First symptom guides diagnosis and prognosis in neurodegenerative diseases—a retrospective study of autopsy proven cases
Funding information
This work was funded by the German Center for Neurodegenerative Diseases (DZNE) and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy—ID 390857198).
Abstract
Background and purpose
Clinical diagnostic criteria for neurodegenerative diseases have been framed based on clinical phenomenology. However, systematic knowledge about the first reported clinical symptoms in neurodegenerative diseases is lacking. Therefore, the aim was to determine the prevalence and clinical implications of the first clinical symptom (FS) as assessed by medical history in neuropathologically proven neurodegenerative diseases.
Methods
Neuropathological diagnoses from the Neurobiobank Munich, Germany, were matched with clinical records for analyses of the diagnostic and prognostic values of FSs.
Results
In all, 301 patients with the neuropathological diagnoses Alzheimer disease (AD), progressive supranuclear palsy (PSP), frontotemporal lobar degeneration (FTLD), Lewy body disease (LBD) including the neuropathologically indistinguishable clinical phenotypes Parkinson disease and dementia with Lewy bodies, multiple system atrophy (MSA) and corticobasal degeneration (CBD) were studied. Memory disturbance was the most common FS in AD (34%), FTLD (19%) and LBD (26%), gait disturbance in PSP (35%) and MSA (27%) and aphasia and personality changes in CBD (20%, respectively). In a model adjusting for prevalence in the general population, AD was predicted by memory disturbance in 79.0%, aphasia in 97.2%, personality changes in 96.0% and by cognitive disturbance in 99.0%. Gait disturbance and tremor predicted LBD in 54.6% and 97.3%, coordination disturbance MSA in 59.4% and dysarthria FTLD in 73.0%. Cognitive FSs were associated with longer survival in AD (12.0 vs. 5.3 years; p < 0.001) and FTLD (8.2 vs. 4.1 years; p = 0.005) and motor FSs with shorter survival in PSP (7.2 vs. 9.7; p = 0.048).
Conclusions
Assessing FSs in neurodegenerative diseases may be beneficial for accuracy of diagnosis and prognosis and thereby may improve clinical care and precision of study recruitment.
INTRODUCTION
Neuropathological examination is the gold standard for diagnosis of neurodegenerative diseases, despite recent advances in the development of in vivo biomarkers [1-4]. This applies for the common neurodegenerative diseases such as Alzheimer’s disease (AD) [3, 5], the neuropathologically defined entity Lewy body disease (LBD) including the neuropathologically indistinguishable clinical diagnoses Parkinson disease (PD) [6, 7] and dementia with Lewy bodies (DLB) [8], as well as for the rarer conditions progressive supranuclear palsy (PSP) [9], frontotemporal lobar degeneration (FTLD) [10-12], multiple system atrophy (MSA) [13] and corticobasal degeneration (CBD) [14]. In clinical settings, the most prominent symptom in early disease stages serves as the guiding symptom in most cases, since several cognitive, behavioral and motor symptoms are present at later stages of neurodegenerative diseases, complicating clinical diagnosing [15, 16].
Whereas there is extensive literature about clinical manifestations of neurodegenerative diseases [3, 6, 8-11, 13, 14, 17, 47, 48], to our knowledge the first evident clinical symptom of the aforementioned diseases has not been systematically assessed to date. In addition, evidence with respect to associations between the first symptom and the course of neurodegenerative diseases is lacking. Hence, the intention was to investigate the distribution of first symptoms in patients with neuropathologically confirmed neurodegenerative diseases. Clinical diagnostic criteria are overlapping and do not allow for an exclusive diagnosis in several cases, in particular in advanced disease stages when several symptoms that are common in different neurodegenerative diseases are present. Therefore, the aim was to analyze a potential added differential diagnostic value of the first clinical symptom. Further, it was intended to investigate associations between the first symptom and survival.
METHODS
Participants
Data from the Neurobiobank Munich (NBM) located at the Center for Neuropathology and Prion Research and from clinical records from the hospital of the Ludwig-Maximilians-Universität München, Munich, and other academic hospitals in Germany were used for this study. All participants with sufficient clinical data who underwent autopsy between 1985 and 2017 and had a neuropathological diagnosis of AD, LBD, PSP (FTLD-tau PSP), FTLD (FTLD-TDP, FTLD-UPS, aFTLD-U, FTLD-IF, BIBD, FTLD-tau PiD, FTLD-ni), MSA or CBD (FTLD-tau CBD [18]) were included. The paper refers to the neuropathologically defined entity Lewy body disease (LBD), which is the corresponding neuropathological diagnosis of both the clinical diagnosis Parkinson disease (PD) and the clinical diagnosis dementia with Lewy bodies (DLB). The neuropathological examination cannot distinguish between the clinical phenotypes PD and DLB.
The study was approved by the Institutional Review Board of the Ludwig-Maximilians-Universität München, Munich, Germany. All NBM cases were collected according to the guidelines of the local ethics committee. The study conforms with the World Medical Association Declaration of Helsinki.
Neuropathological procedures
Brain banking was performed according to established procedures using a standardized protocol [19]. All neuropathological diagnoses were reviewed and confirmed or revised by two senior neuropathologists experienced in neurodegenerative diseases in consensus according to current neuropathological diagnostic criteria [5, 21]. If more than one disease-specific neuropathological change was present, current neuropathological diagnostic criteria and preponderance of pathology determined diagnosis. Neuropathological diagnosis, date of birth, date of death, age at death and sex were transferred to a standardized template.
Clinical evaluation
First clinical symptom and time of onset of the first clinical symptom were extracted from digital and paper medical records and entered in the standardized template. Assessment of the first symptom was based on medical history including third-party information (i.e., relatives, caregivers) as provided in clinical records. Thus, evaluation was based on patient or third-party reported symptoms during a formal clinician-led assessment in the scope of a clinical presentation. For determination of the first symptom and the time of its onset information from medical histories in all available clinical records was taken into account and integrated. The time of onset of the first symptom was defined as the time when the first persistent symptom that was attributed to the disease by the respective clinicians occurred. Speech problems are referred to as dysarthria and language problems as aphasia in the paper. Clinical data were discussed and assessed in consensus by two senior neurologists experienced in the clinical care of neurodegenerative diseases (JV, JL). JV and JL were blinded to neuropathological diagnoses.
Data processing and statistical analyses
A template that merged neuropathological and clinical data was created using FileMaker Pro (Filemaker Inc.). Clinical and demographic features of the study population were analyzed and compared between subgroups using Pearson's chi-squared test for categorical variables and one-way analysis of variance for continuous variables. The Games–Howell test was used for post hoc analysis. The distribution of first symptoms in every neurodegenerative disease was analyzed. Further, the distribution of neurodegenerative diseases for each first clinical symptom was investigated for symptoms with n > 10 in the study population after random matching and adjusting for prevalence of neurodegenerative diseases in the general population. Prevalences in the general population were estimated after a systematic literature search in PubMed (AD 1.45% [22], PSP 0.005% [23-26], FTLD 0.0185% [27], LBD 0.375% [28, 29], MSA 0.0035% [26, 30], CBD 0.006% [31]). To evaluate the differential diagnostic performance of the first clinical symptom for clinical settings with indecision of clinicians between two diseases, the prevalence of first symptoms that occurred n > 10 in the study population was compared pairwise between neurodegenerative diseases using Fisher's exact test or Pearson's chi-squared test, where appropriate. The Benjamini–Hochberg method was applied to adjust for multiple testing. Survival was calculated as the time span between the date of onset of the first clinical symptom and date of death for each individual participant. As numbers for single first clinical symptoms were relatively small, cognitive and motor symptoms were summarized in supergroups. The association of the presence or absence of cognitive or motor symptoms in each neurodegenerative disease with survival was analyzed using Student's t test or the Mann–Whitney U test, where appropriate. For each neurodegenerative disease, the most prevalent supergroup symptom, that is, cognitive symptoms or motor symptoms, was investigated. p values below 0.05 were considered statistically significant. All tests were performed two-sided. The Statistical Package for the Social Sciences (IBM SPSS Statistics, version 25) was used for statistical analysis.
Data availability statement
Data are available upon request.
RESULTS
Participants
The NBM comprised 978 cases at the time of analyses. Of these, 301 individuals with the neuropathological diagnoses AD, PSP, FTLD, LBD (which includes the clinical phenotypes of PD and DLB), MSA or CBD and sufficient medical history data on first symptoms were identified and included in the study. The remaining NBM cases had either a neuropathological diagnosis other than AD, PSP, FTLD, LBD, MSA or CBD, or sufficient clinical information to determine the first clinical symptom was not available. 89 (29.6%) of the included cases had AD, 72 (23.9%) PSP, 47 (15.6%) FTLD, 47 (15.6%) LBD, 26 (8.6%) MSA and 20 (6.6%) CBD. Age at death and survival differed significantly amongst the neurodegenerative diseases. Further details of study population characteristics are provided in Table 1.
| AD (n = 89) | PSP (n = 72) | FTLD (n = 47) | LBD (n = 47) | MSA (n = 26) | CBD (n = 20) | Total (n = 301) | p value | |
|---|---|---|---|---|---|---|---|---|
| Female sex, n (%) | 48 (54.5) | 32 (44.4) | 18 (39.1) | 17 (36.2) | 13 (50.0) | 9 (45.0) | 137 (45.8) | 0.35 |
| Mean age at onset ± SD, years | 63.0 (13.1) | 63.9 (7.6) | 57.9 (12.9) | 63.9 (13.3) | 58.4 (10.5) | 60.1 (7.7) | 62.0 (11.7) | 0.030 1 |
| Mean age at death (SD), years | 74.1 (12.7) | 71.9 (6.2) | 64.0 (11.3) | 77.5 (7.2) | 66.1 (9.2) | 66.5 (8.0) | 71.4 (10.8) | <0.0012 |
| Mean survival (SD), years | 10.9 (7.3) | 8.2 (4.5) | 6.4 (6.6) | 13.8 (11.6) | 7.8 (3.4) | 6.6 (4.1) | 9.5 (7.5) | <0.0013 |
Notes
- Categorical variables were compared between groups using Pearson's chi-squared test and continuous variables using one-way analysis of variance. p values below 0.05 were considered statistically significant and are italicized. For statistically significant between-groups differences the Games–Howell test was performed for pairwise comparisons of differences in means.
- Abbreviations: AD, Alzheimer disease; CBD, corticobasal degeneration; FTLD, frontotemporal lobar degeneration; LBD, Lewy body disease; MSA, multiple system atrophy; PSP, progressive supranuclear palsy.
- 1 No statistically significant differences were found in post hoc pairwise comparisons.
- 2 Mean age of death was statistically significantly greater (i) in AD compared to FTLD, MSA and CBD, (ii) in PSP compared to FTLD and (iii) in LBD compared to PSP, FTLD, MSA and CBD.
- 3 Mean survival was statistically significantly longer (i) in AD compared to PSP, FTLD, MSA and CBD and (ii) in LBD compared to PSP, FTLD, MSA and CBD. There was no statistically significant difference in age at death or survival between AD and LBD.
- LBD refers to the neuropathologically defined entity Lewy body disease that includes the neuropathologically indistinguishable clinical phenotypes of Parkinson disease and dementia with Lewy bodies.
Distribution of first clinical symptoms in neurodegenerative diseases
Thirty-seven first clinical symptoms were extracted from medical records. The detailed distribution of first symptoms of the neurodegenerative diseases studied here is depicted in Figure 1 and Table S1. Memory disturbance was the most common first symptom of patients with AD, LBD and FTLD. Patients with PSP and MSA most frequently presented gait disturbance first. Individuals suffering from CBD most commonly presented equally often with aphasia and personality changes first. There was no statistically significant difference in frequency of the first symptom gait disturbance between the MSA cases with cerebellar (n = 9) and parkinsonian (n = 15) predominance types, as gait disturbance occurred in 2/9 (22.2%) patients with cerebellar and in 4/15 (26.7%) cases with a parkinsonian predominance type (p = 1.0; Fisher's exact test).
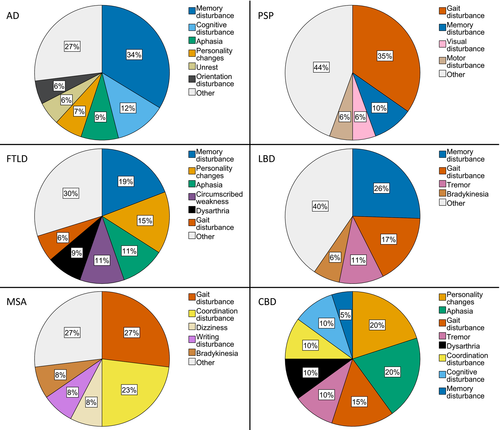
Probabilities for individual neurodegenerative diseases by first clinical symptom in the general population
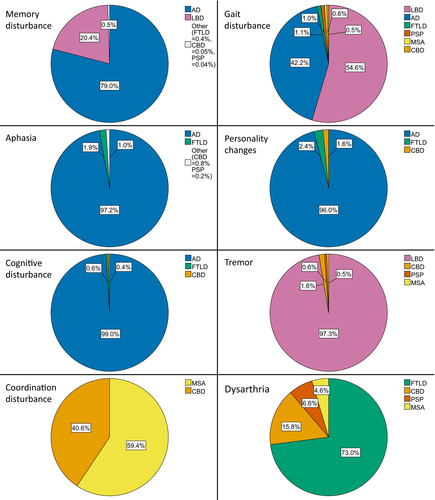
A comprehensive overview of neurodegenerative diagnoses that are predicted by the prevalence adjusted model to follow individual first clinical symptoms in the general population is given in Figure 2. Based on this model, which stipulates that one of the neurodegenerative diseases studied here is the neuropathological diagnosis, the first symptoms memory disturbance, aphasia, personality changes and cognitive disturbance led by far the most frequently to an AD diagnosis in the general population. Gait disturbance and tremor as first symptoms are predicted to be most frequently followed by an LBD diagnosis. Coordination disturbance most commonly precedes MSA and dysarthria FTLD.
Diagnostic accuracy of first symptoms in the study population
Orientation disturbance occurred solely in AD patients as the first clinical symptom (in 5.6%). This results in a 100% specificity and 100% positive predictive value regarding a neuropathological diagnosis of AD in this study. Sensitivity was low (5.6%). The first symptom circumscribed weakness was only present in FTLD patients (in 10.6%) resulting in a 100% specificity and 100% positive predictive value with respect to a neuropathological diagnosis of FTLD. Sensitivity was 10.6%. Dizziness as the first symptom occurred exclusively in PSP (in 4.2%) and MSA (in 7.7%). This leads to a 100% specificity and 100% positive predictive value of dizziness regarding diagnoses of PSP or MSA in this population. Sensitivity was low (5.1%).
Differential diagnostic performance of first clinical symptoms in pairwise comparisons
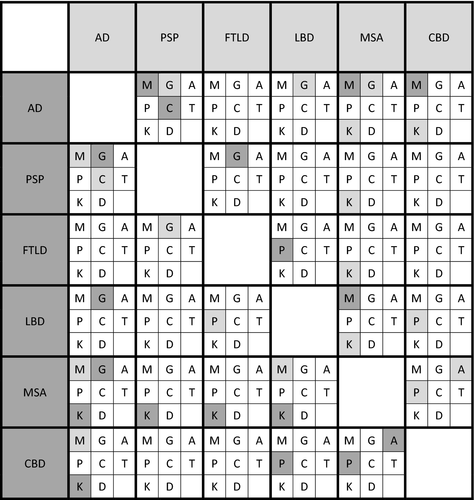
The first symptoms memory disturbance, gait disturbance, aphasia, personality changes, cognitive disturbance and coordination disturbance could distinguish between several neurodegenerative diseases in pairwise comparisons. Tremor and dysarthria as first clinical symptoms did not allow for distinguishing between any neurodegenerative diseases. Details with respect to the differential diagnostic performance of first symptoms in pairwise comparisons are given in Figure 3.
First clinical symptom and survival
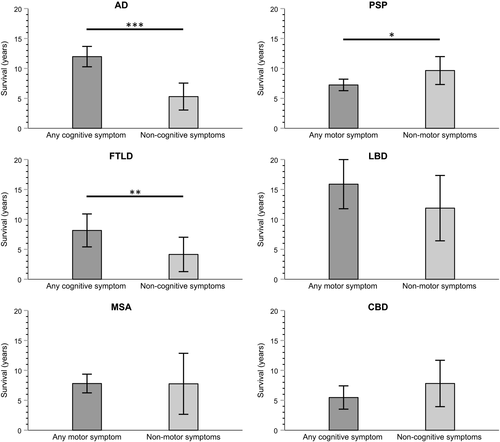
Alzheimer disease patients who showed any cognitive symptom as the earliest symptom (n = 74; 84.1%) had a significantly longer survival compared to AD patients with non-cognitive first symptoms (12.0 vs. 5.3 years; p < 0.001). In PSP, any motor symptom as the clinical first symptom (n = 44; 62.0%) was associated with a shorter survival (7.2 vs. 9.7 years in patients with non-motor first symptoms; p = 0.048). FTLD patients who had any cognitive symptom as the first symptom (n = 25; 56.8%) exhibited a longer survival compared to patients with non-cognitive first symptoms (8.2 vs. 4.1 years; p = 0.005). There was no association between the respective most frequent supergroup symptom (i.e., any cognitive symptom or any motor symptom) and survival in LBD, MSA and CBD (Figure 4).
DISCUSSION
In our study cohort of patients with neuropathologically diagnosed neurodegenerative diseases, memory disturbance was the most frequent first clinical symptom in three of six diseases (AD, FTLD and the neuropathologically defined entity LBD [which includes the clinical phenotypes of PD and DLB]) and gait disturbance in two of six diseases (PSP and MSA). Aphasia and personality changes were most common in CBD.
Unexpectedly, memory disturbance was the most common first symptom in FTLD, a disease with the cardinal symptoms language and behavioral disturbance, whose current diagnostic criteria [10, 11] do not involve an early memory predominant phenotype and in the case of primary progressive aphasia even consider it an exclusionary criterion [10]. Surprisingly memory disturbance was the second most frequent clinical first symptom in PSP. Although PSP is known to cause a variety of phenotypes including cognitive phenotypes, a memory predominant phenotype is not represented in current PSP criteria [9] and was not described in two larger series of neuropathologically confirmed PSP cases [32, 33]. This suggests an added value of assessing the first clinical symptom beyond general clinical presentation and may inspire a very early memory predominant phenotype to be discussed for the diagnostic criteria of FTLD and PSP.
In the model adjusted for prevalences of neurodegenerative diseases in the general population, aphasia as the first clinical symptom is predicted to be by far the most frequent followed by a neuropathological diagnosis of AD. This finding highlights the importance of an atypical AD presentation with a primary progressive aphasia phenotype as a differential diagnosis for an underlying AD pathology [10]. Further, personality changes as the first clinical symptom is predicted to lead substantially more often to a diagnosis of AD than to a diagnosis FTLD that includes a behavioral variant [11]. This seems to render the cardinal symptoms of FTLD, aphasia and behavioral symptoms, not suitable as first clinical symptoms to distinguish between AD and FTLD. This assumption is corroborated by the pairwise comparisons of this study (Figure 3) and emphasizes the need for thorough neuropsychological testing and biomarker evaluation for establishing a diagnosis.
Gait disturbance as the first symptom was predicted to be followed by an AD diagnosis in a relevant percentage whereas tremor was not predicted to be followed by a neuropathological diagnosis of AD in the prevalence adjusted model (Figure 2). This finding is in accordance with a recent study that revealed a bradykinetic profile of motor symptoms in AD [16].
Despite emerging evidence that cognitive disturbance is an integral part of MSA [34], only 4% of MSA patients showed a cognitive symptom, concentration disturbance, as the first clinical symptom. MSA stood out to have by far the smallest portion of cognitive symptoms as the first clinical symptom (Figure 1). In this study, the most common cognitive first symptoms memory disturbance, aphasia and personality changes were never followed by a neuropathological diagnosis of MSA.
Psychosis including hallucinations was very rare and sleep disturbance absent as the first symptom in LBD in this study. This is remarkable, as these symptoms are core clinical features of DLB, besides Parkinson disease one of the two principal phenotypes of LBD, as defined by current diagnostic criteria [8]. Also remarkable, the core clinical features of CBD [14]/CBS (corticobasal syndrome) [9] apraxia, alien limb phenomena, myoclonus and dystonia, did not occur as first clinical symptom in CBD in the current study (Figure 1). These findings may emphasize an added benefit of assessing the first clinical symptom and suggest the use of the first symptom to support differential diagnostic thinking in addition to established diagnostic criteria.
The first symptom orientation disturbance featured a very high accuracy to predict AD, circumscribed weakness to predict FTLD and dizziness to predict PSP or MSA in our study. These symptoms line up with other early clinical symptoms that were shown to be highly accurate for disease prediction in AD: seizures and dysdiadochokinesia [16, 35].
For the common constellation that clinicians hover between two neurodegenerative diagnoses the pairwise comparison of neurodegenerative diseases with respect to the first clinical symptom performed in this study may facilitate accurate diagnosis.
For example, although cognitive phenotypes of PSP and CBD are common [9, 14], memory and cognitive disturbance as the first symptom allowed for the differentiation between AD and PSP and memory disturbance between AD and CBD.
Further, gait disturbance as the first symptom enabled AD to be distinguished from all neurodegenerative diseases with primary motor phenotype (PSP, LBD and MSA) even though motor symptoms are known to be a common and early feature of AD [16, 36]. Whilst motor phenotypes such as amyotrophic lateral sclerosis, CBS and PSP syndromes are common in FTLD [37], gait disturbance as the first symptom allowed for delineation between FTLD and PSP.
Multiple system atrophy could be distinguished from all other diseases except CBD by coordination disturbance as the first symptom. This may reflect the frequent cerebellar phenotype in MSA [13] but also is remarkable as patients may also perceive basal ganglia motor symptoms as coordination problems. The perception of basal ganglia symptoms as coordination disturbance may be the reason that this symptom allows for differentiation between AD and CBD.
Personality changes as first symptom distinguished FTLD from LBD, a differentiation that may be tricky if LBD presents with its primary cognitive phenotype DLB. Additionally, personality changes as the first symptom enabled CBD to be distinguished from LBD and MSA which could be a challenge because of the heterogeneous clinical presentation of CBD [14].
Tremor as the first symptom did not allow for differentiation between any neurodegenerative diseases, interestingly not even between diseases with primary motor manifestation, in particular LBD that includes the clinical phenotype Parkinson disease, and primary cognitive diseases such as AD and FTLD (Figure 3).
Several factors that are associated with survival in neurodegenerative diseases have been identified to date, including genetic factors, demographic data and biomarkers in AD [38, 39] and FTLD [40-42] and genetic factors in PSP [43]. However, to our knowledge there is limited evidence about associations of clinical parameters with survival in neurodegenerative diseases. Clinical parameters bear the advantage of a time- and cost-effective assessment. It is revealed here that any cognitive symptom as the first clinical symptom in AD and FTLD was associated with a longer survival, whereas any motor symptom as the first symptom was associated with a shorter survival in PSP. This knowledge may be useful to estimate prognosis in clinical and research settings by thorough exploration of the first clinical symptom alone.
The strengths of this study are the large size for a clinical-neuropathological correlation study that includes neurodegenerative diseases other than AD, the definite diagnosis of neurodegenerative diseases by neuropathological examination, a comprehensive neuropathological evaluation by two experienced senior neuropathologists in consensus preventing inter-rater variability, the standardized consensual assessment of clinical data, and the standardized neuropathological and clinical evaluation of six common neurodegenerative diseases promoting comparability. Limitations are small numbers in the groups of the rare neurodegenerative diseases MSA and CBD and the retrospective extraction of clinical data from medical records. A prospective investigation including larger numbers for rare neurodegenerative diseases is warranted. In CBD, some of the cases that were attributed the first symptom aphasia could have had speech apraxia which is a common clinical presentation in CBD. A further limitation is that the initial symptoms were not recorded with a standardized questionnaire. Grouping together symptoms into motor and cognitive supergroups could be a potential limitation as specific motor or non-motor features that have previously been shown to be clinical predictors of progression may not be appropriately represented, for example early dysphagia [44] and early autonomic features in PSP [45]. An important question that has to be addressed in future work is whether first symptoms may help to differentiate between specific subtypes of diseases which are challenging to distinguish in the early stages of disease, as has been done recently in a prospective study with small numbers of PSP and CBS patients [46].
In summary, this clinical-neuropathological association study shed light on the distribution of first clinical symptoms in the six frequent neurodegenerative diseases AD, PSP, FTLD, LBD, MSA and CBD. Standardized assessment of the first clinical symptom may facilitate correct diagnosis and prognosis in a time- and cost-effective manner and may prevent patients from more invasive and expensive diagnostic interventions. The clinical implications of first symptoms could be considered in addition to current diagnostic criteria or even inform future revisions. Therefore, awareness of the first clinical symptom in neurodegenerative diseases may improve clinical care and facilitate precise recruitment for clinical studies.
ACKNOWLEDGEMENTS
The authors thank Christian Haass, German Center for Neurodegenerative Diseases (DZNE), Munich, Germany, for proofreading the paper and for providing valuable input. This work was funded by the German Center for Neurodegenerative Diseases (DZNE) and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy—ID 390857198). The altruism of the study participants and their relatives is gratefully acknowledged. Open Access funding enabled and organized by Projekt DEAL.
WOA Institution: LUDWIG-MAXIMILIANS-UNIVERSITAET MUNCHEN
Blended DEAL: Projekt DEAL
CONFLICTS OF INTEREST
Jonathan Vöglein reports no disclosures. Irena Kostova reports no disclosures. Thomas Arzberger reports no disclosures. Sigrun Roeber reports no disclosures. Peer Schmitz reports no disclosures. Mikael Simons reports no disclosures. Viktoria Ruf reports no disclosures. Otto Windl reports no disclosures. Jochen Herms reports no disclosures. Marianne Dieterich reports no disclosures. Adrian Danek reports no disclosures. Günter Höglinger has ongoing research collaborations with Prothena; served/serves as a scientific advisor for Abbvie, Alzprotect, Asceneuron, Biogen, Biohaven, Lundbeck, Novartis, Roche, Sanofi, UCB. Armin Giese reports no disclosures. Johannes Levin reports personal fees from Aesku, personal fees from Bayer Vital, personal fees from Willi Gross Foundation, personal fees from Axon Neuroscience, personal fees from Ionis Pharmaceuticals, personal fees from Roche, non-financial support from Abbvie, compensation from MODAG GmbH for work as CMO outside the submitted work.
AUTHOR CONTRIBUTIONS
Jonathan Vöglein: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); resources (lead); software (lead); supervision (equal); validation (lead); visualization (lead); writing original draft (lead); writing review and editing (lead). Irena Kostova: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); resources (equal); software (equal); writing review and editing (equal). Thomas Arzberger: Data curation (equal); investigation (equal); methodology (equal); resources (equal); writing review and editing (equal). Sigrun Roeber: Data curation (equal); investigation (equal); resources (equal); writing review and editing (equal). Peer Schmitz: Data curation (equal); methodology (equal); resources (equal); software (equal); writing review and editing (equal). Mikael Simons: Investigation (equal); validation (equal); writing review and editing (equal). Viktoria Ruf: Data curation (equal); investigation (equal); resources (equal); writing review and editing (equal). Otto Windl: Data curation (equal); investigation (equal); resources (equal); writing review and editing (equal). Jochen Herms: Data curation (equal); investigation (equal); resources (equal); writing review and editing (equal). Marianne Dieterich: Investigation (equal); resources (equal); writing review and editing (equal). Adrian Danek: Investigation (equal); resources (equal); writing review and editing (equal). GU Höglinger: Investigation (equal); writing review and editing (equal). Armin Giese: Conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); resources (equal); writing review and editing (equal). Johannes Levin: Conceptualization (lead); data curation (equal); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); resources (lead); software (equal); supervision (lead); validation (lead); visualization (lead); writing review and editing (lead).




