Risk of requiring a wheelchair in primary progressive multiple sclerosis: Data from the ORATORIO trial and the MSBase registry
Funding information
This work was financially supported by F. Hoffmann-La Roche Ltd., Basel, Switzerland for the study and publication of the article.
Present address
During completion of the work related to this manuscript, R.Frietas was an employee of F.Hoffman-La Roche Ltd; her current affiliation is Novartis Pharma AG
During completion of the work related to this manuscript, D.Wormser was an employee of F.Hoffman-La Roche Ltd; his current affiliation is Novartis, Basel, Switzerland
Abstract
Background and purpose
Reaching Expanded Disability Status Scale (EDSS) ≥7.0 represents the requirement for a wheelchair. Here we (i) assess the effect of ocrelizumab on time to EDSS ≥7.0 over the ORATORIO (NCT01194570) double-blind and extended controlled periods (DBP+ECP), (ii) quantify likely long-term benefits by extrapolating results, and (iii) assess the plausibility of extrapolations using an independent real-world cohort (MSBase registry; ACTRN12605000455662).
Methods
Post hoc analyses assessing time to 24-week confirmed EDSS ≥7.0 in two cohorts of patients with primary progressive multiple sclerosis (baseline EDSS 3.0–6.5) were investigated in ORATORIO and MSBase.
Results
In the ORATORIO DBP+ECP, ocrelizumab reduced the risk of 24-week confirmed EDSS ≥7.0 (hazard ratio = 0.54, 95% confidence interval [CI]: 0.31–0.92; p = 0.022). Extrapolated median time to 24-week confirmed EDSS ≥7.0 was 12.1 and 19.2 years for placebo and ocrelizumab, respectively (7.1-year delay [95% CI: −4.3 to 18.4]). In MSBase, the median time to 24-week confirmed EDSS ≥7.0 was 12.4 years.
Conclusions
Compared with placebo, ocrelizumab significantly delayed time to 24-week confirmed wheelchair requirement in ORATORIO. The plausibility of the extrapolated median time to reach this milestone in the placebo group was supported by observed real-world data from MSBase. Extrapolated benefits for ocrelizumab over placebo could represent a truly meaningful delay in loss of ambulation and independence.
INTRODUCTION
Approximately 10% to 15% of the multiple sclerosis (MS) population are classified as primary progressive MS (PPMS) [1]. PPMS is distinguished from more common relapsing forms of MS (i.e., relapsing–remitting MS and secondary progressive MS) by gradual worsening of neurological disability from disease onset [1]. Long-term disability is an important outcome for patients with MS [2], and is typically characterized using the Expanded Disability Status Scale (EDSS) [3]. Reaching EDSS ≥7.0 is a key clinical disability milestone, as this represents an inability to walk >5 meters, even with an aid, essentially leading to the requirement for a wheelchair. Progression to EDSS ≥7.0 is associated with a major reduction in patients' quality of life and a treatment cost increase [4].
Ocrelizumab is a humanized monoclonal antibody that selectively targets CD20+ B cells, while preserving the capacity for B-cell reconstitution and preexisting humoral immunity [5, 6]. Ocrelizumab is the first CD20+ B-cell–selective monoclonal antibody approved for the treatment of relapsing MS and PPMS at a twice-yearly dose of 600 mg intravenously [7]. In the Phase III ORATORIO study (NCT01194570) [8] in patients with PPMS, the proportion of patients with 12-week confirmed disability progression (primary endpoint) was lower in patients receiving ocrelizumab (32.9%) versus placebo (39.3%; hazard ratio [HR] = 0.76; 95% confidence interval [CI]: 0.59–0.98; p = 0.03) [8]. In the present analysis, we assessed the effect of ocrelizumab on time to EDSS ≥7.0 in the double-blind period (DBP) and extended controlled period (ECP) of ORATORIO, and extrapolated the potential long-term impact of ocrelizumab on time to requiring a wheelchair. We further assessed the plausibility of the extrapolations, using an independent real-world PPMS cohort from the MSBase registry [9] along with current observed data from the ongoing ORATORIO open-label extension (OLE) [10].
METHODS
ORATORIO trial design and patients
ORATORIO was a Phase III, randomized, parallel-group, double-blind, placebo-controlled trial investigating the efficacy and safety of ocrelizumab in patients with PPMS [8]. Key eligibility criteria included age 18 to 55 years, PPMS diagnosis (2005 revised McDonald criteria [11]), and an EDSS score of 3.0 to 6.5 at screening. ORATORIO consists of three treatment periods: the DBP (≥120 weeks), the ECP (3–9 months; the ECP provided approximately 3 additional months of blinded controlled data and 6 months of controlled follow-up, during which patients were gradually unblinded and entered the OLE period), and the currently ongoing OLE period. All patients completing DBP and ECP were eligible to enter the OLE period. In the ORATORIO DBP+ECP, which lasted approximately 3 years, post hoc analysis was carried out to assess the time to onset of 24-week confirmed EDSS ≥7.0. Consistent with the original analyses [8], patients with an initial disability progression who discontinued treatment early and did not have a subsequent EDSS measurement were imputed as having a 24-week confirmed disability progression event. To further characterize the potential long-term impact of ocrelizumab treatment on the time to 24-week confirmed EDSS ≥7.0, a Weibull regression analysis [12] was used to extrapolate observed data from the ORATORIO DBP+ECP into the future, estimating the time at which 50% of patients were expected to have reached EDSS ≥7.0. The distribution of survival times was approximated by the Weibull distribution, which allows for monotonically increasing or decreasing hazard rates over time [12]. We performed sensitivity analyses using various parametric functions to estimate the delay in median time to requiring a wheelchair in patients treated with ocrelizumab versus placebo; sensitivity analyses were also performed after removal of imputed events. In addition, we analyzed the time to onset of 24-week confirmed EDSS ≥7.0 using data from the OLE period of ORATORIO in patients initially randomized to ocrelizumab (6 years total follow-up [312 weeks]) [10] to provide support for the extrapolation of the ORATORIO DBP+ECP ocrelizumab arm.
The ORATORIO trial protocol (NCT01412333)[8] was approved by the relevant institutional review boards/ethics committees. All patients provided written informed consent. The trial was conducted in accordance the Declaration of Helsinki.
MSBase registry
The MSBase registry (Australian New Zealand Clinical Trials Registry ID: ACTRN12605000455662) is a longitudinal, prospective, international, web-based database collecting standardized clinical outcomes in patients with MS [9]. MSBase was approved by the Melbourne Health Human Research Ethics Committee and by local ethics committees in all participating centers (or exemptions granted, according to local protocols). Written informed consent was provided by patients as required.
Using MSBase, a real-world cohort was created including adults (aged ≥18 years) with PPMS (2005 revised McDonald criteria [11]), a baseline EDSS score of 3.0 to 6.5 (same as ORATORIO), >2 EDSS assessments, and no disease-modifying therapy (DMT) use prior to diagnosis. Kaplan-Meier analyses were used to estimate time to 24-week confirmed EDSS ≥7.0, similar to ORATORIO. Baseline EDSS was the first recorded EDSS in the PPMS untreated state. No imputation was done when EDSS measurements were not available after the initial progression (due to loss to follow-up, death, or analysis data cutoff). Findings from the MSBase cohort were then superimposed onto the Weibull analysis extrapolation from the ORATORIO placebo arm.
Trial registration
The ORATORIO trial was registered in the ClinicalTrials.gov registry (NCT01194570). MSBase registry was registered in the Australian New Zealand Clinical Trials Registry (ACTRN12605000455662).
RESULTS
Patient disposition, demographics, and characteristics
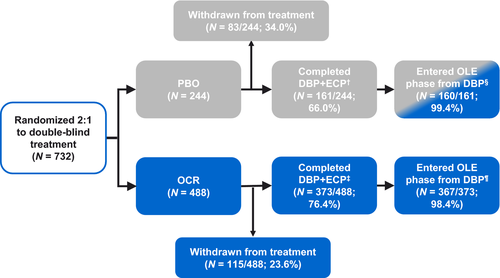
Baseline characteristics from the ORATORIO DBP (n = 732) have been reported previously [8] and are summarized in Table 1. Baseline characteristics were consistent between groups, with a median EDSS of 4.5. All patients (n = 775) in MSBase were treatment-naïve at baseline and remained untreated for most of the study. Baseline characteristics were similar to ORATORIO DBP, with a median EDSS of 4.5. Patient disposition is shown in Figure 1. In the ocrelizumab arm, 98.4% of patients who completed the DBP+ECP of ORATORIO transitioned into the OLE phase (n/N = 367/373; this represents 75.2% of those initially randomized (n/N = 367/488).
| Parameter | ORATORIO ITT | MSBase PPMS real-world cohort, n = 775a | |
|---|---|---|---|
| OCR 600 mg, n = 488 | Placebo, n = 244 | ||
| Age, mean (SD), years | 44.7 (7.9) | 44.4 (8.3) | 43.4 (10.1) |
| Female, n (%) | 237 (48.6) | 124 (50.8) | 437 (56.4) |
| Time since MS symptom onset, median (IQR), years | 6.0 (3.8–8.7) | 5.5 (3.3–8.3) | 5.8 (3.0–10.8) |
| Time since MS diagnosis, median (IQR), years | 1.6 (0.5–4.1) | 1.3 (0.5–3.9) | 0.4 (0–3.9) |
| Score on first eligible EDSS, median (IQR) | 4.5 (3.5–6.0)b | 4.5 (3.5–6.0)b | 4.5 (3.5–6.0)c |
| Distribution of baseline EDSS scores, n (%) | |||
| 2.5 | 1 (0.2) | 1 (0.4) | 0 |
| 3.0 | 41 (8.4) | 19 (7.8) | 111 (14.3) |
| 3.5 | 88 (18.0) | 46 (18.9) | 84 (10.8) |
| 4.0 | 88 (18.0) | 43 (17.6) | 149 (19.2) |
| 4.5 | 44 (9.0) | 25 (10.2) | 63 (8.1) |
| 5.0 | 26 (5.3) | 16 (6.6) | 62 (8.0) |
| 5.5 | 60 (12.3) | 13 (5.3) | 48 (6.2) |
| 6.0 | 76 (15.6) | 56 (23.0) | 141 (18.2) |
| 6.5 | 62 (12.7) | 25 (10.2) | 117 (15.1) |
| 7.0 | 1 (0.2) | 0 | 0 |
| DMT exposure, n (%) | |||
| Ever exposed in 2 years prebaseline | 55 (11.3) | 30 (12.3) | 0 (0) |
| Never exposed in 2 years prebaseline | 433 (88.7) | 214 (87.7) | 775 (100) |
| Time on DMT, mean (SD), yearsd | N/A | N/A | 0.19 (1.04) |
| Time between consecutive EDSS visits, months | |||
| Mean (SD) | 3.0e (N/A) | 3.0e (N/A) | 11.0 (23.9) |
| Median (IQR) | N/A | N/A | 6.8 (3.9–12.2) |
- Abbreviations: DMT, disease-modifying treatment; EDSS, Expanded Disability Status Scale; IQR, interquartile range; ITT, intent to treat; MS, multiple sclerosis; N/A, not applicable; OCR, ocrelizumab; PPMS, primary progressive multiple sclerosis.
- a Based on the MSBase registry definition of PPMS. Patient characteristics described at first recorded EDSS visit after PPMS diagnosis.
- b The baseline EDSS value is the average score of the EDSS assessment at screening and Day 1 visit up to and including the date of randomization. If one of the values is missing, the non-missing values will be used as baseline.
- c Baseline EDSS was the first recorded EDSS in the PPMS untreated state.
- d Between first and last recorded EDSS visits.
- e As per schedule of assessments.
Time to 24-week confirmed EDSS ≥7.0 during the DBP+ECP
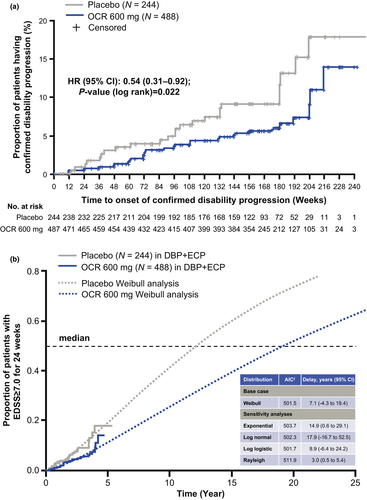
In the ORATORIO DBP+ECP, ocrelizumab reduced the risk of 24-week confirmed EDSS ≥7.0 versus placebo by 46% (HR = 0.54, 95% CI: 0.31–0.92; p = 0.02; Figure 2a).
Extrapolation of time to 24-week confirmed EDSS ≥7.0 from the DBP+ECP
In the ORATORIO DBP+ECP, the extrapolated median time to 24-week confirmed EDSS ≥7.0 was 12.1 years for placebo and 19.2 years for ocrelizumab (Figure 2b). Similar to the placebo arm, the observed median time to 24-week confirmed EDSS ≥7.0 in real-world patients with PPMS from MSBase was 12.4 years (Figure 3). Extending the observation period for time to 24-week confirmed EDSS ≥7.0 in the ocrelizumab arm to include the OLE period of ORATORIO supports the extrapolation from the ORATORIO DBP+ECP for the ocrelizumab group (Figure 3). By comparing the extrapolated median time to 24-week confirmed EDSS ≥7.0 in the ORATORIO DBP+ECP for the placebo and ocrelizumab arms, ocrelizumab is predicted to delay 24-week confirmed EDSS ≥7.0 by 7.1 years (95% CI: −4.3 to 18.4) versus placebo. Sensitivity analyses performed without imputation indicated a similar delay of 7.5 years (data not shown).
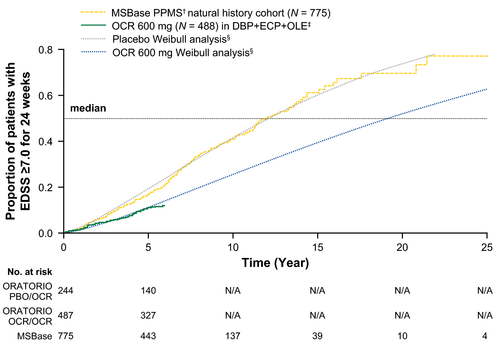
Time to 24-week confirmed EDSS ≥7.0 in MSBase† and the ORATORIO DBP+ECP+OLE,‡ superimposed onto extrapolated results. Patients with a post-baseline EDSS ≥7.0, which is sustained for at least 24 weeks, are considered as having an event. Patients with EDSS ≥7.0 at the time of treatment discontinuation with no further EDSS score are imputed as having an event in ORATORIO but are not imputed in MSBase. †Patients with PPMS with baseline EDSS 3.0–6.5 (N = 775). ‡Data presented up to 312 weeks; OLE data cutoff from January 2019. In ORATORIO, patients with missing baseline EDSS (n = 1) were excluded from the analysis. §Results extrapolated from the DBP+ECP, data cutoff from February 2017.
DBP, double-blind period; ECP, extended controlled period; EDSS, Expanded Disability Status Scale; N/A, not applicable; OCR, ocrelizumab; OLE, open-label extension; PBO, placebo; PPMS, primary progressive multiple sclerosis
DISCUSSION
During the DBP and ECP of the ORATORIO trial, ocrelizumab significantly reduced the risk of requiring a wheelchair by 46% versus placebo. Extrapolated data from the DBP+ECP suggest ocrelizumab treatment delays the median time to requiring a wheelchair by 7.1 years versus placebo, indicating a meaningful long-term benefit for patients with PPMS. Real-world data from the MSBase registry show a similar disability trajectory to that extrapolated from placebo-treated patients in ORATORIO, whereas initial data from the ocrelizumab arm of the ORATORIO OLE period show a similar trajectory to the extrapolation from ocrelizumab-treated patients in the DBP+ECP, supporting the plausibility of these extrapolations. Time to requiring a wheelchair is a well-defined outcome that allows comparisons to be made across studies and across cohorts. Furthermore, it is a clinically meaningful outcome for patients; high levels of disability are associated with a number of physical, emotional, and financial challenges, and an overall reduced quality of life [4, 13, 14]. Delaying time to EDSS ≥7.0 may alleviate some of these burdens. However, it is important to note that due to the restricted eligibility criteria for ORATORIO, patients were generally younger, had a shorter disease duration, and had lower levels of disability than a typical population; therefore, it may be difficult to generalize these results to a broad real-world PPMS population.
One study limitation is that the extrapolations from the ORATORIO DBP+ECP are based on relatively short observed periods, limiting the precision of the long-term extrapolation. Furthermore, the extrapolations are based on the assumption that the risk of disability progression is linear over time, which may not be the case; the disease course of MS is unpredictable and variable across individuals. However, as the study is based specifically on patients with PPMS, it is likely that the disease progression, through the accumulation of clinical deficit and disability, occurs steadily over time, characteristic of a linear model.
An additional limitation is that the data include the ECP of ORATORIO, in which gradual unblinding occurred; therefore, there may have been some bias introduced, for example informative censoring, causing results to be confounded. We cannot exclude the possibility that those patients who discontinued ocrelizumab had a more severe disease course, and thus, the median time to reach EDSS ≥7.0 in ocrelizumab-treated patients may be overestimated. However, 98.4% of patients in the ocrelizumab arm who completed the DBP+ECP of ORATORIO transitioned into the OLE period. This represents 75.2% of those initially randomized (Figure 1), and the low attrition rates in patients who completed the DBP reduces the possibility of selection bias.
Another limitation of this study is that the ORATORIO and MSBase populations could not be directly matched for baseline covariate differences. The MSBase cohort was instead restricted to patients with the same EDSS eligibility criteria as ORATORIO. As a result, the observed disease progression trajectories to EDSS ≥7.0 in MSBase may be affected by potential imbalances in some baseline characteristics between the real-life MSBase cohort and the ORATORIO trial population. However, the populations were comparable for demographics, time since MS symptom onset, and median baseline EDSS scores. The distribution of EDSS 6.0 to 6.5 at baseline was similar between all groups (28.3% in ocrelizumab, 33.2% in placebo, 33.3% in MSBase), further suggesting that the cohorts had similar disability levels at baseline. Additionally, as there are currently no other treatments approved for PPMS, the comparison between the placebo arm and the untreated real-world MSBase cohort remains relevant. Some patients in the MSBase cohort did not remain DMT-free after the baseline EDSS assessment. However, off-label DMT usage remained low, with only 49/775 patients (6.3%) initiating DMTs and a mean of 0.19 years of DMT use between the first and last recorded EDSS visits. Additionally, off-label DMT use was not found to be effective in MSBase [15].
Ocrelizumab significantly delayed the time to requiring a wheelchair in the DBP and ECP of ORATORIO, and the extrapolated median time to reach this disability milestone for the placebo group was similar to that observed in a real-world MSBase cohort. Overall, the effect of ocrelizumab in significantly delaying the time to requiring a wheelchair likely translates to a meaningful long-term benefit for patients with PPMS.
ACKNOWLEDGMENTS
Eleanor Foy (Articulate Science, UK) drafted the manuscript based on input from the authors, funded by F. Hoffmann–La Roche Ltd. The authors had full editorial control of the manuscript and provided final approval. The authors thank all patients, their families, and the investigators who participated in the ORATORIO trial and MSBase registry.
CONFLICT OF INTERESTS
H.B.'s institution (Monash University) has received funding from Biogen, F. Hoffmann-La Roche Ltd., Merck. and Novartis; has carried out contracted research for Novartis, Merck, F. Hoffmann-La Roche Ltd., and Biogen; has taken part in speakers' bureaus for Biogen, Genzyme, F. Hoffmann-La Roche Ltd., and Merck; and has received personal grants from Oxford PharmaGenesis and Biogen (prior to June 30, 2018). T.S. has received compensation for serving on steering committees and advisory boards from Biogen. D.H. received compensation for travel, speaker honoraria. and consultant fees from Biogen, Novartis, Merck, Bayer, Sanofi, Roche, and Teva; as well as support for research activities from Biogen. She was also supported by the Czech Ministry of Education project Progress Q27/LF1. S.H. has received previous travel/conference support and speaking honoraria from Biogen, Sanofi-Genzyme, Merck, Novartis, and Roche. C.S. has received honoraria/research support from Biogen, Merck Serono, Novartis, Roche, Almirall, Amgen, and Teva; and has been supported by the Italian MS Foundation. G.I. has received personal fees from Bayer, Biogen-IDec, Novartis, Sanofi, Merck Serono, Roche, Actelion, Celgene, and Teva. E.K.H. has received honoraria/research support from Biogen, F. Hoffmann-La Roche Ltd., Merck Serono, Novartis, Sanofi Genzyme, and Teva; has served on advisory boards for Actelion, Biogen, Celgene, Merck Serono, Novartis, and Sanofi Genzyme; and has been supported by the Czech Ministry of Education project Progress Q27/LF1. F.G. received honoraria or research funding from Biogen, Genzyme, Novartis, Teva Neurosciences, Mitsubishi, and ONO Pharmaceuticals. A.P. does not declare any competing interests. M.G. received consulting fees from Teva Canada Innovation, Biogen, Novartis, and Genzyme Sanofi; lecture payments from Teva Canada Innovation, Novartis, and EMD; and has also received a research grant from Canadian Institutes of Health Research. R.H. received research grants from Merck, Biogen, and Sanofi; and received honoraria from Merck, Roche, and Sanofi for invited speaker's activities. M.O. does not declare any competing interests. A.L. has received personal compensation for consulting, serving on a scientific advisory board, speaking or other activities from Biogen, Merck Serono, Mylan, Novartis, Roche, Sanofi/Genzyme, and Teva. Her institutions have received research grants from Novartis (in the last 4 years). B.T. received funding for travel and speaker honoraria from Bayer Schering Pharma, CSL Australia, Biogen, and Novartis; and has served on advisory boards for Biogen, Novartis, Roche, and CSL Australia. G.G. received personal compensation in the past for serving as a consultant for AbbVie, Actelion, Atara Bio, Biogen, Celgene, Sanofi Genzyme, Genentech, GlaxoSmithKline, Merck Serono, Novartis, Roche, and Teva; has received personal compensation from Elsevier for serving as an editor on Multiple Sclerosis and Related Disorders; and has received financial support for research activities from Biogen, Roche, Merck, Merck Serono, Novartis, Sanofi Genzyme, and Takeda. L.K.'s institution (University Hospital Basel) received in the last 3 years and used exclusively for research support at the department: steering committee, advisory board, and consultancy fees from Actelion, Alkermes, Almirall, Bayer, Biogen, Celgene/Receptos, df-mp, Excemed, GeNeuro SA, Genzyme, Japan Tobacco, Merck, Minoryx, Mitsubishi Pharma, Novartis, F. Hoffmann-La Roche Ltd., Sanofi-Aventis, Santhera, Teva, and Vianex; and license fees for Neurostatus-UHB products; the Research of the MS Center in Basel has been supported by grants from Bayer, Biogen, Novartis, the Swiss MS Society, the Swiss National Research Foundation, Innoswiss, the European Union, and Roche Research Foundations. S.L.H. serves on the board of trustees for Neurona and on scientific advisory boards for Alector, Annexon, Bionure, and Molecular Stethoscope; and has received travel reimbursement and writing assistance from F. Hoffmann-La Roche Ltd. and Novartis for CD20-related meetings and presentations. X.M. has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Actelion, Alexion, Bayer, Biogen, Celgene, EMD Serono, Genzyme, Immunic, Medday, Merck, Mylan, Nervgen, Novartis, Roche, Sanofi-Genzyme, Teva Pharmaceutical, TG Therapeutics, Excemed, MSIF, and NMSS. L.C. is an employee of F. Hoffmann-La Roche Ltd. R.F. is an employee of F. Hoffmann-La Roche Ltd. F.M. is an employee and shareholder of F. Hoffmann-La Roche Ltd. J.O. is currently an employee and shareholder of F. Hoffmann-La Roche Ltd. During his previous employment he received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Teva, Biogen, Celgene, EMD Serono, MedDay, Novartis, Roche, Sanofi Genzyme, WebMD Global, and Allergan. His research and department were supported by grants from Sanofi Genzyme, Biogen, Novartis, and Roche. E.M.-L.R. is an employee and shareholder of F. Hoffmann-La Roche Ltd. A.S. is an employee and shareholder of F. Hoffmann-La Roche Ltd. Q.W. is an employee of F. Hoffmann-La Roche Ltd. D.W. is an employee of Novartis and shareholder of F. Hoffmann-La Roche Ltd. and Novartis. J.S.W. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Acorda Therapeutics, Alkermes, Brainstorm Cell Therapeutics, EMD Serono, GeNeuro, GW Pharma Ltd., MedDay Pharmaceuticals, NervGen Pharma Corp, Novartis, Roche/Genentech, and Sanofi Genzyme; royalties are received for out-licensed monoclonal antibodies through UTHealth from Millipore Corporation.
AUTHOR CONTRIBUTIONS
Helmut Butzkueven: Conceptualization (lead); investigation (equal); methodology (lead); validation (lead); writing–original draft (equal); writing–review & editing (equal). Tim Spelman: Conceptualization (equal); formal analysis (lead); methodology (equal); validation (equal); writing–original draft (equal); writing–review & editing (equal). Dana Horakova: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Stella Hughes: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Claudio Solaro: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Guillermo Izquierdo: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Eva Kubala Havrdova: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Francois Grand'Maison: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Alexandre Prat: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Marc Girard: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Raymond Hupperts: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Marco Onofrj: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Alessandra Lugaresi: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Bruce Taylor: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Gavin Giovannoni: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Ludwig Kappos: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Stephen L. Hauser: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Xavier Montalban: Conceptualization (supporting); investigation (equal); methodology (supporting); validation (equal); writing–review & editing (equal). Licinio Craveiro: Conceptualization (supporting); methodology (equal); validation (equal); writing–original draft (equal); writing–review & editing (equal). Rita Freitas: Conceptualization (lead); methodology (lead); validation (equal); writing–review & editing (equal). Fabian Model: Conceptualization (lead); formal analysis (equal); methodology (lead); validation (lead); writing–original draft (lead); writing–review & editing (lead). James Overell: Conceptualization (supporting); methodology (equal); validation (equal); writing–original draft (equal); writing–review & editing (equal). Erwan Muros-Le Rouzic: Conceptualization (supporting); methodology (equal); validation (equal); writing–original draft (lead); writing–review & editing (lead). Annette Sauter: Conceptualization (equal); formal analysis (equal); methodology (equal); validation (equal); writing–original draft (equal); writing–review & editing (equal). Qing Wang: Conceptualization (equal); formal analysis (lead); methodology (lead); validation (lead); writing–original draft (lead); writing–review & editing (lead). David Wormser: Conceptualization (lead); methodology (lead); validation (equal); writing–review & editing (equal). Jerry S. Wolinsky: Conceptualization (lead); investigation (equal); methodology (equal); validation (equal); writing–review & editing (equal).
STATISTICAL ANALYSIS
ORATORIO statistical analyses were conducted by Qing Wang (F. Hoffmann-La Roche Ltd., Basel, Switzerland); MSBase statistical analyses were conducted by Tim Spelman (Department of Medicine and Melbourne Brain Centre, The Royal Melbourne Hospital, University of Melbourne, Melbourne, Australia).
Open Research
DATA AVAILABILITY STATEMENT
For ORATORIO, qualified researchers can request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here: https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm. For MSBase, data are available from individual investigators upon request to the corresponding author.




