF noted that there is insufficient evidence for a positive effect of haematopoietic stem cell transplantation (HSCT). Since there are significant morbidities and a mortality risk with HSCT, this treatment should only be considered as a last resort option in specialised CIDP centres [219, 220].
Pharmacological treatment of pain (PICO 12)
- The TF advised assessment and treatment of pain when present in CIDP.
- Assess the cause(s) of the pain, whether neuropathic or nociceptive (especially musculoskeletal) pain. Either might be a consequence of CIDP or unrelated to CIDP. Consider alternative diagnoses mimicking CIDP (such as POEMS, vasculitis, diabetes, amyloidosis, CMT1B) in which neuropathic pain may be even more prevalent.
- For neuropathic pain or dysaesthesia, consider treating according to published guidelines [221, 222]. These recommend tricyclic antidepressants, pregabalin, gabapentin, or serotonin-noradrenaline reuptake inhibitors (duloxetine or venlafaxine) as first line treatments.
Considerations supporting the Good Practice Points (supporting information)
Evidence summary: The prevalence of pain (of any type, but with no alternative cause other than CIDP) at any time during the course of CIDP was estimated as 46% in a systematic review [223] and varied between 7% and 72% in different studies, reviewed by Thakur et al. [224] Neuropathic pain was present in 20% of 79 CIDP patients in the study by Bjelica et al. [225] and an additional 20% had previously taken medication for neuropathic pain. The quality of pain encompassed many different typical symptoms of neuropathic pain such as burning, dysaesthesiae, and others. Non-neuropathic pain in CIDP has not been specifically studied but nociceptive/mechanical pain may be secondary to degenerative changes related to muscle weakness, altered gait and muscle usage patterns, and foot collapse. Radicular pain due to compression of hypertrophic spinal roots has been reported rarely in CIDP [226]. There is low certainty evidence for treatment of pain in CIDP. The use of anti-neuropathic pain drugs in CIDP is described in only a few small uncontrolled series [223, 225]. This limited evidence does not suggest that treatment of neuropathic pain in CIDP should differ from other neuropathic pain conditions. Immune treatment (mostly steroids and/or IVIg), although primarily given to treat motor and sensory deficit, also improved pain in 89% of 46 patients with painful CIDP in pooled uncontrolled small series reviewed by Michaelides et al. [223]. However, this evidence is very low certainty, and pain has not been investigated as an outcome in controlled trials demonstrating efficacy of immune treatments. The TF does not recommend using immune treatment primarily for treating pain. There are no reports on treatment of nociceptive/mechanical pain in CIDP.
Rationale: Despite the absence of evidence of efficacy of pharmacological treatments for neuropathic pain in CIDP, its widespread use in practice in patients with neuropathic pain and CIDP, and their proven efficacy in other neuropathic pain disorders justifies its use in CIDP patients with pain. Drugs for neuropathic pain often cause side-effects, but in patients with severe pain the potential gains were judged to outweigh these. Pain treatment is feasible, acceptable, and reasonably affordable.
Overview of diagnosis and treatment
Recommendations and Good Practice Points
- Clinical: typical CIDP and CIDP variants (Good Practice Points) (Table 1)
- Electrodiagnostic: strongly and weakly supportive of demyelination (recommendations) (Tables 2 and 3)
- Supportive: CSF, imaging (ultrasound, MRI), nerve biopsy and treatment response (Good Practice Points) (PICO 2-4, 6)
- Categories: CIDP and possible CIDP (Table 6)
Treatment of CIDP (3):
For induction treatment
- IVIg or corticosteroids should be considered in typical CIDP and CIDP variants in the presence of disabling symptoms (strong recommendation). Plasma exchange is similarly effective (strong recommendation) but may be less well tolerated and more difficult to administer. The presence of relative contraindications to any of these treatments may influence the choice (weak recommendation). The advantages and disadvantages should be explained to the patient who should be involved in the decision making (Good Practice Point).
- If the objective response is inadequate or the maintenance doses of the initial treatment (IVIg, corticosteroids, or plasma exchange) result in significant side-effects, the other first-line treatment alternatives should be tried before considering combination treatments (strong recommendation). Adding an immunosuppressant or immunomodulatory drug may be considered, but there is no sufficient evidence to recommend any particular drug (Good Practice Point). Treatment decisions should take into account whether there is active disease as evidenced by progression, relapse or demonstration of persistent treatment dependence, and on the other hand determination of deficits that cannot improve due to severe chronic axonal degeneration (Good Practice Point).
- In motor CIDP, IVIg should be considered as the initial treatment (Good Practice Point).
For maintenance treatment
- If the first-line treatment is effective, continuation should be considered until the maximum benefit has been achieved (strong recommendation) and then the dose should be reduced or the interval increased to find the lowest effective maintenance dose (Good Practice Point).
- SCIg and IVIg can both be considered as maintenance treatment in IVIg-responsive patients with active disease (strong recommendation).
- Neuropathic pain should be treated with drugs according to published guidelines on treatment of neuropathic pain (Good Practice Point).
- Advice about foot care, exercise, diet, driving, and life style management should be considered. Depending on the needs of the patient, orthoses, physiotherapy, occupational therapy, psychological support and referral to a rehabilitation specialist should be considered (Good Practice Points). Information about patient support groups should be offered (Good Practice Point).
ACKNOWLEDGEMENTS
The CIDP Guideline Task Force is grateful to the European Academy of Neurology (EAN), the Peripheral Nerve Society (PNS), the GBS/CIDP Foundation International and the GAIN Charity UK for granting unrestricted financial support. Date of EAN approval: May 14, 2021.
CONFLICT OF INTERESTS
P. Y. K. Van den B. reports honoraria, consultancy fees, advisory boards from Pfizer, Genzyme, CSL Behring, LFB, Natus, UCB Pharma, Alnylam Pharmaceuticals. P. A. van D. reports grants from Baxalta, Sanquin, Prinses Beatrix Spierfonds, Talecris. R. D. M. H. reports honoraria, consultancy, travel expenses, and departmental donations from Alnylam, Argenx, Grifols, and CSL Behring. B. A. has nothing to disclose. P. V. fees for consultancy and advisory boards (all to department) from: Annexon, Argenx, Hansa, Immunic, Octapharma, Roche. J. A. A. reports grants from CSL Behring and consultancy from CSL Behring, Grifols, Biopharma, Biotest. S. A. reports grants and honoraria from LFB, Pfizer, CSL Behring. P. H. P. H. B. has nothing to disclose. D. R. C. reports consultancy fees from Acetylon Pharmaceuticals, Alnylam Pharmaceuticals, Annexon Biosciences, Akros Pharma, Biotest Pharmaceuticals, Boehringer Ingelheim, Cigna Health Management, CSL Behring, DP Clinical, Grifols, Karos Pharmaceuticals, Neurocrine Biosciences, Novartis, Octapharma, Pharnext, Sun Pharmaceuticals, Syntimmune, and stock options from Syntimmune. F. E. reports grants from Prinses Beatrix Spierfonds, ZonMw, and consultancy from Astellas, UCB Pharma. H. S. G. reports grants from Prinses Beatrix Spierfonds. T. H. has nothing to disclose. S. K. reports Kakenhi grants from the Ministry of Welfare in Japan. R. A. L. reports consultancy fees from CSL Behring, Grifols, Optioncare, Kedrion, Biotest, Axelacare, Pharnext. M. P. L. reports consultancy fees from Octapharma, CSL Behring and a patent for antiganglioside antibodies licenced to Fujierbo (Johns Hopkins Hospital). E. N.-O. reports consulting fee, honorarium, and advisory/scientific boards from Argenx, Belgium; Astellas, the Netherlands; Baxter/Takeda, Italy, and United States; CSL Behring, Italy, and Switzerland; Grifols, Spain; Janssen, united States; Kedrion, Italy; LFB, France; Novartis, Switzerland; Roche, Switzerland; Sanofi, united States; UCB,UK. L. Q. reports grants and consultancy fees from Grifols, Novartis. Y. A. R. reports grants from LFB and consultancy fees from LFB, CSL Behring. C. S. reports grants from Kedrion, consultancy fees from Airliquidw, Alnylam Pharmaceuticals. H. A. T. reports grants from EU-Tubitak.
AUTHOR CONTRIBUTIONS
Peter Y. K. Van den Bergh: Conceptualization-lead, funding acquisition-lead, methodology-equal, project administration-equal, writing-original draft-lead, writing-review and editing-lead. Pieter A. van Doorn: Conceptualization-equal, methodology-supporting, writing-original draft-supporting, writing-review and editing-supporting. Robert D. M. Hadden: Conceptualization-supporting, funding acquisition-supporting, methodology-supporting, project administration-supporting, writing-original draft-supporting, writing-review and editing-supporting. Bert Avau: Methodology-lead, software-supporting, supervision-supporting, writing-original draft-supporting, writing-review and editing-supporting. Patrick Vankrunkelsven: Methodology-supporting, project administration-supporting, supervision-supporting. Jeffrey A. Allen: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting. Shahram Attarian: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting. Patricia H. Blomkwist: Funding acquisition-supporting, investigation-supporting. David R. Cornblath: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting. Filip Eftimov: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting. H. Stephan Goedee: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting. Thomas Harbo: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting. Satoshi Kuwabara: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting. Richard A. Lewis: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting. Michael P. Lunn: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting. Eduardo Nobile-Orazio: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting. Luis Querol: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting. Yusuf A. Rajabally: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting. Claudia Sommer: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting. Haluk A. Topaloglu: Investigation-supporting, writing-original draft-supporting, writing-review and editing-supporting.